Abstract
Coal gangue is a prevalent solid waste material, and its utilization presents an urgent challenge. This study investigates the impact of incorporating biochar (BC) and polyacrylamide (PAM) into a composite matrix of coal gangue soil (CGS). We conducted incubation experiments to evaluate the physicochemical properties and enzyme activity of CGS with different ratios of BC (1%, 2%, and 5% designated as B1, B2, and B5) and PAM (0.02%, 0.05%, and 0.10% designated as P2, P5, and P10), compared to a control (CK) with no amendments. The results indicate that (1) the lowest bulk density was observed in the B5P10 treatment. The organic carbon content in B5P10 increased by 57.98% compared to the CK. (2) The activities of α-glucosidase, N-acetyl-glucosidase, and alkaline phosphatase in the B5P10 treatment increased by 112.34%, 110.77%, and 52.40%, respectively. The geometric mean values of enzyme activities showed no significant differences among the treatments B2P5, B2P10, B5P2, B5P5, and B5P10, but these were significantly higher than those in the CK. (3) The parameters of pH, active carbon, field capacity, and available phosphorus were identified as the main factors affecting enzyme activity. CGS incorporating 2% BC and 0.05% PAM is recommended for soil reconstruction in mining regions.
Keywords: Biochar, Polyacrylamide, Gangue matrix, Enzyme activity
Subject terms: Forestry, Restoration ecology
Introduction
Large-scale coal mining in northwest China has led to numerous coal gangue dumps. These accumulations occupy extensive land and pose significant environmental challenges, including soil erosion and degradation1, especially in regions with scarce water resources and fragile ecosystems2. To address these challenges, researchers have explored the coal gangue soil (CGS) mixture, which combines coal gangue materials with raw soils such as river sediment and soil parent material. This combination is a promising alternative to natural soil3, enhancing structural stability4, improving nutrient cycling, and optimizing hydrothermal conditions in reconstructed soils, thereby aiding in the ecological restoration of mining-affected areas5. Restoration in arid and semi-arid regions is particularly lengthy and challenging due to the infertile soil conditions. Coal gangue is largely mesoporous, with low specific surface area, limited water-holding capacity, and insufficient available nutrients. Raw soil, on the other hand, often suffers from poor structure and low nutrient content. This necessitates artificial methods to enhance the fertility of CGS and accelerate ecological recovery6. Adding amendments has proven to be an effective management strategy for improving soil quality.
Biochar (BC), derived from various organic materials, is recognized for its high capacity to retain water and exchangeable cations due to its large specific surface area and negative sorption sites. BC has been scientifically validated as an environmentally friendly amendment for reclamation, significantly enhancing soil organic carbon (SOC), total nitrogen (TN), and hydrological properties7,8. Despite its benefits, BC can be dispersed by wind and water, which may limit its long-term retention. In contrast, polyacrylamide (PAM), a synthetic polymer, can effectively mitigate dust dispersion via van der Waals forces, reducing soil particle fluidity9. PAM has also been investigated as a water-saving amendment, enhancing soil water-holding capacity and improving soil structure by promoting clay flocculation, particle binding, and aggregation10,11.
Research highlights the crucial role of soil enzyme activity in biogeochemical reactions, material cycling, and energy flow within soil ecosystems12. Soil hydrolytic enzymes, including β-glucosidase, peptidase, and phosphatases, are essential for organic matter conversion and maintaining carbon and nutrient cycles by participating in mineralization processes7. For example, Oladele et al.13 reported that adding 0.5% BC to sandy clay loam Alfisol significantly increased urease, phosphatase, and catalase activities, enhancing soil nutrient retention. Similarly, Okebalama et al.14 examined the relationship between bioavailability and enzyme activities with varied BC and nutrient additions in farmland. While BC positively influences soil enzyme activity, variability in enzyme response is often attributed to dose-effect relationships15. Cui et al.16 found that adding 1% and 2% BC significantly increased urease and phosphatase activities in saline-alkali soil. Conversely, a 0.15% BC addition to orchard soil decreased enzyme activity17. The effects of PAM on soil properties and function vary. Sadeghi et al.18 demonstrated that adding 0.08% PAM improved soil infiltration. Abu-Hamdeh et al.19 observed that applying PAM at 0.08% increased organic matter and total porosity in chisel-plowed soil. However, Watson et al.20 found that a 0.06% PAM application reduced microbial biomass carbon in incubation trials.
The expense associated with foreign soil is undeniably high due to transportation costs. However, the widespread availability of BC and PAM makes them suitable for improving soil conditions, particularly in mining regions. The combined application of BC and PAM has been recognized as a cost-effective strategy21 that improves soil physical structure22, enhances nutrient utilization23, and promotes plant growth24, making it a viable approach for restoring soil quality in arid and semi-arid regions. Despite these advantages, no research has explored the potential changes in soil enzyme activity from using BC and PAM in rehabilitated mining area soils. Pot experiments offer several benefits, including control over environmental variables, reduced incidental impacts such as heavy rainfall and hurricanes, and decreased experimental maintenance costs. Thus, this study conducted a pot incubation experiment to evaluate the effects of various BC and PAM rates on the physicochemical properties of CGS, aiming to determine the extent to which these amendments influence soil enzyme activity. We hypothesized that the simultaneous application of BC and PAM would enhance enzyme activity in CGS. Our research objectives were (i) to investigate the effects of different BC and PAM rates on CGS physicochemical properties and enzyme activity and (ii) to examine the factors influencing soil enzyme activity due to BC and PAM addition.
Materials and methods
Experimental materials
The coal gangue and raw soil were collected from Yangchangwan in the southern region of Lingwu City, Ningxia Hui Autonomous Region (106°35–106°38′ E, N37°59′–38°03′ N). With an altitude of 1450 m, this area has a climate characterized by infrequent, intense rainfall and generally dry weather. The mean annual temperature is 8.9 °C, with an average annual rainfall of 192.9 mm and an average annual evaporation rate of 1762.9 mm. Coal gangue, characterized by its black colour and cracked appearance, was selected from the gangue dump. The water-soluble salt content in the coal gangue included 0.0228 g/kg Ca2+, 0.0094 g/kg Mg2+, 0.0520 g/kg CO32-, 0.0310 g/kg HCO3-, 0.0185 g/kg Cl⁻, and 0.3571 g/kg SO42-. Near the gangue dump, a soil profile approximately 5 m in depth had formed due to excavation, and raw soil was obtained from the surface layer. The physicochemical properties of the raw soil were as follows: bulk density of 1.25 g/cm3, pH of 8.63, electrical conductivity of 0.13 dS/m, organic carbon content of 2.55 g/kg, TN content of 0.01 g/kg, and total phosphorus content of 5.21 mg/kg. Before the experiment, coal gangue and raw soil were crushed using a polyoxymethylene rod and sieved through a 2 mm mesh. The BC, supplied by Liaoning Golden Future Agriculture Technology Co., Ltd. (Anshan, China), was produced from maize straw through thermal decomposition at 400 °C. The specific gravity (solid density) was 1.5 g/cm3. Key chemical properties of BC include a pH of 9.6, a cation exchange capacity of 23.3 cmol/kg, and a composition of 45.2% C and 1.27% N by weight, with a specific surface area of 8.87 m2/g. Anionic PAM, obtained from Beijing Hanlimiao New Technology Co., Ltd. (Beijing, China), was in the form of fine white particles with diameters between 0.2 and 0.9 mm, and specific gravity was 1.26 g/cm3. Its composition includes 50% C, 8% H, 22% O, and 20% N by weight, a molecular weight of 18 × 10⁶ g/mol, and a degree of hydrolysis of 25%.
Potted incubation experiment
CGS was used as the base matrix, with a coal gangue to raw soil ratio of 1:2. The specific gravity of CGS was 2.38 g/cm3. BC was added at 1%, 2%, and 5% of CGS mass, represented by B1, B2, and B5, respectively. PAM was added at 0.02%, 0.05%, and 0.10% of CGS mass, designated as P2, P5, and P10, respectively. A CK received no amendments. In total, 10 treatments were set up, each with three repetitions. The experimental design is summarized in Table 1.
Table 1.
Experimental design and treatments.
| BC addition | PAM addition | Treatment |
|---|---|---|
| 0 | 0 | CK |
| B1 | P2 | B1P2 |
| P5 | B1P5 | |
| P10 | B1P10 | |
| B2 | P2 | B2P2 |
| P5 | B2P5 | |
| P10 | B2P10 | |
| B5 | P2 | B5P2 |
| P5 | B5P5 | |
| P10 | B5P10 |
The notations 0, B1, B2, B5 indicate biochar addition percentages of 0%, 1%, 2%, and 5%, respectively. Similarly, the notations 0, P2, P5, and P10 indicate polyacrylamide addition percentages of 0%, 0.02%, 0.05%, and 0.1%, respectively. CK represents the blank control.
The mixing of the experimental materials was carried out in three steps. The first step involved preparing CGS by combining gangue and raw soil in a 1:2 ratio in a mixing vessel. The second step included mixing BC, PAM, and a portion of CGS. Specifically, the required amounts of BC and PAM for each treatment and approximately 500 g of CGS were placed in a basin and mixed with a shovel. This mixture was transferred to another basin containing the remaining CGS, followed by thorough mixing using a stirring bar. Finally, the CGS, BC, and PAM mixture was evenly distributed into three plastic pots, each with a diameter of 16 cm and a height of 17 cm, with 2000 g of the mix in each pot. The amended CGS (ACGS) was homogenized and placed in the pots, which were then compacted using a polyoxymethylene bar to ensure uniform compaction. The pots were incubated in a plastic shed and watered every 5 days to maintain appropriate moisture levels. The “small amount and many times” method was applied during each irrigation to prevent matrix loss until slight liquid leakage occurred from the pots. The incubation period for ACGS has spanned 120 days, from June to October. The pots were covered with a perforated film, allowing gas exchange to ensure consistent humidity in the ACGS during the final 30 days.
Sample collection
At the end of the incubation period, undisturbed soil cores were collected using a cutting ring with a diameter of 5 cm and a height of 5 cm to measure the soil moisture characteristics. Additional samples were collected from a 0–10 cm depth. These samples were divided into two portions: one was stored at 4 °C to determine water-soluble chemical components and enzyme activities. In contrast, the other portion was air-dried in cloth bags for subsequent chemical property analysis.
Soil physicochemical properties analyses
The cutting ring method measured bulk density25 (BD) and field water holding capacity26 (FC). Soil pH was determined with a pH meter (Leici PHS-3E, INESA, Shanghai, China) at a soil-to-water ratio 1:2.527. Soil electrical conductivity (EC) was measured directly using a conductometer (Leici DDS-307A, INESA, Shanghai, China) in a soil-to-water ratio 1:528. The dichromate oxidation method27 determined SOC. In contrast, soil active carbon (AC) was analyzed using 0.02 M potassium permanganate29. TN was estimated using the Kjeldahl method27, measured with an automatic Kjeldahl nitrogen determination apparatus (K1306, Shensheng, Shanghai, China). Dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) were extracted with deionized water at a soil-to-water ratio of 1:530, filtered through a 0.45 μm polycarbonate filter membrane, and analyzed using a Multi N/C 3100 Analyzer (Analytik Jena, Germany). Given BC’s potential to absorb inorganic nitrogen and affect nitrogen transformation, TDN was measured instead, as inorganic nitrogen concentration was low31. Available phosphorus (AP) was extracted with 0.5 M sodium bicarbonate and measured by the molybdenum antimony anti-spectrophotometric method27.
Enzyme activities
Soil enzyme activity was determined by measuring either product release or substrate consumption during enzymatic reactions. Specific substrates were added to soil slurries in buffer solutions and incubated for a set duration, following Acosta-Martínez’s method32. The activities of carbon-cycle enzymes—α-glucosidase (AGL), β-glucosidase (BGL), cellobiohydrolase (CBH), N-acetyl-glucosaminidase (NAG)—and nitrogen-cycle enzymes—L-leucine aminopeptidase (LAP) and glycine aminopeptidase (GAP)—were measured, along with alkaline phosphatase (ALP) and arylsulfatase (ASF). Enzyme activities were assessed using para-nitrophenyl (pNP)-α-D-glucopyranoside, pNP-β-D-glucopyranoside, pNP-β-D-cellobioside, 4-pNP-N-acetyl-β-D-glucosaminide, leucine-pNA, glycine-pNA, pNP-phosphate, and potassium 4-nitrophenyl sulfate as substrates. Incubations were carried out at 37 °C, and CK treatments were included. Enzyme activities were expressed as the amount of pNP or pNA (µmol pNP or pNA g-1·h-1) produced per gram of dried soil per unit time33.
To evaluate soil quality, the geometric mean (GME) value of enzyme activity was calculated34:
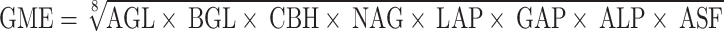 |
Statistical analysis
Data were analyzed using IBM SPSS 21.0 (SPSS, Chicago, IL, USA) and CANOCO 5.0 (Microcomputer Power, Ithaca, NY, USA) software. Diagrams were created in Origin 2022 (OriginLab, Northampton, MA, USA). Differences in soil physicochemical properties and enzyme activities across BC and PAM application rates were examined with a one-way analysis of variance (ANOVA), and significance was assessed using Duncan’s test. Two-way ANOVA was conducted to evaluate the effects of BC, PAM, and their interaction on soil physicochemical properties and enzyme activities, with a significance threshold of p < 0.05. Redundancy analysis (RDA) was used to assess the impact of soil physicochemical properties on enzyme activities. Pearson correlation analysis examined the relationship between ACGS physicochemical properties and enzyme activity.
Results
Soil physicochemical properties
Figure 1 shows that the bulk density values for all treatments with BC and PAM were lower than those of CK. The lowest bulk density was observed in the B5P10 treatment. The highest FC value was also observed in the B5P10 treatment, representing a 25% improvement over CK. Additionally, the pH of B5P10 was significantly lower than the other treatments. The addition of BC notably enhanced the SOC, TN, and AP content. The SOC content in B5P10 showed a 57.98% increase compared to the CK. Treatments including B1P10, B1P5, B2P5, B2P10, and B5P10 resulted in a notable rise in TDN contents.
Fig. 1.
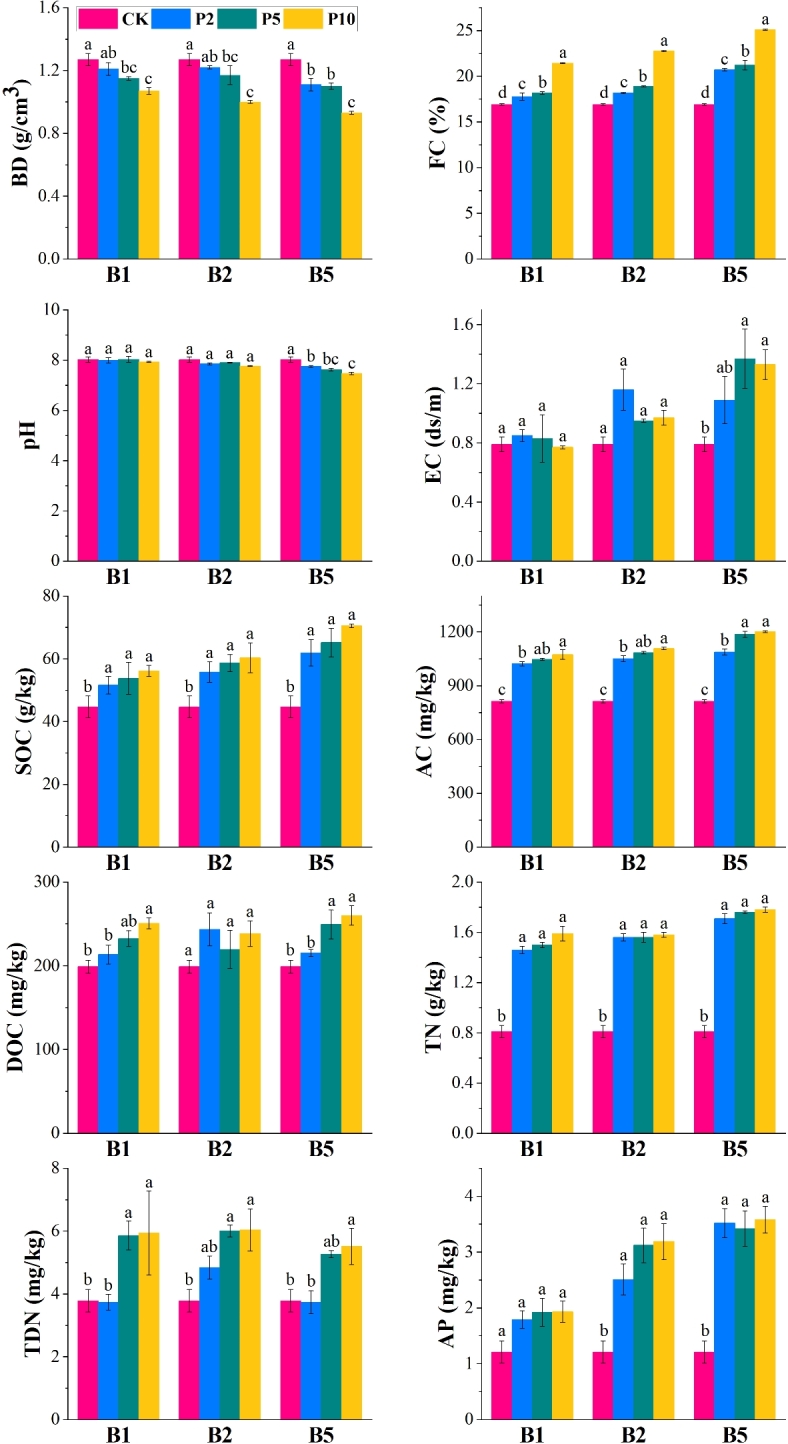
Physicochemical properties of amended coal gangue under various treatments. BD: bulk density; FC: field capacity; SOC: soil organic carbon; AC: active carbon; DOC: dissolved organic carbon; TN: total nitrogen; TDN: total dissolved nitrogen; and AP: available phosphorus. Values are expressed as “mean ± standard error”. No significant differences were observed between values sharing the same letter in the same column (p < 0.05).
Table 2 shows the results of a two-way ANOVA for ACGS physicochemical properties. The addition of BC significantly affected BD, FC, pH, EC, SOC, AC, TN, and AP content in CGS. PAM addition also significantly impacted BD, FC, pH, AC, and TDN. Moreover, the combined application of BC and PAM significantly affected FC.
Table 2.
Results of two-way analysis of variance on amended coal gangue soil physicochemical properties, expressed as F-values with significance levels (**p < 0.01; *p < 0.05).
| Factor | BC (B) | PAM (P) | Interaction (B × P) |
||
|---|---|---|---|---|---|
| Parameter | F | B (sig) | F | P (sig) | F |
| BD | 10.85** | a, b, b, c | 36.76** | a, b, b, c | 1.25 |
| FC | 838.66** | d, c, b, a | 1569.05** | d, c, b, a | 6.52** |
| pH | 34.96** | a, a, b, c | 5.69** | a, b, b, b | 1.19 |
| EC | 10.57** | b, b, ab, a | 0.87 | b, ab, a, a | 1.31 |
| SOC | 8.59** | c, b, b, a | 2.01 | b, a, a, a | 0.12 |
| AC | 56.50** | d, c, b, a | 25.24** | c, b, a, a | 2.87 |
| DOC | 0.36 | b, a, a, a | 2.45 | b, ab, a, a | 1.33 |
| TN | 44.99** | c, b, b, a | 4.00* | b, a, a, a | 1.03 |
| TDN | 1.59 | b, a, a, ab | 9.48** | d, b, a, a | 0.28 |
| AP | 27.07** | c, b, a, a | 0.92 | b, a, a, a | 0.54 |
Different lowercase letters indicate significant (p < 0.05) differences. ‘B (sig)’ signifies significant differences among biochar levels, and ‘P (sig)’ among polyacrylamide levels.
BD: bulk density; FC: field capacity; EC: electrical conductivity; SOC: soil organic carbon; AC: active carbon; DOC: dissolved organic carbon; TN: total nitrogen; TDN: total dissolved nitrogen; and AP: available phosphorus. Different letters in the table represent significant (p < 0.05) differences, following the order a > b > c > d.
Soil enzyme activity
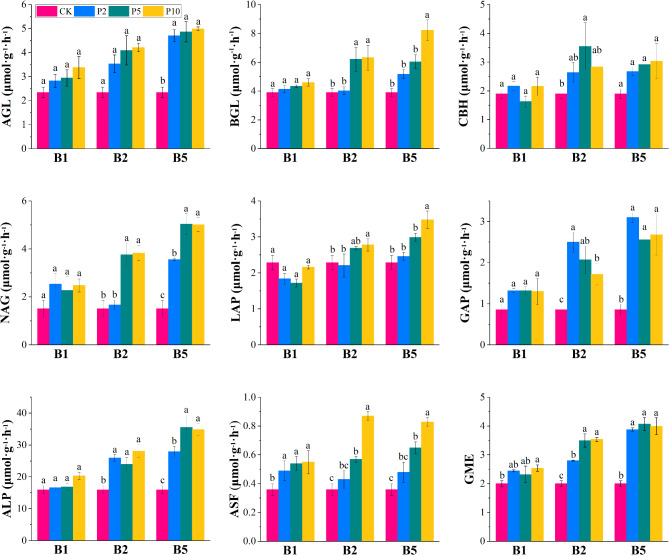
Figure 2 shows the differences in soil enzyme activity across treatments. AGL activity in treatments B5P2, B5P5, and B5P10 was significantly higher than in CK. BGL activity was significantly higher in B2P5, B2P10, and B5P10 than CK. CBH activity showed the greatest increase in B2 and P5 treatments, a significant increase of 186.84% over CK. LAP activity was significantly elevated in B2P10 and B5P10. The addition of BC increased GAP activity, with B5P2 exhibiting a notable increase of 364.46% over CK. Additionally, ALP and ASF activities were enhanced with BC and PAM, reaching maximum increases of 222.34% and 241.67% over CK, respectively. The addition of BC and PAM also increased CGS’s GME, with no significant differences among B2P5, B2P10, B5P2, B5P5, and B5P10, though all were significantly higher than CK.
Fig. 2.
Enzyme activities and the geometric mean of amended coal gangue under different treatments. AGL: α-glucosidase; BGL: β-glucosidase; CBH: cellobiohydrolase; NAG: N-acetyl-glucosaminidase; LAP: L-leucine aminopeptidase; GAP: glycine aminopeptidase; ALP: alkaline phosphatase; and ASF: arylsulfatase. Values sharing the same letter in the same column are not significantly different (p < 0.05).
Table 3 presents the results of two-way ANOVA for ACGS enzyme activities and GME. in ACGS and significantly affected CBH activity. The addition of BC significantly affected the activities of AGL, BGL, NAG, LAP, GAP, ALP, ASF, and GME in the ACGS. PAM addition significantly impacted the activities of BGL, NAG, LAP, and ASF. Furthermore, the combined application of BC and PAM significantly influenced NAG and ASF.
Table 3.
Results of two-way analysis of variance analysis for amended coal gangue soil enzyme activities and geometric mean enzyme expressed as F-values with significance levels (**p < 0.01; *p < 0.05).
| Factor | BC (B) | PAM (P) | Interaction (B × P) |
||
|---|---|---|---|---|---|
| Parameter | F | B (sig) | F | P (sig) | F |
| AGL | 19.10** | c, c, b, a | 1.5 | b, a, a, a | 0.18 |
| BGL | 12.23** | b, b, a, a | 10.18** | b, b, a, a | 2.47 |
| CBH | 5.75* | b, b, a, a | 0.231 | b, a, a, a | 0.99 |
| NAG | 24.82** | c, b, b, a | 9.372** | c, b, a, a | 3.32* |
| LAP | 26.72** | c, b, b, a | 9.292** | b, b, ab, a | 1.53 |
| GAP | 28.37** | c, c, b, a | 2.39 | b, a, a, a | 0.84 |
| ALP | 31.28** | c, c, b, a | 2.61 | b, a, a, a | 1.29 |
| ASF | 6.51** | c, b, a, a | 29.30** | d, c, b, a | 5.75** |
| GME | 69.7** | d, c, b, a | 3.25 | b, a, a, a | 0.10 |
Different lowercase letters indicate significant differences (p < 0.05). B (sig) signifies significant differences among BC application levels, while P (sig) indicates significant differences among polyacrylamide levels.
AGL: α-glucosidase; BGL: β-glucosidase; CBH: cellobiohydrolase; NAG: N-acetyl-glucosaminidase; LAP: L-leucine aminopeptidase; GAP: glycine aminopeptidase; ALP: alkaline phosphatase; ASF: arylsulfatase; and GME: geometric mean enzyme activity.
Relationships between soil physicochemical properties and enzyme activities
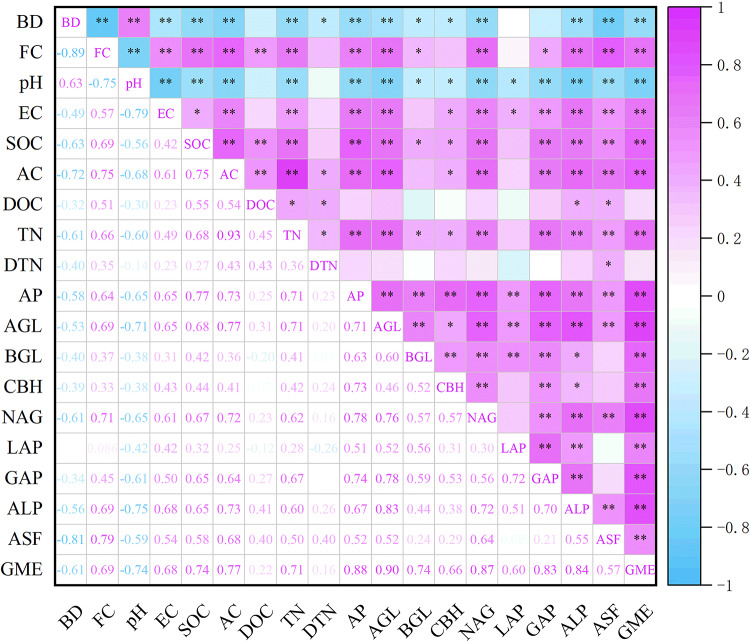
The RDA biplot illustrates the primary physicochemical properties influencing enzyme activity variations (Fig. 3). RDA1 explained 70.8% of the variance, while RDA2 accounted for 2.7%. Key factors affecting enzyme activity included pH, AC, FC, AP, EC, SOC, and TN, contributing to 53.3%, 51.4%, 46.0%, 45.8%, 43.6%, 41.1%, and 35.9% of the variance.
Fig. 3.
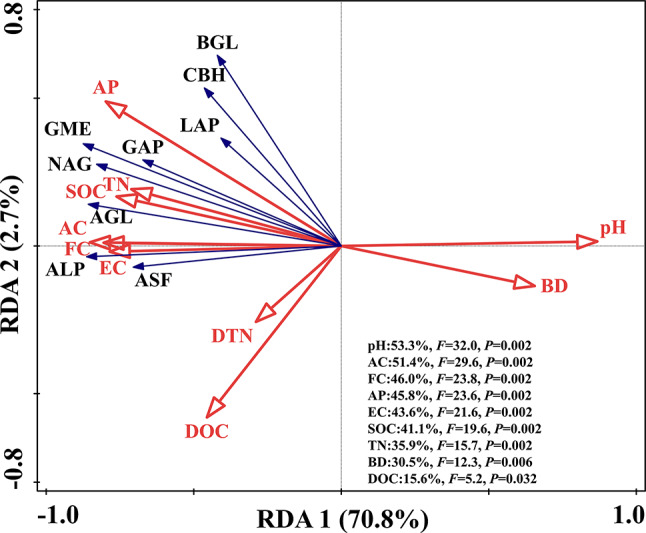
Redundancy analysis results illustrate the relationships between amended coal gangue physicochemical properties, enzyme activities, and geometric mean.
Pearson correlation analysis revealed that enzyme activities and GME were negatively correlated with BD and pH, while they showed positive correlations with FC, EC, SOC, AC, TN, and AP (Fig. 4).
Fig. 4.
Pearson correlation analysis results indicate relationships between amended coal gangue physicochemical properties, enzyme activities, and geometric mean (*p < 0.05, **p < 0.01).
Discussion
Variation of BC and PAM application on enzyme activity of ACGS
Soil enzymes play a critical role in decomposing and transforming soil organic matter and nutrient cycling35. Prior research has demonstrated that BC can enhance microbial activity by expanding growth and reproductive space36, altering the original nutrient composition37, modifying the community composition of microorganisms38, promoting their metabolism, and consequently enhancing enzyme activity. However, incorporating BC does not consistently result in changes in enzymes associated with the carbon cycle. For example, previous studies have indicated that BC can significantly enhance BGL activity, leading to increased enzyme activities following BC application39. Conversely, some research suggests that higher BC doses may reduce enzyme activity40. Wang et al.41 illustrated that lower BC additions (0.5% by mass) can enhance soil enzyme activities linked to β-D-cellobiosidase, BGL, and N-acetyl-β-glucosaminidase, whereas higher additions (5% by mass) exhibit the opposite effect. Our study observed that BC application increased AGL, BGL, and NAG activities. Nevertheless, the activities of CBH showed no significant difference with BC and PAM addition. This finding may relate to enzyme specificity and is supported by Halmi42, who reported no substantial alterations in total cellulose.
Nitrogen cycling in soil is a complex process that depends on multiple enzymes, such as LAP and GAP, which are pivotal for organic nitrogen mineralization. Song et al.43 reported increased LAP activities by applying 10% BC in agricultural lands. However, their research indicated that lower levels of BC (2% and 5% additions) and lower PAM concentrations (0.02% and 0.05% additions) did not significantly impact LAP activity. The reduced LAP activity observed with BC was consistent with findings from Awad et al.21, who reported that the application of 0.2% BC did not increase LAP activities. Intriguingly, minimal amounts of BC and PAM did not affect GAP activity, whereas higher levels of PAM addition (0.05% and 0.1% by mass) reduced GAP activity. This phenomenon could be attributed to the hydrolysis of PAM by specific amino acid enzymes44. As PAM addition increases, enzyme consumption rises, decreasing enzyme activity.
Studies have demonstrated that BC can significantly enhance phosphodiesterase activity45. Adding BC has been shown to increase ALP activity, as evidenced by Jin et al.46. ASF, a crucial enzyme involved in sulfate transformation, exhibits varying responses to BC in prior research. While some studies have shown no significant or minor impact on ASF activity45, others suggest that BC addition can enhance ASF activity34,47. In our study, both BC and PAM profoundly impacted ASF activity. We hypothesize that this effect may be linked to sulfur leaching from coal gangue.
Soil enzymes serve as vital biological indicators for assessing material and energy dynamics and soil quality due to their sensitivity to subtle changes in soil properties resulting from external factors over time7. The geometric mean value of soil enzyme activity is an early indicator of soil quality changes, owing to its correlation with soil physicochemical and biological properties34. Our study observed no significant differences among the treatments B2P5, B2P10, B5P2, B5P5, and B5P10, although all were significantly higher than the CK. Two-factor ANOVA results indicated that both BC and PAM significantly affected GME. Given the substantial quantities of BC and PAM combined, we recommend introducing 2% BC and 0.05% PAM into CGS for soil restoration efforts at the mining site.
The driving factors influencing soil enzyme activity
Soil microorganisms predominantly produce soil enzymes, and factors influencing these microorganisms can significantly impact soil enzyme activity. Consistent with prior research, we identified a significant positive correlation between BD, FC, and enzyme activity, as Xie et al.48 reported. Soil BD is intricately linked to soil aeration and permeability and generally correlates negatively with soil enzyme activity. In this study, we observed that adding BC at 2% and 5% ratios and PAM at 0.05% and 0.1% ratios led to a significant decrease in BD. This trend can be elucidated by the dilution effect stemming from the reduced BD of BC and PAM49. Additionally, PAM’s behavior during water absorption can aid in lowering BD, given its lower density than mineral substances. The reduction in BD resulting from the introduction of BC and PAM can enhance the complexity of the soil pore structure, potentially boosting polymerization and diminishing BC dispersion50. Furthermore, PAM exhibits robust water retention capabilities. Applying PAM to the soil can enhance soil moisture conditions, thereby stimulating the biochemical activity of soil microorganisms and consequently enhancing enzyme activity, as noted by Moghimian et al.51.
Previous studies by Guangming et al.35 have demonstrated that a decrease in pH in alkaline soils can enhance soil enzyme activity, as enzymatic reactions require an appropriate pH environment. BC has been recognized as an effective liming agent, particularly in neutral and acidic soils. However, research on the effects of BC on soils remains relatively limited52. Xu and Wang53 reported that BC application can increase soil pH in acidic soils due to its alkaline properties. In contrast, one researcher54 observed no adverse effects on soil pH in alkaline soils. Our study found that 5% BC combined with 0.02%, 0.05%, and 0.1% PAM decreased the pH of CGS, which is consistent with the results reported by Su et al.55. This can be explained by the difficulty of protonating anionic sites and the soil’s buffering capacity at pH levels above 618. Additionally, soil EC indicates the soil’s nutrient status56. An increase in EC raises nutrient levels in the soil solution, stimulating microbial activity and enhancing enzyme activity.
In our study, enzyme activities were strongly influenced by carbon and nitrogen nutrients, with AC, SOC, and TN accounting for 51.3%, 41.0%, and 35.8% of the total variance, respectively. Regarding carbon morphology, our results indicate that active organic carbon exerts a more substantial influence on enzyme activity than total organic carbon, consistent with findings by Bowles57. BC contains higher carbon and nitrogen content and greater carbon stability, making it useful for increasing soil carbon levels40. In the ACGS, adding 5% BC led to significant increases in SOC and TN. The impact of BC on dissolved organic matter is complex and can be influenced by the application rate, affecting the balance of release and adsorption58.
Regarding coal gangue, dissolved organic matter and inorganic nitrogen derived from the ammonification of organic nitrogen can be released from weathered coal gangue during storage and cultivation59. Our results suggest that coal gangue may contribute significantly to the dissolved organic matter. The dissolved carbon molecules constitute a minor portion of the total BC. In comparison, the chemical fractionation of AC involves the reaction between soil organic matter and a strong oxidizing agent; lower concentrations represent the labile organic carbon fraction. Interestingly, the AC content increased with BC application, indicating that BC comprises a higher proportion of slow-turnover carbon60. Furthermore, adding PAM can increase nitrogen content by releasing N-terminal amino acids21.
Additionally, we observed that DOC was significantly correlated with ALP and ASF, while DTN significantly correlated with ASF. This phenomenon could be attributed to the gradual conversion of total nutrients into water-soluble nutrients61, resulting in a substantial rise in total carbon and nitrogen content, exerting a more pronounced impact on soil enzyme activities during mathematical analysis. This correlation could also be linked to microbial growth, as DOC and DTN primarily supply the nutrients necessary to develop microorganisms in the early stages of culture62. Research has shown that BC promotes the leaching of labile organic matter in soil, stimulating water-soluble carbon levels63. While PAM is too large to penetrate biological cell membranes, small amounts of PAM addition did not affect the organic carbon content in ACGS. As the quantity of PAM increased, a small portion of carbon was released64, leading to increased DOC contents.
Among the various factors influencing enzyme activity, AP is the most significant factor aside from readily oxidizable carbon. The low phosphorus content in ACGS may not meet the metabolic needs of microorganisms, and increasing phosphorus content can enhance enzyme activity, thereby promoting the improvement of various enzyme activities in the soil12. Moreover, lower soil pH can facilitate phosphate dissociation from insoluble complexes, enhancing phosphorus availability in the soil, as He et al.65 demonstrated. These findings contribute to our understanding of the complex interactions between soil enzymes and environmental factors and may have implications for developing sustainable soil practices.
Conclusions
In this study, we investigated the effects of BC and PAM additions on CGS physicochemical properties and enzymatic activity. Our results indicate that adding BC and PAM can increase enzyme activity and GME and improve the quality of CGS. Two-factor analysis revealed that BC significantly influenced BD, field capacity, EC, pH, SOC, AC, TN, and AP in ACGS. Adding 2% and 5% BC significantly increased the activities of AGL, GAP, and ALP. The geometric mean value of enzyme activity indicated that combining 2% BC and 0.05% PAM was the most economical and appropriate ratio to improve soil quality. Furthermore, the RDA highlighted that enzyme activity in ACGS was primarily affected by physical properties and carbon and nitrogen levels. These findings provide a foundational understanding of utilizing BC and PAM in soil enhancement within mining areas. Overall, our study underscores the potential of BC and PAM as effective amendments for improving CGS quality and stimulating enzymatic activity. To the best of our knowledge, no studies have examined the enzymatic activity from applying both BC and PAM in the soil. Therefore, this study provides relatively novel results. However, field trials will be essential since the experiment was limited to indoor conditions. Moreover, the cost-effectiveness of field trials is influenced by various factors, such as labor costs, material transportation costs, and the specific crop cultivated. Subsequent studies focusing on cost analyses should be conducted on a case-by-case basis.
Acknowledgements
The research was supported by the National Key R&D Program of China “Eco-security technology for coal mining bases in the northwestern arid desert regions in China” (2017YFC0504400) and “Study on Geomorphic Reclamation and Earthy Composition in Coal Mining Abandoned Area” (2017YFC0504404).
Author contributions
Q.F.: Writing—review & editing, Supervision. N.L.: Conceptualization, Resources. Y.G.: Funding acquisition, Supervision, Writing—editing. Y.D.: Resources, Supervision. C.Z.: Writing—review & editing.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lv, X. et al. Drivers of spatio-temporal ecological vulnerability in an arid, coal mining region in Western China. Ecol. Indic.106, 105475 (2019). [Google Scholar]
- 2.Xiao, W. et al. Coupling and coordination of coal mining intensity and social-ecological resilience in China. Ecol. Indic.131, 108167 (2021). [Google Scholar]
- 3.Wang, L. et al. Analysis of differences in chemical properties of reconstructed soil under different proportions of topsoil substitute materials. Environ. Sci. Pollut Res.28, 31230–31245 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Du, T. et al. Optimizing the formulation of coal gangue planting substrate using wastes: The sustainability of coal mine ecological restoration. Ecol. Eng.143, 105669 (2020). [Google Scholar]
- 5.Gong, Y. et al. Reclaiming subsidized land: An evaluation of coal gangue interlayers. Adv. Mater. Sci. Eng. 5740659 (2020).
- 6.Fang, L. et al. A long-term study on the soil reconstruction process of reclaimed land by coal gangue filling. Catena195, 104874 (2020). [Google Scholar]
- 7.Genova, G. et al. Analyzing soil enzymes to assess soil quality parameters in long-term copper accumulation through a machine learning approach. Appl. Soil. Ecol.195, 105261 (2024). [Google Scholar]
- 8.Sanchez, E. et al. Assessment of pistachio shell-based biochar application in the sustainable amendment of soil and its performance in enhancing bell pepper (Capsicum annuum L). Growth Sustain.16, 4429 (2024). [Google Scholar]
- 9.Wang, X. et al. Study on the mechanism of polymer inhibiting purplish soil fugitive dust at macro–micro scale in Southwest China. J. Mol. Liq. 396, 123928 (2024). [Google Scholar]
- 10.Szewczuk-Karpisz, K. et al. Hay-based activated biochars obtained using two different heating methods as effective low-cost sorbents: Solid surface characteristics, adsorptive properties and aggregation in the mixed cu(II)/PAM system. Chemosphere250, 126312 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Yang, K. et al. Study on environmental characteristics of sustainable biomimetic soil preparation and its application in gangue reclamation. Constr. Build. Mater.435, 136874 (2024). [Google Scholar]
- 12.He, L. et al. Ecoenzymatic stoichiometry reveals soil P limitation under biochar addition in a reclaimed mine area in Shanxi Province, China. Restor. Ecol.31, e13909 (2023). [Google Scholar]
- 13.Oladele, S. O. Effect of biochar amendment on soil enzymatic activities, carboxylate secretions and upland rice performance in a sandy clay loam Alfisol of Southwest Nigeria. Sci. Afr.4, e00107 (2019). [Google Scholar]
- 14.Okebalama, C. B. et al. Carbon and nutrient cycling responses to repeated application of biochar and NPK fertilizers depend on microenvironmental differences among hierarchical aggregate fractions. Pedobiologia104, 150962 (2024). [Google Scholar]
- 15.Liu, Y. et al. Change in composition and function of microbial communities in an acid bamboo (Phyllostachys praecox) plantation soil with the addition of three different biochars. Ecol. Manag.473, 118336 (2020). [Google Scholar]
- 16.Cui, Q. et al. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ.756, 143801 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Quan, L. et al. Biochar mitigates the effect of nitrogen deposition on soil bacterial community composition and enzyme activities in a Torreya grandis orchard. Ecol. Manag.457, 117717 (2020). [Google Scholar]
- 18.Sadeghi, S. H. et al. The hydrologic behavior of Loess and Marl soils in response to biochar and polyacrylamide mulching under laboratorial rainfall simulation conditions. J. Hydrol.592, 125620 (2021). [Google Scholar]
- 19.Abu-Hamdeh, N. H. et al. Effect of tillage systems and polyacrylamide on soil physical properties and wheat grain yield in arid regions differing in fine soil particles. Archiv Für Acker- Und Pflanzenbau Und Bodenkunde. 65, 182–196 (2019). [Google Scholar]
- 20.Watson, C. et al. Short-term effects of polyacrylamide and dicyandiamide on C and N mineralization in a sandy loam soil. Soil. Use Manag.32, 127–136 (2016). [Google Scholar]
- 21.Awad, Y. M. et al. Carbon and nitrogen mineralization and enzyme activities in soil aggregate-size classes: Effects of biochar, oyster shells, and polymers. Chemosphere198, 40–48 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Kebede, B. et al. Effect of Polyacrylamide integrated with other soil amendments on runoff and soil loss: Case study from northwest Ethiopia. Int. Soil. Water Conserv. Res.10, 487–496 (2022). [Google Scholar]
- 23.Mulualem, T. et al. Dual benefits of polyacrylamide and other soil amendments: Mitigation of soil nutrient depletion and improvement of use-efficiency in Midland agro‐ecology, Ethiopia. Land. Degrad. Dev.33, 2998–3009 (202).
- 24.Abulaiti, A. et al. Application of biochar and polyacrylamide to revitalize coastal saline soil quality to improve rice growth. Environ. Sci. Pollut Res.30, 18731–18747 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Ministry of Agriculture of the People’. s Republic of China of referencing in NY/T 1121.4–2006 Soil Testing Part 4: Method for determination of soil bulk density (2006).
- 26.The Ministry of Agriculture of the People’. s Republic of China of referencing in NY/T 1121.22–2010 Soil Testing Part 22: Cutting ring method for determination of field water-holding capacity in soil (2010).
- 27.Bao, S. D. of referencing in Soil and Agricultural Chemistry Analysis, (ed. Bao. S. D.) 30–86 (Beijing, 2000). [Google Scholar]
- 28.The Ministry of Environmental Protection. People’s Republic of China of referencing in HJ 802–2016 Soil quality-Determination of conductivity- Electrode method (2010).
- 29.Weil, R. R. et al. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agri. 18, 3–17 (2003). [Google Scholar]
- 30.Stefan, A. et al. Leaching of dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) in mor humus as a ected by temperature and pH. Soil. Biol. Biochem.32, 1–10 (2000). [Google Scholar]
- 31.Yu, M. et al. Plant material and its biochar differ in their effects on nitrogen mineralization and nitrification in a subtropical forest soil. Sci. Total Environ.763, 143048 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Acosta-Martínez, V. et al. Long-term soil microbial community and enzyme activity responses to an integrated cropping-livestock system in a semi-arid region. Agric. Ecosyst. Environ.137, 231–240 (2010). [Google Scholar]
- 33.Dick, R. P. of referencing in Methods of Soil Enzymology, (ed. Dick R. P.) 103–210 (Madison, 2011).
- 34.Paz-Ferreiro, J. et al. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fertil. Soils. 48, 511–517 (2012). [Google Scholar]
- 35.Guangming, L. et al. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ.237, 274–279 (2017). [Google Scholar]
- 36.Cen, R. et al. Effect mechanism of biochar application on soil structure and organic matter in semi-arid areas. J. Environ. Manag.286, 112198 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Javeed, H. M. R. et al. Effect of date biochar pyrolyzed at different temperature on physiochemical properties of sandy soil and wheat crop response. Commun. Soil. Sci. Plant. Anal.52, 2110–2124 (2021). [Google Scholar]
- 38.Wang, P. et al. Preparation of two types plant biochars and application in soil quality improvement. Sci. Total Environ.906, 167334 (2024). [DOI] [PubMed] [Google Scholar]
- 39.Ullah, S. et al. Biochar coupled with contrasting nitrogen sources mediated changes in carbon and nitrogen pools, microbial and enzymatic activity in paddy soil. J. Saudi Chem. Soc.24, 835–849 (2020). [Google Scholar]
- 40.Lopes, É. M. G. et al. Biochar increases enzyme activity and total microbial quality of soil grown with sugarcane. Environ. Technol. Innov.21, 101270 (2021). [Google Scholar]
- 41.Wang, X. et al. Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl. Soil. Ecol.96, 265–272 (2015). [Google Scholar]
- 42.Halmi, M. F. A. et al. Effect of two contrasting biochars on soil microbiota in the humid tropics of Peninsular Malaysia. Geoderma395, 115088 (2021). [Google Scholar]
- 43.Song, D. et al. Combined biochar and nitrogen fertilizer change soil enzyme and microbial activities in a 2-year field trial. Eur. J. Soil. Biol.99, 103212 (2020). [Google Scholar]
- 44.Ma, L. et al. Combination of biochar and immobilized bacteria accelerates polyacrylamide biodegradation in soil by both bio-augmentation and bio-stimulation strategies. J. Hazard. Mater.405, 124086 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Wei, Z. et al. Application of biochar in estrogen hormone-contaminated and manure-affected soils: Impact on soil respiration, microbial community and enzyme activity. Chemosphere270, 128625 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Jin, Y. et al. Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: A microcosm incubation study. Chemosphere142, 128–135 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Khadem, A. et al. Biochar application changed arylsulfatase activity, kinetic and thermodynamic aspects. Eur. J. Soil. Biol.95, 103134 (2019). [Google Scholar]
- 48.Xie, X. et al. Response of soil physicochemical properties and enzyme activities to long-term reclamation of coastal saline soil, Eastern China. Sci. Total Environ.607–608, 1419–1427 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Laird, D. A. et al. Impact of biochar amendments on the quality of a typical midwestern agricultural soil. Geoderma158, 443–449 (2010). [Google Scholar]
- 50.Wang, H. et al. Synergic effects of biochar and polyacrylamide amendments on the mechanical properties of silt loam soil under coastal reclamation in China. Catena182, 104152 (2019). [Google Scholar]
- 51.Moghimian, N. et al. Impacts of changes in land use/cover on soil microbial and enzyme activities. Catena157, 407–414 (2017). [Google Scholar]
- 52.Amoah-Antwi, C. et al. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ.722, 137852 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Xu, H. et al. Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol.48, 9391–9399 (2014). [DOI] [PubMed] [Google Scholar]
- 54.de la Rosa, J. M. et al. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a calcic cambisol during a pot experiment of 79 days. Sci. Total Environ.499, 175–184 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Su, C. et al. Biochar can improve the soil quality of new creation farmland on the Loess Plateau. Environ. Sci. Pollut Res.26, 2662–2670 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Kim, H. N. et al. Monitoring of soil EC for the prediction of soil nutrient regime under different soil water and organic matter contents. Appl. Biol. Chem.67, 1–9 (2024). [Google Scholar]
- 57.Bowles, T. M. et al. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil. Biol. Biochem.68, 252–262 (2014). [Google Scholar]
- 58.Feng, Z. et al. Biochar induced changes of soil dissolved organic matter: The release and adsorption of dissolved organic matter by biochar and soil. Sci. Total Environ.783, 147091 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Zhao, L. et al. Characterization of dissolved organic matter derived from coal gangue packed in underground reservoirs of coal mines using fluorescence and absorbance spectroscopy. Environ. Sci. Pollut Res.28, 17928–17941 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Mirsky, S. B. et al. Evaluating soil management using particulate and chemically labile soil organic matter fractions. Soil. Sci. Soc. Am. J.72, 180–185 (2008). [Google Scholar]
- 61.Yang, Y. et al. Soil organic carbon transformation and dynamics of microorganisms under different organic amendments. Sci. Total Environ.750, 141719 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Demisie, W. et al. Effect of biochar on carbon fractions and enzyme activity of red soil. Catena121, 214–221 (2014). [Google Scholar]
- 63.Luo, Y. et al. Pyrolysis temperature during biochar production alters its subsequent utilization by microorganisms in an acid arable soil. Land. Degrad. Dev.29, 2183–2188 (2018). [Google Scholar]
- 64.Entry, J. A. et al. High polyacrylamide application rates do not affect eubacterial structural diversity. Water Air Soil Pollut.224, 1382 (2013). [Google Scholar]
- 65.He, K. et al. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil. Ecol.155, 103674 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.