Abstract
Background
The long-term health consequences of acute bacterial gastroenteritis remain uncertain. We studied the risk of hypertension and reduced kidney function after an outbreak of acute gastroenteritis due to contamination of a regional drinking water supply with Escherichia coli O157:H7 and Campylobacter species.
Methods
A total of 1958 adults with no known history of hypertension or kidney disease before the outbreak participated in a long-term follow-up study. Of the participants, 675 had been asymptomatic during the outbreak, 909 had had moderate symptoms of acute self-limited gastroenteritis, and 374 had had severe symptoms that necessitated medical attention. The outcomes of interest were a diagnosis of hypertension or the presence of reduced kidney function and albuminuria during the follow-up period.
Results
After a mean follow-up of 3.7 years after the outbreak, hypertension was diagnosed in 27.0% of participants who had been asymptomatic during the outbreak and in 32.3% and 35.9% of those who had had moderate and severe symptoms of acute gastroenteritis respectively (trend p = 0.009). Compared with the asymptomatic participants, those with moderate and severe symptoms of gastroenteritis had an adjusted relative risk of hypertension of 1.15 (95% confidence interval [CI] 0.97–1.35) and 1.28 (95% CI 1.04–1.56) respectively. A similar graded association was seen for reduced kidney function, defined as the presence of an estimated glomerular filtration rate below 60 mL/min per 1.73 m2 (trend p = 0.03). No association was observed between gastroenteritis and the subsequent risk of albuminuria.
Interpretation
Acute bacterial gastroenteritis necessitating medical attention was associated with an increased risk of hypertension and reduced kidney function 4 years after infection. Maintaining safe drinking water remains essential to human health, as transient bacterial contaminations may have implications well beyond a period of acute self-limited illness.
Acute bacterial dysentery is a global health concern, particularly in developing countries.1 Escherichia coli O157:H7 and Campylobacter jejuni infections may have long-term health consequences beyond the period of acute illness.2,3,4,5 Receptors for E. coli O157:H7 Shiga toxin are found in the kidney. Exposure to this pathogen may result in substantial loss of nephrons and subsequent hyperfiltration, which can lead to long-term systemic hypertension and reduced kidney function.6,7,8 The most toxic form of E. coli O157:H7 infection is hemolytic uremic syndrome, and the potential for long-term renal dysfunction and hypertension after this condition is well described.2 It is unknown whether bacterial gastroenteritis in the absence of recognized hemolytic uremic syndrome may lead to clinically important long-term renal sequelae. We evaluated the long-term risk of hypertension and reduced kidney function among previously healthy adults following an outbreak of acute gastroenteritis due to the bacterial contamination of a regional water supply in Ontario.
Methods
The methods of this follow-up study have been described elsewhere.9,10 In brief, the municipal water supply in Walkerton, a small rural town in Ontario, became contaminated with bacteria, predominantly E. coli O157:H7 and Campylobacter species, in May 2000. Heavy rainfall had contributed to the surface transport of livestock fecal contaminants into inadequately chlorinated drinking water, supplied from a shallow well.11 Over 2300 people became ill with acute gastroenteritis, 27 cases of hemolytic uremic syndrome were identified, and there were 6 deaths.12 Being the most serious case of water contamination in recent North American history, this event attracted world-wide media attention and sparked public concern about the safety of drinking water.13
Following the outbreak, we invited all people who either lived in the Walkerton area or who had consumed municipal water at the time of the outbreak to attend a clinic and participate in a long-term follow-up study. For this study, it would have been ideal to enroll participants and elicit their acute symptoms immediately after the outbreak. However, because of the unexpected nature of the event, this proved unrealistic for several reasons. First, the community was not affiliated with an academic health centre. Second, time was required to prepare the study protocol, obtain financial support, submit an application for ethical review, and develop local facilities and procedures. Therefore, 2 years elapsed before the first participant was enrolled. A total of 4496 people participated in the study, representing 55% of the town's population and 82% of those who were acutely ill during the outbreak.9 Subsequent analyses confirmed that the demographic characteristics of the study sample were similar to those of the affected population and that potential participation biases (also referred to as response or selection biases) would not lead to overestimates of risk between acute bacterial gastroenteritis and long-term health sequelae.9
According to the study protocol, we asked each of the participants to attend an annual clinic visit 2, 3 and 4 years after the initial outbreak, to complete a standardized questionnaire, undergo a physical examination and provide blood and urine specimens. The design of the questionnaire was guided by questions included in the US Third National Health and Nutrition Examination Survey.14 In order to calculate the glomerular filtration rate (GFR), a question was included asking participants to describe their race or ethnicity (white, black, Native Canadian, Asian or other).
Of the 4496 participants, we excluded those less than 18 years of age (n = 1073), because thresholds for hypertension in children differ from those in adults.15 In addition, we excluded adults with a history of chronic gastrointestinal symptoms (n = 298), hypertension (n = 919), kidney disease (n = 752) or diabetes mellitus (n = 251) present before the outbreak (these conditions were not mutually exclusive; see Appendix 1 for the method of assessment of these conditions). Thus, 1958 adults were included in our analysis.
We were concerned about the accuracy of self-reported acute symptoms of gastroenteritis, given the lag time of at least 2 years between the initial outbreak and the time participants were first questioned about their symptoms. Furthermore, during this period, some participants were eligible for financial compensation from the government, which led to potential recall bias. We had empirical evidence of this phenomenon among 405 participants who had been interviewed by public health officials just after the outbreak and were interviewed again by study personnel 2 years later: as many as 27% recalled acute symptoms that they had previously denied were present.10 Misclassifying participants as unwell if they were truly asymptomatic would bias toward demonstrating no association between acute gastroenteritis and long-term health sequelae. We therefore confirmed self-reported information about each participant's symptoms at the time of the outbreak using historical health records. Trained research assistants used a standardized form to abstract data from both physician and hospital charts for 90% of the study participants. We also obtained electronic records from the regional health unit, which investigated illness at the time of the outbreak.12
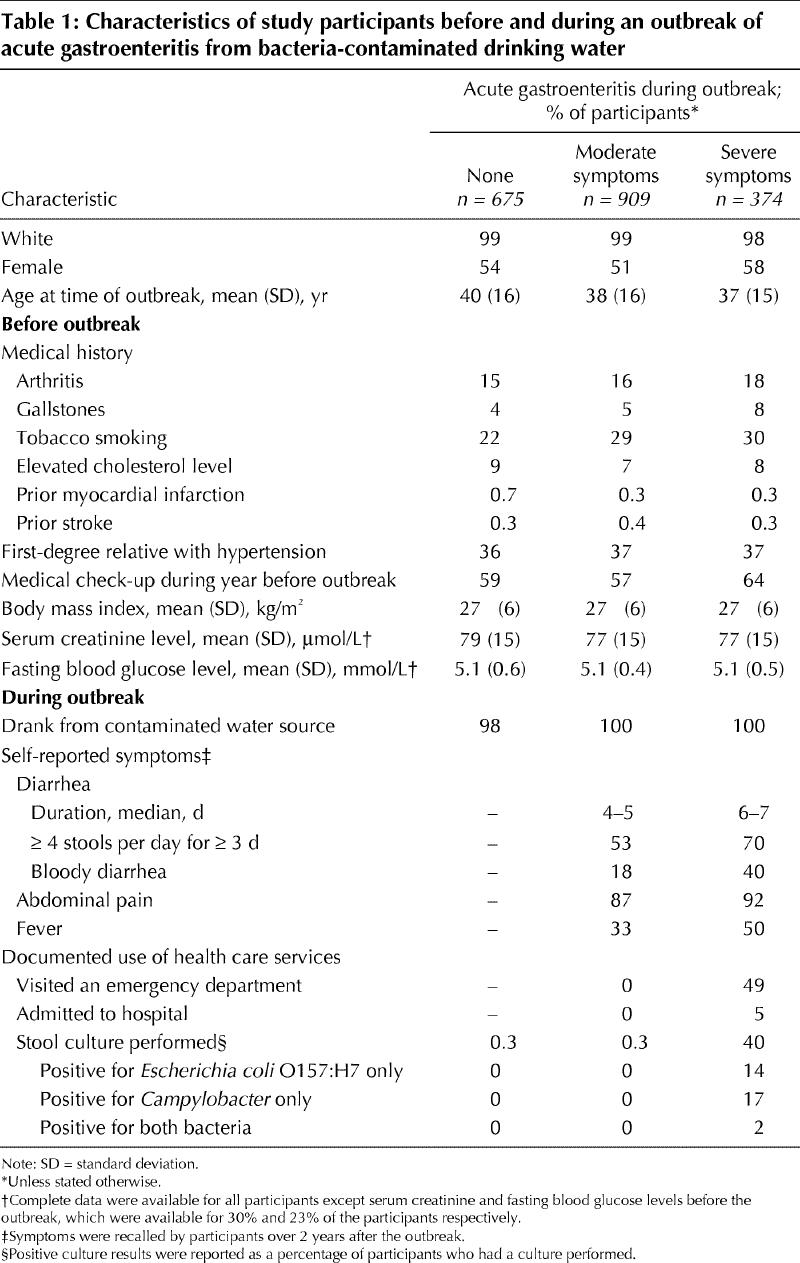
We divided the participants into 3 groups according to the presence and severity of acute gastroenteritis at the time of the outbreak: (a) “none” included participants who had been asymptomatic during the outbreak; (b) “moderate symptoms” included those who had had moderate symptoms of acute self-limited gastroenteritis, which could neither be confirmed nor refuted by prior health records because the participant had not sought medical attention; and (c) “severe symptoms” included those who had had severe symptoms of acute gastroenteritis that necessitated medical attention. Participants with severe symptoms were more likely than those with moderate symptoms to describe bloody diarrhea (40% v. 18%, p < 0.001), prolonged diarrhea (70% v. 53%, p < 0.001) and fever (50% v. 33%, p < 0.001) (Table 1). Forty-nine percent of the participants with severe symptoms had visited an emergency department, where cases of gastroenteritis had been treated conservatively (Table 1). After establishment of the cause of the outbreak, members of the community had been discouraged from submitting stool samples; cultures of stool specimens had been performed in 40% of cases of severe gastroenteritis, of which 14% had yielded positive results for E. coli O157:H7, 17% for Campylobacter and 2% for both bacteria (Table 1).
Table 1
Two sensitivity analyses were performed to categorize acute gastroenteritis by other methods. The first method was based on a history of bloody stools (no symptoms, gastroenteritis, and gastroenteritis with bloody stool). The second method was based on the frequency and duration of diarrhea (no symptoms, gastroenteritis, and gastroenteritis with 4 or more stools per day for 3 or more days). In each case, the point estimate and statistical significance of the association between gastroenteritis and hypertension was similar or exaggerated compared with the results presented herein (data available from the authors upon request).
The primary study outcome was a diagnosis of hypertension, as defined in the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.15 In the absence of diabetes mellitus or renal impairment, hypertension was defined by a mean systolic blood pressure of 140 mm Hg or a mean diastolic blood pressure of 90 mm Hg at any follow-up visit.15 For participants with an estimated GFR below 60 mL/min per 1.73 m2, a level of protein in a 24-hour collection of urine of 300 mg/d, a random urine albumin:creatinine ratio of 22.6 mg/mmol (200 mg/g) or diabetes mellitus, hypertension was defined by a mean systolic blood pressure of 130 mm Hg or a mean diastolic blood pressure of 80 mm Hg.15 During the course of follow-up, a participant was also considered to have hypertension if he or she received a prescription for antihypertensive therapy from a physician not involved in the study.
Blood pressure was measured by trained study personnel at a single clinic, using a validated method.15 At each annual visit, this consisted of measuring the blood pressure of a resting arm, palm facing upward, with the participant seated; measurements were repeated 3 times, at least 1 minute apart. Participants were asked to refrain from smoking 30 minutes before the measurements. The blood pressure cuff size was appropriate for measured arm circumference.15 The first 2 blood pressure readings were taken using a calibrated oscillometric device (Dinamap Pro 100 or 1846SX units, GE Medical Systems Information Technologies); the third auscultatory measurement was performed by study personnel unaware of whether the participant had had gastroenteritis at the time of the outbreak. The mean of the 3 measurements was obtained for each visit.
Secondary study outcomes included the presence of reduced kidney function and micro- or macroalbuminuria. The abbreviated Modification of Diet in Renal Disease (MDRD) equation was used to estimate the GFR;16 a GFR below 60 mL/min per 1.73 m2 was considered indicative of reduced kidney function.17 Using 139 duplicate samples, we calibrated the measurement of serum creatinine used in this study against that of the MDRD core laboratory.18,19 Micro- or macro-albuminuria was defined as a random urine albumin:creatinine ratio of 3.4 mg/mmol (30 mg/g).20
Other outcomes considered were the mean systolic and diastolic blood pressures irrespective of antihypertensive therapy use, estimated GFR and random urine albumin:creatinine ratio at the last study visit.
We standardized the rates of hypertension and other outcomes for age and sex using regional distributions from Canadian census data. Differences between groups, according to the described categories of acute gastroenteritis, were evaluated by crude and age- and sex-adjusted analyses. For categorical outcomes, logistic regression was used to test the significance of adjusted differences across categories. Analysis of variance was conducted to determine crude and adjusted differences for continuous outcomes.
The crude and adjusted relative risk of hypertension after acute gastroenteritis was calculated, along with 95% confidence intervals.21 Participants with no symptoms at the time of the outbreak served as the reference group. We adjusted for the following known risk factors for hypertension, selected a priori, and included them in a multivariate Poisson regression analysis:21 sex, age (in 1-year increments), body mass index (in 1-kg/m2 increments assessed at first clinic visit), family history of hypertension, and self-reported use of a low-salt diet or tobacco smoking before the outbreak. To account for potential differences in the access to health care or health surveillance after the outbreak, we also adjusted for the presence of a health assessment in the year before the outbreak.
All participants provided written informed consent. In 2002, both the University of Western Ontario Research Ethics Board and the Kidney Foundation of Canada Scientific Committee approved the current study protocol.
Results
The characteristics of the study participants before and during the outbreak are presented in Table 1. Of the 1958 participants, 675 had no acute symptoms, 909 had moderate symptoms of gastroenteritis and 374 had severe symptoms. The characteristics of the participants before the outbreak were similar across the 3 groups except for smoking status: fewer asymptomatic participants were smokers (22% v. 29% and 30%, p = 0.003) (Table 1).
Participants were followed for a mean of 3.7 (standard deviation 0.7) years after the outbreak. Overall, 86%, 72% and 75% of the participants returned for annual clinic visits 2, 3 and 4 years after the outbreak, respectively, with no difference in these rates across the 3 symptom groups (p = 0.64).
Hypertension
A total of 492 participants received a diagnosis of hypertension after the outbreak, for an age- and sex-standardized rate of 31.1% over the 3.7 years of follow-up. In most cases (83%), the hypertension was diagnosed only on the basis of blood pressure measurements taken by the study personnel; in 13% of cases, the participant had elevated blood pressure measurements recorded at the study clinic despite having been prescribed antihypertensive therapy after the outbreak; and in the remaining 4% of cases, the participant had normal blood pressure measurements recorded at the study clinic after having begun antihypertensive therapy by their primary physician.
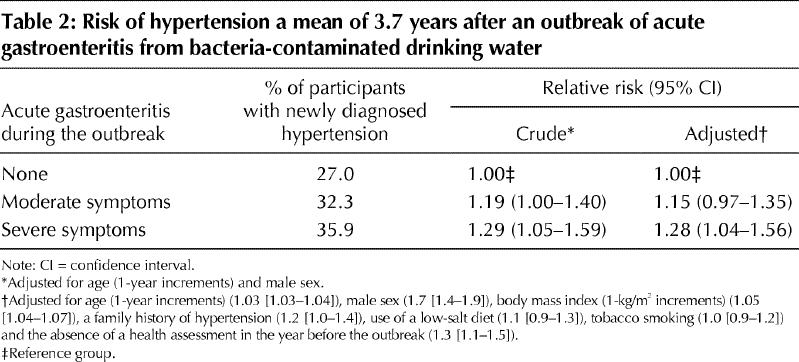
Hypertension was diagnosed in 27.0% of the participants who were asymptomatic during the outbreak, in 32.3% of those who had moderate gastroenteritis and in 35.9% of those who had severe gastroenteritis (p = 0.009) (Fig. 1). Compared with the asymptomatic participants, those with moderate and severe symptoms of gastroenteritis had an adjusted relative risk of a new diagnosis of hypertension of 1.15 (95% confidence interval [CI] 0.97–1.35) and 1.28 (95% CI 1.04–1.56) respectively (Table 2).
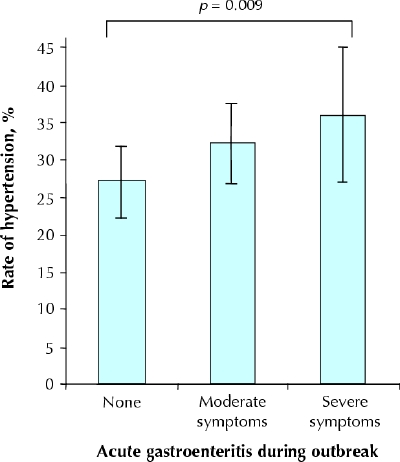
Fig. 1: Age- and sex-standardized rates of newly diagnosed hypertension a mean of 3.7 years after an outbreak of acute gastroenteritis from bacteria-contaminated drinking water. Error bars represent 95% confidence intervals.
Table 2
Among the 1535 participants who attended at least 2 annual study clinic visits, where evidence of hypertension was required on 2 separate occasions, we found that newly diagnosed hypertension was diagnosed in 234 of them, for an age- and sex-standardized rate of 19.4%. The rates were 17.6%, 18.7% and 25.4% among those with no symptoms, moderate gastroenteritis and severe gastroenteritis, respectively (p = 0.01). Compared with the asymptomatic participants, those with moderate and severe symptoms had an adjusted relative risk of a subsequent diagnosis of hypertension of 1.02 (95% CI 0.79–1.32) and 1.49 (95% CI 1.12–1.98) respectively.
Among the 1049 participants who attended all 3 annual clinic visits and who were normotensive at their first visit, 11 were found to have hypertension at both subsequent visits. The respective rates of newly diagnosed hypertension were 0.16%, 1.26% and 1.86% among those with no symptoms, moderate symptoms and severe symptoms of gastroenteritis respectively (p = 0.04).
Among the participants who had no symptoms of gastroenteritis at the time of the outbreak, the mean systolic blood pressure at their last visit, adjusted for age and sex, was 123 mm Hg; the values were significantly higher among those with moderate gastroenteritis (126 mm Hg) and severe gastroenteritis (127 mm Hg) (p = 0.009). The respective diastolic blood pressures were 72, 74 and 74 mm Hg (p = 0.03).
Reduced kidney function and albuminuria
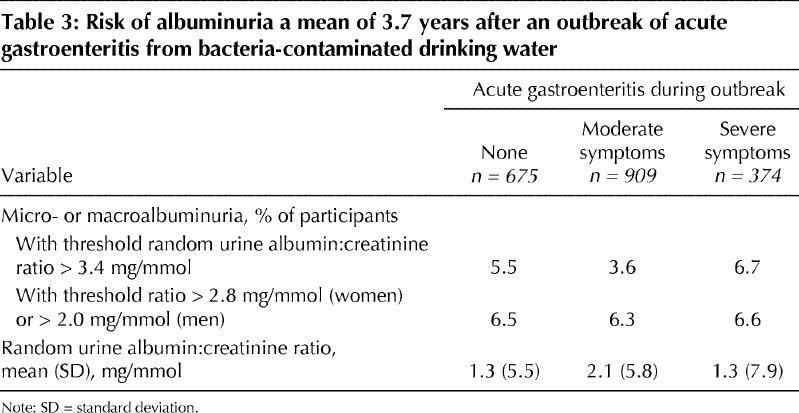
At the last follow-up visit, 38 of the participants were found to have reduced kidney function, of whom 37 had a GFR between 30 and 60 mL/min per 1.73 m2 and 1 had a GFR below 30 mL/min per 1.73 m2. None had end-stage kidney failure. The age- and sex-standardized rate of newly diagnosed reduced kidney function was 4.3% over the 3.7 years of follow-up. The presence of a GFR below 60 mL/min per 1.73 m2 was 2.2% among those with no symptoms, 3.9% among those with moderate gastroenteritis and 6.9% among those with severe gastroenteritis (p = 0.03) (Fig. 2). However, there was no statistical difference between the groups in the mean GFR (p = 0.19), the mean random urine albumin:creatinine ratio (p = 0.57) or the age- and sex-standardized rates of newly diagnosed micro- or macroalbuminuria (p = 0.71) (Table 3).
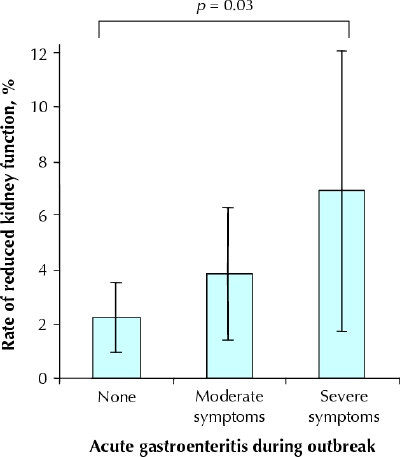
Fig. 2: Age- and sex-standardized rates of newly diagnosed reduced kidney function (defined as glomerular filtration rate < 60 mL/min per 1.73 m2) a mean of 3.7 years after an outbreak of acute gastroenteritis from bacteria-contaminated drinking water. Error bars represent 95% confidence intervals.
Table 3
Interpretation
Nearly 4 years after a major outbreak of acute gastroenteritis from drinking water contaminated with E. coli O157:H7 and Campylobacter bacteria, we observed a relative increase of 33%, or an absolute increase of 9%, in the rate of newly diagnosed hypertension among participants who had experienced severe gastroenteritis during the outbreak. Those who had had acute gastroenteritis had systolic and diastolic blood pressures that were 2 to 4 mm Hg higher than the measurements of participants who had remained asymptomatic during the outbreak. Increased rates of hypertension after bacterial gastroenteritis were also accompanied by evidence of reduced kidney function.
In the general population, the cause of hypertension is multifactorial, influenced by genetic predisposition, diet and lifestyle choices, in addition to any predilection from early kidney damage. We hypothesize that acute enteropathic E. coli and Campylobacter infection may establish a state of chronic inflammation in some people, which could have a number of long-term health consequences.22,23 In addition, those who were acutely ill during the outbreak may have been under the most duress, and detailed psychological assessment may help elucidate the importance of stress as a hypertension mediator.24,25 Biological experiments and human observational studies emphasize the risk of long-term renal sequelae after recovery from hemolytic uremic syndrome, the most toxic form of acute E. coli O157 infection.2,6,8,26,27,28,29 In the current study, we examined the incidence of long-term renal complications after E. coli O157:H7 gastroenteritis, which in some people could be the result of subclinical or unrecognized hemolytic uremic syndrome.30
Large outbreaks of water-borne infections are extremely rare in Western nations. The willingness of the residents in the local community to participate in our study enabled us to obtain detailed information and measurements about their previous and current health. There may never again be an opportunity to systematically study the long-term effects of such a potentially serious widespread outbreak. However, the circumstances surrounding this unique outbreak require the results to be interpreted judiciously.
The number of participants in our study who subsequently had reduced kidney function was small, which limited our ability to conduct relevant multivariate and sensitivity analyses. In addition, the mean estimated GFR and mean random urine albumin:creatinine ratio were not appreciably different after the bacterial gastroenteritis, which suggests that the risk of overt renal disease is not clear. Given the protracted course of most cases of progressive renal disease, a longer follow-up of this cohort could clarify the risk of nephropathy after gastroenteritis due to E. coli O157.31,32,33
Both E. coli O157 and Campylobacter bacteria were present in the drinking water supply, and objective evidence of coinfection was present in some stool cultures. We considered gastroenteritis as a single entity attributable to both pathogens. Although additional analyses may have discerned the rate of hypertension according to stool culture results, the small number of routinely collected specimens at the time of the outbreak limited our ability to do so.
The cohort was assembled 2 years after the outbreak. Selection bias may have influenced the study results, although a previous analysis suggested that any such selection would bias toward demonstrating no association between acute gastroenteritis and long-term sequelae.9
Our data collection after the outbreak depended in part on each participant's ability to recall their health status at the time of the outbreak, which may have been more than 2 years earlier. People seeking financial compensation may be prone to recall acute symptoms that in truth were absent.34 Such misclassification would minimize any true association between acute gastroenteritis and biologically plausible long-term health sequelae. To guard against such a bias, we established a group with self-reported symptoms that were confirmed by medical records: in this group, the observed association with hypertension remained consistent in all of our analyses.
Alternatively, some participants seeking financial compensation who were truly acutely ill during the outbreak may have exaggerated their current symptoms for the purpose of monetary gain. This would exaggerate any true association between bacterial gastroenteritis and long-term sequelae. Although a major concern for self-reported long-term symptoms, we believe this did not influence the observed association with hypertension. We used a standardized and accepted definition of hypertension15 to avoid incorrectly labelling a person as having hypertension. Furthermore, the majority of participants who were found to have hypertension were unaware of its presence at the time of diagnosis.
The outbreak resulted in subsequent detailed surveillance of the health of Walkerton's residents. Although we excluded all people with previously recognized hypertension or risk factors for renal impairment, some participants with newly identified hypertension or reduced kidney function may have had these conditions before the outbreak. Up to 30% of adults may have unrecognized hypertension,15 while others have undiagnosed reduced kidney function.35 As such, the incidence of newly diagnosed hypertension and reduced kidney function probably does not solely represent de novo events arising after the outbreak. Some residents with borderline hypertension or silent renal dysfunction before the outbreak may have been more susceptible to gastrointestinal infection and subsequently had high rates of new diagnoses during follow-up. This would exaggerate any true association between gastroenteritis and these sequelae. However, the equal distribution of relevant health conditions across the 3 exposure groups before the outbreak makes this less likely. The risk of hypertension remained significantly associated with documented symptoms of gastroenteritis even after adjustment for other measured prognostic factors, including health care surveillance before the outbreak. Finally, acute bacterial gastroenteritis was associated with an increased risk of hypertension 4 years after the outbreak among participants who were confirmed to be normotensive by detailed assessment at their first study visit.
In conclusion, acute self-limited bacterial gastroenteritis necessitating medical attention may be an independent risk factor for long-term hypertension and reduced kidney function. Taken together, our findings will help guide future studies assessing the utility of screening and follow-up of patients who recover from acute bacterial gastroenteritis. More importantly, maintaining safe drinking water remains essential to human health,36,37,38,39,40 as transient bacterial contaminations may have implications well beyond a period of acute self-limited illness.41
Main findings
Adults with symptomatic bacterial gastroenteritis from drinking contaminated water were more likely than asymptomatic adults to have newly diagnosed hypertension and reduced renal function during the follow-up period of almost 4 years after infection.
Implications
Acute self-limited bacterial gastroenteritis may be an important causal factor in the pathogenesis of long-term serious health effects such as hypertension and renal impairment.
Supplementary Material
Acknowledgments
We thank Arlene Richards and all the staff and students who assisted with data collection.
Appendix 1.

Footnotes
Fast-tracked article. Published at www.cmaj.ca on May 27, 2005.
This article has been peer reviewed.
Contributors: Amit Garg was the principal author. Amit Garg, Douglas Matsell, Brian Haynes, Marina Salvadori and William Clark all contributed to the conception and design of the study and to the acquisition of data. Amit Garg and Heather Thiessen-Philbrook performed the statistical analysis. All of the authors revised the manuscript for intellectual content and gave their final approval of the version to be published.
The study was supported by a grant from the Kidney Foundation of Canada and the Ontario Ministry of Health and Long-Term Care. These sponsors had no role in the study design, the collection, analysis and interpretation of the data, or the writing of the report. Amit Garg is supported by a Clinician Scientist Award from the Canadian Institutes of Health Research.
Competing interests: None declared.
Correspondence to: Dr. Amit X. Garg, London Kidney Clinical Research Unit, Rm. A01, Westminster Tower, London Health Sciences Centre, 800 Commissioners Rd. E, London ON N6A 4G5; fax 519 685-8072; amit.garg@lhsc.on.ca
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269-76. [DOI] [PubMed]
- 2.Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, et al. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA 2003;290(10):1360-70. [DOI] [PubMed]
- 3.Locht H, Krogfelt KA. Comparison of rheumatological and gastrointestinal symptoms after infection with Campylobacter jejuni/coli and enterotoxigenic Escherichia coli. Ann Rheum Dis 2002;61(5):448-52. [DOI] [PMC free article] [PubMed]
- 4.Hannu T, Mattila L, Rautelin H, Pelkonen P, Lahdenne P, Siitonen A et al. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology (Oxford) 2002;41(3):312-318. [DOI] [PubMed]
- 5.Bremell T, Bjelle A, Svedhem A. Rheumatic symptoms following an outbreak of campylobacter enteritis: a five year follow up. Ann Rheum Dis 1991;50 (12):934-8. [DOI] [PMC free article] [PubMed]
- 6.Hughes AK, Ergonul Z, Stricklett PK, Kohan DE. Molecular basis for high renal cell sensitivity to the cytotoxic effects of shigatoxin-1: upregulation of globotriaosylceramide expression. J Am Soc Nephrol 2002;13(9):2239-45. [DOI] [PubMed]
- 7.Hughes AK, Stricklett PK, Kohan DE. Cytotoxic effect of Shiga toxin-1 on human proximal tubule cells. Kidney Int 1998;54(2):426-37. [DOI] [PubMed]
- 8.Van Setten PA, van Hinsbergh VW, van den Heuvel LP, van der Velden TJ, van de Kar NC, Krebbers RJ et al. Verocytotoxin inhibits mitogenesis and protein synthesis in purified human glomerular mesangial cells without affecting cell viability: evidence for two distinct mechanisms. J Am Soc Nephrol 1997;8(12):1877-88. [DOI] [PubMed]
- 9.Garg AX, Macnab J, Clark WF, Ray JG, Marshall JK, Suri RS, et al. Long-term health sequelae following municipal water contaminated with Escherichia coli O157 and Campylobacter species: population sampling and assessing non-participation biases. Can J Public Health 2005;96(2):125-30. [DOI] [PMC free article] [PubMed]
- 10.Garg AX, Marshall JK, Salvadori M, Thiessen HR, Macnab J, Suri RS, et al. A gradient of acute gastroenteritis to assess risk of long-term health sequelae after drinking bacteria contaminated water. A report from the Walkerton Health Study Investigators; 2005. [DOI] [PubMed]
- 11.Auld H, MacIver D, Klaassen J. Heavy rainfall and waterborne disease outbreaks: the Walkerton example [review]. J Toxicol Environ Health A 2004; 67 (20-22):1879-87. [DOI] [PubMed]
- 12.The investigative report of the Walkerton outbreak of waterborne gastroenteritis May–June 2000. Owen Sound (ON): Bruce-Grey Owen Sound Health Unit; 2000.
- 13.Hrudey S. Drinking-water risk management principles for a total quality management framework. J Toxicol Environ Health A 2004;67(20-22):1555-66. [DOI] [PubMed]
- 14.Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int 2002;61(6):2165-75. [DOI] [PubMed]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42(6):1206-52. [DOI] [PubMed]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130(6):461-70. [DOI] [PubMed]
- 17.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1):S1-266. [PubMed]
- 18.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van LF, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 2002;39(5):920-9. [DOI] [PubMed]
- 19.Garg AX, Clark WF, Salvadori M, Macnab J, Suri RS, Haynes RB, et al; Walkerton Health Study Investigators. Microalbuminuria three years after recovery from Escherichia coli O157 hemolytic uremic syndrome due to municipal water contamination. Kidney Int 2005;67(4):1476-82. [DOI] [PubMed]
- 20.Jones CA, Francis ME, Eberhardt MS, Chavers B, Coresh J, Engelgau M, et al. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis 2002;39(3):445-59. [DOI] [PubMed]
- 21.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159(7):702-6. [DOI] [PubMed]
- 22.Niskanen L, Laaksonen DE, Nyyssonen K, Punnonen K, Valkonen VP, Fuentes R, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension 2004;44(6):859-65. [DOI] [PubMed]
- 23.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA 2003;290(22):2945-51. [DOI] [PubMed]
- 24.Markovitz JH, Matthews KA, Kannel WB, Cobb JL, D'Agostino RB. Psychological predictors of hypertension in the Framingham Study. Is there tension in hypertension? JAMA 1993;270(20):2439-43. [PubMed]
- 25.Reacher M, McKenzie K, Lane C, Nichols T, Kedge I, Iversen A, et al. Health impacts of flooding in Lewes: a comparison of reported gastrointestinal and other illness and mental health in flooded and non-flooded households. Commun Dis Public Health 2004;7(1):39-46. [PubMed]
- 26.Zoja C, Angioletti S, Donadelli R, Zanchi C, Tomasoni S, Binda E, et al. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-kappaB dependent up-regulation of IL-8 and MCP-1. Kidney Int 2002;62(3):846-56. [DOI] [PubMed]
- 27.Schmid DI, Kohan DE. Effect of shigatoxin-1 on arachidonic acid release by human glomerular epithelial cells. Kidney Int 2001;60(3):1026-36. [DOI] [PubMed]
- 28.Pijpers AH, van Setten PA, van den Heuvel LP, Assmann KJ, Dijkman HB, Pennings AH, et al. Verocytotoxin-induced apoptosis of human microvascular endothelial cells. J Am Soc Nephrol 2001;12(4):767-78. [DOI] [PubMed]
- 29.Kodama T, Nagayama K, Yamada K, Ohba Y, Akeda Y, Honda T. Induction of apoptosis in human renal proximal tubular epithelial cells by Escherichia coli verocytotoxin 1 in vitro. Med Microbiol Immunol (Berl) 1999;188(2):73-8. [DOI] [PubMed]
- 30.Lopez EL, Contrini MM, Devoto S, de RM, Grana MG, Aversa L, et al. Incomplete hemolytic-uremic syndrome in Argentinean children with bloody diarrhea. J Pediatr 1995;127(3):364-7. [DOI] [PubMed]
- 31.Mann JF, Gerstein HC, Yi QL, Franke J, Lonn EM, Hoogwerf BJ, et al. Progression of renal insufficiency in type 2 diabetes with and without microalbuminuria: results of the Heart Outcomes and Prevention Evaluation (HOPE) randomized study. Am J Kidney Dis 2003;42(5):936-42. [DOI] [PubMed]
- 32.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004;351(19):1941-51. [DOI] [PubMed]
- 33.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med 1998;339(20):1448-56. [DOI] [PubMed]
- 34.Frueh BC, Gold PB, de Arellano MA. Symptom overreporting in combat veterans evaluated for PTSD: differentiation on the basis of compensation seeking status. J Pers Assess 1997;68(2):369-84. [DOI] [PubMed]
- 35.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, et al. Chronic kidney disease awareness, prevalence, and trends among US adults, 1999 to 2000. J Am Soc Nephrol 2005;16(1):180-8. [DOI] [PubMed]
- 36.Davies JM, Mazumder A. Health and environmental policy issues in Canada: the role of watershed management in sustaining clean drinking water quality at surface sources. J Environ Manage 2003;68(3):273-86. [DOI] [PubMed]
- 37.Krewski D, Balbus J, Butler-Jones D, Haas C, Isaac-Renton J, Roberts K, et al. Managing the microbiological risks of drinking water. J Toxicol Environ Health A 2004;67(20-22):1591-617. [DOI] [PubMed]
- 38.Rizak S, Cunliffe D, Sinclair M, Vulcano R, Howard J, Hrudey S, et al. Drinking water quality management: a holistic approach. Water Sci Technol 2003; 47(9):31-6. [PubMed]
- 39.Blackburn BG, Craun GF, Yoder JS, Hill V, Calderon RL, Chen N, et al. Surveillance for waterborne-disease outbreaks associated with drinking water — United States, 2001–2002. MMWR Surveill Summ 2004;53(8):23-45. [PubMed]
- 40.Barron G, Buchanan S, Hase D, Mainzer H, Ransom MM, Sarisky J. New approaches to safe drinking water. J Law Med Ethics 2002;30(3 Suppl):105-8. [PubMed]
- 41.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217-23. [DOI] [PubMed]
- 42.Jones CA, McQuillan GM, Kusek JW, Eberhardt MS, Herman WH, Coresh J, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis 1998;32(6):992-9. [DOI] [PubMed]
- 43.Canadian Diabetes Association. 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Toronto: The Association; 2003. Available: www.diabetes.ca/cpg2003/chapters.aspx (accessed 2005 Jan 16).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


