INTRODUCTION
The chronic inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC), are recognized as important causes of gastrointestinal disease in children and adults. IBD occurs worldwide, although it is more common in some regions (United States, United Kingdom, and Scandinavia) than in others, with incidence rates of 4 to 10/100,000 persons per year and prevalence rates between 40 to 100/100,000 persons (49).
IBD is most commonly diagnosed between the third and fourth decades of life, with no difference noted between males and females. Approximately 20% of all patients with IBD develop symptoms during childhood (97), with about 5% being diagnosed before 10 years of age (72). About 25% of affected children have a positive family history of IBD. However, there have been no differences noted between normal children and children with IBD with regard to gender, breast feeding, formula intolerance, prior gastrointestinal illness, or emotional stresses (31).
Defining Ulcerative Colitis and Crohn’s Disease
Ulcerative colitis.
UC is a condition in which the inflammatory response and morphologic changes remain confined to the colon. The rectum is involved in 95% of patients, with variable degrees of proximal extension. Inflammation is limited primarily to the mucosa and consists of continuous involvement of variable severity with ulceration, edema, and hemorrhage along the length of the colon. The characteristic histologic findings are acute and chronic inflammation of the mucosa by polymorphonuclear leukocytes and mononuclear cells, crypt abscesses, distortion of the mucosal glands, and goblet cell depletion.
Crohn’s disease.
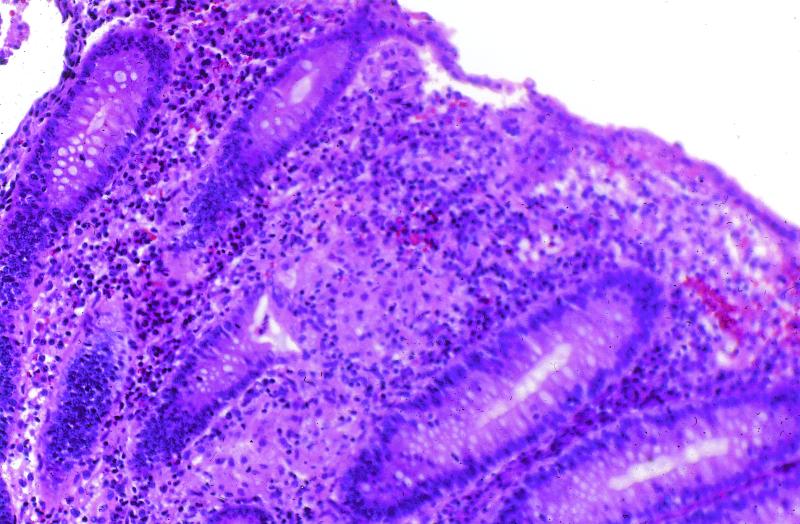
CD, in contrast to UC, can involve any part of the gastrointestinal tract from the oropharynx to the perianal area. Diseased segments frequently are separated by intervening normal bowel, leading to the term “skip areas.” Inflammation can be transmural, often extending through to the serosa, resulting in sinus tracts or fistula formation. Histologic findings include small superficial ulcerations over a Peyer’s patch (aphthoid ulcer) and focal chronic inflammation extending to the submucosa, sometimes accompanied by noncaseating granuloma formation (Fig. 1). The most common location is the ileocecal region, followed by the terminal ileum alone, diffuse small bowel, or isolated colonic disease in decreasing order of frequency.
FIG. 1.
Mildly active Crohn’s colitis with a granuloma within the lamina propria. Hematoxylin and eosin stain; magnification, ×100.
CLINICAL FEATURES
UC and CD are associated with both intestinal and extraintestinal manifestations. Extraintestinal manifestations are usually related to intestinal disease activity and may precede or develop concurrently with intestinal symptoms. While UC and CD have a number of similarities in their clinical presentations, characteristic features are emphasized below.
Intestinal Manifestations
Ulcerative colitis.
The most consistent feature of UC is the presence of blood and mucus mixed with stool, accompanied by lower abdominal cramping which is most intense during the passage of bowel movements. The presence of diarrhea with blood and mucus as opposed to the absence of blood is used clinically to differentiate between UC and irritable bowel syndrome. UC is usually diagnosed earlier after the onset of symptoms than is CD because the presence of gross blood in the stools alerts persons to a gastrointestinal problem. The location of abdominal pain depends on the extent of colonic involvement. Pain is present in the left lower quadrant with distal disease and extends to the entire abdomen with pancolitis. Pediatric patients have a higher frequency of pancolonic involvement, a higher likelihood of proximal extension of disease over time, and a higher risk of colectomy compared to adult patients (71). Abdominal distension, guarding, and rebound tenderness to palpation with decrease in bowel sounds requires close supervision due to the risk of developing toxic megacolon.
Crohn’s disease.
In contrast to UC, the presentation in CD often is subtle, leading to a delay in diagnosis. Gastrointestinal symptoms depend on the location, extent, and severity of involvement. In patients with ileocolonic involvement, abdominal pain is usually postprandial and may be referred to the periumbilical area, especially in children. Examination may localize tenderness to the right lower quadrant, and an inflammatory mass is occasionally felt. Gastroduodenal CD presents with early satiety, nausea, emesis, epigastric pain, or dysphagia. Due to postprandial pain and delay in gastric emptying, patients with gastroduodenal CD often limit their caloric intake to diminish their discomfort. Extensive small bowel disease causes diffuse abdominal pain, anorexia, diarrhea, and weight loss and may result in lactose malabsorption. Physical examination reveals diffuse abdominal tenderness. Clubbing of the distal phalanges is rare but is seen most frequently in children with extensive small bowel disease. Colonic CD may mimic UC, presenting with diarrhea with blood and mucus associated with crampy lower abdominal pain that is often relieved by defecation. Perianal disease is common, as are anal tags, deep anal fissures, and fistulae. Increasing abdominal cramping, distension, and emesis accompanied with borborygmi are signs of progression of the inflammatory process to localized stenosis with partial or complete obstruction.
Extraintestinal Features
Fevers.
Fevers are seen in 40% of patients with IBD at the time of presentation. Fevers can be high spiking on occasion but are usually low grade and chronic and may be unrecognized.
Weight loss.
Weight loss may be a feature of IBD in both adults and children. In children, weight loss or a failure to maintain a normal growth velocity is the commonest systemic feature of IBD and is observed more frequently with CD than with UC.
Delayed growth and sexual maturation in children.
Growth failure (defined as either reduced growth velocity, in centimeters per year, for age or a fall in height percentile from the child’s previous level) and delayed sexual maturation occasionally may be the initial presentation of CD in children (45). Patients also may have a concomitant delay in skeletal maturation, which is evaluated by radiologic determination of the nondominant hand. Delayed growth is more common in CD (60 to 88%) than in UC (6 to 12%), with the greatest frequency found in prepubertal children. Growth delay also can occur as a consequence of chronic corticosteroid use.
Chronic undernutrition resulting from suboptimal enteral intake due to anorexia and abdominal discomfort, as well as increased losses due to a protein-losing enteropathy, is considered to be a major etiologic factor in growth delay. Malabsorption of nutrients is rarely seen unless the patient has had extensive intestinal resection. Other contributing factors include elevated levels of circulating cytokines and low levels of insulin-like growth factor or somatomedin, which are seen in poorly nourished children and increase significantly following treatment (48).
Delayed sexual maturation or arrest of sexual maturation may occur concurrently with growth failure. Some females may also experience secondary amenorrhea due to active disease or weight loss.
Arthralgias and arthritis.
Arthralgias and arthritis occur frequently and occasionally precede intestinal manifestations of IBD. They usually coincide with disease activity and improve with medical treatment of the underlying intestinal inflammation. Two forms of involvement are seen—a peripheral form and an axial form, including ankylosing spondylitis or sacroilitis. The peripheral form is usually pauciarticular, affecting large joints, such as knees, ankles, hips, wrists, and elbows in decreasing order of frequency. Joint deformity is rare, although a destructive granulomatous synovitis has been described in CD. Ankylosing spondylitis is associated with human leukocyte antigen (HLA)-B27 in 50 to 80% of cases compared to over 90% in non-IBD-associated cases (66). Progression is variable and does not appear to correlate with the severity of bowel symptoms.
Mucocutaneous lesions.
Oral aphthoid ulcers occur commonly with IBD. Ulcers usually cause minimal discomfort, although they occasionally cause debilitating pain. They tend to parallel disease activity, and treatment is directed toward the underlying disease. Cutaneous manifestations include erythema nodosum and pyoderma gangrenosum. Erythema nodosum is characterized by the development of painful, indurated, purplish red, ovoid nodules 1 to 3 cm in diameter, most commonly seen over extensor surfaces. Erythema nodosum is more common in CD and usually occurs in association with active intestinal inflammation; improvement coincides with treatment of the bowel disease (69). Pyoderma gangrenosum is a deep severe ulceration of the skin and is an unusual manifestation (<1%) that is usually seen in association with UC. It usually parallels active colonic disease but on occasion is refractory to systemic treatment and requires intensive local therapy such as local administration of corticosteroids, minocycline, dapsone, or clofazimine.
Ophthalmologic complications.
Ocular complications result from IBD itself or chronic corticosteroid therapy. Episcleritis usually is related to disease activity and presents with scleral and conjunctival erythema, a burning sensation, and photophobia. Local corticosteroid drops are usually effective. Iritis and uveitis are associated with the presence of HLA-B27 and typically run a course independent of the bowel disease. They present with eye pain, headache, and blurred vision or may be asymptomatic and detected by slit-lamp examination. Treatment consists of pupillary dilatation, covering the eye to decrease pain and photophobia, and administration of local or systemic corticosteroids. However, long-term use of corticosteroids increases the frequency of posterior subcapsuler cataracts and increased intraocular pressure.
Hepatobiliary disease.
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by fibrosing inflammation and obliteration of the bile ducts. It is usually seen in association with UC. PSC may be asymptomatic and is then detected only because of elevated alkaline phosphatase and γ-glutamyltransferase levels noted during routine blood screening. Patients occasionally present with pruritus and PSC prior to the development of intestinal symptoms from IBD. The course of PSC appears to be unrelated to underlying bowel disease and may progress after a colectomy. PSC is diagnosed by either liver biopsy or an endoscopic retrograde cholangiopancreatogram showing characteristic bile duct changes. Peripheral antineutrophilic cytoplasmic antibodies are positive in most patients with PSC and may be a marker for genetic susceptibility to this disease.
Autoimmune hepatitis in association with IBD also is well documented. Other infectious etiologies including occult viral infections should be excluded. Diagnosis is made following a liver biopsy, and treatment includes corticosteroids and immunosuppressive medications.
Renal disease.
Nephrolithiasis occurs predominantly as uric acid calculi in UC and oxalate calculi in CD. Hypercalciuria from prolonged bed rest or corticosteroid therapy appear to be risk factors. Secondary amyloidosis is extremely rare but has been reported in CD. Obstructive complications may occur in CD due to ureteral compression by inflammatory masses or enterovesicular fistulae.
Bone abnormalities.
Osteopenia (reduced bone mass) can occur both at the onset of IBD and as a complication of prolonged corticosteroid use. Osteopenia is an important potential complication of pediatric IBD since more than 90% of peak bone mass is attained during childhood and adolescence. Failure to attain peak bone mass increases future fracture potential. In the author’s series of 99 children with IBD, low bone mineral density was seen in 33% of children with CD and about half of those patients had severely reduced bone mineral density (z score, >2 standard deviations below mean). In contrast, approximately 10% of patients with UC had low bone mineral density at the lumbar spine (32). Pubertal and postpubertal girls with CD were particularly at risk to have low bone mass. Corticosteroid use was a predictor of low bone mineral density, but other contributing factors remain to be determined. Aseptic or avascular necrosis is rare, and although it is associated with corticosteroid use, the pathogenesis is unclear. Persistent joint pains, especially involving the hips and knees, should prompt consideration of this complication.
DIAGNOSIS
The importance of excluding enteric pathogens before confirming the diagnosis of IBD, or during IBD exacerbations, cannot be overemphasized. Pathogens that may mimic IBD include Salmonella, Shigella, Campylobacter, Aeromonas, Plesiomonas, Yersinia, Eschericha coli O157:H7, Clostridium difficile, Giardia lamblia, Histoplasma, Mycobacterium tuberculosis, and Entamoeba histolytica. Patients with IBD may have concomitant infections at onset, but their symptoms fail to resolve with treatment of the infectious agent or recur within days to weeks. Once enteric infections are excluded, further work-up is initiated. The tests used to diagnose IBD are listed in Table 1.
TABLE 1.
Tests used to diagnose IBD
| Test | Finding |
|---|---|
| Complete blood counts | Microcytic anemia, leukocytosis with band forms, thrombocytosis |
| Acute-phase reactants | Elevated sedimentation rate and serum orosomucoid, and C-reactive protein levels |
| Chemistries | Low serum iron level, hypoalbuminemia, elevated liver enzyme levels |
| Special serologic tests | pANCA, ASCA |
| Stool examinations | Exclude bacterial pathogens, ova and parasites, occult blood, and fecal leukocytes |
| Endoscopic evaluation | Esophagogastroduodenoscopy with biopsy, colonoscopy with biopsy |
| Radiologic evaluation | Upper gastrointestinal tract with small bowel follow-through, enteroclysis (as indicated), barium enema |
Hematologic Tests
Screening tests for IBD include a complete blood count, inflammatory markers, and a metabolic profile that includes liver enzymes. An elevated white blood count with increased band forms, microcytic anemia, and thrombocytosis is suggestive of IBD. Acute-phase reactants like sedimentation rate, C-reactive protein, and serum orosomucoid level are elevated in about 90% of CD pediatric patients, but less frequently in UC. Hypoalbuminemia and a low serum iron level may be seen. Elevated liver enzyme levels should prompt an evaluation for associated liver disease. Newer serologic tests include perinuclear anti-neutrophilic cytoplasmic antibody (P-ANCA) and anti-Saccharomyces cerevisiae antibodies (ASCA). These tests are used to support a diagnosis of IBD or as an aid in distinguishing UC from CD but should not be used to diagnose IBD. ASCA is detected in 44 to 54% of children with CD but, when present, is highly specific (89 to 97%) (100). P-ANCA is detected in 66 to 83% of children with UC and 14 to 19% of children with CD (81, 100). The specificity of the perinuclear staining pattern in UC is confirmed by its disappearance after DNase treatment of the neutrophils.
Endoscopic Evaluation
Once a diagnosis of IBD is entertained, endoscopic examination with biopsies is indicated to establish the diagnosis. In most cases, histologic examination can definitively differentiate between UC and CD, but in some instances of colitis the features may be atypical, leading to a diagnosis of indeterminate colitis.
Radiologic Studies
Radiologic evaluations are usually reserved for patients with CD to assess the involvement of small bowel loops and terminal ileum via a small bowel follow-through X-ray examination. Enteroclysis (direct instillation of dye into the small intestine) is rarely performed due to patient discomfort and because it requires the introduction of a jejunal tube, but it is used in instances where adequate visualization of small bowel loops has not been possible on standard follow-through X-ray examinations. Characteristic features of CD are rigid stenotic segments, skip areas, and sinus tracts or fistulae. Barium enemas are not used to diagnose UC and should not be used in patients with moderate or severe colitis to avoid inducing toxic megacolon. However, barium enemas may be beneficial in delineating stenosis, fistulae, or sinus tracts in patients with CD.
TREATMENT
Medical Management of Ulcerative Colitis
Mild disease.
Oral sulfasalazine alone or in combination with topical medications is used in the treatment of mild disease. Newer 5-aminosalicylic acid medications (mesalamine, olsalazine, and balsalazide) are useful in patients unable to tolerate sulfasalazine due to side effects. Topical preparations including mesalamine and steroid enemas, mesalamine suppositories, and corticosteroid foam also may reduce symptoms in patients with limited distal colonic disease.
Moderate to severe disease.
Patients with significant abdominal cramping, bloody diarrhea, abdominal tenderness, anemia, and hypoalbuminemia need to be hospitalized for close clinical observation and intravenous medications (corticosteroids), fluids, and nutrition. Antispasmodic agents should be avoided because they predispose patients to the development of toxic megacolon. Blood counts and chemistries are closely monitored. Intravenous steroid treatment is continued until abdominal cramping and hematochezia subside. Dietary restrictions, which initially include avoidance of high-fiber, high-residue, and spicy foods, are liberalized as disease activity responds to medical intervention. Concomitant treatment with sulfasalazine/5-aminosalicylic acid preparations is initiated once acute symptoms subside to maintain remission.
Immunosuppressive therapy.
Azathioprine and 6-mercaptopurine are used due to their steroid-sparing effects, since approximately 50% of patients experience adverse effects from corticosteroids. Due to delayed onset of action, these agents are not used to treat acute colitis. Cyclosporine and tacrolimus have been used to treat acute steroid-refractory UC when surgery seemed inevitable. Clinical improvement is seen within 7 to 10 days in patients who achieve remission (15). However, the majority of patients relapse when these agents are withdrawn. Remission can be prolonged by starting azathioprine or 6-mercaptopurine treatment at least 4 weeks prior to discontinuing cyclosporine treatment (92).
Prognosis.
Approximately 25 to 40% of patients with severe UC will eventually require colectomy (36). Patients diagnosed with proctosigmoiditis before they were 21 years of age had a greater risk of progression beyond the splenic flexure and subsequent colectomy than did those in whom the disease remained localized (72). It is generally accepted that patients with extensive colitis require surveillance colonoscopy to detect dysplasia approximately 8 years after diagnosis. After initiation of surveillance colonoscopy, repeat examinations should follow every 1 to 2 years. The risk of developing colon cancer in Swedish patients diagnosed before 15 years of age was 1% after 15 years, 6.5% after 20 years, and 15% after 25 years (20).
Medical Management of Crohn’s Disease
The medical treatment approach for CD is individualized based on the severity of symptoms and degree and site of intestinal involvement.
Corticosteroids.
Corticosteroids (I mg/kg/day) are effective in decreasing disease activity and inducing remission in most patients. However, due to undesirable side effects (cosmetic effects, suppression of linear growth in children, and osteopenia), long-term use of corticosteroids is not recommended. Furthermore, corticosteroids have not proved effective for the maintenance of remission. Budesonide, a potent steroid that undergoes extensive first-pass hepatic metabolism, is useful, but approximately one-third of patients experience adverse effects related to budesonide use.
Sulfasalazine and mesalamine.
Sulfasalazine and mesalamine are used to treat mild to moderate disease and to maintain remission induced by corticosteroids. Sulfasalazine is useful for ileocolonic and colonic disease. About 30% of patients are unable to tolerate sulfasalazine due to side effects, mainly headaches, which can sometimes be averted by starting at a lower dose and gradually increasing the dose as tolerated. Other side effects include hemolytic anemia and pruritic dermatitis. The newer mesalamine preparation, pentasa, has been used for small bowel CD to facilitate steroid withdrawal and to reduce relapse rates following steroid withdrawal (74).
Antibiotics: metronidazole and ciprofloxacin.
Metronidazole and ciprofloxacin are useful in the treatment of mild to moderate disease, particularly in patients with perianal disease and infectious complications. Sensory neuropathy, which may be seen with long-term metronidazole use, usually resolves completely or improves after discontinuation of the drug.
Immunosuppressive therapy.
6-Mercaptopurine and azathioprine are indicated in patients with steroid dependency, extensive small bowel disease, history of previous resections, gastroduodenal disease, and perianal disease especially with refractory fistulae. In a recent study of 95 children with CD, azathioprine/6-mercaptopurine was well tolerated in 82% of patients and led to a steroid reduction in 87% of patients (47). Discontinuation of azathioprine or 6-mercaptopurine was required in 18% of patients due to hypersensitivity reactions (pancreatitis or high fever) or infectious complications. Other side effects such as elevated aminotransferase levels, leukopenia, and gastrointestinal intolerance either respond to dose reduction or resolve spontaneously. Methotrexate has been used to maintain long-term remission in adult patients with CD. Cyclosporine has been used for patients with severe CD with active fistulae.
Biological therapies.
Increased production of inflammatory cytokines, especially tumor necrosis factor alpha (TNF-α), has been described both in histologically normal mucosa and in the inflamed mucosa in CD. Targan et al. showed that a single infusion of 5 mg of a chimeric monoclonal antibody against TNF-α (infliximab) per kg induced a clinical response in 81% and clinical remission in 48% of CD patients compared with 17 and 4%, respectively, in the placebo group (119). Infliximab infusions have since been successfully used to maintain remission as well for the treatment of fistulae in patients with CD (90, 101).
Thalidomide, originally used for its sedative and antiemetic properties, has recently been shown to inhibit TNF-α production by monocytes and other cells. Thalidomide was found to be efficacious in two recent studies of patients with chronically active, steroid-dependent CD. Side effects were mild and dose dependent, with the most common being drowsiness, peripheral neuropathy, edema, and dermatitis (18, 131).
Nutritional intervention.
Nutritional intervention is often used to control disease activity, provide restitution of deficiencies, and provide adequate calories to reverse growth failure in children. Continuous nasogastric infusion or gastrostomy feedings of an elemental or semielemental formula at night may be beneficial. Patients with IBD are given a daily multivitamin and calcium supplementation if their milk intake is suboptimal and oral iron preparations if they have iron deficiency anemia.
Prognosis.
CD is associated with a 54 to 78% rate of relapse within the first 18 to 24 months following the initial diagnosis. Indications for surgery include intractability to medical therapy, suspected perforation or abscess, intestinal obstruction, and hemorrhage. A special circumstance in children is the potential for reversing growth failure, particularly if there is a localized area of resectable disease. However, there is a high incidence of recurrence after surgery with a 34 to 86% relapse in the 3 years following surgery (33).
HYPOTHESES REGARDING PATHOGENESIS
Although many questions remain regarding the etiology of UC and CD, clinical and laboratory studies indicate that both genetic and environmental factors are important. Several theories about the pathogenesis of IBD have been entertained, including an autoimmune response to a luminal or mucosal antigen, a dysfunctional immune response to a commensal bacterium, and an infection with a pathogenic organism which remains in the intestinal tissues and results in a chronic inflammatory response. A variation of the last hypothesis involves persistent abnormalities in immune regulation following an acute infection with a pathogenic organism that is cleared.
Autoimmune Disease
A destructive inflammatory response directed toward a self-antigen such as mucin, goblet cells, colonocytes, or other cells has been proposed as the underlying basis of IBD, particularly in regards to UC (28, 46, 63, 82). As noted above, antibodies against neutrophils have been found in many but not all UC patients (13, 63, 70, 81). However, the titers of antineutrophil antibodies do not seem to correlate with disease activity in UC (98), and their biological significance in UC patients is unclear. Levels of antibodies against a human intestinal tropomyosin isoform also have been reported to be increased in UC patients in some studies (63, 82). In addition, activated complement factors have been noted to colocalize with anticolon antibodies on the luminal surface of the epithelium (70). Exposure to microbial peptides that share immunogenic determinants with self-antigens has been suggested as the trigger for the disruption in immune tolerance to endogenous gut antigens. For example, some studies have implied that antibodies or T cells recognizing mycobacterial heat shock protein HSP65 and human HSP60 may be involved (70). However, other investigations have failed to confirm these findings. In summary, the endogenous antigens which drive the inflammatory reaction and the events which lead to symptomatic disease in UC remain ill defined.
Infection with a Pathogenic Organism
The clinical presentation and histologic appearance of IBD has a number of similarities to gastrointestinal infections by known pathogenic organisms. For instance, acutely CD can appear like gastrointestinal disease due to Yersinia or M. tuberculosis. Infection with other enteric pathogens such as Shigella or Campylobacter can strongly resemble UC. However, this observation may reflect in part the limited mechanisms by which the mucosal immune system in the intestine responds to an insult (70).
Although CD bears the name of the investigator who convincingly distinguished CD from intestinal tuberculosis in 1932, the notion of a mycobacterial infection underlying the pathogenesis of CD has persisted. In particular, M. paratuberculosis, the causative agent of a chronic granulomatous enteritis in ruminants (Johne’s disease), has been considered. M. paratuberculosis has been cultured from several patients with CD, and some investigations have noted increased titers of antibody against mycobacterial antigens in CD patients (24, 42, 123). However, clinical and immunohistochemical studies have failed to provide convincing evidence of a role for mycobacteria in CD (42, 121, 129). In recent years, a number of studies utilizing PCR methods have addressed the mycobacterial hypothesis. The majority of these investigations used primers recognizing IS900, a multicopy genomic DNA insertion element highly specific for M. paratuberculosis. Most of the PCR studies suggest that mycobacterial sequences can be detected in intestinal tissue but that their presence is not specific for CD (42, 129).
Wakefield et al. have suggested that persistent measles infection, particularly when exposure occurs in utero or early in life, may lead to CD (132, 133). This hypothesis proposes that CD is a chronic granulomatous vasculitis in reaction to a persistent infection with measles virus, which exhibits a tropism for the submucosal endothelium of the intestine. Epidemiologic evidence from Sweden has suggested a link between CD and early measles infection (19, 21). In addition, paramyxovirus-like structures have been visualized in the vascular endothelium of CD patients by electron microscopy and measles antigen has been detected in areas of granulomatous inflammation (132, 133). However, other investigators have not been able to confirm an epidemiological link between CD and early measles infection (2a, 25, 37) or to confirm the association by immunohistochemical staining or serologic studies (2, 27, 44).
In summary, despite considerable effort including sensitive PCR-based studies to screen for the presence of microbes, no one particular infectious organism has been definitively associated with IBD. Still, the possibility exists that an as yet unidentified organism that is difficult to detect by current methods is the cause of IBD. However, even if an infectious pathogen is involved, genetic factors clearly are important in determining whether IBD subsequently develops, as discussed below.
Dysregulated Immune Response to Commensal Bacteria
In recent years, a growing body of data has implicated intestinal commensal bacteria in the pathogenesis of IBD and in particular of CD (22, 105, 120). For example, rodent models of IBD exhibit marked reductions in the clinical and histologic signs of intestinal inflammation when the animals are housed in a germ-free environment (6, 16, 23, 113). In human IBD patients, increased levels of mucosal antibodies directed against intestinal bacteria have been observed (105, 120). In addition, the clinical response of CD patients to antibiotics and the beneficial effect of fecal diversion in selected CD patients with perianal disease suggest an involvement of bacteria in the disease symptoms (11, 125, 136). Nonetheless, the precise role of the indigenous gut bacteria in the development and subsequent disease manifestations of IBD remains poorly understood.
Although large numbers of bacteria colonize the lumen of the intestine, several studies including our own have revealed that under normal conditions the intestinal mucosa is relatively free of adherent bacteria (1, 38, 108, 134). However, a number of investigations have suggested that increased numbers of bacterial antigens and nucleic acid sequences are found associated with the intestinal mucosa in IBD (9, 56, 108, 134).
Thus, IBD patients may have alterations in the mucus-epithelial layer which allow a more intimate association of gut bacteria with the mucosa. Potentially, the presence of mucosa-associated indigenous bacteria in these patients produces disease despite the absence of more aggressive pathogenic bacteria. The closer proximity of the bacteria to the epithelium may be a crucial component in the initiation of the cycles of inflammation and changes in intestinal permeability that are characteristic of IBD. Genetic factors may contribute to both the increased penetration of the bacteria or bacterial products as well as the pronounced inflammatory response that occurs.
The identities of the commensal bacteria that may be involved in the pathogenesis of IBD are unclear. Some investigations have pointed to a role for anaerobic bacteria such as Bacteroides or Clostridium spp. (65, 93, 94, 105). For instance, interleukin-10 (IL-10)-deficient mice develop colitis under conventional housing conditions but not under germ-free conditions. At 2 weeks of age, prior to the development of colitis, increased numbers of Clostridium species were noted adhering to the colonic mucosa of the mice (65). Treatment of the mice, before the onset of colitis, with the antibiotic metronidazole, which is active against anaerobic bacteria, prevented disease.
Other studies have suggested an involvement of the gram-negative bacterium E. coli in CD (9, 12, 56, 117, 134). For example, E. coli antibody titers have been observed to be higher in patients with IBD than in controls (117) and E. coli antigens or nucleic acid sequences have been detected with increased frequency in the intestines of patients with CD (9, 56, 134). In addition, Darfeuille-Michaud et al reported that a greater number of E. coli strains harvested from the ilea of patients with CD than of patients without CD exhibited mannose-resistant adherence to the human intestinal cell line Caco-2 (12). Our own data would suggest that some mucosa-associated E. coli strains are capable of altering the permeability of cultured intestinal cell monolayers (96).
The identities of the bacterial antigens that may be implicated in the pathogenesis of CD are unknown. However, cell wall components such as lipopolysaccharide and other bacterial products have strong proinflammatory effects and mediate intestinal inflammation in animal models (104). Furthermore, in one study, bacterial intimin was found to induce mucosal hyperplasia and a prominent T-helper response in the colon in a rodent model (40). In addition, the presence of novel DNA sequence with homology to the ptxR and tetR bacterial transcription factor family has been reported to be associated with CD colonic lesions (116).
Nod2 appears to function in host signaling pathways activated by gram-negative bacterial lipopolysaccharide (44a). Recently, an association of certain Nod2 variants with CD was noted (43a, 80). This finding strongly suggests that interplay between the gram-negative bacterial flora and intestinal innate immune responses is a critical element in the pathogenesis of CD.
ANIMAL MODELS
Although none of the current animal models reproduce human IBD completely, animal models of intestinal inflammation have provided useful insights into the pathogenesis of the intestinal inflammatory response. Animal models of IBD have been referred to as spontaneously occurring or induced (6, 16). Induced models of IBD include (i) animals which have been treated with agents that promote intestinal inflammation, (ii) rodents that have been genetically manipulated through gene targeting or the introduction of transgenes, and (iii) immunodeficient animals into which cell populations that mediate intestinal inflammation have been transferred.
Spontaneously Occurring Models
Spontaneous colitis that develops in cottontop tamarins resembles IBD. In addition, selective breeding of C3H/HeJ mice at Jackson Laboratories (Bar Harbor, Maine) produced a strain of mice referred to as the C3H/HeJBir strain. These mice spontaneously develop inflammation of the cecum and right colon that generally peaks at 3 to 6 weeks of age and resolves by 10 to 12 weeks of age (115). Small lesions at the anorectal junction, however, are common throughout life. The etiology of the colitis remains uncertain, but the C3H/HeJ parenteral strain is particularly sensitive to mucosa-injuring agents such as trinitrobenzene sulfonic acid and dextran sulfate sodium (23).
In addition, a subline of the senescence-accelerated mouse (SAM)P1/Yit strain has been established from AKR/J mice (68). This strain of mice exhibits spontaneous enteric inflammation and skin ulcerations under specific-pathogen-free conditions. The intestinal disease involves the distal part of the small intestine and cecum and histologically appears similar to human CD. Germ-free (SAM)P1/Yit strain mice do not develop intestinal inflammation, but reintroduction of fecal bacteria into the gut induces disease (68).
Induction of IBD with Exogenous Reagents
Luminal agents capable of damaging the intestinal mucosa are able to produce an inflammatory response that may become chronic in genetically susceptible hosts and may mimic some aspects of human IBD. For example, colitis can be induced by administering 5% dextran sulfate sodium in the drinking water, treating the host with indomethacin, or giving an enema containing trinitrobenzene sulfonic acid in ethanol (23). However, the absence of chronicity is a significant shortcoming of acute nonspecific injury models of IBD. Nonetheless, regeneration of the colonic mucosa after repeated cycles of 5% dextran sulfate sodium administration takes several weeks and is a frequent model used for studying mucosal repair mechanisms (16, 23).
IBD in Genetically Altered Rodents
A number of mouse strains generated by gene-targeting methods exhibit intestinal inflammation when housed under conventional conditions. A list of these mouse strains is given in Table 2. Several of these mouse models have targeted mutations that affect cytokine secretion or the CD4+ T-cell population. Cytokines are local mediators of a number of biological processes including cell growth and differentiation as well as inflammatory and immune responses. CD4+ lymphocytes can be classified as Th1-type cells or Th2-type cells on the basis of their cytokine secretion profiles. Th1-type cells are stimulated by IL-12 and produce IL-2, gamma interferon (IFN-γ), and TNF. Th2-type cells secrete IL-4, IL-5, IL-10, and IL-13. In general, Th1 cells are effective inducers of cellular immune responses and Th2 cells support humoral immune responses.
TABLE 2.
Examples of animal IBD models
| Spontaneously occurring |
| C3H/HeJBir mouse strain |
| SAMP1/Yit strain mice |
| Treatment with mucosal-injuring agents |
| Trinitrobenzene sulfonic acid (TNBS) enemas |
| Dextran sulfate sodium (DSS) administration |
| Alteration of cytokine function |
| IL-10 knockout mouse |
| IL-2 knockout mouse |
| TNF ΔARE mice |
| STAT-4 transgenic mice |
| Alteration of T-cell function |
| T-cell receptor α knockout mouse |
| T-cell receptor β knockout mouse |
| HLA-B27 transgenic rat |
| Transfer of CD4+ CD45RBhi cells into SCID or Rag−/− mice |
| Impairment of epithelial barrier function |
| Mutated multidrug-resistant gene mice |
| Intestinal trefoil factor knockout mice |
The cytokine IL-2 stimulates the growth and expansion of T lymphocytes and the activation of other cells, including macrophages. IL-2 knockout mice develop a symptomatic pancolitis with intermittent gastrointestinal bleeding, diarrhea, and often rectal prolapse (23, 102). The colon is grossly thickened and contains mucosal ulcerations. On histopathologic testing, epithelial hyperplasia, crypt distortion and abscesses, and acute and chronic inflammatory cells infiltrating into the lamina propria are noted. Various studies have suggested that CD4+ T cells, rather than B cells, are critical in the pathogenesis of the gut inflammation in IL-2-deficient mice (60).
IL-10 is a cytokine secreted by Th2 cells as well as other cells that inhibits the synthesis of IL-12 and proinflammatory cytokines such as IFN-γ and TNF. IL-10-deficient mice develop a chronic enterocolitis mediated by Th1 cells under conventional housing conditions (23, 51). Histologically, the disease is characterized by excessive regenerative hyperplasia of the mucosa, leading to a marked thickening of the intestinal wall, abnormal crypt and villous architecture, and extensive lymphoplasmocytic and histiocytic infiltration of the lamina propria and submucosa.
AU-rich elements (ARE) in the TNF gene regulate TNF biosynthesis. Mice lacking the ARE produce a more stable TNF mRNA and fail to appropriately repress TNF expression (50). These mice develop CD-like IBD that is presumably related to overexpression of TNF.
Mice with targeted mutations of either T-cell receptor α or T-cell receptor β genes develop colitis with crypt distortion and an inflammatory infiltrate in the lamina propria (75). The pathogenesis of disease in these T-cell receptor knockout mice is unclear, but the extent of disease markedly varies among different inbred strains of mice, suggesting that other susceptibility loci influence disease expression (23).
HLA-B27 transgenic rats with susceptible genetic backgrounds develop chronic colitis and joint inflammation (93, 105). Inflammation is T-cell mediated, particularly by CD4+ T cells, and the presence of commensal bacteria in the intestine appears necessary for disease. Studies suggest that the colitis may result from a loss of tolerance to enteric bacteria (105).
Investigations in other rodent models suggest that a primary defect in macrophage function also can lead to intestinal inflammation. For instance, mice which are lacking the transcription factor STAT-3 have macrophages that fail to be inhibited by IL-10, which generally down-regulates inflammatory responses (118). When STAT-3-deficient mice are treated with lipopolysaccharide, which activates the macrophages to secrete proinflammatory cytokines such as TNF, the mice develop mucosal inflammation (6, 118).
Intestinal epithelial cells are crucial in maintaining the barrier to luminal antigens and bacteria. Investigations using mice generated by gene targeting have shown that epithelial defects in cellular transport, repair, and barrier function also may lead to mucosal inflammation. For instance, mice carrying a mutated multidrug resistance gene lack the ability to pump out small amphiphilic and hydrophobic molecules from within the cell and have an increased sensitivity to certain drugs (85). These mice develop intestinal inflammation that resembles UC, and repletion with normal lymphocytes does not abrogate the colitis. Although both intestinal epithelial cells and lymphocytes express multidrug resistance genes, this observation suggests that the defect in epithelial cell function may be of primary importance (6). Prophylactic treatment with oral antibiotics did prevent colitis in these mice (85). Cadherins are important mediators of epithelial cell adhesion and migration. Mice with altered N-cadherin expression in the intestine develop IBD resembling CD (39). Another example involves the trefoil peptides, which are protease-resistant molecules secreted by mucin cells in the intestine and which have been implicated in mucosal healing. Mice lacking intestinal trefoil factor died of extensive colitis after oral administration of dextran sulfate sodium (67).
Adoptive Transfer of CD4+ Cells into Immunodeficient Mice
The adoptive transfer of CD4+ T cells expressing high levels of CD45RB into SCID mice, which lack B and T cells, results in intestinal inflammation accompanied by diarrhea and weight loss (23). In this model, simultaneous transfer of the CD45RB low-CD4+ T cells prevents the development of inflammation, possibly through the secretion of transforming growth factor β (89).
IMMUNE MEDIATORS OF INTESTINAL INFLAMMATION: LESSONS FROM ANIMAL MODELS
Role of T Cells
CD4+ T cells play a critical role in the mucosal inflammation observed in animal models of IBD. In contrast, CD8+ T cells appear to be of lesser importance. For example, studies with IL-2-deficient mice intercrossed with β2-microglobulin-deficient mice, which lack mature CD8+ T cells, revealed that CD8+ T cells are not necessary for the development of colitis (6). In addition, the significance of CD4+ T cells is demonstrated by the observations that (i) CD4+ T cells are found infiltrating the lamina propria in IBD and (ii) as discussed above, transfer of CD4+ T-cell populations into SCID and Rag−/− mice, which lack mature B and T cells, results in intestinal inflammation.
The T-cell population that appears to mediate the intestinal inflammation in most animal models is an IL-12-producing Th1 cell that secretes IFN-γ and TNF (6, 63, 70). Notably, Th2 cells also may play a role in the intestinal inflammation in some animal models of IBD (73). For example, in experimental colitis resulting from the administration of oxazolone to SJL/J mice, IL-4-producing Th2 cells appear to be important mediators of the inflammation since inflammation is reduced by treating the mice with anti-IL-4 or intercrossing the mice with IL-4 knockout mice (7).
Role of Cytokines
Considerable data point to a prominent role for IL-12 in the development of IBD. For example, in several rodent models of IBD, treatment with anti-IL-12 antibody prevents the development of colitis (6, 109). Further, when intercrossed with IL-12 knockout mice, the TNF ARE knockout mice described above do not develop intestinal inflammation, emphasizing the importance of IL-12 in the initiation of the mucosal inflammation in this mouse model (6).
The binding of IL-12 to its receptor activates a signaling pathway that results in the activation of the transcription factor STAT-4. In the adoptive-transfer model, transfer of T cells from STAT-4-deficient mice into immunodeficient mice results in attenuated disease compared to the transfer of wild-type lymphocytes (109). Further transgenic mice that overexpress STAT-4 develop intestinal inflammation characterized by infiltration with IFN-γ- and TNF-secreting cells (135).
Current data would suggest that IL-12 influences inflammation through both IFN-γ-dependent and IFN-γ-independent mechanisms. While IFN-γ plays a role in mediating the inflammation in animal models of IBD, treatment with anti-IFN-γ is generally a less effective treatment modality than is treatment with anti-IL-12. In addition, IFN-γ knockout mice still develop colitis when exposed to trinitrobenzene sulfonic acid (6). Also, in the adoptive-transfer model, T cells from IFN-γ-deficient mice were demonstrated to produce TNF-α and caused colitis in reconstituted immunodeficient mice (109). These data suggest that other cytokines such as TNF are important in the mucosal inflammatory response in animal models of IBD.
The lymphotoxins alpha and beta are members of the TNF cytokine superfamily and are involved both in the development and maintenance of lymphoid tissues. Administration of soluble lymphotoxin beta receptor immunoglobulin fusion protein attenuated the clinical and histologic disease manifestations in murine models of colitis (64). This study suggests that in addition to TNF, lymphotoxins may play a role in the intestinal inflammation in IBD.
In summary, a number of studies have indicated that defective regulation of Th1 responses, resulting in the unchecked secretion of proinflammatory cytokines such as IFN-γ and TNF, leads to the development of enteritis.
Other Inflammatory Mediators
The secreted factors implicated in the intestinal inflammatory response in IBD are listed in Table 3. Chemokines constitute a large family of chemoattractant peptides that attract and activate leukocytes (26). Chemokines such as IL-8, MCP-1, and ENA-78 are highly expressed in the intestinal mucosa in areas of active CD and UC (62) and may be important mediators of inflammation in IBD.
TABLE 3.
Secreted factors implicated in the intestinal inflammatory response in IBD
| Class | Factor |
|---|---|
| Cytokines | IL-12, TNF, IFN-γ, IL-10, IL-18, IL-15, lymphotoxins alpha and beta, IL-1β |
| Chemokines | IL-8, MCP-1, ENA-78, RANTES |
| Growth factors | Keratinocyte growth factor, vascular endothelial growth factor, epidermal growth factors, trefoil factors |
| Other | Matrix metalloproteinases |
Another potentially significant component in the tissue injury that occurs in IBD is the activation of matrix metalloproteinases. Matrix metalloproteinases are a group of tissue proteases that exhibit preferred cleavage specificity for the N-terminal side of hydrophobic residues (63). Studies have revealed high levels of extracellular matrix metalloproteinases in areas of ulceration in the intestines of humans with IBD (63).
In addition, a number of different growth factors may play an important role in IBD (3). For example, keratinocyte growth factor is produced by gut stromal cells and is activated by proinflammatory cytokines. Keratinocyte growth factor has been implicated in the crypt hyperplasia noted in areas of inflammation in IBD (3, 63).
Conclusions from the Animal Models
In summary, conclusions from studies in the animal models have emphasized the importance of both genetic and environmental factors in the pathogenesis of IBD. In addition, the animal models have revealed that many types of alterations in the immune response can result in intestinal inflammation. However, the importance of CD4+ T cells in mediating intestinal inflammation has been repeatedly shown. Lastly, the animal models strongly suggest that antigens derived from commensal bacteria may drive IBD.
Pathogenesis of Human Disease
A number of studies have suggested that human CD is a Th1-mediated disease and that excessive Th1-cell activity is a critical component of CD (63, 86). For example, CD is characterized by increased numbers of activated T cells in the intestinal mucosa. These T cells have a Th1 profile and secrete IFN-γ. Like IL-12, IL-18 is cytokine involved in Th1-cell development and IFN-γ production. Both IL-12 and IL-18 are found at increased levels in the intestinal mucosa of persons with (76, 88). Also, lamina propria lymphocytes harvested from the intestines of persons with CD have been shown to produce IL-12 (63). In addition to IL-12, TNF clearly is an important mediator of the intestinal inflammation in human CD. As stated above, several clinical trials have shown a chimeric anti-TNF-α antibody to be effective in the treatment of CD, verifying the importance of TNF in the ongoing intestinal inflammation (90, 101, 119, 128). IL-15 is a cytokine with a number of biological activities including the stimulation of T-cell proliferation and migration to sites of inflammation. Increased levels of IL-15 production by CD lamina propria T lymphocytes also have been reported (57).
Another documented feature of CD is increased intestinal permeability. Alterations in intestinal permeability may be an early finding in CD and have been noted in asymptomatic relatives of persons with CD (104).
Less information is available regarding the pathogenesis of UC. However, several studies suggest that UC differs from CD in the profile of cytokines in the intestinal mucosa (86). For example, Th2 cells rather than Th1 cells have been hypothesized to play a prominent role in UC, but only limited data are available to confirm this idea. In support of this notion, though, IL-12 transcripts have been found in the intestinal mucosa of CD patients but not generally in UC (63, 86). Also, the UC intestine has many immunoglobulin G IgG-secreting plasma cells and IgG1 colocalizing with the complement component C3b on the surface of epithelial cells (63). In addition, in UC evidence for an abnormal humoral immune response exists, such as the presence of antibodies to the perinuclear component of neutrophils as mentioned above. Nonetheless, although lamina propria T cells from UC patients have been reported to secrete greater amounts of IL-5 than do those from controls, the production of IL-4 does not appear to be increased in UC, which would be expected with a Th2-cell response (30, 63, 86).
PATHOGENESIS OF EXTRAINTESTINAL MANIFESTATIONS
As outlined above, a number of extraintestinal manifestations of CD and UC have been described. Although the occurrence of extraintestinal symptoms often coincides with intestinal disease activity, the pathogenesis of these manifestations of IBD is not well understood. In the case of arthritis, studies of HLA-B27 transgenic rats suggest that recirculation of memory T cells between the intestine and synovium occurs (14, 23, 105). In support of this idea, adherence of lamina propria lymphocytes to synovial high endothelial venules has been demonstrated. A role for mucosal macrophages transporting antigens from the intestine to the joints also has been postulated. In addition, in the HLA-B27 transgenic rat, intestinal bacteria appear to play a significant role in the pathogenesis of the arthritis (54, 93, 105).
As mentioned above, PSC is a chronic liver disease often associated with UC but also seen in the absence of underlying UC. Although the association of UC with PSC remains poorly understood, both groups of patients have an increased incidence of antineutrophil cytoplasmic antibodies, which may play a role in the disease (91).
GENETICS
Genetic Epidemiology
An increase in the incidence of CD has been observed in nearly all Western countries over the past three decades (4). Two countries, namely, Sweden (55) and the United States (59), have observed a recent leveling off at a high incidence of 6/100,000, while continued increases in incidence have been observed in Copenhagen (77) and Cardiff (122). In contrast, the incidence of UC has leveled off in most countries. For example, in Denmark, the incidence has remained stable for 30 years at between 8 and 9/100,000 inhabitants (52). However, the incidence of UC in Iceland continues to rise and is now among the highest in the world at 12/100,000 (5). While the incidence among African Americans has generally been reported to be lower than that in Caucasian Americans, a recent survey of children in Georgia reported an incidence of CD and UC of 7 to 12/100,000 and 5 to 7/100,000, respectively (79).
Family studies of IBD have demonstrated increased familial aggregation for both CD and UC. The frequency of familial occurrence among unselected individuals with IBD has been reported to be as high as 20 to 30% in referral-based studies, but has ranged between 5 and 10% in population-based surveys (4). The prevalence and relative risk of IBD among relatives of patients with UC or CD can be estimated using either cohort or case-control approaches. The former requires complete information on pedigree structure as well as prevalence of disease in the surrounding population. Using either approach, the relative risk (disease prevalence among first-degree relatives divided by population prevalence) in multiple European populations is approximately 15-fold. The lifetime risk of IBD can most accurately be estimated for parents of IBD patients and was 1.9% in a European cohort (87). Estimates of lifetime risk among siblings and children are more difficult to approximate due to difficulties in correcting for age-adjusted incidence rates, but certainly the lifetime risk is higher than in parents of IBD patients. It is well established that the population prevalence as well as the familial risk is higher among Jews than among non-Jews (137). Among multiply affected families with IBD, 75% are concordant for disease type (that is, families are either pure UC or pure CD) and the remaining 25% have separate members with CD and UC (4).
The familial recurrence risk reflects the contributions of both environmental and genetic factors. Higher concordance rates among monozygotic twins compared to dizygotic twins raised together would implicate genetic contributions to disease pathogenesis. Complete 100% concordance among monozygtic twins would indicate that only genetic factors are required for disease expression. Monozygotic-twin concordance for CD has ranged between 42 and 58%, whereas disease concordance for UC is between 6 and 17% (84, 114, 126). These data indicate that genetic factors are more significant in CD than in UC. The concordance rate among dizygotic twins is 4 to 12% for CD and 0 to 5% for UC, significantly lower than the rates reported among monozygotic twins and of the same order of magnitude as in nontwin siblings. One monozygotic-twin pair with one member having UC and the other having CD has been reported (8). However, the overall paucity in the literature of such discordant twin pairs would suggest that similar but distinct genetic factors contribute to disease pathogenesis for these two diseases.
Genome-Wide Genetic Linkage Studies
The search for disease genes contributing to disease in common, complex genetic disorders such as IBD can include either candidate gene studies or genome-wide searches using genetic linkage. While genetic factors clearly play a role in disease pathogenesis, multiple genes probably contribute to IBD as evidenced by the inability to predict disease occurrence within families with the Mendelian certainty observed for monogenic disorders. The contributions of multiple distinct genes can result from epistatic interactions between two genes (that is, the combined phenotypic effect cannot be predicted from either gene alone), which might require that multiple genetic variants be inherited simultaneously for disease expression to occur. Genetic heterogeneity, where different sets of genes result in similar phenotypic expression in different sets of patients, further complicates attempts to identify disease genes.
Genome-wide searches to identify disease genes have been used successfully to refine the localization of and ultimately identify disease genes for monogenic disorders, such as cystic fibrosis. Genetic markers are typed throughout the genome in order to identify chromosomal regions that are shared more frequently than expected on a statistical basis between affected family members. These regions of increased sharing between family members with the same disease probably contain a disease-contributing genetic variant. Multiple genome-wide searches for IBD genes have been reported, and significant linkages on chromosomes 16, 12, 7, 3, 1, 6, 14, and 19 have been reported in at least one study (10, 17, 35, 43, 61, 95, 107). These studies have varied with respect to the fraction of pure CD, pure UC, and mixed families reported, which reflects in part the differences in referral patterns between centers. For the most part, many more pure-CD families have been studied, and among these linkages, the best established is the linkage observed among pure-CD families to the pericentromeric region of chromosome 16. Fewer UC families have been studied, and UC is likely to be a less genetic disorder than CD. However, significantly greater evidence for linkage was observed for UC than CD on chromosome 12, which may explain the less consistent replication observed for this region compared to chromosome 16. The presence of mixed families, having one member with CD and another member with UC, suggests that at least some genetic variants will contribute to both diseases.
As stated above, recent reports have linked certain Nod2 variants to CD (43a, 80). The Nod2 gene is located within the peak region of linkage on chromosome 16, which has been established as a susceptibility locus for CD but not UC (43). The Nod2 variants associated with CD include a frameshift mutation due to a cytosine insertion, 3020InsC, that is expected to encode a truncated Nod2 protein lacking the 33 C-terminal amino acids. Nod2 has homology to plant disease resistance gene products and has been implicated in NF-κB activation. NF-κB is a transcription factor involved in cellular inflammatory responses (44a). While the identification of CD-linked Nod2 variants undoubtedly will provide novel insights into the pathogenesis of CD, a minority of CD patients appear to have the Nod2 variants described to date. In addition, some asymptomatic persons (about 5% of the general population) also have the disease-associated Nod2 variants. Consequently, these data suggest the importance of additional genetic loci in the development of CD.
Linkage Studies in the Chromosome 6p Region
In addition to the pericentromeric region of chromosome 16, significant evidence for linkage to the chromosome 6p region has been reported for CD as well as for UC. The chromosome 6p region contains the major histocompatibility complex, which includes the HLA genes as well as numerous other immune-associated genes, including the TNF, gene. Thus, linkage to this region is not surprising, given the chronic inflammatory nature of these disorders (34, 138). However, in contrast to type I diabetes mellitus, where genetic linkage studies are dominated by the chromosome 6p linkage, the linkage evidence here for IBD is one among several equally significant linkages. This indicates that non-major histocompatibility complex genes are at least equally significant in disease pathogenesis compared to the contribution of MHC genes.
The TNF gene represents an ideal candidate gene for IBD. As detailed above, increased expression of TNF in mice results in a clinical picture of ileitis and arthritis (50) and anti-TNF treatments have proven to be highly effective therapies for CD (90, 101, 119, 128). Multiple genetic variants in the promoter region of the TNF gene have been associated with increased transcriptional activity of mRNA (41, 110). Association studies with various TNF promoter variants have demonstrated conflicting results with respect to IBD; however, a recent Japanese study has demonstrated positive associations of novel upstream promoter variants of the TNF gene with CD (78). The small numbers of study patients have limited many of the reported case-control association studies, and definitive conclusions on the association with disease await the completion of much larger studies.
Association Studies of HLA Class II Genes and IBD
At present, there are no universally observed associations between any single specific intragenic risk allele and IBD. Given that many genuine disease associations will confer only modestly increased relative risks, very large studies are required to definitively establish disease associations. Completion of the human genomic sequence is currently being followed by large-scale efforts to resequence the genome (in multiple individuals) in order to establish common allelic variants in all expressed genes. Establishing which of these intragenic molecular variants have direct consequences on gene function and regulation represents a broad central goal of genetic research efforts.
Given the enormous genetic variation present in the major histocompatibility complex and the central role of these genes in the immune response, the HLA class II genes are candidates for association with IBD. In particular, a large number of case-control association studies have tested HLA-DR gene associations with CD and UC. Interpretation of these studies is complicated by the fact that earlier studies (which comprise a large fraction of studies reported in the literature) used broad serologic subtypes, which have subsequently been shown to correspond to multiple DNA-based genotypes. For example, the serologic subtype DR4 corresponds to DNA genotypes DRB1*0401 through DRB1*0419 and serologic subtype DR2 corresponds to DNA genotypes DRB1*1501 through DRB1*1504 and DRB1*1601 through DRB1*1606. Furthermore, within HLA DR2, the DRB1*15 subtypes have been reported to be positively associated with UC whereas the DRB1*16 subtypes are negatively associated with UC (112).
Given these limitations in interpreting the present literature, the large majority of reported association studies in UC have reported a positive association with HLA DR2 and a negative association with HLA DR4. While the most significant associations between UC and HLA DR2 have been reported in Japanese populations (with an odds ratio of 4.92), a significant positive association was observed in pooled Caucasian population studies (odds ratio, 1.51; 95% confidence interval, 1.20 to 1.90) as well (112). While the number of studies performing DNA genotyping has been smaller, positive contributions to the DR2 association were found to be made by both the DRB1*1501 and DRB1*1502 genotypes (112). Positive associations with DRB1*0103 (29, 106) and DR9 (112) also have been reported. A list of associations is given in Table 4.
TABLE 4.
HLA class II associations with IBD
| HLA definition
|
Association with disease
|
||
|---|---|---|---|
| Serologic specificity | DNA genotypes | UC | CP |
| DR103 | DRB1*0103 | Positive | |
| DR2a | DRB1*1501–DRB1*1504 | Positive to DR2 overall | Negative association with DR2 |
| DRB1*1601–DRB1*1606 | Positive to DR15, negative to DR16; positive to DRB1*1501, DRB1*1502 | ||
| DR7 | DRB1*0701 | Positive | |
| DR9 | DRB1*0901 | Positive | |
| DR52c | DRB3*0301 | Positive | |
Serologic subtype DR2 can be subtyped as DR15 or DR16.
In contrast, in a meta-analysis of CD studies, the HLA DR2 subtype was negatively associated with disease, and the DR7, DRB3*0301 and DQ4 subtypes were positively associated with disease (112). The very consistent observations of opposite associations of HLA-DR2 between CD and UC suggest that some disease associations at the class II genes represent phenotype-modifying factors rather than a primary pathogenic response to specific antigens required for disease expression.
HLA Association Studies and Extraintestinal Manifestations of Disease
Significant HLA associations have been identified with specific extraintestinal manifestations of IBD, with the best characterized being the association of ankylosing spondylitis with HLA-B27. Ankylosing spondylitis has been associated with both UC and CD (124). Differences in endogenous peptide presentation by HLA-B27 subtypes might be relevant in the disease pathogenesis. HLA associations here possibly involve arthritic peptides and molecular mimicry, but proof that ankylosing spondylitis is an autoimmune disease attributable to cross-reactivity between bacteria and HLA-B27 is still lacking (127).
HLA associations also have been reported for peripheral arthropathies associated with IBD. For example, the two subtypes of IBD-associated peripheral arthropathies (type I, pauciarticular arthropathy, typically affecting large joints, lasting less than 5 weeks, and associated with intestinal relapses; type II, polyarticular aruthropathy, causing persistent, long-lasting symptoms that are independent of intestinal course) have distinct HLA associations (83).
In addition, while less well established, there is some evidence to support positive associations of multiple class II haplotypes among patients manifesting both IBD and PSC (130).
Genetics and Environmental Factors: Effect of Tobacco on Disease Phenotype
Just as genetic factors will probably include modifiers that affect disease phenotype (expression as UC versus CD), environmental factors will affect specific disease features as well. Of these environmental factors, by far, the best characterized is tobacco exposure (99). Tobacco use is protective against the development of UC; while less consistently observed, it has been positively associated with CD. Specifically, active smokers were less likely to develop UC than were nonsmokers (odds ratio, 0.53; 95% confidence interval, 0.24 to 1.14). In addition, the risk was decreased in passive smokers, i.e., persons whose parents had smoked (odds ratio, 0.50; 95% confidence interval, 0.25 to 1.00) (103). The protective effect of tobacco against developing UC is similar to that observed for celiac disease, a chronic inflammatory disorder of the proximal intestine. The duration of the protective effect against developing UC in one study appears to be limited to 3 years; this would explain the frequently noted observation that UC is a disease of ex-smokers. In contrast, the protective effect of tobacco use against developing celiac disease appears to be longer-lived (111).
This effect of tobacco is not limited to inflammation of the intestine; the odds of having PSC also was significantly decreased among current smokers (the estimated odds of having PSC in current smokers compared with never-smokers was 0.13). Therefore, the related disorders UC and PSC are both diseases of nonsmokers. However, the association between UC and PSC does not fully explain the association between nonsmoking and PSC (58).
The effect of tobacco on disease expression holds within multiply affected families with IBD as well (53). Specifically, in a European cohort, within pure-CD multiplex families, the frequency of tobacco use was 64%, whereas within pure-UC families, 31% of affected individuals used tobacco. Interestingly, this trend held for mixed families having one member with CD and another with UC; among members of mixed families with CD, the frequency of tobacco use was 64%, compared to 23% among individuals with UC.
CONCLUSIONS
IBD is a common disease that causes considerable morbidity in the United States and Europe. In recent years, important advances have been made in our understanding of the immune mediators of intestinal inflammation. This information has led to new therapeutic approaches to IBD. Furthermore, considerable data point to the significance of genetic factors, which may predispose patients to a dysregulated immune response, in the development of IBD. The commensal bacterial flora also appears to be a critical element, particularly with respect to CD. While the precise role of the commensal bacteria and the genetic factors involved in the pathogenesis IBD remain ill defined, research in the next decade promises to yield fresh insights into these areas.
REFERENCES
- 1.Amann, R. I., L. Krumholz, and D. A. Stahl. 1990. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 172:762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andus, T., and V. Gross. 2000. Etiology and pathophysiology of inflammatory bowel disease—environmental factors. Hepatogastroenterology 47:29–43. [PubMed] [Google Scholar]
- 2a.Anonymous. 1998. WHO concludes that measles viruses are not associated with Crohn’s disease. Commun. Dis. Rep. Wkly. 8:75–78. [PubMed] [Google Scholar]
- 3.Beck, P. L., and D. K. Podolsky. 1999. Growth factors in inflammatory bowel disease. Inflamm. Bowel Dis. 5:44–60. [DOI] [PubMed] [Google Scholar]
- 4.Binder, V. 1998. Genetic epidemiology of inflammatory bowel disease. Dig. Dis. 16:351–355. [DOI] [PubMed] [Google Scholar]
- 5.Bjornsson, S., J. H. Johannsson, and E. Oddsson. 1998. Inflammatory bowel disease in Iceland, 1980–1989. A retrospective nationwide epidemiologic study. Scand. J. Gastroenterol. 33:71–77. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg, R. S., L. J. Saubermann, and W. Strober. 1999. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr. Opin. Immunol. 11:648–656. [DOI] [PubMed] [Google Scholar]
- 7.Boirivant, M., U. Fuse, A. Chu, and W. Stober. 1998. Oxazolone colitis: a murine model of T helper cell type-2 colitis treatable with antibodies to interleukin-4. J. Exp. Med. 188:1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breslin, N. P., A. Todd, C. Kilgallen, and C. O’Morain. 1997. Monozygotic twins with Crohn’s disease and ulcerative colitis: a unique case report. Gut 41:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartun, R. W., H. J. Van Kruiningen, C. A. Pedersen, M. M. Berman. 1993. An immunocytochemical search for infectious agents in Crohn’s disease. Mod. Pathol. 6:212–219. [PubMed] [Google Scholar]
- 10.Cho, J. H., D. L. Nicolae, L. H. Gold, C. T. Fields, M. C. LaBuda, P. M. Rohal, M. R. Pickles, L. Qin, Y. Fu, J. S. Mann, B. S. Kirschner, E. W. Jabs, J. Weber, S. B. Hanauer, and S. R. Bayless. 1998. Identification of susceptibility loci for inflammatory bowel disease on chromosomes 1p, 3q, and 4q: evidence for epistasis between 1p and IBD1. Proc. Natl. Acad. Sci. USA 95:7502–7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombel, J. F., M. Lemann, M. Cassagnou, Y. Bouhnik, B. Duclos, J. L. Dupas, B. Notteghem, and J. Y. Mary. 1999. A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn’s Disease. Am. J. Gastroenterol. 94:674–678. [DOI] [PubMed] [Google Scholar]
- 12.Darfeuille-Michaud, A. D., C. Neut, N. Barnich, E. Lederman, P. Di Martino, P. Desreumaux, L. Gambiez, B. Joly, A. Cortot, and J. F. Colombel. 1998. Presence of adherent Escherichia coli strains in the ileal mucosa of patients with Crohn’s disease. Gastroenterology 115:1405–1413. [DOI] [PubMed] [Google Scholar]
- 13.Das, K. M. 1999. Relationship of extraintestinal involvements in inflammatory bowel disease: new insights into autoimmune pathogenesis. Dig. Dis. Sci. 44:1–13. [DOI] [PubMed] [Google Scholar]
- 14.De Vos, M., F. De Keyser, H. Mielants, C. Cuvelier and E. Veys. 1998. Bone and joint diseases in inflammatory bowel disease. Aliment. Pharmacol. Ther. 12:397–404. [DOI] [PubMed] [Google Scholar]
- 15.D’Haens, G., L. Lemmens, K. Geboes, L. Vandeputte, F. Van Acker, L. Mortelmans, M. Peeters, S. Vermeire, F. Penninckx, F. Nevens, M. Hiele, and P. Rutgeerts. 2001. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology 120:1323–1329. [DOI] [PubMed] [Google Scholar]
- 16.Dielman, L. A., A. S. Pena, S. G. M. Meuwissen, and E. P. Van Rees. 1997. Role of animal models for the pathogenesis and treatment of inflammatory bowel disease. Scand. J. Gastroenterol. 32(Suppl. 223):99–104. [PubMed] [Google Scholar]
- 17.Duerr, R. H., M. M. Barmada, L. Zhang, R. Pfutzer, and D. E. Weeks. 2000. High-density genome scan in Crohn’s disease shows confirmed linkage to chromosome 14q11–12. Am. J. Hum. Genet. 66:1857–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrenpreis, E. D., S. V. Kane, L. B. Cohen, R. D. Cohen, and S. B. Hanauer. 1999. Thalidomide therapy for patients with refractory Crohn’s disease: an open-label trial. Gastroenterology 117:1271–1277. [DOI] [PubMed] [Google Scholar]
- 19.Ekbom, A., A. J. Wakefield, M. Zack, and H. O. Adami. 1994. Perinatal measles infection and subsequent Crohn’s disease. Lancet 344:508–510. [DOI] [PubMed] [Google Scholar]
- 20.Ekbom, A., G. C. Helmick, M. Zack, L. Holmberg, and H. O. Adami. 1992. Survival and causes of death in patients with inflammatory bowel disease: a population based study. Gastroenterology 103:954–960. [DOI] [PubMed] [Google Scholar]
- 21.Ekbom, A., P. Daszak, W. Kraaz, and A. J. Wakefield. 1996. Crohn’s disease after in-utero measles virus exposure. Lancet 348:515–517. [DOI] [PubMed] [Google Scholar]
- 22.Elson, C. O. 2000. Commensal bacteria as targets in Crohn’s disease. Gastroenterology 119:254–257. [DOI] [PubMed] [Google Scholar]
- 23.Elson, C. O., R. B. Sartor, G. S. Tennyson, and R. H. Riddell. 1995. Experimental models of inflammatory bowel disease. Gastroenterology 109:1344–1367. [DOI] [PubMed] [Google Scholar]
- 24.Engstrand L. 1995. Mycobacterium paratuberculosis and Crohn’s disease. Scand. J. Infect. Dis. Suppl. 98:27–29. [PubMed] [Google Scholar]
- 25.Feeney, M., A. Ciegg, P. Winwood, and J. Snook. 1997. A case-control study of measles vaccination and inflammatory bowel disease. The East Dorset Gastroenterology Group. Lancet 350:764–766. [DOI] [PubMed] [Google Scholar]
- 26.Feng, L. 2000. Role of chemokines in inflammation and immunoregulation. Immunol. Res. 21:203–210. [DOI] [PubMed] [Google Scholar]
- 27.Fisher, N. C., L. Yee, P. Nightingale, R. McEwan, and J. A. Gibson. 1997. Measles virus serology in Crohn’s disease. Gut 41:66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folwaczny, C., N. Noehl, K. Tschop, S. P. Endres, W. Heldwein, K. Loeschke, and H. Fricke, 1997. Goblet cell autoantibodies in patients with inflammatory bowel disease and their first-degree relatives. Gastroenterology 113:101–106. [DOI] [PubMed] [Google Scholar]
- 29.Forcione, D. G., B. Sands, K. J. Isselbacher, A. Rustgi, D. K. Podolsky, and S. Pillai. 1996. An increased risk of Crohn’s disease in individuals who inherit the HLA class II DRB3*0301 allele. Proc. Natl. Acad. Sci. USA 93:5094–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuss, I. J., M. Neurath, M. Bonrivant, et al. 1996. Disparate CD4+ T lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-γ whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 157:1261–1270. [PubMed] [Google Scholar]
- 31.Gilat, T. M., and J. S. Langman. 1987. Childhood factors in the pathogenesis of inflammatory bowel disease: an international cooperative study. Scand. J. Gastroenterol. 22:1009–1024. [DOI] [PubMed] [Google Scholar]
- 32.Gokhale, R., M. J. Favus, T. Karrison, M. S. Sutton, B. Rich, and B. S. Kirschner. 1998. Bone mineral density assessment in children with inflammatory bowel disease. Gastroenterology 114:902–911. [DOI] [PubMed] [Google Scholar]
- 33.Grand, R. J., J. Ramakrishna, and K. A. Calenda. 1996. Therapeutic strategies for pediatric Crohn’s disease. Clin. Investig. Med. 19:373–380. [PubMed] [Google Scholar]
- 34.Hampe, J., S. H. Shaw, R. Saiz, N. Leysens, A. Lantermann, S. Mascheretti, N. J. Lynch, A. J. MacPherson, S. Bridger, S. van Deventer, P. Stokkers, P. Morin, M. M. Mirza, A. Forbes, J. E. Lennard-Jones, C. G. Mathew, M. E. Curran, and S. Schreiber. 1999. Linkage of inflammatory bowel disease to human chromosome 6p. Am. J. Hum. Genet. 65:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hampe, J., S. Schreiber, S. H. Shaw, K. F. Lau, S. Bridger, A. J. Macpherson, L. R. Cardon, H. Sakul, T. J. Harris, A. Buckler, J. Hall, P. Stokkers, S. J. van Deventer, P. Nurnberg, M. M. Mirza, J. C. Lee, J. E. Lennard-Jones, C. G. Mathew, and M. E. Curran. 1999. A genome-wide analysis provides evidence for novel linkages in inflammatory bowel disease in a large European cohort. Am. J. Hum. Genet. 64:808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanauer, S. B. 1996. Inflammatory bowel disease, p. 705–715. In J. C. Bennett and F. Plum (ed.), Cecil textbook of medicine, 20th ed. The W. B. Saunders Co., Philadelphia, Pa.
- 37.Haslam, N., J. F. Mayberry, A. B. Hawthorne, R. G. Newcombe, G. K. Holmes, and C. S. Probert. 2000. Measles, month of birth, and Crohn’s disease. Gut 47:801–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendrickson, B. A., J. Guo, R. M. Laughlin, Y. Chen, and J. Alverdy. 1999. Increased type 1 fimbrial expression among Escherichia coli isolates in the murine cecum following catabolic stress. Infect. Immun. 67:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hermiston, M. L., and J. L. Gordon. 1995. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 270:1203–1207. [DOI] [PubMed] [Google Scholar]
- 40.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588–591. [DOI] [PubMed] [Google Scholar]
- 41.Higuchi, T., N. Seki, S. Kamizono, A. Yamada, A. Kimura, H. Kato, and K. Itoh. 1998. Polymorphism of the 5′-flanking region of the human tumor necrosis factor (TNF)-alpha gene in Japanese. Tissue Antigens 51:605–612. [DOI] [PubMed] [Google Scholar]
- 42.Hubbard, J., and C. M. Surawicz. 1999. Etiological role of mycobacterium in Crohn’s disease: an assessment of the literature. Dig Dis. 17:6–13. [DOI] [PubMed] [Google Scholar]
- 43.Hugot, J. P., P. Laurent-Puig, C. Gower-Rousseau, J. M. Olson, J. C. Lee, L. Beaugerie, I. Naom, et al. 1996. Mapping of a susceptibility locus for Crohn’s disease on chromosome 16. Nature 379:821–823. [DOI] [PubMed] [Google Scholar]
- 43a.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O’Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411:599–603. [DOI] [PubMed] [Google Scholar]
- 44.Iizuka, M., M. Chiba, M. Yukawa, T. Nakagomi, T. Fukushima, S. Watanabe, and O. Nakagomi. 2000. Immunohistochemical analysis of the distribution of measles related antigen in the intestinal mucosa in inflammatory bowel disease. Gut 46:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Inohara, N., Y. Ogura, F. F. Chen, A. Muto, and G. Nuñez. 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem. 276:2551–2554. [DOI] [PubMed] [Google Scholar]
- 45.Kanof, M. E., A. M. Lake, and T. M. Bayless. 1988. Decreased height velocity in children and adolescents before the diagnosis of Crohn’s disease. Gastroenterology 95:1523–1527. [DOI] [PubMed] [Google Scholar]
- 46.Khoo, U. Y., I. Bjarnason, A. Donaghy, R. Williams, and A. Macpherson. 1995. Antibodies to colonic epithelial cells from the serum and colonic mucosal washings in ulcerative colitis. Gut 37:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirschner, B. S. 1998. Safety and efficacy of azathioprine (AZA) and 6-mercaptopurine (6-MP) in the treatment of children and adolescents with inflammatory bowel disease. Gastroenterology. 115:813–821. [DOI] [PubMed] [Google Scholar]
- 48.Kirschner, B. S., and M. S. Sutton. 1986. Somatomedin-C levels in growth-impaired children and adolescents with chronic inflammatory bowel disease. Gastroenterology 91:830–836. [DOI] [PubMed] [Google Scholar]
- 49.Kirsner, J. B., and R. G. Shorter. 1982. Recent developments in “non-specific” inflammatory bowel disease. N. Engl. J. Med. 306:775–785. [DOI] [PubMed] [Google Scholar]
- 50.Kontoyiannis, D., M. Pasparakis, T. T. Pizarro, F. Cominelli, and G. Kollias. 1999. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10:387–398. [DOI] [PubMed] [Google Scholar]
- 51.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274. [DOI] [PubMed] [Google Scholar]
- 52.Langholz, E., P. Munkholm, O. H. Nielsen, S. Kreiner, and V. Binder. 1991. Incidence and prevalence of ulcerative colitis in Copenhagen county from 1962 to 1987. Scand. J. Gastroenterol. 26:1247–1256. [DOI] [PubMed] [Google Scholar]
- 53.Lee, J. C. W., and J. E. Lennard-Jones. 1996. Inflammatory bowel disease in 67 families each with three or more affected first-degree relatives. Gastroenterology 111:587–596. [DOI] [PubMed] [Google Scholar]
- 54.Lichtman, S. N., J. Wang, R. B. Sartor, C. Zhang, D. Bender, F. G. Dalldorf, and J. H. Schwab. 1995. Reactivation of arthritis induced by small bowel bacterial overgrowth in rats: role of cytokines, bacteria, and bacterial polymers. Infect. Immun. 63:2295–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindberg, E., and G. Jarnerot. 1991. The incidence of Crohn’s disease is not decreasing in Sweden. Scand. J. Gastroent. 26:495–450. [DOI] [PubMed] [Google Scholar]
- 56.Liu, Y., J. Van Kruningen, A. N. West, et al. 1995. Immunocytochemical evidence of listeria, Escherichia coli and streptococcus antigens in Crohn’s disease. Gastroenterology 108:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu, Z., K. Geboes, S. Colpaert, G. R. D’Haens, P. Rutgeerts, and J. I. Ceuppens. 2000. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J. Immunol. 164:3608–3615. [DOI] [PubMed] [Google Scholar]
- 58.Loftus, E. V., Jr., W. J. Sandborn, W. J. Tremaine, D. W. Mahoney, A. R. Zinsmeister, K. P. Offord, and L. J. Melton III. 1996. Primary sclerosing cholangitis is associated with nonsmoking: a case-control study. Gastroenterology 110:1496–1502. [DOI] [PubMed] [Google Scholar]
- 59.Loftus, E. V., M. D. Silverstein, W. J. Sandborn, W. J. Tremaine, W. S. Harmsen, and A. R. Zinsmeister. 1998. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology 114:1161–1168. [DOI] [PubMed] [Google Scholar]
- 60.Ma, A., M. Datta, E. Margosian, J. Chen, and I. Horak. 1995. T cells, but not B cells, are required for bowel inflammation in interleukin 2-deficient mice. J. Exp. Med. 182:1567–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma, Y., J. D. Ohmen, Z. Li, L. G. Bentley, C. McElree, S. Pressman, S. R. Targan, N. Fischel-Ghodsian, J. I. Rotter, and H. A. Yang. 1999. A genome-wide search identifies potential new susceptibility loci for Crohn’s disease. Inflamm. Bowel Dis. 5:271–278. [DOI] [PubMed] [Google Scholar]
- 62.MacDermott, R. P. 1999. Chemokines in inflammatory bowel disease. J. Clin. Immunol. 19:266–272. [DOI] [PubMed] [Google Scholar]
- 63.MacDonald, T. T., G. Monteleone, and S. L. F. Pender. 2000. Recent developments in the immunology of inflammatory bowel disease. Scand. J. Immunol. 51:2–9. [DOI] [PubMed] [Google Scholar]
- 64.Mackay, F., J. L. Browning, P. Lawton, S. A. Shah, M. Comiskey, A. K. Bhan, E. Mizoguchi, C. Terhorst, and S. J. Simpson. 1998. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology 115:1464–1475. [DOI] [PubMed] [Google Scholar]
- 65.Madsen, K. L., J. S. Doyle, M. M. Tavernini, L. D. Jewell, R. P. Rennie, and R. N. Fedorak. 2000. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterology 118:1094–1105. [DOI] [PubMed] [Google Scholar]
- 66.Mallas, E. G., P. Mackintosh, P. Asquith, and W. T. Cooke. 1976. Histocompatibility antigens in inflammatory bowel disease. Their clinical significance and their association with arthropathy with special reference to HLA-B27(W27). Gut 17:906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mashimo, H., D. C. Wu, D. K. Podolsky, and M. C. Fishman. 1996. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274:262–265. [DOI] [PubMed] [Google Scholar]
- 68.Matsumoto, S., Y. Okabe, H. Setoyama, K. Takayama, J. Ohtsuka, H. Funahashi, A. Imaoka, Y. Okada, and Y. Umesaki. 1998. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut 43:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mayer, L., and H. Janowitz. 1988. Extraintestinal manifestations of inflammatory bowel disease, p.299–317. In J. B. Kirsner and E. G. Shorter (ed.), Inflammatory bowel disease. Lea & Febiger, Philadelphia, Pa.
- 70.Merger, M., and K. Croitoru. 1998. Infections in the immunopathogenesis of chronic inflammatory bowel disease. Semin. Immunol. 10:69–78. [DOI] [PubMed] [Google Scholar]
- 71.Michener, W. M., G. Whelan, R. L. Greenstreet, and R. G. Farmer. 1982. Comparison of clinical features of Crohn’s disease and ulcerative colitis with onset in childhood or adolescence. Clevel. Clin. Q. 49:13–16. [DOI] [PubMed] [Google Scholar]
- 72.Mir-Madjlessi, S. H., W. M. Michener, and R. G. Farmer. 1986. Course and prognosis of idiopathic ulcerative proctosigmoiditis in young patients. J. Pediatr. Gastroenterol. Nutr. 5:570–576. [PubMed] [Google Scholar]
- 73.Mizoguchi, A., E. Mizoguchi, and A. K. Bhan. 1999. The critical role of interleukin-4 but not interfeon gamma in the pathogenesis of colitis in T-cell receptor α mutant mice. Gastroenterology 116:320–326. [DOI] [PubMed] [Google Scholar]
- 74.Modigliani, R., J. F. Colombel, J. L. Dupas, M. Dapoigny, V. Costil, M. Veyrac, B. Duclos, J. C. Soule, J. P. Gendre, J. P. Galmiche, O. Danne, G. Cadiot, H. Lamouliatte, J. Belaiche, and J. Y. Mary. 1996. Mesalamine in Crohn’s disease with steroid-induced remission: effect on steroid withdrawal and remission maintenance. Gastroenterology 110:688–693. [DOI] [PubMed] [Google Scholar]
- 75.Monbaerts, P., E. Mizoguchi, M. J. Grusby, L. H. Glimcher, A. K. Bhan, and S. Tonegawa. 1993. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 75:274–282. [DOI] [PubMed] [Google Scholar]
- 76.Monteleone, G., F. Trapasso, T. Parrello, L. Biancone, A. Stella, R. Iuliano, F. Luzza, A. Fusco, and F. Pallone. 1999. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J. Immunol. 163:143–147. [PubMed] [Google Scholar]
- 77.Munkholm, P., E. Langholz, O. H. Nielsen, S. Kreiner, and V. Binder. 1992. Incidence and prevalence of Crohn’s disease in the county of Copenhagen, 1962–1987: a sixfold increase in incidence. Scand. J. Gastroenterol. 27:609–614. [DOI] [PubMed] [Google Scholar]
- 78.Negoro, K., Y. Kinouchi, N. Hiwatashi, S. Takahashi, S. Takagi, J. Satoh, T. Shimosegawa, and T. Toyota. 1999. Crohn’s disease is associated with novel polymorphisms in the 5′-flanking region of the tumor necrosis factor gene. Gastroenterology 117:1062–1068. [DOI] [PubMed] [Google Scholar]
- 79.Ogunbi, S. O., J. A. Ransom, K. Sullivan, B. T. Shoen, and B. D. Gold. 1998. Inflammatory bowel disease in African-American children living in Georgia. J. Pediatr. 133:103–107. [DOI] [PubMed] [Google Scholar]
- 80.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J.-P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nuñez, and J. H. Cho. 2001. Susceptibility to Crohn’s disease by a frameshift mutation in Nod2, resulting in lipopolysaccharide hyporesponsiveness. Nature 411:603–606. [DOI] [PubMed] [Google Scholar]
- 81.Olives, J. P., A. Breton, J. P. Hugot, F. Oksman, C. Johannet, J. Ghisolfi, J. Navarro, and J. P. Cezard. 1997. Antineutrophil cytoplamic antibodies in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 25:142–148. [DOI] [PubMed] [Google Scholar]
- 82.Onuma, E. K., P. S. Amenta, K. Ramaswamy, J. J. Lin, and K. M. Das. 2000. Autoimmunity in ulcerative colitis (UC): a predominant colonic mucosal B cell response against human tropomyosin isoform 5. Clin. Exp. Immunol. 121:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Orchard, T. R., S. Thiyagaraja, K. I. Welsh, B. P. Wordsworth, J. S. Gaston Hill, and D. P. Jewell. 2000. Clinical phenotype is related to HLA genotype in the peripheral arthropathies of inflammatory bowel disease. Gastroenterology 118:274–278. [DOI] [PubMed] [Google Scholar]
- 84.Orholm, M., V. Binder, T. I. A. Sorensen, and K. Kyvik. 1996. Inflammatory bowel disease in a Danish twin register. Gut 39(Suppl. 3):A187. [Google Scholar]
- 85.Panwala, C. M., J. C. Jones, and J. L. Viney. 1998. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistant gene, mdr1a, spontaneously develop colitis. J. Immunol. 161:5733–5744. [PubMed] [Google Scholar]
- 86.Papadakis, K. A., and S. R. Targan. 2000. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 51:289–298. [DOI] [PubMed] [Google Scholar]
- 87.Peters, M., H. Nevens, F. Baert, M. Hiele, A. M. de Meyer, R. Vlietinck, and P. Rutgeerts. 1996. Familial aggregation in Crohn’s disease: increased age-adjusted risk and concordance in clinical characteristics. Gastroenterology 111:597–603. [DOI] [PubMed] [Google Scholar]
- 88.Pizarro, T. T., M. H. Michie, M. Bentz, J. Woraratanadharm, M. F. Smith, Jr., E. Foley, C. A. Moskaluk, S. J. Bickston, and F. Cominelli. 1999. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J. Immunol. 162:6829–6835. [PubMed] [Google Scholar]
- 89.Powie, F., J. Carlino, M. W. Leach, S. Mauze, and R. I. Coffman. 1996. A critical role for transforming growth factor-β but not interleukin-4 in the suppression of T helper type 1-mediated colitis by CD4Rb low CD4+ T cells. J. Exp. Med. 183:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Present, D. H., P. Rutgeerts, S. Targan, S. B. Hanauer, L. Mayer, R. A. Van Hogezand, D. K. Podolsky, B. E. Sands, T. Braakman, K. L. DeWoody, T. F. Schaible, and S. J. H. Van Deventer. 1999. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N. Engl. J. Med. 340:1398–1405. [DOI] [PubMed] [Google Scholar]
- 91.Raj, V., and D. R. Lichtenstein. 1999. Hepatobiliary manifestations of inflammatory bowel disease. Gastroenterol. Clin. North Am. 28:491–513. [DOI] [PubMed] [Google Scholar]
- 92.Ramakrishna, J., N. Langhans, K. Calenda, et al. 1996. Combined use of cyclosporine and azathioprine or 6-mercaptopurine in pediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 22:296–302. [DOI] [PubMed] [Google Scholar]
- 93.Rath, H. C., H. H. Herfarth, J. S. Ikeda, W. B. Grenther, T. E. Hamm, Jr., E. Balish, J. D. Taurog, R. E. Hammer, K. H. Wilson, and R. B. Sartor. 1996. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J. Clin. Investig. 98:945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rath, H. C., K. H. Wilson, and R. B. Sartor. 1999. Differential induction of colitis and gastritis in HLA-B27 transgenic rats selectively colonized with Bacteroides vulgatus or Escherichia coli. Infect. Immun. 67:2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rioux, J. D., M. S. Silverberg, M. J. Daly, A. H. Steinhart, R. S. McLeod, A. M. Griffiths, T. Green, T. S. Brettin, V. Stone, S. B. Bull, A. Bitton, C. N. Williams, G. R. Greenberg, Z. Cohen, E. S. Lander, T. J. Hudson, and K. A. Siminovitch. 2000. Search in Canadian families with inflammatory bowel disease reveals two novel susceptibility loci. Am. J. Hum. Genet. 66:1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rocha, F., R. Laughlin, M. W. Musch, B. A. Hendrickson, E. B. Chang, and J. Alverdy. 2001. Surgical stress shifts the intestinal Escherichia coli population to that of a more adherent phenotype: role in barrier regulation. Surgery 130:65–73. [DOI] [PubMed] [Google Scholar]
- 97.Rogers, B. M. G., L. M. Clark, and J. B. Kirsner. 1971. The epidemiologic and demographic characteristics of inflammatory bowel disease: an analysis of a computerized file of 1400 patients. J. Chronic Dis. 24:743–773. [DOI] [PubMed] [Google Scholar]
- 98.Roozendaal, C., K. Pogany, E. J. Hummel, G. Horst, G. Dijkstra, G. F. Nelis, P. C. Limburg, J. H. Kleibeuker, and C. G. Kallenberg. 1999. Titres of anti-neutrophil cytoplasmic antibodies in inflammatory bowel disease are not related to disease activity. Q. J. Med. 92:651–658. [DOI] [PubMed] [Google Scholar]
- 99.Rubin, D. T., and S. B. Hanauer. 2000. Smoking and inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 12:855–862. [DOI] [PubMed] [Google Scholar]
- 100.Ruemmele, F. M., S. R. Targan, G. Levy, M. Dubinsky, J. Braun, and E. G. Seidman. 1998. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology 115:822–829. [DOI] [PubMed] [Google Scholar]
- 101.Rutgeerts, P., G. D’Haens, S. R. Targan, E. Vasiliauskas, S. B. Hanauer, D. H. Present, L. Mayer, R. A. Van Hogezand, T. Braakman, K. L. DeWoody, T. F. Schaible, and S. J. H. Van Deventer. 1999. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 117:761–769. [DOI] [PubMed] [Google Scholar]
- 102.Sadlack, B., H. Merz, H. Schorle, A. Schimpl, A. C. Feller, and I. Horak. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75:253–261. [DOI] [PubMed] [Google Scholar]
- 103.Sandler, R. S., D. P. Sandler, C. W. McDonnell, and J. I. Wurzelmann. 1992. Childhood exposure to environmental tobacco smoke and the risk of ulcerative colitis. Am. J. Epidemiol. 135:603–608. [DOI] [PubMed] [Google Scholar]
- 104.Sartor, R. B. 1995. Current concepts of etiology and pathogenesis of ulcerative colitis and Crohn’s Disease. Gastroenterol. Clin. North Am. 24:475–507. [PubMed] [Google Scholar]
- 105.Sartor, R. B. 1997. Review article: Role of the enteric microflora in the pathogenesis of intestinal inflammation and arthritis. Aliment. Pharmacol. Ther. 11(Suppl. 3):17–23. [DOI] [PubMed] [Google Scholar]
- 106.Satsangi, J., K. L. Welsh, M. Bunce, C. Julier, J. M. Farrant, J. L. Bell, and D. P. Jewell. 1996. Contribution of genes of the major histocompatibility complex to susceptibility and disease phenotype in inflammatory bowel disease. Lancet 347:1212–1217. [DOI] [PubMed] [Google Scholar]
- 107.Satsangi, J., M. Parkes, E. Louis, M. Lathrop, J. Bell, and D. P. Jewell. 1996. Two stage genome-wide search in inflammatory bowel disease provides evidence for susceptibility loci on chromosomes 3, 7 and 12. Nat. Genet. 14:199–202. [DOI] [PubMed] [Google Scholar]
- 108.Schultsz, C., F. M. Van Den Berg, F. W. Ten Kate, G. N. J. Tytgat, and J. Dankert. 1999. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology 117:1089–1097. [DOI] [PubMed] [Google Scholar]
- 109.Simpson, S. J., S. Shah, M. Comiskey, Y. P. de Jong, B. Wang, E. Mizoguchi, A. K. Bhan, and C. Terhorst. 1998. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin12/signal transducer and activator of transcription (Stat)-4 pathway but is not conditional on interferon-γ expression by T cells. J. Exp. Med. 187:1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Skoog, T., F. M. van’t Hooft, B. Kallin, S. Jovinge, S. Boquist, J. Nilsson, P. Eriksson, and A. Hamsten. 1999. A common functional polymorphism (C→A substitution at position −863) in the promoter region of the tumour necrosis factor-alpha (TNF-alpha) gene associated with reduced circulating levels of TNF-alpha. Hum. Mol. Genet. 8:1443–1449. [DOI] [PubMed] [Google Scholar]
- 111.Snook, J. A., L. Dwyer, C. Lee-Elliott, S. Khan, D. W. Wheeler, and D. S. Nicholas. 1996. Adult coeliac disease and cigarette smoking. Gut 39:60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stokkers, P. C. F., P. H. Reitsma, G. N. J. Tytgat, and S. J. H. van Deventer. 1999. HLA-DR and -DQ phenotypes in inflammatory bowel disease: a meta-analysis. Gut 45:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Strober, W., B. R. Ludviiksson, and I. J. Fuss. 1998. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn disease. Ann. Intern. Med. 128:848–856. [DOI] [PubMed] [Google Scholar]
- 114.Subhani, J., S. M. Montgomery, R. E. Pounder, and A. J. Wakefield. 1998. Concordance rates of twins and siblings in inflammatory bowel disease (IBD). Gut 42(Suppl. 1):A40. [Google Scholar]
- 115.Sundberg, J. P., C. O. Elson, H. Bedigian, and E. H. Birkenmeier. 1994. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology 107:1726–1735. [DOI] [PubMed] [Google Scholar]
- 116.Sutton, C. L., J. Kim, A. Yamane, H. Dalwadi, B. Wei, C. Landers, S. R. Targan, and J. Braun. 2000. Identification of a novel bacterial sequence associated with Crohn’s disease. Gastroenterology 119:23–31. [DOI] [PubMed] [Google Scholar]
- 117.Tabaqchali, S., D. P. O’Donoghue, and K. A. Bettelheim. 1978. Escherichia coli antibodies in patients with inflammatory bowel disease. Gut 19:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takeda, K., T. Kaisho, N. Terada, and S. Akira. 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10:34–49. [DOI] [PubMed] [Google Scholar]
- 119.Targan, S. R., S. B. Hanauer, S. J. van Deventer, L. Mayer, D. H. Present, T. Braakman, K. L. DeWoody, T. F. Schaible, and P. J. Rutgeerts. 1997. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N. Engl. J. Med. 337:1029–1035. [DOI] [PubMed] [Google Scholar]
- 120.Thayer, W. R., Jr., and V. Chitnavis. 1994. The case for an infectious etiology. Med. Clin. North Am. 78:1233–1247. [DOI] [PubMed] [Google Scholar]
- 121.Thomas, G. A., G. L. Swift, J. T. Green, R. G. Newcombe, C. Braniff-Mathews, J. Rhodes, S. Wilkinson, G. Strohmeyer, and G. Kreuzpainter. 1998. Controlled trial of antituberculous chemotherapy in Crohn’s disease: a five year follow up study. Gut 42:497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomas, G. A. O., D. Millar-Jones, J. Rhodes, G. M. Roberts, G. T. Williams, and J. F. Mayberry. 1995. Incidence of Crohn’s disease in Cardiff over 60 years. 1986–1990. An update. Eur. J. Gastroenterol. Hepatol. 7:401–405. [PubMed] [Google Scholar]
- 123.Thompson, D. E. 1994. The role of mycobacteria in Crohn’s disease. J. Med. Microbiol. 41:74–94. [DOI] [PubMed] [Google Scholar]
- 124.Tiwana, H., C. Wilson, R. S. Walmsley, A. J. Wakefield, M. S. Smith, N. L. Cox, M. J. Hudson, and A. Ebringer. 1997. Antibody responses to gut bacteria in ankylosing spondylitis, rheumatoid arthritis, Crohn’s disease and ulcerative colitis. Rheumatol. Int. 17:11–16. [DOI] [PubMed] [Google Scholar]
- 125.Turumen, U., M. Fakkila, V. Yaltonen, et al. 1993. Longterm outcome of ciprofloxacin treatment in severe perianal or fistulous Crohn’s disease. Gastroenterology 104:A793. [Google Scholar]
- 126.Tysk, C., E. Linkberg, G. Jarnerot, and B. Floderus-Myrhed. 1988. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins: a study of heritability and the influence of smoking. Gut 29:990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van der Linden, S., and D. van der Heijde. 2000. Clinical aspects, outcome assessment, and management of ankylosing spondylitis and postenteric reactive arthritis. Curr. Opin. Rheumatol. 12:263–268. [DOI] [PubMed] [Google Scholar]
- 128.van Deventer, S. J. H. 2000. Immunotherapy of Crohn’s disease. Scand. J. Immunol. 51:18–22. [DOI] [PubMed] [Google Scholar]
- 129.van Kruiningen, H. J. 1999. Lack of support for a common etiology in Johne’s disease of animals and Crohn’s disease in humans. Inflamm. Bowel Dis. 5:183–191. [DOI] [PubMed] [Google Scholar]
- 130.van Milligen de Wit, A. W., S. J. van Deventer, and G. N. Tytgat. 1995. Immunogenetic aspects of primary sclerosing cholangitis: implications for therapeutic strategies. Am. J. Gastroenterol. 90:893–900. [PubMed] [Google Scholar]
- 131.Vasiliauskas, E. A., L. Y. Kam, M. T. Abreu-Martin, P. V. Hassard, K. A. Papadakis, H. Yang, J. B. Zeldis, and S. R. Targan. 1999. An open label pilot study of low dose thalidomide in chronically active, steroid dependent Crohn’s disease. Gastroenterology 117:1278–1287. [DOI] [PubMed] [Google Scholar]
- 132.Wakefield, A. J., A. Ekbom, A. P. Dhillon, R. M. Pittilo, and R. E. Pounder. 1995. Crohn’s disease: pathogenesis and persistent measles virus infection. Gastroenterology 108:911–916. [DOI] [PubMed] [Google Scholar]
- 133.Wakefield A. J., S. M. Montgomery, and R. E. Pounder. 1999. Crohn’s disease: the case for measles virus. Ital. J. Gastroenterol. Hepatol. 31:247–254. [PubMed] [Google Scholar]
- 134.Walmsley, R. S., A. Anthony, R. Sim, R. E. Pounder, and A. J. Wakefield. 1998. Absence of Escherichia coli, Listeria monocytogenes and Klebsiella pneumoniae antigens within inflammatory bowel disease tissues. J. Clin. Pathol. 51:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wirtz S., S. Finotto, S. Kanzler, et al. 1999. Chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transferr by TNF-plus IFN-gamma-producing CD4+ T cells that respond to bacterial antigens. J. Immunol. 162:1884–1888. [PubMed] [Google Scholar]
- 136.Yamamoto, T., R. N. Allan, and M. R. Keighley. 2000. Effect of fecal diversion alone on perianal Crohn’s disease. World J. Surg. 24:1258–1262. [DOI] [PubMed] [Google Scholar]
- 137.Yang, H., C. McElree, M. P. Roth, F. Shanahan, S. R. Targan, and J. I. Rotter. 1993. Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews. Gut 34:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang, H., S. E. Plevy, K. Taylor, D. Tyan, N. Fischel-Ghodsian, C. McElree, S. R. Targan, and J. I. Rotter. 1999. Linkage of Crohn’s disease to the major histocompatibility complex region is detected by multiple non-parametric analyses. Gut 44:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]