Abstract
This article from the American Society for Gastrointestinal Endoscopy (ASGE) provides a full description of the methodology used to inform the final guidance outlined in the accompanying summary and recommendations article for strategies to manage endoscopically placed gastrostomy tubes. This article was developed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework and specifically addresses the utility of PEG versus interventional radiology–guided gastrostomy (IR-G), the need for withholding antiplatelet and anticoagulant medications, appropriate timing to initiate tube feedings, and appropriate selection of the gastrostomy technique in patients with malignant dysphagia. In patients needing enteral access, the ASGE suggests PEG as the preferred technique for initial gastrostomy over IR-G. The ASGE recommends that tube feeding can be safely started within 4 hours of the gastrostomy without the need for an intentional delay. The ASGE suggests that a PEG can be performed without the need to withhold antiplatelet medications. In patients on anticoagulants who need to undergo PEG placement, the ASGE suggests that the periprocedural management of anticoagulants should be based on a multidisciplinary discussion with the patient regarding the risk of bleeding versus cardiovascular adverse events. In patients with malignant dysphagia, either transoral “Pull” PEG or transcutaneous “Direct” PEG can be performed for initial enteral access.
This guideline document was prepared by the Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy using the best available scientific evidence and considering a multitude of variables including, but not limited to, adverse events, patients’ values, and cost implications. The purpose of these guidelines is to provide the best practice recommendations that may help standardize patient care, improve patient outcomes, and reduce variability in practice.
We recognize that clinical decision-making is complex. Guidelines, therefore, are not a substitute for a clinician's judgment. Such judgments may, at times, seem contradictory to our guidance due to many factors that are impossible to fully consider by guideline developers. Any clinical decisions should be based on the clinician’s experience, local expertise, resource availability, and patient values and preferences.
This article is not a rule and should not be construed as establishing a legal standard of care, or as encouraging, advocating for, mandating, or discouraging any particular treatment. Our guidelines should not be used in support of medical complaints, legal proceedings, and/or litigation, as they were not designed for this purpose.
Introduction
Enteral access for long-term nutrition is often required for patients with dysphagia or the inability to ensure adequate volitional intake of food. In such patients with an intact and functional GI tract, a gastrostomy is the preferred means to facilitate enteral nutrition.1 Although creating a gastrostomy and placing a feeding tube through it remains a commonly performed procedure, it is associated with the risk of adverse outcomes.2
An endoscopic technique for creation of a gastrostomy and placement of a feeding tube is a widely accepted method for achieving enteral access. In addition to the PEG technique, radiological and surgical techniques are also often used. The first PEG was performed in infants in 1979 to avoid laparotomy.3,4 Over time, the techniques for enteral access have been refined, indications have expanded, and data regarding outcomes have become available. The use of gastrostomy tubes has also increased, especially in older patients with significant comorbidities.5,6
There are 3 frequently used techniques for endoscopic gastrostomies: transoral “Pull” PEG, transoral “Push” PEG, and transcutaneous “Direct” PEG, which have previously been described in detail.1 Briefly, the “Pull” PEG technique is described by Gauderer et al,4 which uses a wire loop to pull the feeding tube through the abdominal access site.1 The transoral “Push” PEG technique uses the Sacks-Vine technique,1,7 during which the tube is advanced over the guidewire at the patient’s mouth. “Direct” PEGs are “Introducer” PEGs using the Russell technique with Seldinger dilators, T-fasteners to secure the stomach to the abdominal wall, and a peel-away catheter.1,8
Endoscopists play an essential role in the placement and management of feeding tubes. Currently, there are no guidelines specific to gastrostomy tubes from any of the U.S.-based professional GI societies. Therefore, the American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice (SOP) Committee aimed to develop guidelines on gastrostomy tubes. These guidelines follow the Population, Intervention, Comparison, Outcome (PICO) format and use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. In formulating these guidelines, we conducted extensive literature reviews, including a formal systematic review of literature and meta-analyses.
Aims and scope
The aim of this ASGE guideline is to provide evidence-based recommendations on the management of feeding tubes placed through a gastrostomy in patients requiring enteral access. This article, subtitled “Methodology and review of evidence,” provides a detailed account of the evidence synthesis process that ultimately led to our recommendations, summarized in the article subtitled “Summary and recommendations.”9
This guideline addressed the following clinical PICO questions using the GRADE format.
-
(1)
In patients with normal foregut anatomy needing initial enteral access, is PEG or interventional radiology–guided percutaneous gastrostomy (IR-G) the preferred modality of choice?
-
(2)
In patients undergoing PEG, should antiplatelets or anticoagulants be held before the procedure to prevent continuing bleeding or other adverse events?
-
(3)
In patients who undergo PEG placement, should tube feeds be initiated early or be intentionally delayed?
-
(4)
In patients with malignant dysphagia requiring gastrostomy placement, is transoral “Pull” PEG or transcutaneous “Direct” gastrostomy the preferred modality to reduce the risk of implantation metastasis?
Methodology
Overview
This article was prepared by the SOP Committee of the ASGE and is a continuation of our society’s effort to produce evidence-based clinical guidelines using the GRADE framework.10 GRADE is a transparent process for preparing and presenting summaries of evidence with the goal of making evidence-based clinical practice recommendations.9, 10, 11, 12 The recommendations in this article were based on up-to-date meta-analyses of the available literature for each clinical question. The quality or certainty in the evidence and strength of recommendations were based on the GRADE framework.
Evidence profiles were created with the help of GRADE methodologists, and a GRADE panel was held in March 2023, where the evidence was reviewed and recommendations were generated. In developing our recommendations, we took into consideration the certainty in the evidence, benefits and harms of different management options, feasibility, patient values and preferences, resources utilization, direct cost, cost-effectiveness, and health equity. The final wording of the recommendations including direction and strength was approved by all members of the panel and the ASGE governing board. Stronger recommendations are typically stated as “we recommend…,” whereas weaker recommendations are indicated by phrases such as “we suggest….” The meanings and interpretations of various qualities of evidence and the implications of corresponding recommendations are summarized in Table 1.
Table 1.
Categories of quality of evidence and corresponding meaning and interpretation along with implications on various stakeholders
| Quality of evidence | Meaning | Interpretation |
|---|---|---|
| High | We are confident that the true effect lies close to that of the estimate of the effect. | Further research is very unlikely to change our confidence in the estimate of the effect. |
| Moderate | We are moderately confident in the estimate of the effect; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. | Further research is likely to have an impact on our confidence in the estimate of the effect and may change the estimate. |
| Low | Our confidence in the estimate of the effect is limited; the true effect may be substantially different from the estimate of the effect. | Further research is very likely to have an impact on our confidence in the estimate of the effect and is likely to change the estimate. |
| Very low | We have very little confidence in the estimate of the effect; the true effect is likely to be substantially different from the estimate of the effect. | Any estimate of the effect is very uncertain. |
| Implications for | Strong recommendation | Conditional recommendation |
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | Most individuals in this situation would want the suggested course of action, but many would not. |
| Clinicians | Most individuals should receive the test. Formal decision aids are not likely to be needed to help individual patients make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with his or her values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their values and preferences. |
| Policymakers | The recommendation can be adopted as policy in most situations. Compliance with this recommendation according to the guideline could be used as a quality criterion or performance indicator. | Policymaking will require substantial debate and involvement of various stakeholders. |
Formulation of clinical questions
Clinical questions were conceptualized by the authors of the documents and members of the ASGE SOP committee and approved by the Governing Board. The questions followed the “PICO” format: (P) population, (I) intervention, (C) comparator, and (O) outcomes of interest. For all clinical questions, relevant outcomes were identified a priori and rated from not important to critical through a consensus process.
Literature search and study selection criteria
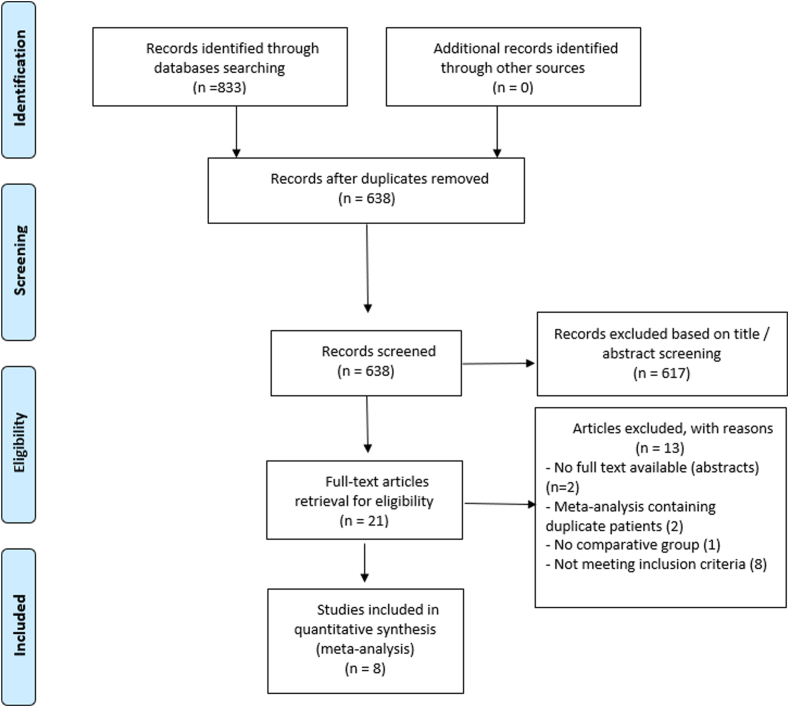
To inform the guideline panel, comprehensive electronic searches of PubMed, Embase, Web of Science, Ovid, and Cochrane EBM Reviews were designed and carried out by an experienced health research librarian. In addition, the reference sections of any relevant meta-analyses or manuscripts were manually reviewed. The searches were limited to prospective and retrospective studies published in English language. To be included, studies were required to meet all inclusion criteria (English language, prospective or retrospective comparative study, and 1 or more outcomes of interest assessed), and studies were excluded if any exclusion criteria were met (case report or review, case series, cohort study with no comparator arm, comparative study with n < 10 patients included, and missing or incomplete data).
The full search strategy is provided in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19, Figure 20, Figure 21, Figure 22, Figure 23, Figure 24, Figure 25. The library was uploaded into Covidence (Covidence, Melbourne, VIC, Australia), and 3 reviewers (D.R.K., N.C., W.M.A.) screened titles and abstracts for eligibility, with the first 2 independent assessments being used to determine inclusion or exclusion in the next stage, and with disagreements being resolved by consensus. Full-text screening then took place in a similar fashion.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram for records reviewed for endoscopic versus radiological gastrostomy tube in patients with normal foregut anatomy. PEG-J, PEG-jejunostomy; PIG, peroral image–guided gastrostomy.
Figure 2.
Risk of 30-day all-cause mortality with PEG and interventional radiology–guided gastrostomy. CI, Confidence interval; OR, odds ratio; PRG, percutaneous radiological gastrostomy.
Figure 3.
Risk of malfunction of feeding tube with PEG and interventional radiology–guided gastrostomy. CI, Confidence interval; OR, odds ratio; PRG, percutaneous radiological gastrostomy.
Figure 4.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram for records reviewed for holding antiplatelet or anticoagulant medication before placement of a PEG tube.
Figure 5.
Forest plot of studies evaluating bleeding risk in patients with aspirin use compared with those not given aspirin. CI, Confidence interval; OR, odds ratio.
Figure 6.
Forest plot of studies evaluating bleeding risk in patients with clopidogrel use compared with those not given clopidogrel. CI, Confidence interval; OR, odds ratio.
Figure 7.
Forest plot of studies evaluating bleeding risk in patients with dual antiplatelet therapy (DAPT) use compared with those not given DAPT. CI, Confidence interval; OR, odds ratio.
Figure 8.
Forest plot of studies evaluating bleeding risk in patients with anticoagulant use compared with those not given anticoagulants. CI, Confidence interval; OR, odds ratio.
Figure 9.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram for search strategy for assessing optimal timing of tube feed initiation after PEG tube placement.
Figure 10.
Risk of 30-day all-cause mortality with early and delayed tube feed initiation for all studies. CI, Confidence interval.
Figure 11.
Risk of 30-day all-cause mortality with early and delayed tube feed initiation for randomized controlled trial studies. CI, Confidence interval; RR, relative risk ratio.
Figure 12.
Short-term (<72 hours) mortality with early and delayed tube feed initiation for all studies. CI, Confidence interval.
Figure 13.
Short-term (<72 hours) mortality with early and delayed tube feed initiation for randomized controlled trial studies. CI, Confidence interval; RR, relative risk ratio.
Figure 14.
Adverse events with early and delayed tube feed initiation for all studies. CI, Confidence interval.
Figure 15.
Adverse events with early and delayed tube feed initiation for randomized controlled trial studies. CI, Confidence interval; RR, relative risk ratio.
Figure 16.
Vomiting with early and delayed tube feed initiation for all studies. CI, Confidence interval.
Figure 17.
Vomiting with early and delayed tube feed initiation for randomized controlled trial studies. CI, Confidence interval; RR, relative risk ratio.
Figure 18.
Aspiration for early and delayed tube feed initiation for all studies. CI, Confidence interval.
Figure 19.
Peristomal leak for early and delayed tube feeds for all studies. CI, Confidence interval.
Figure 20.
Wound infection for early and delayed tube feeds for all studies. CI, Confidence interval.
Figure 21.
Wound infection for early and delayed tube feeds for randomized controlled trial studies. CI, Confidence interval; RR, relative risk ratio.
Figure 22.
Stomatitis rates for early and delayed tube feeds for all studies. CI, Confidence interval.
Figure 23.
Stomatitis rates for early and delayed tube feeds for randomized controlled trial studies. CI, Confidence interval; RR, relative risk ratio.
Figure 24.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram for the search strategy for assessing risk of implantation metastasis.
Figure 25.
Incidence of implantation metastasis with transoral “Pull” PEG and “Direct” gastrostomy without the transoral passage of feeding tube. CI, Confidence interval; OR, odds ratio.
Studies assessing outcomes of percutaneous endoscopic jejunostomy, PEG with jejunal extension or IR-G with jejunal extension, feeding tube exchange through pre-existing gastrostomies, or studies examining pediatric patients were excluded. Noncomparative studies were also excluded.
Data extraction and statistical analysis
Data were abstracted using a standardized form. Meta-analysis was carried out with DerSimonian and Laird random effects models. The Cochrane I2 statistic was used to measure and report statistical heterogeneity. Publication bias was assessed visually using funnel plots. For all the eligible studies, we used the Newcastle-Ottawa scale and the Qumseya scale to assess the quality and risk of bias performed by 3 independent authors (D.R.K., N.C., W.M.A.).13, 14, 15 Statistical analyses were performed using RStudio version 1.2.1335 (Integrated Development Environment for R, Boston, Mass, USA).
Certainty in evidence (quality of evidence)
The certainty in the evidence was determined using the GRADE framework, beginning with study design (randomized controlled trials [RCTs] vs observational studies) and then assessing methodological limitations, inconsistency, indirectness, imprecision, publication bias, potential large study effects, dose-response gradients, and plausible confounding. The final quality of evidence ranges from very low to high (Table 1). The results of the meta-analyses and the assessments above were used to prepare a GRADE evidence profile for each clinical question using the GRADEpro GDT application (http://gdt.guidelinedevelopment.org/app).
Panel composition and conflict of interest management
The synthesized evidence was presented at a GRADE panel that took place in person in March 2023 and included the SOP committee members, an interventional radiologist (R.C.Z.), PEG content experts (J.C.F., S.A.M.), nutrition specialist (A.Y.K.), GRADE methodologists (N.F., M.D.), and patient representatives. All members were asked to disclose their respective conflicts of interest based on the relevant ASGE policies, available at https://www.asge.org/forms/conflict-of-interest-disclosure and https://www.asge.org/docs/default-source/about-asge/mission-and-governance/asge-conflict-of-interest-and-disclosure-policy.pdf.
Results
Question 1: In patients with normal foregut anatomy needing initial enteral access, is PEG or IR-G the preferred modality of choice?
Recommendation 1. In patients with normal foregut anatomy requiring enteral access, the ASGE suggests PEG over IR-G as the initial approach for gastrostomy (conditional recommendation and low quality of evidence)
For this question, a systematic review of published literature comparing PEG and IR-G as the initial intervention for enteral access was performed. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram detailing study screening and inclusion is provided in Figure 1. Outcomes of interest included all-cause mortality at 30 days, malfunction of a feeding tube, colon perforation, peritonitis, technical failure, bleeding, and aspiration (Table 2).
Table 2.
Comparative outcomes of PEG and interventional radiologist-guided gastrostomy
| Outcome | Studies | OR | 95% CI | P value | I2 (%) | Cochrane Q | Publication bias |
|---|---|---|---|---|---|---|---|
| 30-day mortality | 20 | 0.57 | 0.37-0.87 | .01 | 30.30 | 0.1 | 0.56 |
| Colon perforation | 8 | 0.92 | 0.25-3.33 | .87 | 0 | 0.42 | 0.01∗ |
| Peritonitis | 11 | 0.6 | 0.28-1.23 | .14 | 0 | 0.81 | 0.47 |
| Bleeding | 15 | 0.8 | 0.31-2 | .59 | 0 | 0.48 | 0.11 |
| Technical failure | 14 | 2.52 | 0.92-6.89 | .06 | 61 | 0.003 | 0.005 |
| Peristomal infection | 21 | 0.92 | 0.65-1.29 | .63 | 24.50 | 0.15 | 0.22 |
| Aspiration pneumonia | 9 | 0.58 | 0.28-1.20 | .11 | 0 | 0.72 | 0.45∗ |
| Tube malfunction | 20 | 0.5 | 0.27-0.92 | .02 | 84.20 | 0.001 | 0.09 |
CI, Confidence interval; OR, odds ratio.
Egger’s regression test should not be used for publication bias when study numbers are less than 10.
A recently published high-quality systematic review and meta-analysis, which assessed 33 studies comparing outcomes in 275,117 patients who underwent a PEG and 192,691 patients who underwent IR-G, was identified and updated to include recently published studies.11 While updating the above meta-analysis,11 2 other studies were noted to meet selection criteria.16,17 Of these, 1 did not provide data in a usable format. The primary investigators were contacted to obtain raw data for further assessment.17
The above meta-analysis also included 3 large, nationwide studies that used administrative databases to identify patients and compare outcomes.18, 19, 20 As part of the GRADE process, these 3 studies were excluded for the initial analysis and used only as supportive evidence. However, for a subset of serious adverse outcomes that have a low incidence (specifically, perforation of colon, and peritonitis), the panel concurred that single-center retrospective studies with modest sample sizes were likely underpowered to detect statistically meaningful differences. For these critical but less common adverse outcomes, data from the large nationwide studies were included in the analysis.
All-cause mortality at 30 days
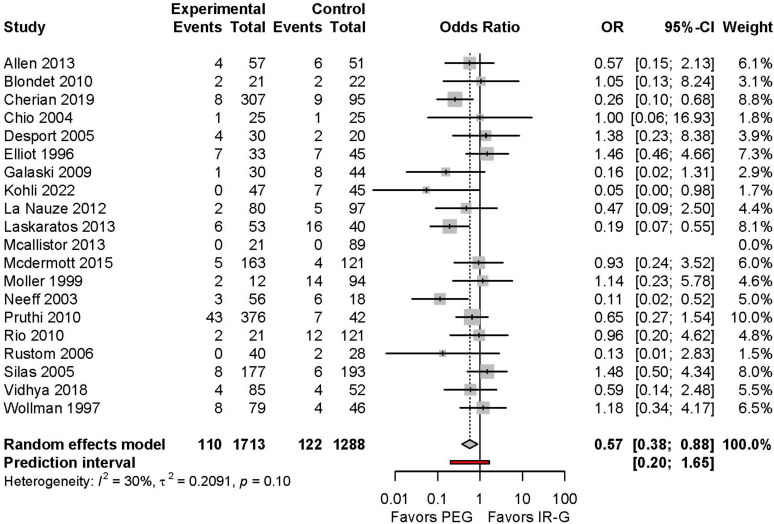
For this outcome, 20 studies met the selection criteria.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 Notably, 3 large, nationwide studies that analyzed administrative databases were excluded for the initial evaluation.18, 19, 20 The quality of evidence was rated down due to the retrospective nature of most of the studies. All-cause 30-day mortality was 6.4% (110 of 1713) in patients who underwent PEG and 9.5% (122 of 1288) in patients who underwent IR-G (odds ratio [OR], 0.57; 95% confidence interval [95% CI], 0.37-0.87; P = .01; Fig. 2). The certainty of evidence was rated as low because comparative data specific to overall severity of comorbidities were available in only some of the source studies.11,18,19
Of note, a recently published meta-analysis that included the 3 large administrative databases also reached a similar conclusion and demonstrated a lower odds of all-cause 30-day mortality with PEG than with IR-G (OR, 0.73, 95% CI, 0.58-0.93; P = .01).11
The expert panel included an interventional radiologist who noted that some of the source studies were published many years ago and that the IR-G techniques have evolved over time and become safer. However, we found no studies that demonstrate a meaningful reduction in mortality over time with IR-G.
Malfunction of a feeding tube
For this outcome, 20 studies were reviewed.16,23, 24, 25, 26,29,30,33, 34, 35, 36, 37, 38,41, 42, 43, 44, 45, 46, 47 Notably, 3 large administrative databases were excluded for the initial evaluation.18, 19, 20 The quality of evidence was rated down due to the retrospective design of the studies. Further, the definition of tube malfunction varied across different studies and included tube dislodgment, clogging of the tube, need for replacement, peristomal erythema, and pain at the site of gastrostomy. Because of the varying definition, evidence was rated down further for quality. The incidence of tube-related malfunction was 11.9% (274 of 2302) for PEG versus 19.3% (398 of 2060) for IR-G (OR, 0.51; 95% CI, 0.28-0.92; P = .02, Fig. 3).
Of note, the recently published meta-analysis,11 which included 3 large administrative databases, also reached a similar conclusion and demonstrated a lower incidence of tube malfunction with PEG than with IR-G (OR, 0.56; 95% CI, 0.33-0.95; P = .03).
The interventional radiologist in the expert panel noted that the most frequently used gastrostomy tubes in IR-G are typically 18F in diameter. These are thinner than feeding tubes placed during PEG, which are typically 20F to 24F.27 The larger diameter of the PEG feeding tube is very likely associated with a lower risk of clogging of the tube. Hence, this difference may not necessarily be solely due to technique but also due to the size/diameter of the tube. The panel also included a patient representative who had experienced tube-related malfunction with an IR-G but noted no adverse experience with a subsequent PEG.
Perforation of the colon
For this outcome, 8 studies met the selection criteria.17,23,27,32,40,41,43,48 The quality of evidence was rated down due to publication bias as well as the retrospective nature of the source studies. The incidence of colon perforation was 0.5% (4 of 774) with PEG and 2.1% (14 of 672) with IR-G (OR, 0.92; 95% CI, 0.25-3.33; P = .87). It was noted that the incidence of colon perforation is so low that it is unlikely to be detected in retrospective single-center studies with small sample sizes, which are very likely underpowered to assess less common events. Further, the panel noted that colon perforation is a very serious adverse outcome that has a critical impact on patient morbidity and mortality.
For this outcome, the recently published meta-analysis,11 which assessed 10 studies including 3 large administrative databases,18, 19, 20 was also included. The incidence of colon perforation was 0.1% (310 of 272,866) with PEG and 0.2% (390 of 190,851) with IR-G (OR, 0.6; 95% CI, 0.49-0.75; P = .008).
The interventional radiologist on the panel noted that the techniques for performing IR-G have improved over the past many years and there could be a reduction in the incidence of colon perforation with newer techniques. The panel also noted that multiple interventions are performed during a PEG to minimize the risk of colon perforation. These include transillumination, one-to-one indentation, and a safe-track needle technique to ensure the absence of any intervening colon between the anterior abdominal wall and the stomach.1,49 These techniques are not available with IR-G. The panel suggested that these interventions be performed routinely during a PEG procedure.
Peritonitis
For this outcome, 11 studies were assessed.23,25,26,30,33,35,36,38,40,42,43 The quality of evidence was rated down due to the retrospective nature of the source studies. The incidence of peritonitis was 1% (9 of 896) with PEG versus 2.7% (17 of 636) with IR-G (OR, 0.6; 95% CI, 0.28-1.23; P = .14).
Like the risk of colon perforation, the panel noted that peritonitis is a critical adverse outcome, but retrospective, single-center studies with modest sample sizes are likely to be underpowered to analyze this outcome in a statistically appropriate manner. Hence, for this outcome, the previously published meta-analysis, which included 3 large administrative databases, was also reviewed. The meta-analysis reported that the incidence of peritonitis was 1.9% with PEG and 2.7% with IR-G (OR, 0.71; 95% CI, 0.63-0.81; P < .001).
Postprocedure bleeding
For this analysis, 15 observation studies that met selection criteria were included.17,23,24,26,27,30,31,35,38, 39, 40, 41, 42, 43, 44 The quality of evidence was rated down due to the retrospective nature of the source studies. Bleeding was defined as clinically significant bleeding requiring either blood transfusion or therapeutic intervention. A small amount of bleeding during skin incision was not included as a significant outcome.
The incidence of bleeding was noted to be 0.8% (13 of 1553) with PEG versus 1.8% (17 of 939) with IR-G (OR, 0.8; 95% CI, 0.31-2; P = .59). A secondary analysis that included the recent meta-analysis also demonstrated no statistically significant difference in the incidence of bleeding between PEG and IR-G.
Of note, the source studies did not clearly differentiate between patients who stopped antiplatelet/anticoagulants versus those who did not. Those data are analyzed in PICO 2 in this article.
Technical failure
Technical failure was defined as the inability to create a gastrostomy and place a feeding tube on the initial attempt. Patients who underwent a successful PEG/IR-G after failure of the initial attempt with the other technique were analyzed based on the initial intervention.
For this outcome, 14 studies were reviewed.21,22,27,30,31,35, 36, 37,39,42,44,48,50,51 The quality of evidence was rated down for inconsistency as well as the retrospective nature of the source studies. The incidence of technical failure was 6.1% (73 of 1196) with PEG versus 2.6% (18 of 705) with IR-G (OR, 2.52; 95% CI, 0.92-6.89; P = .06). The secondary analysis using the recent meta-analysis also demonstrated no significant difference in technical failure between PEG and IR-G.
Infection at the PEG tube site
The incidence of peristomal infections was reported in 21 studies.16,24, 25, 26,30, 31, 32, 33, 34, 35, 36,38, 39, 40, 41, 42, 43, 44, 45, 46, 47 The quality of evidence was rated down due to the retrospective nature of the source studies. The incidence of peristomal infection was 7.7% (191 of 2487) with PEG versus 7.8% (173 of 2204) with IR-G (OR, 0.92; 95% CI, 0.65-1.29; P = .63). The secondary analysis using the recent meta-analysis also demonstrated no significant difference in peristomal infections between PEG and IR-G.
Current ASGE guidelines recommend the administration of periprocedural antibiotics in patients undergoing initial gastrostomy to minimize the risk of peristomal infections.52 Older interventional radiology guidelines did not specifically mandate the use of periprocedural antibiotics during IR-G due to lack of the transoral passage of the feeding tube and perceived reduction in bacterial contamination.53 However, the most recent guideline from the Society of Interventional Radiology recommends periprocedural antibiotics with IR-G irrespective of the technique used.54
Aspiration pneumonia
The incidence of aspiration pneumonitis was reported in 9 studies.26,27,31,35,36,38,39,43 The quality of evidence was rated down due to the retrospective nature of these source studies. The incidence of aspiration pneumonia was 1.2% (12 of 976) with PEG and 3.4% (17 of 495) with IR-G (OR, 0.58; 95% CI, 0.28-1.21; P = .11). The secondary analysis using the recently published meta-analysis also demonstrated no significant difference in the incidence of aspiration pneumonia with PEG and IR-G.
Cost, cost-effectiveness, and equity
There were no studies that assessed cost-effectiveness. In terms of cost, direct comparisons for the procedure or sedation were not available. As an indirect assessment, the work relative value unit (RVU) is 3.56 for PEG and 3.93 for IR-G. However, the work RVUs do not provide a complete assessment of cost. Data from non-U.S. studies (one each from Canada and Australia) were reviewed but were not used for the final analysis.11,23,26 There were no specific concerns related to equity because IR-G and PEG are both invasive procedures that are frequently performed at large and small community hospitals.
Certainty of evidence
For most of the outcomes, the risk of bias was judged to be serious because definitions of outcomes were not uniformly characterized and all source studies did not provide comparative data regarding the prevalence of comorbidities in the patients in each arm. There were concerns with imprecision in studies due to low event rates in some outcomes as well as wide CIs. There were no issues with indirectness. The quality of evidence was rated down to “low” due to the retrospective study design of the source studies, risk of bias, and imprecision in estimates of the incidence.
Discussion
Noting the low quality of evidence; comparable rates of technical success with PEG and IR-G; concern for serious adverse outcomes such as death, colon perforation, and peritonitis associated with IR-G; and input from expert radiologists and patient advocates, the ASGE suggests PEG over IR-G as the modality of choice for initial enteral access. The panel notes that in institutions where IR-G is frequently performed or PEG is unavailable, IR-G can be considered an appropriate method for enteral access.
Question 2: In patients undergoing PEG, should antiplatelet or anticoagulant agents be held before the procedure to prevent continuing bleeding or other adverse events?
Recommendation 2a: In patients on antiplatelets, including dual antiplatelet therapy (DAPT), who need to undergo PEG placement, the ASGE suggests against routine withholding of antiplatelets (conditional recommendation and very low quality of evidence).
Recommendation 2b: In patients on anticoagulants who need to undergo PEG placement, the ASGE suggests that the periprocedural management of anticoagulants should be based on a multidisciplinary discussion including the patient regarding the risk of bleeding versus cardiovascular events (conditional recommendation and very low quality of evidence).
Discontinuing antithrombotic medications can pose a significant risk to high-risk patients.55, 56, 57 Guidelines on withholding antithrombotic medications, including antiplatelet medications and anticoagulants, are critical to minimize mortality and morbidity associated with PEG placement. As such, we performed a systematic review of published literature comparing PEG placement with or without antithrombotic medications using Ovid MEDLINE and Embase for all studies published through September 2022. Outcomes included bleeding, mortality, risk of cardiovascular events, adverse events, transfusions, and cost.
Only 1 study was identified that directly compared outcomes in patients who held their antithrombotics versus those who did not.58 Given the limited nature of this study and to better understand the risk of bleeding, the search was expanded to include studies evaluating patients who were on any antithrombotic medications during their hospitalization or at the time of PEG placement, versus patients who were never on any of these medications. The PRISMA diagram detailing study screening and inclusion is provided in Figure 4. We identified 4 retrospective cohort studies, from which patient level data on bleeding outcomes were extracted.59, 60, 61, 62 No usable data were available on mortality, risk of cardiovascular events, overall adverse events, transfusions, hospitalization, and cost. Qumseya scores on quality were 6 to 7 for the studies included.13
The definition of bleeding varied between different studies. One study stratified their data specifically on PEG-related bleeding. However, all studies reported “significant bleeding,” defined as some variation of bleeding requiring endoscopic intervention, transfusion of blood products, hemoglobin drop of ≥2 g/dL, or clinical signs of active bleeding.
In the single retrospective study in which patients’ antithrombotic medications were held, 272 patients with antiplatelet medications were included (199 held, 73 continued) and the outcomes of patients on single antiplatelet medications (aspirin or clopidogrel) and DAPT medications were pooled. No specific dosing of aspirin was given in this study. Only 1 bleeding event was noted when antiplatelets were held and none when continued.58 As such, no significant increase in the rate of bleeding was noted in this study (single study OR, 0.90; 95% CI, 0.036-22.35; P = .95).
We then identified 4 nonrandomized respective cohort studies and extracted patient level data of 4708 patients to compare the risk of bleeding with or without any antithrombotic medications given during the hospitalization (Table 3).59, 60, 61, 62 Only 1 study specifically reported data in which PEG placement was done without stopping the antithrombotic medications,60 and 1 study reported data with the aspirin continued62; the other studies reported cases in which only antithrombotic medications were given sometime during the periprocedural period.
Table 3.
Summary outcomes of significant bleeding with PEG placement on the medication listed versus control
| Medication administered | Studies | OR | 95% CI | P value | I2 (%) | Cochrane Q |
|---|---|---|---|---|---|---|
| Single antiplatelet | ||||||
| Aspirin | 4 | 1.39 | 0.56-3.44 | .32 | 31.80 | 0.69 |
| Clopidogrel | 4 | 1.26 | 0.18-8.81 | .72 | 59.30 | 0.12 |
| Dual antiplatelet | 2 | 0.75 | 0.15-3.59 | .25 | 0 | 0.81 |
| Anticoagulation | 2 | 1.56 | 0.00-853.51 | .53 | 48.80 | 0.16 |
Publication bias was not reported given the low number of studies.
CI, Confidence interval; OR, odds ratio.
When evaluating antiplatelets, the use of aspirin alone did not significantly increase the rate of bleeding after PEG (4 studies, OR, 1.39; 95% CI, 0.56-3.44; P = .32; Fig. 5).59, 60, 61, 62 Similarly, patients on clopidogrel did not have an increased rate of bleeding compared with patients not on clopidogrel (Fig. 6; 4 studies, OR, 1.26; 95% CI, 0.18-8.81; P = .72).59, 60, 61, 62 We also extracted data for patients receiving DAPT and found no increased rate of bleeding (Fig. 7; 2 studies, OR, 0.75; 95% CI, 0.16-3.59; P = .25).59,60 Anticoagulants, which included only therapeutic heparin or warfarin, also did not increase the rate of bleeding, with data only available from 2 studies (Fig. 8; OR, 1.56; 95% CI, 0.00-853.51; P = .53).59,60 Very limited data were available on novel oral anticoagulants, so these were not included in the current guideline.
Certainty of evidence
The certainty of the evidence was low throughout. All studies were rated down for indirectness because they were subgroup analyses or reanalysis of patient-level data. Studies were rated down for imprecision given the low number of outcomes, with bleeding rates of 5.1% or less. In addition, the wide CI in the 2 studies reporting outcomes in patients on anticoagulation further rated down the precision. Overall, the certainty of evidence was considered very low.
Discussion
The panel noted that beyond the risks for cardiovascular events, there are other risks or disadvantages associated with holding antithrombotic medications. This includes keeping patients on nasal feeds, increasing length of stay, and delays in transfer to longer-term acute care facilities. In addition, consistent with expert opinion, the bleeding events were not life-threatening. The patient advocate also preferred bleeding over a cardiovascular event. No data were available on patient values, cost-effectiveness, and equity. No data were available on maneuvers to reduce bleeding such as tightening bumper for a short duration immediately after placement.
Given the totality of evidence, a conditional recommendation was drafted suggesting against the routine withholding of antiplatelet medications, including DAPT. Conversely, given the low quality of evidence, the ASGE suggests that the management of anticoagulant medications should be based on a multidisciplinary discussion regarding the competing risks of bleeding (with continuation of medications) and cardiovascular adverse events (with stopping medications). As standard of care for shared medical decision-making, the patient or legal guardian should be a part of these discussions.
Question 3: In patients who undergo PEG placement, should tube feeds be initiated early or intentionally delayed?
Recommendation 3. In patients who undergo PEG placement, the ASGE recommends that PEG tubes may be used for feeding early (within 4 hours) over routine delays in initiation (strong recommendation and low to moderate quality of evidence).
For this question, a systematic review of published literature comparing intentionally delayed versus early tube feed initiation after PEG placement was performed. The PRISMA diagram detailing study screening and inclusion is provided in Figure 9. For this, we used Ovid MEDLINE and Embase for all studies published through September 27, 2022. We used search terms and subheadings including enteral nutrition, enteral feeding, tube feeding, nutritional support, postpyloric, enteric, enteral, transpyloric, endoscopy, GI, digestive system, gastrostomy, PEG, interval, early, timing, initiation, delayed, and immediate (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19, Figure 20, Figure 21, Figure 22, Figure 23, Figure 24, Figure 25).
Outcomes of interest included all-cause mortality at 30 days, short-term mortality (<72 hours), adverse events, vomiting, aspiration, peristomal leak, wound infection, and PEG site stomatitis.
We identified 4 RCTs, which included 290 patients, 145 of whom underwent intentionally delayed tube feed initiation after PEG placement and 145 underwent early tube feed initiation.63, 64, 65, 66 All studies had Qumseya scores of ≥7.13 Early tube feed initiation was defined variously within 1 hour,63 within 3 hours,66 at 3 hours,65 and at 4 hours64 after PEG tube placement. Intentionally delayed tube feed initiation was performed at 24 hours in 3 of the studies63, 64, 65 and the day after PEG placement in 1 study.66 We additionally identified 4 non-RCT comparative studies comprising 3 retrospective cohorts67, 68, 69 and 1 prospective observational study,70 which included 829 total additional patients, 371 of whom underwent early feeding and 458 underwent delayed feeding. For these non-RCT comparative studies, early tube feed initiation was defined at 3 hours,70 before 4 hours,68,69 or at or before 4 hours after PEG placement.67 Tube feed initiation was considered delayed if performed at or later than 4 hours,68 more than 4 hours,67 between 4 and 24 hours,69 or 16 to 24 hours after PEG placement.70
For the meta-analysis, we defined early tube feed initiation at or before 4 hours after PEG tube placement and delayed initiation over 4 hours after PEG placement. Hence, 1 retrospective cohort71 that considered early tube feed initiation before 24 hours and delayed after 24 hours was excluded from our analysis. Outcomes were measured for both the RCTs alone and the RCTs and non-RCTs combined (total 516 early and 603 delayed initiations of feeding) (Tables 4 and 5). Definitions/criteria for clinical adverse events were not standardized or consistently reported for the included studies. Tube feed type and administration (aside from timing) was also not standardized or consistently reported.
Table 4.
Comparative outcomes of early and delayed tube feed initiation for all studies
| Outcome | Studies | OR | 95% CI | P value | I2 (%) |
|---|---|---|---|---|---|
| 30-day mortality | 5 | 0.83 | 0.51-1.35 | .45 | 0 |
| Short-term (<72 hours) mortality | 3 | 1.06 | 0.24-4.80 | .94 | 8 |
| Adverse events | 8 | 0.97 | 0.69-1.35 | .85 | 0 |
| Vomiting | 4 | 1.42 | 0.73-2.77 | .30 | 0 |
| Aspiration | 6 | 4.53 | 0.73-28.06 | .10 | 0 |
| Peristomal leak | 6 | 0.50 | 0.16-1.59 | .24 | 3 |
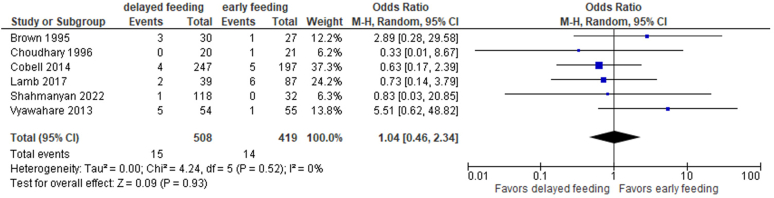
| Wound infection | 6 | 1.04 | 0.46-2.34 | .93 | 0 |
| Stomatitis | 5 | 0.90 | 0.15-5.51 | .91 | 49 |
CI, Confidence interval; OR, odds ratio.
Table 5.
Comparative outcomes of early and delayed tube feed initiation for all randomized controlled trials
| Outcome | Studies | RR | 95% CI | P value | I2 (%) |
|---|---|---|---|---|---|
| 30-day mortality | 2 | 0.93 | 0.06-14.00 | .79 | 0 |
| Short-term (<72 hours) mortality | 2 | 1.78 | 0.03-95.63 | .31 | 0 |
| Adverse events | 4 | 1.02 | 0.23-4.47 | .96 | 25 |
| Vomiting | 2 | 1.30 | 0.00-1819.05 | .72 | 0 |
| Wound infection | 2 | 1.34 | 0.00-304187.64 | .81 | 8 |
| Stomatitis | 2 | 0.26 | 0.01-9.04 | .1 | 0 |
CI, Confidence interval; OR, odds ratio; RR, relative risk ratio.
Two meta-analyses of RCTs72,73 were not used in our analysis because they both included 2 RCTs of likely overlapping patients, 1 in abstract form with 77 patients74 and 1 in manuscript form with 80 patients from the same hospital and study time period.63
Mortality
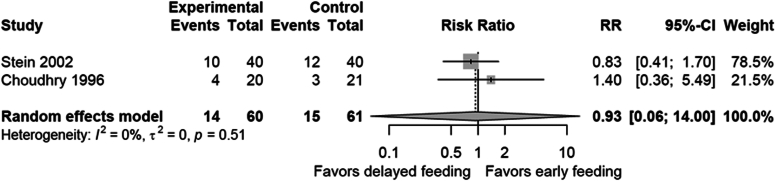
For all-cause mortality at 30 days, data from 2 RCT and 3 non-RCT studies63,65,67,69,70 demonstrated similar mortality (9.3% for delayed vs 12% for early, OR, 0.83; 95% CI, 0.51-1.35; P = .45) (Fig. 10). When including only the 2 RCTs,63,65 mortality rates were statistically comparable (23.3% for delayed vs 24.6% for early, relative risk ratio [RR], 0.93; 95% CI, 0.06-14.00; P = .79) (Fig. 11). While assessing the certainty of evidence, we rated down for imprecision.
Short-term (<72 hours) mortality was examined in 3 studies,63,65,67 which demonstrated no statistically significant difference (1.3% for delayed vs 1.6% for early, OR, 1.06; 95% CI, 0.24-4.8; P = .94) (Fig. 12). When considering only the 2 RCTs,63,65 no significant mortality difference was noted (6.7% vs 3.3%, RR, 1.78; 95% CI, 0.03-95.63; P = .31) (Fig. 13). The evidence was rated down for imprecision.
Adverse events
All 8 studies examined adverse events and demonstrated no difference in the incidence of adverse events (15.4% for delayed vs 18.4% for early, OR, 0.97; 95% CI, 0.69-1.35; P = .85) (Fig. 14). When including only the 4 RCTs, no statistically significant difference was observed (12.4% vs 12.4%, RR 1.02; 95% CI, 0.23-4.47; P = .96) (Fig. 15). The evidence was rated down for imprecision.
Vomiting
Two RCTs63,65 and 2 non-RCTs67,69 reported the incidence of vomiting. There was no difference based on data from all the 4 studies combined (6.6% for delayed vs 4.6% for early, OR, 1.42; 95% CI, 0.73-2.77; P = .3) (Fig. 16), or for only the RCTs (8.3% vs 6.6%, RR, 1.30; 95% CI, 0.00-1819.1; P = .72) (Fig. 17). This was rated down for imprecision.
Aspiration
Only 1 aspiration event, which occurred in the delayed group, was reported in the 3 RCTs that examined this adverse event.63, 64, 65 Hence, a meta-analysis of RCTs was not performed. For the 6 non-RCT and RCT studies that compared aspiration rates,63, 64, 65,67, 68, 69 all aspiration events occurred in the delayed arm, but this difference was not statistically significant (0.8% vs 0%, OR, 4.53; 95% CI, 0.73-28.06; P = .1) (Fig. 18). The data were rated down for imprecision. This was noted to have a strong association due to a large effect.
Peristomal leak
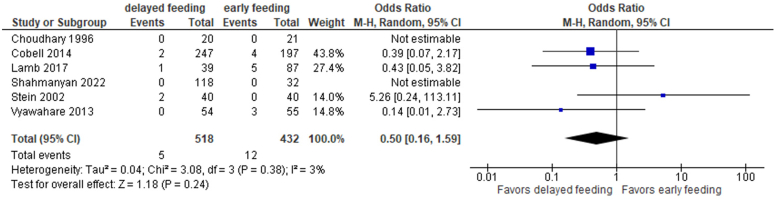
Two RCTs examined peristomal leak.63,65 One study reported 2 events in the delayed arm, whereas the other did not have any peristomal leaks occurring in either study arm. Therefore, a meta-analysis was only performed using the 6 combined RCT and non-RCT studies that reported peristomal leaks63,65,67, 68, 69, 70 (Fig. 19). Although more peristomal leaks were noted in the early feeding group (2.8% vs 1%), this difference was small and not significant (OR, 0.5; 95% CI, 0.16-1.59; P = .24).
PEG site stomatitis
The differentiation between stomatitis and infection at the site of the gastrostomy is unclear, because no definitions were provided in the source studies. Stomatitis at the PEG site was inferred as inflammation in the absence of infection, whereas infection was inferred as confirmed or suspected infection of the PEG site. Because these were reported separately in some studies,67 these were analyzed separately.
Stomatitis rates were reported for 2 RCTs63,65 and 3 non-RCTs.67,68,70 No difference was seen (1.9% for delayed vs 1.4% for early, OR, 0.9; 95% CI, 0.15-5.51; P = .91) (Fig. 22) in all studies. A nonsignificant increase in stomatitis rates was noted for the early versus delayed groups (4.9% vs 0%, RR, 0.26; 95% CI, 0.01-9.04; P = .1) when including only the RCTs (Fig. 23). The data were rated down due to imprecision.
Wound infection
Rates of wound infection in 6 non-RCT and RCT studies65, 66, 67, 68, 69, 70 were also similar (3.0% for delayed vs 3.3% for early, OR, 1.04; 95% CI, 0.46-2.34; P = .93) (Fig. 20), as were rates of wound infections when considering the 2 RCTs only65,66 (6% vs 4.2%, RR: 1.34; 95% CI, 0.0-304,188; P = .81) (Fig. 21). The data were rated down due to imprecision.
Discussion
The panelists noted a misconception that the delayed initiation of tube feeds allows the stoma site to heal. Instead, the stoma site takes at least several weeks to heal.75 Another concern with early tube feed initiation has been that gastric wall injury during PEG placement may transiently decrease gastric motility, resulting in increased gastric residual volume.63 Although several studies included in our analysis reported gastric residual volume as an outcome, the routine measurement of gastric residuals is of unclear clinical significance and may lead to unnecessary tube feed discontinuation.76, 77, 78, 79 In addition, the measurement of gastric residual volume and the definition of high gastric residuals are not standardized and varied between the studies that reported them. The panel agreed that routine gastric residual measurement should not be performed, and this was not included in our analysis.
Theoretically, earlier tube feed initiation may allow patients to reach goal rates quicker, resulting in earlier discharge, decreased length of hospitalization, and decreased health care cost. There were no studies that compared cost-effectiveness, time to reach goal tube feed rate, or hospital length of stay. The panel noted that the difference in nutritional status is likely clinically insignificant between early and delayed tube feed initiation, but no studies reported this.
For patient value assessment, our patient advocate emphasized a desire to have tube feeding initiated as soon as possible to not delay or interrupt nutrition therapy and felt reassured that there were no increased adverse events noted for earlier tube feed initiation. The expert panel also noted a theoretical benefit of reducing the risk of ileus with earlier tube feed initiation.
Overall, the evidence was of low to moderate quality. Given the lack of a significant difference in outcomes between the early and delayed groups in RCTs, the theoretical benefits of earlier tube feed initiation, input from the experts on the panel, and the patient value assessment, the panel made a strong recommendation for early initiation of tube feed after PEG tube placement.
The panel emphasized that although earlier tube initiation after PEG tube placement is recommended, there are clinical situations where tube feed initiation after PEG tube placement may not be clinically appropriate, such as patients on vasopressors, with severe ileus or obstruction, or patients with bowel ischemia. In addition, although intentional delay is not recommended, early tube feed initiation after PEG tube placement may not always be feasible due to delays related to the logistics of initiating tube feeds. The panel noted that in most cases, although it was not studied specifically, medications may be given immediately via the PEG after placement, unless there are contraindications to enteral medications.
Question 4: In patients with malignant dysphagia requiring gastrostomy placement, is transoral “Pull” PEG or transcutaneous “Direct” gastrostomy the preferred modality to reduce the risk of implantation metastasis?
Recommendation 4. In patients with malignant dysphagia requiring gastrostomy placement, the ASGE suggests either a transoral “Pull” PEG or transcutaneous “Direct” PEG (conditional recommendation and very low quality of evidence).
Creation of a gastrostomy and placement of a feeding tube in patients with malignant etiology of dysphagia are associated with implantation metastasis at the site of the gastrostomy.80 Recent literature has suggested an association between implantation metastasis and use of the “Pull” PEG technique (Ponsky method) or the “Push” PEG technique (Sacks-Vine method), both of which entail the transoral passage of the feeding tube. The incidence of implantation metastasis is reportedly lower when using techniques that do not require the transoral passage of the feeding tube: “Direct” endoscopic gastrostomy (which use the “Introducer” PEG technique) or IR-G.27,81, 82, 83
For this question, a systematic review of published literature was performed. A recently published systematic review and meta-analysis from Siu et al82 was reviewed. This meta-analysis included case reports and hence was deemed unsuitable for primary analysis. Consequently, this meta-analysis was updated. The PRISMA diagram detailing study screening and inclusion is provided in Fig. 24.
Implantation metastasis with transoral “Pull” PEG and “Direct” gastrostomy (“Direct” PEG and IR-G)
The incidence of implantation metastasis was reported in 11 retrospective studies.16,29,84, 85, 86, 87, 88, 89, 90, 91, 92 The quality of evidence was rated down due to the retrospective study design of the source studies, publication bias, and imprecision in estimates of the incidence of implantation metastasis given low event rates. Further, there was 1 study with an outsize impact on the forest plot due to relatively higher incidence of metastasis within a small cohort.16
The incidence of implantation metastasis was 0.6% (7 of 1093) versus 0.07% (1 of 1436) with transoral “Pull” PEG and “Direct” gastrostomy (PEG and IR-G), respectively (OR, 3.34; 95% CI, 0.36-30.70; P = .52, Fig. 25).
Comparative outcomes of “Direct” PEG and IR-G
Published literature suggests that a “Direct” PEG and IR-G can be performed in patients with malignant etiology of dysphagia to minimize risk of implantation metastasis because both gastrostomy techniques obviate the need for the transoral passage of the feeding tube. There was only 1 study that compared “Direct” PEG and IR-G in patients unable to undergo a transoral “Pull/Push” PEG. This single-center study noted comparable technical success between “Direct” PEG and IR-G.27 However, all-cause 30-day mortality was 0% with “Direct” PEG and 15.6% (7 of 45) with IR-G. The only serious adverse outcome, perforation of the colon, was noted with IR-G. Procedure times were shorter and dosage of sedatives was higher with “Direct” PEG than with IR-G.
Cost, cost-effectiveness, and equity
There were no studies assessing cost and cost-effectiveness.
Certainty of evidence
The risk of bias was judged to be very serious, and there were concerns with imprecision due to extremely low event rates. The method of diagnosing implantation metastasis was not uniformly defined in the source studies. The quality of evidence was rated down to “very low” because of the retrospective study design of the source studies, risk of bias, and imprecision in estimates of the incidence.
Discussion
In addition to concerns with the quality of the data, the experts noted that mechanical translocation of malignant cells during the transoral passage of the gastrostomy tube is not the only putative etiology of implantation metastasis. Available literature also suggests hematological spread of malignant cells leading to implantation metastasis.93 Implantation metastasis has also been reported with a “Direct” PEG as well as IR-G even though there is no transoral passage of the feeding tube.94,95
Further, the panel expressed significant concerns regarding the inequitable access to “Direct” PEG because most centers preferentially perform “Pull” PEG.83 In many centers, expertise to perform “Direct” PEG is not available and IR-G may be associated with adverse outcomes. Hence, the panel refrained from making a recommendation against use of transoral “Pull” PEGs in patients with aerodigestive malignancies.
The risk of implantation metastasis should be discussed with the patient during the informed consent process.96 In centers where IR-G or “Direct” PEG is available, these options should be offered. Further, the site of the gastrostomy should be examined for implantation metastasis. Finally, better quality studies examining this outcome are needed to better delineate the risk of implantation metastasis.
Guideline update
ASGE guidelines are reviewed for updates approximately every 5 years or in the event that new data may influence a recommendation. Updates follow the same ASGE guideline development process.
Disclosure
Dr Kohli has been a consultant for and has received a research grant from Olympus Corporation of the Americas. Dr Cosgrove is a consultant for Olympus Corporation of the Americas; is a consultant for and has received food and beverage from Boston Scientific Corporation; and has received food and beverage from Ambu Inc. Dr Abidi is a consultant for and has received food and beverage from Ambu Inc, Apollo Endosurgery US Inc, and CONMED Corporation; has received research support from GI Dynamics; and has received food and beverage from Olympus America Inc, AbbVie Inc, Boston Scientific Corporation, RedHill Biopharma Inc, and Salix Pharmaceuticals. Dr Machicado is a consultant for Mauna Kea Technologies, Inc and has received food and beverage from Mauna Kea Technologies, Inc and Boston Scientific Corporation. Dr Forbes is a consultant for Boston Scientific Corporation, PENTAX of America, Inc, AstraZeneca, and Pendopharm Inc; is on the speaker bureau for PENTAX of America, Inc and Boston Scientific Corporation; and has received research support from PENTAX of America, Inc. Dr Marya is a consultant for Boston Scientific Corporation and has received food and beverage from Boston Scientific Corporation and Apollo Endosurgery US Inc. Dr Thiruvengadam has received a grant from Boston Scientific Corporation. Dr Thosani is a consultant for and has received travel compensation and food and beverage from PENTAX of America, Inc and Boston Scientific Corporation; is a speaker for and has received travel compensation and food and beverage from AbbVie Inc; and is a consultant for Ambu Inc. Dr Ngamruengphong is a consultant for Boston Scientific Corporation and has received food and beverage from Medtronic, Inc, Boston Scientific Corporation, PENTAX of America, Inc, and Ambu Inc. Dr Elhanafi has received food and beverage from Medtronic, Inc, Nestle HealthCare Nutrition Inc, Ambu Inc, Salix Pharmaceuticals, Takeda Pharmaceuticals USA, Inc, and Merit Medical Systems Inc. Dr Sheth has consulted for Janssen Research & Development, LLC. Dr McClave is an educational consultant and is on the speaker’s bureau for Nestle; is on the speaker’s bureau for Abbott; and is on the advisory board for Avanos. Dr Qumseya is a consultant for and has received food and beverage from Medtronic, Inc; is a consultant for Assertio Management, LLC; is a speaker for Castle Biosciences; and has received food and beverage from FUJIFILM Health care Americas Corporation and Boston Scientific Corporation. All other authors disclosed no financial relationships.
Acknowledgments
We would like to thank Leonard Amato and Aaron Zelin, patient representatives from the National Foundation of Swallowing Disorders, for participating in our panel, as well as Drs Michael Bartel, Hala Fatima, and Kevin Waschke for their review of this article. This guideline was funded exclusively by the American Society for Gastrointestinal Endoscopy; no outside funding was received to support the development of this guideline.
Footnotes
Divyanshoo Rai Kohli, Natalie Cosgrove, and Wasif M. Abidi contributed equally to the article.
This article was reviewed and approved by the Governing Board of the American Society for Gastrointestinal Endoscopy.
References
- 1.ASGE Technology Committee. Kwon R.S., Banerjee S., Desilets D., et al. Enteral nutrition access devices. Gastrointest Endosc. 2010;72:236–248. doi: 10.1016/j.gie.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 2.ASGE Standards of Practice Committee. Coelho-Prabhu N., Forbes N., Thosani N.C., et al. Adverse events associated with EGD and EGD-related techniques. Gastrointest Endosc. 2022;96:389–401.e1. doi: 10.1016/j.gie.2022.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Ponsky J.L. Percutaneous endoscopic gastrostomy: after 40 years. Gastrointest Endosc. 2021;93:1086–1087. doi: 10.1016/j.gie.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Gauderer M.W., Ponsky J.L., Izant R.J. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872–875. doi: 10.1016/s0022-3468(80)80296-x. [DOI] [PubMed] [Google Scholar]
- 5.Folwarski M., Klek S., Brzeziński M., et al. Prevalence and trends in percutaneous endoscopic gastrostomy placement: results from a 10-year, nationwide analysis. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.906409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendiratta P., Tilford J.M., Prodhan P., Curseen K., Azhar G., Wei J.Y. Trends in percutaneous endoscopic gastrostomy placement in the elderly from 1993 to 2003. Am J Alzheimers Dis Other Demen. 2012;27:609–613. doi: 10.1177/1533317512460563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foutch P.G., Woods C.A., Talbert G.A., Sanowski R.A. A critical analysis of the Sacks-Vine gastrostomy tube: a review of 120 consecutive procedures. Am J Gastroenterol. 1988;83:812–815. [PubMed] [Google Scholar]
- 8.Russell T.R., Brotman M., Norris F. Percutaneous gastrostomy. A new simplified and cost-effective technique. Am J Surg. 1984;148:132–137. doi: 10.1016/0002-9610(84)90300-3. [DOI] [PubMed] [Google Scholar]
- 9.ASGE Standards of Practice Committee. Kohli D.R., Amateau S.K., Desai M., et al. American Society for Gastrointestinal Endoscopy guideline on management of post-liver transplant biliary strictures: summary and recommendations. Gastrointest Endosc. 2023;97:607–614. doi: 10.1016/j.gie.2022.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Schünemann H., Brożek J., Guyatt G., Oxman A., editors. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013. GRADE handbook. https://gdt.gradepro.org/app/handbook/handbook.html. Accessed March 6, 2023. [Google Scholar]
- 11.Kohli D.R., Radadiya D.K., Patel H., Sharma P., Desai M. Comparative outcomes of endoscopic and radiological gastrostomy tube placement: a systematic review and meta-analysis with GRADE analysis. Ann Gastroenterol. 2022;35:592–602. doi: 10.20524/aog.2022.0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amateau S.K., Kohli D.R., Desai M., et al. American Society for Gastrointestinal Endoscopy guideline on management of post-liver transplant biliary strictures: methodology and review of evidence. Gastrointest Endosc. 2023;97:615–637.e11. doi: 10.1016/j.gie.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Qumseya B.J. Quality assessment for systematic reviews and meta-analyses of cohort studies. Gastrointest Endosc. 2021;93:486–494.e1. doi: 10.1016/j.gie.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Hartling L., Hamm M., Milne A., et al. Decision rules for application of the newcastle-Ottawa scale [Internet]. Validity and Inter-Rater Reliability Testing of Quality Assessment Instruments [Internet]. Agency for Healthcare Research and Quality (US) 2012. https://www.ncbi.nlm.nih.gov/books/NBK92291/ Available at: [PubMed]
- 15.Duvvuri A., Bandla H., Thoguluva V.C., et al. Comparing accuracy of high-risk features for detecting advanced neoplasia in pancreatic cystic lesions: a systematic review and meta-analysis. Ann Gastroenterol. 2021;34:743–750. doi: 10.20524/aog.2021.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forner D., Mok F., Verma N., et al. Placement technique impacts gastrostomy tube-related complications amongst head and neck cancer patients. Oral Oncol. 2022;130 doi: 10.1016/j.oraloncology.2022.105903. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed Elfadil O., Linch F.B., Seegmiller S.L., Hurt R.T., Mundi M.S., Neisen M.J. Safety and effectiveness of radiologic and endoscopic percutaneous gastrostomy placement: a randomized study. JPEN J Parenter Enteral Nutr. 2022;46:1808–1817. doi: 10.1002/jpen.2365. [DOI] [PubMed] [Google Scholar]
- 18.Kohli D.R., Kennedy K.F., Desai M., Sharma P. Comparative safety of endoscopic vs radiological gastrostomy tube placement: outcomes from a large, nationwide veterans affairs database. Am J Gastroenterol. 2021;116:2367–2373. doi: 10.14309/ajg.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 19.Kohli D.R., Kennedy K.F., Desai M., Sharma P. Safety of endoscopic gastrostomy tube placement compared with radiologic or surgical gastrostomy: nationwide inpatient assessment. Gastrointest Endosc. 2020;93:1077–1085.e1. doi: 10.1016/j.gie.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Maasarani S., Khalid S.I., Creighton C., et al. Outcomes following percutaneous endoscopic gastrostomy versus fluoroscopic procedures in the Medicare population. Surg Open Sci. 2021;3:2–7. doi: 10.1016/j.sopen.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen J.A., Chen R., Ajroud-Driss S., et al. Gastrostomy tube placement by endoscopy versus radiologic methods in patients with ALS: a retrospective study of complications and outcome. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:308–314. doi: 10.3109/21678421.2012.751613. [DOI] [PubMed] [Google Scholar]
- 22.Blondet A., Lebigot J., Nicolas G., et al. Radiologic versus endoscopic placement of percutaneous gastrostomy in amyotrophic lateral sclerosis: multivariate analysis of tolerance, efficacy, and survival. J Vasc Interv Radiol. 2010;21:527–533. doi: 10.1016/j.jvir.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Cherian P., Blake C., Appleyard M., Clouston J., Mott N. Outcomes of radiologically inserted gastrostomy versus percutaneous endoscopic gastrostomy. J Med Imaging Radiat Oncol. 2019;63:610–616. doi: 10.1111/1754-9485.12932. [DOI] [PubMed] [Google Scholar]
- 24.Desport J.C., Mabrouk T., Bouillet P., Perna A., Preux P.M., Couratier P. Complications and survival following radiologically and endoscopically-guided gastrostomy in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:88–93. doi: 10.1080/14660820410021258. [DOI] [PubMed] [Google Scholar]
- 25.Elliott L.A., Sheridan M.B., Denyer M., Chapman A.H. PEG -- is the E necessary? A comparison of percutaneous and endoscopic gastrostomy. Clin Radiol. 1996;51:341–344. doi: 10.1016/s0009-9260(96)80112-7. [DOI] [PubMed] [Google Scholar]
- 26.Galaski A., Peng W.W., Ellis M., Darling P., Common A., Tucker E. Gastrostomy tube placement by radiological versus endoscopic methods in an acute care setting: a retrospective review of frequency, indications, complications and outcomes. Can J Gastroenterol. 2009;23:109–114. doi: 10.1155/2009/801925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohli D.R., Smith C., Chaudhry O., Desai M., DePaolis D., Sharma P. Direct percutaneous endoscopic gastrostomy versus radiological gastrostomy in patients unable to undergo transoral endoscopic pull gastrostomy. Dig Dis Sci. 2023;68:852–859. doi: 10.1007/s10620-022-07569-7. [DOI] [PubMed] [Google Scholar]
- 28.Laskaratos F.M., Walker M., Walker M., et al. Predictive factors for early mortality after percutaneous endoscopic and radiologically-inserted gastrostomy. Dig Dis Sci. 2013;58:3558–3565. doi: 10.1007/s10620-013-2829-0. [DOI] [PubMed] [Google Scholar]
- 29.McAllister P., MacIver C., Wales C., et al. Gastrostomy insertion in head and neck cancer patients: a 3 year review of insertion method and complication rates. Br J Oral Maxillofac Surg. 2013;51:714–718. doi: 10.1016/j.bjoms.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Neeff M., Crowder V.L., McIvor N.P., Chaplin J.M., Morton R.P. Comparison of the use of endoscopic and radiologic gastrostomy in a single head and neck cancer unit. ANZ J Surg. 2003;73:590–593. doi: 10.1046/j.1445-2197.2003.t01-1-02695.x. [DOI] [PubMed] [Google Scholar]
- 31.Pruthi D., Duerksen D.R., Singh H. The practice of gastrostomy tube placement across a Canadian regional health authority. Am J Gastroenterol. 2010;105:1541–1550. doi: 10.1038/ajg.2009.756. [DOI] [PubMed] [Google Scholar]
- 32.Rio A., Ellis C., Shaw C., et al. Nutritional factors associated with survival following enteral tube feeding in patients with motor neurone disease. J Hum Nutr Diet. 2010;23:408–415. doi: 10.1111/j.1365-277X.2010.01057.x. [DOI] [PubMed] [Google Scholar]
- 33.Rustom I.K., Jebreel A., Tayyab M., England R.J.A., Stafford N.D. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: a comparison study in head and neck cancer patients. J Laryngol Otol. 2006;120:463–466. doi: 10.1017/S0022215106000661. [DOI] [PubMed] [Google Scholar]
- 34.Silas A.M., Pearce L.F., Lestina L.S., et al. Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: a comparison of indications, complications and outcomes in 370 patients. Eur J Radiol. 2005;56:84–90. doi: 10.1016/j.ejrad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Vidhya C., Phoebe D., Dhina C., Jayne S., Robert F. Percutaneous endoscopic gastrostomy (PEG) versus radiologically inserted gastrostomy (RIG): a comparison of outcomes at an Australian teaching hospital. Clin Nutr ESPEN. 2018;23:136–140. doi: 10.1016/j.clnesp.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Wollman B., D’Agostino H.B. Percutaneous radiologic and endoscopic gastrostomy: a 3-year institutional analysis of procedure performance. AJR Am J Roentgenol. 1997;169:1551–1553. doi: 10.2214/ajr.169.6.9393163. [DOI] [PubMed] [Google Scholar]
- 37.Chiò A., Galletti R., Finocchiaro C., et al. Percutaneous radiological gastrostomy: a safe and effective method of nutritional tube placement in advanced ALS. J Neurol Neurosurg Psychiatry. 2004;75:645–647. doi: 10.1136/jnnp.2003.020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Nauze R.J., Collins K., Lyon S., et al. Outcomes of percutaneous endoscopic gastrostomy versus radiologically inserted gastrostomy tube insertion at a tertiary hospital. e-SPEN J. 2012;7:e144–e148. [Google Scholar]
- 39.ProGas Study Group Gastrostomy in patients with amyotrophic lateral sclerosis (ProGas): a prospective cohort study. Lancet Neurol. 2015;14:702–709. doi: 10.1016/S1474-4422(15)00104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Möller P., Lindberg C.G., Zilling T. Gastrostomy by various techniques: evaluation of indications, outcome, and complications. Scand J Gastroenterol. 1999;34:1050–1054. doi: 10.1080/003655299750025174. [DOI] [PubMed] [Google Scholar]
- 41.Clayton S., DeClue C., Lewis T., et al. Radiologic versus endoscopic placement of gastrostomy tube: comparison of indications and outcomes at a tertiary referral center. South Med J. 2019;112:39–44. doi: 10.14423/SMJ.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 42.Cosentini E.P., Sautner T., Gnant M., Winkelbauer F., Teleky B., Jakesz R. Outcomes of surgical, percutaneous endoscopic, and percutaneous radiologic gastrostomies. Arch Surg. 1998;133:1076–1083. doi: 10.1001/archsurg.133.10.1076. [DOI] [PubMed] [Google Scholar]
- 43.Grant D.G., Bradley P.T., Pothier D.D., et al. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol. 2009;34:103–112. doi: 10.1111/j.1749-4486.2009.01889.x. [DOI] [PubMed] [Google Scholar]
- 44.Laasch H.U., Wilbraham L., Bullen K., et al. Gastrostomy insertion: comparing the options--PEG, RIG or PIG? Clin Radiol. 2003;58:398–405. doi: 10.1016/s0009-9260(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 45.MacLean A.A., Alvarez N.R., Davies J.D., Lopez P.P., Pizano L.R. Complications of percutaneous endoscopic and fluoroscopic gastrostomy tube insertion procedures in 378 patients. Gastroenterol Nurs. 2007;30:337–341. doi: 10.1097/01.SGA.0000296252.70834.19. [DOI] [PubMed] [Google Scholar]
- 46.Park S.K., Kim J.Y., Koh S.J., et al. Complications of percutaneous endoscopic and radiologic gastrostomy tube insertion: a KASID (Korean Association for the Study of Intestinal Diseases) study. Surg Endosc. 2019;33:750–756. doi: 10.1007/s00464-018-6339-1. [DOI] [PubMed] [Google Scholar]
- 47.Righetti J., Morris S., Fotoohi M., Selva D.L., Zehr T., Kozarek R. Is percutaneous radiologic gastrostomy safer than percutaneous endoscopic gastrostomy? Am J Interv Radiol. 2021;5:16. [Google Scholar]
- 48.Singh R.R., Nah S.A., Roebuck D.J., et al. Double-blind randomized clinical trial of percutaneous endoscopic gastrostomy versus radiologically inserted gastrostomy in children. Br J Surg. 2017;104:1620–1627. doi: 10.1002/bjs.10687. [DOI] [PubMed] [Google Scholar]
- 49.Westaby D., Young A., O’Toole P., Smith G., Sanders D.S. The provision of a percutaneously placed enteral tube feeding service. Gut. 2010;59:1592–1605. doi: 10.1136/gut.2009.204982. [DOI] [PubMed] [Google Scholar]
- 50.Thornton F.J., Fotheringham T., Alexander M., Hardiman O., McGrath F.P., Lee M.J. Amyotrophic lateral sclerosis: enteral nutrition provision--endoscopic or radiologic gastrostomy? Radiology. 2002;224:713–717. doi: 10.1148/radiol.2243010909. [DOI] [PubMed] [Google Scholar]
- 51.Barkmeier J.M., Trerotola S.O., Wiebke E.A., et al. Percutaneous radiologic, surgical endoscopic, and percutaneous endoscopic gastrostomy/gastrojejunostomy: comparative study and cost analysis. Cardiovasc Intervent Radiol. 1998;21:324–328. doi: 10.1007/s002709900269. [DOI] [PubMed] [Google Scholar]
- 52.ASGE Standards of Practice Committee. Khashab M.A., Chithadi K.V., Acosta R.D., et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81–89. doi: 10.1016/j.gie.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Itkin M., DeLegge M.H., Fang J.C., et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE) J Vasc Interv Radiol. 2011;22:1089–1106. doi: 10.1016/j.jvir.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Chehab M.A., Thakor A.S., Tulin-Silver S., et al. Adult and pediatric antibiotic prophylaxis during vascular and IR procedures: a Society of Interventional Radiology practice parameter update endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J Vasc Interv Radiol. 2018;29:1483–1501.e2. doi: 10.1016/j.jvir.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Weimar C., Cotton D., Sha N., et al. Discontinuation of antiplatelet study medication and risk of recurrent stroke and cardiovascular events: results from the PRoFESS study. Cerebrovasc Dis. 2013;35:538–543. doi: 10.1159/000351144. [DOI] [PubMed] [Google Scholar]
- 56.Thim T., Johansen M.B., Chisholm G.E., et al. Clopidogrel discontinuation within the first year after coronary drug-eluting stent implantation: an observational study. BMC Cardiovasc Disord. 2014;14:100. doi: 10.1186/1471-2261-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jneid H., Anderson J.L., Wright R.S., et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:645–681. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Lee C., Im J.P., Kim J.W., et al. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy: a multicenter, retrospective study. Surg Endosc. 2013;27:3806–3815. doi: 10.1007/s00464-013-2979-3. [DOI] [PubMed] [Google Scholar]
- 59.Singh D., Laya A.S., Vaidya O.U., Ahmed S.A., Bonham A.J., Clarkston W.K. Risk of bleeding after percutaneous endoscopic gastrostomy (PEG) Dig Dis Sci. 2012;57:973–980. doi: 10.1007/s10620-011-1965-7. [DOI] [PubMed] [Google Scholar]
- 60.Thosani N., Rashtak S., Kannadath B.S., et al. Bleeding risk and mortality associated with Uninterrupted antithrombotic therapy during percutaneous endoscopic gastrostomy tube placement. Am J Gastroenterol. 2021;116:1868–1875. doi: 10.14309/ajg.0000000000001348. [DOI] [PubMed] [Google Scholar]
- 61.Richter-Schrag H.J., Richter S., Ruthmann O., Olschewski M., Hopt U.T., Fischer A. Risk factors and complications following percutaneous endoscopic gastrostomy: a case series of 1041 patients. Can J Gastroenterol. 2011;25:201–206. doi: 10.1155/2011/609601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel H., Gaduputi V., Sakam S., Kumar K., Chime C., Balar B. Serotonin reuptake inhibitors and post-gastrostomy bleeding: reevaluating the link. Ther Clin Risk Manag. 2015;11:1283–1289. doi: 10.2147/TCRM.S87044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stein J., Schulte-Bockholt A., Sabin M., Keymling M. A randomized prospective trial of immediate vs. next-day feeding after percutaneous endoscopic gastrostomy in intensive care patients. Intensive Care Med. 2002;28:1656–1660. doi: 10.1007/s00134-002-1473-5. [DOI] [PubMed] [Google Scholar]
- 64.McCarter T.L., Condon S.C., Aguilar R.C., Gibson D.J., Chen Y.K. Randomized prospective trial of early versus delayed feeding after percutaneous endoscopic gastrostomy placement. Am J Gastroenterol. 1998;93:419–421. doi: 10.1111/j.1572-0241.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 65.Choudhry U., Barde C.J., Markert R., Gopalswamy N. Percutaneous endoscopic gastrostomy: a randomized prospective comparison of early and delayed feeding. Gastrointest Endosc. 1996;44:164–167. doi: 10.1016/s0016-5107(96)70134-7. [DOI] [PubMed] [Google Scholar]
- 66.Brown D.N., Miedema B.W., King P.D., Marshall J.B. Safety of early feeding after percutaneous endoscopic gastrostomy. J Clin Gastroenterol. 1995;21:330–331. doi: 10.1097/00004836-199512000-00020. [DOI] [PubMed] [Google Scholar]
- 67.Cobell W.J., Hinds A.M., Nayani R., et al. Feeding after percutaneous endoscopic gastrostomy: experience of early versus delayed feeding. South Med J. 2014;107:308–311. doi: 10.1097/SMJ.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 68.Shahmanyan D., Lawrence J.C., Lollar D.I., et al. Early feeding after percutaneous endoscopic gastrostomy tube placement in patients who require trauma and surgical intensive care: a retrospective cohort study. JPEN J Parenter Enteral Nutr. 2022;46:1160–1166. doi: 10.1002/jpen.2303. [DOI] [PubMed] [Google Scholar]
- 69.Lamb L.C., Jayaraman V., Montgomery S.C., Umer A., Shapiro D.S., Feeney J.M. Immediate tube feeding after percutaneous endoscopic gastrostomy: early return to goal tube feeds without added complications. Conn Med. 2017;81:75–79. [PubMed] [Google Scholar]
- 70.Vyawahare M.A., Shirodkar M., Gharat A., Patil P., Mehta S., Mohandas K.M. A comparative observational study of early versus delayed feeding after percutaneous endoscopic gastrostomy. Indian J Gastroenterol. 2013;32:366–368. doi: 10.1007/s12664-013-0348-8. [DOI] [PubMed] [Google Scholar]
- 71.Wesley S., Samuels N., Williams K., et al. Early versus late tube feeding initiation after PEG tube placement: does time to feeding matter? Injury. 2021;52:1198–1203. doi: 10.1016/j.injury.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Szary N.M., Arif M., Matteson M.L., Choudhary A., Puli S.R., Bechtold M.L. Enteral feeding within three hours after percutaneous endoscopic gastrostomy placement: a meta-analysis. J Clin Gastroenterol. 2011;45:e34–e38. doi: 10.1097/MCG.0b013e3181eeb732. [DOI] [PubMed] [Google Scholar]
- 73.Bechtold M.L., Matteson M.L., Choudhary A., Puli S.R., Jiang P.P., Roy P.K. Early versus delayed feeding after placement of a percutaneous endoscopic gastrostomy: a meta-analysis. Am J Gastroenterol. 2008;103:2919–2924. doi: 10.1111/j.1572-0241.2008.02108.x. [DOI] [PubMed] [Google Scholar]
- 74.Schulte-Bockholt A., Sabin M., Rosenstock U., et al. Immediate versus next day PEG feeding: a randomized prospective study in ICU/Intermediate care patients. Gastroenterology. 1998;28:1656–1660. [Google Scholar]
- 75.Prosser B. Common issues in PEG tubes--what every fellow should know. Gastrointest Endosc. 2006;64:970–972. doi: 10.1016/j.gie.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 76.Wang Z., Ding W., Fang Q., Zhang L., Liu X., Tang Z. Effects of not monitoring gastric residual volume in intensive care patients: a meta-analysis. Int J Nurs Stud. 2019;91:86–93. doi: 10.1016/j.ijnurstu.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Yasuda H., Kondo N., Yamamoto R., et al. Monitoring of gastric residual volume during enteral nutrition. Cochrane Database Syst Rev. 2021;9 doi: 10.1002/14651858.CD013335.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reignier J., Mercier E., Le Gouge A., et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309:249–256. doi: 10.1001/jama.2012.196377. [DOI] [PubMed] [Google Scholar]
- 79.Montejo J.C., Miñambres E., Bordejé L., et al. Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med. 2010;36:1386–1393. doi: 10.1007/s00134-010-1856-y. [DOI] [PubMed] [Google Scholar]
- 80.Gleeson F.C., Lee J.H., Dewitt J.M. Tumor seeding associated with selected gastrointestinal endoscopic interventions. Clin Gastroenterol Hepatol. 2018;16:1385–1388. doi: 10.1016/j.cgh.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 81.Cappell M.S. Risk factors and risk reduction of malignant seeding of the percutaneous endoscopic gastrostomy track from pharyngoesophageal malignancy: a review of all 44 known reported cases. Am J Gastroenterol. 2007;102:1307–1311. doi: 10.1111/j.1572-0241.2007.01227.x. [DOI] [PubMed] [Google Scholar]
- 82.Siu J., Fuller K., Nadler A., et al. Metastasis to gastrostomy sites from upper aerodigestive tract malignancies: a systematic review and meta-analysis. Gastrointest Endosc. 2020;91:1005–1014.e17. doi: 10.1016/j.gie.2019.12.045. [DOI] [PubMed] [Google Scholar]
- 83.ASGE Training Committee 2013-2014. Enestvedt B.K., Jorgensen J., Sedlack R.E., et al. Endoscopic approaches to enteral feeding and nutrition core curriculum. Gastrointest Endosc. 2014;80:34–41. doi: 10.1016/j.gie.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 84.Akkersdijk W.L., van Bergeijk J.D., van Egmond T., et al. Percutaneous endoscopic gastrostomy (PEG): comparison of push and pull methods and evaluation of antibiotic prophylaxis. Endoscopy. 1995;27:313–316. doi: 10.1055/s-2007-1005699. [DOI] [PubMed] [Google Scholar]
- 85.Retes F.A., Kawaguti F.S., de Lima M.S., et al. Comparison of the pull and percutaneous endoscopic gastrostomy techniques in patients with head and neck cancer. United European Gastroenterol J. 2017;5:365–373. doi: 10.1177/2050640616662160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ehrsson Y.T., Langius-Eklöf A., Bark T., Laurell G. Percutaneous endoscopic gastrostomy (PEG) - a long-term follow-up study in head and neck cancer patients. Clin Otolaryngol Allied Sci. 2004;29:740–746. doi: 10.1111/j.1365-2273.2004.00897.x. [DOI] [PubMed] [Google Scholar]
- 87.Hiki N., Maetani I., Suzuki Y., et al. Reduced risk of peristomal infection of direct percutaneous endoscopic gastrostomy in cancer patients: comparison with the pull percutaneous endoscopic gastrostomy procedure. J Am Coll Surg. 2008;207:737–744. doi: 10.1016/j.jamcollsurg.2008.06.335. [DOI] [PubMed] [Google Scholar]
- 88.Köhler G., Kalcher V., Koch O.O., Luketina R.R., Emmanuel K., Spaun G. Comparison of 231 patients receiving either “pull-through” or “push” percutaneous endoscopic gastrostomy. Surg Endosc. 2015;29:170–175. doi: 10.1007/s00464-014-3673-9. [DOI] [PubMed] [Google Scholar]
- 89.Teich N., Selig L., Liese S., et al. Usage characteristics and adverse event rates of the direct puncture and pull techniques for percutaneous endoscopic gastrostomy in patients with malignant tumors of the upper aerodigestive tract. Endosc Int Open. 2018;6:E29–E35. doi: 10.1055/s-0043-121879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walton G.M. Complications of percutaneous gastrostomy in patients with head and neck cancer--an analysis of 42 consecutive patients. Ann R Coll Surg Engl. 1999;81:272–276. [PMC free article] [PubMed] [Google Scholar]
- 91.Yang Y., Schneider J., Düber C., Pitton M.B. Comparison of fluoroscopy-guided Pull-type percutaneous radiological gastrostomy (Pull-type-PRG) with conventional percutaneous radiological gastrostomy (Push-type-PRG): clinical results in 253 patients. Eur Radiol. 2011;21:2354–2361. doi: 10.1007/s00330-011-2194-3. [DOI] [PubMed] [Google Scholar]
- 92.Strijbos D., Keszthelyi D., Gilissen L.P.L., et al. Percutaneous endoscopic versus radiologic gastrostomy for enteral feeding: a retrospective analysis on outcomes and complications. Endosc Int Open. 2019;07:E1487–E1495. doi: 10.1055/a-0953-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Otsuka I. Cutaneous metastasis after Surgery, injury, Lymphadenopathy, and peritonitis: possible Mechanisms. Int J Mol Sci. 2019;20:3286. doi: 10.3390/ijms20133286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Francesca V., Giuseppina D.C., Federica G., et al. Risk of tumor implantation in percutaneous endoscopic gastrostomy in the upper aerodigestive tumors. Acta Biomed. 2018;89:117–121. doi: 10.23750/abm.v89i8-S.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rowell N.P. Tumor implantation following percutaneous endoscopic gastrostomy insertion for head and neck and oesophageal cancer: review of the literature. Head Neck. 2019;41:2007–2015. doi: 10.1002/hed.25652. [DOI] [PubMed] [Google Scholar]
- 96.ASGE Standards of Practice Committee. Storm A.C., Fishman D.S., Buxbaum J.L., et al. American Society for Gastrointestinal Endoscopy guideline on informed consent for GI endoscopic procedures. Gastrointest Endosc. 2022;95:207–215.e2. doi: 10.1016/j.gie.2021.10.022. [DOI] [PubMed] [Google Scholar]