INTRODUCTION
Bacterial endophthalmitis is an infection of the interior of the eye that, despite appropriate therapeutic intervention, frequently results in visual loss. Recently, research has begun to elucidate the molecular and cellular events that contribute to the damage that occurs in intraocular infection. This review discusses the epidemiology and therapuetic challenges of endophthalmitis, as well as current findings from the analysis of bacterial and host interactions in the pathogenesis of eye infections. Based on these and related studies, a model for the molecular pathogenesis of endopthalmitis is advanced. A more comprehensive understanding of the contributions of the molecular and cellular interactions in endophthalmitis will likely unveil possible therapeutic targets designed to ameliorate the infection and preserve vision.
BACTERIAL ENDOPHTHALMITIS
Endophthalmitis is an ocular inflammation resulting from the introduction of an infectious agent into the posterior segment of the eye. During infection, irreversible damage to delicate photoreceptor cells of the retina frequently occurs. Despite aggressive therapeutic and surgical intervention, endophthalmitis generally results in partial or complete loss of vision, often within a few days of inoculation. Infectious agents generally gain access to the posterior segment of the eye following one of three routes: (i) as a consequence of intraocular surgery (postoperative), (ii) following a penetrating injury of the globe (posttraumatic), or (iii) from hematogenous spread of bacteria to the eye from a distant anatomical site (endogenous). Although uncommon, endophthalmitis can also result from keratitis, an infection of the cornea which, if left untreated, can result in corneal perforation and intraocular seeding of organisms (117).
Postoperative Endophthalmitis
Postoperative endophthalmitis has been reported following nearly every type of ocular surgery. It occurs most frequently following cataract surgery—the most commonly performed type of ocular surgery. The overall incidence of post-cataract surgery endophthalmitis in the United States, using modern techniques of phacoemulsification and intraocular lens implantation, is about 0.1% (1, 60, 68, 116). The incidence following other types of intraocular surgery has been reported to range between 0.05 and about 0.37% (1, 60, 68). In general, those procedures with a higher risk for acute postoperative endophthalmitis (secondary intraocular lens implantation and penetrating keratoplasty [corneal transplantation]) are those with a greater potential for wound leaks with subsequent intraocular bacterial contamination.
The etiologic agents of acute postoperative endophthalmitis are generally microorganisms of the eyelid margin and preocular tear film. Although preoperative topical antimicrobial agents can decrease colony counts in the tear film, they do not sterililize the area. In one study, culture of aqueous fluid immediately following cataract surgery revealed a 9% culture-positive rate (39). Presumably, low inoculum levels and/or low pathogenicity combined with the innate ocular defenses against infection explain the low rate of clinical infection despite the relatively high prevalence of microorganisms in the eye following surgery.
In most series from the United States, coagulase-negative staphylococci are responsible for about 70% of post-cataract surgery endophthalmitis, followed by Staphylococcus aureus, viridans group streptococci, other gram-positive microorganisms, and gram-negative microorganisms (1, 55, 126). Enterococci are notable among the gram-positive microorganisms both for prevalence and severity of disease (35).
Outcomes following appropriate surgical management are related to a number of factors—most importantly to the level of visual function at the time of clinical presentation. In the Endophthalmitis Vitrectomy Study (EVS), a National Institutes of Health-funded multicenter prospective study, eyes presenting with a visual acuity of only light perception achieved a final vision of 20/40 in 33% of cases, even though the most efficacious management protocol was used. If the presenting vision was better than light perception, over 60% of eyes achieved 20/40 or better vision utilizing the same treatment protocol (35).
Postoperative endophthalmitis may also occur weeks to years following surgery. This delayed infection is likely due either to sequesteration of low-virulence organisms introduced at the time of surgery or to delayed inoculation of organisms. In the former case, Propionibacterium acnes is the most common microorganism encountered, and clinically evident low-to moderate-grade inflammation may occur weeks to months after surgery (6, 26, 112). In cases with delayed inoculation of microorganisms, organisms gain access to the eye through either wound abnormalities, suture tracks, or filtering blebs. The most common clinical situation involves antecedent glaucoma filtering surgery (125, 150). In addition to reflecting the colonization of the preocular tear film, delayed infection of this type is associated with a higher prevalence of streptococcal species.
Posttraumatic Endophthalmitis
Penetrating ocular injuries are accompanied by infection at a much higher rate than occurs with surgery. In most series of penetrating injury cases, from 3 to 17% of eyes develop microbial endophthalmitis (67, 87, 96, 140). The broad prevalence range is due to factors such as frequency within the series of intraocular foreign bodies, distribution of trauma causes, and management strategies. Most authorities agree that the three most important risk factors for posttraumatic endophthalmitis are the presence of an intraocular foreign body, delay in closure of the globe, and location and extent of laceration or rupture of the globe.
Posttraumatic-endophthalmitis-associated isolates include a greater variety of organisms than those following ocular surgery and include bacteria derived from the environment. Bacillus cereus is ranked second behind staphylococci in prevalence and some cases are polymicrobial (15, 75, 96, 114, 140).
Endogenous Endophthalmitis
Endogenous endophthalmitis results from the introduction of organisms into the posterior segment of the eye as a result of hematogenous spread from a remote primary site of infection. Endogenous endophthalmitis is relatively rare, accounting for only 2 to 8% of all endophthalmitis cases (51, 97, 107). Populations at greatest risk include immunocompromised patients or those on immunosuppressive therapy, patients with prolonged indwelling devices, and intravenous drug abusers (27, 119). Common causes of endogenous bacterial endophthalmitis include S. aureus, B. cereus, and gram-negative organisms, including Escherichia coli, Neisseria meningitidis, and Klebsiella spp. (27, 81, 107, 142). Bacillus spp. are a primary bacterial cause of endogenous endophthalmitis in intravenous drug abusers and are most likely seeded from contaminated injection paraphernalia and drug solutions (27, 51, 119). The most common etiological agent of all cases of endogenous endophthalmitis is the opportunistic fungus Candida albicans (107).
ENDOPHTHALMITIS: A THERAPEUTIC CHALLENGE
Successful treatment of microbial endophthalmitis must take into account the unique challenges posed by the delicate anatomy and physiology of ocular tissues. Inflammation-induced opacity of the cornea, anterior chamber, lens, and/or vitreous impedes formation of a clear image on the retina. Inflammation-mediated damage to the trabecular meshwork and/or ciliary body may produce blinding glaucoma or ocular hypotony. Most critically, damage to the neurosensory retina and retinal pigment epithelium may destroy the basic photochemical process of vision. The center of the macula (that portion of the retina responsible for central vision) is an area only about 500 μm in diameter.
While the retina has a rich blood supply, the vitreous (about 4 to 5 ml in volume) and the anterior chamber (about 2 ml in volume) are avascular and isolated from the systemic circulation by the blood-ocular fluid barrier (131, 133, 134). These unique anatomic features represent a barrier for the delivery of not only cellular and humoral mediators of host immunity but also antimicrobial or anti-inflammatory agents administered systemically. A second problem lies in the sensitivity of the retinal photoreceptor cells and other retinal cells directly adjacent to the vitreous. Such cells are highly sensitive not only to the offending pathogen and the resulting inflammatory response but also to high doses of antimicrobial agents administered locally to treat the infection (23, 29, 36, 152).
Choice of Antimicrobial Agent
When endophthalmitis is initially suspected, the pathogen is not typically known, so the choice of antimicrobial agent must be made empirically. Unfortunately, clinical features of infection and culture results often do not correlate adequately to guide the choice of antibiotics upon presentation (65). Furthermore, despite coverage with broad-spectrum antibiotics, visual loss remains a common result (36, 98). Outcome of endophthalmitis management is likely due to several factors, including the responsible pathogen, the patient’s age, the duration between injury and treatment, the therapy chosen, and the condition of the eye upon presentation (43). Clinical and experimental studies have firmly established that delay in therapy will result in poor visual outcome, especially in severe cases of endophthalmitis (4, 42, 74, 77, 91, 107).
The recommended management of bacterial endophthalmitis includes direct injection of antibiotics into the vitreous (10, 19, 36, 145). Systemic antibiotics have also been used concurrently for bacterial endophthalmitis (1, 107), although some potentially effective antibiotics (vancomycin and aminoglycosides) do not penetrate readily into the vitreous (38), due in part, to the protective effect of the blood-ocular fluid barrier. Intraocular inflammation increases the permeability of the blood-ocular fluid barrier, enhancing penetration of systemic antibiotics into the vitreous cavity (38). However, intravitreal levels achieved vary substantially, frequently falling below the MICs for many ocular pathogens.
Because of variable penetration into the vitreous cavity of aminoglycosides, vancomycin, and cephalosporins (the traditional mainstays of antimicrobial therapy in bacterial endophthalmitis), the EVS evaluated their clinical efficacy in a post-cataract surgery endophthalmitis controlled trial. Systemic antibiotics, as used in the study, did not enhance visual outcomes when used in combination with intravitreal administration (36). Based on that study, parenteral antimicrobial agents are not recommended for eyes that would have satisfied criteria for EVS eligibility.
This recommendation against systemic antimicrobial therapy does not apply to eyes following other types of ocular surgery, trauma, or suspected endogenous endophthalmitis. Clinicians are also always at liberty to modify EVS recommendations based on modifying clinical signs or new therapeutic information. Although new data suggest that fluoroquinolones penetrate into the inflamed and noninflamed vitreous better than other classes of antibiotics, they have not been subjected to rigorous, blinded clinical trials (40, 93). Despite this, fluoroquinolones in particular are currently used by many clinicians in combination with intravitreal antibiotics in management of some severe endophthalmitis cases. Systemic antibiotics remain an integral part of the therapeutic approach to endogenous endophthalmitis where there is concomitant bacteremia.
Intravitreal administration of antibiotics is a key component of the clinical management of exogenous bacterial endophthalmitis. Known intravitreal levels of antibiotics can be directly and immediately achieved and remain above the MICs for most pathogens for protracted periods of time. The three most commonly utilized antibiotics for intravitreal administration include 1.0 mg of vancomycin, 0.4 mg of amikacin, and 2.2 mg of ceftazidime. Both vancomycin and amikacin were included in the EVS protocol. Antibiotic susceptibilities of bacterial isolates recovered in the EVS are summarized in Table 1 (55). Virtually all post-cataract surgery endophthalmitis isolates are sensitive to one or both agents (55). Many clinicians prefer to substitute ceftazidime for amikacin because of the well-recognized destructive retinal microvasculitis which may occur as a dose-dependent toxicity of aminoglycosides (23, 29, 36). The spectra and sensitivities of ceftazidime and amikacin are similar for most ocular isolates. Therefore, the two most commonly utilized combinations are vancomycin and amikacin or vancomycin and ceftazidime.
TABLE 1.
Antibiotic susceptibilities of bacterial isolates recovered in the EVS (55)
| Organism(s) | Total no. of isolates recovered | % Isolates susceptible to:
|
||
|---|---|---|---|---|
| Amikacin | Ceftazidime | Vancomycin | ||
| Coagulase-negative staphylococci | 226 | 86.1 | 62.1 | 100 |
| S. aureus | 32 | 81.3 | 77.4 | 100 |
| Streptococci | 29 | 14.8 | 92.6 | 100 |
| Enterococci | 7 | 0 | 0 | 100 |
| Miscellaneous gram-positive organisms | 10 | 77.8 | 66.7 | 100 |
| Gram-negative organisms | 19 | 89.5 | 89.5 | 0 |
| Total | 323 | 77.6 | 66.7 | 95.9 |
Despite broad-spectrum antibacterial activity, fluoroquinolones are not widely used for direct intraocular administration, due in part to concerns regarding possible toxicity (129, 152). The emergence of antibiotic-resistant organisms, particularly vancomycin-resistant gram-positive pathogens, such as Enterococcus faecalis and potentially S. aureus, may affect future therapeutic design.
Anti-Inflammatory Agents
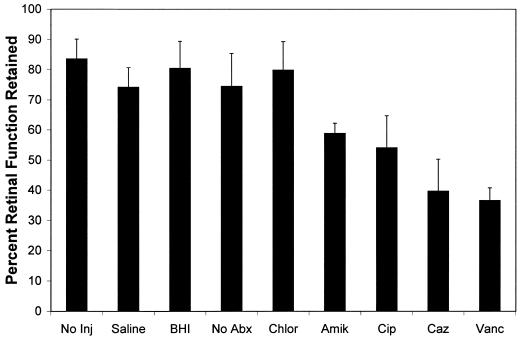
Although an ocular inflammatory response is vital for the clearance of organisms during infection, this response can induce bystander damage to sensitive neurologic tissues. The ocular inflammatory response to intravitreal gram-positive organisms is induced by growing organisms as well as metabolically inactive organisms, whole cell walls, and cell wall components (20, 24, 89). Intravitreal injection of gram-negative lipopolysaccharide induces inflammatory-cell infiltration and protein leakage into the aqueous humor (57, 92, 100). Antibiotic-induced release of cell walls or their components may therefore exacerbate intraocular inflammation during endophthalmitis treatment. As shown in Fig. 1, sterile filtrates of Bacillus subtilis treated with cell wall-active antibiotics induced ocular inflammation and resulted in loss of retinal response (B. D. Jett, J. Chodosh, and M. S. Gilmore, Abstr. Assoc. Res. Vis. Ophthalmol. Meet., abstr. 4038, 1996). The phenomenon of antibiotic-induced inflammation is similarly well-established in animal models of otitits media and meningitis (69, 108, 113, 127). For meningitis, adjunctive use of corticosteroids has been shown to effectively suppress inflammation (108, 141). For treatment of endophthalmitis, however, reports on the benefits of corticosteroid administration have been contradictory, and as a result, the use of intravitreal steroids to treat bacterial endophthalmitis remains controversial. In experimental models of bacterial endophthalmitis, concomitant administration of dexamethasone was reported to be beneficial (62, 85, 101, 102, 123, 154), had no effect (4, 30, 62, 70, 118), or was detrimental (88) to infection outcome. Despite these conflicting results, dexamethasone is frequently used as an adjunct to antibiotic therapy in endophthalmitis.
FIG. 1.
Loss of retinal responsiveness following injection of B. subtilis culture filtrates. Mid-logarithmic-phase B. subtilis was exposed to various antibiotics for 3 h. Antibiotic-treated cultures were sterilized by passage through 0.22-μm-pore-size filters prior to injection of 0.1 ml into mid-vitreous of New Zealand White rabbits. Electroretinography was performed on postinjection days 1, 3, 5, and 7, and results are expressed as a percentage of retinal function retained compared to the baseline. Control eyes included those receiving no injection: (No Inj), surgical control (saline injection) (Saline), sterile broth (uninoculated brain heart infusion) (BHI), and no antibiotic treatment (No Abx). Electroretinography losses during the 1-week course were averaged for each group and compared using the Student t test. Values and error bars represent means + standard errors of the means for four or more eyes per group. Abbreviations for antibiotics: Chlor, chloramphenicol (500 μg/ml); Amik, amikacin (100 μg/ml); Cip, ciprofloxacin (25 μg/ml); Caz, ceftazidime (500 μg/ml); Vanc, vancomycin (250 μg/ml).
Vitrectomy
Although intravitreal antibiotic therapy can provide effective bacterial killing during endophthalmitis, vitrectomy is an appealing adjunct to management. Vitrectomy (surgical cutting and aspiration of vitreous contents and replacement with balanced salt solution) debrides the vitreous cavity of bacteria, inflammatory cells, and other toxic debris; promotes better diffusion of antibiotics; removes inflammatory membranes; permits earlier visualization of the retina; and may speed recovery of vision (87). Vitrectomy has been shown to improve visual outcome in severe postoperative EVS-eligible cases (36). An ongoing debate exists concerning the appropriate timing for vitrectomy in traumatized eyes. However, most reports agree that vitrectomy should be performed without delay in severe cases of endophthalmitis, especially those involving intraocular foreign bodies (2, 66). Microorganisms may be recovered from extracted vitreous, but the microbiologic yield does not appear to be enhanced over that recovered from a needle tap (54).
CORRELATIONS BETWEEN BACTERIAL VIRULENCE AND VISUAL OUTCOME
Because of the limited immune response that occurs within the eye, a wide range of organisms cause intraocular infection, and as a result, a wide spectrum of symptoms are associated with endophthalmitis. Symptoms of endophthalmitis range from a relatively painless anterior chamber inflammation, such as that typically caused by Staphylococcus epidermidis; to an indolent and protracted intraocular infection caused by P. acnes; to an explosive ocular and periorbital infection caused by B. cereus (1, 26, 114). Differences in disease outcome have been ascribed to toxin production by the offending organism. However, clinical reports also document severe infection by nontoxigenic organisms, such as coagulase-negative staphylococci or Bacillus species other than B. cereus (34, 58, 77, 84, 111). The reason for this discrepancy is likely rooted in precisely what constitutes an intraocular toxin. Recent experiments employing molecular biological techniques to generate specific mutations show that bacterial protein toxins contribute to intraocular tissue damage in some types of bacterial endophthalmitis but not in others (16, 17, 22, 62, 63; B. D. Jett et al., Abstr. Assoc. Res. Vis. Ophthalmol. Meet.; M. C. Booth, L. M. Pence, and M. S. Gilmore, Abstr. Assoc. Res. Vis. Ophthalmol. Meet., abstr. 1386, 1999). The gram-positive cell wall and its constituents are also capable of inciting significant intraocular inflammation (20) and therefore should be regarded as nonclassical intraocular toxins.
B. cereus Toxins
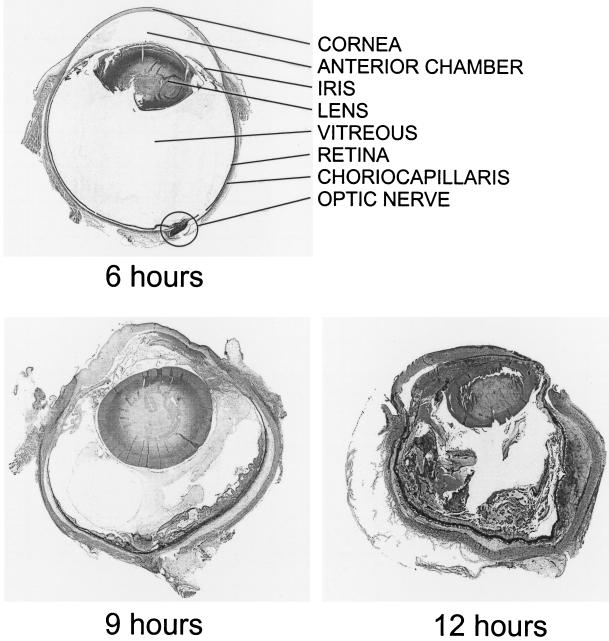
One of the most explosive and devastating forms of bacterial endophthalmitis is caused by B. cereus. A comprehensive review reported that only 9% of posttraumatic cases caused by B. cereus and other Bacillus species resulted in a final visual acuity of 20/70 or better. Seventy percent of eyes lost all useful vision, including 48% of cases resulting in evisceration or enucleation (31). Despite aggressive drug and/or surgical intervention, B. cereus endophthalmitis typically results in migration of organisms throughout the eye and a rapid and severe intraocular inflammatory response, resulting in loss of functional vision, if not the eye, within 24 to 48 h (Fig. 2). This consequence suggests that even if the infected eye is rendered sterile by antibiotics, ocular damage continues to occur. B. cereus produces a number of cytolysins and enzymes that could contribute to the rapid course and severity of endophthalmitis, including hemolysins, lipases, enterotoxins, and proteases (48).
FIG. 2.
Rapid nature of B. cereus endophthalmitis. Approximately 100 CFU of a clinical endophthalmitis isolate of B. cereus was injected intravitreally at 0 h (22). Whole eyes were harvested for histological analysis at 6, 9, and 12 h postinfection. The progression of experimental B. cereus endophthalmitis mimics that of the natural clinical course, including rapid influx of inflammatory cells into the posterior and anterior segment and dissolution of retinal architecture during the later stages of infection. At 6 h postinfection, little inflammation is observed on gross histological examination. By 9 h postinfection, extensive inflammation is seen in the posterior and anterior segment. Corneal and conjunctival edema and dissolution of retinal layers are apparent. By 12 h, the posterior and anterior segments are engorged with inflammatory cells and fibrin, and the architecture of the globe has begun to degrade. All sections were stained with hematoxylin-eosin.
One B. cereus pore-forming toxin, hemolysin BL, has been analyzed in terms of its role as a virulence factor in endophthalmitis (11, 22). Hemolysin BL is a tripartite toxin, with hemolytic, dermonecrotic, and emetic activities. Although purified hemolysin BL and crude B. cereus supernatants caused retinal tissue damage in an in vitro retinal button toxicity assay (11), a specific role for hemolysin BL in intraocular infection was not established. Using a B. cereus allelic-replacement hemolysin BL-deficient mutant, we demonstrated that hemolysin BL made a detectable contribution only very early in experimental B. cereus endophthalmitis but did not affect the overall course of infection. Intraocular inflammation and retinal toxicity occurred irrespective of the presence of hemolysin BL, implying the contribution of other factors to pathogenicity (22).
Considering reported activities against other types of mammalian cells and tissues, additional B. cereus toxins, such as cereolysin AB (48, 49), cereolysin O (120), or collagenase (124), could be involved in endophthalmitis pathogenesis. Sphingomyelinase and phospholipase C comprise the cytolytic unit known as cereolysin AB, the activity of which results from the sequential action of each enzyme on cell membranes (48, 49). Cereolysin O is a thiol-activated cytolysin that binds to cholesterol as an initial step prior to cell lysis, an activity that may extend to cells of the posterior segment of the eye (120). Collagenolytic B. cereus strains have been isolated from cases of endophthalmitis (124). Collagen is a major constituent of the vitreous and, therefore, a potential intraocular target for this enzyme. Although the activities of these toxins may expand to intraocular cells and tissues, their roles in vivo during endophthalmitis have yet to be determined. In vivo testing of isogenic mutants defective in these toxins similar to those constructed for the analysis of hemolysin BL in endophthalmitis will unambiguously define the contributions of other B. cereus toxins to disease.
E. faecalis Cytolysin and Aggregation Substance
E. faecalis is the causative agent in 4 to 8% of postoperative endophthalmitis cases and is isolated most frequently from infected filtering blebs following glaucoma surgery (34, 35, 80). Clinically, the visual outcome in cases of E. faecalis endophthalmitis is usually poor, with only 15% of E. faecalis endophthalmitis cases resulting in a final visual acuity of 20/200 or better (34, 35, 80). E. faecalis has become a key public health concern because of the emergence of antibiotic resistance to most, and in some cases all, useful antibiotics (59). Vancomycin resistance in E. faecalis ocular isolates has yet to be reported.
E. faecalis strains frequently harbor conjugative plasmids that encode a cytolysin which effectively lyses both eukaryotic and prokaryotic cells (48). The cytolysin consists of a large lytic subunit (CylLL) and a small lytic subunit (CylLS). Each subunit has lanthionine-type posttranslational modifications, as are found among lantibiotic class bacteriocins, but are unique among bacterial toxins. Both cytolysin subunits are secreted through a dedicated ATP-binding cassette transporter, CylB, and are proteolytically processed by a subtilisin-class serine protease, CylA, which renders the cytolysin subunits active against prokaryotic and eukaryotic cells (48). Epidemiologic analyses of E. faecalis clinical isolates demonstrated an enrichment for the cytolysin phenotype among endophthalmitis-derived isolates, suggesting its importance in endophthalmitis (18).
An experimental model of E. faecalis endophthalmitis was used to analyze the contribution of the cytolysin in disease (62, 63). Cytolysin-producing E. faecalis caused more fulminant and destructive changes in retinal architecture and a precipitous decline in retinal function, compared to noncytolytic transposon-insertion E. faecalis mutants. Transmission electron microscopy showed retinal tissue damage occurring several hours before microscopic detection in eyes infected with the cytolytic strain (62, 63). Cytolysin-production by E. faecalis also contributed to treatment failures during infection. Experimental endophthalmitis caused by noncytolytic E. faecalis was resolved with intravitreal ampicillin, gentamicin, and dexamethasone, while similar infections caused by isogenic cytolytic strains were refractory to treatment (62, 63). These results suggest that in cases of endophthalmitis caused by cytolysin-producing E. faecalis, treatment success may require targeting of the cytolysin as an adjunct to therapy.
In addition to destructive intraocular changes, E. faecalis has been observed to localize in close proximity to retinal tissues and vitreal structures, regardless of their cytolytic phenotype. One adhesin, aggregation substance, was found to be a virulence-enhancing factor in experimental E. faecalis endocarditis (25). In the experimental endophthalmitis model, enterococci attached to membranous vitreous structures, but distinct localization patterns and the course and severity of disease were independent of the aggregation substance phenotype, suggesting a role for other adhesion factors in disease (61).
S. aureus Global Regulation and Toxins
S. aureus is a leading cause of post-traumatic and postoperative endophthalmitis. Like E. faecalis and B. cereus, the clinical outcome of S. aureus endophthalmitis is frequently poor, resulting in visual outcomes with final visual acuities of 20/400 or worse in more than 50% of reported cases (3, 68, 99, 103, 110, 121). The increased incidence of multiple antibiotic resistance in clinical isolates threatens to further increase the rate of treatment failures for S. aureus.
S. aureus secretes several extracellular enzymes and toxins, many of which have been implicated in the pathogenesis of disease in nonocular systems. These virulence factors are coordinately controlled by quorum-sensing systems that act at the transcriptional level, namely, agr (accessory gene regulator) and sar (staphylococcal accessory regulator) (105). In general, S. aureus secretes cell wall-associated products and adhesins (e.g., clumping factor, fibronectin-binding protein, and protein A) during the logarithmic phase of growth, while most extracellular virulence factors (e.g., toxins, proteases, and lipases) are secreted postexponentially. With a few exceptions, regulatory mutants in agr or sar (or both) produce cell wall-associated proteins throughout the entire growth cycle but do not produce most extracellular toxins (105). In nearly every experimental comparison of the virulence of wild-type S. aureus with global regulatory mutants, the virulence of the global regulatory mutants is reduced or abolished (105).
Global regulatory mutants have been analyzed in experimental models of S. aureus endophthalmitis. The virulence of an isogenic mutant harboring a Tn551 insertion into the agr regulatory locus (agr) was compared with its wild-type strain in both the rabbit and rat (16, 45). Eyes infected with the wild-type strain showed a more rapid decine in retinal responsiveness and a more intense intraocular inflammatory response than eyes infected with the agr strain. Focal retinal destruction was observed in eyes infected with the wild-type strain at 36 h postinfection, while eyes infected with the agr strain appeared normal (16). An extension of these studies analyzed whether the sar global regulatory system contributed to virulence. The rate of retinal destruction in eyes infected with the sar mutant was similar to that for infections with the wild-type strain. Therefore, the sar locus alone did not modify the overall virulence of S. aureus in endophthalmitis. However, the combined effect of insertional mutations in both sar and agr led to near-complete attenuation of virulence (17).
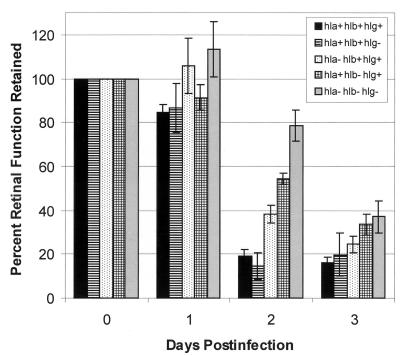
While these studies highlight the importance of globally regulated toxins in S. aureus endophthalmitis pathogenesis, the contribution of individual toxins to virulence remained unclear. Gamma-toxin and leukocidin, both two-component toxins active against several inflammatory cell types, were highly toxic when injected intravitreally, causing significant retinal destruction and inflammation (122). However, the concentrations of toxins produced by S. aureus during active intraocular infection were unknown, and therefore, whether the toxin concentrations used in this study were of physiological relevance is unclear. Experimental models of S. aureus corneal infection have shown that alpha-toxin, a membrane-damaging hemolysin, is the primary virulence factor in corneal epithelial tissue destruction during keratitis (21). To determine the individual contributions of not only alpha-toxin but also gamma-hemolysin and beta-toxin (a sphingomyelinase) in intraocular disease, the degrees of virulence of isogenic mutants deficient in each toxin locus were compared in the experimental model of S. aureus endophthalmitis (M. C. Booth et al., Abstr.). The absence of gamma-toxin had no positive effect on retinal function compared to that of wild-type infection on postinfection day 2 (Fig. 3). Supersac et al. (136) demonstrated significant ocular inflammation in eyes intravitreally infected with a gamma-hemolysin-deficient mutant, supporting the lack of a central role for this toxin in endophthalmitis pathogenesis. However, destruction of the retina in eyes infected with either the alpha-toxin- or the beta-toxin-deficient strain was significantly attenuated compared to eyes infected with wild-type S. aureus on postinfection day 2, suggesting that alpha-toxin and beta-toxin contributed to the decrease in retinal function observed. There was also cumulative effect of the absence of all three toxins on retinal function. Retinal function in eyes infected with an alpha-, beta-, and gamma-toxin-deficient triple mutant was preserved to a higher degree than that observed with wild-type S. aureus or single-toxin-deficient mutants on postinfection day 2 (Fig. 3). On postinfection day 3, differences in disease severity became less clear. Marked reductions in retinal responsiveness in all strains tested occurred, demonstrating the effect of cumulative damage or suggesting the importance of additional factors to virulence in the later stages of infection. Taken together, these data demonstrate that several globally regulated toxins, notably alpha-toxin and beta-toxin, are important in retinal destruction during S. aureus endophthalmitis (M. C. Booth et al., Abstr.). Novel therapeutics designed to inactivate global regulation of S. aureus during the early stages of infection may therefore be more effective in arresting tissue damage than the targeting of individual toxins.
FIG. 3.
Contribution of specific membrane-damaging toxins to decreases in retinal function during experimental S. aureus endophthalmitis. Approximately 100 CFU of the following S. aureus strains was injected into the vitreous at day 0 (16, 17): hla+hlb+hlg+, wild-type strain; hla+hlb+hlg−, isogenic mutant lacking gamma-hemolysin; hla−hlb+hlg+, isogenic mutant lacking alpha-toxin; hla+hlb−hlg+, isogenic mutant lacking beta-toxin; and hla−hlb−hlg−, isogenic mutant lacking alpha-toxin, beta-toxin, and gamma-hemolysin (strains provided by Tim Foster, Trinity College). Data at each postinfection day are presented as percentages of the B-wave amplitude of retinal function retained compared to baseline and sham-injected controls. Electroretinography losses were averaged for each group and compared using the Student t test. Values and error bars represent means ± standard errors of the means for four or more eyes per group.
Bacterial Behavior in the Eye
Another aspect of virulence that has yet to be investigated extensively is that of bacterial behavior in the eye during infection. Experimental models have shown that, in general, the course and severity of S. aureus or E. faecalis endophthalmitis correlated with intraocular growth rates (16, 17, 20, 22, 61, 62, 63). For these organisms, maximal inflammation is observed shortly after they reach the stationary phase of intraocular growth. In contrast, eyes infected with B. cereus exhibit an almost immediate inflammatory response despite the low number of organisms at the earliest stages of infection, several hours before the growing organisms reached maximal numbers in the eye (20, 22).
In terms of intraocular localization during infection, S. aureus and E. faecalis were recovered exclusively in the vitreous (20). In contrast, B. cereus was observed within retinal tissues 6 h postinfection, in aqueous humor 9 h postinfection, and in all ocular tissues 12 h postinfection (20). B. cereus is a motile organism, whereas S. aureus and E. faecalis are not. Preliminary studies indicate that motile B. cereus is significantly more virulent than nonmotile B. cereus (M. C. Callegan, S. T. Kane, and M. S. Gilmore, Abstr. Assoc. Res. Vis. Ophthalmol. Meeting, abstr. 1857, 2000), suggesting a possible role for this trait in pathogenicity.
Bacterial Cell Walls and Their Components
The majority of studies on the inflammogenicity of gram-positive organisms have focused on the contribution of the cell wall. The inflammatory capacity of gram-positive cell walls and their components (peptioglycan, lipoteichoic acid, and capsular polysaccharide) is well-documented in a number of in vivo and in vitro systems (14, 32, 53, 71, 72, 143, 144). Metabolically inactive organisms, cell walls, and individual components have also been shown to stimulate inflammatory cell chemotaxis, cytokine production, and cellular toxicity in experimental ocular systems (20, 44). In the vitreous, peptidoglycan is highly inflammogenic, inciting rapid influx of inflammatory cells into the posterior segment in a manner similar to that of gram-negative lipopolysaccharide (57).
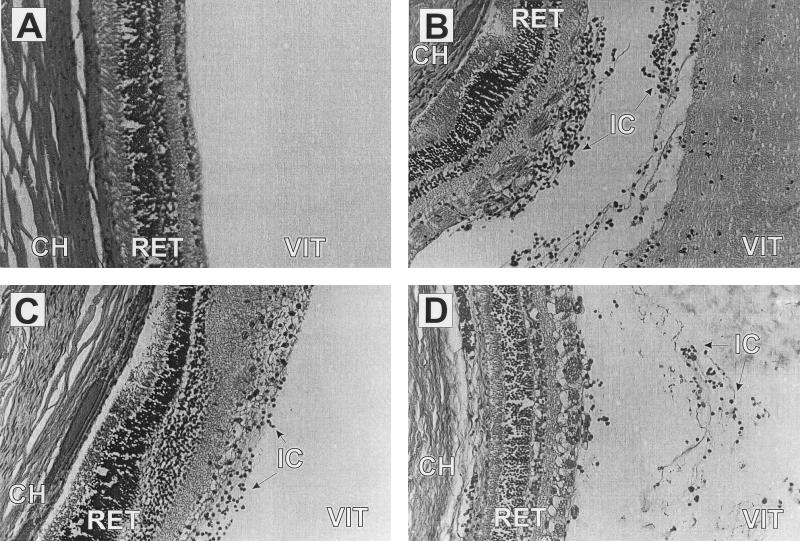
Comparisons of the inflammogenicity of cell walls from several gram-positive species demonstrate that cell walls of Bacillus sp. are most stimulatory, while P. acnes cell walls are minimally stimulatory (56, 130, 151). Since gram-positive cell walls are cross-linked to various degrees (106), the extent to which differences in the tertiary cell wall configurations account for the clincal variability observed with ocular infections is not known. Recently, we analyzed the intraocular inflammogenicity of B. cereus, S. aureus, and E. faecalis to determine whether these differences were species specific (20). Overall, neither metabolically inactive organisms nor purified sacculi caused significant reductions in retinal responsiveness, but these preparations caused significant intraocular inflammation (Fig. 4). The inflammogenicity of metabolically inactive organisms was greater than that of purified sacculi, indicating a role for extractable cell wall-associated components in stimulating intraocular inflammation (20). Significant differences in relative intraocular inflammogenicty between these organisms were not found, however. The effect of fragmentation of the peptidoglycan, as occurs with organisms exposed to cell wall-active antibiotics, was not explored and may affect the outcome of these experiments.
FIG. 4.
Histological analysis of intravitreal injection of metabolically inactive B. cereus, S. aureus, and E. faecalis. Metabolically inactive B. cereus (106 CFU), S. aureus (106 CFU), and E. faecalis (108 CFU) were injected into the vitreous. Injection inocula were chosen based upon the number of viable organisms present when early signs of infection were observed in the natural infection (20). Representative eyes were harvested at 72 h postinfection. Vitreous was sham injected (A) or injected with B. cereus (B), E. faecalis (C), or S. aureus (D). Injection of metabolically inactive organisms (B to D) caused extensive influx of inflammatory cells and fibrin accumulation in the posterior chamber by 72 h postinfection. Retinal layers remained intact. All sections were stained with hematoxylin-eosin. Magnification, ×270. Abbreviations: CH, choriocapillaris; RET, retina; VIT, vitreous; IC, inflammatory cells.
INTRAOCULAR HOST RESPONSE: IMPLICATIONS FOR DISEASE SEVERITY
As noted earlier, the eye must maintain a clear visual tract for normal visual function. Anatomical and functional characteristics render the eye an immunologically privileged site, affording protection against excessive bystander damage caused by the host immune response (132). Several factors account for ocular immune privilege, which limits inflammatory responses in order to avoid bystander damage to the retina. Ocular immune privilege has been extensively studied in the setting of the adaptive immune response (94). Although an adaptive response probably does not occur during the ordinarily acute course of endophthalmitis, the findings in this field likely have important implications for the innate immune response to intraocular microorganisms.
Blood-Ocular Fluid Barrier
Under normal physiological conditions, the blood-ocular fluid barrier ensures proper functioning of intraocular tissues and is essential for immune privilege (28). The blood-ocular fluid barrier, consisting of inner and outer blood-retina barriers and the blood-aqueous humor barrier, limits the influx of macromolecules into aqueous humor, vitreous, and the retinal intercellular spaces. The inner blood-retina barrier is formed by tight junctions between the endothelial cells and basement membrane of retinal capillaries and retinal pericytes, which control the blood supply for the inner retinal layers, preventing leakage of plasma constituents into the vitreous. The tight junctions between retinal pigment epithelial cells constitute the outer blood-retina barrier and control the blood supply to retinal photoreceptor cells and the choriocapillaris. The blood-aqueous humor barrier is formed by the iris and ciliary epithelium and thus divides the highly perfused iris from its neighboring compartments, the anterior chamber and the anterior vitreous (28).
The importance of these barriers for retinal function becomes evident in diseases in which this compartmentalization is compromised, such as occurs in diabetic retinopathy (8). During inflammation these barriers break down (90). While the blood-ocular fluid barriers furnish the retina and other intraocular tissues with a controlled supply of blood and nutrients, these tissues also maintain an immune-privileged environment by supplying increasing levels of certain cytokines, such as transforming growth factor β (50, 137), α-melanocyte-stimulating hormone (138), and vasoactive intestinal peptide (139). Although these factors share immunosuppressive properties and have been detected in healthy aqueous humor, it is unknown to what degree these cytokines regulate the immune response in endophthalmitis.
ACAID
Several laboratories have documented the process of ocular antigen presentation and its immunological consequences in great detail in the context of anterior chamber-associated immune deviation (ACAID) (52, 73, 79, 95). Ocular antigen presenting cells, namely, macrophages and dendritic cells, are found in the iris and the choriocapillaris (86, 128). Cells residing in the iris are likely candidates for antigen sampling of the anterior chamber, and antigen presenting cells appear to travel via the trabecular meshwork and the venous circulation toward the spleen (135). In the posterior segment, astrocytes and retinal pigment epithelial cells of the retina, and dendritic cells and macrophages of the choriocapillaris appear to be capable of presenting antigen (82), and the posterior compartment of the eye also displays typical features of immune privilege (64).
The impact of ACAID in intraocular bacterial infection is unclear. In most cases of postoperative endophthalmitis, bacteria enter the eye via the anterior chamber, where antigen presentation may initially occur. Therefore, antigen presenting cells residing in the iris are the most likely to first encounter these pathogens. This process may be facilitated by the mild inflammatory reaction in the anterior segment resulting from tissue manipulation of surgery. However, the duration of “immunological silence” between the introduction of organisms and immune activation in the early phase of endophthalmitis may be long enough to afford a growth advantage to the organism. Considering the immunosuppressive environment of the anterior chamber and the frequency of microbial contamination during surgery (12), it is in fact puzzling why intraocular infection is relatively rare. Retinal and uveal antigen presenting cells may not have access to antigen under physiological conditions but may function in this capacity upon activation during the later stages of infection, if the microbes have gained access to the posterior segment. Presumably, it is when the defense mechanisms of immune privilege are overwhelmed that the fulminant inflammation typical of severe cases of endophthalmitis occurs.
Complement and Proinflammatory Cytokines
Studies addressing the roles of complement and proinflammatory cytokines in bacterial endophthalmitis are limited. Experiments with systemically decomplemented guinea pigs revealed the importance of vitreal complement components in inhibiting bacterial growth (5, 46). The host defenses of partially decomplemented guinea pigs were impaired following intravitreal challenge with S. aureus, S. epidermidis, and Pseudomonas aeruginosa. Host defenses were restored as complement levels returned to normal (5, 46).
Interleukin-1 (IL-1) is produced by a number of cell types in response to various inflammatory and infectious stimuli (146). IL-1 initially mediates the acute-phase response, inducing other inflammatory mediators such as prostaglandins, phospholipase A2, collagenases and other proinflammatory cytokines (IL-6 and tumor necrosis factor [TNF]). In the eye, IL-1 is produced by corneal epithelial cells, Mueller cells, and ciliary body cells in response to different uveitogenic stimuli (33). Intravitreal injection of recombinant IL-1β induces the breakdown of the blood-retina barrier and leukocyte recruitment into the intraocular tissue (9).
Like IL-1, IL-6 is produced by numerous cell types and is induced by other cytokines (IL-1, gamma-interferon, and TNF alpha [TNF-α]) (5). IL-6 is crucial for the production of acute-phase proteins such as C-reactive protein and fibrinogen by the liver and promotes B- and T-cell differentiation (147). In the eye, IL-6 is produced primarily by retinal pigment epithelial cells (13). Although the role of IL-6 in ocular infection is not known, it is known to play a local role in negative feedback on IL-1 and TNF-α production (115). There is a strong correlation between IL-6 concentrations and leukocyte count in bacterial meningitis (149).
A variety of immune cells are able to produce TNF-α in response to microbial infection. In the eye, TNF-α is produced by astrocytes and by histiocyte-like cells in the iris ciliary body (7, 153). The effects of TNF-α include the induction of apoptosis, major histocompatibility complex upregulation, and pyrogenicity. In terms of its role in inflammation, TNF-α is strong activator of nuclear factor kappa B (NF-κB), a universally present transcriptional regulator that regulates the expression of chemokines, growth factors, and cell adhesion molecules. Aside from initiating the acute-phase response in immune cells, NF-κB induces a number of neuroprotective events (83). TNF-α injected intravitreally provokes an intraocular inflammatory reaction, albeit to a lesser degree than IL-1β, and together they act synergistically with IL-1β (41). The importance of IL-8 has been demonstrated in experimental uveitis (148). IL-8 promotes the recruitment of polymorphonuclear leukocytes (109), and because dense neutrophil infiltration is a characteristic feature of endophthalmitis, its involvement in intraocular infection is probable but has not yet been determined.
Few studies have adressed the role of cytokines in intraocular bacterial infection. Jett et al. (B. D. Jett, D. W. Parke, and M. S. Gilmore, Abstr. Assoc. Res. Vis. Ophthalmol. Meet., abstr. 3768, 1998) compared the kinetics of of IL-1, IL-6, and TNF-α induction in ocular tissues during gram-positive bacterial infection in rats. IL-1β and IL-6 were detected at the highest levels beginning as early as 6 h postinfection in B. cereus-, B. subtilis-, and E. faecalis-infected eyes. These cytokine levels peaked from 12 to 24 h. Cytokine induction in Bacillus-infected eyes occurred earlier and to a greater extent than that in E. faecalis-infected eyes. B. subtilis produced cytokine induction patterns similar to those of B. cereus. B. subtilis is commonly though of as a nontoxigenic organism, suggesting that the intraocular host response may be more dependent on the degree of inflammogenicity of the infectious agent rather than on toxin production (B. D. Jett et al., Abstr. Assoc. Res. Vis. Ophthalmol. Meet., abstr. 3768, 1998). In experimental S. aureus endophthalmitis, Giese et al. (47) detected proinflammatory cytokines at maximal levels at 24 h, which correlated with clinical observations of peaks in disease severity and inflammatory cell infiltration. However, while both studies demonstrate differences in proinflammatory cytokine gene induction by different bacterial species, the relationship between cytokine induction and endophthalmitis severity remains an open question.
Cellular Infiltration
A characteristic histopathological feature of endophthalmitis is a dense polymorphonuclear infiltrate of vitreous cavity, trabecular meshwork, anterior and posterior lens capsule, ciliary body, retina, and the choroids (37). Depending on the infecting organism, the onset of infiltration can be delayed and the infiltration can be comparatively limited, as in the case of S. epidermidis endophthalmitis (104), or more fulminant, as in B. cereus, S. aureus, or E. faecalis endophthalmitis (20). Considering the host of potentially retinotoxic products of neutrophils, such as oxyradicals, it is possible that these molecules contribute to retinal toxicity during bacterial endophthalmitis, as is the case in anatomically analogous infections, such as meningitis (78).
The role of T lymphocytes in endophthalmitis has not been established, but it is possible that they play important roles in regulating the immune response in this infection in this setting, as has been observed for bacterial keratitis (76).
MODEL OF ENDOPHTHALMITIS PATHOGENESIS
A picture of the molecular and cellular events that occur in the evolution of endophthalmitis is only beginning to emerge. However, much can be learned by drawing on observations made in broadly analagous systems, including studies on the pathogenesis of meningitis. As with meningitis, bacteria that gain access into the interior of the eye can replicate largely unimpeded by the immune system as a result of vascular compartmentalization and active suppression mechanisms. During growth, toxin production by virulent organisms results in loss of retinal function at a rate that appears to be organism dependent. The specific effects of bacterial toxins on tissues and cells of the posterior segment have not been well studied. In addition to toxins, the mere presence of certain organisms in these immunologically privileged areas stimulates intraocular inflammation. Cell envelopes, fragments of peptidoglycan, and teichoic acid or lipopolysaccharide are likely shed into these spaces during intraocular growth or antibiotic killing. These components, as well as the growing organisms themselves, may come in contact with and stimulate resident immune cells to produce proinflammatory cytokines or other immune mediators. The production of soluble immune mediators initiates a cascade of inflammatory events, including increased permeabilty of the blood-ocular fluid barrier, with influx of additional soluble mediators and recruitment of phagocytic inflammatory cells to the site of infection. Inflammatory cells may in turn produce more inflammatory cytokines, in addition to toxic enzymes and reactive oxygen species involved in phagocytosis. During the later stages of protracted endophthalmitis, lymphocytes may migrate into inflamed intraocular tissues, and an immunoglobulin response may result. The ultimate result is disruption of retinal architecture and death of nonregenerating retinal photoreceptor cells and a significant intraocular inflammatory response which can exacerbate the harmful effects of bacterial growth and toxin production by causing bystander damage.
While this scenario may pertain to untreated infections with virulent organisms, intraocular infection with nontoxigenic organisms such as S. epidermidis or P. acnes may also follow a similar course, albeit at a lower and possibly more immunologically controlled rate. However, because nontoxigenic organisms, such as B. subtilis, can cause as fulminant an intraocular infection as virulent organisms, the differences in infection severity may lie in not only whether the organism produces toxins but also to what extent the organism incites intraocular inflammation or undermines immune privilege.
CONCLUSIONS AND PERSPECTIVES
A more detailed understanding of the interactions between offending organisms and the intraocular host response is needed to more effectively treat endophthalmitis and improve visual outcome. Clinical evidence shows that while antibiotics effectively kill intraocular organisms and anti-inflammatory agents supress the intraocular inflammatory response, these drugs have no effect on the toxins or inflammation-derived enzymes that directly affect retinal function or architecture. As more information becomes available with respect to the natural course of different types of endophthalmitis, several steps in the evolution of infection may emerge as new therapeutic opportunities. Therapeutic targets of the bacterium may include global regulation of virulence factors, inactivating specific retinal toxins, blocking bacterial motility, or blocking bacterial tissue attachment and colonization. Potential therapuetic targets among the myriad of host responses include the production of proinflammatory cytokines, recruitment of inflammatory cells, and production of toxic inflammatory by-products. Further research in this area will delineate which of these are among the most viable options.
Acknowledgments
We thank Mary C. Booth, James Chodosh, Daniel J. J. Carr, Keeta Gilmore, Wolfgang Haas, Lynn E. Hancock, Brett Shepard, Phil Coburn, Scott Kane, and D. Clay Cochran. We also acknowledge Jerry Y. Niederkorn (University of Texas Southwest Medical Center, Dallas) for critical review of the manuscript and Tim Foster (Trinity College, Dublin, Ireland) for providing S. aureus isolates used in in vivo endophthalmitis studies.
Portions of the work presented in this review were supported by U.S. Health Service grants EY08289, EY12985, and EY12190 and grants from Fight for Sight-Prevent Blindness America, University of Oklahoma Alumni Research Foundation, and Research to Prevent Blindness, Inc.
REFERENCES
- 1.Aaberg, T. M., H. W. Flynn, J. Schiffman, and J. Newton. 1998. Nosocomial acute-onset postoperative endophthalmitis survey: a 10-year review of incidence and outcomes. Ophthalmology 105:1004–1010. [DOI] [PubMed] [Google Scholar]
- 2.Abu el-Asrar, A. M., S. A. Al-Amro, A. A. Al-Mosallam, and S. Al-Obeidan. 1999. Post-traumatic endophthalmitis: causative organisms and visual outcome. Eur. J. Ophthalmol. 9:21–31. [DOI] [PubMed] [Google Scholar]
- 3.Affeldt, J. C., H. W. Flynn, R. K. Forster, S. Mandelbaum, J. G. Clarkson, and G. D. Jarus. 1987. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology 94:407–413. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar, H. E., T. A. Meredith, C. Drews, H. Grossniklaus, A. D. Sawant, and S. Gardner. 1996. Comparative treatment of experimental Staphylococcus aureus endophthalmitis. Am. J. Ophthalmol. 121:310–317. [DOI] [PubMed] [Google Scholar]
- 5.Aizuss, D. H., B. J. Mondino, H. L. Sumner, and B. A. Dethlefs. 1985. The complement system and host defense against Pseudomonas endophthalmitis. Investig. Ophthalmol. Vis. Sci. 26:1262–1266. [PubMed] [Google Scholar]
- 6.Aldave, A. J., J. D. Stein, V. A. Deramo, G. K. Shah, D. H. Fischer, and J. I. Maguire. 1999. Treatment strategies for postoperative Propionibacterium acnes endophthalmitis. Ophthalmology 106:2395–2401. [DOI] [PubMed] [Google Scholar]
- 7.Aloisi, F., A. Care, G. Borsellino, P. Gallo, S. Rosa, A. Bassani, A. Cabibbo, U. Testa, G. Levi, and C. Peschle. 1992. Production of hemolymphopoeietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1β and tumor necrosis factor α. J. Immunol. 149:2358–2366. [PubMed] [Google Scholar]
- 8.Antonetti, D. A., E. Lieth, A. J. Barber, and T. W. Gardner. 1999. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin. Ophthalmol. 14:240–248. [DOI] [PubMed] [Google Scholar]
- 9.Bamforth, S. D., S. L. Lightman, and J. Greenwood. 1997. Ultrastructural analysis of interleukin-1 beta-induced leukocyte recruitment to the rat retina. Investig. Ophthalmol. Vis. Sci. 38:25–35. [PubMed] [Google Scholar]
- 10.Baum, J., G. A. Peyman, and M. Barza. 1982. Intravitreal administration of antibiotic in the treatment of bacterial endophthalmitis. III. Consensus. Surv. Ophthalmol. 26:204–206. [DOI] [PubMed] [Google Scholar]
- 11.Beecher, D. J., J. S. Pulido, N. P. Barney, and A. C. Wong. 1995. Extracellular virulence factors in Bacillus cereus endophthalmitis: methods and implication of involvement of hemolysin BL. Infect. Immun. 63:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigi, B., W. Westlake, E. Mangelschots, B. Chang, W. Rich, and T. Riordan. 1997. Peroperative microbial contamination of anterior chamber aspirates during extracapsular cataract extraction and phacoemulsification. Br. J. Ophthalmol. 81:953–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benson, M. T., L. Sheperd, R. C. Rees, and I. G. Rennie. 1991. Production of interleukin-6 by human retina pigment epithelium in vitro and its regulation by other cytokines. Curr. Eye Res. 11:173–179. [DOI] [PubMed] [Google Scholar]
- 14.Bhakdi, S., T. Klonisch, P. Nuber, and W. Fischer. 1991. Stimulation of monokine production by lipoteichoic acids. Infect. Immun. 59:4614–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boldt, H. C., J. S. Pulido, C. F. Blodi, J. C. Folk, and T. A. Weingeist. 1989. Rural endophthalmitis. Ophthalmology 96:1722–1726. [DOI] [PubMed] [Google Scholar]
- 16.Booth, M. C., R. V. Atkuri, S. K. Nanda, J. J. Iandolo, and M. S. Gilmore. 1995. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Investig. Ophthalmol. Vis. Sci. 36:1828–1836. [PubMed] [Google Scholar]
- 17.Booth, M. C., A. L. Cheung, K. L. Hatter, B. D. Jett, M. C. Callegan, and M. S. Gilmore. 1997. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect. Immun. 65:1550–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth, M. C., K. L. Hatter, D. Miller, J. Davis, R. Kowalski, D. W. Parke, J. Chodosh, B. D. Jett, M. C. Callegan, R. Penland, and M. S. Gilmore. 1998. Molecular epidemiology of Staphylococcus aureus and Enterococcus faecalis in endophthalmitis. Infect. Immun. 66:356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brod, R. D., and H. W. Flynn. 1993. Endophthalmitis: current approaches to diagnosis and therapy. Curr. Opin. Infect. Dis. 6:628–637. [Google Scholar]
- 20.Callegan, M. C., M. C. Booth, B. D. Jett, and M. S. Gilmore. 1999. Pathogenesis of gram-positive bacterial endophthalmitis. Infect. Immun. 67:3348–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callegan, M. C., L. S. Engel, J. M. Hill, and R. J. O’Callaghan. 1994. Corneal virulence of Staphylococcus aureus: roles of alpha-toxin and protein A in pathogenesis. Infect. Immun. 62:2478–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callegan, M. C., B. D. Jett, L. E. Hancock, and M. S. Gilmore. 1999. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect. Immun. 67:3357–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campochiaro, P. A., and J. I. Lim. 1994. Aminoglycoside toxicity in the treatment of endophthalmitis. Arch. Ophthalmol. 112:48–53. [DOI] [PubMed] [Google Scholar]
- 24.Chen, J., Y. Fujino, and T. Takahashi. 1999. Experimental uveitis induced by intravitreal or intravenous lipoteichoic acid in rabbits. Jpn. J. Ophthalmol. 43:368–374. [DOI] [PubMed] [Google Scholar]
- 25.Chow, J. W., L. A. Thal, M. B. Perri, J. A. Vazquez, S. M. Donabedian, D. B. Clewell, and M. J. Zervos. 1993. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 37:2474–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark, W. L., P. K. Kaiser, H. W. Flynn, A. Belfort, D. Miller, and D. M. Meisler. 1999. Treatment strategies and visual acuity outcomes in chronic postoperative Propionibacterium acnes endophthalmitis. Ophthalmology 106:1665–1670. [DOI] [PubMed] [Google Scholar]
- 27.Cowan, C. L., W. M. Madden, G. F. Hatem, and J. C. Merritt. 1987. Endogenous Bacillus cereus panophthalmitis. Ann. Ophthalmol. 19:65–68. [PubMed] [Google Scholar]
- 28.Cunha-Vaz, J. G. 1997. The blood-ocular barriers: past, present, and future. Doc. Ophthalmol. 93:149–157. [DOI] [PubMed] [Google Scholar]
- 29.D’Amico, D. J., L. Caspers-Velers, J. Libert, E. Shanks, M. Schrooyen, L. A. Hanninen, and K. R. Kenyon. 1985. Comparative toxicity of intravitreal aminoglycoside antibiotics. Am. J. Ophthalmol. 100:264–275. [DOI] [PubMed] [Google Scholar]
- 30.Das, T., S. Jalali, V. K. Gothwal, S. Sharma, and T. J. Naduvilath. 1999. Intravitreal dexamethasone in exogenous bacterial endophthalmitis: results of a prospective randomised study. Br. J. Ophthalmol. 83:1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David, D. B., G. R. Kirkby, and B. A. Noble. 1994. Bacillus cereus endophthalmitis. Br. J. Ophthalmol. 78:577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeKimpe, S. J., M. Kengatharan, C. Thiemermann, and J. R. Vane. 1995. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92:10359–10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Vos, A. F., V. N. A. Klaren, and A. Kijlstra. 1994. Expression of multiple cytokines and IL-1RA in the uvea and retina during endotoxin-iduced uveitis in the rat. Investig. Ophthalmol. Vis. Sci. 35:3873–3883. [PubMed] [Google Scholar]
- 34.Driebe, W. T., S. Mandelbaum, R. K. Forster, L. K. Schwartz, and W. W. Culbertson. 1986. Pseudophakic endophthalmitis. Diagnosis and management. Ophthalmology 93:442– 448. [DOI] [PubMed] [Google Scholar]
- 35.Endophthalmitis Vitrectomy Study. 1996. Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy Study. Am. J. Ophthalmol. 122:830–846. [DOI] [PubMed] [Google Scholar]
- 36.Endophthalmitis Vitrectomy Study. 1995. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch. Ophthalmol. 113:1479–1496. [PubMed] [Google Scholar]
- 37.Engstrom, R. E., B. J. Mondino, B. J. Glasgow, H. Pitchekian-Halabi, and S. A. Adamu. 1991. Immune response to Staphylococcus aureus endophthalmitis in a rabbit model. Investig. Ophthalmol. Vis. Sci. 32:1523–1533. [PubMed] [Google Scholar]
- 38.Ferencz, J. R., E. I. Assia, L. Diamantstein, and E. Rubinstein. 1999. Vancomycin concentration in the vitreous after intravenous and intravitreal administration for postoperative endophthalmitis. Arch. Ophthalmol. 117:1023–1027. [DOI] [PubMed] [Google Scholar]
- 39.Ferro, J. F., M. de-Pablos, M. J. Logrono, L. Guisasola, and F. Aizpuru. 1997. Postoperative contamination after using vancomycin and gentamicin during phacoemulsification. Arch. Ophthalmol. 115:165–170. [DOI] [PubMed] [Google Scholar]
- 40.Fiscella, R. G., T. K. Nguyen, M. J. Cwik, B. A. Phillpotts, S. M. Friedlander, D. C. Alter, M. J. Shapiro, N. P. Blair, and J. P. Gieser. 1999. Aqueous and vitreous penetration of levofloxacin after oral administration. Ophthalmology 106:2286–2290. [DOI] [PubMed] [Google Scholar]
- 41.Fleisher, L. N., J. B. Ferrell, and M. C. McGahan. 1992. Synergistic uveitic effects of tumor necrosis factor-alpha and interleukin-1 beta. Investig. Ophthalmol. Vis. Sci. 33:2120–2127. [PubMed] [Google Scholar]
- 42.Forster, R. K. 1992. Experimental postoperative endophthalmitis. Trans. Am. Ophthalmol. Soc. 90:505–559. [PMC free article] [PubMed] [Google Scholar]
- 43.Foster, R. E., J. A. Martinez, T. G. Murray, P. E. Rubsamen, H. W. Flynn, and R. K. Forster. 1996. Useful visual outcomes after treatment of Bacillus cereus endophthalmitis. Ophthalmology 103:390–397. [DOI] [PubMed] [Google Scholar]
- 44.Fox, A., M. E. Hammer, P. Lill, T. G. Burch, and G. Burrish. 1984. Experimental uveitis elicited by peptidoglycan-polysaccharide complexes, lipopolysaccharide, and muramyl dipeptide. Arch. Ophthalmol. 102:1063–1067. [DOI] [PubMed] [Google Scholar]
- 45.Giese, M. J., J. A. Berliner, A. Riesner, E. A. Wagar, and B. J. Mondino. 1999. A comparison of the early inflammatory effects of an agr−/sar− versus a wild type strain of Staphylococcus aureus in a rat model of endophthalmitis. Curr. Eye Res. 18:177–185. [DOI] [PubMed] [Google Scholar]
- 46.Giese, M. J., B. J. Mondino, B. J. Glasgow, H. L. Sumner, S. A. Adamu, H. P. Halabi, and H. J. Chou. 1994. Complement system and host defense against staphylococcal endophthalmitis. Investig. Ophthalmol. Vis. Sci. 35:1026–1032. [PubMed] [Google Scholar]
- 47.Giese, M. J., H. L. Sumner, J. A. Berliner, and B. J. Mondino. 1998. Cytokine expression in a rat model of Staphylococcus aureus endophthalmitis. Investig. Ophthalmol. Vis. Sci. 39:2785–2790. [PubMed] [Google Scholar]
- 48.Gilmore, M. S., M. C. Callegan, and B. D. Jett. 1999. Enterococcus faecalis cytolysin and Bacillus cereus bi- and tri-component toxins, p.419–434. In J. E. Alouf and Freer J. H. (ed.), The comprehensive sourcebook of bacterial protein toxins, 2nd ed. Academic Press, London, United Kingdom.
- 49.Gilmore, M. S., A. L. Cruz-Rodz, M. Leimeister-Wachter, J. Kreft, and W. Goebel. 1989. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J. Bacteriol. 171:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Granstein, R. D., R. Staszewski, T. L. Knisely, E. Zeira, R. Nazareno, M. Latina, and D. M. Albert. 1990. Aqueous humor contains transforming growth factor-beta and a small (less than 3500 daltons) inhibitor of thymocyte proliferation. J. Immunol. 144:3021–3027. [PubMed] [Google Scholar]
- 51.Greenwald, M. J., L. G. Wohl, and C. H. Sell. 1986. Metastatic bacterial endophthalmitis: a comtemporary reappraisal. Surv. Ophthalmol. 31:81–101. [DOI] [PubMed] [Google Scholar]
- 52.Griffith, T. S., T. Brunner, S. M. Fletcher, D. R. Green, and T. A. Ferguson. 1995. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270:1189–1192. [DOI] [PubMed] [Google Scholar]
- 53.Gupta, D., Y. P. Jin, and R. Dziarski. 1995. Peptidoglycan induces transcription and secretion of TNF-alpha and activation of lyn, extracellular signal-regulated kinase, and rsk signal transduction proteins in mouse macrophages. J. Immunol. 155:2620–2630. [PubMed] [Google Scholar]
- 54.Han, D. P., S. R. Wisniewski, S. F. Kelsey, B. H. Doft, M. Barza, and P. R. Pavan. 1999. Microbiologic yields and complication rates of vitreous needle aspiration versus mechanized vitreous biopsy in the Endophthalmitis Vitrectomy Study. Retina 19:98–102. [DOI] [PubMed] [Google Scholar]
- 55.Han, D. P., S. R. Wisniewski, L. A. Wilson, M. Barza, A. K. Vine, B. H. Doft, and S. F. Kelsey. 1996. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am. J. Ophthalmol. 122:1–17. [DOI] [PubMed] [Google Scholar]
- 56.Heumann, D., C. Barras, A. Severin, M. P. Glauser, and A. Tomasz. 1994. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect. Immun. 62:2715–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howes, E. L., P. W. Cole, T. M. Adair, V. K. Cruse, and M. Pollycove. 1994. Cellular and vascular responses in acute experimental ocular inflammation. Investig. Ophthalmol. Vis. Sci. 35:4031–4038. [PubMed] [Google Scholar]
- 58.Huebner, J., and D. A. Goldmann. 1999. Coagulase-negative staphylococci: role as pathogens. Annu. Rev. Med. 50:223–236. [DOI] [PubMed] [Google Scholar]
- 59.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Javitt, J. C., D. A. Street, J. M. Tielsch, Q. Wang, M. M. Kolb, O. Schien, A. Sommer, M. Bergner, and E. P. Steinberg. 1994. National outcomes of cataract extraction. Retinal detachment and endophthalmitis after outpatient cataract surgery. Ophthalmology 101:100–106. [DOI] [PubMed] [Google Scholar]
- 61.Jett, B. D., R. V. Atkuri, and M. S. Gilmore. 1998. Enterococcus faecalis localization in experimental endophthalmitis: role of plasmid-encoded aggregation substance. Infect. Immun. 66:843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jett, B. D., H. G. Jensen, R. V. Atkuri, and M. S. Gilmore. 1995. Evaluation of therapeutic measures for treating endophthalmitis caused by isogenic toxin-producing and toxin-nonproducing Enterococcus faecalis strains. Investig. Ophthalmol. Vis. Sci. 36:9–15. [PubMed] [Google Scholar]
- 63.Jett, B. D., H. G. Jensen, R. E. Nordquist, and M. S. Gilmore. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 60:2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang, L. Q., and J. W. Streilein. 1996. Immune privilege extended to allogeneic tumor cells in the vitreous cavity. Investig. Ophthalmol. Vis. Sci. 32:224–228. [PubMed] [Google Scholar]
- 65.Johnson, M. W., B. H. Doft, S. F. Kelsey, M. Barza, L. A. Wilson, C. C. Barr, S. R. Wisniewski, and the Endophthalmitis Vitrectomy Sudy Group. 1997. The Endophthalmitis Vitrectomy Study: relationship between clinical presentation and microbiologic spectrum. Ophthalmology 104:261–272. [DOI] [PubMed] [Google Scholar]
- 66.Jonas, J. B., and W. M. Budde. 1999. Early versus late removal of retained intraocular foreign bodies. Retina 19:193–197. [DOI] [PubMed] [Google Scholar]
- 67.Jonas, J. B., H. L. Knorr, and W. M. Budde. 2000. Prognostic factors in ocular injuries caused by intraocular or retrobulbar foreign bodies. Ophthalmology 107:823–828. [DOI] [PubMed] [Google Scholar]
- 68.Kattan, H. M., H. W. Flynn, S. D. Pflugfelder, C. Robertson, and R. K. Forster. 1991. Nosocomial endophthalmitis survey: current incidence of infection after intraocular surgery. Ophthalmology 98:227–238. [PubMed] [Google Scholar]
- 69.Kawana, M., C. Kawana, and G. S. Giebink. 1992. Penicillin treatment accelerates middle ear inflammation in experimental pneumococcal otitis media. Infect. Immun. 60:1908–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim, I. T., K. H. Chung, and B. S. Koo. 1996. Efficacy of ciprofloxacin and dexamethasone in experimental Pseudomonas endophthalmitis. Korean J. Ophthalmol. 10:8–17. [DOI] [PubMed] [Google Scholar]
- 71.Kim, Y. S., S. Kennedy, and M. G. Tauber. 1995. Toxicity of Streptococcus pneumoniae in neurons, astrocytes, and microglia in vitro. J. Infect. Dis. 171:1363–1368. [DOI] [PubMed] [Google Scholar]
- 72.Kim, Y. S., and M. G. Tauber. 1996. Neurotoxicity of glia activated by gram-positive bacterial products depends on nitric oxide production. Infect. Immun. 64:3148–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kosiewicz, M. M., P. Alard, and J. W. Streilein. 1998. Alterations in cytokine production following intraocular injection of soluble protein antigen: impairment in IFN-gamma and induction of TGF-beta and IL-4 production. J. Immunol. 161:5382–5390. [PubMed] [Google Scholar]
- 74.Kresloff, M. S., A. A. Castellarin, and M. A. Zarbin. 1998. Endophthalmitis. Surv. Ophthalmol. 43:193–224. [DOI] [PubMed] [Google Scholar]
- 75.Kunimoto, D. Y., T. Dat, S. Sharma, S. Jalai, A. B. Majji, U. Gopinathan, S. Athmanathan, and T. N. Rao. 1999. Microbiologic spectrum and susceptibility of isolates: part II. Posttraumatic endophthalmitis. Am. J. Ophthalmol. 128:242–244. [DOI] [PubMed] [Google Scholar]
- 76.Kwon, B., and L. D. Hazlett. 1997. Association of CD4+ T-cell dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa ocular infection. J. Immunol. 159:6283–6290. [PubMed] [Google Scholar]
- 77.Lam, S. R., R. G. Devenyi, A.R. Berger, and W. Dunn. 1999. Visual outcome following penetrating globe injuries with retained intraocular foreign bodies. Can. J. Ophthalmol. 34:389–393. [PubMed] [Google Scholar]
- 78.Leib, S. L., Y. S. Kim, L. L. Chow, R. A. Sheldon, and M. G. Tauber. 1996. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J. Clin. Investig. 98:2632–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li, X. Y., L. T. D’Orazio, and J. Y. Niederkorn. 1996. Role of Th1 and Th2 cells in anterior chamber-associated immune deviation. Immunology 89:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mandelbaum, S., and R. K. Forster. 1987. Endophthalmitis associated with filtering blebs. Int. Ophthalmol. Clin. 27:107–111. [DOI] [PubMed] [Google Scholar]
- 81.Margo, C. E., R. N. Mames, and J. R. Guy. 1994. Endogenous Klebsiella endophthalmitis: report of two cases and review of the literature. Ophthalmology 101:1298–1301. [DOI] [PubMed] [Google Scholar]
- 82.Matsuhara, T., G. Pararajasegaram, G. S. Wu, and N. A. Rao. 1999. Retinal microglia differentially express phenotypic markers of antigen-presenting cells in vitro. Investig. Ophthalmol. Vis. Sci. 40:3186–3193. [PubMed] [Google Scholar]
- 83.Mattson, M. P., C. Culmsee, Z. Fu, and S. Camandola. 2000. Roles of nuclear factor κB in neuronal survival and plasticity. J. Neurochem. 74:443–456. [DOI] [PubMed] [Google Scholar]
- 84.Maucour, M. F., C. Brugniart, A. Ducasse, L. Brasme, and O. Bajolet. 1999. Bacillary endophthalmitis. Four case reports. J. Fr. Ophtalmol. 22:371–376. [PubMed] [Google Scholar]
- 85.Maxwell, D. P., B. D. Brent, J. G. Diamond, and L. Wu. 1991. Effect of intravitreal dexamethasone on ocular histopathology in a rabbit model of endophthalmitis. Ophthalmology 98:1370–1375. [DOI] [PubMed] [Google Scholar]
- 86.McMenamin, P. G., I. Holthouse, and P. G. Holt. 1992. Class II major histocompatibility complex (Ia) antigen-bearing dendritic cells within the iris and ciliary body of the rat eye: distribution, phenotype and relation to retinal microglia. Immunology 77:385–393. [PMC free article] [PubMed] [Google Scholar]
- 87.Meredith, T. A. 1999. Posttraumatic endophthalmitis. Arch. Ophthalmol. 117:520–521. [DOI] [PubMed] [Google Scholar]
- 88.Meredith, T. A., H. E. Aguilar, C. Drews, A. Sawant, S. Gardner, L. A. Wilson, and H. E. Grossniklaus. 1996. Intraocular dexamethasone produces a harmful effect on treatment of experimental Staphylococcus aureus endophthalmitis. Trans. Am. Ophthalmol. Soc. 94:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merino, G., Y. Fujino, and R. K. Hanashiro. 1998. Lipoteichoic acid as an inducer of acute uveitis in the rat. Investig. Ophthalmol. Vis. Sci. 39:1251–1256. [PubMed] [Google Scholar]
- 90.Metrikin, D. C., C. A. Wilson, B. A. Berkowitz, M. K. Lam, G. K. Wood, and R. M. Peshock. 1995. Measurement of blood-retinal barrier breakdown in endotoxin-induced endophthalmitis. Investig. Ophthalmol. Vis. Sci. 36:1361–1370. [PubMed] [Google Scholar]
- 91.Mittra, R. A., and W. F. Mieler. 1999. Controversies in the management of open-globe injuries involving the posterior segment. Surv. Ophthalmol. 44:215–225. [DOI] [PubMed] [Google Scholar]
- 92.Mo, J. S., A. Matsukawa, S. Ohkawara, and M. Yoshinaga. 1999. Role and regulation of IL-8 and MCP-1 in LPS-induced uveitis in rabbits. Exp. Eye Res. 68:333–340. [DOI] [PubMed] [Google Scholar]
- 93.Morlet, N., G. G. Graham, B. Gatus, A.J. McLachlan, C. Salonikas, D. Naidoo, I. Goldberg, and C. M. Lam. 2000. Pharmacokinetics of ciprofloxacin in the human eye: a clinical study and population pharmacokinetic analysis. Antimicrob. Agents Chemother. 44:1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niederkorn, J. Y. 1999. Anterior chamber-associated immune deviation. Chem. Immunol. 73:59–69. [DOI] [PubMed] [Google Scholar]
- 95.Niederkorn, J. Y., and J. W. Streilein. 1983. Alloantigens placed into the anterior chamber of the eye induce specific suppression of delayed-type hypersensitivity but normal cytotoxic T lymphocyte and helper T lymphocyte responses. J. Immunol. 131:2670–2674. [PubMed] [Google Scholar]
- 96.O’Brien, T. P., and S. Choi. 1995. Trauma-related ocular infections. Int. Ophthalmol. Clin. N. Am. 8:667–679. [Google Scholar]
- 97.Okada, A. A., R. P. Johnson, W. C. Liles, D. J. D’Amico, and A. S. Baker. 1994. Endogenous bacterial endophthalmitis: report of a ten-year retrospective study. Ophthalmology 101:832–838. [PubMed] [Google Scholar]
- 98.Okhravi, N., H. M. A. Towler, P. Hykin, M. Matheson, and S. Lightman. 1997. Assessment of a standard treatment protocol on visual outcome following presumed bacterial endophthalmitis. Br. J. Ophthalmol. 81:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olson, J. C., H. W. Flynn, R. K. Forster, and W. W. Culbertson. 1983. Results in the treatment of postoperative endophthalmitis. Ophthalmology 90:692–699. [DOI] [PubMed] [Google Scholar]
- 100.Ozturk, F., E. Kurt, U. U. Inan, L. Emiroglu, S. S. Ilker, and G. Sobaci. 1999. Effect of propolis on endotoxin-induced uveitis in rabbits. Jpn. J. Ophthalmol. 43:285–289. [DOI] [PubMed] [Google Scholar]
- 101.Park, S. S., N. Samiy, K. Ruoff, D. J. D’Amico, and A. S. Baker. 1995. Effect of intravitreal dexamethasone in treatment of pneumococcal endophthalmitis in rabbits. Arch. Ophthalmol. 113:1324–1329. [DOI] [PubMed] [Google Scholar]
- 102.Park, S. S., R. V. Vallar, C.H. Hong, S. Von Gunten, K. Ruoff, and D. J. D’Amico. 1999. Intravitreal dexamethasone effect on intravitreal vancomycin elimination in endophthalmitis. Arch. Ophthalmol. 117:1058–1062. [DOI] [PubMed] [Google Scholar]
- 103.Phillips, W. B., T. P. Wong, R. L. Bergren, M. A. Friedberg, and W. E. Benson. 1994. Late onset endophthalmitis associated with filtering blebs. Ophthalmic Surg. 25:88–91. [PubMed] [Google Scholar]
- 104.Pleyer, U., B. J. Mondino, S. A. Adamu, H. Pitchekian-Halabi, R. E. Engstrom, and B. J. Glasgow. 1992. Immune response to Staphylococcus epidermidis-induced endophthalmitis in a rabbit model. Investig. Ophthalmol. Vis. Sci. 33:2650–2663. [PubMed] [Google Scholar]
- 105.Projan, S. J., and R. P. Novick. 1999. The molecular basis of pathogenicity, p.55–82. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingstone, New York, N.Y.
- 106.Rogers, H. J., H. R. Perkins, and J. B. Ward. 1980. Peptidoglycan, p.191–214. In H. J. Rogers, H. R. Perkins, and J. B. Ward (ed.), Microbial cell walls and membranes. Chapman and Hall, New York, N.Y.
- 107.Romero, C. F., M. K. Rai, C. Y. Lowder, and K. A. Adal. 1999. Endogenous endophthalmitis: case report and brief review. Am. Fam. Phys. 60:510–514. [PubMed] [Google Scholar]
- 108.Rooney, P., G. Bilbe, O. Zak, and T. O’Reilly. 1995. Dexamethasone treatment of lipopolysaccharide-induced meningitis in rabbits that mimics magnification of inflammation following antibiotic therapy. J. Med. Microbiol. 43:37–44 [DOI] [PubMed] [Google Scholar]
- 109.Rot, A. 1992. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration, Immunol. Today 13:291–294. [DOI] [PubMed] [Google Scholar]
- 110.Rowsey, J. J., D. L. Newsom, D. J. Sexton, and W. K. Harms. 1982. Endophthalmitis: current aproaches. Ophthalmology 89:1055–1066. [DOI] [PubMed] [Google Scholar]
- 111.Roy, M., J. C. Chen, M. Miller, D. Boyaner, O. Kasner, and E. Edelstein. 1997. Epidemic Bacillus endophthalmitis after cataract surgery I: acute presentation and outcome. Ophthalmology 104:1768–1772. [DOI] [PubMed] [Google Scholar]
- 112.Samson, C. M., and C. S. Foster. 2000. Chronic postoperative endophthalmitis. Int. Ophthalmol. Clin. 40:57–67. [DOI] [PubMed] [Google Scholar]
- 113.Sato, K., M. K. Quartey, C. L. Liebeler, and G. S. Giebink. 1995. Timing of penicillin treatment influences the course of Streptococcus pneumoniae-induced middle ear inflammation. Antimicrob. Agents Chemother. 39:1896–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schemmer, G. R., and W. T. Driebe. 1987. Post-traumatic Bacillus cereus endophthalmitis. Arch. Ophthalmol. 105:342–344. [DOI] [PubMed] [Google Scholar]
- 115.Schindler, R., J. Mancilla, S. Endres, R. Ghorbani, S. C. Clark, and C. A. Dinarello. 1990. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 75:40–47. [PubMed] [Google Scholar]
- 116.Schmitz, S., H. B. Dick, F. Krummenaur, and N. Pfeiffer. 1999. Endophthalmitis in cataract surgery: results of a German survey. Ophthalmology 106:1869–1877. [DOI] [PubMed] [Google Scholar]
- 117.Scott, I. U., H. W. Flynn, W. Feuer, S. C. Pflugfelder, E. C. Alfonso, R. K. Forster, and D. Miller. 1996. Endophthalmitis associated with microbial keratitis. Ophthalmology 103:1864–1870. [DOI] [PubMed] [Google Scholar]
- 118.Shah, G. K., J. D. Stein, S. Sharma, A. Sivalingam, W. E. Benson, C. D. Regillo, G. C. Brown, and W. Tasman. 2000. Visual outcomes following the use of intravitreal steroids in the treatment of postoperative endophthalmitis. Ophthalmology 107:486–489. [DOI] [PubMed] [Google Scholar]
- 119.Shamsuddin, D., C. U. Tuazon, C. Levy, and J. Curtin. Bacillus cereus panophthalmitis: source of the organism. Rev. Infect. Dis. 4:97–103. [DOI] [PubMed]
- 120.Shany, S., A. W. Bernheimer, P. S. Grushoff, and K. W. Kim. 1974. Evidence for membrane cholesterol as the common binding site for cereolysin, streptolysin O, and saponin. Mol. Cell. Biochem. 3:179–186. [DOI] [PubMed] [Google Scholar]
- 121.Shrader, S. K., J. D. Band, C. B. Lauter, and P. Murphy. 1990. The clinical spectrum of endophthalmitis: incidence, predisposing factors, and features influencing outcome. J. Infect. Dis. 162:115–120. [DOI] [PubMed] [Google Scholar]
- 122.Siqueira, J. A., C. Speeg-Schatz, F. I. Freitas, J. Sahel, H. Monteil, and G. Prevost. 1997. Channel-forming leucotoxins from Staphylococcus aureus cause severe inflammatory reactions in a rabbit eye model. J. Med. Microbiol. 46:486–494. [DOI] [PubMed] [Google Scholar]
- 123.Smith, M. A., J. A. Sorenson, G. D’Aversa, S. Mandelbaum, I. Udell, and W. Harrison. 1997. Treatment of experimental methicillin-resistant Staphylococcus epidermidis endophthalmitis with intravitreal vancomycin and intravitreal dexamethasone. J. Infect. Dis. 175:462–466. [DOI] [PubMed] [Google Scholar]
- 124.Soderling, E. and K. U. Pauino. 1981. Conditions of production and properties of the collagenolytic enzymes by two Bacillus strains from dental plaque. J. Peridontal Res. 16:513–523. [PubMed] [Google Scholar]
- 125.Soltau, J. B., R. F. Rothman, D. L. Budenz, D. S. Greenfield, W. Feuer, J. M. Liebmann, and R. Ritch. 2000. Risk factors for glaucoma filtering bleb infections. Arch. Ophthalmol. 118:338–342. [DOI] [PubMed] [Google Scholar]
- 126.Speaker, M. G., F. A. Milch, M. K. Shah, W. Eisner, and B. N. Kreiswirth. 1991. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology 98:639–650. [DOI] [PubMed] [Google Scholar]
- 127.Spellerberg, B. and E. I. Tuomanen. 1994. The pathophysiology of pneumococcal meningitis. Ann. Med. 26:411–418. [DOI] [PubMed] [Google Scholar]
- 128.Steptoe, R. J., P. G. Holt, and P. G. McMenamin. 1995. Functional studies of major histocompatibility class II-positive dendritic cells and resident tissue macrophages isolated from the rat iris. Immunology 85:630–637. [PMC free article] [PubMed] [Google Scholar]
- 129.Stevens, S. X., B. D. Fouraker, and H. G. Jensen. 1991. Intraocular safety of ciprofloxacin. Arch. Ophthalmol. 109:1737–1743. [DOI] [PubMed] [Google Scholar]
- 130.Stimpson, S. A., R. R. Brown, S. K. Anderle, D. G. Klapper, R. L. Clark, W. J. Cromartie, and J. H. Schwab. 1986. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect. Immun. 51:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Streilein, J. W. 1999. Immunoregulatory mechanisms of the eye. Prog. Retinal Eye Res. 18:357–370. [DOI] [PubMed] [Google Scholar]
- 132.Streilein, J. W. 1999. Regional immunity and ocular immune privilege. Chem. Immunol. 73:11–38. [DOI] [PubMed] [Google Scholar]
- 133.Streilein, J. W. 1997. Regulation of ocular immune responses. Eye 11:171–175. [DOI] [PubMed] [Google Scholar]
- 134.Streilein, J. W., B. R. Ksander, and A. W. Taylor. 1997. Immune deviation in relation to ocular immune privilege. J. Immunol. 158:3557–3560. [PubMed] [Google Scholar]
- 135.Streilein, J. W., and J. Y. Niederkorn. 1981. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J. Exp. Med. 153:1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Supersac, G., Y. Piemont, M. Kubina, G. Prevost, and T. J. Foster. 1998. Assessment of the role of gamma-toxin in experimental endophthalmitis using a hlg-deficient mutant of Staphylococcus aureus. Microb. Pathog. 24:241–251. [DOI] [PubMed] [Google Scholar]
- 137.Tanihara, H., M. Yoshida, M. Matsumoto, and N. Yoshimura. 1993. Identification of transforming growth factor-β expressed in cultured human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 34:413–419. [PubMed] [Google Scholar]
- 138.Taylor, A. W., J. W. Streilein, and S. W. Cousins. 1992. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr. Eye Res. 11:1199–1206. [DOI] [PubMed] [Google Scholar]
- 139.Taylor, A. W., J. W. Streilein, and S. W. Cousins. 1994. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J. Immunol. 153:1080–1086. [PubMed] [Google Scholar]
- 140.Thompson, S. T., L. M. Parver, C. L. Enger, W. F. Meiler, and P. E. Liggett. 1993. Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. Ophthalmology 100:1468–1474. [DOI] [PubMed] [Google Scholar]
- 141.Townsend, G. C., and W. M. Scheld. 1996. The use of corticosteroids in the management of bacterial meningitis in adults. J. Antimicrob. Chemother. 37:1051–1061. [DOI] [PubMed] [Google Scholar]
- 142.Tseng, C. Y., P. Y. Liu, Z. Y. Shi, Y. J. Lau, B. S. Hu, J. M. Shyr, W. S. Tsai, and Y. H. Lin. 1996. Endogenous endophthalmitis due to Escherichia coli: case report and review. Clin. Infect. Dis. 22:1107–1108. [DOI] [PubMed] [Google Scholar]
- 143.Tuomanen, E., H. Liu, B. Hengstler, O. Zak, and A. Tomasz. 1985. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 151:859–868. [DOI] [PubMed] [Google Scholar]
- 144.Tuomanen, E., A. Tomasz, B. Hengstler, and O. Zak. 1985. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J. Infect. Dis. 151:535–540. [DOI] [PubMed] [Google Scholar]
- 145.Vahey, J. B. and H. W. Flynn. 1991. Results in the management of Bacillus endophthalmitis. Ophthalmic Surg. 22:681–686. [PubMed] [Google Scholar]
- 146.Van Deuren, M., A. S. Dofferhoff, and J. W. Van der Meer. 1992. Cytokines and the response to infection. J. Pathol. 168:349–356. [DOI] [PubMed] [Google Scholar]
- 147.Van Snick, J. 1990. Interleukin-6: an overview. Annu. Rev. Immunol. 8:252–278. [DOI] [PubMed] [Google Scholar]
- 148.Verma, M. J., N. Mukaida, U. Vollmer-Conna, K. Matsushima, A. Lloyd, and D. Wakefield. 1999. Endotoxin-induced uveitis is partially inhibited by anti-IL-8 antibody treatment. Investig. Ophthalmol. Vis. Sci. 40:2465–2470. [PubMed] [Google Scholar]
- 149.Waage, A., A. Halstensen, R. Shalaby, P. Brandtzaeg, P. Kierulf, and T. Espevik. 1989. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J. Exp. Med. 170:1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Waheed, S., D.C. Ritterband, D. S. Greenfield, J. M. Liebmann, J. A. Seedor, and R. Ritch. 1998. New patterns of infecting organisms in late bleb-related endophthalmitis: a ten year review. Eye 12:910–915. [DOI] [PubMed] [Google Scholar]
- 151.Webster, G. F., J. J. Leyden, R. A. Musson, and S. D. Douglas. 1985. Susceptibility of Propionibacterium acnes to killing and degradation by human neutrophils and monocytes in vitro. Infect. Immun. 49:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wiechens, B., D. Neumann, J. B. Grammer, U. Pleyer, J. Hedderich, and G. I. Duncker. 1998–1999. Retinal toxicity of liposome-incorporated and free ofloxacin after intravitreal injection in rabbit eyes. Int. Ophthalmol. 22:133–143. [DOI] [PubMed] [Google Scholar]
- 153.Yoshida, M., N. Yoshimura, M. Hangai, H. Tanihara, and Y. Honda. 1994. Interleukin-1α and interleukin-1β and tumor necrosis factor gene expression in endotoxin-induced uveitis. Investig. Ophthalmol. Vis. Sci. 35:1107–1113. [PubMed] [Google Scholar]
- 154.Yoshizumi, M. O., G. C. Lee, R. A. Equi, I. T. Kim, H. Pitchekian-Halabi, S. A. Adamu, and B. J. Mondino. 1998. Timing of dexamethasone treatment in experimental Staphylococcus aureus endophthalmitis. Retina 18:130–135. [DOI] [PubMed] [Google Scholar]