INTRODUCTION
Moraxella (Branhamella) catarrhalis, formerly called Neisseria catarrhalis or Micrococcus catarrhalis, is a gram-negative, aerobic diplococcus frequently found as a commensal of the upper respiratory tract (124, 126; G. Ninane, J. Joly, P. Piot, and M. Kraytman, Letter, Lancet ii:149, 1997). Over the last 20 to 30 years, the bacterium has emerged as a genuine pathogen and is now considered an important cause of upper respiratory tract infections in otherwise healthy children and elderly people (48, 108, 132, 168). Moreover, M. catarrhalis is an important cause of lower respiratory tract infections, particularly in adults with chronic obstructive pulmonary disease (COPD) (48, 108, 168). In immunocompromized hosts, the bacterium can cause a variety of severe infections including pneumonia, endocarditis, septicemia, and meningitis (48, 63, 72). In addition, hospital outbreaks of respiratory disease due to M. catarrhalis have been described (188, 200), now establishing the bacterium as a nosocomial pathogen. Because M. catarrhalis has long been considered a harmless commensal (48, 124, 126), relatively little is known about its pathogenic characteristics and virulence factors, although developments in this field of research have accelerated over the past 5 years.
The emergence of M. catarrhalis as a pathogen in the last decade, together with the increasing prevalence of β-lactamase-producing strains, has renewed interest in this bacterial species. In this review, we will summarize important features of this organism, focusing on microbial epidemiology, virulence, immunity, and clinical and molecular-pathogenic aspects of infections caused by this organism.
TAXONOMY
In the past, M. catarrhalis was considered a nonpathogenic member of the resident flora of the nasopharynx. It was one of the species belonging to the so-called nongonococcal, nonmeningococcal neisseriae, considered to be members of the normal flora. The name of the species has caused considerable confusion. The bacterium was first described in 1896 (98) and was called Micrococcus catarrhalis. Later it was renamed Neisseria catarrhalis. In 1963, Berger showed that the original genus Micrococcus catarrhalis actually contained two distinct species, Neisseria cinerea and N. catarrhalis (16). These species could be separated based on the results of nitrate and nitrite reduction and tributyrin conversion testing. Because of the wide phylogenetic separation between N. catarrhalis and the so-called “true” Neisseria species, observed by a variety of a methods, the bacterium was moved to the new genus Branhamella in honour of Sara E. Branham (49). In 1984, B. catarrhalis was reassigned to the genus Moraxella as Moraxella (Branhamella) catarrhalis (34). This genus now contains both coccoid and rod-shaped bacteria, which are genetically related. The position of M. catarrhalis in the prokaryotic kingdom is shown in Fig. 1. DNA sequencing has substantiated the validity of the current taxonomic classification (84, 191). Many scientists preferred the name Branhamella catarrhalis, and in several recent publications this name is primarily used. As a means of resolving this semantic problem, Catlin (47) has proposed the formation of a new family, Branhamaceae, to accommodate the genera Moraxella and Branhamella. However, comparison of 16S rDNA sequences of Moraxella spp. and these from bacterial species in related genera has demonstrated the close relation of M. catarrhalis to M. lacunata subsp. lacunata and to a “false” Neisseria species, N. ovis. In addition, M. catarrhalis appears to be more closely related to Acinetobacter spp. than to Neisseria spp. (84). On the basis of these results, the latter authors conclude that there is no rationale for a separate Branhamella genus. Consequently, M. catarrhalis is the currently preferred name for this bacterial species.
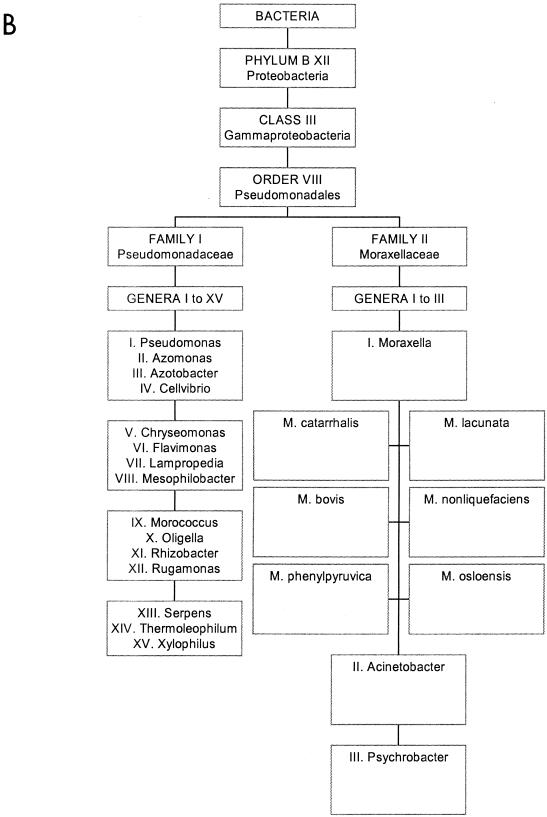
FIG. 1.
The bacterial species M. catarrhalis and its close relatives. (A) Position of the γ proteobacteria in the prokaryotic kingdom. M. catarrhalis is within this class of organisms. (B) More detailed positioning of M. catarrhalis in the order Pseudomonales. Panel A reprinted from reference 106 with permission of the publisher; the data in panel B are derived from Bergey’s Manual of Determinative Bacteriology, 8th ed. (R. E. Buchanan and N. E. Gibbons, ed.), The Williams & Wilkins Co. Baltimore, Md. (web-accessible version).
ISOLATION AND IDENTIFICATION
Isolation of M. catarrhalis from clinical specimes, e.g., sputum, can be complicated by the presence of nonpathogenic neisseriae. Selective agar media have been used to isolate M. catarrhalis with some success. For example, acetazolamide, which reduces the growth of Neisseria species when used under aerobic conditions, and the antimicrobial components vancomycin, trimethoprim, and amphotericin B may be included in an agar medium to inhibit the growth of the normal flora (71, 227).
Over the years, the following criteria have been used to unambiguously distinguish M. catarrhalis from other bacterial species: Gram stain; colony morphology; lack of pigmentation of the colony on blood agar; oxidase production; DNase production; failure to produce acid from glucose, maltose, sucrose, lactose, and fructose; growth at 22°C on nutrient agar; failure to grow on modified Thayer-Martin medium; and, finally, reduction of nitrate and nitrite (76, 214). However, it has been shown that growth at 22°C and failure to grow on modified Thayer-Martin medium are not reliable parameters for the correct identification of M. catarrhalis (76). Also, Jönsson et al. (128) showed that colony morphology, Gram stain, and oxidase production were an insufficient group of characteristics to permit correct and final identification of M. catarrhalis in cultures derived from sputum samples. It should be noted, however, that the Gram stain still plays a crucial role both in the isolation of the bacterium from clinical material (e.g., sputum) and in its subsequent identification. In typical Gram stains, M. catarrhalis presents itself as a gram-negative diplococcus with flattened abutting sides. The bacterium has a tendency to resist destaining. Colonies on blood agar are nonhemolytic, round, opaque, convex, and greyish white. The colony remains intact when pushed across the surface of the agar. The bacteria are oxidase positive, but additional tests are needed for routine identification. Positive reactions for DNase production, reduction of nitrate and nitrite, and tributyrin hydrolysis are valuable differentiating characteristics (48, 214, 217). According to Catlin (48), the identity of M. catarrhalis is best confirmed by positive reactions in at least three of these differentiating tests, since none of them is 100% sensitive or specific by itself.
Modern DNA technology has opened new avenues for the detection of M. catarrhalis in clinical materials (e.g., middle ear effusion) without the need for bacterial culture. In particular, PCR tests for M. catarrhalis have been both designed and used for clinical purposes, with direct detection of M. catarrhalis DNA by PCR being concordant with culture and endotoxin detection. However, DNA assays yield significantly more positive results than does culture when, for instance, middle ear effusions are analyzed, which suggests superior sensitivity of the DNA amplification assays (70). The clinical relevance of PCR has been validated extensively in the chinchilla model for otitis media. This animal model was instrumental in demonstrating the quick and effective effusion-mediated clearance of DNA and dead M. catarrhalis bacteria from the middle ear cleft, implying that in this case a positive PCR result was indicative of the presence of viable bacteria (193). Moreover, PCR has also been reliably used for the detection of mixed infections in the same experimental-infection model (11), thereby substantiating the applicability of multiplex PCR approaches for the detection of mixed bacterial infections, e.g., with M. catarrhalis, Haemophilus influenzae, and Streptococcus pneumoniae in a single amplification assay. This approach has even been successful for culture-negative effusion (194). Recently, clinical evaluation of a multiplex PCR specific for the above three pathogens plus Alloiococcus otitidis demonstrated that reliable DNA amplification-based diagnostics of middle ear effusions can be performed in a single working day (113). Furthermore, the sensitivity of the PCR tests corresponds to six or seven genome equivalents, making PCR an unrivaled diagnostic assay (195). However, it has to be emphasized that the technical demands of PCR are still beyond the capacities of many routine microbiology laboratories, although improvements in robotics and other forms of laboratory automation are fast bridging the current gap between theory and practice.
EPIDEMIOLOGY
For several reasons, epidemiological studies of M. catarrhalis are difficult. Practical typing systems have become available only recently, and the lack of reliable serological tests is at least partly to blame for this problem. Moreover, the clinical interest in M. catarrhalis is only relatively recent, and many laboratories did not report M. catarrhalis as a pathogen, especially when a well-recognized pathogen (e.g., S. pneumoniae or H. influenzae) was present as well. In addition, as mentioned above, the isolation of M. catarrhalis from sputa is complicated by the presence of nonpathogenic neisseriae. Thus, the use of selective agar media could be advantageous (71, 227).
Conventional and Molecular Typing Systems
Several phenotyping strategies have been described for epidemiological typing of M. catarrhalis, although none of these has been accepted internationally. Serological typing of lipopolysaccharide (LPS) (230), isoelectric focusing of β-lactamase proteins (179), and electrophoretic profiling of outer membrane proteins (13) have been described but have never been used in large-scale studies. Besides these and some other regularly employed phenotyping procedures (66, 190), other methods based on nucleic acid polymorphism have become available more recently. Comparison of restriction endonuclease analysis with phenotyping (57) has indicated that restriction endonuclease analysis of genomic DNA can be used successfully for delineation of disease outbreaks (134, 188). Also, macrorestriction enzymes and pulsed-field gel electrophoresis have been used to shed light on matters of epidemiological concern (237, 254). An example of the use of PFGE to document patient-to-patient transmission is shown in Fig. 2. The use of strain-specific DNA probes has also been documented (14, 243). Recent studies identified amplified fragment length polymorphism analysis and automated ribotyping as useful typing procedures (32, 235). Moreover, when these two strategies were used, complement-resistant strains of M. catarrhalis were found to form a distinct clonal lineage within the species (Fig. 3) (32, 235). These observations are in agreement with earlier studies identifying M. catarrhalis as a genetically heterogeneous species from which successful clones occasionally proliferate (85). Expansion of such competitive types has also been documented during distinct periods and in particular geographic regions (157). Frequent horizontal gene transfer seems possible (31, 150), and in relation to the observations made for complement resistance, it may be postulated that other phenotypic traits could be acquired through cross-species gene acquisition. For instance, Bootsma et al. (31) demonstrated that the barrier between M. catarrhalis and gram-positive microorganisms may be occasionally crossed by antimicrobial resistance genes.
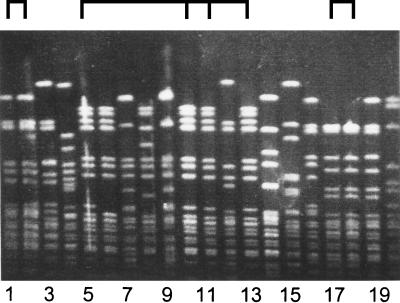
FIG. 2.
Pulsed-field gel electrophoresis of SpeI-digested M. catarrhalis DNA. Twenty nosocomial isolates were analyzed, which resulted in the identification of several clusters of indiscriminate strains. As indicated at the top, isolates 1 and 2 and isolates 17 and 18 are identical, which fits well with the fact that the strains were isolated from the same patients on separate occasions. Strains 5, 10, 11, and 13 were isolated from different patients hospitalized during overlapping time intervals in the same pediatric department. It was concluded that patient-to-patient transmission occurred in this setting.
FIG. 3.
Dendrogram constructed on the basis of RiboPrint pattern types obtained for 13 complement-sensitive and 2 complement-resistant strains of M. catarrhalis (235). Selected were those RiboPrint patterns that are representative of the diverse genogroups that could be identified. The resistant strains appear to be a more homogeneous group (only 2 closely related types encountered among 47 strains) than are the complement-sensitive strains. The tree was constructed in the BioNumerics program developed by Applied Maths (Kortrijk, Belgium). On the basis of Pearson coefficients and unweighted pair group method using arithmetic averages (UPGMA), patterns were normalized using molecular size markers coanalyzed during pattern creation. The 40 to 100 scale above the dendrogram indicates the percent identity between fingerprints compared. The fully automated RiboPrinter has been developed and marketed by Qualicon, a Dupont subsidiary (Warwick, United Kingdom).
Carriage
The M. catarrhalis carriage rate in children is high (up to 75%) (89, 230, 231). In contrast, the carriage rate of M. catarrhalis in healthy adults is very low (about 1 to 3%) (69; T. Ejlartsen, Letter, Eur. J. Clin. Microbiol. Infect. Dis., 10:89, 1991). This inverse relationship between age and colonization has been known since 1907 (9) and is still present today (81; C. Hol, C. M. Verduin, E. van Dijke, J. Verhoef, and H. van Dijk, Letter, Lancet 341:1281, 1993). At present, there is no good explanation for the difference in rates of colonization between children and adults; one explanation may be the age-dependent development of secretory immunoglobulin A (IgA). Remarkably, IgG antibody levels do not correlate with the state of colonization or with lower respiratory tract infection with M. catarrhalis in children (82). Interestingly, nasopharyngeal carriage rates are significantly higher in winter and autumn than in spring and summer (230).
Monthly or bimonthly sampling of the nasopharynges of children (n = 120) by Faden et al. (89) revealed the presence of M. catarrhalis in 77.5% of subjects at least once during the first 2 years of life. Furthermore, these authors showed a clear relationship between the frequency of colonization and the development of otitis media. A small Japanese study revealed that colonization in children attending a day care center is highly dynamic (254). Although clusters of genotypes could be discerned and seemed to persist for periods of 2 to 6 weeks, frequent changes in the nature of individual colonizing strains of M. catarrhalis were observed. Of note, rates of isolation of M. catarrhalis are much higher in fall and winter than in spring and summer. This seasonal difference in isolation is less pronounced with S. pneumoniae or H. influenzae (230) but is quite common in viral infections.
Other authors have described a relationship between the frequency of colonization and the occurrence of upper respiratory infection (40, 196, 230, 231). Klingman et al. (139) investigated the colonization of the respiratory tract of patients with bronchiectasis. A subset of these patients was repeatedly colonized with different M. catarrhalis strains. The patients were colonized with the same strain for an average of 2.3 months as determined by restriction fragment length polymorphism patterns, and colonization with a new strain did not correlate with changes in clinical status. Although not studied in detail, there are indications that adults with chronic lung disease are colonized at a higher rate than are healthy adults (169).
DISEASES IN CHILDHOOD
M. catarrhalis is now considered an important pathogen in respiratory tract infections, both in children and in adults with underlying COPD. Occasionally, the bacterium causes systemic disease, e.g., meningitis and sepsis (2, 48, 59, 75). Bacteremia due to M. catarrhalis should be considered especially in febrile children with an underlying immune dysfunction and an upper respiratory tract infection (2). In addition, M. catarrhalis may be the single cause of sinusitis, otitis media, tracheitis, bronchitis, pneumonia, and, less commonly, ocular infections in children. In children, nasopharyngeal colonization often precedes the development of M. catarrhalis-mediated disease (89). Below we summarize the clinical features of childhood disease.
Sinusitis
Sinus development is a process that may take up to 20 years, although the ethmoid and maxillary sinuses are already present at birth; the development of sphenoid and frontal sinuses starts in the first few years of life (55). Sinusitis is a very common infection in early childhood, accounting for about 5 to 10% of upper respiratory tract infections (239, 240; E. R. Wald, Editorial, Pediatr. Ann. 27:787–788, 1998). It is often underdiagnosed in children because the symptoms are nonspecific. In addition, physical examination and radiology are of little value in young children, and an etiologic diagnosis requires culturing an aspirate of sinus secretions (28). In acute sinusitis (where symptoms are present for 10 to 30 days) and subacute disease (30 to 120 days), S. pneumoniae, H. influenzae, and M. catarrhalis are the most frequently isolated bacterial pathogens (27, 28, 46, 239, 240; Wald, Editorial). S. pneumoniae is found in 30 to 40% of patients, while H. influenzae and M. catarrhalis each account for approximately 20% of cases. Interestingly, in children with asthma, the same distribution of bacterial pathogens is found (238), although Goldenhersch et al. (103) isolated M. catarrhalis as the predominant pathogen in subacute or chronic sinusitis (symptoms present for more than 30 days) in children with respiratory allergy. It has been suggested that there is a possible underestimation of isolation rates for M. catarrhalis, since the bacterium stops growing in environments with reduced oxygen concentrations, a condition frequently present during sinusitis and otitis media (39, 204). This would indicate an even greater role for M. catarrhalis in the etiology of these infectious diseases.
Otitis Media
Acute otitis media (AOM) is a very frequent infection in children: before the age of 1 year, around 50% of children have experienced at least one period of AOM. This proportion rises to 70% at the age of 3 years (136, 222; M. L. Kabongo, Letter, Am. Fam. Physician 40:34, 39, 1989). Undoubtedly, it is the most serious and frequent infection caused by M. catarrhalis in children, and as such M. catarrhalis causes tremendous morbidity and requires the widespread use of antibiotics (20, 58, 88, 89, 97, 136, 137, 230). While not frequently encountered as a pathogen, M. catarrhalis has been recognized as a specific pathogen in AOM for nearly 70 years (109). Since 1980, a marked increase has been reported in the isolation of M. catarrhalis from middle-ear exudates (26, 141, 155, 213). This increase in M. catarrhalis isolation to approximately 15 to 20% (187) has been accompanied by the appearance of β-lactamase-producing strains, which now account for approximately 90 to 95% of isolates. However, the exact magnitude of this apparent increase in isolation rates may not have been adequately measured yet (155), since tympanocentesis and culture of middle ear fluid are not performed routinely. Patel et al. (187) cultured the middle ear fluids of 99 children with AOM and isolated S. pneumoniae, nontypeable H. influenzae, and M. catarrhalis from 39, 30, and 25% of subjects, respectively. Again, the isolation rates for M. catarrhalis might be an underestimation, given the relatively anaerobic environment of the middle ear during infection (8). In a study using PCR, M. catarrhalis DNA was detected in 46.4% of pediatric chronic middle ear effusion specimens (n = 97), compared to 54.6% for H. influenzae DNA and 29.9% for S. pneumoniae DNA (194). A large percentage (48%) of specimens was PCR positive and culture negative, whereas all culture-positive specimens were also PCR positive. It is very unlikely that the PCR-positive yet culture-negative specimens reflect the persistence of DNA from old infections (10, 193, 194). The severity of symptoms and numbers of bacteria in middle ear fluid appear to be lower for M. catarrhalis than for S. pneumoniae or H. influenzae (87).
Lower Respiratory Tract Infections
Although lower respiratory tract infections in children are a common cause of morbidity and even mortality among children worldwide, obtaining a microbiological diagnosis is notoriously difficult. Most studies use combinations of serological and conventional microbiological (e.g., culture- or PCR-based) methods. Many of these methods have only been used within a research setting and are not always reliable or readily available to clinicians. As a consequence, data concerning the role of M. catarrhalis in lower respiratory tract infections are not conclusive. Lower respiratory tract infections due to M. catarrhalis appear to be relatively rare during childhood, with most infections occurring in children below the age of 1 year (35). Korppi et al. (140) have investigated the seroconversion to M. catarrhalis in patients who were hospitalized with middle (laryngitis, tracheitis, bronchitis) and lower respiratory tract infections. They found seroconversion in only 4 (5%) of 76 children who had M. catarrhalis-positive nasopharyngeal aspirate cultures compared to 4 (1%) of 373 children who had negative cultures. According to their results, M. catarrhalis is not a likely cause of these infections in children. However, in contrast to these findings, several other studies have indeed implicated M. catarrhalis in lower respiratory tract infections in children. First, M. catarrhalis has been isolated in pure culture from secretions obtained by tracheal aspiration in neonates, infants, and children with pneumonia (15, 21, 107, 155). Underlying bronchopulmonary dysplasia has been suggested as a predisposing factor in these cases (15, 60). Second, in a prospective study combining microbiological and clinical criteria, M. catarrhalis was identified as a significant respiratory pathogen in children (35). Third, both local and systemic antibody responses to M. catarrhalis infection have been documented in several studies (25, 51, 90, 91, 101, 102). Pneumonia in children can be complicated by bacteremia with M. catarrhalis (59, 123, 224). For example, Ioannidis et al. (123) have presented data on 58 cases of M. cattarhalis bacteremia, including cases in 28 children younger than 12 years. Most patients (ca. 70%) had an underlying disease (malignancy and/or neutropenia, underlying respiratory tract disorder), and an associated respiratory tract infection was identified in half of the patients. In children with bacteremia, skin lesions such as purpuric and petechial rash were frequent. Of 58 patients, 12 died (21%), including 4 of 5 patients with endocarditis and 4 of 7 patients who did not receive therapy. In conclusion, although the current literature does not provide a definite answer, the available data suggest that M. catarrhalis can be involved in lower respiratory tract infections in children.
Other Infections
M. catarrhalis has been implicated as a cause of bacterial tracheitis in childhood (23, 36, 86, 155; V. K. Wong and W. H. Mason, Letter, Pediatr. Infect. Dis. J. 6:945–946, 1987), for which preceding viral infection has been considered a significant predisposing factor (Wong and Mason, Letter). In addition, a role for this microorganism has been suggested in conjunctivitis and keratitis (152, 155), although reports on ocular infections have been rare (1, 152, 247; R. L. Bergren, W. S. Tasman, R. T. Wallace, and L. J. Katz, Letter, Arch. Ophthalmol. 111:1169–1170, 1993). Finally, one case of fatal meningitis due to M. catarrhalis has been reported (63).
INFECTIONS IN ADULTS
M. catarrhalis has been associated with a variety of clinical syndromes in adults; the most frequent are discussed in more detail below. It has to be emphasized, however, that M. catarrhalis can also manifest itself as a pathogen in the nosocomial setting. A rare but very serious and frequently lethal infection with M. catarrhalis appears to be endocarditis (123, 180, 219).
Laryngitis
M. catarrhalis is the most common bacterial species isolated from adult patients with laryngitis. Schalén et al. (209, 210) found that of 40 adults with this disease, 22 were infected by M. catarrhalis (55%), compared to 0 of 40 healthy adults. Even so, the exact role of M. catarrhalis, either as an innocent bystander or as a causal microorganism in the pathogenesis of adult laryngitis, is not fully understood.
Bronchitis and Pneumonia
M. catarrhalis is not a common cause of lower respiratory tract infections in healthy adults. However, the bacterium causes pulmonary infections in three separate clinical settings (169): (i) in COPD patients, (ii) pneumonia in the elderly, and (iii) as a nosocomial respiratory tract pathogen.
M. catarrhalis is a common cause of exacerbations in COPD (35, 43, 64, 83, 108, 160, 165, 178, 182, 192, 208). In COPD and otitis media, only S. pneumoniae and nontypeable H. influenzae are isolated more often than M. catarrhalis, yet the frequency of isolation of M. catarrhalis from sputa has risen during the past 10 to 15 years (35, 64, 160). This rise cannot be ascribed only to an increased awareness in the laboratory (64). One study has shown M. catarrhalis to be the single most isolated pathogen in COPD (218). Sarubbi et al. (208) reviewed all respiratory tract cultures (n = 16,627) performed over a period of 42 months and identified M. catarrhalis in 2.7% (n = 457) of these cultures. In this study, M. catarrhalis was found to be the second most commonly isolated respiratory tract pathogen after nontypeable H. influenzae but ranking before S. pneumoniae. In addition, these and many other authors (62, 64, 69, 192, 208, 251, 252) demonstrated striking seasonality, with winter and spring being the periods with the greatest incidence of M. catarrhalis isolation. This pattern is not found with S. pneumoniae or H. influenzae (64, 69). Preceding viral respiratory tract infection caused by respiratory syncytial virus, for example, could be a factor in the seasonal variations which have been observed with M. catarrhalis infections, although this hypothesis remains untested (69, 229).
The typical clinical picture of an M. catarrhalis respiratory infection is that of tracheobronchitis, presenting with cough and production of purulent sputum. Pneumonia caused by M. catarrhalis tends to be a relatively mild disease. It differs from bronchitis by the presence of mostly lower-lobe infiltrates on chest X rays (108, 182, 218, 252). High fever, pleuritic pain, and toxic states are uncommon, as are empyema and bacteremia (108, 182, 192, 218, 252). Collazos et al. (59) reviewed 15 cases of bacteremic pneumonia due to M. catarrhalis that had been reported in the literature. These cases (nine in adults and six in children) were similar in both characteristics and clinical symptoms to those described for patients with bronchitis or pneumonia without bacteremia. The mortality rate for these bacteremic cases was 13.3%. An even larger review by Ioannidis et al. in 1995 (123) described the clinical spectrum of M. catarrhalis bacteremia in 58 patients. Predisposing factors were present in more than 70% of the patients and included neutropenia, malignancy, and respiratory impairment, either alone or in combination. In this study, maculopapular rash appeared to be a relatively rare symptom and was most frequently seen in patients with neutropenia. Mortality was high (29%) among patients with underlying respiratory disease, and the infection was more severe when the patient was coinfected with other respiratory tract pathogens. The overall mortality related to respiratory infection appears to be relatively low (around 10% [12, 108, 252]). Even so, M. catarrhalis pneumonia often occurs in patients with end-stage pulmonary or malignant disease, and the short-term mortality in some patient categories is as high as 45% (252). Most patients are elderly (older than 65 years), and 90 to 95% of patients have underlying cardiopulmonary disease (12, 108, 252), with COPD being present in the majority of cases. Many patients appear to be malnourished (252). A large percentage (>70%) are smokers or exsmokers (69). Men appear to be at greater risk than women, although this observation could be confounded by, for instance, smoking habits (12, 59, 69, 108, 182).
Research into the colonization and infection of bronchiectasis patients with M. catarrhalis over time has indicated that a subset of patients (around 20%) appeared to be chronically colonized with M. catarrhalis, sometimes consecutively with four different strains. A causal relation between isolation of the bacterium and exacerbations could not be proven, although its presence in a large proportion of patients suggests a causal role (139).
An additional organism can also be isolated from about 40 to 50% of sputum cultures; in most cases, S. pneumoniae or H. influenzae are isolated (12, 108, 182, 192). For several reasons, it is important to define the role of M. catarrhalis in these mixed infections, particularly with respect to the adequate management of patients and specific antibiotic therapy. In a mixed infection with S. pneumoniae, for example, should treatment for M. catarrhalis be considered at all, or can antibiotic treatment be targeted at the pneumococcus alone?
Nosocomial Infections
That nosocomial infections could be caused by M. catarrhalis has been suggested by several investigators (15, 19, 35, 60, 67, 107, 108, 188, 200). In the past it has been difficult to confirm the spread of the organism among hospitalized patients, because of the lack of a reliable typing system. Furthermore, because of the mildness of the disease, nosocomial spread can be overlooked or simply disregarded. Patterson et al. (188) used restriction endonuclease analysis to confirm an outbreak in a hospital unit. Strains from five patients and two staff members yielded identical genotype patterns when this technique was used. During the investigation of another putative outbreak, immunoblotting with normal human serum was combined with restriction endonuclease analysis to type M. catarrhalis strains. Six M. catarrhalis isolates from a cluster of infections involving five patients in a respiratory unit were shown to be identical to each other and different from other, unrelated strains from the same institution (200). Both methods provided good discrimination between strains, but they were not always in complete agreement (166, 200). Thus, the use of more than one typing technique was recommended. Another useful option would be restriction endonuclease analysis with several enzymes rather than just one. Clear vehicles of bacterial dissemination have not yet been identified in the clinical setting. However, Ikram et al. (122) found the nosocomial spread of M. catarrhalis to be common, especially in respiratory wards. They also showed that considerable contamination of the environment with M. catarrhalis may occur, implying a possible aerosol-mediated mode of dissemination. Thus, important questions remain to be answered with regard to the nosocomial spread of M. catarrhalis, including the identification of the reservoir of infection and the mode(s) of transmission. Person-to-person transmission (122, 161, 188, 200) and spread from environmental sources (44, 122) have been implicated in nosocomial transmission on the basis of circumstantial evidence; of possible significance is the observation that the bacterium is able to survive in expectorated sputum for at least 3 weeks (44). Nursery schools are sites where frequent exchanges of strains may occur (254). Preliminary data do reveal that this may indeed be important in the epidemiology of M. catarrhalis carriage (unpublished observations).
ANTIMICROBIAL SUSCEPTIBILITY
Apart from its almost universal β-lactamase-mediated resistance to penicillins and its inherent resistance to trimethoprim, M. catarrhalis remains universally sensitive to most antibiotics used in the treatment of respiratory infections (18, 119, 159). A recent large international study, the Alexander Project 1996–1997, revealed that 100% of isolates were susceptible to amoxicillin-clavulanic acid, cefixime, chloramphenicol, ciprofloxacin, and ofloxacin (92). For some antibiotics (cefaclor, ceftriaxone, and doxycyclin) a small increase (<0.5%) in the incidence of resistant strains was noted over the years. The clinical relevance of this increase is still unknown. Of note, strains that produce β-lactamase are expected to be resistant to penicillin, ampicillin, amoxicillin, and piperacillin (18, 33, 99, 130).
β-Lactamase Production
Before 1970, no M. catarrhalis isolate was observed to produce β-lactamase (48, 245); the first β-lactamase-positive strain was isolated in 1976 (245). By 1980, however, 75% of M. catarrhalis isolates from the United States produced β-lactamase (244). By 1990, about 80% of respiratory M. catarrhalis isolates from the United States (130) and over 90% of isolates from England and Scotland were positive for β-lactamase (99). Recent studies from Australia, Europe and the United States all noted β-lactamase production in over 90% of isolates (74, 95, 154, 223, 231, 242, 251). Walker et al. (242) investigated trends in antibiotic resistance of M. catarrhalis isolates (n = 375) in a single hospital over a 10-year period (1984 to 1994). During this period, the number of isolates showing β-lactamase production increased from 30 to 96%. Moreover, a trend toward reduced susceptibility to four β-lactam antibiotics, penicillin G, ceftriaxone, amoxicillin-clavulanic acid, and imipenem, but not cefamandole, was observed (although this was not clinically relevant). For of penicillin and ceftriaxone, this trend was due to an increased frequency of β-lactamase-positive isolates. However, the increase in the MIC of amoxicillin-clavulanic acid and imipenem was not due to the increased frequency of β-lactamase-positive strains but occurred mainly within the group of β-lactamase-positive strains. These observations indicate either (i) a selection for more efficient β-lactamases, (ii) a more efficient production of a β-lactamase, or (iii) selection for additional resistance determinants. Given the high percentage of strains that produce β-lactamase and despite the fact that successful amoxicillin treatment of patients infected with β-lactamase-positive M. catarrhalis has been reported, clinicians should assume that all isolates of M. catarrhalis are resistant to amoxicillin, ampicillin, piperacillin, and penicillin (73, 147).
In M. catarrhalis two types of β-lactamases can be found that are phenotypically identical: the BRO-1 and BRO-2 types. Both are membrane associated, and they differ by only a single amino acid. The enzymes are encoded by chromosomal genes, and these genes can be relatively easily transferred from cell to cell by conjugation (159, 245). Fortunately, both enzymes are readily inactivated by β-lactamase inhibitors, and all isolates are still susceptible to amoxicillin in combination with clavulanic acid (119, 159). BRO-1 is associated with higher MICs than is BRO-2; the difference is attributed to the production of more enzyme as a consequence of the higher transcriptional activity of the BRO-1 gene. BRO-1 is the most common enzyme and is present in ca. 90% of β-lactamase-positive strains (159, 245). Recent studies have shown that the β-lactamase of M. catarrhalis is lipidated, suggesting a gram-positive origin. M. catarrhalis is the first gram-negative bacterial species possessing such a lipidated BRO-type β-lactamase (31). This adds to the complexity of the dissemination of antibiotic resistance traits among M. catarrhalis strains in the sense that there seems to be a possibility for the acquisition of genes even from the gram-positive gene pool. The G+C content of the BRO genes provides additional proof of their non-Moraxella origin and suggests a recent acquisition event. The lack of a genetic barrier between gram-negative and -positive bacterial species is a reason for clinical concern, and additional research on the mechanism of DNA uptake by M. catarrhalis is certainly warranted.
β-Lactamase from M. catarrhalis not only protects the bacteria producing the enzyme but also is thought to inactivate penicillin therapy of concomitant infections by serious airway pathogens such as S. pneumoniae and/or nontypeable H. influenzae (37, 38, 42, 115). This phenomenon is referred to as the indirect pathogenicity of M. catarrhalis. Indeed, in such circumstances, treatment failures have been reported (187, 230), demonstrating the importance of reporting not only pure but also mixed cultures positive for M. catarrhalis (246).
CELL WALL STRUCTURES
Lipooligosaccharides
Lipooligosaccharide (LOS) is an important virulence factor of gram-negative bacteria. M. catarrhalis LOS appears to be semirough, meaning that it contains only one repeating O antigen at best (96, 118). In addition, it appears to be more antigenically conserved among strains than does the LOS of other gram-negative bacteria (171). This suggests that it will probably not serve as a useful basis for a typing system (71). Even so, Vaneechoutte et al. were able to distinguish three LOS types, A, B, and C, by enzyme-linked immunosorbent assay; these types accounted for more that 95% of all strains. Type A represents the great majority of strains (61%), with types B and C containing more limited numbers of strains (29 and 5%, respectively); 5% of strains remain unidentified (228). The various types can be discriminated by biophysical differences imposed by the presence of serotype-specific LOS structures (Fig. 4) (79, 118). There is a common polysaccharide inner core in serotypes A, B, and C, which can, at least in part, explain the existing cross-reactivity between the serotypes. The antigenic specificities of the three serotypes are caused by differences in terminal sugars of one of the branches (118). Moreover, a structural overlap was documented with the LPS moieties from species of the Neisseria and Haemophilus groups. The LOS of serotype B and C contain oligosaccharide chains of variable length. This could be due to phase-variable expression of the biosynthetic genes, as suggested by the presence of tandem repeats (189). Another explanation offered is that variations in the activity of enzymes involved in cell wall assembly (influenced by environmental factors or growth rate, for example) result in a different oligosaccharide (118). LOS is also present in culture supernatants of M. catarrhalis as a part of subcellular elements called blebs. These small vesicles may facilitate the distribution of LOS in the host environment. Whether these structures serve some physiological function is currently unknown. LOS serotype A, once adequately detoxified, can be used as a vaccine when conjugated to a protein carrier (120). Increases in the levels of anti-LOS IgG are observed on immunization. The increased levels of antibodies enhanced clearance of bacteria from the lungs of mice after an aerosol-mediated M. catarrhalis infection. Detoxified M. catarrhalis LOS conjugated to the high-molecular-weight (HMW) surface protein of nontypeable H. influenzae provides a potentially very interesting bivalent vaccine (see also below).
FIG. 4.
Schematic structure of the LOS moieties that cover the surface of the M. catarrhalis cells. Three main serotypes, A, B, and C, can be discerned, which differ in the nature of the R group. Abbreviations: D-Galp, 𝒹-galactose phosphate; Kdo, 2-keto-3-deoxyoctonate; GlcpNac, N-acetyllactosamine.
Peptidoglycan
Regarding the peptidoglycan, the studies by Keller et al. (135) indicate that M. catarrhalis organisms have a multilayered peptidoglycan architecture. This peptidoglycan layer was shown to be responsible for the extraordinary capacity of the organism to trigger various functional capacities of macrophages. Secretion of tumor necrosis factor and nitrite metabolism plus the cells’ tumoricidal activity were clearly enhanced. This triggering capacity could be, at least partially, an explanation for the low virulence of M. catarrhalis. It seems as if peptidoglycan is involved in some sort of suicidal activity, and further studies into the basic mechanisms of this phenomenon are certainly needed.
Outer Membrane Proteins
In contrast to other nonenteric gram-negative bacterial species, the outer membrane protein (OMP) profiles of different M. catarrhalis strains show a high degree of similarity. Using sucrose gradient purification of M. catarrhalis outer membranes and sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Murphy and coworkers identified eight major proteins, designated OMPs A through H, ranging from 21 to 98 kDa (13, 170, 177). OMPs C and D appeared to be two different stable forms of the same protein, the CD protein. The strong degree of similarity of OMP profiles explains why serotyping systems on the basis of OMP profiles are of little epidemiological use. On the other hand, these well-conserved surface proteins could be interesting vaccine candidates. In recent years the genes for several of these outer membrane proteins have been mapped and characterized in more detail.
In addition to OMPs A to H, Murphy and Klingman described a novel OMP, designated HMW-OMP or ubiquitous surface protein UspA (138). UspA has recently been shown to be encoded by two different genes, which share the coding potential for a homologous, internal protein domain of more than 90% amino acid sequence homology (4). Additional UspA-like genes have been discovered (144). Mutation in the UspA1-encoding gene resulted in an attenuated phenotype: adherence capacities of the deletion mutant were significantly decreased (3). Furthermore, it was demonstrated that UspA2 is essential for complement resistance (3, 144). Protein purification studies provided proof that the UspA1 protein binds specifically to HEp-2 cells and has an affinity for fibronectin (163). The UspA2 protein, on the other hand, preferentially binds to vitronectin. Both purified proteins are immunogenic in mice, and immunized animals clear bacteria from their lungs more rapidly than do nonimmunized mice (163). Genetic studies have shown that intraspecies variability in the genes can be attributed mainly to variation in regions of repetitive DNA in the genes (61). In addition, electron microscopy of M. catarrhalis strains has revealed that the UspA1 and UspA2 proteins present as “lollipop-shaped” structures protruding from the bacterial surface (114). Interestingly, the structure of the Yersinia adhesin (YadA) protein of Yersinia enterocolitica, a protein involved in adhesion and defence against complement-mediated killing, has a very similar overall structural organization and function (114, 207). Moreover, many related genes have been identified in the genomes of a wide variety of bacterial species, suggesting that the proteins serve essential and universally required functions. Although the UspA1 and UspA2 antigens currently are the best-studied M. catarrhalis proteins, their vaccine potential still is matter of ongoing investigations (see also below).
The heat-modifiable CD OMP could be cloned and expressed in Escherichia coli. The gene appeared to be strictly conserved among M. catarrhalis strains (175). Homology was found with the porin F protein (OprF) of Pseudomonas spp. (R. de Mot and J. Vanderleyden, Letter, Mol. Microbiol. 13:379–380, 1994) and with an OmpA-like protein from Acinetobacter spp. (184), indicating transspecies conservation that is generally associated with functional importance. The CD protein was found to be involved in binding purified human mucin from the nasopharynx and middle ear but not in binding mucin from the saliva and tracheobronchial mucin (22). The CD protein is a potential vaccine candidate (253), and antibodies raised in mice enhanced clearance in a pulmonary challenge model (176).
M. catarrhalis expresses both transferrin and lactoferrin receptors on its surface, named transferrin-binding proteins A and B (TbpA and TbpB) and lactoferrin-binding proteins A and B (LbpA and LbpB) (30), respectively. These proteins are partially homologous at the genetic level. In addition, homologous proteins of the lactoferrin-binding proteins as well as the transferrin-binding proteins are found in Neisseria spp., Haemophilus spp., and other gram-negative bacteria. These proteins provide the cell with the capacity to acquire iron by sequestering it from host carrier proteins (5, 45, 78). The receptors themselves appear to be significant virulence factors, since mutation analysis of the transferrin receptor has demonstrated an impaired growth capacity for the mutated strain. The receptors are also immunogenic and may be interesting vaccine candidates (54, 255). Several genes are associated with this iron acquisition machinery, with some functioning as associate receptors and others functioning as facilitating factors (148). Molecular knockout of the gene for transferrin-binding protein TbpB revealed that in the presence of a TbpB-specific monoclonal antibody and human complement, the mutant resisted killing, in contrast to the wild type, which was rapidly killed (149). However, the epitope recognized by the monoclonal antibody was surface expressed in only one of three clinical isolates.
The OMP E antigen appears to be of low immunogenicity, but it does possess universally surface-expressed epitopes in different M. catarrhalis strains (24). It is a relatively highly conserved protein, for which no definite function has yet been defined, although it may have a function in the uptake of nutrients (i.e., fatty acids) by the bacterium (173). In addition, this recent study found an increased sensitivity to complement-mediated killing in a knockout mutant of OMP E.
Important goals for present and future investigations are to determine the antigenic variability of OMPs, to find antigens that generate protective antibodies, and to determine the precise function of these proteins in the pathogenesis of diseases caused by the bacterium.
Pericellular Structures
The attachment of bacteria to mucosal epithelial cells is often mediated by pili or fimbriae. Some studies have provided evidence for the expression of pili by M. catarrhalis (156), whereas others have been unable to demonstrate their presence (7, 110). Consequently, some strains may be pilus positive whereas others have been proven to lack pili (7, 202). Pili are composed of polymerized protein subunits called pilins. Marrs and Weir (156) found several characteristics that point to the presence of type 4 (MePhe) pili in M. catarrhalis. In addition, electron microscopic data revealed that besides pili similar to those of type 4, an additional non-type 4 class of pili exists. Elucidation of the prevalence and role of these pili in the pathogenesis and host response to M. catarrhalis requires further study (171), although preliminary studies have already revealed that fimbriated bacteria bind more efficiently to lower bronchial epithelial cells than nonfimbriated bacteria do (201).
Capsule
The presence of a polysaccharide capsule has been previously suggested (7). Capsules are considered to be an important virulence factor in both gram-positive and gram-negative bacteria. Unlike the situation in many other bacterial pathogens, the capsule is not detectable when colonies of M. catarrhalis are examined on agar plates. More research is necessary to definitely demonstrate the presence of a capsule and to define its role, if any, in virulence.
VIRULENCE
In general, the pathogenicity and virulence of a microorganism are determined by its ability to avoid host defense mechanisms. Smith (215) recognizes five cardinal requirements for a bacterium to be virulent: (i) binding, colonization, and infection of mucous surfaces; (ii) entry into host tissues; (iii) multiplication in the in vivo environment; (iv) interference with host defense mechanisms; and (v) production of damage to the host. Relatively little is known about the precise virulence traits of M. catarrhalis. Below, information will be provided on bacterial adherence and models of infection, whereas complement resistance will be presented as an example for some of these general virulence features. It has to be emphasized, however, that a complete insight into the full virulence gene repertoire is still lacking. For example, it has been demonstrated on the basis of DNA hybridisation studies that M. catarrhalis harbors homologues of phase-variable H. influenzae virulence genes (189). The precise nature of these genes has yet to be elucidated, which implies that on the basis of relatively straightforward cloning experiments, several new virulence genes could well be identified in the near future.
Adherence
It is noteworthy that only a small number of studies on the precise interaction between M. catarrhalis receptors and human antigens have been undertaken. An elegant study was presented by Reddy et al. (198). Using a purified middle ear mucin glycoprotein, they showed that only the CD protein of M. catarrhalis was capable of establishing a specific interaction with the sialo version of the human protein. A follow-up study from the same laboratory revealed immense heterogeneity in the interaction between upper respiratory tract pathogens and human mucins (22). In this study, the CD protein of M. catarrhalis was shown to specifically attach to the mucin molecules from the nasopharynx and middle ear but not to mucin from the saliva and tracheobronchial mucin. Interactions such as these represent the first steps in the process of bacterial colonization and infection. The general mechanism of cellular adherence of M. catarrhalis to host cell surfaces has been studied by Rikitomi et al. (202). The presence or absence of fimbriae did not influence the capacity of the bacterium to adhere or to cause hemagglutination. Indeed, the mechanisms of binding appeared different for adherence and hemagglutination. Another study found no differences between the source of the isolate (blood or lungs) and hemagglutination (129). Furthermore, these investigators showed that attachment was not determined primarily by lectin-carbohydrate interactions. In contrast to the findings of these investigators, an in vitro adherence study with HEp-2 cell cultures demonstrated that strains derived from infections adhere more efficiently than do mere colonizers (94). In addition, data from this latter study on experimental periodate treatment suggested that bacterial adherence in this artificial system appears to be mediated by microbial carbohydrate moieties. Of interest, adherence of M. catarrhalis appeared to be stimulated by neutrophil defensins, peptides with broad-spectrum antimicrobial activity, released from activated neutrophils during inflammation, suggesting that defensin-mediated adherence contributes to persistence of infection, for instance in COPD patients (104).
A recent study by Ahmed et al. (6) investigated the influence of charge on adherence. Although bacteria and epithelial cells are both negatively charged, interaction between the negatively charged surface of M. catarrhalis cells and positively charged domains called microplicea on pharyngeal epithelial cells was found. More research, especially into the role of proteins like fibronectin, vitronectin, and plasminogen in adhesion, is needed. The contradictory nature of some of the current observations only strengthens this suggestion.
Animal Models
The low virulence of M. catarrhalis in laboratory animals has hampered protection experiments and pathogenicity studies in rats and mice. Although several studies have been conducted with different animal species, reports describing a reliable infection model are scarce. A reproducible, but rather artificial, model was presented by Lee et al. (J. C. Lee, J. C. Hamel, D. Staperd, and C. W. Ford, Abstr. 93rd Gen. Meet. Am. Soc. Microbiol., p. 50, abstr. B-137, 1993), who were able to isolate live M. catarrhalis from a specific mouse strain, C3H/HeN. Bacteria, suspended in brain heart infusion broth supplemented with 8% brewer’s yeast and 0.2% Tween 80, were inoculated via an intraperitoneal route. Infection resulted in high mortality and facilitated antibiotic efficacy studies. In another murine model (236), designed to study phagocytic responses and clearance mechanisms after endotracheal challenge with M. catarrhalis, a high influx of polymorphonuclear leukocytes into the lungs was noted. Bacteria were cleared from the lungs within 24 to 48 h, and the animals remained healthy. A deficiency in complement component C5 resulted in a minor delay in clearance. A similar and most frequently used animal model is a mouse model for the study of pulmonary clearance of M. catarrhalis (226). This model consisted of transoral inoculation of bacteria into the lungs under anesthesia and operative exposure of the trachea. Enumeration of viable bacteria in the lungs involved aseptic removal and homogenization of the lungs, followed by serial dilution and plating on agar media. This model permits an evaluation of the interaction of bacteria with lower respiratory tract epithelium and the precise assessment of pathologic changes in the lungs. As an example, using this model, MacIver et al. (151) obtained evidence that immunization with M. catarrhalis-derived outer membrane vesicles gives rise to a systemic IgG antibody response which is accompanied by enhanced clearance of M. catarrhalis from the lungs, Kyd et al. (143) used a model of mucosal immunization involving direct inoculation of killed bacteria into the Peyer’s patch followed by an intratracheal booster with dead M. catarrhalis. Enhanced clearance of bacteria from the lungs was observed, correlating with higher levels of specific IgA and IgG in serum and bronchoalveolar lavage fluid. A clear disadvantage of the above models is their complex and invasive nature, requiring operation techniques and general anesthesia. In addition, the clearance of M. catarrhalis from the lungs of mice is relatively rapid (within 6 to 24 h), most probably as a result of the low virulence of M. catarrhalis for laboratory animals. Moreover, since M. catarrhalis inhabits the upper respiratory tract, inhalation models are preferred over intraperitoneal, endotracheal, or transoral inoculation models. An initial report describing a putatively effective and reproducible inhalation model in mice was recently published. Moreover, passive and active immunization studies in this animal model documented improved pulmonary clearance of M. catarrhalis bacteria (120, 121).
Useful infection models in rats have been described only in the past 2 years. A purulent otitis media could be induced in Sprague-Dawley rats, for instance (248). This infection progressed in a relatively mild fashion, lasting for about 1 week. On immunization, a protection rate of 50% or more was induced. Using the same model, a clear increase in the density of goblet cells in the middle ear up to 60 days after inoculation of bacteria was found, suggesting a highly increased mucosal secretory capacity (50). In another rat model, inhalation of heat-killed M. catarrhalis cells clearly affected the laryngeal mucosa (125), resulting in a clinical syndrome reminiscent of laryngotracheitis in children. The studies mentioned above suggest that rats may provide an infection model that is more interesting than was previously thought.
Inoculation of M. catarrhalis into the middle ear of chinchillas and gerbils gave rise to effusion, but no live bacteria could be recovered from the middle ear after 24 h (77). Later studies, however, revealed the feasibility of studying otitis media in the chinchilla model. Although chinchillas are not generally available, PCR would not have reached it current state of applicability without the studies in these animals (10, 11, 193).
In conclusion, despite several drawbacks, mouse models of pulmonary clearance appear useful in studies of the effects of vaccination with several M. catarrhalis antigens on clearance of the bacteria. The rat models appear promising. Suitable animal models to study the pathogenicity of infection by M. catarrhalis in any detail are not yet available. This is primarily because rodents, the best accessible laboratory animals, tend to resist infection with this microorganism.
Complement Resistance
Complement resistance is considered an important virulence factor of many gram-negative bacteria, which may explain why gram-negative bacteria isolated from the blood are largely complement resistant; these strains are also especially successful in establishing animal models of infection (41, 203). In general, rough strains of gram-negative bacteria, producing LPS devoid of O-specific side chains, are highly susceptible to C5b-9-mediated killing whereas smooth strains, which synthesize complete LPS, are often complement resistant (221). Since the LPS of M. catarrhalis is of the rough type (96), it presumably does not play a major role in complement resistance. However, Zaleski et al. recently showed that inactivation of galE, a gene encoding a UDP-glucose-4-epimerase involved in the biosynthesis of the Galα1-4Galβ1-4Glc LOS epitope, results in enhanced susceptibility to serum-mediated killing (256). Apparently, deviant LOS structures render strains more susceptible to complement attack. The structural details facilitating these interactions are still unknown. Given that complement resistance is considered an important virulence factor of neisseriae (68, 127, 153, 199), the similarity between members of the neisseriae and M. catarrhalis makes complement resistance and the underlying mechanism an important subject for further study.
The clinical relevance of complement resistance was shown for a group of strains isolated from the sputa of elderly persons (167). Complement resistance can be considered a virulence factor of M. catarrhalis: the majority of strains (89%) isolated from lower respiratory tract infections are resistant to complement-mediated killing, whereas strains from the upper respiratory tract of children are mostly sensitive (58%) (117; Hol et al., Letter). Several other authors have tested M. catarrhalis strains for complement resistance (39, 51, 129, 216; R. E. Winn and S. L. Morse, Abstr. 84th Annu. Meet. Am. Soc. Microbiol., p. 28, 1984). Complement-resistant strains inhibit the terminal pathway of complement, i.e., formation of the membrane attack complex of complement (233). The binding of human vitronectin, an inhibitor of the terminal pathway of complement, appears to play a crucial role in complement resistance of M. catarrhalis (234). In Fig. 5 the binding of human vitronectin to complement-resistant and complement-sensitive strains of M. catarrhalis is shown. HMW-OMP, also known as ubiquitous surface protein A (composed of two separate proteins, UspA1 and UspA2), appears to play a major role (C. M. Verduin, H. J. Bootsma, C. Hol, A. Fleer, M. Jansze, K. L. Klingman, T. F. Murphy, and H. Van Dijk, Abstr. 95th Gen. Meet. Am. Soc. Microbiol., p. 189, abstr. B-137, 1995). Indeed, it was shown that vitronectin binds to UspA2 (163). Furthermore, a UspA2 mutant strain of M. catarrhalis was sensitive to complement-mediated killing, whereas the parent strain and an isogenic mutant with a mutation in UspA1 were resistant (3).
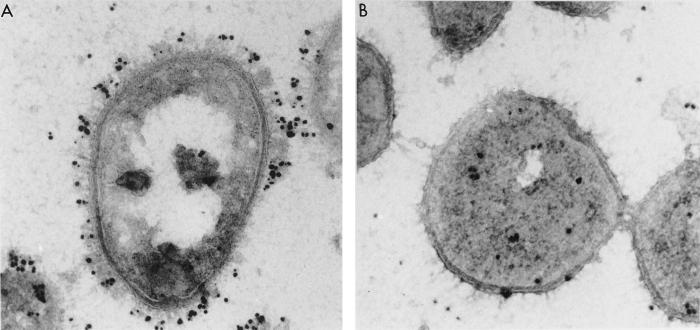
FIG. 5.
Electron micrographs of the binding of human vitronectin to complement-resistant (left) and complement-sensitive (right) strains of M. catarrhalis. Immunogold labeling using antibodies specific for human vitronectin revealed that this protein is effectively bound to the resistant cells whereas the sensitive strain fails to bind a significant quantity of the human matrix protein. Note that the vitronectin protein seems to be attached toward the boundaries of the cellular matrix, which may correlate with the protruded orientation of the vitronectin-binding ubiquitous surface proteins (UspA).
Another study (112) suggested that OMP CopB/OMP B2 is involved in the resistance of M. catarrhalis to killing by normal human serum. An isogenic mutant not expressing CopB was killed by normal human serum, whereas the wild-type parent strain survived. In addition, the researchers showed that the CopB- mutant strain was less able to survive in the lungs of mice (112). Recently, it has been shown that inactivation of the CopB-encoding gene inhibits iron acquisition from lactoferrin and transferrin (5), although this may be due to an indirect effect (29). In addition, CopB had significant homology to TonB-dependent OMPs, among which is the N. gonorrhoeae outer membrane protein FrpB. These proteins bind to and transport several ligands from the environment into the intracellular compartment of the bacterium. These functions are controlled by the TonB proteins, which are thought to be involved in energy transduction. Yet another OMP, OMP E, has also been shown to be involved in complement resistance. An M. catarrhalis OMP E knockout mutant showed a clear increase in serum sensitivity (173).
We conclude that complement resistance in M. catarrhalis probably is a highly multifactorial process, from the perspectives of both the host and the pathogen. Within the near future, additional bacterial genes involved in the defense against the complement system may be discovered, requiring a major research effort to integrate the individual contributions of all the different molecules into an overall mechanistic scheme.
IMMUNITY
M. catarrhalis-related immunology is a rather confusing area of the literature. M. catarrhalis infections are restricted to mucosal surfaces and are not systemic. Therefore, the correlation between systemic antibody responses and protection against this type of infections is not as straightforward as with systemic infections caused by other species of gram-negative bacteria. In addition, technical differences in the assays used in different studies may account for the lack of consistent results (90, 102). In general, surface structures of the bacterium are the main target for an antibody response, and the recognition of major targets for a protective antibody response is of clear importance for the development of an efficacious vaccine.
Many aspects of immunity to respiratory tract infections caused by M. catarrhalis are still unknown; they may include local factors as mucociliary clearance, aerodynamics, alveolar macrophage activity, complement-mediated killing, and surfactant activity. These factors play important roles in host defense against oropharyngeal pathogens (225). The development of an inflammatory response or specific antibody response may, however, augment these host defense mechanisms (225). As an example, in COPD patients, local host defense against respiratory pathogens is relatively poor, and although M. catarrhalis is not a normal inhabitant of the upper respiratory tract in adults (69; Ejlerten, Letter), infections caused by M. catarrhalis are frequent in these patients. This points to an important role for these local defense mechanisms in nonspecific clearance of this bacterium.
Bacterial clearance and phagocytic cell responses have been shown to differ among bacterial species. Streptococci, M. catarrhalis, and nontypeable H. influenzae appeared to be removed from the lungs of mice through various mechanisms (186). It was found that, compared to other species, M. catarrhalis was cleared relatively slowly from the lungs, and a more pronounced, 400-fold increase in numbers of polymorphonuclear leukocytes in the lungs was observed (186). In addition, it was shown that there was stimulation of adherence of M. catarrhalis by neutrophil defensins, peptides with broad-spectrum antimicrobial activitity that are released from activated neutrophils during inflammation, suggesting that defensin-mediated adherence contributes to persistence of infection, for instance in COPD patients (104).
Below we will summarize the literature covering the antibody responses of humans to whole bacteria and several different antigens of M. catarrhalis.
Antibody Responses to Whole Bacteria
Several authors have investigated antibody responses to M. catarrhalis in different patient cohorts. Chapman et al. (51) showed that 18 (90%) of 20 adult patients with lower respiratory tract infections due to M. catarrhalis, as defined by strict clinical criteria, had bactericidal antibodies in their convalescent-phase sera whereas only (37%) of 19 had bactericidal antibody present in their acute-phase sera. Black and Wilson (25) obtained essentially the same results in a larger-scale study dealing with IgG antibodies in acute- and convalescent-phase and control sera from adults with bronchopulmonary disease. Likewise, an enzyme-linked immunosorbent assay study showed that 10 of 19 children with AOM to M. catarrhalis had an increase in serum IgG antibody titers to the bacterium (146). Comparable results were found in the study of Faden et al. (90): 8 of 14 young children (younger than 2 years) with otitis media (57%) showed a rise in the levels of serum antibody to their own M. catarrhalis isolate (90). In a recent study of infants with otitis media, a specific IgG response (mainly IgG1 and IgG3) was detected in 10 of 12 children aged 8 months or older compared to 1 of 6 younger children. In addition, immunoblotting revealed four immunodominant OMPs, UspA, CopB, TbpB, and a protein of 60 kDa, probably OMP CD (158).
Antibodies to M. catarrhalis are very low or absent in children younger than 1 year, and the development of an antibody response in children, especially of the IgG3 subclass, correlates with a decrease in colonization. In addition, antibodies to OMPs of M. catarrhalis, mainly of the IgG3 subclass, appear around the age of 4 years (56, 102). Furthermore, failure to produce significant levels of IgG3 antibodies against M. catarrhalis predisposes to infection with the bacterium (100). In addition, all adults appeared to have antibodies to M. catarrhalis (56, 102). Data gathered during studies focusing on single-protein responses indicate that normal children and adults develop a systemic, M. catarrhalis-specific IgG response that may be protective. Again, this may in part explain the differences between children and adults when the colonization rate is considered (9, 52, 56, 89, 102; Ejlersten, Letter).
Lipooligosaccharide Immunogenicity
With regard to LOS, it was reported that antibody responses to these surface structures constitute a major part of the humoral immune response during infection with M. catarrhalis. This antibody response is not serotype specific but is directed to common epitopes of the LOS of different M. catarrhalis serotypes (185, 197). Hence, M. catarrhalis LOS may be of interest for evaluation as a possible vaccine candidate. The usefulness of the LOS surface structures for the development of a vaccine requires more knowledge about the role of these structures in the pathogenesis of disease and the accompanying immune response, although preliminary results are already promising (105, 120).
Immunogenicity of Outer Membrane Proteins
Much research nowadays is focused on the identification and characterization of OMPs of M. catarrhalis as suitable vaccine candidates. OMP B1, CopB/OMP B2, LbpB, OMP CD, OMP E, OMP G, TbpB, and UspA have all been mentioned as potential vaccine candidates (53, 111, 158, 168, 172, 173, 175, 212, 253, 255). In contrast, no antibody response to TbpA or LbpA could be detected in convalescent-phase sera from patients with pulmonary infections, limiting their role as vaccine candidates (255). Hansen and coworkers showed that CopB/OMP B2 is a target for antibodies that increase pulmonary clearance in the mouse (111). Another study has demonstrated that CopB is essentially well conserved and that most strains react with CopB-specific monoclonal antibodies (212). However, certain regions in the protein show interstrain variability; therefore, if this protein is to be developed into a candidate vaccine, only its conserved regions should be targeted. Sethi et al. (211) found a predominant antibody response to a minor 84-kDa OMP, designated OMP B1, OMP CD does not appear to be an immunodominant antigen, as indicated by the fact that there is an absence of a new antibody response to this protein after exacerbation of M. catarrhalis infection in COPD patients. Furthermore, the high degree of sequence conservation suggests that there is no immune selective pressure. Still, the purified antigen could be a promising vaccine candidate (174, 253). Helminen et al. (111) also presented evidence that UspA may be a target for protective antibodies in humans. Chen et al. (53) immunized mice with purified UspA and subsequently challenged these mice intratracheally with M. catarrhalis. Six hours after challenge, approximately 50% fewer bacteria were isolated from the lungs of the immunized mice than from the lungs of the nonimmunized control mice. In addition, antibodies induced complement-dependent bacterial killing of heterologous M. catarrhalis strains (53). The finding that IgG3 is a major contributing factor in the immune response to M. catarrhalis was confirmed by a study by Chen et al. of the immune response of healthy adults and children to UspA1 and UspA2 (52). In a small cohort of children suffering from otitis media, antibodies specific for UspA1 and UspA2 could be identified (206). The amounts of these specific antibodies varied strongly with age (205). IgG antibody titers to UspA were low during the first 2 years of life and reached a maximum only during adulthood, whereas no specific IgA to UspA could be detected in nasopharyngeal secretions of young children. Considering the age-dependent differences in antibody prevalence, the question on whether to vaccinate against M. catarrhalis remains relevant.
Local Antibody Response
Only a few investigators have studied the development of antibody responses in the middle ear fluid of children with otitis media (89, 90, 133, 220). IgG and IgA appeared to be produced locally in the majority of patients, but antibodies derived from serum were also detected in middle ear fluids of patients with otitis media. Faden et al. (90) showed that middle ear fluid IgG, IgM, or IgA antibody was produced in 100, 29, and 71% of the children, respectively. Of interest, many children with local antibodies in their middle ear fluid did not develop a systemic antibody response. Local antibodies may play an important role in the recovery from and prevention of AOM (102).
In a study focusing on local IgA antibodies to UspA in the nasopharyngeal secretions of children colonized by M. catarrhalis, no response was detected (205).
Since M. catarrhalis is a primarily mucosal pathogen, more detailed studies of local immune responses are urgently needed, and the role of IgA antibodies in resistance to M. catarrhalis infection clearly needs more attention.
Vaccines
The development of vaccines for the prevention of M. catarrhalis-mediated disease is currently a hot topic. The most promising vaccine candidates have recently been reviewed by McMichael (162). Most of the molecules that have raised people’s hopes have been described in the previous section of this review and need not be reiterated here. However, the combination of data that is available in today’s literature suggests that the development of an M. catarrhalis vaccine is well under way: animal models of infection have been developed and described, and several vaccine candidate molecules have been studied with respect to prevalence and genetic conservation among different isolates. Although new candidate molecules are regularly brought forward (93), relatively little or nothing is known about the optimal routes for vaccine delivery or whether there is a need for adjuvants. A recent study, using a rat model, suggests that the mucosal route of delivery for M. catarrhalis is more effective than systemic immunization (142). However, no decisive data are available. It will probably still take more than a decade before the first vaccines for genuine clinical use will become available.
CONCLUDING REMARKS
It has become evident over the past decades that M. catarrhalis has significant pathogenic potential. Classical antibiotic treatment alleviates the clinical burden, but in the end, only effective vaccination may prevent the development of disease. For example, AOM is a major cause of morbidity in early childhood and is responsible for an estimated 25 million physician visits and $3.8 billion of medical expenditures annually. Since M. catarrhalis is one of the three major pathogens in AOM, vaccination could constitute a significant and cost-effective health benefit. Many potential vaccination strategies have been suggested over the years, but clinical trials have not yet been conducted. Effective immunity seems to be acquired during the first 10 years of life. The group of children that suffers from recurrent otitis media caused by M. catarrhalis may be problematic; for these patients, vaccination may in the end provide an important relief. For the future, identification of important physiological response regulators may be helpful in the identification of novel therapeutic targets (164). In addition, M. catarrhalis genomics should be strengthened: knowledge of the whole genome sequence, preferably for both complement-resistant and complement-susceptible isolates, should be instrumental in the recognition of immunologically relevant genes and regulators. At the time of writing this review, only a gross genetic map is available (181). The genome sequence itself is still eagerly awaited.
In conclusion, over the past two decades M. catarrhalis has evolved from an emerging to a well-established pathogen. Indeed, β-lactamase-producing isolates appear to be widespread, and this may play an important role in the therapy of infections, particularly in the treatment of mixed infections. If novel, specific, and effective modes of disease treatment or prevention (including vaccination) are to be developed for M. catarrhalis infections, then further research into the fundamental nature of M. catarrhalis pathogenicity will be required.
Acknowledgments
We acknowledge John Hays for reading and improving our manuscript. Margriet Jansze, Marly Kools-Sijmons, Cindy van der Schee, and Henri Verbrugh are thanked for their continuous support in both thought and action.
REFERENCES
- 1.Abbott, M. 1992. Neisseriaceae and Moraxella sp.: the role of related microorganisms associated with conjunctivitis in the newborn. Int. J. STD AIDS 3:212–213. [DOI] [PubMed] [Google Scholar]
- 2.Abuhammour, W. M., N. M. Abdel-Haq, B. I. Asmar, and A. S. Dajani. 1999. Moraxella catarrhalis bacteremia: a 10-year experience. South. Med. J. 92:1071–1074. [DOI] [PubMed] [Google Scholar]
- 3.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. L. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun. 66:3113–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aebi, Ć., I. Maciver, J. L. Latimer, L. D. Cope, M. K. Stevens, S. E. Thomas, G. H. McCracken, Jr., and E. J. Hansen. 1997. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun. 65:4367–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aebi, C., B. Stone, M. Beucher, L. D. Cope, I. Maciver, S. E. Thomas, G. H. McCracken, Jr., P. F. Sparling, and E. J. Hansen. 1996. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect. Immun. 64:2024–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed, K., T. Nakagawa, Y. Nakano, G. Martinez, A. Ichinose, C. H. Zheng, M. Akaki, M. Aikawa, and T. Nagatake. 2000. Attachment of Moraxella catarrhalis occurs to the positively charged domains of pharyngeal epithelial cells. Microb. Pathog. 28:203–209. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed, K., N. Rikitomi, A. Ichinose, and K. Matsumoto. 1991. Possible presence of a capsule in Branhamella catarrhalis. Microbiol. Immunol. 35:361–366. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. 1994. Otitis media bacteriology and immunology. Pediatr. Infect. Dis. J. 13(Suppl.):20–22. [Google Scholar]
- 9.Arkwright, J. A. 1907. On the occurence of the Micrococcus catarrhalis in normal and catarrhal noses and its differentiation from the other Gram-negative diplococci. J. Hyg. 7:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aul, J. J., K. W. Anderson, R. M. Wadowsky, W. J. Doyle, L. A. Kingsley, J. C. Post, and G. D. Ehrlich. 1998. Comparative evaluation of culture and PCR for the detection and determination of persistence of bacterial strains and DNAs in the Chinchilla laniger model of otitis media. Ann. Otol. Rhinol. Laryngol. 107:508–513. [DOI] [PubMed] [Google Scholar]
- 11.Bakaletz, L. O., G. J. White, J. C. Post, and G. D. Ehrlich. 1998. Blinded multiplex PCR analyses of middle ear and nasopharyngeal fluids from chinchilla models of single- and mixed-pathogen-induced otitis media. Clin. Diagn. Lab. Immunol. 5:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barreiro, B., L. Esteban, E. Prats, E. Verdaguer, J. Dorca, and F. Manresa. 1992. Branhamella catarrhalis respiratory infections. Eur. Respir. J. 5:675–679. [PubMed] [Google Scholar]
- 13.Bartos, L. C., and T. F. Murphy. 1988. Comparison of the outer membrane proteins of 50 strains of Branhamella catarrhalis. J. Infect. Dis. 158:761–765. [DOI] [PubMed] [Google Scholar]
- 14.Beaulieu, D., S. Scriver, M. G. Bergeron, D. E. Low, T. R. Parr, Jr., J. E. Patterson, A. Matlow, and P. H. Roy. 1993. Epidemiological typing of Moraxella catarrhalis by using DNA probes. J. Clin. Microbiol. 31:736–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg, R. A., and D. L. Bartley. 1987. Pneumonia associated with Branhamella catarrhalis in infants. Pediatr. Infect. Dis. J. 6:569–573. [DOI] [PubMed] [Google Scholar]
- 16.Berger, U. 1963. Die anspruchslosen Neisserien. Ergeb. Microbiol. Immunitaetsforsch. Exp. Ther. 36:97–167. [Google Scholar]
- 17.Reference deleted.
- 18.Berk, S. L., and J. H. Kalbfleisch. 1996. Antibiotic susceptibility patterns of community-acquired respiratory isolates of Moraxella catarrhalis in western Europe and in the USA. The Alexander Project Collaborative Group. J. Antimicrob. Chemother. 38(Suppl. A):85–96. [DOI] [PubMed] [Google Scholar]
- 19.Berk, S. L., and A. Verghese. 1989. Emerging pathogens in nosocomial pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 8:11–14. [DOI] [PubMed] [Google Scholar]
- 20.Berman, S. 1995. Otitis media in children. N. Engl. J. Med. 332:1560–1565. [DOI] [PubMed] [Google Scholar]
- 21.Berner, R., R. F. Schumacher, M. Brandis, and J. Forster. 1996. Colonization and infection with Moraxella catarrhalis in childhood. Eur. J. Clin. Microbiol. Infect. Dis. 15:506–509. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein, J. M., and M. Reddy. 2000. Bacteria-mucin interaction in the upper aerodigestive tract shows striking heterogeneity: implications in otitis media, rhinosinusitis, and pneumonia. Otolaryngol. Head Neck Surg. 122:514–520. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein, T., R. Brilli, and B. Jacobs. 1998. Is bacterial tracheitis changing? A 14-month experience in a pediatric intensive care unit. Clin. Infect. Dis. 27:458–462. [DOI] [PubMed] [Google Scholar]
- 24.Bhushan, R., C. Kirkham, S. Sethi, and T. F. Murphy. 1997. Antigenic characterization and analysis of the human immune response to outer membrane protein E of Branhamella catarrhalis. Infect. Immun. 65:2668–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black, A. J., and T. S. Wilson. 1988. Immunoglobulin G (IgG) serological response to Branhamella catarrhalis in patients with acute bronchopulmonary infections. J. Clin. Pathol. 41:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block, S. L. 1997. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr. Infect. Dis. J. 16:449–456. [DOI] [PubMed] [Google Scholar]
- 27.Bluestone, C. D. 1986. Otitis media and sinusitis in children. Role of Branhamella catarrhalis. Drugs 31(Suppl. 3):132–141. [DOI] [PubMed] [Google Scholar]
- 28.Blumer, J. 1998. Clinical perspectives on sinusitis and otitis media. Pediatr. Infect. Dis. J. 17(8 Suppl.):S68–S72. [DOI] [PubMed] [Google Scholar]
- 29.Bonnah, R. A., H. Wong, S. M. Loosmore, and A. B. Schryvers. 1999. Characterization of Moraxella (Branhamella) catarrhalis 1bpB, 1bpA, and lactoferrin receptor orf3 isogenic mutants. Infect. Immun. 67:1517–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnah, R. A., R. Yu, and A. B. Schryvers. 1995. Biochemical analysis of lactoferrin receptors in the Neisseriaceae: identification of a second bacterial lactoferrin receptor protein. Microb. Pathog. 19:285–297. [DOI] [PubMed] [Google Scholar]
- 31.Bootsma, H. J., P. C. Aerts, G. Posthuma, T. Harmsen, J. Verhoef, H. van Dijk, and F. R. Mooi. 1999. Moraxella (Branhamella) catarrhalis BRO beta-lactamase: a lipoprotein of gram-positive origin? J. Bacteriol. 181:5090–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bootsma, H. J., H. G. van der Heide, S. van de Pas, L. M. Schouls, and F. R. Mooi. 2000. Analysis of Moraxella catarrhalis by DNA typing: evidence for a distinct subpopulation associated with virulence traits. J. Infect. Dis. 181:1376–1387. [DOI] [PubMed] [Google Scholar]
- 33.Bootsma, H. J., H. van Dijk, J. Verhoef, A. Fleer, and F. R. Mooi. 1996. Molecular characterization of the BRO beta-lactamase of Moraxella (Branhamella) catarrhalis. Antimicrob. Agents Chemother. 40:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bovre, K. 1984. The Genus Moraxella, p.296–303. In N. R. Krieg and J. G. Holt (ed.), Bergey’s manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, Md. [Google Scholar]
- 35.Boyle, F. M., P. R. Georghiou, M. H. Tilse, and J. G. McCormack. 1991. Branhamella (Moraxella) catarrhalis: pathogenic significance in respiratory infections. Med. J. Aust. 154:592–596. [DOI] [PubMed] [Google Scholar]
- 36.Brook, I. 1997. Aerobic and anaerobic microbiology of bacterial tracheitis in children. Pediatr. Emerg. Care 13:16–18. [DOI] [PubMed] [Google Scholar]
- 37.Brook, I. 1986. Direct and indirect pathogenicity of Branhamella catarrhalis. Drugs 31(Suppl. 3):97–102. [DOI] [PubMed] [Google Scholar]
- 38.Brook, I. 1991. In vitro susceptibility vs. in vivo efficacy of various antimicrobial agents against the Bacteroides fragilis group. Rev. Infect. Dis. 13:1170–1180. [DOI] [PubMed] [Google Scholar]
- 39.Brorson, J. E., A. Axelsson, and S. E. Holm. 1976. Studies on Branhamella catarrhalis (Neisseria catarrhalis) with special reference to maxillary sinusitis. Scand. J. Infect. Dis. 8:151–155. [DOI] [PubMed] [Google Scholar]
- 40.Brorson, J. E., and B. E. Malmvall. 1981. Branhamella catarrhalis and other bacteria in the nasopharynx of children with longstanding cough. Scand. J. Infect. Dis. 13:111–113. [DOI] [PubMed] [Google Scholar]
- 41.Brown, E. J., K. A. Joiner, and M. M. Frank. 1983. The role of complement in host resistance to bacteria. Springer Semin. Immunopathol. 6:349–360. [DOI] [PubMed] [Google Scholar]
- 42.Budhani, R. K., and J. K. Struthers. 1998. Interaction of Streptococcus pneumoniae and Moraxella catarrhalis: investigation of the indirect pathogenic role of beta-lactamase-producing moraxellae by use of a continuous-culture biofilm system. Antimicrob. Agents Chemother. 42:2521–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabello, H., A. Torres, R. Celis, M. El-Ebiary, J. Puig de la Bellacasa, A. Xaubet, J. Gonzalez, C. Agusti, and N. Soler. 1997. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur. Respir. J. 10:1137–1144. [DOI] [PubMed] [Google Scholar]
- 44.Calder, M. A., M. J. Croughan, D. T. McLeod, and F. Ahmad. 1986. The incidence and antibiotic susceptibility of Branhamella catarrhalis in respiratory infections. Drugs 31(Suppl. 3):11–16. [DOI] [PubMed] [Google Scholar]
- 45.Campagnari, A. A., T. F. Ducey, and C. A. Rebmann. 1996. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect. Immun. 64:3920–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cappelletty, D. 1998. Microbiology of bacterial respiratory infections. Pediatr. Infect. Dis. J. 17(8 Suppl.):S55–S61. [DOI] [PubMed] [Google Scholar]
- 47.Catlin, B. W. 1991. Branhamaceae fam. nov., a proposed family to accommodate the genera Branhamella and Moraxella. Int. J. Syst. Bacteriol. 41:320–323. [Google Scholar]
- 48.Catlin, B. W. 1990. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin. Microbiol. Rev. 3:293–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catlin, B. W. 1970. Transfer of the organism named Neisseria catarrhalis to Branhamella gen. nov. Int. J. Syst. Bacteriol. 20:155–159. [Google Scholar]
- 50.Caye-Thomasen, P., A. Hermansson, M. Tos, and K. Prellner. 2000. Middle ear secretory capacity after acute otitis media caused by Streptococcus pneumoniae, Moraxella catarrhalis, non-typeable or type B Haemophilus influenzae. A comparative analysis based on goblet cell density. Acta Otolaryngol. Suppl. 543:54–55. [PubMed] [Google Scholar]
- 51.Chapman, A. J., Jr., D. M. Musher, S. Jonsson, J. E. Clarridge, and R. J. Wallace, Jr. 1985. Development of bactericidal antibody during Branhamella catarrhalis infection. J. Infect. Dis. 151:878–882. [DOI] [PubMed] [Google Scholar]
- 52.Chen, D., V. Barniak, K. R. VanDerMeid, and J. C. McMichael. 1999. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect. Immun. 67:1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen, D., J. C. McMichael, K. R. VanDerMeid, D. Hahn, T. Mininni, J. Cowell, and J. Eldridge. 1996. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect. Immun. 64(6):1900–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen, D., J. C. McMichael, K. R. VanDerMeid, A. W. Masi, E. Bortell, J. D. Caplan, D. N. Chakravarti, and V. L. Barniak. 1999. Evaluation of a 74-kDa transferrin-binding protein from Moraxella (Branhamella) catarrhalis as a vaccine candidate. Vaccine 18:109–118. [DOI] [PubMed] [Google Scholar]
- 55.Cherry, J. D., and J. P. Dudley. 1992. Sinusitis, p.142–148. In R. D. Feigin and J. D. Cherry (ed.), Textbook of pediatric infectious diseases, 3rd ed., vol. 1. The W. B. Saunders Co., Philadelphia, Pa. [Google Scholar]
- 56.Christensen, J. J. 1999. Moraxella (Branhamella) catarrhalis: clinical, microbiological and immunological features in lower respiratory tract infections. APMIS Suppl. 88:1–36. [PubMed] [Google Scholar]
- 57.Christensen, J. J., J. Ursing, and B. Bruun. 1994. Genotypic and phenotypic relatedness of 80 strains of Branhamella catarrhalis of worldwide origin. FEMS Microbiol Lett. 119:155–159. [DOI] [PubMed] [Google Scholar]
- 58.Cohen, R. 1997. The antibiotic treatment of acute otitis media and sinusitis in children. Diagn. Microbiol. Infect. Dis. 27:35–39. [DOI] [PubMed] [Google Scholar]
- 59.Collazos, J., J. de Miguel, and R. Ayarza. 1992. Moraxella catarrhalis bacteremic pneumonia in adults: two cases and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 11:237–240. [DOI] [PubMed] [Google Scholar]
- 60.Cook, P. P., D. W. Hecht, and D. R. Snydman. 1989. Nosocomial Branhamella catarrhalis in a paediatric intensive care unit: risk factors for disease. J. Hosp. Infect. 13:299–307. [DOI] [PubMed] [Google Scholar]
- 61.Cope, L. D., E. R. Lafontaine, C. A. Slaughter, C. A. Hasemann, Jr., C. Aebi, F. W. Henderson, G. H. McCracken, Jr., and E. J. Hansen. 1999. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J. Bacteriol. 181:4026–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dabernat, H., J. L. Avril, and Y. Boussougant. 1990. In-vitro activity of cefpodoxime against pathogens responsible for community-acquired respiratory tract infections. J Antimicrob Chemother. 26(Suppl. E):1–6. [DOI] [PubMed] [Google Scholar]
- 63.Daoud, A., F. Abuekteish, and H. Masaadeh. 1996. Neonatal meningitis due to Moraxella catarrhalis and review of the literature. Ann. Trop. Paediatr. 16:199–201. [DOI] [PubMed] [Google Scholar]
- 64.Davies, B. I., and F. P. Maesen. 1988. The epidemiology of respiratory tract pathogens in southern Netherlands. Eur. Respir. J. 1:415–420. [PubMed] [Google Scholar]
- 65.Reference deleted.
- 66.Denamur, E., N. Picard-Pasquier, C. Mura, B. Picard, J. Orfila, and R. Krishnamoorthy. 1991. Comparison of molecular epidemiological tools for Branhamella catarrhalis typing. Res. Microbiol. 142:585–589. [DOI] [PubMed] [Google Scholar]
- 67.Denamur, E., G. Suermondt, A. Debroca, C. Defouilloy, G. Laurans, J. F. Muir, and J. Orfila. 1989. Nosocomial pulmonary infections caused by Branhamella catarrhalis in intensive care units. Agressologie 30:251–253. [PubMed] [Google Scholar]
- 68.Densen, P. 1989. Interaction of complement with Neisseria meningitidis and Neisseria gonorrhoeae. Clin. Microbiol Rev. 2(Suppl.):S11–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DiGiovanni, C., T. V. Riley, G. F. Hoyne, R. Yeo, and P. Cooksey. 1987. Respiratory tract infections due to Branhamella catarrhalis: epidemiological data from Western Australia. Epidemiol. Infect. 99:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dingman, J. R., M. G. Rayner, S. Mishra, Y. Zhang, M. D. Ehrlich, J. C. Post, and G. D. Ehrlich. 1998. Correlation between presence of viable bacteria and presence of endotoxin in middle-ear effusions. J. Clin. Microbiol. 36:3417–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doern, G. V. 1990. Branhamella catarrhalis: phenotypic characteristics. Am. J. Med. 88:33S–35S. [DOI] [PubMed] [Google Scholar]
- 72.Doern, G. V. 1986. Branhamella catarrhalis—an emerging human pathogen. Diagn. Microbiol. Infect. Dis. 4:191–201. [DOI] [PubMed] [Google Scholar]
- 73.Doern, G. V., A. B. Brueggemann, G. Pierce, T. Hogan, H. P. Holley, Jr., and A. Rauch. 1996. Prevalence of antimicrobial resistance among 723 outpatient clinical isolates of Moraxella catarrhalis in the United States in 1994 and 1995: results of a 30-center national surveillance study. Antimicrob. Agents Chemother. 40:2884–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doern, G. V., R. N. Jones, M. A. Pfaller, and K. Kugler. 1999. Haemophilus influenzae and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY antimicrobial Surveillance Program (United States and Canada, 1997). Antimicrob. Agents Chemother. 43:385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doern, G. V., M. J. Miller, and R. E. Winn. 1981. Branhamella (Neisseria) catarrhalis systemic disease in humans. Case reports and review of the literature. Arch. Intern. Med. 141:1690–1692. [PubMed] [Google Scholar]
- 76.Doern, G. V., and S. A. Morse. 1980. Branhamella (Neisseria) catarrhalis: criteria for laboratory identification. J. Clin. Microbiol. 11:193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doyle, W. J. 1989. Animal models of otitis media: other pathogens. Pediatr. Infect. Dis. J. 8(1 Suppl.):S45–S47. [PubMed] [Google Scholar]
- 78.Du, R. P., Q. Wang, Y. P. Yang, A. B. Schryvers, P. Chong, M. H. Klein, and S. M. Loosmore. 1998. Cloning and expression of the Moraxella catarrhalis lactoferrin receptor genes. Infect. Immun. 66:3656–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edebrink, P., P. E. Jansson, G. Widmalm, T. Holme, and M. Rahman. 1996. The structures of oligosaccharides isolated from the lipopolysaccharide of Moraxella catarrhalis serotype B, strain CCUG 3292. Carbohydr. Res. 295:127–146. [DOI] [PubMed] [Google Scholar]
- 80.Reference deleted.
- 81.Ejlertsen, T., E. Thisted, F. Ebbesen, B. Olesen, and J. Renneberg. 1994. Branhamella catarrhalis in children and adults. A study of prevalence, time of colonisation, and association with upper and lower respiratory tract infections. J. Infect. 29:23–31. [DOI] [PubMed] [Google Scholar]
- 82.Ejlertsen, T., E. Thisted, P. A. Ostergaard, and J. Renneberg. 1994. Maternal antibodies and acquired serological response to Moraxella catarrhalis in children determined by an enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 1:464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eller, J., A. Ede, T. Schaberg, M. S. Niederman, H. Mauch, and H. Lode. 1998. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest 113:1542–1548. [DOI] [PubMed] [Google Scholar]
- 84.Enright, M. C., P. E. Carter, I. A. MacLean, and H. McKenzie. 1994. Phylogenetic relationships between some members of the genera Neisseria, Acinetobacter, Moraxella, and Kingella based on partial 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 44:387–391. [DOI] [PubMed] [Google Scholar]
- 85.Enright, M. C., and H. McKenzie. 1997. Moraxella (Branhamella) catarrhalis—clinical and molecular aspects of a rediscovered pathogen. J. Med. Microbiol. 46:360–371. [DOI] [PubMed] [Google Scholar]
- 86.Ernst, T. N., and M. Philp. 1987. Bacterial tracheitis caused by Branhamella catarrhalis. Pediatr. Infect. Dis. J. 6:574. [DOI] [PubMed] [Google Scholar]
- 87.Faden, H., J. Bernstein, L. Brodsky, J. Stanievich, and P. L. Ogra. 1992. Effect of prior antibiotic treatment on middle ear disease in children. Ann. Otol. Rhinol. Laryngol. 101:87–91. [DOI] [PubMed] [Google Scholar]
- 88.Faden, H., L. Duffy, R. Wasielewski, J. Wolf, D. Krystofik, and Y. Tung. 1997. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J. Infect. Dis. 175:1440–1445. [DOI] [PubMed] [Google Scholar]
- 89.Faden, H., Y. Harabuchi, and J. J. Hong. 1994. Epidemiology of Moraxella catarrhalis in children during the first 2 years of life: relationship to otitis media. J. Infect. Dis. 169:1312–1317. [DOI] [PubMed] [Google Scholar]
- 90.Faden, H., J. Hong, and T. Murphy. 1992. Immune response to outer membrane antigens of Moraxella catarrhalis in children with otitis media. Infect. Immun. 60:3824–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faden, H., J. J. Hong, and N. Pahade. 1994. Immune response to Moraxella catarrhalis in children with otitis media: opsonophagocytosis with antigen-coated latex beads. Ann. Otol. Rhinol. Laryngol. 103:522–524. [DOI] [PubMed] [Google Scholar]
- 92.Felmingham, D., and R. N. Gruneberg. 2000. The Alexander Project 1996–1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J. Antimicrob. Chemother. 45:191–203. [DOI] [PubMed] [Google Scholar]
- 93.Fitzgerald, M., R. Mulcahy, S. Murphy, C. Keane, D. Coakley, and T. Scott. 1999. Transmission electron microscopy studies of Moraxella (Branhamella) catarrhalis. FEMS Immunol. Med. Microbiol. 23:57–66. [DOI] [PubMed] [Google Scholar]
- 94.Fitzgerald, M., S. Murphy, R. Mulcahy, C. Keane, D. Coakley, and T. Scott. 1999. Tissue culture adherence and haemagglutination characteristics of Moraxella (Branhamella) catarrhalis. FEMS Immunol. Med. Microbiol. 24:105–114. [DOI] [PubMed] [Google Scholar]
- 95.Fluit, A. C., F. J. Schmitz, M. E. Jones, J. Acar, R. Gupta, and J. Verhoef. 1999. Antimicrobial resistance among community-acquired pneumonia isolates in Europe: first results from the SENTRY antimicrobial surveillance program 1997. SENTRY Participants Group. Int. J. Infect. Dis. 3:153–156. [DOI] [PubMed] [Google Scholar]
- 96.Fomsgaard, J. S., A. Fomsgaard, N. Hoiby, B. Bruun, and C. Galanos. 1991. Comparative immunochemistry of lipopolysaccharides from Branhamella catarrhalis strains. Infect. Immun. 59:3346–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Froom, J., L. Culpepper, M. Jacobs, R. A. DeMelker, L. A. Green, L. van Buchem, P. Grob, and T. Heeren. 1997. Antimicrobials for acute otitis media? A review from the International Primary Care Network. Br. Med. J. 315:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frosch, P., and W. Kolle. 1896. Die Mikrokokken, p.154–155. In C. Flugge (ed.), Die Mikroorganism, 3rd ed., vol. 2. Verlag von Vogel, Leipzig, Germany. [Google Scholar]
- 99.Fung, C. P., M. Powell, A. Seymour, M. Yuan, and J. D. Williams. 1992. The antimicrobial susceptibility of Moraxella catarrhalis isolated in England and Scotland in 1991. J. Antimicrob. Chemother. 30:47–55. [DOI] [PubMed] [Google Scholar]
- 100.Goldblatt, D., G. K. Scadding, V. J. Lund, A. M. Wade, M. W. Turner, and J. P. Pandey. 1994. Association of Gm allotypes with the antibody response to the outer membrane proteins of a common upper respiratory tract organism, Moraxella catarrhalis. J. Immunol. 153:5316–5320. [PubMed] [Google Scholar]
- 101.Goldblatt, D., N. D. Seymour, R. J. Levinsky, and M. W. Turner. 1990. An enzyme-linked immunosorbent assay for the determination of human IgG subclass antibodies directed against Branhamella catarrhalis. J. Immunol. Methods 128:219–225. [DOI] [PubMed] [Google Scholar]
- 102.Goldblatt, D., M. W. Turner, and R. J. Levinsky. 1990. Branhamella catarrhalis: antigenic determinants and the development of the IgG subclass response in childhood. J. Infect. Dis. 162:1128–1135. [DOI] [PubMed] [Google Scholar]
- 103.Goldenhersh, M. J., G. S. Rachelefsky, J. Dudley, J. Brill, R. M. Katz, A. S. Rohr, S. L. Spector, S. C. Siegel, P. Summanen, E. J. Baron, et al. 1990. The microbiology of chronic sinus disease in children with respiratory allergy. J. Allergy Clin. Immunol. 85:1030–1039. [DOI] [PubMed] [Google Scholar]
- 104.Gorter, A. D., P. S. Hiemstra, S. de Bentzmann, S. van Wetering, J. Dankert, and L. van Alphen. 2000. Stimulation of bacterial adherence by neutrophil defensins varies among bacterial species but not among host cell types. FEMS Immunol. Med. Microbiol. 28:105–111. [DOI] [PubMed] [Google Scholar]
- 105.Gu, X. X., J. Chen, S. J. Barenkamp, J. B. Robbins, C. M. Tsai, D. J. Lim, and J. Battey. 1998. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect. Immun. 66:1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gupta, R. S. 2000. The phylogeny of proteobacteria: relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol. Rev. 24:367–402. [DOI] [PubMed] [Google Scholar]
- 107.Haddad, J., A. Le Faou, U. Simeoni, and J. Messer. 1986. Hospital-acquired bronchopulmonary infection in premature infants due to Branhamella catarrhalis. J. Hosp. Infect. 7:301–302. [DOI] [PubMed] [Google Scholar]
- 108.Hager, H., A. Verghese, S. Alvarez, and S. L. Berk. 1987. Branhamella catarrhalis respiratory infections. Rev. Infect. Dis. 9:1140–1149. [DOI] [PubMed] [Google Scholar]
- 109.Hart, V. K. 1927. The bacteriology of acute ears. Laryngoscope 37:56–61. [Google Scholar]
- 110.Hellio, R., M. Guibourdenche, E. Collatz, and J. Y. Riou. 1988. The envelope structure of Branhamella catarrhalis as studied by transmission electron microscopy. Ann. Inst. Pasteur Microbiol. 139:515–525. [DOI] [PubMed] [Google Scholar]
- 111.Helminen, M. E., I. Maciver, J. L. Latimer, J. Klesney-Tait, L. D. Cope, M. Paris, G. H. McCracken, Jr., and E. J. Hansen. 1994. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J. Infect. Dis. 170:867–872. [DOI] [PubMed] [Google Scholar]
- 112.Helminen, M. E., I. Maciver, M. Paris, J. L. Latimer, S. L. Lumbley, L. D. Cope, G. H. McCracken, Jr., and E. J. Hansen. 1993. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival in vivo. J. Infect. Dis. 168:1194–1201. [DOI] [PubMed] [Google Scholar]
- 113.Hendolin, P. H., A. Markkanen, J. Ylikoski, and J. J. Wahlfors. 1997. Use of multiplex PCR for simultaneous detection of four bacterial species in middle ear effusions. J. Clin. Microbiol. 35:2854–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hol, C., E. E. Van Dijke, C. M. Verduin, J. Verhoef, and H. van Dijk. 1994. Experimental evidence for Moraxella-induced penicillin neutralization in pneumococcal pneumonia. J. Infect. Dis. 170:1613–1616. [DOI] [PubMed] [Google Scholar]
- 116.Reference deleted.
- 117.Hol, C., C. M. Verduin, E. E. Van Dijke, J. Verhoef, A. Fleer, and H. van Dijk. 1995. Complement resistance is a virulence factor of Branhamella (Moraxella) catarrhalis. FEMS Immunol. Med. Microbiol. 11:207–211. [DOI] [PubMed] [Google Scholar]
- 118.Holme, T., M. Rahman, P. E. Jansson, and G. Widmalm. 1999. The lipopolysaccharide of Moraxella catarrhalis structural relationships and antigenic properties. Eur. J. Biochem. 265:524–529. [DOI] [PubMed] [Google Scholar]
- 119.Hoogkamp-Korstanje, J. A., S. I. Dirks-Go, P. Kabel, W. L. Manson, E. E. Stobberingh, R. W. Vreede, and B. I. Davies. 1997. Multicentre in-vitro evaluation of the susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis to ciprofloxacin, clarithromycin, co-amoxiclav and sparfloxacin. J. Antimicrob. Chemother. 39:411–414. [DOI] [PubMed] [Google Scholar]
- 120.Hu, W. G., J. Chen, J. F. Battey, and X. X. Gu. 2000. Enhancement of clearance of bacteria from murine lungs by immunization with detoxified lipooligosaccharide from Moraxella catarrhalis conjugated to proteins. Infect. Immun. 68:4980–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu, W. G., J. Chen, F. M. Collins, and X. X. Gu. 1999. An aerosol challenge mouse model for Moraxella catarrhalis. Vaccine 18:799–804. [DOI] [PubMed] [Google Scholar]
- 122.Ikram, R. B., M. Nixon, J. Aitken, and E. Wells. 1993. A prospective study of isolation of Moraxella catarrhalis in a hospital during the winter months. J. Hosp. Infect. 25:7–14. [DOI] [PubMed] [Google Scholar]
- 123.Ioannidis, J. P., M. Worthington, J. K. Griffiths, and D. R. Snydman. 1995. Spectrum and significance of bacteremia due to Moraxella catarrhalis. Clin. Infect. Dis. 21:390–397. [DOI] [PubMed] [Google Scholar]
- 124.Jawetz, E., J. L. Melnich, and E. A. Adelberg. 1976. Review of medical microbiology, 12th ed., p.183. Lange Medical Publications, Los Altos, Calif.
- 125.Jecker, P., A. McWilliam, S. Napoli, P. G. Holt, R. Pabst, M. Westhofen, and J. Westermann. 1999. Acute laryngitis in the rat induced by Moraxella catarrhalis and Bordetella pertussis: number of neutrophils, dendritic cells, and T and B lymphocytes accumulating during infection in the laryngeal mucosa strongly differs in adjacent locations. Pediatr. Res. 46:760–766. [DOI] [PubMed] [Google Scholar]
- 126.Johnson, M. A., W. L. Drew, and M. Roberts. 1981. Branhamella (Neisseria) catarrhalis—a lower respiratory tract pathogen? J. Clin. Microbiol. 13:1066–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Joiner, K. A., K. A. Warren, C. Hammer, and M. M. Frank. 1985. Bactericidal but not nonbactericidal C5b-9 is associated with distinctive outer membrane proteins in Neisseria gonorrhoeae. J. Immunol. 134:1920–1925. [PubMed] [Google Scholar]
- 128.Jonsson, I., B. Eriksson, and A. Krook. 1990. Minimal criteria for identification of Moraxella (Branhamella) catarrhalis. Apmis 98:954–956. [PubMed] [Google Scholar]
- 129.Jordan, K. L., S. H. Berk, and S. L. Berk. 1990. A comparison of serum bactericidal activity and phenotypic characteristics of bacteremic, pneumonia-causing strains, and colonizing strains of Branhamella catarrhalis. Am. J. Med. 88:28S–32S. [DOI] [PubMed] [Google Scholar]
- 130.Jorgensen, J. H., G. V. Doern, L. A. Maher, A. W. Howell, and J. S. Redding. 1990. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the United States. Antimicrob. Agents Chemother. 34:2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reference deleted.
- 132.Karalus, R., and A. Campagnari. 2000. Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes Infect. 2:547–559. [DOI] [PubMed] [Google Scholar]
- 133.Karjalainen, H., M. Koskela, J. Luotonen, E. Herva, and P. Sipila. 1990. Antibodies against Streptococcus pneumoniae, Haemophilus influenzae and Branhamella catarrhalis in middle ear effusion during early phase of acute otitis media. Acta Otolaryngol. 109:111–118. [DOI] [PubMed] [Google Scholar]
- 134.Kawakami, Y., I. Ueno, T. Katsuyama, K. Furihata, and H. Matsumoto. 1994. Restriction fragment length polymorphism (RFLP) or genomic DNA of Moraxella (Branhamella) catarrhalis isolates in a hospital. Microbiol. Immunol. 38:891–895. [DOI] [PubMed] [Google Scholar]
- 135.Keller, R., J. E. Gustafson, and R. Keist. 1992. The macrophage response to bacteria. Modulation of macrophage functional activity by peptidoglycan from Moraxella (Branhamella) catarrhalis. Clin. Exp. Immunol. 89:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klein, J. O. 1994. Lessons from recent studies on the epidemiology of otitis media. Pediatr. Infect. Dis. J. 13:1031–1034. [DOI] [PubMed] [Google Scholar]
- 137.Klein, J. O. 1999. Management of acute otitis media in an era of increasing antibiotic resistance. Int. J. Pediatr. Otorhinolaryngol. 49(Suppl. 1):S15–S17. [DOI] [PubMed] [Google Scholar]
- 138.Klingman, K. L., and T. F. Murphy. 1994. Purification and characterization of a high-molecular-weight outer membrane protein of Moraxella (Branhamella) catarrhalis. Infect. Immun. 62:1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klingman, K. L., A. Pye, T. F. Murphy, and S. L. Hill. 1995. Dynamics of respiratory tract colonization by Branhamella catarrhalis in bronchiectasis. Am. J. Respir. Crit. Care Med. 152:1072–1078. [DOI] [PubMed] [Google Scholar]
- 140.Korppi, M., M. L. Katila, J. Jaaskelainen, and M. Leinonen. 1992. Role of Moraxella (Branhamella) catarrhalis as a respiratory pathogen in children. Acta Paediatr. 81:993–996. [DOI] [PubMed] [Google Scholar]
- 141.Kovatch, A. L., E. R. Wald, and R. H. Michaels. 1983. Beta-lactamase-producing Branhamella catarrhalis causing otitis media in children. J. Pediatr. 102:261–264. [DOI] [PubMed] [Google Scholar]
- 142.Kyd, J., and A. Cripps. 2000. Identifying vaccine antigens and assessing delivery systems for the prevention of bacterial infections. J. Biotechnol. 83:85–90. [DOI] [PubMed] [Google Scholar]
- 143.Kyd, J., A. John, A. Cripps, and T. F. Murphy. 1999. Investigation of mucosal immunisation in pulmonary clearance of Moraxella (Branhamella) catarrhalis. Vaccine 18:398–406. [DOI] [PubMed] [Google Scholar]
- 144.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reference deleted.
- 146.Leinonen, M., J. Luotonen, E. Herva, K. Valkonen, and P. H. Makela. 1981. Preliminary serologic evidence for a pathogenic role of Branhamella catarrhalis. J. Infect. Dis. 144:570–574. [DOI] [PubMed] [Google Scholar]
- 147.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luke, N. R., and A. A. Campagnari. 1999. Construction and characterization of Moraxella catarrhalis mutants defective in expression of transferrin receptors. Infect. Immun. 67:5815–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luke, N. R., T. A. Russo, N. Luther, and A. A. Campagnari. 1999. Use of an isogenic mutant constructed in Moraxella catarrhalis to identify a protective epitope of outer membrane protein B1 defined by monoclonal antibody 11C6. Infect. Immun. 67:681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Luna, V. A., S. Cousin, Jr., W. L. Whittington, and M. C. Roberts. 2000. Identification of the conjugative mef gene in clinical Acinetobacter junii and Neisseria gonorrhoeae isolates. Antimicrob. Agents Chemother. 44:2503–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.MacIver, I., M. Unhanand, G. H. McCracken, Jr., and E. J. Hansen. 1993. Effect of immunization of pulmonary clearance of Moraxella catarrhalis in an animal model. J. Infect. Dis. 168:469–472. [DOI] [PubMed] [Google Scholar]
- 152.Macsai, M. S., D. S. Hillman, and J. B. Robin. 1988. Branhamella keratitis resistant to penicillin and cephalosporins. Case report. Arch. Ophthalmol. 106:1506–1507. [DOI] [PubMed] [Google Scholar]
- 153.Mandrell, R. E., J. J. Kim, C. M. John, B. W. Gibson, J. V. Sugai, M. A. Apicella, J. M. Griffiss, and R. Yamasaki. 1991. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J. Bacteriol. 173:2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Manninen, R., P. Huovinen, and A. Nissinen. 1997. Increasing antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in Finland. J. Antimicrob. Chemother. 40:387–392. [DOI] [PubMed] [Google Scholar]
- 155.Marchant, C. D. 1990. Spectrum of disease due to Branhamella catarrhalis in children with particular reference to acute otitis media. Am. J. Med. 88:15S–19S. [DOI] [PubMed] [Google Scholar]
- 156.Marrs, C. F., and S. Weir. 1990. Pili (fimbriae) of Branhamella species. Am. J. Med. 88:36S–40S. [DOI] [PubMed] [Google Scholar]
- 157.Martinez, G., K. Ahmed, C. H. Zheng, K. Watanabe, K. Oishi, and T. Nagatake. 1999. DNA restriction patterns produced by pulsed-field gel electrophoresis in Moraxella catarrhalis isolated from different geographical areas. Epidemiol. Infect. 122:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mathers, K., M. Leinonen, and D. Goldblatt. 1999. Antibody response to outer membrane proteins of Moraxella catarrhalis in children with otitis media. Pediatr. Infect. Dis. J. 18:982–988. [DOI] [PubMed] [Google Scholar]
- 159.McGregor, K., B. J. Chang, B. J. Mee, and T. V. Riley. 1998. Moraxella catarrhalis: clinical significance, antimicrobial susceptibility and BRO beta-lactamases. Eur. J. Clin. Microbiol. Infect. Dis. 17:219–234. [DOI] [PubMed] [Google Scholar]
- 160.McLeod, D. T., F. Ahmad, S. Capewell, M. J. Croughan, M. A. Calder, and A. Seaton. 1986. Increase in bronchopulmonary infection due to Branhamella catarrhalis. Br. Med. J. 292:1103–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McLeod, D. T., F. Ahmad, M. J. Croughan, and M. A. Calder. 1986. Bronchopulmonary infection due to B. catarrhalis. Clinical features and therapeutic response. Drugs 31(Suppl. 3):109–112. [DOI] [PubMed] [Google Scholar]
- 162.McMichael, J. C. 2000. Progress toward the development of a vaccine to prevent Moraxella (Branhamella) catarrhalis infections. Microbes Infect. 2:561–568. [DOI] [PubMed] [Google Scholar]
- 163.McMichael, J. C., M. J. Fiske, R. A. Fredenburg, D. N. Chakravarti, K. R. VanDerMeid, V. Barniak, J. Caplan, E. Bortell, S. Baker, R. Arumugham, and D. Chen. 1998. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect. Immun. 66:4374–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mibus, D. J., B. J. Mee, K. F. McGregor, C. D. Garbin, and B. J. Chang. 1998. The identification of response regulators of Branhamella catarrhalis using PCR. FEMS Immunol. Med. Microbiol. 22:351–354. [DOI] [PubMed] [Google Scholar]
- 165.Miravitlles, M., C. Espinosa, E. Fernandez-Laso, J. A. Martos, J. A. Maldonado, and M. Gallego. 1999. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest 116:40–46. [DOI] [PubMed] [Google Scholar]
- 166.Morgan, M. G., H. McKenzie, M. C. Enright, M. Bain, and F. X. Emmanuel. 1992. Use of molecular methods to characterize Moraxella catarrhalis strains in a suspected outbreak of nosocomial infection. Eur. J. Clin. Microbiol. Infect. Dis. 11:305–312. [DOI] [PubMed] [Google Scholar]
- 167.Murphy, S., M. Fitzgerald, R. Mulcahy, C. Keane, D. Coakley, and T. Scott. 1997. Studies on haemagglutination and serum resistance status of strains of Moraxella catarrhalis isolated from the elderly. Gerontology 43:277–282. [DOI] [PubMed] [Google Scholar]
- 168.Murphy, T. F. 1996. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol. Rev. 60:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Murphy, T. F. 1998. Lung infections. 2. Branhamella catarrhalis: epidemiological and clinical aspects of a human respiratory tract pathogen. Thorax 53:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Murphy, T. F. 1990. Studies of the outer membrane proteins of Branhamella catarrhalis. Am. J. Med. 88:41S–45S. [DOI] [PubMed] [Google Scholar]
- 171.Murphy, T. F. 1989. The surface of Branhamella catarrhalis: a systematic approach to the surface antigens of an emerging pathogen. Pediatr. Infect. Dis. J. 8(1 Suppl.):S75–S77. [PubMed] [Google Scholar]
- 172.Murphy, T. F., and L. C. Bartos. 1989. Surface-exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect. Immun. 57:2938–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murphy, T. F., A. L. Brauer, N. Yuskiw, and T. J. Hiltke. 2000. Antigenic structure of outer membrane protein E of Moraxella catarrhalis and construction and characterization of mutants. Infect. Immun. 68:6250–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Murphy, T. F., C. Kirkham, E. DeNardin, and S. Sethi. 1999. Analysis of antigenic structure and human immune response to outer membrane protein CD of Moraxella catarrhalis. Infect. Immun. 67:4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Murphy, T. F., C. Kirkham, and A. J. Lesse. 1993. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol. Microbiol. 10:87–97. [DOI] [PubMed] [Google Scholar]
- 176.Murphy, T. F., J. M. Kyd, A. John, C. Kirkham, and A. W. Cripps. 1998. Enhancement of pulmonary clearance of Moraxella (Branhamella) catarrhalis following immunization with outer membrane protein CD in a mouse model. J. Infect. Dis. 178:1667–1675. [DOI] [PubMed] [Google Scholar]
- 177.Murphy, T. F., and M. R. Loeb. 1989. Isolation of the outer membrane of Branhamella catarrhalis. Microb. Pathog. 6:159–174. [DOI] [PubMed] [Google Scholar]
- 178.Murphy, T. F., and S. Sethi. 1992. Bacterial infection in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 146:1067–1083. [DOI] [PubMed] [Google Scholar]
- 179.Nash, D. R., R. J. Wallace, Jr., V. A. Steingrube, and P. A. Shurin. 1986. Isoelectric focusing of beta-lactamases from sputum and middle ear isolates of Branhamella catarrhalis recovered in the United States. Drugs 31(Suppl. 3):48–54. [DOI] [PubMed] [Google Scholar]
- 180.Neumayer, U., H. K. Schmidt, K. P. Mellwig, and G. Kleikamp. 1999. Moraxella catarrhalis endocarditis: report of a case and literature review. J. Heart Valve Dis. 8:114–117. [PubMed] [Google Scholar]
- 181.Nguyen, K. T., E. J. Hansen, and M. A. Farinha. 1999. Construction of a genomic map of Moraxella (Branhamella) catarrhalis ATCC 25238 and physical mapping of virulence-associated genes. Can. J. Microbiol. 45:299–303. [PubMed] [Google Scholar]
- 182.Nicotra, B., M. Rivera, J. I. Luman, and R. J. Wallace, Jr. 1986. Branhamella catarrhalis as a lower respiratory tract pathogen in patients with chronic lung disease. Arch. Intern. Med. 146:890–893. [PubMed] [Google Scholar]
- 183.Reference deleted.
- 184.Ofori-Darko, E., Y. Zavros, G. Rieder, S. A. Tarle, M. Van Antwerp, and J. L. Merchant. 2000. An OmpA-like protein from Acinetobacter spp. stimulates gastrin and interleukin-8 promoters. Infect Immun. 68:3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oishi, K., H. Tanaka, F. Sonoda, S. Borann, K. Ahmed, Y. Utsunomiya, K. Watanabe, T. Nagatake, M. Vaneechoutte, G. Verschraegen, and K. Matsumoto. 1996. A monoclonal antibody reactive with a common epitope of Moraxella (Branhamella) catarrhalis lipopolysaccharides. Clin. Diagn. Lab. Immunol. 3:351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Onofrio, J. M., A. N. Shulkin, P. J. Heidbrink, G. B. Toews, and A. K. Pierce. 1981. Pulmonary clearance and phagocytic cell response to normal pharyngeal flora. Am. Rev. Respir. Dis. 123:222–225. [DOI] [PubMed] [Google Scholar]
- 187.Patel, J. A., B. Reisner, N. Vizirinia, M. Owen, T. Chonmaitree, and V. Howie. 1995. Bacteriologic failure of amoxicillin-clavulanate in treatment of acute otitis media caused by nontypeable Haemophilus influenzae. J. Pediatr. 126:799–806. [DOI] [PubMed] [Google Scholar]
- 188.Patterson, T. F., J. E. Patterson, B. L. Masecar, G. E. Barden, W. J. Hierholzer, Jr., and M. J. Zervos. 1988. A nosocomial outbreak of Branhamella catarrhalis confirmed by restriction endonuclease analysis. J. Infect. Dis. 157:996–1001. [DOI] [PubMed] [Google Scholar]
- 189.Peak, I. R., M. P. Jennings, D. W. Hood, M. Bisercic, and E. R. Moxon. 1996. Tetrameric repeat units associated with virulence factor phase variation in Haemophilus also occur in Neisseria spp. and Moraxella catarrhalis. FEMS Microbiol. Lett. 137:109–114. [DOI] [PubMed] [Google Scholar]
- 190.Peiris, V., and J. Heald. 1992. Rapid method for differentiating strains of Branhamella catarrhalis. J. Clin. Pathol. 45:532–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pettersson, B., A. Kodjo, M. Ronaghi, M. Uhlen, and T. Tonjum. 1998. Phylogeny of the family Moraxellaceae by 16S rDNA sequence analysis, with special emphasis on differentiation of Moraxella species. Int. J. Syst. Bacteriol. 48:75–89. [DOI] [PubMed] [Google Scholar]
- 192.Pollard, J. A., R. J. Wallace, Jr., D. R. Nash, J. I. Luman, and R. W. Wilson. 1986. Incidence of Branhamella catarrhalis in the sputa of patients with chronic lung disease. Drugs 31(Suppl. 3):103–108. [DOI] [PubMed] [Google Scholar]
- 193.Post, J. C., J. J. Aul, G. J. White, R. M. Wadowsky, T. Zavoral, R. Tabari, B. Kerber, W. J. Doyle, and G. D. Ehrlich. 1996. PCR-based detection of bacterial DNA after antimicrobial treatment is indicative of persistent, viable bacteria in the chinchilla model of otitis media. Am. J. Otolaryngol. 17:106–111. [DOI] [PubMed] [Google Scholar]
- 194.Post, J. C., R. A. Preston, J. J. Aul, M. Larkins-Pettigrew, J. Rydquist-White, K. W. Anderson, R. M. Wadowsky, D. R. Reagan, E. S. Walker, L. A. Kingsley, et al. 1995. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA 273:1598–1604. [PubMed] [Google Scholar]
- 195.Post, J. C., G. J. White, J. J. Aul, T. Zavoral, R. M. Wadowsky, Y. Zhang, R. A. Preston, and G. D. Ehrlich. 1996. Development and validation of a multiplex PCR-based assay for the upper respiratory tract bacterial pathogens Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Mol. Diagn. 1:29–39. [DOI] [PubMed] [Google Scholar]
- 196.Prellner, K., P. Christensen, B. Hovelius, and C. Rosen. 1984. Nasopharyngeal carriage of bacteria in otitis-prone and non-otitis-prone children in day-care centres. Acta Otolaryngol. 98:343–350. [DOI] [PubMed] [Google Scholar]
- 197.Rahman, M., T. Holme, I. Jonsson, and A. Krook. 1995. Lack of serotype-specific antibody response to lipopolysaccharide antigens of Moraxella catarrhalis during lower respiratory tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 14:297–304. [DOI] [PubMed] [Google Scholar]
- 198.Reddy, M. S., T. F. Murphy, H. S. Faden, and J. M. Bernstein. 1997. Middle ear mucin glycoprotein: purification and interaction with nontypable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol. Head Neck Surg. 116:175–180. [DOI] [PubMed] [Google Scholar]
- 199.Rice, P. A. 1989. Molecular basis for serum resistance in Neisseria gonorrhoeae. Clin. Microbiol. Rev. 2(Suppl.):S112–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Richards, S. J., A. P. Greening, M. C. Enright, M. G. Morgan, and H. McKenzie. 1993. Outbreak of Moraxella catarrhalis in a respiratory unit. Thorax 48:91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rikitomi, N., K. Ahmed, and T. Nagatake. 1997. Moraxella (Branhamella) catarrhalis adherence to human bronchial and oropharyngeal cells: the role of adherence in lower respiratory tract infections. Microbiol. Immunol. 41:487–494. [DOI] [PubMed] [Google Scholar]
- 202.Rikitomi, N., B. Andersson, K. Matsumoto, R. Lindstedt, and C. Svanborg. 1991. Mechanism of adherence of Moraxella (Branhamella) catarrhalis. Scand. J. Infect. Dis. 23:559–567. [DOI] [PubMed] [Google Scholar]
- 203.Roantree, R., and I. Rantz. 1960. A study of the relationship of the normal bactericidal activity of human serum to bacterial infection. J. Clin. Investig. 39:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ruuskanen, O., and T. Heikkinen. 1994. Otitis media: etiology and diagnosis. Pediatr. Infect. Dis. J. 13(Suppl. 1):S23–S26; discussion S50–S54. [PubMed] [Google Scholar]
- 205.Samukawa, T., N. Yamanaka, S. Hollingshead, K. Klingman, and H. Faden. 2000. Immune responses to specific antigens of Streptococcus pneumoniae and Moraxella catarrhalis in the respiratory tract. Infect. Immun. 68:1569–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Samukawa, T., N. Yamanaka, S. Hollingshead, T. F. Murphy, and H. Faden. 2000. Immune response to surface protein A of Streptococcus pneumoniae and to high-molecular-weight outer membrane protein A of Moraxella catarrhalis in children with acute otitis media. J. Infect. Dis. 181:1842–1845. [DOI] [PubMed] [Google Scholar]
- 207.Sandt, C. H., and C. W. Hill. 2000. Four different genes responsible for nonimmune immunoglobulin-binding activities within a single strain of Escherichia coli. Infect. Immun. 68:2205–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sarubbi, F. A., J. W. Myers, J. J. Williams, and C. G. Shell. 1990. Respiratory infections caused by Branhamella catarrhalis. Selected epidemiologic features. Am. J. Med. 88:9S–14S. [DOI] [PubMed] [Google Scholar]
- 209.Schalén, L., P. Christensen, I. Eliasson, S. Fex, C. Kamme, and C. Schalén. 1985. Inefficacy of penicillin V in acute laryngitis in adults. Evaluation from results of double-blind study. Ann. Otol. Rhinol. Laryngol. 94:14–17. [DOI] [PubMed] [Google Scholar]
- 210.Schalén, L., P. Christensen, C. Kamme, H. Miorner, K. I. Pettersson, and C. Schalén. 1980. High isolation rate of Branhamella catarrhalis from the nasopharynx in adults with acute laryngitis. Scand. J. Infect. Dis. 12:277–280. [DOI] [PubMed] [Google Scholar]
- 211.Sethi, S., S. L. Hill, and T. F. Murphy. 1995. Serum antibodies to outer membrane proteins (OMPs) of Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect. Immun. 63:1516–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sethi, S., J. M. Surface, and T. F. Murphy. 1997. Antigenic heterogeneity and molecular analysis of CopB of Moraxella (Branhamella) catarrhalis. Infect. Immun. 65:3666–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shurin, P. A., C. D. Marchant, C. H. Kim, G. F. Van Hare, C. E. Johnson, M. A. Tutihasi, and L. J. Knapp. 1983. Emergence of beta-lactamase-producing strains of Branhamella catarrhalis as important agents of acute otitis media. Pediatr. Infect. Dis. J. 2:34–38. [DOI] [PubMed] [Google Scholar]
- 214.Singh, S., K. M. Cisera, J. D. Turnidge, and E. G. Russell. 1997. Selection of optimum laboratory tests for the identification of Moraxella catarrhalis. Pathology 29:206–208. [DOI] [PubMed] [Google Scholar]
- 215.Smith, H. 1995. The state and future of studies on bacterial pathogenicity, p.335–357. In J. A. Roth, C. A. Bolin, K. A. Brogden, C. Minion, and M. J. Wannemueller (ed.), Virulence of bacterial pathogens, 2nd ed. American Society for Microbiology, Washington, D.C.
- 216.Soto-Hernandez, J. L., S. Holtsclaw-Berk, L. M. Harvill, and S. L. Berk. 1989. Phenotypic characteristics of Branhamella catarrhalis strains. J. Clin. Microbiol. 27:903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Speeleveld, E., J. M. Fossepre, B. Gordts, and H. W. Van Landuyt. 1994. Comparison of three rapid methods, tributyrine, 4-methylumbelliferyl butyrate, and indoxyl acetate, for rapid identification of Moraxella catarrhalis. J. Clin. Microbiol. 32:1362–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sportel, J. H., G. H. Koeter, R. van Altena, A. Lowenberg, and W. G. Boersma. 1995. Relation between beta-lactamase producing bacteria and patient characteristics in chronic obstructive pulmonary disease (COPD). Thorax 50:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stefanou, J., A. V. Agelopoulou, N. V. Sipsas, N. Smilakou, and A. Avlami. 2000. Moraxella catarrhalis endocarditis: case report and review of the literature. Scand. J. Infect. Dis. 32:217–218. [DOI] [PubMed] [Google Scholar]
- 220.Takada, R., Y. Harabuchi, T. Himi, and A. Kataura. 1998. Antibodies specific to outer membrane antigens of Moraxella catarrhalis in sera and middle ear effusions from children with otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 46:185–195. [DOI] [PubMed] [Google Scholar]
- 221.Taylor, P. W. 1995. Resistance of bacteria to complement, p.49–64. In J. A. Roth, C. A. Bolin, K. A. Brogden, C. Minion, and M. J. Wannemueller (ed.), Virulence of bacterial pathogens, 2nd ed. American Society for Microbiology, Washington, D.C.
- 222.Teele, D. W., J. O. Klein, and B. Rosner. 1989. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J. Infect. Dis. 160:83–94. [DOI] [PubMed] [Google Scholar]
- 223.Thornsberry, C., M. E. Jones, M. L. Hickey, Y. Mauriz, J. Kahn, and D. F. Sahm. 1999. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in the United States, 1997–1998. J. Antimicrob. Chemother. 44:749–759. [DOI] [PubMed] [Google Scholar]
- 224.Thorsson, B., V. Haraldsdottir, and M. Kristjansson. 1998. Moraxella catarrhalis bacteraemia. A report on 3 cases and a review of the literature. Scand. J. Infect. Dis. 30:105–109. [DOI] [PubMed] [Google Scholar]
- 225.Toews, G. B., E. J. Hansen, and R. M. Strieter. 1990. Pulmonary host defenses and oropharyngeal pathogens. Am. J. Med. 88:20S–24S. [DOI] [PubMed] [Google Scholar]
- 226.Unhanand, M., I. Maciver, O. Ramilo, O. Arencibia-Mireles, J. C. Argyle, G. H. McCracken, Jr., and E. J. Hansen. 1992. Pulmonary clearance of Moraxella catarrhalis in an animal model. J. Infect. Dis. 165:644–650. [DOI] [PubMed] [Google Scholar]
- 227.Vaneechoutte, M., G. Verschraegen, G. Claeys, and A. M. van den Abeele. 1988. Selective medium for Branhamella catarrhalis with acetazolamide as a specific inhibitor of Neisseria spp. J. Clin. Microbiol. 26:2544–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vaneechoutte, M., G. Verschraegen, G. Claeys, and A. M. Van Den Abeele. 1990. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J. Clin. Microbiol. 28:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Van Hare, G. F., and P. A. Shurin. 1987. The increasing importance of Branhamella catarrhalis in respiratory infections. Pediatr. Infect. Dis. J. 6:92–94. [DOI] [PubMed] [Google Scholar]
- 230.Van Hare, G. F., P. A. Shurin, C. D. Marchant, N. A. Cartelli, C. E. Johnson, D. Fulton, S. Carlin, and C. H. Kim. 1987. Acute otitis media caused by Branhamella catarrhalis: biology and therapy. Rev. Infect. Dis. 9:16–27. [DOI] [PubMed] [Google Scholar]
- 231.Varon, E., C. Levy, F. De La Rocque, M. Boucherat, D. Deforche, I. Podglajen, M. Navel, and R. Cohen. 2000. Impact of antimicrobial therapy on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, and Branhamella catarrhalis in children with respiratory tract infections. Clin. Infect. Dis. 31:477–481. [DOI] [PubMed] [Google Scholar]
- 232.Reference deleted.
- 233.Verduin, C. M., M. Jansze, C. Hol, T. E. Mollnes, J. Verhoef, and H. van Dijk. 1994. Differences in complement activation between complement-resistant and complement-sensitive Moraxella (Branhamella) catarrhalis strains occur at the level of membrane attack complex formation. Infect. Immun. 62:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Verduin, C. M., M. Jansze, J. Verhoef, A. Fleer, and H. Van Dijk. 1994. Complement resistance in Moraxella (Branhamella) catarrhalis is mediated by a vitronectin-binding surface protein. Clin. Exp. Immunol. 97:50. [Google Scholar]
- 235.Verduin, C. M., M. Kools-Sijmons, J. van der Plas, J. Vlooswijk, M. Tromp, H. van Dijk, J. Banks, H. Verbrugh, and A. van Belkum. 2000. Complement-resistant Moraxella catarrhalis forms a genetically distinct lineage within the species. FEMS Microbiol. Lett. 184:1–8. [DOI] [PubMed] [Google Scholar]
- 236.Verghese, A., E. Berro, J. Berro, and B. W. Franzus. 1990. Pulmonary clearance and phagocytic cell response in a murine model of Branhamella catarrhalis infection. J. Infect. Dis. 162:1189–1192. [DOI] [PubMed] [Google Scholar]
- 237.Vu-Thien, H., C. Dulot, D. Moissenet, B. Fauroux, and A. Garbarg-Chenon. 1999. Comparison of randomly amplified polymorphic DNA analysis and pulsed-field gel electrophoresis for typing of Moraxella catarrhalis strains. J. Clin. Microbiol. 37:450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wald, E. R. 1992. Microbiology of acute and chronic sinusitis in children. J. Allergy Clin. Immunol. 90:452–456. [DOI] [PubMed] [Google Scholar]
- 239.Wald, E. R. 1998. Sinusitis. Pediatr. Ann. 27:811–818. [DOI] [PubMed] [Google Scholar]
- 240.Wald, E. R. 1992. Sinusitis in children. N. Engl. J. Med. 326:319–323. [DOI] [PubMed] [Google Scholar]
- 241.Reference deleted.
- 242.Walker, E. S., C. L. Neal, E. Laffan, J. H. Kalbfleisch, S. L. Berk, and F. Levy. 2000. Long-term trends in susceptibility of Moraxella catarrhalis: a population analysis. J. Antimicrob. Chemother. 45:175–182. [DOI] [PubMed] [Google Scholar]
- 243.Walker, E. S., R. A. Preston, J. C. Post, G. D. Ehrlich, J. H. Kalbfleisch, and K. L. Klingman. 1998. Genetic diversity among strains of Moraxella catarrhalis: analysis using multiple DNA probes and a single-locus PCR-restriction fragment length polymorphism method. J. Clin. Microbiol. 36:1977–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wallace, R. J., Jr., D. R. Nash, and V. A. Steingrube. 1990. Antibiotic susceptibilities and drug resistance in Moraxella (Branhamella) catarrhalis. Am. J. Med. 88:46S–50S. [DOI] [PubMed] [Google Scholar]
- 245.Wallace, R. J., Jr., V. A. Steingrube, D. R. Nash, D. G. Hollis, C. Flanagan, B. A. Brown, A. Labidi, and R. E. Weaver. 1989. BRO-beta-lactamases of Branhamella catarrhalis and Moraxella subgenus Moraxella, including evidence for chromosomal β-lactamase transfer by conjugation in B. catarrhalis, M. nonliquefaciens, and M. lacunata. Antimicrob. Agents Chemother. 33:1845–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wardle, J. K. 1986. Branhamella catarrhalis as an indirect pathogen. Drugs 31(Suppl. 3):93–96. [DOI] [PubMed] [Google Scholar]
- 247.Weiss, A., J. H. Brinser, and V. Nazar-Stewart. 1993. Acute conjunctivitis in childhood. J. Pediatr. 122:10–14. [DOI] [PubMed] [Google Scholar]
- 248.Westman, E., A. Melhus, S. Hellstrom, and A. Hermansson. 1999. Moraxella catarrhalis-induced purulent otitis media in the rat middle ear. Structure, protection, and serum antibodies. Apmis 107:737–746. [PubMed] [Google Scholar]
- 249.Reference deleted.
- 250.Reference deleted.
- 251.Wood, G. M., B. C. Johnson, and J. G. McCormack. 1996. Moraxella catarrhalis: pathogenic significance in respiratory tract infections treated by community practitioners. Clin. Infect. Dis. 22:632–636. [DOI] [PubMed] [Google Scholar]
- 252.Wright, P. W., and R. J. Wallace, Jr. 1989. Pneumonia due to Moraxella (Branhamella) catarrhalis. Semin. Respir. Infect. 4:40–46. [PubMed] [Google Scholar]
- 253.Yang, Y. P., L. E. Myers, U. McGuinness, P. Chong, Y. Kwok, M. H. Klein, and R. E. Harkness. 1997. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol. Med. Microbiol. 17:187–199. [DOI] [PubMed] [Google Scholar]
- 254.Yano, H., M. Suetake, A. Kuga, K. Irinoda, R. Okamoto, T. Kobayashi, and M. Inoue. 2000. Pulsed-field gel electrophoresis analysis of nasopharyngeal flora in children attending a day care center. J. Clin. Microbiol. 38:625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yu, R. H., R. A. Bonnah, S. Ainsworth, and A. B. Schryvers. 1999. Analysis of the immunological responses to transferrin and lactoferrin receptor proteins from Moraxella catarrhalis. Infect. Immun. 67:3793–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zaleski, A., N. K. Scheffler, P. Densen, F. K. Lee, A. A. Campagnari, B. W. Gibson, and M. A. Apicella. 2000. Lipooligosaccharide P(k) (Galα1-4Galβ1-4Glc) epitope of Moraxella catarrhalis is a factor in resistance to bactericidal activity mediated by normal human serum. Infect. Immun. 68:5261–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]