Abstract
Objective
Vaginal bleeding is a relatively common problem during pregnancy, with up to 25% of women experiencing some level of bleeding or spotting during pregnancy. Although not always a concern, pregnancy-related vaginal bleeding may detrimentally impact maternal and fetal health, and can arise at any stage during the pregnancy, usually linked with preterm labor, miscarriage, placental abnormalities, or implantation bleeding. As vaginal bleeding may compromise newborn health outcomes, for example, leading to low birth weight and neonatal intensive care unit (NICU) hospitalizations, it is imperative to understand the etiology of the bleed and effectively manage the patient’s condition. Thus, the aim of this study was to evaluate the prognostic value of vaginal bleeding as an indicator for at-risk pregnancies, aiming to elucidate its association with perinatal outcomes and pregnancy complications.
Methods
Consecutive pregnant female patients, aged 16–47 years, were included in this retrospective cohort study, and categorized into those with or without pregnancy-associated bleeding. Between-group differences and correlations in pregnancy complications and perinatal outcomes were statistically analyzed.
Results
Among a total of 441 patients, the incidence of vaginal bleeding was 74.4%, predominantly occurring in the first trimester (67.1%). No significant association was found between bleeding and maternal age, parity, abortion history, or total number of pregnancies. Educational level varied, with 64.9% of moderate education level experiencing bleeding. Pre-pregnancy comorbidities, such as diabetes, hypertension, and hypothyroidism, were not significantly correlated with bleeding. However, a history of infertility and progesterone treatment during pregnancy was significantly associated with bleeding episodes. An increased risk for NICU admissions and conditions such as respiratory distress in neonates was found, alongside a significant association with post-delivery bleeding and premature labor in the later trimesters.
Conclusions
Vaginal bleeding during pregnancy was not found to be significantly associated with maternal age, parity, abortion history, or total pregnancies. Nonetheless, the findings suggest a significant relationship between bleeding episodes and a history of infertility or progesterone treatment during pregnancy. Additionally, pre-pregnancy comorbidities, including diabetes, hypertension, and hypothyroidism were not associated with pregnancy bleeding. However, the latter was associated with increased risk of post-delivery bleeding, premature labor, and neonatal complications, such as respiratory distress and NICU admissions.
Keywords: Vaginal bleeding in pregnancy, Maternal outcomes, Perinatal outcomes, Pregnancy complications, Early intervention, Diagnostic approaches in pregnancy, Treatment options for pregnancy bleeding, Pregnancy risk indicators
Introduction
Vaginal bleeding during pregnancy represents a clinical spectrum ranging from mild spotting to severe hemorrhage, with significant implications for pregnancy outcomes. Its prevalence varies throughout the gestational phases, each characterized by specific etiologies and associated risks. Effective management and early intervention in cases of vaginal bleeding are paramount in safeguarding both maternal and fetal health1,2
This bleeding can arise from a multitude of factors, differentiated by the gestational period. Early in pregnancy, the integrity of local and systemic hemostatic mechanisms within the uterus is essential for maintaining pregnancy. Dysregulation of these mechanisms, including the aberrant expression of tissue factors by cytotrophoblasts or the local generation of thrombin and soluble fms-like tyrosine kinase-1, can precipitate bleeding episodes.1–4
While implantation bleeding is considered a physiological event, occurring in approximately 25% of pregnancies as the zygote attaches to the uterine lining, other causes, such as ectopic pregnancies, miscarriages, and molar pregnancies, are also associated with pathological risks.4–9 Moreover, bleeding may emanate from non-pregnancy related pathologies, including cervical or vaginal disorders, such as erosions, polyps, fibroids, cancer, or trauma.1–5
According to the current body of literature, about 15–25% of pregnancies are reported to have one form of vaginal bleeding in the first trimester and the majority of these cases were demonstrated to have an increased risk of unfavorable pregnancy outcomes, most notably: miscarriage, premature delivery, low birth weight and even stillbirth.10–14 This type of bleeding usually stems from subchorionic hematoma and ectopic pregnancy.11,12 In turn, these conditions may have a detrimental impact on the health of the mother and the fetus. Similarly, studies on the impact of decidual hemorrhage in relation to premature delivery found that vaginal bleeding may signify chorioamnionitis, which is detrimental to fetal development.15,16
During the second trimester, vaginal bleeding is usually associated with placental abruption, placenta previa, or an incompetent cervix, which can lead to preterm birth. 17 At a later stage, during the third trimester of pregnancy, vaginal bleeding may be observed due to placental abruption, placenta previa, or vasa previa, in addition to cervical changes or uterine fibroids. 17
The primary aim of the present study was to systematically review instances of vaginal bleeding during pregnancy and their impact on gestational progression and outcomes. Secondary objectives included the evaluation of associated complications and identification of potential risk factors, with the overall aim of enhancing early diagnosis and follow-up care for pregnant women at elevated risk of adverse outcomes, emphasizing the need for tailored clinical approaches.
Patients and methods
Study design and population
In this retrospective cohort study, the medical records of pregnant women who visited a private clinic in Beirut between 2019 and 2023 were reviewed. Sample size was calculated using Slovin’s formula to ensure a 95% confidence level and a 5% margin of error. Patients were consecutively selected based on their medical records meeting inclusion and exclusion criteria, and non-bleeding controls were selected from the same clinic population.
The selection approach aimed to ensure that the participants had similar healthcare access and socioeconomic backgrounds, to help minimize confounding variables and enhance the comparability between bleeding and non-bleeding groups. All participants provided verbal informed consent to the general use of their data for research purposes (including the present retrospective study), as part of routine clinical practice on first presentation to the clinic. The study was conducted in accordance with the ethical standards of the Rafic Hariri University Hospital’s Institutional Review Board Committee, which granted approval on 2 February 2024 (registration overseen by GY). Due to the prevailing circumstances in Lebanon, a formal approval number was not issued, however, all necessary ethical guidelines and standards were strictly adhered to, ensuring the integrity and ethical compliance of the research. In addition, the study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, 18 and with the 1975 Declaration of Helsinki, as revised in 2013. All patient details were de-identified to ensure anonymity.
Inclusion and exclusion criteria
Inclusion criteria comprised pregnant women aged 16–47 years, with or without vaginal bleeding during pregnancy, and with documented pregnancy outcomes. Patients with coagulopathies, incomplete records, multiple gestations, or ectopic pregnancies were excluded from the study.
Data collection
Demographic details, medical histories, symptoms, and outcomes were extracted from patient records. The primary focus was on bleeding during pregnancy, while secondary outcomes included delivery type, neonatal intensive care unit (NICU) admissions, and post-delivery complications.
Statistical analyses
Categorical data are presented as n (%) prevalence or incidence, and continuous data are presented as mean ± SD. Data were analyzed using IBM SPSS software, version 27 (IBM Corp., Armonk, NY, USA). Descriptive statistics were applied for demographic and incidence analysis. Comparative analyses between groups were performed with χ2-test, Fisher’s exact test, or Mann–Whitney U-test. A P-value <0.05 was considered to be statistically significant.
Results
A total of 441 pregnant women were included in the study, and preliminary findings revealed that 328 out of the 441 patients (74.4%) experienced bleeding, predominantly in the first trimester. Variability in bleeding duration was shown among these patients, with intermittent bleeding being the most reported (139 patients [42.4%]; Figure 1).
Figure 1.
Characteristics of bleeding during pregnancy in 328 patients.
The incidence of pregnancy-related vaginal bleeding was most pronounced during the first trimester, with 67.1% (220/328) of cases occurring during this period. This was followed by the second trimester at 20.1% (66/328) and the third trimester at 12.8% (42/328).
Demographic characteristics
Demographic factors in patients who did or did not experience pregnancy bleeding are summarized in Table 1. The overall study cohort predominantly resided in city areas (91.8%), with the majority being Lebanese (66.0%), followed by Syrian (13.4%), Palestinian (7.0%), and other nationalities (13.6%). No statistically significant differences in bleeding incidence were observed based on location of residence or nationality (P = 0.626 and 0.593, respectively). A notable finding was the significant association between educational level and the incidence of bleeding (P = 0.003), with a higher prevalence among women with moderate education (64.9%) versus those with low (22.3%) or high education levels (12.8%).
Table 1.
Demographic characteristics in patients with or without bleeding during pregnancy.
| Characteristic | Pregnancy bleeding |
Total (n = 441) | Statistical significance a | |
|---|---|---|---|---|
| No (n = 113) | Yes (n = 328) | |||
| Residence location | P = 0.626 | |||
| City | 105 (92.9%) | 300 (91.5%) | 405 (91.8%) | |
| Urban | 8 (7.1%) | 28 (8.5%) | 36 (8.2%) | |
| Education level | P = 0.003 | |||
| Low | 9 (8.0%) | 73 (22.3%) | 82 (18.6%) | |
| Moderate | 89 (78.8%) | 213 (64.9%) | 302 (68.5%) | |
| High | 15 (13.3%) | 42 (12.8%) | 57 (12.9%) | |
| Nationality | P = 0.593 | |||
| Lebanese | 79 (69.9%) | 212 (64.6%) | 291 (66.0%) | |
| Syrian | 12 (10.6%) | 47 (14.3%) | 59 (13.4%) | |
| Palestinian | 9 (8.0%) | 22 (6.7%) | 31 (7.0%) | |
| Other | 13 (11.5%) | 47 (14.3%) | 60 (13.6%) | |
Data presented as n (%) prevalence.
Statistically significant difference at P < 0.05 (χ2-test or Mann–Whitney U-test).
Medical/obstetric history and obstetric and pre-pregnancy comorbidities
Comparative data regarding medical/obstetric histories and pre-pregnancy comorbidities in patients with or without pregnancy bleeding are summarized in Table 2.
Table 2.
Medical history and pre-pregnancy comorbidities in patients with or without bleeding during pregnancy.
| Characteristic | Pregnancy bleeding |
Total (N = 441) | Statistical significance | ||
|---|---|---|---|---|---|
| No (n = 113) | Yes (n = 328) | ||||
| Body mass index, kg/m2 | 22–32 | 1 (0.9%) | 3 (0.9%) | 4 (0.9%) | P = 0.893 a |
| 32–35 | 44 (38.9%) | 136 (41.5%) | 180 (40.8%) | ||
| >35 | 68 (60.2%) | 189 (57.6%) | 257 (58.3%) | ||
| Age, years | Mean ± SD | 28.45 ± 5.03 | 28.47 ± 4.83 | 28.46 ± 4.87 | P = 0.977 a |
| Min–Max | 16–47 | 18–45 | 16–47 | ||
| Parity | Mean ± SD | 2.20 ± 1.40 | 2.03 ± 1.29 | 2.07 ± 1.32 | P = 0.222 a |
| Min–Max | 0–7 | 0–7 | 0–7 | ||
| History of abortion | Mean ± SD | 0.74 ± 1.18 | 0.66 ± 0.91 | 0.68 ± 0.99 | P = 0.448 a |
| Min–Max | 0–4 | 0–6 | 0–6 | ||
| Gravida | Mean ± SD | 2.95 ± 2.25 | 2.69 ± 1.87 | 2.76 ± 1.97 | P = 0.232 a |
| Min–Max | 1–11 | 1–13 | 1–13 | ||
| History of infertility | No | 89 (78.8%) | 206 (63.0%) | 295 (67.0%) | P = 0.002 a |
| Yes | 24 (21.2%) | 121 (37.0%) | 145 (33.0%) | ||
| Previous normal vaginal delivery | No | 45 (39.8%) | 162 (49.4%) | 207 (46.9%) | P = 0.079 a |
| Yes | 68 (60.2%) | 166 (50.6%) | 234 (53.1%) | ||
| Previous cesarean section | No | 68 (60.2%) | 171 (52.1%) | 239 (54.2%) | P = 0.139 a |
| Yes | 45 (39.8%) | 157 (47.9%) | 202 (45.8%) | ||
| Previous dilation and curettage | No | 106 (93.8%) | 307 (93.6%) | 413 (93.7%) | P = 0.938 a |
| Yes | 7 (6.2%) | 21 (6.4%) | 28 (6.3%) | ||
| Treatment with progesterone | No | 89 (78.8%) | 16 (4.9%) | 105 (23.8%) | P < 0.001 a |
| Yes | 24 (21.2%) | 312 (95.1%) | 336 (76.2%) | ||
| Urinary tract infection | No | 104 (92.0%) | 286 (87.2%) | 390 (88.4%) | P = 0.165 a |
| Yes | 9 (8.0%) | 42 (12.8%) | 51 (11.6%) | ||
| Diabetes | No | 112 (99.1%) | 321 (97.9%) | 433 (98.2%) | P = 0.686b |
| Yes | 1 (0.9%) | 7 (2.1%) | 8 (1.8%) | ||
| Anemia | No | 68 (60.2%) | 181 (55.2%) | 249 (56.5%) | P = 0.380 a |
| Yes | 45 (39.8%) | 147 (44.8%) | 192 (43.5%) | ||
| Hypothyroid | No | 113 (100.0%) | 325 (99.1%) | 438 (99.3%) | P = 0.573b |
| Yes | 0 (0.0%) | 3 (0.9%) | 3 (0.7%) | ||
| Hypertension | No | 113 (100.0%) | 322 (98.2%) | 435 (98.6%) | P = 0.345b |
| Yes | 0 (0.0%) | 6 (1.8%) | 6 (1.4%) | ||
Data presented as n (%) prevalence, mean ± SD or min–max.
Statistically significant difference at P < 0.05.
χ2-test or bFisher’s exact test.
Body Mass Index (BMI) distribution among participants was notably varied, with significant representation across different BMI categories, yet no statistically significant correlation to bleeding incidence was observed based on BMI (P = 0.893). Age, parity, abortion history, and total number of pregnancies did not significantly correlate with bleeding incidence, suggesting these factors do not independently predict bleeding during pregnancy.
A significant correlation was found between history of infertility and the incidence of vaginal bleeding during pregnancy (P = 0.002), with 37.0% of patients who experienced bleeding reporting a history of infertility, compared with 21.2% who did not (Table 2). However, no statistically significant association was revealed between the incidence of vaginal bleeding during pregnancy and history of normal vaginal deliveries or Cesarean sections (P = 0.079 and 0.139, respectively). The study also found no significant impact of a history of dilation and curettage on the occurrence of bleeding during pregnancy (P = 0.938). A notable finding was the strong association between progesterone treatment and the incidence of bleeding, where progesterone treatment, administered during pregnancy, was found to be significantly associated with a higher incidence of vaginal bleeding (95.1% of patients who experienced bleeding during pregnancy had undergone progesterone treatment versus 4.9% who did not report bleeding; P < 0.001).
No statistically significant associations were found between pre-pregnancy comorbidities, including urinary tract infections, diabetes, anemia, hypothyroidism, or hypertension, and the incidence of bleeding during pregnancy (P = 0.165, 0.686, 0.380, 0.573, and 0.345, respectively). Of note, the majority of participants, regardless of bleeding status, did not present with these conditions (Table 2).
Associated symptoms
Data on pregnancy bleeding, bleeding-associated symptoms and pregnancy complications are summarized in Table 3.
Table 3.
Bleeding associated symptoms and complications in patients with or without bleeding during pregnancy.
| Characteristic | Pregnancy bleeding |
Total (N = 441) | Statistical significance | ||
|---|---|---|---|---|---|
| No (n = 113) | Yes (n = 328) | ||||
| Pain (associated with bleeding) | No | 73 (64.6%) | 86 (26.2%) | 159 (36.1%) | P < 0.001 a |
| Yes | 40 (35.4%) | 242 (73.8%) | 282 (63.9%) | ||
| Spotting red | No | 113 (100%) | 42 (12.8%) | 150 (34.0%) | P < 0.001 b |
| Yes | 0 (0%) | 286 (87.2%) | 291 (66.0%) | ||
| Spotting brownish | No | 113 (100%) | 39 (11.9%) | 146 (33.1%) | P < 0.001 a |
| Yes | 0 (0%) | 289 (88.1%) | 295 (66.9%) | ||
| Clots | No | 113 (100%) | 272 (82.9%) | 383 (86.8%) | P < 0.001 b |
| Yes | 0 (0%) | 56 (17.1%) | 58 (13.2%) | ||
| Hematoma at ultrasound | No | 109 (96.5%) | 281 (85.7%) | 390 (88.4%) | P = 0.002 b |
| Yes | 4 (3.5%) | 47 (14.3%) | 51 (11.6%) | ||
| Hematoma at ultrasound | No | 109 (96.5%) | 281 (85.7%) | 390 (88.4%) | P = 0.019 a |
| <1 cm | 1 (0.9%) | 5 (1.5%) | 6 (1.4%) | ||
| 1 cm | 2 (1.8%) | 33 (10.1%) | 35 (7.9%) | ||
| >1 cm | 1 (0.9%) | 9 (2.7%) | 10 (2.3%) | ||
| Gestational diabetes | No | 111 (98.2%) | 315 (96.0%) | 426 (96.6%) | P = 0.374b |
| Yes | 2 (1.8%) | 13 (4.0%) | 15 (3.4%) | ||
| Pre-eclampsia | No | 113 (100%) | 319 (97.3%) | 432 (98.0%) | P = 0.120b |
| Yes | 0 (0.0%) | 9 (2.7%) | 9 (2.0%) | ||
Data presented as n (%) prevalence.
Statistically significant difference at P < 0.05.
χ2-test or bFisher’s exact test.
Statistically significant associations were found between bleeding during pregnancy and the symptoms of pain, spotting, and clot presence (all P < 0.001). Pain was reported significantly more among women with bleeding, indicating its strong association with this condition. Ultrasound revealed hematomas more frequently in women with bleeding, offering critical diagnostic insight. Of those who bled, 14.3% had a hematoma, while only 3.5% of women without bleeding had this finding (P = 0.002). When categorized by size, hematomas of 1 cm were present in 10.1% of women who bled, >1 cm in 2.7%, and <1 cm in 1.5% versus 1.8%, 0.9% and 0.9%, respectively, in those who did not bleed during pregnancy (P = 0.019). No statistically significant association was found between pregnancy bleeding and pre-eclampsia or diabetes.
NICU admissions and their association with bleeding during pregnancy
Analysis of data related to NICU admissions in connection with bleeding during pregnancy is summarized in Table 4. Specifically, a significantly higher incidence of NICU admissions, and a significantly higher incidence of respiratory distress leading to these admissions, was shown in neonates born to mothers who experienced pregnancy bleeding versus those who did not (P = 0.002 and 0.006, respectively).
Table 4.
Association between bleeding during pregnancy and admission to the neonatal intensive care unit (NICU).
| Characteristic | Pregnancy bleeding |
Total (N = 441) | Statistical significance | ||
|---|---|---|---|---|---|
| No (n = 113) | Yes (n = 328) | ||||
| NICU admission | No | 104 (92.0%) | 259 (79.0%) | 363 (82.3%) | P = 0.002 a |
| Yes | 9 (8.0%) | 69 (21.0%) | 78 (17.7%) | ||
| Cause of NICU admission: bilirubin | No | 109 (96.5%) | 309 (94.2%) | 418 (94.8%) | P = 0.465b |
| Yes | 2 (1.8%) | 21 (6.4%) | 23 (5.2%) | ||
| Cause of NICU admission: cardiac | No | 113 (100.0%) | 325 (99.1%) | 438 (99.3%) | P = 0.573b |
| Yes | 0 (0.0%) | 3 (0.9%) | 3 (0.7%) | ||
| Cause of NICU admission: respiratory distress | No | 111 (98.2%) | 290 (90.9%) | 401 (90.9%) | P = 0.006 b |
| Yes | 2 (1.8%) | 38 (11.6%) | 40 (9.1%) | ||
| Cause of NICU admission: hypoglycemia | No | 112 (99.1%) | 327 (99.7%) | 439 (99.5%) | P = 0.447b |
| Yes | 1 (0.9%) | 1 (0.3%) | 2 (0.5%) | ||
Data presented as n (%) prevalence.
Statistically significant difference at P < 0.05.
χ2-test or bFisher’s exact test.
Post-natal outcomes associated with bleeding during pregnancy
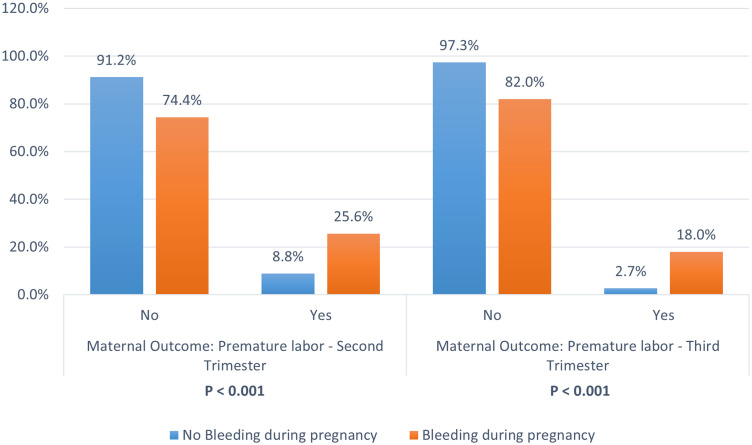
Data regarding bleeding during pregnancy and post-natal outcomes are summarized in Table 5, with a nuanced relationship found between bleeding during pregnancy and post-natal outcomes. Differences in rates of normal vaginal deliveries, Cesarean sections, and abortions between patients who did or did not experience bleeding during pregnancy did not reach statistical significance (P = 0.064). However, significant increases were noted in post-delivery bleeding and premature labor during the second and third trimesters. Among those who experienced pregnancy bleeding, 25.6% had premature labor in the second trimester, compared with 8.8% % of patients who did not bleed during pregnancy (P < 0.001). In the third trimester, patients who experienced pregnancy bleeding had significantly higher rates of pre-term labor, than those with no bleeding (18.0% versus 2.7%, P < 0.001; Figure 2).
Table 5.
Post-natal outcomes in patients with or without bleeding during pregnancy.
| Characteristic | Pregnancy bleeding |
Total (N = 441) | Statistical significance | ||
|---|---|---|---|---|---|
| No (n = 113) | Yes (n = 328) | ||||
| Obstetric outcome | Normal vaginal delivery | 66 (58.4%) | 159 (48.5%) | 225 (51.0%) | P = 0.064 a |
| Cesarean-section | 46 (40.7%) | 154 (47.0%) | 200 (45.4%) | ||
| Abortion | 1 (0.9%) | 15 (4.6%) | 16 (3.6%) | ||
| Postpartum hemorrhage | No | 106 (93.8%) | 278 (84.8%) | 384 (87.1%) | P = 0.013 a |
| Yes | 7 (6.2%) | 50 (15.2%) | 57 (12.9%) | ||
| Anemia post delivery | No | 109 (96.5%) | 318 (97.0%) | 427 (96.8%) | P = 0.761b |
| Yes | 4 (3.5%) | 10 (3.0%) | 14 (3.2%) | ||
| Dehiscence | No | 113 (100.0%) | 324 (98.8%) | 437 (99.1%) | P = 0.576b |
| Yes | 0 (0.0%) | 4 (1.2%) | 4 (0.9%) | ||
| Placenta low laying at ultrasound | No | 112 (99.1%) | 318 (21.0%) | 430 (97.5%) | P = 0.303b |
| Yes | 1 (0.2%) | 10 (2.3%) | 11 (2.5%) | ||
| Placenta previa at ultrasound | No | 113 (100.0%) | 326 (99.4%) | 439 (99.5%) | P = 1.000b |
| Yes | 0 (0.0%) | 2 (0.6%) | 2 (0.5%) | ||
| Maternal outcome: premature labor – second trimester | No | 103 (91.2%) | 244 (74.4%) | 347 (78.7%) | P < 0.001 a |
| Yes | 10 (8.8%) | 84 (25.6%) | 94 (21.3%) | ||
| Maternal outcome: premature labor – third trimester | No | 110 (97.3%) | 269 (82.0%) | 379 (85.9%) | P < 0.001 b |
| Yes | 3 (2.7%) | 59 (18.0%) | 62 (14.1%) | ||
Data presented as n (%) prevalence.
Statistically significant difference at P < 0.05.
χ2-test or bFisher’s exact test.
Figure 2.
Relationship between bleeding during pregnancy and premature labor in 441 patients (patients with pregnancy bleeding, n = 328; patients without pregnancy bleeding, n = 113).
Discussion
The findings of the present study, coupled with a comprehensive review of the literature, underscore the necessity of an integrated approach in managing bleeding during pregnancy. Such an approach should be finely tailored to address the distinct risks and outcomes observed across diverse patient profiles. The prevalence of bleeding during pregnancy identified in the present study was 74.4% of total participants, a figure that significantly exceeds previously reported rates. This discrepancy suggests potential selection bias or that the present study population represents a uniquely high-risk group. 19 Notably, the prevalence of pregnancy bleeding is most pronounced during the first trimester (67.1% of patients with bleeding in the present study), a period often associated with implantation and other benign causes of bleeding, as corroborated by existing literature.19–24 However, the present findings reveal a more complex scenario, where bleeding episodes extend into later pregnancy stages. Specifically, 20.1% of bleeding cases occurred during the second trimester and 12.8% occurred during the third trimester. These rates underscore the need to monitor and manage bleeding throughout pregnancy, as later-stage bleeding is often associated with serious complications, such as placental abruption, placenta previa, and preterm labor, thus supporting the evolving understanding of bleeding etiologies as pregnancy progresses.3–5,9
The timing of miscarriages, primarily before the 13th week, has been shown in recent studies and underscores the importance of early clinical intervention. 25 Although miscarriage timing is a relevant factor in the context of vaginal bleeding, this was not within the scope of the present study. Future research should aim to evaluate the association between vaginal bleeding and miscarriage rates, particularly in the first trimester, to better understand these relationships. The peak of bleeding episodes occurring between the 5th and 8th weeks further emphasizes this need. 26 The critical role of gestational age and the visualization of embryonic cardiac activity in improving pregnancy outcomes has been shown in previously published studies, with literature suggesting a significant decrease in miscarriage rates upon the detection of such activity.27,28
The present findings elucidate the relationship between bleeding during pregnancy and various adverse outcomes, which further reinforces some intricacies involved in obstetric care. As shown in previous studies, the visualization of fetal cardiac activity during bleeding episodes notably reduces the risk of miscarriage, 29 emphasizing the prognostic value of ultrasound evaluations. Additionally, women who experience bleeding but continue their pregnancy past viability are at an elevated risk of delivering low birth weight infants and facing increased perinatal morbidity and mortality.30,31
The impacts of light versus heavy bleeding in early pregnancy have been reported, 32 with associated consequential risks, such as fetal growth restriction and preterm premature rupture of membranes. 26 The stark contrast in premature labor rates between those with and without bleeding episodes in the present study highlights the significance of bleeding as a risk factor for adverse pregnancy outcomes, a finding echoed by multiple studies.19,33,34
Contrary to expectations, demographic factors, including educational attainment, have shown a nuanced association with bleeding episodes in the present and previous studies.35,36 This observation suggests that broader socio-economic considerations should inform prenatal care strategies. The findings of the present study revealed no significant associations between vaginal bleeding during pregnancy and factors such as maternal age, parity, abortion history, or total pregnancies. This aligns with existing literature, suggesting that these demographic factors do not consistently correlate with the incidence of vaginal bleeding in pregnancy. 37
Interestingly, a lower educational level correlates with a higher incidence of bleeding, hinting at the potential influence of health literacy, access to care, or adherence to prenatal guidelines on health outcomes.36,38 This correlation is supported by studies from China and Denmark that found an inverse relationship between socio-economic status and spontaneous miscarriages.38,39
The mean age of participants experiencing bleeding was 28.47 ± 4.83 years, with no statistically significant difference in age-related bleeding incidence. The link between a history of infertility and an increased incidence of bleeding in the present study underscores the potential complications associated with pregnancies conceived through assisted reproductive technologies.19,40–44
The association between progesterone treatment and bleeding, as observed in the present study, requires nuanced interpretation.24,40,45–47 This finding may reflect the symptomatic management use of progesterone rather than a direct causative relationship, aligning with the current understanding of its role in pregnancy. This is consistent with the work of Stephenson et al., who highlighted that women with a history of recurrent pregnancy loss often benefit from progesterone treatment, which may influence bleeding patterns. 48
No significant association was found between gestational diabetes and bleeding,32,46,49 nor between pre-eclampsia and bleeding,46,50–52 indicating a complex interplay between these conditions and bleeding during pregnancy. These insights highlight the multifaceted nature of bleeding during pregnancy, calling for a deeper investigation into these relationships. Symptoms such as pain, spotting, clots, and hematomas, emerged as significant indicators of bleeding risk, 49 emphasizing the importance of symptom-based surveillance in prenatal care. The association of bleeding with hematomas on ultrasound also underscores the importance of vigilant monitoring for these symptoms as indicators of potential bleeding complications, guiding both clinical management and patient care strategies.
The present study found no significant association between the prevalence of low-lying placenta or placenta previa and vaginal bleeding during pregnancy, consistent with the findings of previous studies,19,31 underscoring the diagnostic value of ultrasound in managing these cases. Moreover, the observed higher frequency of hematomas in women who bled, in the present and previous studies,5,31,46 indicates the relevance of ultrasound in clinical decision-making.
The relationship between bleeding during pregnancy and the mode of delivery, 47 as well as the significant correlation between antepartum bleeding and post-delivery complications, including NICU admissions,19,25,53 found in the present and previously published studies, highlights the profound impact of bleeding on neonatal outcomes. The increased incidence of respiratory distress as a cause of NICU admission further highlights pregnancy bleeding as a notable risk factor for respiratory conditions in neonates necessitating specialized care, and underscores the critical need for vigilant prenatal monitoring and intervention in such cases. Thus, comprehensive monitoring and management strategies to mitigate the risks to neonatal health outcomes associated with bleeding during pregnancy are urgently required.
Strengths and limitations
The present study is characterized by several strengths that enhance its credibility and the reliability of its findings. First and foremost, the participant pool comprised all patients who visited a private clinic over a duration of 4 years, specifically for consultations related to vaginal bleeding during pregnancy. This comprehensive approach enabled us to establish clear inclusion and exclusion criteria, ensuring a focused and relevant study cohort. Secondly, the study boasts an acceptable sample size, comprising 441 pregnant women. This number allows for a robust statistical analysis, enhancing the generalizability of the results. Thirdly, a thorough assessment was conducted for each patient, including a review of their previous medical and obstetric-gynecological history (such as abortions and uterine surgeries), current gestational data (including screening and analytical values, as well as a history of obstetric complications), and information regarding the delivery and the newborn.
Despite these strengths, the study is not without limitations. A primary constraint is its retrospective nature, which means the analysis was confined to information available in digitized clinical histories, potentially affecting the depth and breadth of the analyzed data. Secondly, the scope of the study was limited to visits to a private clinic, meaning the follow-up of gestation and subsequent analysis of obstetric history were conducted exclusively within this facility. Consequently, data on patients who received prenatal care at different centers is lacking, though this issue was partially mitigated by retrieving some lost data through external monitoring and collecting medical histories from the centers of delivery. Moreover, the present study sample included patients aged 16–47 years, and it is worth noting that pregnancies at age extremities (<18 and >35 years) tend to be associated with an increased risk of miscarriage. In addition, the study included episodes of vaginal bleeding during pregnancy, regardless of gestational age. Nonetheless, it remains important to acknowledge that the outcomes for mothers and newborns may differ from one trimester to another. Indeed, third trimester bleeding is more linked with pathological etiologies, such as placenta previa or accreta spectrum, compared with first-trimester bleeding, which tends to be physiological. Furthermore, most of the studied patients were city residents (91.8%), which does not necessarily represent the whole Lebanese population
Given the high prevalence of first-trimester bleeding observed and its significant potential implications for pregnancy outcomes, there is a compelling case for extending this research. Conducting a prospective study would allow for the identification, establishment, and control of potential risk factors that may contribute to poor gestational outcomes. This future direction could provide invaluable insights into preventing adverse effects associated with early pregnancy bleeding, thereby enhancing maternal and neonatal health
Conclusion
This study affirms established obstetric research findings while probing crucial management and implication questions of pregnancy bleeding. The study calls for enhanced monitoring and specific interventions for symptomatic bleeding or risk factors, such as infertility and low educational attainment. The findings of the present study revealed no significant associations between vaginal bleeding during pregnancy and maternal age, parity, abortion history, or total pregnancies. Nonetheless, the findings suggest a significant relationship between bleeding episodes and a history of infertility or progesterone treatment during pregnancy. Pre-pregnancy comorbidities, including diabetes, hypertension, and hypothyroidism, were not associated with bleeding. However, pregnancy bleeding was found to be associated with increased incidence of post-delivery bleeding, premature labor, and neonatal complications, such as respiratory distress and NICU admissions. From this perspective, the findings underscore the critical need for vigilant monitoring and possibly earlier intervention for pregnancies complicated by bleeding, to mitigate adverse maternal and neonatal outcomes.
Acknowledgement
We would like to extend our sincere thanks to Mr. Hamza Nakib for his invaluable contributions in enhancing the tables and correcting the mistakes in the manuscript.
Author contributions: Maroun Matar: Conceptualization, supervision, data curation, reviewing the manuscript, visualization; validation.
Georges Yared: Conceptualization, supervision, data curation, reviewing the manuscript, visualization; validation.
Christopher Massaad: Conceptualization; investigation; visualization; writing, review and editing; supervision; formal analysis.
Kariman Ghazal: Conceptualization, methodology, data curation, analysis and writing, visualization; validation.
The Authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Kariman Ghazal https://orcid.org/0000-0002-3199-631X
Data availability statement
The data supporting the findings of this study are available upon request from the corresponding author.
References
- 1.Kamble PD, Bava AK, Shukla M, et al. First trimester bleeding and pregnancy outcome. Int J Reprod Contracept Obstet Gynecol 2017; 6: 1484–1487. [Google Scholar]
- 2.Tank PD. Early pregnancy complications. In: Bhide A, Arulkumaran S, Damania KR, et al. (eds) Arias’ Practical Guide to High Risk Pregnancy and Delivery: A South Asian Perspective. 4th ed. New Delhi: Elsevier, 2015, pp. 104–110. [Google Scholar]
- 3.Lockwood CJ, Krikun G, Rahman M, et al. The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin Thromb Hemost 2007; 33: 111–117. [DOI] [PubMed] [Google Scholar]
- 4.Norwitz ER. Defective implantation and placentation: Laying the blueprint for pregnancy complications. Reprod Biomed Online 2006; 13: 591–599. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists (ACOG). ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol 2019; 133: 1. [DOI] [PubMed] [Google Scholar]
- 6.Rana P, Kazmi I, Singh R, et al. Ectopic pregnancy: a review. Arch Gynecol Obstet 2013; 288: 747–757. [DOI] [PubMed] [Google Scholar]
- 7.Wang PS, Rodgers SK, Horrow MM. Ultrasound of the first trimester. Radiol Clin North Am 2019; 57: 617–633. [DOI] [PubMed] [Google Scholar]
- 8.Silver RM. Placental abruption. In: Berghella V. (ed) Maternal-Fetal Evidence Based Guidelines. 3rd ed. Boca Raton: CRC Press, 2018, pp. 219–233. [Google Scholar]
- 9.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response – A review. Placenta 2003; 24: S21–S27. [DOI] [PubMed] [Google Scholar]
- 10.Sayyad H, Jadoon H, Gul M, et al. Pregnancy outcome in first trimester bleed. Pakistan Journal of Medical & Health Sciences 2023; 17: 666. [Google Scholar]
- 11.Bhatu JJ, Prajapati DS. A study of feto-maternal outcome in bleeding per vaginum in first trimester of pregnancy. Int J Reprod Contracept Obstet Gynecol 2020; 9: 1191–1195. [Google Scholar]
- 12.Umeshchandra S, Patil LD. A study of maternal and foetal outcome in pregnant women with history of first trimester vaginal bleeding. Journal of Evolution of Medical and Dental Sciences 2022; 11: 728–737. [Google Scholar]
- 13.Saraswat L, Bhattacharya S, Maheshwari A, et al. Maternal and perinatal outcome in women with threatened miscarriage in the first trimester: a systematic review. BJOG 2010; 117: 245–257. [DOI] [PubMed] [Google Scholar]
- 14.Hackney D, Glantz J. Vaginal bleeding in early pregnancy and preterm birth: systemic review and analysis of heterogeneity. J Matern Fetal Neonatal Med 2011; 24: 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velez Edwards DR, Baird DD, Hasan R, et al. First-trimester bleeding characteristics associate with increased risk of preterm birth: data from a prospective pregnancy cohort. Hum Reprod 2012; 27: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yakıştıran B, Yüce T, Söylemez F. First trimester bleeding and pregnancy outcomes: case-control study. International Journal of Women’s Health and Reproduction Sciences 2016; 4: 4–7. [Google Scholar]
- 17.Lal AK, Watson WJ. Placenta previa and placental abruption. In: Winn HN, Chervenak FA, Romerao R. (eds) Clinical Maternal-Fetal Medicine. 3rd ed. London: CRC Press, 2012, pp. 9.1–9.8. [Google Scholar]
- 18.Von Elm E, Altman DG, Egger M; STROBE Initiative et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 19.Amirkhani Z, Akhlaghdoust M, Abedian M, et al. Maternal and perinatal outcomes in pregnant women with first trimester vaginal bleeding. J Family Reprod Health 2013; 7: 57–61. [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 25th ed. New York: McGraw-Hill Education, 2018. [Google Scholar]
- 21.Bala N, Kaur N, Shifali A, et al. A study of maternal outcome in first trimester bleeding. Int J Reprod Contracept Obstet Gynecol 2020; 9: 2104–2112. [Google Scholar]
- 22.Chhabra SA, Tickoo C, Kalra P. Perinatal outcome in cases with bleeding during first and early second trimester. World Academy of Science, Engineering and Technology, International Journal of Medical, Health, Biomedical, Bioengineering and Pharmaceutical Engineering 2014; 8: 61–66. [Google Scholar]
- 23.Tiwari S, Jose T, Saraswat M. Pregnancy outcomes in first trimester vaginal bleeding. Int J Sci Res 2017; 6: 398–400. [Google Scholar]
- 24.Kanmaz AG, Inan AH, Beyan E, et al. The effects of threatened abortions on pregnancy outcomes. Ginekol Pol 2019; 90: 195–200. [DOI] [PubMed] [Google Scholar]
- 25.Kortsmit K, Nguyen AT, Mandel MG, et al. Abortion Surveillance – United States, 2020. Morbidity and Mortality Weekly Report Surveillance Summary 2022; 71(No. SS-10): 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasan R, Baird DD, Herring AH, et al. Patterns and predictors of vaginal bleeding in the first trimester of pregnancy. Ann Epidemiol 2010; 20: 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prieto B, Adiego B, Suela J, et al. Prenatal screening and diagnosis of genetic abnormalities: SEGO, SEQCML, AEDP consensus recommendations. Adv Lab Med 2020; 1: 20200043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Royal College of Obstetricians and Gynaecologists. Antepartum Haemorrhage: Green-top Guideline No. 63, https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/antepartum-haemorrhage-green-top-guideline-no-63/ (2011, accessed 15 August 2021).
- 29.Tongsong T, Srisomboon J, Wanapirak C, et al. Pregnancy outcome of threatened abortion with demonstrable fetal cardiac activity: a cohort study. J Obstet Gynaecol (Tokyo 1995) 1995; 21: 331–335. [DOI] [PubMed] [Google Scholar]
- 30.Lindblad Wollmann C, Ahlberg M, Saltvedt S, et al. Risk of repeat cesarean delivery in women undergoing trial of labor: a population-based cohort study. Acta Obstet Gynecol Scand 2018; 97: 1524–1529. [DOI] [PubMed] [Google Scholar]
- 31.Al-Memar M, Vaulet T, Fourie H, et al. First-trimester intrauterine hematoma and pregnancy complications. Ultrasound Obstet Gynecol 2020; 55: 536–545. [DOI] [PubMed] [Google Scholar]
- 32.Naert M. Association between first-trimester subchorionic hematomas and pregnancy loss in singleton pregnancies. Doctoral Dissertation, Icahn School of Medicine at Mount Sinai, New York, NY, USA, 2020. [DOI] [PubMed]
- 33.Patel NG, Patel MS, Shah SR, et al. Study of outcome of pregnancy in patients with first-trimester bleeding per vaginum. International Journal of Advances in Medicine 2017; 3: 230–233. [Google Scholar]
- 34.De Sutter P, Bontinck J, Schutysers V, et al. First-trimester bleeding and pregnancy outcome in singletons after assisted reproduction. Hum Reprod 2006; 21: 1907–1911. [DOI] [PubMed] [Google Scholar]
- 35.Kortekaas JC, Kazemier BM, Keulen JKJ, et al. Risk of adverse pregnancy outcomes of late-and postterm pregnancies in advanced maternal age: a national cohort study. Acta Obstet Gynecol Scand 2020; 99: 1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farooq MU, Shah MAR, Yaseen MR. Mother schooling and malnutrition among children of rural-urban Pakistan. Epidemiology, Biostatistics, and Public Health 2022; 16: 10.2427/12978. [DOI] [Google Scholar]
- 37.Zhao H, He W, Yang Z. A pairwise and network meta‐analysis comparing the efficacy and safety of progestogens in threatened abortion. Int J Gynaecol Obstet 2022; 156: 383–393. [DOI] [PubMed] [Google Scholar]
- 38.Norsker FN, Espenhain L, A Rogvi S, et al. Socioeconomic position and the risk of spontaneous abortion: a study within the Danish National Birth Cohort. BMJ Open 2012; 2: e001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng D, Li C, Wu T, et al. Factors associated with spontaneous abortion: a cross-sectional study of Chinese populations. Reprod Health 2017; 14: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipilä P, Hartikainen-Sorri AL, Oja H, et al. Perinatal outcome of pregnancies complicated by vaginal bleeding. Br J Obstet Gynaecol 1992; 99: 959–963. [DOI] [PubMed] [Google Scholar]
- 41.Kavyashree HS, Rajeshwari K. A study on pregnancy outcome in patients with first trimester vaginal bleeding. Int J Reprod Contracept Obstet Gynecol 2019; 8: 820–824. [Google Scholar]
- 42.Shivanagappa M, Sagar SG, Manoli N. Ultrasound evaluation of vaginal bleeding in first trimester of pregnancy: a comparative study with clinical examination. Int J Sci Stud 2015; 3: 202–206. [Google Scholar]
- 43.Yasmin H, Mohsin H, Korejo R. Preterm deliveries in women presenting with threatened abortion. J Surg Pak 2015; 20: 5–9. [Google Scholar]
- 44.Helmerhorst FM, Perquin DA, Donker D, et al. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ 2004; 328: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coomarasamy A, Williams H, Truchanowicz E, et al. A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med 2015; 373: 2141–2148. [DOI] [PubMed] [Google Scholar]
- 46.Eaton JL, Zhang X, Kazer RR. First-trimester bleeding and twin pregnancy outcomes after in vitro fertilization. Fertil Steril 2016; 106: 140–143. [DOI] [PubMed] [Google Scholar]
- 47.Saraswat L, Bhattacharya S, Maheshwari A, et al. Maternal and perinatal outcome in women with threatened miscarriage in the first trimester: a systematic review. BJOG 2010; 117: 245–257. [DOI] [PubMed] [Google Scholar]
- 48.Stephenson MD, McQueen D, Winter M, et al. Luteal start vaginal micronized progesterone improves pregnancy success in women with recurrent pregnancy loss. Fertil Steril 2017; 107: 684–690.e2. [DOI] [PubMed] [Google Scholar]
- 49.Tuuli MG, Norman SM, Odibo AO, et al. Perinatal outcomes in women with subchorionic hematoma: a systematic review and meta-analysis. Obstet Gynecol 2011; 117: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 50.Bell MJ. A historical overview of preeclampsia-eclampsia. J Obstet Gynecol Neonatal Nurs 2010; 39: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maynard SE, Karumanchi SA. Preeclampsia. In: Mount DB, Pollak MR. (eds) Molecular and genetic basis of renal disease. 1st ed. St. Louis, Mo, USA: W.B. Saunders, 2008. W.B. Saunders, pp. 441–451. [Google Scholar]
- 52.Abalos E, Cuesta C, Grosso AL, et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013; 170: 1–7. [DOI] [PubMed] [Google Scholar]
- 53.Hasan R, Baird DB, Herring AH, et al. Association between first-trimester vaginal bleeding and miscarriage. Obstet Gynecol 2009; 114: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available upon request from the corresponding author.