Abstract
Neutrophils are the first responders among peripheral immune cells to infiltrate the central nervous system following a traumatic brain injury (TBI), triggering neuroinflammation that can exacerbate secondary tissue damage. The precise molecular controls that dictate the inflammatory behavior of neutrophils post-TBI, however, remain largely elusive. Our comprehensive analysis of the molecular landscape surrounding the trauma in TBI mice has revealed a significant alteration in the abundance of β2 integrin (ITGB2), predominantly expressed by neutrophils and closely associated with immune responses. Using the fluid percussion injury (FPI) mouse model, we investigated the therapeutic efficacy of Rovelizumab, an agent that blocks ITGB2. The treatment has demonstrated significant improvements in neurologic function in TBI mice, attenuating blood–brain barrier permeability, mitigating oxidative stress and inflammatory mediator release, and enhancing cerebral perfusion. Moreover, ITGB2 blockade has effectively limited the adherence, migration, and infiltration of neutrophils, and has impeded the formation of neutrophil extracellular traps (NETs) upon their activation. Finally, it was demonstrated that ITGB2 mediates these effects mainly through its interaction with intercellular adhesion molecule-1 (ICAM 1) of endotheliocyte. These findings collectively illuminate ITGB2 as a crucial molecular switch that governs the adverse effects of neutrophils post-TBI and could be targeted to improve clinical outcome in patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-025-02071-9.
Keywords: Traumatic brain injury, Neuroinflammation, β2 integrin, Neutrophil extracellular traps, Intercellular adhesion molecule-1
Introduction
Traumatic brain injury (TBI) is a serious public health issue that poses a significant threat to human health and well-being, and it is one of the leading causes of death and disability worldwide [1, 2]. TBI can be categorized into primary and secondary brain injuries. Within minutes of mechanical trauma, a series of cellular and biochemical changes occur in the brain, including calcium overload, inflammatory responses, oxidative stress, endothelial dysfunction, and apoptosis, which in turn lead to secondary brain injury [3–7].
When primary brain injury is beyond intervention, controlling and reducing secondary brain injury becomes a focal point and challenge in clinical and scientific research. Literature has reported that changes in the immune system after TBI have been proven to play an important role in the occurrence and development of secondary injury [8]. An increasing number of studies have found that persistent and excessive secondary inflammatory damage hinders neuroprotection and neurorepair, which is not conducive to the recovery of neurological function after TBI [9, 10]. Therefore, there is an urgent need to explore strategies to efficiently control and mitigate the excessive activation of inflammatory cells.
Neutrophils, as an essential part of the innate immune system, play a significant role in the inflammatory response following TBI [11, 12]. Dennis et al. reported that neutrophils are the first peripheral immune cells to enter the central nervous system (CNS) after TBI and can reach a peak within 24 to 72 h post-injury [13]. In the wake of TBI, there is a significant release of inflammatory factors and chemokines (IL-1β, TNF-α, IL-6, CXCL1-5, and CXCL8-10) from the injured brain tissue, which are potent chemoattractants for the recruitment and activation of neutrophils [14]. At the same time, the neutrophils that infiltrate the site of injury exacerbate the inflammatory response by discharging a range of detrimental molecules, such as reactive oxygen species (ROS) [15], lipocalin 2 [16], IL-17A [17], and MMP9 [18]. The synergistic release of these factors intensifies the inflammatory cascade, leading to the formation of a severe inflammatory storm. Correspondingly, previous studies have shown that inhibiting peripheral leukocyte activation after TBI could confer long-term neuroprotection and improve the outcome of TBI, suggesting that neutrophils trigger neuroinflammation that can exacerbate secondary tissue damage after TBI [19, 20].
Concurrently, in the recent years, there has been a growing focus on the study of neutrophil extracellular traps (NETs), which encompasses the ejection of granular proteins into the cellular cytoplasm, the extrusion of decondensed chromatin along with histone modifications, and the release of cytoplasmic proteins into the extracellular milieu, which may constitute a novel form of cellular demise [21]. Neutrophil elastase, NADPH oxidase, MPO, and citrullination of histones are required for NETs formation [22]. With the deepening of research, Ma et al. found that activating neutrophil elastase downregulates the Sepina3n aggravating blood–brain barrier (BBB) disruption after TBI [23]. Additionally, the involvement of NETs in the pathogenesis of TBI is supported by observations of 1) NETs can directly lead to the death of endothelial cells [24], 2) NETs aggravate neuronal endoplasmic reticulum stress and apoptosis via TLR9 after TBI [25], 3) NETs contribute to coagulopathy after TBI [26], 4) NETs facilitate sympathetic hyperactivity by polarizing microglia toward M1 phenotype after TBI [27]. In contrast, the inhibition of NETs could ameliorate neuroinflammation, neuronal apoptosis and NLRP1-dependent neuronal pyroptosis in TBI mice [28, 29].
In TBI, the role of neutrophil activation in inflammatory response and secondary brain injury has been elucidated in detail. However, the precise molecular controls that dictate the inflammatory behavior of neutrophils post-TBI remain largely elusive. We intend to explore the targets that mediate the adhesion and infiltration of neutrophils and take interventions to reduce the infiltration of peripheral immune cells, thereby breaking the inflammatory cascade, and effectively controlling the excessive inflammatory response after TBI. To this end, we performed an unbiased genome-wide transcript sequencing of brain injured tissue surgically removed from mice with TBI. We identified β2 integrin (ITGB2) as a master gene in triggering brain inflammation. We employed an agent for ITGB2 blockade and demonstrated its efficacy in preclinical models.
Methods
The animal study was approved by the Ethics Committee of Tianjin Medical University General Hospital, and all experimental procedures were strictly compliant with the ARRIVE Guidelines. Human sample collection was carried out in strict accordance with protocols endorsed by the Institutional Review Board of Tianjin Medical University General Hospital (Tianjin, China). Written informed consent was obtained from each patient or legal surrogate in accordance with the Helsinki declaration.
Reagents
Rovelizumab (Med Chem Express, Shanghai, China), Monoclonal anti-mouse lymphocyte antigen 6 complex locus G antibody (Anti-Ly6G) (RRID: AB_469157) was purchased from Invitrogen (MA, USA). Anti-mouse intercellular adhesion molecule-1 antibody (Anti-ICAM 1) (RRID: AB_1107661) and Mouse IgG1 isotype control antibody (RRID: AB_1107784) were purchased from Bio X Cell (Lebanon, USA). Primary antibodies against ITGB2 (RRID: AB_11005054), NOX2 (RRID: AB_3304390) and Iba 1 (RRID: AB_3148646) were purchased from NOVUS (Centennial, USA). Primary antibodies against GFAP (RRID: AB_880202), 8-hydroxy-2’-deoxyguanosine (8-OHDG) (RRID:AB_940049), Claudin-5 (RRID: AB_11157940), MPO (RRID: AB_2864724), Ly6G (RRID: AB_2923218), H3cit (RRID: AB_304752), H3 (RRID: AB_302613) were purchased from Abcam (Cambridge, UK). Primary antibody against NeuN (RRID: AB_2904530) was purchased from Cell Signaling Technologies (Danvers, MA, USA). Primary antibodies against CD31 (RRID: AB_2161028), MPO (RRID: AB_2250866) and VE-Cadherin (RRID: AB_2077789) were purchased from R&D (Minneapolis, USA). Primary antibodies against APC anti-Ly6G (RRID: AB_1877163), Percp-cy5.5 anti-Ly6G (RRID: AB_1877271) and AP-Cy7 anti-CD11b (RRID: AB_830642) were purchased from Biolegend (San Diego, CA, USA). Primary antibody against Occludin (RRID: AB_2533977) was purchased from ThermoFisher (MA, USA). Primary antibodies against ICAM1 (RRID: AB_3674626), GAPDH (RRID: AB_2769570), PAD4 (RRID: AB_2763639), MMP9 (RRID: AB_2862974) and MMP2 (RRID: AB_2766854) were purchased from Abclonal (Wuhan, CHINA). Syndecan-1 ELISA kit (Abcam), IL-6, IL-1β, and TNF-α quantizing ELISA kit (Abclonal), Dihydroethidium (Med Chem Express), DCFH-DA (Solarbio, Beijing, China), Evans blue (Sigma Aldrich, St. Louis, MO, USA), Tunel (Beyotime), MPO activity kit(Sigma-Aldrich), MPO-DNA ELISA kit (Roche, Basel, Switzerland), Rhodamine 6G (Sigma-Aldrich), FITC-dextran (2,000,000 Da, Sigma-Aldrich), Neutrophil Isolation Kit (Solarbio), N-formylmethionylleucylphenylalanine (Med Chem Express), Peroxidase-labeled anti-DNA mAb (Sigma-Aldrich), Pierce ™ protein A/G magnetic bead (ThermoFisher), Cell Tracker™ CM-DiI (Invitrogen).
Animals, TBI model, and experimental design
Male C57BL/6 J mice, aged 8 weeks and weighing between 20–22 g, sourced from HFK Bioscience Corporation (Beijing, China), were maintained in a controlled environment with a 12-h light–dark cycle, regulated temperature and humidity, and provided with ad libitum access to food and water. The procedures to establish fluid percussion injury (FPI) mouse model have been previously described [10]. Briefly, a 3-mm-diameter cranial opening was made, preserving the dura mater, located 2.0 mm posterior to the bregma and 2.0 mm lateral to the midline. This opening facilitated the rapid injection of saline at a precise pressure using an FPI device (Custom Design & Fabrication, Richmond, VA, USA). The injury was induced by regulating the pressure to 1.9 ± 0.2 atm. Immediately after TBI, the mice were administered intravenously with 100μL of (1) vehicle saline, (2) Rovelizumab (4 mg/kg body weight). Additionally, mice received an intraperitoneal administration of (3) Anti-Ly6G (0.1 mL of 1 mg/mL), (4) IgG Isotyoe Control (0.1 mL of 1 mg/mL), (5) Anti-ICAM 1 (0.1 mL of 1 mg/mL) 24 h before TBI. The sham group was included, which underwent all procedural steps except for the actual FPI strike.
Human brain tissue samples
The study encompassed three cases of TBI, sourced from individuals who had endured such trauma and were subjected to Cranial Injury Debridement to remove the damaged neural areas within a 24-h window from the onset of symptoms. Concurrently, for the establishment of a control group, three brain tissue samples were acquired from donors who had been declared brain-dead. Inclusion criteria were patients with 1) confirmation of TBI within a 6-h timeframe post-injury via computed tomography (CT) or magnetic resonance imaging (MRI) of the head; 2) age 18–80 years. Exclusion criteria were patients with 1) spinal cord injury or other extracranial injuries; 2) previous TBI or other neurological diseases such as ischemic and hemorrhagic stroke; 3) active or recent infection alongside ongoing treatments with anti-inflammatory or immunosuppressive medications; 4) malignant tumor; 5) severe liver/kidney failure; 6) hematological, rheumatic, or immune disorders. Demographic and clinical information were extracted from electronic medical records.
Transcriptome RNA sequencing and bioinformatics analyses
A total of 15 brain tissues were submitted to the RNA sequencing process, including TBI/Sham (10/5) mice from different time nodes. Total RNA was extracted using MagBeads Total RNA Extraction Kit (ThermoFisher) following the manufacturer’s instructions and quality checked by an Agilent 2100 Bioanalyzer (Agilent technologies, Santa Clara, CA, US). RNA sequencing was further performed using the Illumina NovaSeq6000 platform after completion of library construction by steps such as isolation of mRNA, fragmentation, first-strand cDNA synthesis, second-strand cDNA synthesis, end repair, 3 ‘end plus A, ligation adapters and enrichment. The high-throughput RNA sequencing service was provided by Biotechnology Corporation (Shanghai, China).
After data quality control and low-abundance transcript filtering, RNA read counts were normalized to variance-stable transformed values and performed gene differential expression analysis using the “DESeq2” R package. Differentially expressed genes (DEGs) between TBI and Sham were identified with the criteria of the absolute value of log2(Fold Change) > 1 and false discovery rate (FDR) < 0.05. Using the R package “cluster profile”, the TBI-associated genes were screened, following that, gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were carried out. a protein–protein interaction (PPI) network was constructed to screen for pivotal genes based on the String online database (https://cn.string-db.org).
Western blotting
Western blotting was carried out in the manner previously described [10]. Protein extraction from brain tissues of each experimental group was performed using a lysis buffer that included protease and phosphatase inhibitors. The brain tissues were homogenized, allowing proteins to be extracted into the supernatant fraction, which was then quantified using the bicinchoninic acid assay. Specific primary antibodies were applied to polyvinylidene fluoride (PVDF) membranes and incubated overnight at 4 °C. Except for GAPDH, which was prepared at 1:10 000 dilution, the remaining antibodies were prepared at a ratio of 1:1000 dilution. Next day, the membranes underwent three rounds of washing to remove unbound antibodies. Subsequently, the membranes were incubated for 1 h at room temperature with secondary antibodies (Zhongshan Golden Bridge, Beijing, China), which were prepared at a ratio of 1:5000 dilution. The immunoblots were then developed using an enhanced chemiluminescence detection system and visualized with the ChemiDoc Touch Imaging System (Bio-Rad, Hercules, CA, USA). The intensity of the protein bands was analyzed densitometrically using ImageJ software (version 1.53, National Institutes of Health), providing a quantitative assessment of protein expression levels.
Immunofluorescence (IF)
For IF staining, frozen sections of brain tissue (10 μm thick) were rewarmed for 10 min and washed three times with PBS for 5 min each. Then the sections were permeabilized for 30 min at room temperature with 0.3% Triton X-100 and blocked for 1 h at 37℃ with 3% bovine serum albumin (BSA) to prevent non-specific antibody binding. After that, the sections were incubated overnight at 4℃ with primary antibodies, which were prepared at a ratio of 1:200 dilutions. Next day, the sections were rinsed three times with PBS for 5 min to remove any unbound primary antibodies before incubation for 1 h at room temperature with the respective secondary antibodies (ThermoFisher, MA, USA), which were prepared at a ratio of 1:500 dilution. After the final washes with PBS, the sections were prepared for nuclear staining by adding DAPI. The fluorescence signals were then examined using a fluorescence microscope (Olympus BX-61, Tokyo, Japan) and a laser scanning confocal microscopy (Olympus FV1000). Among them, for the coronal sections of mouse brain, our fields of view were selected in the cortex around the trauma, as shown in Fig. 3H. The red arrow in the lesion area in the H&E staining diagram indicates the trauma center, and the black box indicates the cortex around the lesion. The captured IF images were subsequently analyzed using ImageJ software to quantify fluorescence intensity and distribution.
Fig. 3.
Effect of Rovelizumab on oxidative stress at 3 days after TBI. A-B Representative western blotting bands and densitometric quantifications of NOX2 (n = 6/group); C-E The changes in inflammatory cytokines (IL-6、IL-1β and TNF-α) after brain injury and the effects of Rovelizumab were evaluated using ELISA (n = 6/group); F-G Flow cytometry was used to detect the expression and quantification of DCFH-DA in neutrophils (Ly6G +) at 3 days after TBI; H Schematic diagram of HE staining of coronal sections of the brain of TBI mice. The black boxes in the H&E-stained illustration show the perilesional cortex, and the red region shows the lesioned area; I-J Representative microphotographs of immunostaining and quantitative analysis of DHE/MPO/DAPI. Scale bar = 50 µm (n = 6/group); K-L Representative microphotographs of immunohistochemical staining and quantitative analysis of 8-OHDG/MPO/DAPI. Scale bar = 20 µm (n = 6/group). All data are shown as mean ± SD
Neurological function assessment
Prior to the induction of FPI, a modified neurological severity scores (mNSS) functional test and rotarod test were performed, and it was repeated on the 1st, 3rd, 5th, and 7th days after TBI. The procedures used were based on previous research [30]. All testers were well-trained and blinded to the experimental treatment assignment.
Evans blue (EB) dye extravasation
To assess vascular leakage and brain edema resulting from FPI, the mice were administered 100 µL of a 2% EB solution via the tail vein. Two hours after injection, the mice were euthanized and subjected to perfusion with PBS. Subsequently, the brains were extracted and examined under a stereoscope, focusing on both the horizontal and coronal planes of the tissue. The cerebellum and olfactory bulb were meticulously removed, and the resulting half-brains were weighed. These half-brains were then homogenized in 1 mL of formamide (Aladdin, Shanghai, China) and incubated at a temperature of 60 °C for an entire night. For 30 min, the brain homogenates were centrifuged at 16,000 g. The supernatant, containing the EB, was carefully transferred to a 96-well plate, with 200 µL aliquoted per well in duplicate. The presence of EB in the supernatant was quantified using a microplate reader, which was preheated to ensure accurate measurements at an optical density (OD) of 620 nm.
Hematoxylin and Eosin (H&E) Staining and Nissl Staining
For H&E and Nissl staining, paraffin sections of brain tissue were stained using a hematoxylin and eosin staining kit (Solarbio) and Nissl Stain Kit (Methyl Violet Method) (Solarbio) as per the manufacturer’s guidelines after the dewaxing process. Finally, the sections were mounted using neutral glue and examined under a light microscope (Olympus BX-46).
Enzyme-Linked Immunosorbent Assay (ELISA)
The citrated blood samples (0.32% final concentration) were drawn from the apex of TBI mice, cell free plasma (CFP) was then obtained for syndecan-1 measurement using an ELISA kit and the protein concentrations of IL-6, IL-1β, and TNF-α in mouse brain lysates were measured with mouse IL-6, IL-1β, and TNF-α quantizing ELISA kit according to the manufacturer instructions. The measured OD values were converted into concentration values.
Flow cytometry
To quantify neutrophils (CD11b+Ly6G+) and its ROS content, we isolated cellular components from brain tissue and circulating blood to perform flow cytometry analysis as we previously described [31]. Briefly, brains were harvested and homogenized with 70 µm nylon cell strainers (BD Biosciences, Franklin Lakes, NJ) in cold PBS. Cell suspensions were centrifuged at 2000 rpm for 5 min, and cell pellets were collected. After centrifugation, the cell pellets were resuspended in 5 ml of 30% Percoll (Cytiva, Shanghai, CHINA) and centrifuged at 700 g for 10 min. Cell pellets were harvested on the bottom of the tube and washed once with 5 ml 1% BSA solution for staining. On the other hand, blood samples were subjected to centrifugation at 150 g for a duration of 20 min. This process effectively separated the blood components into distinct layers: an upper plasma layer, a lower red blood cell layer, and a thin intermediate layer rich in leukocytes. The white blood cells, present at the interface, were meticulously harvested from the leukocyte layer. Subsequently, red blood cell lysis buffer (Solarbio) was introduced, following the manufacturer’s recommended protocol to purify the white blood cell population. The protocol of cell staining followed the manual’s instructions. Flow cytometry was performed using a FACS LSR II flow cytometer (Beckon Dickinson) and data were analyzed by Flow Jo version 10 (flowjo.com).
Dihydroethidium (DHE) staining
For DHE staining, the frozen sections of brain tissue (10 μm thick) were recooled and washed three times with PBS buffer, then working solution containing 5 µM DHE was added and incubated at 37° C for half an hour for fluorescent probe loading. After appropriate rinsing, the cells were incubated with primary antibody to MPO (diluted 1:200) at 4° C overnight. The next day, sections were washed three times with PBS for 5 min to remove unbound primary antibodies and then incubated for 1 h at room temperature with the corresponding secondary antibody (ThermoFisher, MA, USA) at a dilution ratio of 1:500. After a final wash with PBS, DAPI was added for nuclear staining. The fluorescence signals were observed by fluorescence microscope (Olympus BX-61). The acquired IF images were analyzed using ImageJ software to quantify fluorescence intensity and distribution.
Cerebral blood flow
Cerebral cortical blood flow was monitored at 3 days post-TBI using a non-invasive laser speckle imaging technique. The system utilized a PeriCam PSI System (Perimed), which features a 70 mW integrated laser and a high-resolution CCD camera with a 1388 × 1038-pixel array, as detailed in a recent publication [32]. Briefly, mice were first sedated using isoflurane to ensure a non-invasive and stress-free monitoring environment. They were then positioned on a temperature-controlled stereotaxic frame (RWD Life Science, San Diego, CA, USA) with the skull exposed. The CCD camera was positioned 10 cm directly above the mouse skull, allowing it to capture images of cerebral blood flow within a defined scanning area of 2.0 × 2.0 cm. The resulting images were analyzed using PIMSoft software (version 1.5, Perimed). This software calculated the ipsilateral cortical blood flow, measured in perfusion units (PU), and presented the data as mean perfusion values.
Immunohistochemistry (IHC) staining
For IHC staining, the paraffin Sects. (10 μm thick) were boiled for 30 min in sodium citrate buffer (0.01 mol/L, pH 6.0) (Solarbio) for antigen retrieval after a series of dewaxing and hydration. After cooling, the slides were blocked to prevent nonspecific binding by incubating them in a solution of PBS containing 0.3% Triton X-100 and 3% BSA for 1.5 h at room temperature. Subsequently, the sections were incubated with primary antibody against MPO (diluted 1:200) at 4 °C overnight. Next day, the slides were washed with PBS and then incubated with secondary antibody (Gene Tech, Shanghai, China) for 1 h. Following another round of PBS washes, the slides were developed using a 3,3’-diaminobenzidine (DAB) solution for the peroxidase reaction, which produces a visible precipitate at the antigen site. The sections were then counterstained with hematoxylin, dehydrated through a series of graded ethanol solutions, and cleared with xylene. The slides were mounted with neutral glue and examined under an optical microscope (Olympus BX-46). Among them, for the coronal sections of mouse brain, our fields of view were selected in the cortex around the trauma, as shown in Fig. 3H. The IHC images were analyzed using ImageJ software to quantify staining intensity and distribution.
In vivo two-photon microscopy
Mice were anesthetized and maintained on a heating plate to ensure their body temperature was kept constant at 37 ± 0.5 °C. A precise craniotomy was then performed over the right somatosensory cortex, positioned 2.5 mm lateral and 1.5 mm posterior to the bregma, using a high-speed micro drill. A sterile cover glass was carefully placed over the cranial window and affixed with dental cement to create a stable imaging portal. The mice were subsequently imaged through these cranial windows using an upright multiphoton laser-scanning microscope (Olympus FluoView FVMPE-RS), which allowed for high-resolution, in vivo observation of the cerebral cortex. To detect leukocyte, Rhodamine 6G (0.1 mL of 20 μM) was injected i.v. into mice [33]. Blood vessels were visualized by i.v. injection of FITC-dextran (0.1 mL of 10 mg/mL) [34].
Tunel staining
As previously described [35], the frozen sections of brain tissue (10 μm thick) were blocked by BSA (3%) in PBS with 0.3% Triton X-100. After incubation overnight at 4℃ with primary antibody against NeuN (diluted 1:200), Tunel staining was performed by incubating the sections in the mixture of Tunel staining solution and Alexa Fluor 555 donkey anti-mouse IgG (Thermo Fisher) for 1 h at room temperature. After the final washes with PBS, the sections were prepared for nuclear staining by adding DAPI. The fluorescence signals were then examined using a fluorescence microscope (Olympus BX-61). The captured IF images were subsequently analyzed using ImageJ software to quantify fluorescence intensity and distribution.
Quantification of NETs content
For MPO activity, as described previously [28]. Briefly, the brain cortex from the side of injury was homogenized and centrifuged, and the precipitate was resuspended in potassium phosphate buffer containing 0.5% cetyltrimethylammonium bromide (Sigma-Aldrich). Then 40μL of the supernatant was mixed with 100μL of tetramethylbenzidine solution (Sigma-Aldrich) and incubated in a 96-well plate in the dark for 10 min. The presence of MPO in the samples was quantified by measuring the absorbance at 450 nm using a spectrophotometer. The entire process was conducted by two researchers who were unaware of the experimental group assignment.
For quantification of MPO-DNA complexes, initially, the cortex samples from the side of injury were homogenized and centrifuged. After overnight coating with anti-MPO antibody at 4 ℃, 96-well plates were then blocked with 5% BSA for 2 h at room temperature. Then 50 μL of tenfold dilution of the mouse brain homogenates and 80 μL of incubation buffer containing a peroxidase-labeled anti-DNA mAb were mixed and added to the wells. The plates were then incubated at room temperature for 2 h. After washing, the plate was developed with 100 μL ABST substrate. After a 30-min incubation in the dark, the absorbance (OD) at 405 nm was measured, indicating the presence of MPO-DNA complexes. The entire process was conducted by two researchers who were unaware of the experimental group assignment.
Co-immunoprecipitation
Briefly, the cortex samples from the side of injury were homogenized and centrifuged. The supernatants were incubated with Pierce ™ protein A/G magnetic beads (incubated with 1 µg anti-mouse ITGB2 antibody for 1 h at room temperature) overnight at 4 °C. The supernatants were discarded, and the magnetic beads were washed thrice with lysis buffer. Then, each sample was dissolved in 1 × loading buffer (containing DTT), heated in a 100 °C dry heater for 10 min and performed western blotting experiments.
Cell lines, cell culture, and experimental design
Human Cortical Microvessels Endothelial Cells/D3 (hCMEC/D3) (ATCC, Manassas, VA, USA) maintained in Endothelial Growth Medium-2 (Lonza) supplemented with 1% penicillin/streptomycin. Cells were cultured at 37 °C in a humidified atmosphere with 5% CO2, and the medium was changed every 2–3 days. To evaluate neutrophil adhesion and migration in vitro, (1) Vehicle saline, (2) Rovelizumab (10 µM), (3) IgG Isotype Control (0.1 mL of 1 mg/Ml), (4) Anti-ICAM 1 (0.1 mL of 1 mg/mL) were added to the culture medium for the management of Polymorphonuclear neutrophil (PMN).
PMN isolation, adhesion, and transmigration assays
PMNs were isolated from peripheral venous blood of healthy individuals using a Neutrophil Isolation Kit. The adherence of PMNs to treated hCMEC/D3 was analyzed as previous described [36]. In brief, PMNs were first labeled using the fluorescent probe Cell Tracker™ CM-DiI per the manufacturer’s instructions. The labeled cells (5 × 104 cells/well) were then added to treated hCMEC/D3 and co-incubated in a 24-well plate at 37 °C for 1 h. After the incubation period, non-adherent PMNs were washed away, and the adherent cells were fixed with a 4% paraformaldehyde solution. The fixed PMNs were then visualized under a fluorescence microscope (Olympus FV1000) and quantified using the NIH ImageJ software. Additionally, The PMN trans-endothelial migration was measured as described previously [37]. Briefly, hCMEC/D3 were cultured on 24-well transwell inserts (3-µm pore) until confluent. They were then subjected to the designated treatments for a period of three hours at 37 °C. Post-treatment, the Cell Tracker-labeled PMNs were introduced onto the hCMEC/D3 monolayer within the transwell inserts. These were then placed in a medium containing N-formylmethionylleucylphenylalanine (fMLP) (1 μM) for 1 h at 37 °C.The media from the bottom chambers of the transwells were harvested, and the number of fluorescently positive cells was determined using a FACS LSR II flow cytometer.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Variance homogeneity was evaluated using the F-test, and the normality of the quantitative data was assessed with the Kolmogorov–Smirnov test. When data were normally distributed with equal variances, t-tests were applied for pairwise comparisons, and one-way or repeated measures analysis of variance (ANOVA) was utilized for multiple group analyses, followed by Tukey’s post-hoc test. Non-parametric comparisons between groups were conducted using the Kruskal-Walli’s test. The statistical analyses were conducted using SPSS software (version 26.0, IBM Corporation). A p-value below 0.05 was recognized as statistically significant.
Results
TBI increases the expression of ITGB2 preferentially in neutrophil in patients and mice
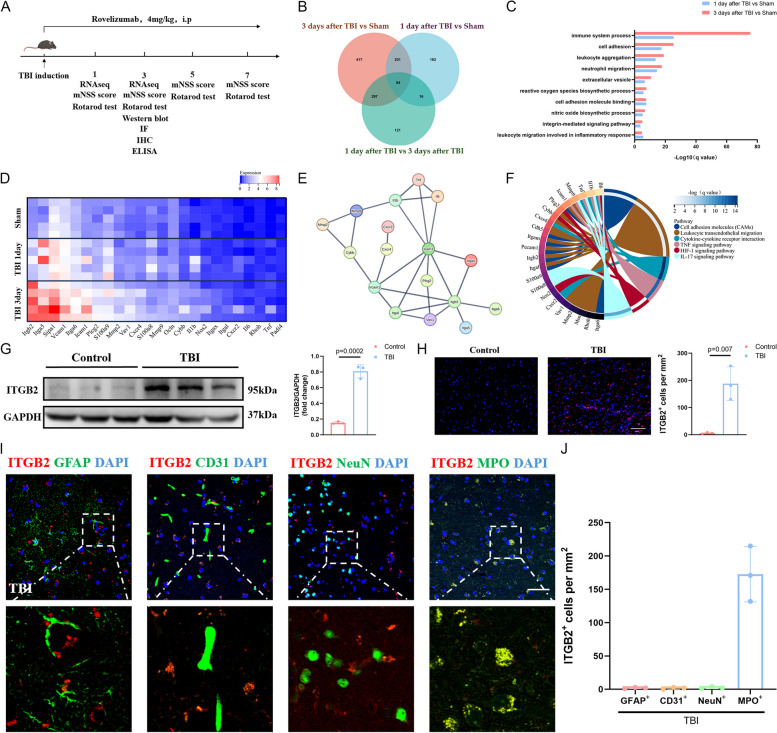
To probe the molecular events in brain edema after TBI, we performed transcriptome sequencing of 10 brain trauma tissues from the mice with TBI. In contrast to the 5 sham mice brain tissue examined simultaneously, brain trauma tissue developed a distinct gene expression pattern (Fig. 1B). The divergent gene profiles between sham and mice with TBI involve pathways catalyzing immune system process, cell adhesion, leukocyte aggregation and neutrophil migration (Fig. 1C).
Fig. 1.
Increased ITGB2 content in brain tissue after TBI was associated with infiltrated neutrophils. A Schematic diagram of the experimental design; B Comparisons of the DEGs in brain tissues among 1 and 3 days after TBI and Sham mice. Each number indicates the unique or common DEGs between comparisons; C Selected top 10 Gene Ontology enrichment in brain tissue at 1 and 3 days after TBI versus Sham mice; D The expression profile of top genes in brain tissue at 1 and 3 days after TBI and Sham mice (n = 5/group); E Protein–Protein Interaction (PPI) network analysis with the significantly regulated genes; F The circos graph of Kyoto Encyclopedia of Genes and Genomes enrichment pathways associated with the above genes in data set of 3 days after TBI versus Sham mice; G Representative western blotting bands and densitometric quantifications of ITGB2 between TBI patients and Control (n = 3/group); H Representative microphotographs of immunostaining and quantitative analysis of ITGB2 between TBI patients and Control. Scale bar = 50 µm (n = 3/group); I-J Immunostaining of ITGB2 expression in Astrocytes (GFAP+ cells), Endotheliocytes (CD31+ cells), Neurons (NeuN+ cells) and Neutrophils (MPO+ cells) in TBI patients trauma brain tissue (n = 3/group). Scale bar = 20 µm. All data are shown as mean ± SD
Of note, there are 84 common DEGs that changed during TBI progression in mice (Fig. 1B), among which we analyzed the expression profile of the top 25 genes that was most relevant to the immune response. ITGB2 was most abundantly expressed among these genes (Fig. 1D). The PPI network of the above genes was shown in Fig. 1E, of which ITGB2 displayed superior interactions with other molecules and may deserve more attention. Additionally, KEGG enrichment analysis of these genes in the data set of 3 days after TBI versus Sham mice revealed that these genes were mainly associated with cell adhesion molecules (CAMs), leukocyte trans endothelial migration, and others (Fig. 1F).
To verify the important role of ITGB2 in the pathological process after TBI, we performed protein quantification of brain tissue from three donors and three TBI patients undergoing hematoma evacuation surgery and found that ITGB2 expression was significantly increased after trauma (Fig. 1 G-H). All three patients had severe TBI with Glasgow Coma Scale (GCS) scores ranging from 3 to 8. The demographic and clinical characteristics of the patients and controls are presented in Supplementary Table 1. On the other hand, in situ immunostaining of TBI patients brain tissue shows that ITGB2 is predominantly expressed by neutrophil, whereas its expression on astrocyte, endotheliocyte and neurons was relatively low (Fig. 1 I-J).
Rovelizumab ameliorated brain injury and improved neurological outcomes after TBI
ITGB, β2 Integrin, is a vital component of the leukocyte integrin family, facilitating lymphocyte circulation and homing, cellular adhesion, and signaling processes initiated at the cell surface [38]. Rovelizumab, however, could bind ITGB2 and has activity against ITGB2 complexes including the ITGAM-ITGB2 heterodimer, which is reported to be used in research on multiple sclerosis (MS), hemorrhagic shock, myocardial infarction (MI) and stroke [39, 40].We therefore utilized Rovelizumab to explore its therapeutic effects on TBI. Firstly, the inhibitory effect of Rovelizumab on ITGB2 was observed in TBI mice (Fig. 2 A-B). And then, Rovelizumab significantly improved the neurological function of TBI mice, as measured by rotarod test (Fig. 2C) and mNSS scores (Fig. 2D). On the other hand, The EB dye extravasation test was performed to evaluate the FPI-induced vascular leakage. 3 days after TBI, the damage to the BBB caused by TBI was significantly ameliorated by Rovelizumab (Fig. 2 E–F). Moreover, from the H&E staining, there was an intuitive sense of the degree of damage to the cortex. The H&E staining indicated that the Rovelizumab treatment shrunk the trauma focus (Fig. 2G). Nissl staining was employed to determine the number of neurons in the peripheral areas of the trauma focus. Rovelizumab treatment significantly increased living neurons, as compared with mice receiving vehicle buffer (Fig. 2 H-I). These results demonstrated that Rovelizumab effectively ameliorated brain injury and improved neurological outcomes after TBI.
Fig. 2.
Effect of Rovelizumab on neurological function, vascular leakage and changes in pathological morphology at 3 days after TBI. A-B Representative western blotting bands and densitometric quantifications of ITGB2 (n = 6/group); C-D Neural function was evaluated using rotarod test and mNSS scores (n = 13/group); E–F FPI-induced vascular leakage was measured via EB dye extravasation (n = 6/group); G Morphological changes were observed using H&E staining; H-I The number of surviving neurons around the wound focus were observed via Nissl staining (n = 6/group). All data are shown as mean ± SD
Rovelizumab inhibits the respiratory burst of neutrophils and inflammatory response following TBI
The respiratory burst, a critical component of the immune system’s first line of defense, particularly in neutrophils, is characterized by the production of a surge of reactive oxygen species (ROS) via NADPH oxidase (NOX2) upon immune activation [41]. This ROS surge can lead to oxidative stress, a key factor in the pathophysiology of TBI [42]. ITGB2, a molecule central to immune cell adhesion and signaling, may play a significant role in this process. Our study found that Rovelizumab-treated mice exhibited reduced NOX2 content (Fig. 3 A-B). Concurrently, we measured levels of IL-6, IL-1β, and TNF-α and observed that Rovelizumab diminished the levels of these inflammatory factors post-TBI (Fig. 3 C-E). DCFH-DA, a commonly used reagent for detecting cellular ROS, was employed to measure ROS content in neutrophils isolated from mouse brains via flow cytometry [43]. The results indicated an increase in ROS content in neutrophils after TBI, a trend that Rovelizumab effectively inhibited (Fig. 3 F-G). Additionally, DHE staining, used to assess intracellular superoxide anion (O2-) levels, showed a reduction in neutrophils following Rovelizumab treatment (Fig. 3 I-J). Furthermore, we examined the DNA damage marker 8-OHDG, caused by ROS-induced oxidative stress in neutrophils, and found that Rovelizumab significantly mitigated neutrophil damage post-TBI (Fig. 3 K-L). Therefore, it was clear that ITGB2 blockade inhibited oxidative stress in neutrophils and inflammatory response after TBI.
Rovelizumab ameliorated endothelial dysfunction and cortical blood flow after TBI
We first investigated the impact of TBI on endothelial dysfunction, which is characterized by reduction in Nitric Oxide (NO) availability, disruption of the glycocalyx and adherens junctions, the loss of vascular integrity and increased permeability, as well as the expression of adhesion molecules [6, 44]. Western blotting showed that Rovelizumab increased the expressions of VE-Cadherin, Occludin and Claudin-5 that declined after TBI, which are key molecules localized at junctions between adjacent endotheliocyte to maintain the barrier integrity [45]. We also found Rovelizumab could reduce the expression of ICAM 1 (Fig. 4 A-E). Moreover, Rovelizumab intervention had significantly decreased plasma syndecan-1 shedding (Fig. 4F) and increased NO production (Fig. 4G). More importantly, Rovelizumab increased cortical blood flow (Fig. 4 H-I), as compared with TBI mice. IF staining further supported above point (Fig. 4 J-K). Taken together, these results demonstrated that Rovelizumab could effectively attenuate BBB damage.
Fig. 4.
Effect of Rovelizumab on BBB disruption at 3 days after TBI. A-E Representative western blotting bands and densitometric quantifications of Claudin-5, Occludin, ICAM 1 and VE-Cadherin (n = 6/group); F The endothelial glycocalyx disruption was assessed by measuring plasma syndecan-1 (n = 6/group); G Plasma NO content was measured using commercially available kits. (n = 6/group); H-I Representative photographs of laser speckle contrast imaging and quantitative analyses of cortical blood flow. PU, perfusion unit (n = 6/group); J-K Representative microphotographs of immunostaining and quantitative analysis of CD31/Claudin-5/DAPI. Scale bar = 20 µm (n = 6/group). All data are shown as mean ± SD
The protective effect of Rovelizumab may be mediated in part by the involvement of neutrophils
Based on in situ immunostaining results demonstrating predominant ITGB2 expression in neutrophils and the protective effects of ITGB2 blockade following TBI, we investigated the critical role of neutrophils in the pathological processes. To explore this further, we utilized Anti-Ly6G to deplete neutrophils in mice [46]. Our findings showed that neutrophil depletion significantly reduced the number of circulating neutrophils 3 days after TBI. At the same time, treatment with Rovelizumab also mitigated the activation of the peripheral immune response, reducing the circulating neutrophil count through its protective effects (Fig. 5 A-B). We next quantified ITGB2 expression after neutrophil depletion and observed a significant reduction in ITGB2 content in the brains of TBI mice (Fig. 5 C-D). Neutrophil depletion also inhibited the brain’s inflammatory response, as evidenced by the reduced levels of inflammatory factors (Fig. 5 E–G). Furthermore, staining results revealed a substantial increase in neutrophil infiltration into the brain during the acute phase following TBI, which was reversed by both Rovelizumab and Anti-Ly6G interventions (Fig. 5 H-I). Additionally, in-vivo two-photon microscopy confirmed the adhesion and aggregation of neutrophils in the cerebral blood vessels of TBI mice. Our observations revealed a marked increase in leukocyte adhesion to the cerebral blood vessels in TBI mice, which was attenuated by treatment with Rovelizumab and Anti-Ly6G (Fig. 5 J-K). These protective effects were associated with significant preservation of neural function, as evidenced by a reduction in neuronal apoptosis (Fig. 5 L-M). However, a comparative analysis of neutrophil depletion and Rovelizumab treatment revealed no significant differences between the two interventions (Fig. 5), suggesting that Rovelizumab’s protective effects on neuronal survival are, at least in part, mediated through neutrophil-driven inflammatory pathways.
Fig. 5.
The protective effect of Rovelizumab may be mediated in part by the involvement of neutrophils. A-B Representative flow images of circulating blood neutrophils and quantitative analysis (n = 4/group); C-D Representative western blotting bands and densitometric quantifications of ITGB2 (n = 6/group); E–G The changes in inflammatory cytokines (IL-6、IL-1β and TNF-α) after brain injury and the treatments intervention, which were evaluated using ELISA (n = 6/group); H-I Representative microphotographs of immunohistochemical staining and quantitative analysis of MPO. Scale bar = 50 µm (n = 5/group); J-K Representative two-photon fluorescence images and quantitative analysis of adherent leukocyte. Blood vessels are shown in green (FITC-Dextran), and rhodamine6G+ fluorescence leukocytes are shown in red. Scale bar = 40 µm (n = 5/group); L-M Representative microphotographs of immunostaining and quantitative analysis of NeuN/Tunel/DAPI. Scale bar = 50 µm (n = 6/group). All data are shown as mean ± SD
ITGB2 blockade inhibited NETs formation at 3 days after TBI
To confirm the formation of NETs in the brain tissue of mice 3 days after TBI, western blotting revealed significantly elevated protein levels of Ly6G, PAD4, MMP9, MMP2, and citrullinated histone H3 (H3Cit) in the cortex surrounding the trauma site (Fig. 6 A-F). Furthermore, we observed a notable increase in MPO content and MPO-DNA complex levels in the cortical regions at 3 days post-TBI (Fig. 6 G, I). Double immunofluorescence (IF) staining for MPO and H3Cit further confirmed that the number of H3Cit-positive neutrophils in the contused cortex was significantly higher at 3 days after TBI (Fig. 6 H, J). When ITGB2 was blocked using Rovelizumab, the expression of Ly6G, PAD4, MMP9, MMP2, and H3Cit in the brain was reduced, as was the content of MPO and the MPO-DNA complex in the cortical homogenates of TBI mice. Additionally, the presence of H3Cit-positive neutrophils in the injured cortex was also diminished, indicating that ITGB2 blockade effectively inhibited NETs formation at 3 days following TBI.
Fig. 6.
ITGB2 blockade inhibited NETs formation following TBI. A-F Representative western blotting bands and densitometric quantifications of PAD4, MMP9, MMP2, Ly6G and H3cit (n = 6/group); G Quantification of MPO activity in the brain of mice (n = 8/group); H, J Representative microphotographs of immunohistochemical staining and quantitative analysis of MPO/H3cit/DAPI. Scale bar = 20 µm (n = 6/group); I Quantitative analyses of cortex MPO-DNA complexes (n = 8/group). All data are shown as mean ± SD
ITGB2 mainly interacts with ICAM1 of endotheliocyte to mediate the adhesion and migration of neutrophils
To further determine the underlying mechanism by which ITGB2 regulates neutrophil trans endothelial migration, we utilized immunoprecipitation to separate ITGB2 and its interacting proteins, subsequently employing immunoblotting to detect proteins that may bind to ITGB2. Notably, Intercellular adhesion molecule-1 (ICAM 1) was identified as a binding partner of ITGB2 (Fig. 7 A), and its increased expression after TBI has been well-documented (Fig. 4 A, D). To confirm the pivotal role of ICAM 1 in neutrophil infiltration, we utilized Anti-ICAM 1 to counteract the effects of ICAM 1. We initially assessed the levels of ITGB2 and ICAM 1 in mouse brains following administration of Anti-ICAM 1, observing a significant reduction in their expression (Fig. 7 B-D). Utilizing in vivo two-photon microscopy, we confirmed that treatment with Anti-ICAM 1 lessened leukocyte adhesion and aggregation within cerebral blood vessels after TBI (Fig. 7 E–F). Further, in situ immunostaining of the peritraumatic cortex in TBI mice showed ICAM 1 expression across endotheliocytes, astrocytes, and microglia, while ITGB2 in neutrophils predominantly engaged with ICAM1 on endotheliocytes, not on microglia or astrocytes (Fig. 7 G). In vitro experiments, where we co-cultured hCMEC/D3 with human blood PMNs, revealed that both Rovelizumab and Anti-ICAM1 treatments effectively lowered the adhesion of PMNs to the endothelium and their migration across it (Fig. 7 H-J). Taken together, ITGB2 regulates neutrophil adhesion and migration mainly by interacting with ICAM 1 on endotheliocytes.
Fig. 7.
ITGB2 interacts with ICAM1 of endotheliocyte to mediate the adhesion and migration of neutrophils. A The mutual binding of ITGB2 and ICAM 1 was assessed by co-immunoprecipitation; B-D Representative western blotting bands and densitometric quantifications of ITGB2 and ICAM 1 (n = 6/group); E–F Representative two-photon fluorescence images and quantitative analysis of adherent leukocyte. Blood vessels are shown in green (FITC-Dextran), and rhodamine6G+ fluorescence leukocytes are shown in red. Scale bar = 40 µm (n = 5/group); G Representative microphotographs of immunostaining of ICAM 1 and ITGB2 in Endotheliocytes (CD31+ cells), Astrocytes (GFAP+ cells) and Microglia (Iba 1+ cells). Scale bar = 20 µm (n = 6/group); H-J The adhesion and transmigration of PMNs to endotheliocyte. Scale bar = 20 µm (n = 6/group). All data are shown as mean ± SD
Anti-ICAM1 may help to improve the outcome of TBI
Behavioral experiments revealed that treatment with Anti-ICAM 1 could ameliorate neurological deficits in TBI mice, with a noticeable enhancement in motor function (Fig. 8 A-B). Additionally, Anti-ICAM 1 led to a reduction in MPO content (Fig. 8 C), MPO-DNA complex content (Fig. 8 D), and the number of H3Cit-positive neutrophils (Fig. 8 E–F) in the brain. Concurrently, it increased the expression of tight junction proteins in the brains of TBI mice (Fig. 8 G-H), indicating an improvement in endothelial dysfunction. Collectively, these findings indicate that Anti-ICAM1 may help to improve the outcome of TBI.
Fig. 8.
Anti-ICAM1 may help to improve the outcome of TBI. A-B Neural function was evaluated using rotarod test and mNSS scores (n = 12/group); C Quantification of MPO activity in the brain of mice (n = 8/group); D Quantitative analyses of cortex MPO-DNA complexes (n = 8/group); E–F Representative microphotographs of immunohistochemical staining and quantitative analysis of MPO/H3cit/DAPI. Scale bar = 20 µm (n = 6/group); G-H Representative microphotographs of immunostaining and quantitative analysis of CD31/Claudin-5/DAPI. Scale bar = 50 µm (n = 6/group).All data are shown as mean ± SD
Discussion
The post-TBI inflammatory response is intricately woven, involving an array of inflammatory mediators and cellular interactions [9]. Key to this process is the activation of the immune system, the liberation of inflammatory factors, and the breach of the BBB [47, 48]. After injury, the compromised BBB permits heightened invasion and engagement of inflammatory cells, including macrophages, neutrophils, and lymphocytes, which subsequently discharge factors such as IL-1β and TNF-α [49, 50]. These cytokines spark inflammation within the affected neural regions and systemically. Gaining insights into these dynamics is essential for crafting therapeutic interventions designed to mitigate secondary neurological damage and enhance patient prognoses.
After profiling the brain tissues extracted from mice with TBI, we successfully documented the emerging molecular landscape during the acute phase post-trauma. TBI rapidly and significantly alters the transcriptomic profile of the impacted brain areas. Notably, most genes and signaling pathways associated with cell death and immune response were upregulated, with significant changes observed as early as 1 day after the injury, and such alterations persisted throughout the development of cerebral edema and its subsequent progression. Additionally, the landscape shows that TBI provoke cell adhesion, leukocyte trans endothelial migration, cytokine-cytokine receptor interaction and immune reactions by up-regulating ITGB2, ITGA5, SIPA1, VCAM 1 and ICAM 1. The finding led us to ponder which of these genes/pathways are critical for initiating the immune response and neuronal cell death. ITGB2, a well-characterized protein involved in leukocyte adhesion and migration [38], was notably upregulated at 3 days post-TBI, coinciding with the peak of leukocyte infiltration and the intensification of neuroinflammation. In contrast, SIPA1, which regulates Rho GTPase activity and cell migration [51, 52], was gradually upregulated at 1 and 3 days post-TBI. Although SIPA1 is involved in cell proliferation and migration, its precise role in immune responses and leukocyte behavior in TBI remains unclear. The delayed pattern of SIPA1 upregulation does not correlate as closely with the acute inflammatory phase, which is critical for understanding the immediate consequences of TBI. Therefore, we focused on ITGB2, capturing the peak of the inflammatory response and its influence on brain injury and repair. This observation is further validated by a clear increase in ITGB2 expression in human brain tissues affected by trauma, emphasizing its central role in the injury response.
Here, we deployed Rovelizumab, an agent that targets ITGB2 by binding to it and neutralizing the activity of ITGB2-containing complexes [39], to explore its therapeutic potential of ITGB2 inhibition following TBI. Our findings demonstrated that the administration of Rovelizumab significantly mitigated the severity of neurological impairments, arrested the decline in motor performance, reduced the heightened permeability of the BBB, and curbed vascular leakage associated with TBI. Furthermore, Rovelizumab was instrumental in diminishing excessive oxidative stress and inflammation, easing endothelial dysfunction, preserving the integrity of tight junctions, and enhancing cerebral blood flow perfusion. These results suggest that ITGB2 blockade may confer substantial therapeutic benefits during the recovery phase following TBI.
On the other hand, by immunolocalization we found the preferential ITGB2 expression on neutrophil, suggesting that neutrophil may serve as a vector for the biological effects of ITGB2. The presence and behavior of neutrophils in TBI and Cerebral Ischemia Reperfusion Injury are marked by complexity and diversity, correlating positively with the extent of trauma incurred [53]. Neutrophil infiltration is a critical component of the inflammatory response and involves a sophisticated migratory process from the bloodstream to the BBB. During this migration, elastase (NE) released by neutrophils significantly disrupts the binding between cadherins, leading to an increase in BBB permeability [54, 55]. Additionally, matrix metalloproteinases (MMPs), including MMP-2, MMP-3, and MMP-9, which are derived from neutrophils, can degrade and remodel the extracellular matrix [56], playing a crucial role in the migration of immune cells [57, 58]. Furthermore, the formation of NETs by activated neutrophils within the brain tissue can directly lead to the demise of endotheliocyte [24]. Overall, neutrophil-mediated degradation of the BBB facilitates the infiltration of additional immune cells, thereby triggering an excessive inflammatory response. An overactive immune system can lead to nonselective tissue damage.
Notably, the meninges have been identified as the closest immunologically active tissue to the brain [59]. Meningeal leukocytes are capable of sensing fluctuations in peripheral blood and cerebrospinal fluid (CSF) to support brain homeostasis [60]. The cranial-dural interface is characterized by a dense network of neutrophils [61]. Recent studies have reported that neuroimmune circuits mediated by neutrophils, localized at the meningeal-brain interface, are responsible for sensing social threats and integrating the internal state of the organism to support the negative regulation of fear responses and defensive behaviors [62]. Therefore, neutrophils entering the brain via the meningeal compartment may play a significant role in maintaining host behavioral homeostasis. In this study, we mainly focused on inhibiting the adhesion and infiltration of neutrophils in cerebral blood vessels, protecting the damaged blood–brain barrier, and thus inhibiting excessive neuroinflammation. Whether neutrophils in the meningeal compartment play their immunomodulatory role deserves further study.
Within the scope of our research, we also utilized Anti-Ly6G, a technique for neutrophil depletion, as an intervention for TBI [46]. Our findings indicate that the neutrophil depletion led to a reduction in circulating hyperplastic neutrophils during the acute phase following TBI. Concurrently, Rovelizumab, exerting its protective influence, also diminishes the count of circulating neutrophils. Both interventions were observed to reduce leukocyte adhesion within cerebral blood vessels, neutrophil infiltration into brain tissue, and neuronal apoptosis, as evidenced by two-photon microscopy and immunohistochemical staining. Notably, no significant discrepancy in therapeutic efficacy was detected between ITGB2 blockade and neutrophil depletion, implying that the neuroprotective effects of Rovelizumab may be mediated in part through pathways affected by neutrophil inflammation. Collectively, these findings suggest that ITGB2 blockade curtails neutrophil adhesion, aggregation, and trans endothelial migration subsequent to TBI, thereby safeguarding neurological function.
Additionally, neutrophils that infiltrate the brain tissue may become activated, leading to the release of NETs. NETs, complex webs of extracellular double-stranded DNA intertwined with histones, neutrophil elastase, MPO, and cathepsin, are intricately woven to capture and neutralize pathogens [63]. Nonetheless, a recent investigation has detected NETs in brain regions subjected to contusion, linking their presence to elevated intracranial pressure (ICP) and the poor prognosis of TBI patients [64]. A plethora of experimental studies have illuminated the detrimental impact of NETs on neuronal and glial cell function and the integrity of the BBB, culminating in adverse sequelae within the CNS [65, 66]. Moreover, the involvement of NETs in the inflammatory and coagulation cascades triggered by TBI has been well-documented and curbing it has been shown to ameliorate coagulation status and enhance clinical outcomes. Collectively, these insights advocate for the consideration of NETs as a promising therapeutic horizon in TBI management, where their suppression could offer a strategic advantage in mitigating TBI-related inflammation, coagulopathy, and endothelial dysfunction, thereby potentially enhancing patient prognosis. Here, we have provided evidence of the presence of NETs in the brain tissue of mice with TBI through the colocalization of NET-specific markers, such as H3cit and MPO, coupled with the quantitative detection of MPO-DNA complexes. However, Rovelizumab significantly reduced the formation of NETs in TBI mice, suggesting its potential as a therapeutic intervention for mitigating the pathological processes associated with TBI.
Finally, we delved into the intricate mechanisms by which ITGB2 regulates neutrophil trans endothelial migration, a process that entails the cooperative action of multiple molecular players. Phillips et al. discovered that selectins, expressed on activated endothelial cells, can initiate the adhesion cascade of neutrophils by binding to CD15a ligands on their surface [67]. Wolach et al. identified ICAM 1, ICAM 2, and PECAM1 as key mediators in neutrophil adhesion and migration across the endothelium [68]. In this study, we show that ITGB2 interacts with ICAM-1, which plays a role in key physiological processes such as cell signaling, activation, immune response, and inflammation [69, 70]. ICAM 1 is markedly upregulated following TBI. Studies have shown that ICAM1 is expressed on microglia, astrocytes and endotheliocytes in response to inflammatory stimuli [71–73]. By immunolocalization, we revealed that neutrophil ITGB2 mainly interacted with ICAM1 on endotheliocytes. Additionally, by employing Anti-ICAM 1 to counteract ICAM 1’s effect, we have confirmed it could enhance neurological function, suppress the adhesion and migration of neutrophils, prevent the formation of NETs, and safeguard endothelial function.
Consistent with our findings, Saikia et al. demonstrated that ICAM-1 deletion using CRISPR/Cas9 technology significantly attenuated blood–brain barrier (BBB) damage after TBI, reduced leukocyte infiltration in the brain, and promoted functional recovery after TBI [74]. Their study provides an alternative mechanism for neutrophil recruitment across the endothelium to the injured brain, namely that ICAM-1 regulates leukocyte migration via the paxillin/FAK-dependent Rho GTPase pathway. This is an extremely important addition to our understanding of the mechanisms involved in neutrophil recruitment. Notably, the experimental design of Saikia et al. shares numerous similarities with our study, including the use of a fluid impact model in mice and the evaluation of behavior along similar postoperative timelines. Both studies highlight the critical role of ICAM-1 in the inflammatory response following TBI and suggest that targeting ICAM-1 could be a promising therapeutic approach to mitigate the detrimental effects of TBI.
The characterization of the molecular landscape of brain after TBI and the identification of ITGB2 as a prominent regulator for inflammatory cascade fundamentally increase our understanding of propagation of neuroinflammation. The screening of the ITGB2 blockade Rovelizumab and its proof of validity open a potential therapeutic avenue not only for TBI but also for other acute brain injuries such as intracerebral hemorrhage and acute ischemic stroke. Nonetheless, the study is not without limitations. Firstly, while our research on ITGB2 has primarily centered on its role in neutrophils, as a member of the leukocyte integrin family, ITGB2 likely exerts regulatory influences over a spectrum of immune cells. The intricacies of these effects remain largely uncharted territory. Secondly, the genomic landscape of TBI mouse brain diverges in some respects from that of the human brain, and there may be genes that are more related to immune response that change after TBI, potentially unveiling new avenues for therapeutic intervention. Thirdly, all of the strategies used to block ITGB2 or ICAM 1 in this study were systemic. To clearly verify the role of ITGB2 in neutrophils, we should knock out ITGB2 in neutrophils, but this part of verification was not completed due to the limitation of experimental conditions. Lastly, our study focused solely on male mice. Given that females may show differences in their treatment response to TBI, this limitation restricts the generalizability of our findings. Future studies should consider including female subjects to provide a more comprehensive understanding of the treatment response to TBI, which is essential for translating our findings into clinical practice..
Conclusion
In summary, our study presents novel findings that ITGB2 levels are elevated in the brain following TBI. Pharmacological approaches targeting ITGB2 regulating neutrophil infiltration and curtailing the formation of NETs, may be utilized as potential treatment options for TBI and deserve further investigations in future studies.
Supplementary Information
Acknowledgements
We would like to thank all the participants in the study.
Abbreviations
- TBI
Traumatic brain injury
- CNS
Central nervous system
- BBB
Blood-brain barrier
- ROS
Reactive oxygen species
- MMP
Matrix metalloproteinases
- ICAM 1
Intercellular adhesion molecule 1
- NETs
Neutrophil extracellular traps
- ITGB2
β2 Integrin
- DEGs
Differentially expressed genes
- FPI
Fluid percussion injury
- IL
Interleukin
- TNF
Tumor growth factors
- CXCL
Chemokine (C-X-C motif) ligand (CXCL) family
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- FDR
False discovery rate
- EB
Evans Blue
- PPI
Protein-protein interaction
- OD
Optical density
- IF
Immunofluorescence
- IHC
Immunohistochemistry
- GO
Gene Oncology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- Mnss
Modified Neurological Severity Score
- NOX2
NADPH oxidase enzyme
- DHE
Dihydroethidium
- 8-OHDG
8-Hydroxydeoxyguanosin
- Anti-Ly6G
Lymphocyte antigen 6 complex locus G-specific antibody
- NO
Nitric oxide
- ANOVA
Analysis of variance
- hCMEC/D3
Human Cortical Microvessels Endothelial Cells/D3
- PMNs
Polymorphonuclear leukocytes
- H3Cit
Citrullinated histone H3
- PECAM-1
Platelet endothelial cell adhesion molecule-1
- NE
Neutrophil elastase
- ICP
Intracranial pressure
- PU
Perfusion units
- H&E
Hematoxylin and Eosin Staining
- EB
Evans Blue
- BSA
Bovine serum albumin
- ELISA
Enzyme-Linked Immunosorbent Assay
- GCS
Glasgow Coma Scale
Authors’ contributions
S.Z., J.Z., T.L. and X.X. conceived, designed and supervised the study. L.L., R.P., C.W. and X.C. developed methodology. L.L., R.P., C.W., X.C., D.G., S.G., B.C. and Y.L. performed experiments, L.L., T.L., H.J., J.W., K.L., X.Z., J.X. and Y.W. interpreted the results, carried data analysis and prepared the figures and tables. C.H., X.L., Y.C., J.X. and F.L. provided technical support. L.L. wrote the manuscript. S.Z., J.Z. T.L. and X.X. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (grant 81930031, 82471331, 82001317, 82071674, 82301547, 82401604), the Taishan Scholar Youth Expert Program of Shandong Province (grant tsqn202408398), the Scientific Research Program of Tianjin Education Commission (grant 2024KJ248), the Beijing Hospitals Authority Youth Program (grant QML20230801), and the Beijing-Tianjin-Hebei Basic Research Collaboration Project (22JCZXJC00160).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This human subject study was approved by the Ethics Committees of Tianjin Medical University General Hospital. All participants (or legal representatives) were informed of the study protocol and signed the consent form in accordance with the Helsinki declaration. All animal care and experimental procedures were approved by the Ethics Committee of Tianjin Medical University General Hospital, Tianjin and were conducted in strict accordance with the ARRIVE Guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Li, Ruilong Peng, Cong Wang and Xin Chen contributed equally to this work.
Contributor Information
Xin Xu, Email: xuxindoc@hotmail.com.
Tuo Li, Email: ytdzlt3528@tmu.edu.cn.
Jianning Zhang, Email: jianningzhang@hotmail.com.
Shu Zhang, Email: gloria523@163.com.
References
- 1.Mollayeva T, Mollayeva S, Colantonio A. Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat Rev Neurol. 2018;14(12):711–22. [DOI] [PubMed] [Google Scholar]
- 2.Ashina H, Eigenbrodt AK, Seifert T, Sinclair AJ, Scher AI, Schytz HW, et al. Post-traumatic headache attributed to traumatic brain injury: classification, clinical characteristics, and treatment. Lancet Neurol. 2021;20(6):460–9. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Sun D, Zhao Z, Fang J, Li M, Lv C, et al. Neutrophil membrane-derived nanoparticles protect traumatic brain injury via inhibiting calcium overload and scavenging ROS. J Nanobiotechnol. 2024;22(1):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron. 2017;95(6):1246–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Zhou XM, Wu LY, Liu GJ, Xu WD, Zhang XS, et al. Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J Neuroinflammation. 2020;17(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Li F, Bai X, Jia H, Wang C, Li P, et al. Circulating extracellular vesicles from patients with traumatic brain injury induce cerebrovascular endothelial dysfunction. Pharmacol Res. 2023;192:106791. [DOI] [PubMed] [Google Scholar]
- 7.Ge Y, Wu X, Cai Y, Hu Q, Wang J, Zhang S, et al. FNDC5 prevents oxidative stress and neuronal apoptosis after traumatic brain injury through SIRT3-dependent regulation of mitochondrial quality control. Cell Death Dis. 2024;15(5):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Gao W, Cheng S, Yin D, Li F, Wu Y, et al. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation. 2017;14(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T, Li L, Peng R, Hao H, Zhang H, Gao Y, et al. Abrocitinib Attenuates Microglia-Mediated Neuroinflammation after Traumatic Brain Injury via Inhibiting the JAK1/STAT1/NF-κB Pathway. Cells. 2022;11(22):3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YW, Li S, Dai SS. Neutrophils in traumatic brain injury (TBI): friend or foe? J Neuroinflammation. 2018;15(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120(5):1368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13(3):171–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson NH, Hadad R, Taylor RR, Rodríguez Pilar J, Salazar O, Llompart-Pou JA, et al. Inflammatory Biomarkers of Traumatic Brain Injury. Pharmaceuticals (Basel). 2022;15(6):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, et al. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52(1):78–84. [DOI] [PubMed] [Google Scholar]
- 16.Jang E, Lee S, Kim JH, Kim JH, Seo JW, Lee WH, et al. Secreted protein lipocalin-2 promotes microglial M1 polarization. Faseb j. 2013;27(3):1176–90. [DOI] [PubMed] [Google Scholar]
- 17.Xu XJ, Ge QQ, Yang MS, Zhuang Y, Zhang B, Dong JQ, et al. Neutrophil-derived interleukin-17A participates in neuroinflammation induced by traumatic brain injury. Neural Regen Res. 2023;18(5):1046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPherson CA, Merrick BA, Harry GJ. In vivo molecular markers for pro-inflammatory cytokine M1 stage and resident microglia in trimethyltin-induced hippocampal injury. Neurotox Res. 2014;25(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu N, Han J, Li Y, Jiang Y, Shi SX, Lok J, et al. Recombinant annexin A2 inhibits peripheral leukocyte activation and brain infiltration after traumatic brain injury. J Neuroinflammation. 2021;18(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei P, Wang K, Luo C, Huang Y, Misilimu D, Wen H, et al. Cordycepin confers long-term neuroprotection via inhibiting neutrophil infiltration and neuroinflammation after traumatic brain injury. J Neuroinflammation. 2021;18(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89. [DOI] [PubMed] [Google Scholar]
- 22.Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The Neutrophil. Immunity. 2021;54(7):1377–91. [DOI] [PubMed] [Google Scholar]
- 23.Ma X, Niu X, Zhao J, Deng Z, Li J, Wu X, et al. Downregulation of Sepina3n Aggravated Blood-Brain Barrier Disruption after Traumatic Brain Injury by Activating Neutrophil Elastase in Mice. Neuroscience. 2022;503:45–57. [DOI] [PubMed] [Google Scholar]
- 24.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE. 2012;7(2):e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mi L, Min X, Shi M, Liu L, Zhang Y, Zhu Y, et al. Neutrophil extracellular traps aggravate neuronal endoplasmic reticulum stress and apoptosis via TLR9 after traumatic brain injury. Cell Death Dis. 2023;14(6):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin J, Wang F, Tian J, Zhao X, Dong J, Wang N, et al. Neutrophil extracellular traps contribute to coagulopathy after traumatic brain injury. JCI Insight. 2023;8(6):e141110. [DOI] [PMC free article] [PubMed]
- 27.Qu X, Hou X, Zhu K, Chen W, Chen K, Sang X, et al. Neutrophil extracellular traps facilitate sympathetic hyperactivity by polarizing microglia toward M1 phenotype after traumatic brain injury. Faseb j. 2023;37(9):e23112. [DOI] [PubMed] [Google Scholar]
- 28.Shi G, Liu L, Cao Y, Ma G, Zhu Y, Xu J, et al. Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1α/ASK1/JNK signaling pathway in mice with traumatic brain injury. J Neuroinflammation. 2023;20(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Y, Shi M, Liu L, Zuo Y, Jia H, Min X, et al. Inhibition of neutrophil extracellular trap formation attenuates NLRP1-dependent neuronal pyroptosis via STING/IRE1α pathway after traumatic brain injury in mice. Front Immunol. 2023;14:1125759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Li Z, Yao Y, Jin WN, Wood K, Liu Q, et al. Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc Natl Acad Sci U S A. 2017;114(3):E396-e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, Li L, Peng R, Liu C, Liu X, Liu Y, et al. Brain-derived extracellular vesicles mediate systemic coagulopathy and inflammation after traumatic brain injury. Int Immunopharmacol. 2024;130:111674. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Wang C, Wu Y, Houck K, Hilton T, Zhou A, et al. Conformation-dependent blockage of activated VWF improves outcomes of traumatic brain injury in mice. Blood. 2021;137(4):544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerrot D, Kerroch M, Placier S, Vandermeersch S, Trivin C, Mael-Ainin M, et al. Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. Am J Pathol. 2011;179(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. 2020;11(1):2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Wu T, Han X, Wan J, Jiang C, Chen W, et al. Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice. J Cereb Blood Flow Metab. 2017;37(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ge X, Li W, Huang S, Yin Z, Yang M, Han Z, et al. Increased miR-21-3p in Injured Brain Microvascular Endothelial Cells after Traumatic Brain Injury Aggravates Blood-Brain Barrier Damage by Promoting Cellular Apoptosis and Inflammation through Targeting MAT2B. J Neurotrauma. 2019;36(8):1291–305. [DOI] [PubMed] [Google Scholar]
- 37.Yan M, Zhang X, Chen A, Gu W, Liu J, Ren X, et al. Endothelial cell SHP-2 negatively regulates neutrophil adhesion and promotes transmigration by enhancing ICAM-1-VE-cadherin interaction. Faseb j. 2017;31(11):4759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kijas JM, Bauer TR Jr, Gäfvert S, Marklund S, Trowald-Wigh G, Johannisson A, et al. A missense mutation in the beta-2 integrin gene (ITGB2) causes canine leukocyte adhesion deficiency. Genomics. 1999;61(1):101–7. [DOI] [PubMed] [Google Scholar]
- 39.Qualls JE, Murray PJ. A double agent in cancer: stopping macrophages wounds tumors. Nat Med. 2010;16(8):863–4. [DOI] [PubMed] [Google Scholar]
- 40.Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, et al. Hu23F2G, an antibody recognizing the leukocyte CD11/CD18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153(2):223–33. [DOI] [PubMed] [Google Scholar]
- 41.El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273(1):180–93. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Wu X, Wang J, Shi Y, Hu Q, Cui W, et al. Adiponectin/AdiopR1 signaling prevents mitochondrial dysfunction and oxidative injury after traumatic brain injury in a SIRT3 dependent manner. Redox Biol. 2022;54:102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffo A, Bosco N, Pagano A, Balestrazzi A, Macovei A. Noninvasive Methods to Detect Reactive Oxygen Species as a Proxy of Seed Quality. Antioxidants (Basel). 2023;12(3):626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludewig P, Winneberger J, Magnus T. The cerebral endothelial cell as a key regulator of inflammatory processes in sterile inflammation. J Neuroimmunol. 2019;326:38–44. [DOI] [PubMed] [Google Scholar]
- 45.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16(2):209–21. [DOI] [PubMed] [Google Scholar]
- 46.Cai P, Luo H, Xu H, Zhu X, Xu W, Dai Y, et al. Recombinant ADAMTS 13 Attenuates Brain Injury After Intracerebral Hemorrhage. Stroke. 2015;46(9):2647–53. [DOI] [PubMed] [Google Scholar]
- 47.Navarrete C, García-Martín A, Correa-Sáez A, Prados ME, Fernández F, Pineda R, et al. A cannabidiol aminoquinone derivative activates the PP2A/B55α/HIF pathway and shows protective effects in a murine model of traumatic brain injury. J Neuroinflammation. 2022;19(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciryam P, Gerzanich V, Simard JM. Interleukin-6 in Traumatic Brain Injury: A Janus-Faced Player in Damage and Repair. J Neurotrauma. 2023;40(21–22):2249–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H, Zheng J, Xu S, Fang Y, Wu Y, Zeng J, et al. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation. 2021;18(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shan J, Shi R, Hazra R, Hu X. Regulatory T lymphocytes in traumatic brain injury. Neurochem Int. 2024;173:105660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minato N, Hattori M. Spa-1 (Sipa1) and Rap signaling in leukemia and cancer metastasis. Cancer Sci. 2009;100(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyata M, Rikitake Y, Takahashi M, Nagamatsu Y, Yamauchi Y, Ogita H, et al. Regulation by afadin of cyclical activation and inactivation of Rap1, Rac1, and RhoA small G proteins at leading edges of moving NIH3T3 cells. J Biol Chem. 2009;284(36):24595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mu Q, Yao K, Syeda MZ, Wan J, Cheng Q, You Z, et al. Neutrophil Targeting Platform Reduces Neutrophil Extracellular Traps for Improved Traumatic Brain Injury and Stroke Theranostics. Adv Sci (Weinh). 2024;11(21):e2308719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86(4):1245–57. [DOI] [PubMed] [Google Scholar]
- 55.Allport JR, Ding H, Collins T, Gerritsen ME, Luscinskas FW. Endothelial-dependent mechanisms regulate leukocyte transmigration: a process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J Exp Med. 1997;186(4):517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bui TM, Yalom LK, Ning E, Urbanczyk JM, Ren X, Herrnreiter CJ, et al. Tissue-specific reprogramming leads to angiogenic neutrophil specialization and tumor vascularization in colorectal cancer. J Clin Invest. 2024;134(7):e174545. [DOI] [PMC free article] [PubMed]
- 57.Hayashi T, Kaneko Y, Yu S, Bae E, Stahl CE, Kawase T, et al. Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res. 2009;1280:172–7. [DOI] [PubMed] [Google Scholar]
- 58.Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65(4):702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rebejac J, Eme-Scolan E, Arnaud Paroutaud L, Kharbouche S, Teleman M, Spinelli L, et al. Meningeal macrophages protect against viral neuroinfection. Immunity. 2022;55(11):2103-17.e10. [DOI] [PubMed] [Google Scholar]
- 60.Rua R, McGavern DB. Advances in Meningeal Immunity. Trends Mol Med. 2018;24(6):542–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ, et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021;373(6553):eabf7844. [DOI] [PMC free article] [PubMed]
- 62.Wu B, Meng L, Zhao Y, Li J, Tian Q, Pang Y, et al. Meningeal neutrophil immune signaling influences behavioral adaptation following threat. Neuron. 2025;113(2):260–276.e8. [DOI] [PubMed]
- 63.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–47. [DOI] [PubMed] [Google Scholar]
- 64.Vaibhav K, Braun M, Alverson K, Khodadadi H, Kutiyanawalla A, Ward A, et al. Neutrophil extracellular traps exacerbate neurological deficits after traumatic brain injury. Sci Adv. 2020;6(22):eaax8847. [DOI] [PMC free article] [PubMed]
- 65.Kim SW, Lee H, Lee HK, Kim ID, Lee JK. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun. 2019;7(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanhai Z, Bin Q, Shengjun Z, Jingbo L, Yinghan G, Lingxin C, et al. Neutrophil extracellular traps, released from neutrophil, promote microglia inflammation and contribute to poor outcome in subarachnoid hemorrhage. Aging (Albany NY). 2021;13(9):13108–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillips ML, Schwartz BR, Etzioni A, Bayer R, Ochs HD, Paulson JC, et al. Neutrophil adhesion in leukocyte adhesion deficiency syndrome type 2. J Clin Invest. 1995;96(6):2898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolach B, Gavrieli R, Wolach O, Stauber T, Abuzaitoun O, Kuperman A, et al. Leucocyte adhesion deficiency-A multicentre national experience. Eur J Clin Invest. 2019;49(2):e13047. [DOI] [PubMed] [Google Scholar]
- 69.Haydinger CD, Ashander LM, Tan ACR, Smith JR. Intercellular Adhesion Molecule 1: More than a Leukocyte Adhesion Molecule. Biology (Basel). 2023;12(5):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65(6):961–71. [DOI] [PubMed] [Google Scholar]
- 71.Li H, Liu P, Zhang B, Yuan Z, Guo M, Zou X, et al. Acute ischemia induces spatially and transcriptionally distinct microglial subclusters. Genome Med. 2023;15(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L, Xu Z, Jia Z, Cai S, Wu Q, Liu X, et al. Modulating mTOR-dependent astrocyte substate transitions to alleviate neurodegeneration. Nat Aging. 2025. 10.1038/s43587-024-00792-z. [DOI] [PubMed]
- 73.Guerra-Espinosa C, Jiménez-Fernández M, Sánchez-Madrid F, Serrador JM. ICAMs in Immunity, Intercellular Adhesion and Communication. Cells. 2024;13(4):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saikia BB, Bhowmick S, Malat A, Preetha Rani MR, Thaha A, Abdul-Muneer PM. ICAM-1 Deletion Using CRISPR/Cas9 Protects the Brain from Traumatic Brain Injury-Induced Inflammatory Leukocyte Adhesion and Transmigration Cascades by Attenuating the Paxillin/FAK-Dependent Rho GTPase Pathway. J Neurosci. 2024;44(11):e1742232024. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.