Abstract
Though biofilms were first described by Antonie van Leeuwenhoek, the theory describing the biofilm process was not developed until 1978. We now understand that biofilms are universal, occurring in aquatic and industrial water systems as well as a large number of environments and medical devices relevant for public health. Using tools such as the scanning electron microscope and, more recently, the confocal laser scanning microscope, biofilm researchers now understand that biofilms are not unstructured, homogeneous deposits of cells and accumulated slime, but complex communities of surface-associated cells enclosed in a polymer matrix containing open water channels. Further studies have shown that the biofilm phenotype can be described in terms of the genes expressed by biofilm-associated cells. Microorganisms growing in a biofilm are highly resistant to antimicrobial agents by one or more mechanisms. Biofilm-associated microorganisms have been shown to be associated with several human diseases, such as native valve endocarditis and cystic fibrosis, and to colonize a wide variety of medical devices. Though epidemiologic evidence points to biofilms as a source of several infectious diseases, the exact mechanisms by which biofilm-associated microorganisms elicit disease are poorly understood. Detachment of cells or cell aggregates, production of endotoxin, increased resistance to the host immune system, and provision of a niche for the generation of resistant organisms are all biofilm processes which could initiate the disease process. Effective strategies to prevent or control biofilms on medical devices must take into consideration the unique and tenacious nature of biofilms. Current intervention strategies are designed to prevent initial device colonization, minimize microbial cell attachment to the device, penetrate the biofilm matrix and kill the associated cells, or remove the device from the patient. In the future, treatments may be based on inhibition of genes involved in cell attachment and biofilm formation.
INTRODUCTION
Biofilms have been described in many systems since Van Leeuwenhoek examined the “animalcules” in the plaque on his own teeth in the seventeenth century, but the general theory of biofilm predominance was not promulgated until 1978 (37). This theory states that the majority of bacteria grow in matrix-enclosed biofilms adherent to surfaces in all nutrient-sufficient aquatic ecosystems and that these sessile bacterial cells differ profoundly from their planktonic (floating) counterparts (37). The data on which this theory is predicated came mostly from natural aquatic ecosystems, in which direct microscopic observations and direct quantitative recovery techniques showed unequivocally that more than 99.9% of the bacteria grow in biofilms on a wide variety of surfaces. This predominance of biofilms was established in all natural ecosystems except deep groundwater and abyssal oceans, and we now realize that these sessile populations account for most physiological processes in these ecosystems (40).
Because bacterial biofilms cause very serious problems in industrial water systems, the people who manage these systems have been the first to develop methods to sample sessile bacteria and develop strategies to control their costly depredations. Biofilm samplers, which are fitted into the walls of industrial pipes and vessels, are now widely used in industrial systems, and the biocides used to protect industrial installations are routinely tested for their efficacy in killing sessile bacteria.
This consensus that bacteria grow preferentially in matrix-enclosed biofilms in natural and industrial systems was not immediately accepted in the medical and dental areas in spite of the universal acceptance of dental plaque as a type of biofilm. However, new methods for the direct examination of biofilms soon showed that the organisms that cause many device-related and other chronic infections actually grow in biofilms in or on these devices (39). Gradually, important intellectual syntheses began to be made.
Once we concede that bacteria lack a complex nervous system that could enable them to determine their location vis-à-vis the animal body, we deduce that they have certain basic survival strategies that they employ wherever they are. In natural and industrial systems, they form biofilms, within which they are protected from antibacterial chemicals (including natural antibiotics), environmental bacteriophages, and phagocytic amoebae. For these reasons, it should come as no surprise that chronic biofilm infections resist antibiotic therapy and are phenomenally resistant to host clearance mechanisms such as antibodies and phagocytes.
For many centuries humans have suffered from acute bacterial infections (e.g., plague), in which planktonic cells of specialized pathogens mounted life-threatening attacks on our bodies. We have countered with vaccines and antibiotics, and these acute diseases are now largely under some measure of control. However, organisms that have been successful for millions of years in the environment (e.g., Pseudomonas and Legionella spp.) are now mounting successful attacks on our health care facilities. Obviously, they make full use of the biofilm strategy that has protected them so well in their native habitats. Compromised individuals, who might not have survived in earlier times, are especially susceptible to this new cohort of “environmental” pathogens that have invaded our homes and schools just as they have invaded our hospitals.
BIOFILMS DEFINED
Our definition of biofilm has evolved over the last 25 years. Marshall in 1976 (129) noted the involvement of “very fine extracellular polymer fibrils” that anchored bacteria to surfaces. Costerton et al. (37) observed that communities of attached bacteria in aquatic systems were found to be encased in a “glycocalyx” matrix that was found to be polysaccharide in nature, and this matrix material was shown to mediate adhesion. Costerton et al., in 1987 (41), stated that biofilm consists of single cells and microcolonies, all embedded in a highly hydrated, predominantly anionic exopolymer matrix. Characklis and Marshall in 1990 (28) went on to describe other defining aspects of biofilms, such as the characteristics of spatial and temporal heterogeneity and involvement of inorganic or abiotic substances held together in the biofilm matrix.
Costerton et al., in 1995 (40), emphasized that biofilms could adhere to surfaces and interfaces and to each other, including in the definition microbial aggregates and floccules and adherent populations within pore spaces of porous media. Costerton and Lappin-Scott (38) at the same time stated that adhesion triggered expression of genes controlling production of bacterial components necessary for adhesion and biofilm formation, emphasizing that the process of biofilm formation was regulated by specific genes transcribed during initial cell attachment. For example, in studies of Pseudomonas aeruginosa, Davies and Geesey (47) have shown that the gene (algC) controlling phosphomannomutase, involved in alginate (exopolysaccharide) synthesis, is upregulated within minutes of adhesion to a solid surface. Recent studies have shown that algD, algU, rpoS, and the genes controlling polyphosphokinase synthesis are all upregulated in biofilm formation and that as many as 45 genes differ in expression between sessile cells and their planktonic counterparts (E. Pulcini, J. Costerton, and K. Sauer, personal communication).
A new definition for biofilm must therefore take into consideration not only readily observable characteristics, i.e., cells irreversibly attached to a surface or interface, embedded in a matrix of extracellular polymeric substances which these cells have produced, and including the noncellular or abiotic components, but also other physiological attributes of these organisms, including such characteristics as altered growth rate and the fact that biofilm organisms transcribe genes that planktonic organisms do not.
The new definition of a biofilm is a microbially derived sessile community characterized by cells that are irreversibly attached to a substratum or interface or to each other, are embedded in a matrix of extracellular polymeric substances that they have produced, and exhibit an altered phenotype with respect to growth rate and gene transcription. This definition will be useful, because some bacterial populations that fulfilled the earlier criteria of a biofilm, which involved matrix formation and growth at a surface, did not actually assume the biofilm phenotype. These “nonbiofilm” populations, which include colonies of bacteria growing on the surface of agar, behave like planktonic cells “stranded” on a surface and exhibit none of the inherent resistance characteristics of true biofilms. We can now speak of biofilm cells within matrix-enclosed fragments that have broken off from a biofilm on a colonized medical device and now circulate in body fluids with all the resistance characteristics of the parent community.
HOW MICROORGANISMS FORM BIOFILMS
Now that we concede that bacteria form biofilms in essentially the same manner in whatever ecosystem they inhabit, it is important that we take full advantage of the elegant studies of this process that fill the environmental and industrial microbiology literature. The scientific and engineering community has already examined biofilm formation in some detail and has published a couple of books (30, 113) on this subject. Many aspects of biofilm formation are counterintuitive, and it may be useful to summarize these issues, so that the medical community does not repeat this work.
Perhaps the first surprise, for the medical community, is that bacteria form biofilms preferentially in very high shear environments (i.e., rapidly flowing milieus). Planktonic bacteria can adhere to surfaces and initiate biofilm formation in the presence of shear forces that dwarf those of heart valves and exceed Reynolds numbers of 5,000 (30). The Reynolds number is a dimensionless number describing the turbulent flow of a liquid; if this number is high, turbulent flow exists; if it is low, laminar flow conditions prevail. Engineers speculate that turbulent flow enhances bacterial adhesion and biofilm formation by impinging the planktonic cells on the surface, but whatever the mechanism, biofilms form preferentially at high-shear locations in natural and industrial systems.
Studies of bacterial adhesion with laboratory strains of bacteria, many of which had been transferred thousands of times and lost their ability to adhere, first indicated that very smooth surfaces might escape bacterial colonization. Subsequent studies with “wild” and fully adherent bacterial strains showed that smooth surfaces are colonized as easily as rough surfaces and that the physical characteristics of a surface influence bacterial adhesion to only a minor extent (40). Once a biofilm has formed and the exopolysaccharide matrix has been secreted by the sessile cells, the resultant structure is highly viscoelastic and behaves in a rubbery manner (197). When biofilms are formed in low-shear environments, they have a low tensile strength and break easily, but biofilms formed at high shear are remarkably strong and resistant to mechanical breakage.
BIOFILM EXAMINATION AND MEASUREMENT
Our understanding of biofilms has developed as the methods for biofilm examination and characterization have evolved. Much of the early investigative work on biofilms relied heavily on the scanning electron microscope. This technique utilizes graded solvents (alcohol, acetone, and xylene) to gradually dehydrate the specimen prior to examination, since water of hydration is not compatible with the vacuum used with the electron beam. This dehydration process results in significant sample distortion and artifacts; the extracellular polymeric substances, which are approximately 95% water (28), will appear more as fibers than as a thick gelatinous matrix surrounding the cells.
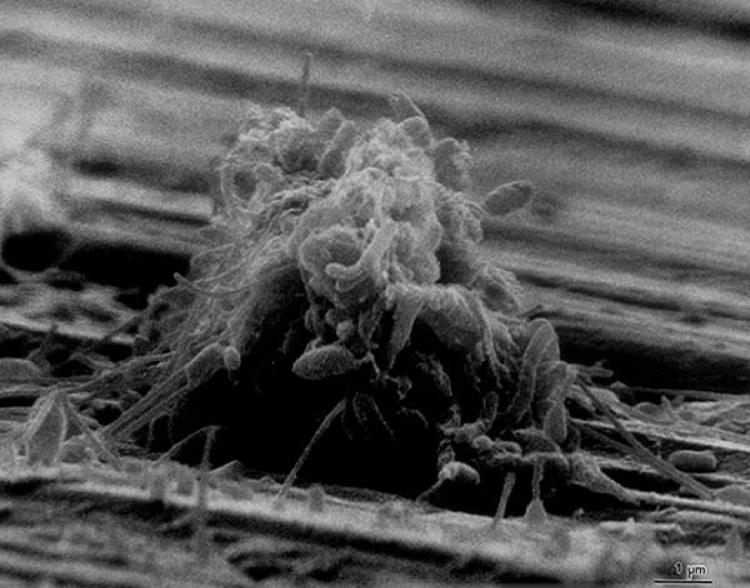
The use of transmission electron microscopy and specific polysaccharide stains like ruthenium red allowed researchers both to identify the nature of these extracellular fibers in biofilms and to better elucidate their association with the cells. Electron microscopy has been used for the examination and characterization of biofilms on medical devices (160, 187) and in human infections (66, 147). Because of its excellent resolution properties, the electron microscope will, in spite of its limitations, continue to be an important tool for the biofilm scientist. Figure 1 shows a typical scanning electron microscope image of a biofilm.
FIG. 1.
Scanning electron micrograph of a biofilm on a metal surface from an industrial water system.
The development of the confocal laser scanning microscope (CLSM) in the 1980s provided researchers with the ability to examine biofilms in situ without the limitations encountered with the scanning electron microscope, albeit at lower magnifications. The trade-off in resolution was more than offset by the ability to examine the biofilm matrix unaltered and intact.
The use of both CLSM and epifluorescence microscopy requires that the organisms in the biofilms be stained with fluorescent stains. These stains are designed to emit light at specific wavelengths and can be used to probe specific cellular functions. For example, nucleic acid stains such as DAPI (4′,6′-diamidino-2-phenylindole), acridine orange, and Syto 9 will stain the DNA and RNA of all cells regardless of their viability. Other stains have been developed for probing cell viability. Propidium iodide is taken up only by cells with damaged cytoplasmic membranes, and 5-cyano-2,3-ditolyl tetrazolium chloride is taken up and reduced to 5-cyano-2,3-ditolyl tetrazolium chloride-formazan only by cells that have a functioning cytochrome system. Using a suite of such stains allows the biofilm researcher to quantify all the cells and determine which ones are viable.
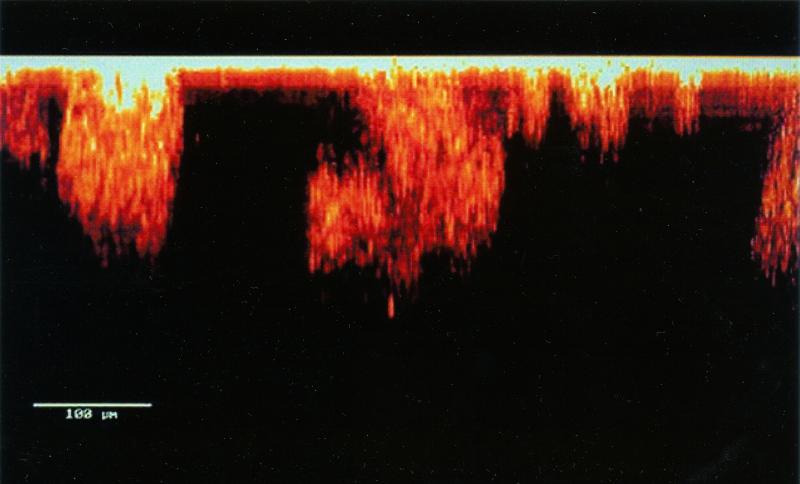
Fluorescent antisera and fluorescent in situ hybridization probes may enable us to identify specific organisms within a mixed biofilm community. Green fluorescent protein, a constitutively produced, plasmid-mediated molecule, can allow biofilms to be examined noninvasively, without fixation or staining (18). A confocal laser scanning microscopic image of a biofilm is shown in Fig. 2.
FIG. 2.
Confocal laser scanning micrograph of a biofilm, showing cell clusters and water channels. Reproduced with the permission of Paul Stoodley.
In more common use are techniques that rely on removal of the biofilms or biofilm-associated organisms from the substratum by some type of mechanical force, such as vortexing or sonication, prior to examination and measurement. The most commonly used procedure for measurement of biofilms is the viable plate count procedure, in which the resuspended and dispersed biofilm cells are plated onto a solid microbiological medium, incubated, and counted.
Table 1 lists several of the methods that have been used by clinical microbiologists for the recovery and measurement of clinically relevant biofilms on indwelling medical devices. For most of these techniques, a determination of the recovery efficiency of the method (i.e., the percentage of cells that are actually recovered from the biofilm) is needed. Methods that allow a determination of biofilm cell count in the implanted device without necessitating device removal, such as the endoluminal brush technique, could provide a distinct advantage for the clinical practitioner, potentially alleviating the need for device removal when the device is found not to contain intraluminal biofilms. These methods all rely on the quantification of biofilm cells as a measurement of total biofilm accumulation. Other methods have been used by biofilm researchers for measuring biofilms, including total protein (139), absorbance at either 550 nm (88) or 950 nm (201), tryptophan fluorescence (4), endotoxin (164), and total ATP (R. W. Walter and L. M. Cooke, paper no. 410, presented at the National Association of Corrosion Engineers Annual Conference, 1997). Any of these methods could be investigated for the measurement of clinically relevant biofilms.
TABLE 1.
Methods that have been used for measurement of biofilms on catheters
| Method | Basic protocola | Advantage | Limitation(s) | Reference |
|---|---|---|---|---|
| Roll-plate | Roll the catheter tip over the surface of a blood agar plate | Easy to use | Examines only catheter outer surface, inaccurate | 126 |
| Vortex, then viable count | Catheter section in PBS is vortexed then cultured on different media | Measures intraluminal and extraluminal biofilm | Recovery efficiency unknown | 202 |
| Sonicate, vortex, then viable count | Catheter section in TSB, sonicate then vortex, then culture on blood agar | Measures intraluminal and extraluminal biofilm | Recovery efficiency unknown | 178 |
| Sonicate, vortex, homogenize, then viable count | Catheter section in PBS, sonicate/vortex repeatedly, then homogenize and culture on blood agar | Recovery efficiency determined | Measures intraluminal biofilm only | 53 |
| Acridine orange direct staining | Following roll-plate method, catheter section is stained with acridine orange | Allows direct examination of catheter | Method does not allow quantification | 224 |
| Endoluminal brush | Brush is introduced into the implanted catheter, removed, placed into PBS, sonicated, and plated | Allows examination of indwelling catheter | Effect of procedure on patient and recovery efficiency unknown | 102 |
| Alginate swab | Swab introduced into the implanted catheter, removed, then streaked over a blood agar plate | Allows examination of indwelling catheter | Effect of procedure on patient and recovery efficiency unknown | 25 |
PBS, phosphate-buffered saline; TSB, Trypticase soy broth.
It should be obvious to the reader at this point that any method that sets out to estimate the efficacy of a treatment against biofilms should use biofilms and not planktonic cells to do so. Standard NCCLS broth microdilution methods for susceptibility testing cannot accurately estimate antimicrobial efficacy against biofilms, because these techniques are based on the exposure of planktonic organisms to the antimicrobial agent. However, a number of apparatuses have been developed for this purpose, as shown in Table 2. All of the model systems presented have been shown to provide useful information on biofilm processes, and several of these systems have been used to determine the efficacy of various antimicrobial agents against biofilm-associated organisms. Key parameters that may affect the rate and extent of biofilm formation in a model system, and which therefore should be considered in model system design, are given in Table 3.
TABLE 2.
Apparatuses that have been used for growing and testing biofilms
| Apparatus | Organism(s) tested | Flow dynamics | Substratum | Method for removing and quantifying biofilm | Reference |
|---|---|---|---|---|---|
| Modified Robbins device | Pseudomonas pseudomallei | Batch/mixing | Silastic disks | Method of removal not given; viable count | 208 |
| Calgary biofilm device | P. aeruginosa, S. aureus, E. coli | Batch/mixing | Plastic pegs | Sonicate peg, then viable count | 26 |
| Disk reactor | Gram-negative bacteria | Batch/mixing | Teflon coupons | Sonicate, vortex, homogenize, then viable or direct count | 54 |
| CDC biofilm reactor | Gram-negative bacteria | Continuous/open system | Needleless connectors (plastic) | Sonicate, vortex, homogenize, then viable or direct count | 144 |
| Perfused biofilm fermentor | Candida albicans | Continuous/open system | Cellulose-acetate filters | Shake in sterile water, then viable count | 11 |
| Model bladder | Gram-negative bacteria | Continuous/open system | Urinary catheters | Direct examination by SEM or TEMa or by chemical analysis | 195 |
SEM, scanning electron microscopy; TEM, transmission electron microscopy.
TABLE 3.
Factors to consider in the development of a model biofilm system
| Medium | Inoculum | Hydrodynamics | Substratum |
|---|---|---|---|
| Composition, temperature, presence of antimicrobial agents | Identity of organism, no. of cells | Flow rate, presence of shear, batch vs. open system, retention time | Roughness, chemistry, conditioning films |
BIOFILM ULTRASTRUCTURE
Biofilms were perceived as unstructured accretions of bacterial cells, surrounded by the cells' exopolysaccharide matrices, for the first decade (1978 to 1990) following the discovery of the importance and ubiquity of biofilms. These perceptions were based on flawed techniques for direct observation, in that electron microscopy required complete dehydration of the highly hydrated biofilm matrices and in that light microscopy was badly distorted by out-of-focus effects. CLSM was invented in the 1950s, but it was never used to study bacteria because the whole field was fixated on the planktonic phenotype. CLSM produces optical slices of complex structures, so that out-of-focus effects are removed, and it requires no sample preparation, so that living organisms can be observed if fluorescence can be introduced in order to visualize the cells. The first examination of living biofilms using CLSM produced a whole series of revelations that are the basis of current biofilm concepts.
Foremost has been the observation that developed biofilms are not structurally homogeneous monolayers of microbial cells on a surface. Rather, they can be described as heterogeneous in both time and space (116). The basic building block or structural unit of the biofilm is the microcolony, and an elucidation of basic biofilm processes, such as quorum sensing, antimicrobial resistance, and detachment, may hinge on an understanding of the physiological interactions of microcolonies within a developed biofilm.
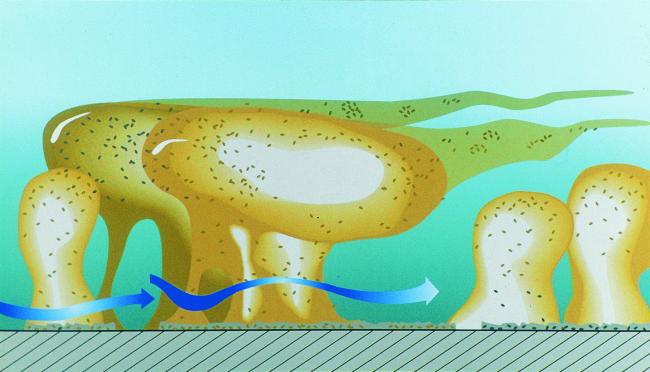
Figure 3 shows a mixed-species biofilm grown on a metal surface in a laboratory potable-water reactor system. Note both the heterogeneous nature and the presence of individual microcolonies within this biofilm. Living, fully hydrated biofilms are composed of cells (±15% by volume) and of matrix material (±85% by volume), and the cells are located in matrix-enclosed “towers” and “mushrooms” (Fig. 4). Open water channels are interspersed between the microcolonies that contain the sessile cells (115), and physical techniques have shown that the bulk water of these systems enters these channels to produce convective flow (50).
FIG. 3.
Mixed-species heterotrophic biofilm grown on stainless steel in a potable-water biofilm reactor containing Pseudomonas aeruginosa, Klebsiella pneumoniae, and Flavobacterium spp. This image of a biofilm was obtained, after staining with 4′,6′-diamidino-2-phenylindole, with a Zeiss Axioskop 2 epifluorescence microscope and the Zeiss deconvolution system.
FIG. 4.
Biofilm structure cartoon. Copyright Center for Biofilm Engineering, Montana State University, Bozeman, Mont. Reprinted with permission.
With CLSM, direct observations of living biofilms, ranging from single-species laboratory biofilms to complex multispecies communities growing in natural ecosystems, have shown that this basic community structure is universal, with some minor variations. It is difficult to illustrate the dynamic dimensions that are very important in biofilms by using printed work and two-dimensional figures, but we can use the image of a forest of rubbery towers, each of which is attached to the colonized surface. The direct examination of biofilms in high-shear environments (197) has shown that each microcolony is deformed by these forces, to form a tadpole shape that oscillates in the bulk fluid.
The structural characteristic of biofilms that has the greatest impact on the outcome of chronic bacterial infections, such as native valve endocarditis, is the tendency of individual microcolonies to break off and/or detach when their tensile strength is exceeded. This detachment of preformed microcolonies containing sessile cells in the antibiotic-resistant biofilm phenotype poses a very serious risk of infective emboli in the first capillary bed that is encountered. This shedding of microcolonies from preformed biofilms on heart valves can lead to stroke or to severe pulmonary sequelae, and its consequences are well recognized by the clinical community.
RESISTANCE TO ANTIMICROBIAL AGENTS
The nature of biofilm structure and the physiological attributes of biofilm organisms confer an inherent resistance to antimicrobial agents, whether these antimicrobial agents are antibiotics, disinfectants, or germicides. Table 4 shows the dramatic differences in susceptibility of planktonic and biofilm organisms to antimicrobial agents. Mechanisms responsible for resistance may be one or more of the following: (i) delayed penetration of the antimicrobial agent through the biofilm matrix, (ii) altered growth rate of biofilm organisms, and (iii) other physiological changes due to the biofilm mode of growth.
TABLE 4.
Susceptibility of planktonic and biofilm bacteria to selected antibiotics
| Reference | Organism | Antibiotic | MIC or MBC of planktonic phenotype (μg/ml) | Concn effective against biofilm phenotype (μg/ml) |
|---|---|---|---|---|
| 215 | S. aureus NCTC 8325-4 | Vancomycin | 2 (MBC) | 20a |
| 26 | Pseudomonas aeruginosa ATCC 27853 | Imipenem | 1 (MIC) | >1,024b |
| 26 | E. coli ATCC 25922 | Ampicillin | 2 (MIC) | 512b |
| 208 | P. pseudomallei | Ceftazidime | 8 (MBC) | 800c |
| 114 | Streptococcus sanguis 804 | Doxycycline | 0.063 (MIC) | 3.15d |
Concentration required for 99% reduction.
Minimal biofilm eradication concentration.
Concentration required for ∼99% reduction.
Concentration required for >99.9% reduction.
Delayed Penetration of the Antimicrobial Agent
Antimicrobial molecules must diffuse through the biofilm matrix in order to inactivate the encased cells. The extracellular polymeric substances constituting this matrix present a diffusional barrier for these molecules by influencing either the rate of transport of the molecule to the biofilm interior or the reaction of the antimicrobial material with the matrix material. Suci et al. (198) demonstrated a delayed penetration of ciprofloxacin into Pseudomonas aeruginosa biofilms; what normally required 40 s for a sterile surface required 21 min for a biofilm-containing surface. Hoyle et al. (83) found that dispersed bacterial cells were 15 times more susceptible to tobramycin than were cells in intact biofilms. DuGuid et al. (57) examined Staphylococcus epidermidis susceptibility to tobramycin and concluded that the organization of cells within biofilms could in part explain the resistance of this organism to this antimicrobial agent.
Other studies have examined antimicrobial agent penetration and interaction with the extracellular polymeric substance material of biofilms. Hatch and Schiller (79) showed that a 2% suspension of alginate isolated from P. aeruginosa inhibited diffusion of gentamicin and tobramycin, and this effect was reversed by using alginate lyase. Souli and Giamarellou (181) demonstrated the ability of S. epidermidis slime to hinder the antimicrobial susceptibility of Bacillus subtilis to a large number of agents. Not all antimicrobial agents were equally affected; glycopeptides such as vancomycin and teicoplanin were significantly affected, whereas agents such as rifampin, clindamycin, and the macrolides were either unaffected or minimally affected. Another study (74) examined the diffusion of several antimicrobial agents (ceftazidime, cefsulodin, piperacillin, gentamicin, and tobramycin) through synthetic and naturally produced alginate gels and found that beta-lactam antibiotics diffused into the matrix more rapidly than did aminoglycosides. Aminoglycosides were found to initially bind to the alginates, but diffusion increased after an 80- to 100-min lag period.
Altered Growth Rate of Biofilm Organisms
Another proposed mechanism for biofilm resistance to antimicrobial agents is that biofilm-associated cells grow significantly more slowly than planktonic cells and, as a result, take up antimicrobial agents more slowly. Using a method of cell culture designed to determine the effect of growth rate apart from other biofilm processes, Evans et al. (63) found that the slowest growing Escherichia coli cells (in biofilms) were the most resistant to cetrimide. At growth rates higher than 0.3 per h, biofilm and planktonic cells were equally susceptible. Another study showed that S. epidermidis biofilm growth rates strongly influenced susceptibility; the faster the rate of cell growth, the more rapid the rate of inactivation by ciprofloxacin (56). Anwar et al. (5) found that older (10-day-old) chemostat-grown P. aeruginosa biofilms were significantly more resistant to tobramycin and piperacillin than were younger (2-day-old) biofilms. A dosage of 500 μg of piperacillin plus 5 μg of tobramycin per ml completely inactivated both planktonic and young (2-day-old) biofilm cells. Older (10-day-old) biofilm cell counts were reduced only approximately 20% by exposure to this dose. Similar results have been observed with several different combinations of bacteria and antimicrobial agents (2, 32, 51).
Other Physiological Changes Due to Biofilm Mode of Growth
Gram-negative bacteria respond to nutrient limitation and other environmental stresses by synthesizing sigma factors. In E. coli, those sigma factors that are under the control of the rpoS regulon regulate the transcription of genes whose products mitigate the effects of stress. By studying E. coli biofilms formed by strains with and without the rpoS gene, Adams and McLean (1) found that the rpoS+ E. coli biofilms had higher densities and a higher number of viable organisms. Since rpoS is activated during slow growth of this organism, it appears that conditions that elicit the slowing of bacterial growth, such as nutrient limitation or build-up of toxic metabolites, favor the formation of biofilms. Nutrient limitation and increases in toxic metabolite concentrations might be particularly acute within the depths of established biofilms. Tresse et al. (203) found that agar-entrapped E. coli cells were more resistant to an aminoglycoside as oxygen tensions were decreased. They suggested that the effect was due to lowered uptake of the antibiotic by the oxygen-starved cells. Dagostino et al. (42) proposed that initial bacterial association with a surface may result in the repression or induction of genes, which in turn results in a number of physiological responses.
HUMAN INFECTIONS INVOLVING BIOFILMS
Koch's postulates state that (i) the organism is regularly found in the lesions of the disease, (ii) it can be isolated in pure culture on artificial media, (iii) inoculation of this culture produces a similar disease in experimental animals, and (iv) the organism can be recovered from the lesions of these animals (49). The question of whether biofilms are etiological agents of disease in many cases cannot be proven according to Koch's postulates. Nickel and Costerton (147) studied coagulase-negative staphylococci (CoNS) in chronic prostatitis and were able to detect these organisms in biopsies from infected individuals. Nevertheless, they concluded that it was not possible to state definitively that these organisms were the cause of the infection. All that could be stated was that there was an association between the presence of the organisms and the disease. For several of the diseases discussed in this section, such as periodontitis, native valve endocarditis, and cystic fibrosis, that association is stronger. For others, such as otitis media, the association is less well established. A discussion of several noted infectious diseases for which the biofilm link has been suggested follows.
Native Valve Endocarditis
Native valve endocarditis (NVE) is a condition that results from the interaction between the vascular endothelium, generally of the mitral, aortic, tricuspid, and pulmonic valves of the heart, and bacteria or fungi circulating in the bloodstream (118). The diversity of organisms causing NVE is quite extensive. Tunkel and Mandell (204) noted that of 2,345 cases of infective endocarditis, 56% were caused by streptococci (including viridans streptococci, enterococci, pneumococci, and Streptococcus bovis), 25% by staphylococci (19% coagulase positive and 6% CoNS), and the balance by gram-negative bacteria and fungi (Candida and Aspergillus spp.). These organisms gain access to the bloodstream primarily via the oropharynx, gastrointestinal tract, and genitourinary tract.
Normally, microorganisms adhere poorly to intact endothelium. However, when the endothelium is damaged, nonbacterial thrombotic endocarditis (NBTE), in which the thrombus is an accumulation of platelets, fibrin, and occasionally red blood cells, will develop at the point of injury. Durack (59) induced NBTE formation in rabbits by leaving a polyethylene catheter in place in contact with the aortic valve. Fibronectin, secreted by endothelial cells, platelets, and fibroblasts in response to a vascular injury, has been identified in thrombotic lesions of heart valves. Fibronectin can simultaneously bind to fibrin, collagen, human cells, and bacteria (118).
Several bacteria have fibronectin receptors, including Staphylococcus aureus and several species of Streptococcus (118). Lowrance et al. (119, 120) showed in an animal model that Streptococcus sanguis binds to the fibronectin molecule and that low-fibronectin-binding mutants of S. sanguis are less virulent than the high-binding strains. Several of the streptococci also produce high-molecular-weight dextrans that promote adherence to the surface of the thrombus in NBTE (166). Dall et al. (43) showed that dextranase blocked microbial adhesion in experimental animals. Inoculum size may also be important, depending on the species. Gram-negative bacteria do not adhere as well as gram-positive organisms, and induction of endocarditis in laboratory animals requires a much higher inoculum of gram-negative bacteria than of gram-positive organisms (96).
Early work by Durack showed that bacteria would localize in sites of NBTE within 30 min of injection into a rabbit containing a polyethylene catheter (59). Though most of the bacteria were ingested by white blood cells that were stuck to the edges of the NBTE, some bacteria were not ingested and adhered to the edge of the vegetation. Within hours these bacteria had begun to multiply. Bacterial microcolonies developed in the platelet-fibrin matrix, primarily where there were few white blood cells. Several bacterial colonies eventually (after 24 h) developed fibrin capsules and were thus protected from the white blood cells. It appeared to the authors that the movement of the white blood cells was hindered by the fibrin. Durack and Beeson (58) also showed that most of the metabolic activity of the biofilm bacteria was on the surface; colonies deeper in the thrombus were inactive. Also, they observed that the majority of bacteria in a vegetation enter a resting state within 2 days of infection.
Biofilms on native heart valves may result in valve tissue damage or production of emboli. Ferguson et al. (66), in studies of rabbits infected with staphylococci, found that bacteria penetrated into the connective tissue of the aortic valve, structurally damaging it. Release of cells or clumps of cells and NBTE components into the bloodstream may also occur as a result of NVE biofilms. These emboli may cause serious complications throughout the body. Fungi, because they produce bulky, friable vegetations, more frequently produce emboli. Stiles and Friesinger (196) noted that fungal biofilms may exceed 2 cm in diameter and the rate of clinically apparent emboli was higher in fungi than in bacteria. Rohmann et al. (168) found that embolic events were more common in patients with vegetations larger than 10 mm in diameter.
NVE may be detected either indirectly, by a combination of clinical symptoms and identification of organisms in the bloodstream, or by observing the vegetations via imaging techniques. One such imaging technique in common use is echocardiography. However, though it may be a good technique for documenting the presence or absence of biofilms, the use of echocardiography as a routine method for establishing diagnosis is not recommended. Approximately half of patients with clinical criteria examined in a study by Stewart et al. (185) demonstrated vegetative lesions by echocardiography. These findings were confirmed by others (22, 121). Berger et al. (15) noted that the limit of detection for biofilms on infected valves is a diameter of approximately 3 mm. However, Rohmann et al. (168) found that monitoring vegetation size with transesophageal cardiography, particularly in culture-negative patients, may help to assess the efficacy of antimicrobial treatment.
Most medical practitioners recommend prophylactic antibiotics when patients with a high risk of endocarditis undergo dental and other invasive procedures. This treatment consists of 3 g of amoxicillin taken orally 1 h before a procedure and then 1.5 g 6 h later (166). This treatment would be expected to kill planktonic organisms in the bloodstream prior to attachment. Once the biofilm is established on the heart valves, treatment is much less effective due to a combination of mass transfer limitations and inherent resistance of biofilm organisms.
Depending on the organism involved, various antibiotic therapies have been used. Penicillin is the normal treatment for streptococcal endocarditis, and it may be supplemented with gentamicin to produce synergistic killing. Treatment may be increased when complications such as large vegetation size occur. Other antibiotics or combinations of antibiotics are used for other organisms. Dall et al. (43) found that addition of dextranase as an adjuvant to penicillin prevented microbial adhesion and facilitated penicillin sterilization of infected valves in experimental animals. Joly et al. (96) found that antibiotic treatment was more successful when serum antibiotic levels were held at least 10-fold higher than the minimal bactericidal concentration (MBC) through the entire dosing regimen. Sandoe et al. (172) successfully treated Staphylococcus capitus endocarditis with vancomycin and rifampin for prolonged treatment. Perrotta and Fiore (156) found that Streptococcus bovis endocarditis was successfully resolved by using penicillin G together with streptomycin (6 days), followed by imipenem (4 days).
Candida endocarditis has been treated successfully with fluconazole (212). Rohmann et al. (168) investigated the effect of antibiotic treatment on vegetation size using transesophageal echocardiography in 183 patients monitored over a 76-week period. The reduction in vegetation size as a result of treatment was as follows: vancomycin, 45%; ampicillin, 19%; and penicillin, 5%. Penicillinase-resistant drugs resulted in a 15% increase, and cephalosporin resulted in a 40% increase. These results underline the importance of closely monitoring the biofilm size over the course of the treatment, especially since embolic events are more common for larger vegetations. Another treatment approach is to surgically remove the vegetation from the infected valve, a procedure termed vegetectomy (86).
Clearly, the formation of biofilms on native heart valves (termed vegetations by the medical community) is a well-documented biofilm process. However, there are still important questions that must be addressed. What threshold number of microorganisms in the bloodstream is required to develop a biofilm? Could in vitro studies be developed that will more accurately predict the efficacy of antimicrobial agents in vivo? Can bacteria that are ingested by leukocytes survive to colonize a sterile NBTE site?
Otitis Media
Otitis media (OM) is a disease of the middle ear that involves the inflammation of the mucoperiosteal lining. OM is a very common childhood disease, may be acute or chronic, and is caused by a number of different organisms, including Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, group A beta-hemolytic streptococci, enteric bacteria, Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, and other organisms (65). Mixed cultures may also be isolated (73). Stenfors and Raisanen (184) quantified the bacteria in middle ear effusions collected from patients with OM. They found counts ranging between 105 and 109 per ml of effusion material. In certain cases of chronic OM, the middle ear may contain a highly viscous fluid (OM with effusion) (73). Under these conditions, the implantation of tympanostomy tubes is performed to alleviate pressure build-up and hearing loss.
Tympanostomy tubes are subject to contamination, and biofilms will build up on their inner surfaces. Biedlingmaier et al. (16) investigated the colonization of Armstrong-style silicone, fluoroplastic, ionized modified silicone and silver oxide-coated Armstrong-style silicone tubes by Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis in Trypticase soy broth. They found that all three organisms developed biofilms on the Armstrong silicone and the silver oxide-coated Armstrong-style silicone tubes. P. aeruginosa also developed biofilms on the fluoroplastic tubes. Only the ionized silicone tubes remained free of contamination and biofilms.
Saidi et al. (170) investigated biofilm formation on tubes implanted into the ears of guinea pigs inoculated with S. aureus. In this study, the tube materials investigated included silicone, silver oxide-impregnated silicone, fluorplastic, silver oxide-impregnated fluorplastic, and ion-bombarded silicone. The tubes were left in place for 10 days, fixed, and examined by scanning electron microscopy. The results of this study showed that all of the materials contained attached bacteria, though the ion-bombarded silicone had fewer cells, which did not appear to have formed a biofilm.
Gourin and Hubbell (75) investigated the efficacy of silver oxide-impregnated silastic tympanostomy tubes inserted into the ears of 630 patients with chronic OM in preventing postoperative otorrhea (drainage from the ear) in a prospective nonrandomized clinical study. They found that the use of the treated tympanostomy tubes resulted in a lower incidence of postoperative otorrhea after the first postoperative week. The authors opined that the silver oxide prevented adherence and colonization of selected bacteria to the tube but probably had no effect on the established infection in the middle ear.
The fact that biofilm organisms are significantly more resistant to antimicrobial agents has already been discussed. An additional consideration in the case of biofilms of otitis media is that there is very low penetration of antibiotics into the middle ear fluid. Krause et al. (107) compared concentrations of amoxicillin, cefaclor, erythromycin-sulfisoxazole, and trimethoprim-sulfamethoxazole in middle ear fluid and serum of children with serous OM. For samples collected 15 to 240 min after administration of a single oral dose, levels of antibiotic in the middle ear fluid were always significantly lower than those in the serum. Also, certain antibiotics, such as erythromycin, were never detected at all in the middle ear fluid.
Kondoh and Hashiba (106) evaluated the efficacy of several macrolide antibiotics, i.e., clarithromycin, erythromycin, and midacamycin, against biofilms of P. aeruginosa growing on Teflon in a minimal medium for a 7-day exposure period. Both clarithromycin and erythromycin inhibited biofilm formation, as evidenced by decreases in total protein, alginate, and hexose on Teflon beads. However, the planktonic bacterial levels were unaffected by the treatments, and the authors proposed that the inhibitory effects were due to factors other than bactericidal activity. Both clarithromycin and erythromycin inhibited biofilm formation at 1/20 of the MIC. Since this concentration can be achieved in sputum and nasal discharges, there is a good probability that these antimicrobial agents would be effective against biofilm diseases caused by P. aeruginosa, including OM.
With the exception of a single report by Hayes et al. (J. D. Hayes, R. Veeh, X. Wang, J. W. Costerton, J. C. Post, and G. D. Ehrlich, abstr. 186, Am. Soc. Microbiol. Biofilm 2000 Conf., 2000), there is very little evidence for the development of biofilms on mucosal surfaces of the middle ear in OM. In this study, the authors used scanning electron microscopy to provide evidence of H. influenzae biofilms on the middle ear mucosal surfaces of chinchillas that had been injected with a culture of this organism. Recent unpublished work with the chinchilla model of OM, in collaboration with Ehrlich and Post, clearly shows biofilm formation by both scanning electron microscopy and CLSM.
Chronic Bacterial Prostatitis
The prostate gland may become infected by bacteria that have ascended from the urethra or by reflux of infected urine into the prostatic ducts emptying into the posterior urethra (52). Once the bacteria enter the prostatic duct and ascini, they multiply rapidly and elicit a host response. As long as the infection is in the early acute stages, the bacteria can easily be eradicated with antibiotic therapy (146). If these bacteria persist, they can form sporadic microcolonies and biofilms that adhere to the epithelial cells of the duct system. Organisms isolated in cases of chronic bacterial prostatitis include E. coli (most common isolate), Klebsiella, enterobacteria, Proteus, Serratia, Pseudomonas aeruginosa, CoNS, coryneforms, and Enterococcus faecalis (52). In another study, Nickel and Costerton (151) isolated E. coli, P. aeruginosa, Bacteroides spp., Gardnerella spp., Corynebacterium spp., and CoNS.
Much of our understanding of the probable role of biofilms in chronic bacterial prostatitis has come either from studies employing animal models (148, 150) or from biopsies collected from men with prostatitis (147, 151). Nickel et al. (150) inoculated the prostates of rats with a culture of 108 E. coli organisms per ml by means of a sterile catheter. Rats were sacrificed after 1, 3, and 7 days and weekly for 8 weeks, and biopsy samples of prostates were collected. These samples were examined by either scanning electron microscopy or transmission electron microscopy. Samples were also sonicated and plated onto MacConkey agar. They demonstrated that bacteria were present in glycocalyx-encased microcolonies and appeared to be firmly adherent to the ductal and acinar mucosal layers.
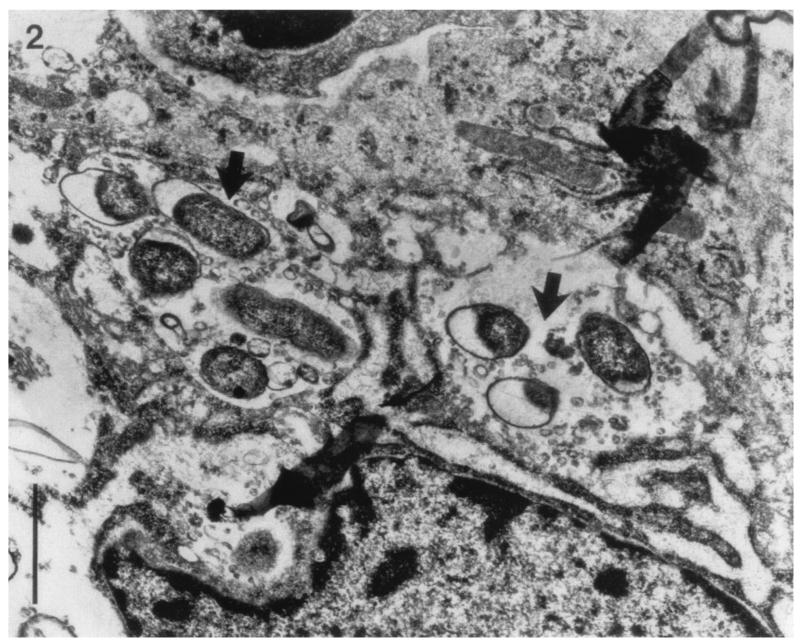
Nickel and Costerton (151) evaluated 20 men with a history of chronic bacterial prostatitis. Biopsies were collected from infected prostates, processed aseptically, and plated onto nutrient agar. Histological specimens were also examined by scanning electron microscopy and transmission electron microscopy. The authors showed evidence of bacterial attachment to the ductal walls, especially for P. aeruginosa. Nickel and Costerton (147) were also able to demonstrate, using needle biopsies, sporadic microcolonies of CoNS in the intraductal space. The microcolonies were enveloped in a dehydrated slime matrix. Transmission electron microscopy portrayed bacterial biofilms very clearly, as shown in Fig. 5.
FIG. 5.
Transmission electron micrograph of a prostatic duct in an area of focal chronic inflammation from a patient with an E. coli chronic prostatitis. Arrows point to bacterial microcolonies amid inflammatory cells and debris. These bacteria were cultured from both expressed prostatic secretions and tissue biopsies obtained 4 weeks after antibiotics were discontinued. Bar, 1 μm. Reprinted from reference 151 with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Domingue and Hellstrom (52) state that treatment failures are common in prostatitis, probably as a result of the local environment surrounding the infecting organisms and the fact that these organisms have produced a biofilm. Once bacteria infect the prostate, they produce a glycocalyx and become inactive. With this change in metabolism, the cells can become more resistant to antimicrobial agents (146). Nickel and Costerton (151) presented a study of chronic bacterial prostatitis in 20 men whose symptoms did not resolve with long-term courses of antibiotic therapy. The dosage regimens of these antibiotics had been determined by culture and sensitivity testing in the laboratory. They found that it took significantly longer (96 h) to grow bacteria from sonicated tissue biopsy samples than to grow bacteria cultured from patients with cystitis. This observation lends support to the conclusion that organisms growing in the tissues as biofilms have an altered metabolism.
In light of the fact that prostatitis is apparently caused by biofilm-associated organisms, Nickel et al. (146) have suggested that a recommended treatment regimen might be to deliver higher antibiotic concentrations directly to the biofilm within the prostatic ducts.
Cystic Fibrosis
Cystic fibrosis (CF), a chronic disease of the lower respiratory system, is the most common inherited disease. In this condition, the normal mucociliary clearance system that cleanses the bronchopulmonary epithelium of inhaled particles depends on an upward directional flow of a mucus layer on the tips of cilia that move freely in the underlying watery layer. In CF there is a net deficiency of water, which hinders the upward flow of the mucus layer. Decreased secretion and increased absorption of electrolytes lead to dehydration and thickening of secretions covering the respiratory epithelium (104).
According to May et al. (131), 70% of patients with CF are defective in the cystic fibrosis transmembrane conductance regulator protein (CFTR), which results in altered secretions in the secretory epithelia. The hyperviscous mucus that is produced is thought to increase the incidence of bacterial lung infections in CF patients. According to Govan and Deretic (76), the CF gene, which encodes the CFTR, has been identified. The CFTR functions as a chloride ion channel protein. Chloride ion transport is severely impaired when the CFTR is defective in CF patients. Staphylococcus aureus is usually the first pulmonary isolate from these patients (131). It can normally be controlled by antibiotics. S. aureus and H. influenzae infections usually predispose the CF-affected lung to colonization with P. aeruginosa. Burkholderia cepacia has also been shown to infect the lungs of CF patients with lethal consequences, but it has never attained the 80% colonization rate of P. aeruginosa (76).
The exact mechanism of P. aeruginosa colonization of the lungs of patients with CF is not known. There is evidence that enhanced pseudomonal receptors on the respiratory epithelia may be responsible; impaired mucociliary clearance is another possibility (76). During initial colonization, the organisms are nonmucoid. Persistence of the organism in the lungs of patients with CF ultimately will result in a mucoid phenotype (104). There is no clear interval between the initial colonization by P. aeruginosa and conversion to mucoid forms; it may take several months to years. The variable timing of the emergence indicates that this is caused by random mutations, followed by selection of mucoid strains in the lungs of patients with CF (76).
This mucoid phenotype was first observed by Lam et al. (110) in postmortem specimens of infected lung tissue and bronchoscopy material from infected patients. The mucoid material was shown to be a polysaccharide material, later identified as alginate. The conditions that trigger the conversion to the mucoid phenotype have been investigated. Hoyle et al. (84) demonstrated, using a chemostat and modified Robbins device, that mucoid exopolysaccharide was transiently produced following adherence of P. aeruginosa. May et al. (131) noted that several in vitro conditions, such as nutrient limitation, the addition of surfactants, and suboptimal levels of antibiotics, may result in mucoidy. Mucoidy is even elicited by addition of ethanol to the medium, indicating that this phenotype may be a response to dehydration.
Mathee et al. (130) showed that biofilms of P. aeruginosa challenged with either activated human peripheral blood polymorphonuclear leukocytes (PMNs) or hydrogen peroxide (a product released in low levels by PMNs) yielded about 0.1% mucoid colonies, while unchallenged biofilms produced none. Alginate was overproduced by all the mucoid colonies. They hypothesized that activated PMNs and the release of toxic products such as hydrogen peroxide could play a role in the generation of mucoid organisms during the inflammatory response.
The sputum from the lungs of patients with CF is usually filled with large numbers of PMNs, and the inflammatory defense mechanisms in the lungs of patients with CF against mucoid P. aeruginosa are usually dominated by PMNs and antibodies (130). In contrast to P. aeruginosa, B. cepacia does not generally produce alginate-like compounds, though some investigators have reported the production of other exopolysaccharides. Mucoid colonial morphology in B. cepacia is rare in both environmental and clinical strains. The presence of biofilms or microcolonies of Burkholderia has not been reported for patients colonized solely by this organism (76).
A question posed by a number of investigators is why mucoid P. aeruginosa infections are so recalcitrant and resistant to immune system clearance. Koch and Hoiby (104) stated that the biofilm mode of growth protects the organisms from antimicrobial agents and host defenses. The alginate layer of mucoid strains appears to prevent antibody coatings and blocks the immunological determinants required for opsonic phagocytosis (90, 91, 131, 135). Mucoid strains are apparently more resistant to nonopsonic phagocytosis than are nonmucoid strains (90, 131). There is evidence that the alginate may promote adherence of the mucoid strains to epithelial cells in the pulmonary tract, thereby inhibiting clearance. In vivo experiments with infected rats confirmed this; mucoid P. aeruginosa strains were less rapidly removed from the pulmonary tract than were nonmucoid strains (131).
Another mechanism for persistence and survival was proposed by Cochrane et al. (33). Using rats that had been artificially infected with agar beads containing P. aeruginosa, they found that the bacteria within these beads produced elevated levels of high-molecular-weight iron-regulated membrane proteins that can function as receptors for iron-siderophore complexes. These molecules aid in the scavenging of low levels of iron from the bloodstream. A host defense mechanism against pathogenic organisms is to restrict available iron in order to limit this essential bacterial nutrient. By producing iron-scavenging compounds, the organisms are better able to survive in the host.
Anwar et al. (6) also suggested that biofilm age was a critical factor in P. aeruginosa survival. In their experimental system, older biofilm cells of this organism were less susceptible to either whole blood or serum than were either younger biofilms or planktonic organisms.
The possibilities for successful treatment of CF may ultimately hinge on early antimicrobial treatment to prevent or delay chronic infection with P. aeruginosa. Koch and Hoiby (104) noted that early treatment with oral ciprofloxacin and inhaled colistin could postpone chronic infection with P. aeruginosa for several years. They also suggested that a vaccine against this organism might be effective in preventing initial colonization of the lungs of patients with CF.
Periodontitis
Periodontal diseases, infections involving the supporting tissues of teeth, range from mild and reversible inflammations of the gums (gingiva) to chronic destruction of periodontal tissues (gingiva, periodontal ligament, and alveolar bone). Chronic periodontitis may lead to exfoliation of the teeth (112). The channel between the tooth root and the gingiva (gum), termed the subgingival crevice, is the primary site of periodontal infection and will deepen into a periodontal pocket with the progression of the disease (112).
Moore et al. (140) characterized the organisms isolated from patients with moderate periodontal disease and found that Fusobacterium nucleatum, Peptostreptococcus micros, Eubacterium timidum, Eubacterium brachy, Lactobacillus spp., Actinomyces naeslundii, Pseudomonas anaerobius, Eubacterium sp. strain D8, Bacteroides intermedius, Fusobacterium sp., Selenomonas sputigena, Eubacterium sp. strain D6, Bacteroides pneumosintes, and Haemophilus aphrophilus were all positively correlated with gingivitis. They concluded that the predominant organisms in the subgingival areas of patients with moderate periodontitis are not found in healthy patients.
Lamont and Jenkinson (112) and Socransky and Haffajee (180) noted that Porphyromonas gingivalis is the primary agent responsible for periodontitis. Omar et al. (154) examined subgingival plaque in adult patients with periodontitis and showed that spirochetes and cocci tended to increase in these areas. Dzink et al. (60) found that the predominant microflorae of active lesions in subgingival areas were Fusobacterium nucleatum, Wolinella recta, Bacteroides intermedius, Bacteroides forsythus, and Bacteroides gingivalis (Porphyromonas gingivalis). Marsh (128) noted that the predominant flora, even between sites in the same subject, is highly diverse, though periodontitis is clearly a polymicrobic infection.
Proteinaceous conditioning films, called acquired pellicle, develop on the exposed surfaces of enamel almost immediately after cleaning of the tooth surface within the oral cavity. The pellicle comprises albumin, lysozyme, glycoproteins, phosphoproteins, lipids, and gingival crevice fluid (128). Within hours of pellicle formation, single cells of primarily gram-positive cocci and rod-shaped bacteria from the normal oral flora colonize these surfaces. The pioneer species are predominantly streptococci, actinomycetes, and smaller numbers of Haemophilus (128). These organisms have the ability to bind directly to the pellicle through the production of extracellular glucans (105). After several days, actinomycetes predominate, and the characteristic polysaccharide matrix of a biofilm begins to develop (128).
Organisms associating with and attaching to cells in this early biofilm do so by a process called coaggregation. Coaggregation is cell-to-cell recognition whereby organisms in the biofilm can recognize and adhere to genetically distinct bacteria by means of adhesins. These adhesins recognize protein, glycoprotein, or polysaccharide receptors on oral surfaces, including other cell types (105). A climax biofilm community, termed plaque, will develop within 2 to 3 weeks if the plaque is left undisturbed, with 50- to 100-μm-thick biofilms developing (112). In addition to matrix polysaccharides, there will be polymers of salivary origin (128).
Plaque that becomes mineralized with calcium and phosphate ions is termed calculus or tartar (176). In addition to development on the tooth surfaces (within fissures), plaque can develop more extensively in protected areas, including approximal areas (between the teeth) and the gingival crevice (between the tooth and gum). As the plaque mass increases in these protected areas, the beneficial buffering and antimicrobial properties of the saliva are less able to penetrate and protect the tooth enamel, leading to dental caries or periodontal disease (128). In support of this, Corbet and Davies (35) reviewed data showing that control of supragingival plaque by professional tooth cleaning and personal efforts would prevent gingival inflammation and adult periodontitis.
Within the subgingival crevice, the primary source of nutrients for the developing biofilm is gingival crevice fluid, a serum exudate that bathes the gingival crevice. This fluid provides proteins, glycoproteins, and other nutrients. Bacterial nutrients may also originate from saliva and the host diet (especially fermentable carbohydrates) (128). Though there is a constant flow of air through the oral cavity, the tooth surface rapidly becomes anaerobic on colonization with microorganisms. Marsh (128) noted that redox potential (Eh) fell from >+200 mV to −30 mV within 2 days of colonization and to <−150 mV after 7 days. The Eh of the gingival crevice is usually lower than that of other sites around a healthy tooth. Bradshaw et al. (20) used a model system oral biofilm and demonstrated that anaerobes increased in proportion to aerobes with increasing biofilm age. They showed that mixed cultures can protect obligate anaerobes in the biofilms from the toxic effects of oxygen.
As the organisms develop biofilms in the subgingival crevice, they produce proteolytic enzymes that damage tissue directly or interfere with host defenses (128). Collagenase and hyaluronidase are also present and capable of degrading collagen. Breakdown of the fiber barrier system may occur, and the lesion may then progress to one that may attack the supporting structures of the tooth (176). Gram-negative organisms also produce endotoxins that may result in inflammation (176). Lamont et al. (111) demonstrated that Porphyromonas gingivalis was capable of invading epithelium cells in a laboratory assay, eliciting invasion mechanisms similar to those of other pathogens. In their assay, none of the serum concentrations used affected the invasive ability of the organism. Serum was used to simulate crevicular fluid.
The control of periodontitis is rooted in the removal of established biofilms (plaque) from the subgingival areas, in combination with supplemental antimicrobial agents. Quirynen et al. (159) found that chlorhexidine rinses after mechanical cleaning significantly improved gum health, as measured by a reduction in probing depth of the gingival crevice. Kinniment et al. (101) found that pathogens such as P. gingivalis and F. nucleatum were inhibited within laboratory oral biofilms by treatment with chlorhexidine, in support of the findings by Quirynen. Reynolds et al. (163) found that subgingival irrigation with chlorhexidine during ultrasonic scaling provided a significant improvement in probing depth compared to that of the untreated control group. Jeong et al. (92) found that root planing plus a mixture of tetracycline and citric acid-containing gel was most effective in decreasing pocket depth. In this case, the root planing consisted of mechanically removing plaque and calculus from the exposed root surfaces. Citric acid acted as a chelating agent to remove mineral deposits on the root surfaces.
Clearly, there is an association between the occurrence of biofilms and infection in certain human diseases. The organisms responsible, the extracellular components of the biofilm, the nature of the required conditioning film, and the mode of pathogenicity vary from one disease condition to the next. In every case discussed, however, there are certain underlying processes that are unchanging: production of an extracellular matrix polymer, resistance to antimicrobial agents that increases with biofilm age, and resistance to immune system clearance.
BIOFILMS ON MEDICAL DEVICES
Because the criteria for the biofilm mode of growth are quite broad, as has been discussed, the environments suitable for microorganisms to colonize and establish biofilms are practically limitless. Costerton et al. (39) provided a partial listing of medical devices that have been shown to become colonized by biofilms. Biofilms of various medical devices have been studied extensively over the last 20 years, though much of the published research used very basic tools, such as viable culture techniques and scanning electron microscopy, to characterize the microbial diversity and visualize the biofilms. For certain devices, such as urinary catheters and contact lenses, research has also elucidated the susceptibility of various materials to bacterial adhesion and biofilm formation.
A description follows of the biofilms on specific devices: prosthetic heart valves, central venous catheters, urinary (Foley) catheters, contact lenses, intrauterine devices, and dental unit water lines.
Prosthetic Heart Valves
Two major groups of prosthetic heart valves are currently used, mechanical valves and bioprostheses (tissue valves) (21). The rates of prosthetic valve endocarditis (PVE), or microbial infection of the valve and surrounding tissues of the heart, are similar for both types of valves (21). Estimates of the rate of PVE range from 0.5% (77) to 1 and 4% (55). The surgical implantation of the prosthetic valve results in tissue damage, leading to the accumulation of platelets and fibrin at the suture site and on the device. As is the case with NVE, there is a greater susceptibility for initial microbial colonization in these locations (55).
Illingworth et al. (87) noted that PVE is predominantly caused by colonization of the sewing cuff fabric of the prosthetic valve by microorganisms. Karchmer and Gibbons (98) added that the microorganisms will commonly invade the valve annulus into which the prosthetic valve has been sewn, potentially leading to a separation between the valve and the tissue and resulting in leakage.
Though the etiologic agents of PVE are generally identified by blood culture, transesophageal echocardiography is also used to detect biofilms (55). Organisms responsible for PVE differ depending on whether the infection can be classified as early or late. CNS are the predominant early colonizers (77, 98), probably resulting from initial contamination of the surgical site during the procedure. For late PVE, which by definition is from 12 months onward following the valve replacement, the organisms responsible may be streptococci, CoNS, enterococci, S. aureus, gram-negative coccobacilli, or fungi (98). Hancock (77) also noted that viridans group streptococci were the most common organism isolated during late PVE.
There still remain important questions to be answered regarding PVE, such as rate of colonization in vivo, rate of detachment, and physiology of biofilm organisms in the nutritionally rich environment of the heart. Techniques that could enable investigators to visualize and quantify biofilms on valves either in vivo or following removal and model systems that can be used to grow biofilms on mechanical valves are needed.
Central Venous Catheters
Maki (123) noted that central venous catheters (CVCs) pose a greater risk of device-related infection than does any other indwelling medical device, with infection rates of 3 to 5%. Catheters may be inserted for administration of fluids, blood products, medications, nutritional solutions, and hemodynamic monitoring (68). Biofilms have been shown by scanning electron microscopy and transmission electron microscopy to be universally present on CVCs and may be associated with either the outside of the catheter or the inner lumen (160). Organisms that colonize the CVC originate either from the skin insertion site, migrating along the external surface of the device, or from the hub, due to manipulation by health care workers, migrating along the inner lumen (62, 162). Because the device is in direct contact with the bloodstream, the surface becomes coated with platelets, plasma, and tissue proteins such as albumin, fibrinogen, fibronectin, and laminin (162). These materials act as conditioning films; S. aureus adheres to proteins such as fibronectin, fibrinogen, and laminin, and S. epidermidis adheres only to fibronectin (162). The organisms may also produce adhesins.
Rupp et al. (169) investigated the role of S. epidermidis-produced adhesins in an animal model. The adhesins examined were polysaccharide intercellular adhesin and hemagglutinin. They found that wild-type organisms adhered in greater numbers to CVCs and produced higher rates of infection than did polysaccharide intercellular adhesin and hemagglutinin knockout strains. Murga et al. (144) showed that gram-negative organisms also adhered in vitro more extensively to materials that had been conditioned with freshly drawn human blood.
Colonization and biofilm formation may occur within 3 days of catheterization (3). Raad et al. (160) also showed that catheters in place for less than 10 days tended to have more extensive biofilm formation on the external surface of the catheter; for longer-term catheters (up to 30 days), biofilms were more extensive on the internal lumen. Organisms colonizing CVCs include CoNS, S. aureus, P. aeruginosa, Klebsiella pneumoniae, Enterococcus faecalis, and Candida albicans (62, 162).
Biofilms on CVCs have routinely been detected by a semiquantitative procedure termed the roll-plate technique, in which the distal tip of the catheter is removed aseptically and rolled over the surface of a nonselective medium. Quantification of the biofilm on the catheter tip is dependent on the number of organisms that are recovered by contact with the agar surface. A number of investigators have used this procedure to quantify biofilms and determine the relationship between biofilm formation and bloodstream infection (3, 9, 36, 124). However, this technique will not detect organisms on the inner lumen of the catheter and is unable to detect more than 1,000 CFU per tip.
Raad et al. (161) observed that the roll-plate technique has a low diagnostic sensitivity and low predictive value for catheter-related bacteremia. They attempted to enhance biofilm quantification by using sonication plus vortexing of catheter tips and found that a level of 104 CFU per tip was predictive of a catheter-related septicemia. Anaissie et al. (3) studied catheters collected from patients and quantified the biofilms using either the roll-plate, sonication, or scanning electron microscope method. The biofilms were quantified by scanning electron microscopy by measuring the total area of the outer and inner luminal surfaces covered by biofilms. They defined colonization as either ≥15 CFU/tip in catheters by the roll-plate technique or ≥100 CFU/tip in catheters by the sonication technique.
Zufferey et al. (224) directly stained catheter tips with acridine orange after the tips had been processed by the roll-plate technique. The cells in the biofilm were not quantified by this procedure; a positive or negative result was reported. They found good agreement between the two techniques, and the acridine orange staining technique provided more rapid results.
Regardless of the technique used to quantify biofilms, any attempt to relate the occurrence of biofilms with infection should take into consideration the method of blood sampling. Duplicate blood samples should ideally be drawn peripherally (from a vein rather than through the CVC) to ascertain that the organisms in the blood sample have not originated from the device biofilms during sampling (162).
Urinary Catheters
Urinary catheters are tubular, latex, or silicone devices that are inserted through the urethra into the bladder to measure urine output, collect urine during surgery, prevent urinary retention, or control urinary incontinence (99). One study (222) found that the percentage of patients undergoing indwelling urinary catheterization was 13.2% for hospital patients, 4.9% for nursing homes, and 3.9% for patients receiving home care. The Foley catheter has an inflatable balloon near the tip that holds the catheter in place in the bladder, and the catheter, once installed, is connected to a drainage tube and collection bag (189).
Catheter systems may be open or closed systems. In open systems, the catheter drains into an open collection container; in closed systems, the catheter empties into a securely fastened plastic collecting bag (99). In open systems, catheters quickly become contaminated, and patients commonly develop urinary tract infections within 4 days (99). Patients with closed systems are much less susceptible to urinary tract infections, and the urine from the patient can remain sterile for 10 to 14 days in approximately half the patients (99). Regardless of whether the system is open or closed, Stickler noted that 10 to 50% of patients undergoing short-term catheterization (up to 7 days) develop infections, whereas essentially all patients undergoing long-term catheterization (greater than 28 days) will develop urinary tract infections (189). McLean et al. further noted that the risk of catheter-associated infection increases by approximately 10% for each day the catheter is in place (134).
The organisms that attach to the catheter and develop the biofilm originate from one of several sources: (i) organisms are introduced into the urethra or bladder as the catheter is inserted, (ii) organisms gain entry through the sheath of exudate that surrounds the catheter, or (iii) organisms travel intraluminally from the inside of the tubing or collection bag (99). Rogers et al. (167) used a model bladder system to determine the impact of leg bag design on the ascending and descending contamination rate of the urinary drainage system and showed that all leg bag designs supported biofilms and were the primary reservoir for contamination of catheters. McLean et al. noted that the ascent up the catheter to the bladder occurred within 1 to 3 days (134).
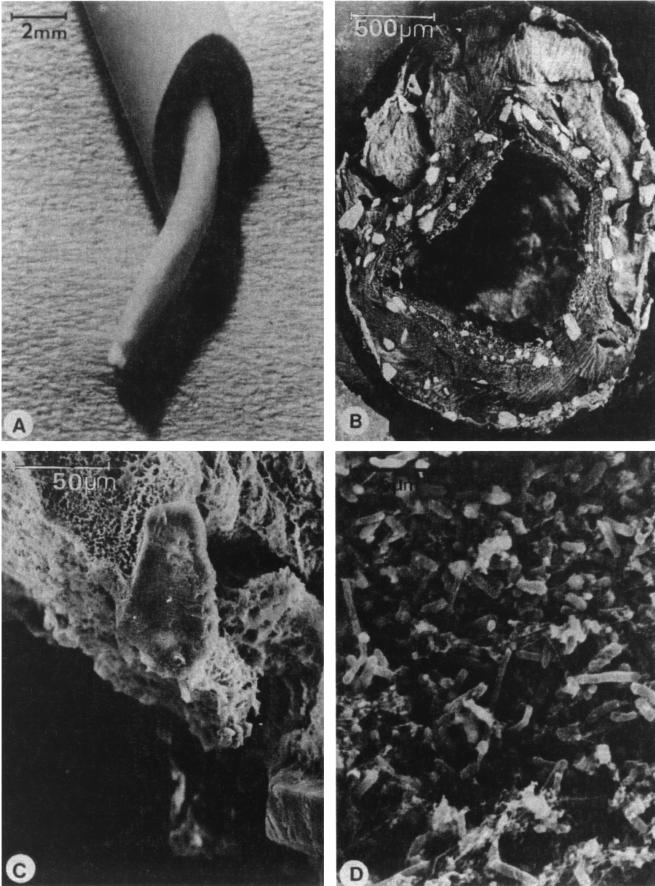
Evidence for biofilm formation on catheters comes from both in vivo and in vitro studies. The scanning electron microscope and transmission electron microscope have been used to document biofilms on urinary catheters removed from patients (152, 189, 194). Figure 6 shows a well-developed urinary catheter biofilm. Stickler (189) noted one study that measured 109 P. aeruginosa cells per cm2 of luminal catheter surface.
FIG. 6.
(A) Cut section of a urinary catheter collected from a patient, revealing a worm-like structure occluding the lumen; (B) low-power scanning electron micrograph of a freeze-fractured cross-section of a blocked catheter; (C) crystalline formations on the outer surface of a freeze-dried preparation of material blocking the catheter; (D) fixed and critical-point-dried specimen showing that, below their crystalline coats, the catheter casts are composed of a mass of cocci and bacilli. Reprinted from reference 190 with permission of the publisher (W. B. Saunders).
Ganderton et al. (71) measured biofilm thickness on silicone and silicone-coated Foley catheters collected from patients undergoing long-term catheterization and found that thicknesses ranged up to approximately 200 μm, with an occasional catheter containing biofilms between 200 and 500 μm. That study also measured biofilm plate counts as high as 108 per cm2 in long-term catheters. However, they found that biofilm thickness and plate counts were quite variable and that there was no clear relationship between duration of catheter use and extent of biofilm formation. For example, the thickest biofilm observed (490 μm) was from a catheter in place for 42 days; biofilm organisms isolated were E. coli (6.5 × 107 per cm2) and K. pneumoniae (4.6 × 106 per cm2). One of the thinnest biofilms observed was from a 41-day catheter colonized by Morganella morganii (2.4 × 107 per cm2) and diphtheroids (2.8 × 105 per cm2). The average maximal biofilm thickness measured was only 10 μm and was quite patchy.
Ladd et al. (109) proposed a rapid method for the detection of biofilms on Foley catheters based on malachite green staining of acridine orange-prestained specimens and validated the method using P. aeruginosa-colonized catheters. They found that the malachite green stain minimized the autofluorescence of the latex catheter surfaces and allowed more reliable counting. These investigators also found that there was no significant difference between catheter biofilms counted directly on the catheter surface and biofilms quantified by sonication and viable plating. Evidence has also been provided that, at least in the case of P. aeruginosa, urinary catheter biofilms produce quorum-sensing molecules in situ and in vitro, providing further evidence for developed biofilm communities in these systems (193).
Initially, catheters are colonized by single species, such as S. epidermidis, Enterococcus faecalis, E. coli, or Proteus mirabilis. As the catheter remains in place, the number and diversity of organisms increase. Mixed communities develop, containing such organisms as Providencia stuartii, P. aeruginosa, Proteus mirabilis, and Klebsiella pneumoniae (189). Other organisms isolated from urinary catheter biofilms include M. morganii, Acinetobacter calcoaceticus (194), and Enterobacter aerogenes (192). Nickel et al. (152) also noted that it appeared that only a small percentage of the different morphological types observed by scanning electron microscopy and transmission electron microscopy could be grown by culturing. It is possible that at least a percentage of the organisms in these biofilms may not be culturable or cannot compete with the more rapidly growing organisms commonly isolated on complex media.
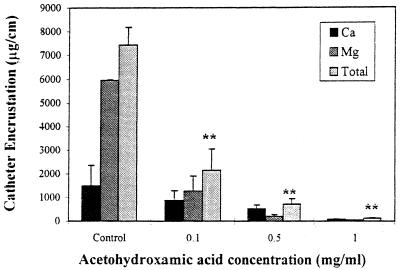
Urinary catheter biofilms are unique in that certain of the component organisms may alter the local pH through the production of urease, which hydrolyzes the urea of the urine to form free ammonia. The ammonia, in turn, will raise the local pH and allow precipitation of minerals such as calcium phosphate (hydroxyapatite) and magnesium ammonium phosphate (struvite). These minerals will then deposit in the catheter biofilms (205), forming what is termed a mineral encrustation. Stickler et al. (194) presented a case study of a person whose urinary catheter was completely blocked within 4 to 5 days; X-ray microanalysis of the biofilms in this catheter showed that it contained elevated levels of calcium, magnesium, and phosphorus. The primary urease-producing organisms in urinary catheters are P. mirabilis, M. morganii, P. aeruginosa, K. pneumoniae, and Proteus vulgaris (194, 205). Studies have shown further that mineral encrustations are observed only in catheters containing these organisms (192, 187).
Contact Lenses
Contact lenses have been classified according to material of construction, design, wear schedule, and frequency of disposal. Soft contact lenses are made of either hydrogel or silicone and are designed to allow oxygen to diffuse through the lens material to provide oxygen to the cornea. Hard contact lenses are constructed of polymethylmethacrylate and move with each blink, allowing oxygen-containing tears to flow underneath the lens (46). Bacteria adhere readily to both types of lenses (46, 137, 182, 183).
Miller and Ahearn (137) examined initial attachment of P. aeruginosa to hydrophilic contact lenses (hydrogels) and found that the rate of adherence varied depending on water content and polymer composition. Though these were initial adhesion studies, only 2 h in duration, they observed extracellular matrix polymers by transmission electron microscopy and ruthenium red staining. The degree of attachment was found to depend on a number of factors, including the nature of the substrate, pH, electrolyte concentration, ionic charge of the polymer, and bacterial strain tested. Their results showed that there was greater adherence to hydrophobic surfaces and to lenses composed of nonionic polymers.
Stapleton et al. (183) also observed greater adhesion of P. aeruginosa to low-water-content nonionic lenses than to ionic lenses. They found that maximal adhesion occurred after 45 min and did not increase for contact periods as long as 24 h. Miller et al. (136) also showed that P. aeruginosa adhesion was enhanced by mucin, lactoferrin, lysozyme, immunoglobulin A, bovine serum albumin, and mixtures of these molecules, though exposure to human tears resulted in both an increase and decrease in adherence depending on the lens formulation tested. These investigators noted that the data would not allow an accurate prediction of how these molecules would perform under in situ conditions.
Organisms that have been shown to adhere to contact lenses include P. aeruginosa, S. aureus, S. epidermidis, Serratia spp., E. coli, Proteus spp., and Candida spp. (46). An established biofilm (extensive exopolymer matrix) was demonstrated by scanning electron microscopy of a lens removed from a patient with keratitis caused by P. aeruginosa (182). McLaughlin-Borlace et al. (133) also provided evidence of biofilms on the surfaces of 20 contact lens samples collected from patients with a clinical diagnosis of microbial keratitis. In several cases the biofilms contained multiple species of bacteria or bacteria and fungi.
Biofilms have also been shown to develop on contact lens storage cases (46, 133, 218). In fact, the lens case has been implicated as the primary source of organisms for contaminated lens disinfectant solutions and lenses (133). One study found that 80% of asymptomatic lens users had contaminated storage cases (133). These investigators found that bacterial biofilms were present on 17 of 20 storage cases examined, a significantly greater percentage than the percentage of lenses containing biofilms. They also isolated the identical organism from the lens case and the corneas of infected patients for 9 of 12 samples examined. Additionally, studies have found that the protozoan Acanthamoeba may be a component of these biofilms (46, 133) These organisms feed on the biofilm bacteria and may also be a cause of microbial keratitis.
Intrauterine Devices
Two types of intrauterine devices (IUDs) are commonly used: IUDs made of a nonabsorbable material, such as polyethylene, impregnated with barium sulfate, and IUDs that release a chemically active substance, such as copper or a progestational agent. IUDs generally have a tail that facilitates locating the device for removal. These tails are composed of a plastic monofilament surrounded by a nylon sheath. One particular model, the Dalkon Shield, has a tail composed of a bundle of 200 to 400 such monofilaments surrounded by a sheath (31).
IUD use has been shown to result in pelvic inflammatory disease (31, 117, 219). IUDs removed from asymptomatic women have been shown to be heavily contaminated with S. epidermidis, enterococci, and anaerobic lactobacilli (219). Marrie and Costerton (127) also isolated Lactobacillus plantarum, S. epidermidis, Corynebacterium spp., group B streptococci, Micrococcus spp., Candida albicans, S. aureus, and Enterococcus spp. In addition, IUDs removed from women with pelvic inflammatory disease may also contain beta-hemolytic streptococci, S. aureus, E. coli, and some anaerobic bacteria (219).
Evidence for biofilms on IUDs has been demonstrated by scanning electron microscopy and transmission electron microscopy (12, 89, 127) and by culture on complex media (127, 200, 219). Using the scanning electron microscope, Marrie and Costerton also demonstrated the presence of human leukocytes and cellular debris in the biofilms (127).
The tail portion of the IUD may be a primary source of contamination. One study found that approximately half of the IUD samples that had no tail protruding into the cervix were sterile. Another study determined that contamination was heaviest on the distal portions of the tail, which is directly exposed to the vaginal flora (12). Tatum et al. (200) proposed that the tail of the Dalkon Shield IUD could act as a wick to allow bacteria to travel by capillary action and enter the endometrial cavity. They showed, using dye uptake and bacterial cultures in vitro, that this could happen. Also, after disinfecting the outer surface of Dalkon Shield IUDs collected from patients, they found that 86% were positive for culturable bacteria.
An interesting finding of a study by Bank and Williamson (12) was that no PMNs were observed within multifilament tails collected from asymptomatic women. PMNs will rapidly attach to nylon fibers and quickly become immobilized, significantly limiting their ability to travel up the interfibrillar spaces of the Dalkon Shield tail. The implication is that the bacteria, once inside the tail, would be relatively protected from attack by phagocytes, which normally maintain sterility in the uterus. This may explain why organisms readily populate IUD tails, even in asymptomatic patients. However, Jaques et al. (89) presented evidence using a rabbit model that the tail does not influence the rate of colonization of the IUD. Devices inserted through the vagina were colonized rapidly (within 2 weeks), while those inserted surgically remained uncolonized even after 8 weeks.
Development of a reproducible nonanimal model system for growing and evaluating IUD biofilms might allow a clearer understanding of the rate of biofilm formation and the importance of different materials, contaminating organisms, and treatments which could control the process.
Dental Unit Water Lines
Unlike other medical devices discussed previously, dental water systems are not indwelling devices, and the public health significance of biofilms in these systems is unclear. However, because dental procedures may expose patients and dental professionals to opportunistic and pathogenic organisms originating from the various components of the dental unit, there may be potential for human impact. Hence, we include a discussion of biofilms in these systems.
Dental units are equipped with small-bore flexible plastic tubing that supplies water to different hand pieces, such as the air-water syringe, the ultrasonic scaler, and the high-speed hand piece. Units may be supplied with municipal water or from separate reservoirs containing either distilled or sterile water (13). Elevated bacterial counts have been found in water collected from these systems (13, 70, 132, 217). For example, Furuhashi and Miyamae (70) found that bacterial counts increased from usually less than 40 per ml in the incoming municipal water supply to between 103 and 105 per ml in water collected from the three-way syringe. They noted that the air-turbine hand piece and cup water filler also had elevated counts. Mayo et al. (132) found that water from stagnant lines (unused for 48 h) contained counts as high as 107 per ml. Organisms generally isolated from these units include Pseudomonas spp., Flavobacterium spp., Acinetobacter spp., Moraxella spp., Achromobacter spp. (199), Methylobacterium spp. (213), Rhodotorula spp., hyphomycetes (Cladosporium spp., Aspergillus spp., and Penicillium spp.), Bacillus spp., Streptococcus spp., CoNS, Micrococcus spp., and Corynebacterium spp. (138). Legionella pneumophila has also been isolated from these systems (8, 23, 155).
Evidence for biofilms in these systems has come from a number of studies, using both scanning electron microscopy (143, 173, 213) and viable plating of organisms isolated from the dental unit components (199). Whitehouse et al. (213) observed a variety of bacteria embedded in an apparent polysaccharide matrix. They also cultured the same organisms from both tubing biofilm samples and water samples, and numbers were similar in both types of samples. Santiago et al. (173) also observed amebic trophozoites and cysts and, in one biofilm sample, nematodes by transmission electron microscopy. Using plate count techniques following mechanical removal of biofilms from tubing sections, Tall et al. (199) isolated greater than 104 CFU per cm2 of tubing. They also found a positive correlation between biofilm and water counts. They showed that by 180 days of exposure, the entire surface of the dental unit water line was covered by a thick, multiple layer of extracellular polymeric substances.
Dental suction systems such as saliva ejectors have also been shown to harbor biofilms containing both mixed skin flora and aquatic bacteria. In one study (14), 25% of 35 dental unit suction systems were culture positive; deposits on the inner lumen of evacuation lines were composed of bacterial microcolonies and an extensive extracellular matrix containing collagen and fibrin.
Fortunately, water-borne outbreaks as a result of contamination have been very few. One exception was a case cited in which two cancer patients undergoing dental work contracted a P. aeruginosa infection 3 to 5 days following the dental procedures. In both cases, infection (swelling) occurred in the area where the matrix band had been used. Pyocin typing demonstrated that the organisms cultured from the patient and water lines were identical. However, the authors noted the possibility that the organisms of concern may have originated from the patient and not the water system (177).
Important issues remain, such as survival and transmission of pathogens (other than Legionella, e.g., nontuberculous Mycobacterium spp., E. coli O157:H7, and Cryptosporidium sp.) in dental unit systems and the effect of other disinfectants, such as monochloramine and ozone, in both preventing and controlling biofilms.
RELATIONSHIP BETWEEN BIOFILM FORMATION AND DISEASE
It is clear from epidemiologic evidence that biofilms have a role in infectious diseases, both for specific conditions such as cystic fibrosis and periodontitis and in bloodstream and urinary tract infections as a result of indwelling medical devices. The process may be particularly relevant for immunocompromised patients, who lack the ability to combat invading organisms. Beyond the evidence, however, the exact processes by which biofilm-associated organisms elicit disease in the human host are only poorly understood at best. Suggested mechanisms include the following: (i) detachment of cells or cell aggregates from indwelling medical device biofilms, resulting in bloodstream or urinary tract infections, (ii) production of endotoxins, (iii) resistance to the host immune system, and (iv) provision of a niche for the generation of resistant organisms (through resistance plasmid exchange). A basis for each of these proposed mechanisms follows.
Detachment of Cells or Cell Aggregates
Cells may detach individually from biofilms as a result of cell growth and division within the biofilms, or cell aggregates or clusters may detach or be sloughed from the biofilm. Though detachment has not been well characterized for medical device biofilms, some aspects of the process can be considered universal for all biofilms. Laboratory studies have shown that an increase in shear stress, as would occur during changes in direction or rate of flow, will result in an increase in the rate of cell erosion from the biofilm (29). It has also been shown that detachment of cells or aggregates may be related to changes in substrate concentration (27). Davies et al. (48) also showed that acyl-homoserine lactone molecules could mediate both biofilm architecture and detachment. Regardless of the reason, detached cells could conceivably cause an infection. Bloodstream and urinary tract infections could conceivably result from very small numbers of bacteria.
Production of Endotoxins
In addition to the direct effects of cell detachment or antimicrobial resistance, gram-negative bacteria within biofilms of indwelling medical devices will produce endotoxins, which may in turn elicit an immune response in the patient. Several studies have measured endotoxin levels of biofilms (82, 164, 207). Vincent et al. (207) showed that bacterial counts within biofilms on hemodialyzer tubing correlated with endotoxin levels. However, none of these studies documented the levels or kinetics of endotoxin release from the biofilms.
Resistance to the Host Immune System
Shiau and Wu (179) found that extracellular slime produced by S. epidermidis interfered with macrophage phagocytic activity. Meluleni et al. (135) showed that the opsonic antibodies made by patients with chronic cystic fibrosis were ineffective in mediating phagocytosis and elimination of bacterial cells growing in biofilm microcolonies. Ward et al. (211) used a rabbit model to show that bacterial growth within a biofilm on an implanted peritoneal device was unaffected by the vaccinated animal's immune system (in terms of phagocytosis). The vaccinated animals had a 1,000-fold-higher titer of the antibody, but it appears that the antibodies did not reach the surface of bacterial cells within the biofilms. Yasuda et al. (220) found that E. coli cells grown in a biofilm and then resuspended were as sensitive to phagocytosis as normal (nonbiofilm) bacteria but were less sensitive to the killing activity of the human polymorphonuclear leukocytes in vitro. They hypothesized that the increase in resistance was the result of an increase in resistance of the biofilm bacteria to killing by active oxygen species in the polymorphonuclear leukocytes. These results lead to the conclusion that organisms detaching from a biofilm on a medical device or other infection could overcome the immune system more readily to cause an infection.
Provision of a Niche for the Generation of Resistant Organisms
It has been shown that bacteria can exchange plasmids by conjugation within biofilms, and resistance factors may be carried on a plasmid. Hausner and Wuertz (80) showed plasmid transfer between E. coli and Alcaligenes eutrophus in laboratory-grown biofilms. Roberts et al. (165) also demonstrated conjugation between different genera in an oral biofilm. The physical proximity of cells within microcolonies in biofilms would be expected to favor conjugation over the same process among suspended (planktonic) organisms. This was in fact demonstrated by Ehlers and Bouwer (61), who showed that conjugation rates between different species of Pseudomonas were significantly higher in biofilms (transconjugant/recipient ratio, approximately 10−2) than for the same organisms under planktonic conditions (transconjugant/recipient ratio, approximately 10−7). This could be especially relevant in the case of indwelling medical device biofilms, where resistant organisms could be spread from patient to patient on the hands of health care workers.
INTERVENTION STRATEGIES
When the inherent resistance of biofilms to industrial biocides was first discovered, this property was attributed to a limitation in mass transfer conferred by the matrix material (41). However, it was soon revealed that the matrix of a biofilm limits diffusion only when the diffusing molecule actually reacts with the matrix material (186). The biofilm phenotype is remarkably resistant to antibacterial agents, including antibiotics (149), and biofilm cells are also remarkably resistant to the bactericidal effects of metal ions, including copper and silver. Wild strains of many different species of bacteria colonize the surfaces of these metals very avidly, and some of the thickest and most luxuriant clinically relevant biofilms have formed naturally on the copper wires of IUDs (127).
Extensive attempts to control biofilm formation in industrial systems by manipulation of the metallurgy and the surface characteristics of pipes and vessels have all failed. We can expect an equal lack of success if we take this approach with medical devices. Industry currently relies on mechanical cleaning and oxidative biocides; the former removes biofilms, and the latter gradually dissolves the biofilm matrix material and eventually kills the sessile cells. As Winston Churchill is supposed to have said, “Those who do not understand history are doomed to repeat it!”
That being said, intervention strategies currently used for biofilm control will either (i) prevent initial device contamination, (ii) minimize initial microbial cell attachment to the device, (iii) penetrate the biofilm matrix and kill the biofilm-associated cells, or (iv) remove the device. The following specific treatments have been proposed for several of the medical devices already discussed.
Prosthetic Heart Valves
Generally, antibiotics are prescribed for prolonged periods (up to 8 weeks, depending on the antimicrobial agent prescribed and organism to be treated) (55), though it has been noted that relatively few patients can be cured by antimicrobial therapy alone (77). Illingworth et al. (87) described a silver-coated sewing cuff from a St. Jude mechanical heart valve which was designed to prevent microorganism attachment and colonization. The coating (termed silzone) was a dense layer of metallic silver deposited on the individual fibrils designed to inhibit attachment. These authors implanted this fabric material into a guinea pig artificially infected with Staphylococcus epidermidis. By measuring inflammation, they showed that the silzone-coated fabric produced less inflammation than uncoated fabric.
To document the efficacy of this approach, Carrel et al. (24) described both in vitro studies with a number of organisms and a case study of a patient who received a silver-coated St. Jude valve. However, Kjaergard et al. (103) found that prosthetic valve endocarditis could not be prevented by implantation of the St. Jude silver-coated valves and ultimately replaced the patient's infected valve with an aortic homograph. They noted that the St. Jude silver-coated valve may be effective in vitro but that it does not protect the tissues surrounding the prosthesis; this position was supported by Cook et al. (34).
Central Venous Catheters
Several strategies for controlling biofilms on CVCs have been suggested, including using topical antimicrobial ointments, minimizing the length of catheterization, using in-line filtration of intravenous fluids, using a surgically implanted cuff to the catheter, coating the inner lumen with antimicrobial agents, and (as a last resort) removing the device (123). Maki and Band (122) found that topical antimicrobial agents provided only modest protection against catheter-related infections, and this protective effect was primarily for peripheral venous catheters in place for more than 4 days.
Freeman and Gould (69) found that 0.05% sodium metabisulfite added to the introphic agents delivered to the left atrial system with a catheter acted as an intravenous antiseptic and eliminated left atrial colonization and endocarditis. The same basic approach was used by Wiernikowski et al. (214), except that sterile saline was used as the locking agent; the time to infection was increased twofold by use of this treatment. A subcutaneous collagen cuff impregnated with silver has been tested and found in some studies to prevent bloodstream infection (68, 125, 162), though Raad (162) noted that this treatment was ineffective for catheters in place for more than 10 days. The silver acts as a biocidal agent to prevent the attachment and growth of bacteria.
Another approach for controlling biofilms on CVCs has been to impregnate the catheter with either silver salts or antibiotics. Table 5 compares a number of these catheters and their efficacy in preventing biofilm formation and bloodstream infection. Veenstra et al. (206) reviewed the results of 13 different clinical studies (2,830 catheters) in which antibiotic-impregnated catheters were compared with untreated catheters. They concluded that central venous catheters impregnated with chlorhexidine combined with silver sulfadiazine were effective in reducing the incidence of catheter colonization and catheter-related bloodstream infections in patients at high risk for catheter-related infections. Darouiche (44) reviewed the various CVC treatments incorporating silver and found that silver-chelated collagen cuffs were threefold less likely to be colonized and fourfold less likely to cause bloodstream infection than uncuffed catheters, that CVCs coated with silver alone were clinically ineffective, that CVCs coated with chlorhexidine and silver sulfadiazine provide short-lived protection, since the internal lumen of the catheter is not treated, and that silver ionophoretic CVCs have been shown to be protective against Staphylococcus aureus in a rabbit model system, though clinical studies have yet to be done.
TABLE 5.
Antimicrobial coating treatments that have been evaluated for the control of biofilms on central venous catheters (CVCs)
| Reference | Treatmenta | Evaluation criteriab | Resultsc |
|---|---|---|---|
| 45 | MR compared to CS | Patient study, 356 MR-CVC compared to 382 CS-CVC, avg D = 8.4, roll tip or sonication | MR, 7.9% colonized, 0.3% BSI; CS, 22.8% colonized, 3.4% BSI; MR significantly more effective |
| 124 | CS compared to untreated control | Patient study, 208 CS-CVC compared to 195 untreated control-CVC, avg D = 6, roll tip | CS, 13.5% colonized, 1.0% BSI; control, 24.1% colonized, 4.7% BSI; CS significantly more effective |
| 97 | TC compared to untreated control | Patient study, 97 TC-CVC compared to 81 untreated control-CVC, avg D = 7, roll tip | TC, 2.06% colonized, no BSI; control, 13.6% colonized, no BSI; TC significantly more effective |
| 10 | Silver ions compared to untreated control | Patient study, 34 SI-CVC compared to 33 untreated control-CVC, avg D = 4.49 (SI) and 4.06 (control), sonication | SI, 52.9% colonized; control, 57.6% colonized; no significant difference |
| 19 | Silver(s) compared to untreated control | Patient study, 86 S-CVC compared to 79 untreated control-CVC, median D = 9 (S) and 8 (control), roll tip | S, 14% colonized, 4.65% CAI; control, 22.8% colonized, 16.5% CAI; S significantly more effective |
| 7 | Heparin compared to untreated control | Patient study, 13 H-CVC compared to 19 untreated control-CVC, D not given, semiquantitative and quantitative method | H, 31% colonized, no BSI; control, 74% colonized, 26.3% BSI; H significantly more effective |
| —d | Ciprofloxacin compared to untreated control | Laboratory study, P. aeruginosa in a flow cell, D = 300 min | >50% reduction in attachment compared to control |
MR, minocycline plus rifampin; CS, chlorhexidine plus silver sulfadiazine; TC, tridecylmethylammonium chloride plus cephalosporin.
D, duration of catheter insertion (in days); SI, silver ion; S, silver; H, heparin. Roll tip method or sonication method used for quantification of catheter colonization.
BSI, bloodstream infection; CAI, catheter-associated infection.
Reference: C. Kwok, S. Hendricks, C. Wan, J. D. Bryers, B. D. Ratner, and T. Horbett, Proc. 23rd Int. Symp. Controlled Release Bioactive Mat., p. 230-231, 1996.
Urinary Catheters
Control strategies that have been used to inhibit biofilm formation on urinary catheters include antimicrobial ointments and lubricants, bladder instillation or irrigation, antimicrobial agents in the collection bags, impregnating the catheter with antimicrobial agents (silver oxide), and using systemic antibiotics for prophylaxis in catheterized patients (99). Sedor and Mulholland (175) also noted that the material of catheter construction might also be important; silicone catheters obstruct less often than latex, Teflon, or silicone-coated latex in patients prone to catheter encrustation.
Darouiche (44) reviewed the efficacy of various types of silver-coated indwelling medical devices. Two categories of treated bladder (urinary) catheters were discussed, those coated with silver oxide and those coated with silver hydrogel. With the silver oxide-coated catheters, there have been mixed results in human clinical trials. With the silver hydrogel-coated catheters, one prospective study indicated that the incidence of bacteriuria was reduced by 30% for patients who received the treated catheters, and this effect apparently was due to protection from gram-positive bacteria or yeasts. Saint and Lipsky (171) reviewed eight different randomized, controlled trials and found that silver alloy catheters were significantly better than untreated catheters, while silver oxide catheters were not. They opined that silver alloy catheters could be considered for patients at highest risk for developing serious consequences from a urinary tract infection, though other investigators have questioned their conclusions (174).
Table 6 provides a list of treatments which have been evaluated against biofilms on urinary catheter biofilms. Figure 7 also shows the effect of the urease inhibitor acetohydroxamic acid on Proteus mirabilis encrustations on silicone catheters.
TABLE 6.
Examples of selected urinary catheter biofilm control treatments
| Reference | Treatment | Results |
|---|---|---|
| 190 | Systemic ciprofloxacin therapy in catheterized patient | Inhibited catheter plugging for 10 wk |
| 191 | Latex catheter coated with either silicone, hydrogel, or silver-hydrogel in in vitro assay | Rate of swarming by Proteus mirabilis over catheter surface slowest (most effective) on silicone, highest (least effective) on hydrogel |
| 142 | Catheters coated with either hydrogel, silver, silicone, or Teflon in model bladder system containing Proteus mirabilis | Silicone provided longest time to blockage (most effective); hydrogel-silver had the shortest time to blockage (least effective); none completely resisted colonization |
| 141 | Urease inhibitors (acetohydroxamic acid [1 mg/ml] and fluorofamide [1 mg/ml]) in model bladder system containing Proteus mirabilis | Both treatments lowered calcium and magnesium concentrations on catheter surface and reduced urine pH from 9.1 to 7.6 |
| 188 | Chlorhexidine (200 μg/ml); mandelic acid (1%); lactic acid (1%); mandelic acid + lactic acid (0.5% each); and providone iodine (1%) in model bladder system containing Citrobacter diversus, P. aeruginosa, and Enterobacter faecalis | Mandelic acid and mandelic acid + lactic acid reduced biofilms for all three organisms |
| 145 | Tobramycin (200 μg/ml) in model system containing P. aeruginosa | Treatment prolonged lag phase and slowed biofilm formation rate |
| 149 | Tobramycin (1,000 μg/ml) in model system containing silko-latex catheter discs and P. aeruginosa | Treatment resulted in 3-log reduction of biofilm; planktonic cells completely inactivated at 50 μg/ml |
| 153 | Systemic amdinocillin at increasing concns in catheterized rabbit model system | Ineffective at levels below 400 mg/kg |
| 157 | Ciprofloxacin-loaded liposomes in hydrogel coatings on silicone catheters in a catheterized rabbit model inoculated with E. coli | Treatment increased time to urinary tract infection (50%) compared to untreated hydrogel-coated catheter |
| 95 | Various gram-positive and gram-negative bacteria exposed to catheter segments containing a nitrofurazone matrix coating in an in vitro assay | All strains except P. aeruginosa inhibited to some extent by the treatment |
| 94 | Various multidrug-resistant gram-positive and gram-negative bacteria exposed to catheter segments containing a nitrofurazone coating in an in vitro assay | All strains except vancomycin-resistant enterococci inhibited by the treatment |
FIG. 7.
Effect of acetohydroxamic acid, a urease inhibitor, on the encrustation of silicone catheters by Proteus mirabilis biofilms. Each value is the mean calculated from three replicated experiments. ∗∗, significant difference (P < 0.01) from the control values (analysis of variance). The mean values for the log of the number of viable cells per milliliter of urine at 24 h were 8.02 (control), 8.16 (0.01 mg of acetohydroxamic acid per ml), 8.20 (0.5 mg/ml), and 8.09 (1.0 mg/ml). Reprinted from reference 141 with permission of Springer-Verlag Co.
Contact Lenses
Several studies have compared the efficacy of contact lens storage and cleaning solutions against bacterial biofilms on lens storage cases. Wilson et al. (218) compared quaternary ammonium compounds, chlorhexidine-gluconate, and hydrogen peroxide and found that 3% hydrogen peroxide was most effective in inactivating bacterial biofilm organisms that were 24 h old (Serratia marcescens, P. aeruginosa, S. epidermidis, or Streptococcus pyogenes). Biofilms of C. albicans, on the contrary, were highly resistant to all treatments, including hydrogen peroxide. Another study found that sodium salicylate was effective in decreasing initial bacterial adherence to lenses and cases (64). However, one study found that biofilms could be detected on contact lenses removed from patients with microbial keratitis whose lens storage cases were treated according to the manufacturer's instructions with disinfectants such as hydrogen peroxide and chlorine release systems (133). Gandhi et al. (72) showed that Serratia marcescens could grow in chlorhexidine disinfectant solutions.
Further research is needed to determine the efficacy of disinfectant solutions against model system biofilms and natural biofilms on contact lenses that have been removed from patients with an active infection.
Dental Unit Water Lines
Flushing has been suggested as one treatment for reducing the planktonic bacterial load that originates from the tubing biofilms (177); however, several studies have shown that flushing alone is ineffective in significantly decreasing bacterial contamination (132, 173, 216). Mills et al. (138) suggested that povidone iodine be used to reduce microbial contamination. They demonstrated that treated tubing samples contained between 4 and 5 logs fewer bacteria per ml initially following treatment with povidine iodine, but the levels returned to pretreatment levels within 22 days. Fiehn and Henriksen (67) showed that treatment with 0.5 to 1 ppm free chlorine for 10 min each day reduced normal bacterial counts by about 2 logs from pretreatment levels. When chlorination was discontinued, the counts continued to increase. However, Murdoch-Kinch et al. (143) found that chlorination (1:10 bleach) of systems already contaminated with biofilms was ineffective in removing them.
The problem may lie in the fact that dental unit water lines are very small in diameter, present a very high surface-to-volume ratio and relatively low flow rates, and are ideal for colonization with aquatic bacteria, leading to biofilm formation. Other methods of treatment, such as use of separate, sterile water supplies and filtration, have also been suggested (143).
Novel and Unproven Strategies
Numerous biofilm control strategies have been proposed. Because of concerns with device compatibility or effects on the patient, many such treatments cannot be considered for medical devices. Nevertheless, several merit further investigation. Zips et al. (223) showed that ultrasound treatment removed up to 95% of Pseudomonas diminuta attached to ultrafiltration membranes. Huang et al. (85) demonstrated the efficacy of ultrasound against P. aeruginosa biofilms on steel and also showed that this treatment improved the efficacy of gentamicin against the same biofilms. Blenkinsopp et al. (17) found that low-strength electrical fields (plus or minus 12 V cm−1) combined with a low current density (plus or minus 2.1 mA cm−2) enhanced the efficacy of several commercial biocides at levels below the threshold for efficacy against planktonic cells, as shown in Fig. 8.
FIG. 8.
(A) Effect of a low-strength electric field with a low current density followed by biocide application (arrows) on P. aeruginosa colonization (mean, n = 2). At 24 h, glutaraldehyde (5 ppm) (open and solid squares) or kathon (1 ppm) (open and solid triangles) was applied to both electrified and control devices. (B) Effect of biocides on an established (24-h) P. aeruginosa biofilm in the presence and absence of a low-strength electric field with a low current density. Glutaraldehyde (5 ppm) (open and solid squares) or quaternary ammonium compound (10 ppm) (open and solid diamonds) was supplied to both electrified and control devices for 24 h (mean, n = 2). The electrified devices are represented by solid symbols. Reprinted from reference 17 with permission of the American Society for Microbiology.
In light of the fact that biofilms comprise both cells and extracellular polymeric substances, treatments that either eradicate or penetrate the extracellular polymeric substances might also be effective. For example, Johansen et al. (93) showed that a mixture of enzymes was effective in eradicating laboratory-grown biofilms of several different organisms. Though the extracellular polymeric substance matrix of biofilms may be highly variable, especially between different organisms, it might be possible to identify the polysaccharides for a specific organism in a biofilm and treat the biofilm with that enzyme. Hatch and Schiller (79) showed that alginate lyase allowed more effective diffusion of gentamicin and tobramycin through alginate, the biofilm polysaccharide of P. aeruginosa.
Davies et al. (48) showed that signaling molecules (acyl-homoserine lactones) were involved in biofilm architecture and detachment, and it has been suggested that novel treatments might be based on disruption of these quorum-sensing systems (48, 78, 193). In addition, since younger biofilms are more susceptible to antimicrobial agents than are older biofilms of the same organism (as already discussed), development of noninvasive techniques that detect early biofilm formation might result in greater success in their treatment and removal. A number of laboratories are currently attempting to elucidate the genes that are activated or repressed during initial biofilm formation. In the future, treatments that inhibit the transcription of these genes might be able to completely inhibit biofilms.
CONCLUSIONS
Bacterial cells have grown in the biofilm phenotype for billions of years, as a part of their successful strategy to colonize most of this planet and most of its life forms. We have only recognized this distinct phenotype as the predominant mode of bacterial growth for the last two decades. Initially, biofilms attracted our attention because we could detect them by direct methods, and our approach to understanding them was necessarily descriptive and somewhat academic. During the past 5 years, studies of this distinct phenotype have provided a rational explanation for this pattern of gene expression, and biofilms can now be defined in genetic terms.
The medical importance of these scientific and engineering studies now resides not in scholarly interest, but in our ability to explain the characteristics of device-related and other chronic infections and to design strategies to counter their refractory nature. All of the chronic infections described in this review share the fundamental characteristics of all bacterial biofilms. Their distinct phenotype makes them resistant to antibacterial agents, and their matrix makes them resistant to the antibacterial molecules and cells mobilized by the host. While many biofilm infections are “stealthy,” in that they develop slowly and initially produce few symptoms, they may be very damaging because they promote immune complex sequelae and act as reservoirs for acute exacerbations.
In surveying the extent of this current invasion of our health care facilities by “environmental” pathogens, we have heretofore depended on the direct demonstration of matrix-enclosed biofilms on the colonized device or in the infected tissue. This diagnostic feature is unequivocal and useful, but we should now begin to examine any infection that is refractory to antibiotic therapy and to host defenses in terms of the genes that are expressed to produce the refractory bacterial phenotype. Furthermore, we must begin to use the biofilm phenotype of each chronic pathogen in the development of new vaccines and antibiotics aimed at biofilm-specific targets and in studies of the causes and means of controlling this burgeoning group of diseases.
Acknowledgments
We express our appreciation to J. Michael Miller for helpful suggestions and encouragment and to the following individuals for providing articles or figures for this paper: Kieth Broome, John Dart, Harvey Holmes, Shannon Mills, Marc Mittelman, Curtis Nickel, David Stickler, and Paul Stoodley. Elizabeth White of the CDC also provided assistance with the Zeiss deconvolution system.
REFERENCES
- 1.Adams, J. L., and R. J. C. McLean. 1999. Impact of rpoS deletion on Escherichia coli biofilms. Appl. Environ. Microbiol. 65:4285-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amorena, B., E. Gracia, M. Monzon, J. Leiva, C. Oteiza, M. Perez, J.-L. Alabart, and J. Hernandez-Yago. 1999. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J. Antimicrob. Chemother. 44:43-55. [DOI] [PubMed] [Google Scholar]
- 3.Anaissie, E., G. Samonis, D. Kontoyiannis, J. Costerton, U. Sabharwal, G. Bodey, and I. Raad. 1995. Role of catheter colonization and infrequent hematogenous seeding in catheter-related infections. Eur. J. Clin. Microbiol. Infect. Dis. 14:135-137. [DOI] [PubMed] [Google Scholar]
- 4.Angell, P., A. A. Arrage, M. W. Mittelman, and D. C. White. 1993. On line, non-destructive biomass determination of bacterial biofilms by fluorometry. J. Microbiol. Methods 18:317-327. [Google Scholar]
- 5.Anwar, H., J. L. Strap, K. Chen, and J. W. Costerton. 1992. Dynamic interactions of biofilms of mucoid Pseudomonas aeruginosa with tobramycin and piperacillin. Antimicrob. Agents Chemother. 36:1208-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anwar, H., J. L. Strap, and J. W. Costerton. 1992. Susceptibility of biofilm cells of Pseudomonas aeruginosa to bactericidal actions of whole blood and serum. FEMS Microbiol. Lett. 92:235-242. [DOI] [PubMed] [Google Scholar]
- 7.Appelgren, P., U. Ransjo, L. Bindslev, and O. Larm. 1995. Does surface heparinisation reduce bacterial colonisation of central venous catheters? Lancet 345:130.. [DOI] [PubMed] [Google Scholar]
- 8.Atlas, R. M., J. F. Williams, and M. K. Huntington. 1995. Legionella contamination of dental-unit waters. Appl. Environ. Microbiol. 61:1208-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aufwerber, E., S. Ringertz, and U. Ransjo. 1991. Routine semiquantitative cultures and central venous catheter-related bacteremia. APMIS 99:627-630. [DOI] [PubMed] [Google Scholar]
- 10.Bach, A., E. Heinrich, A. Frick, H. Schmidt, B. Bottiger, and E. Martin. 1999. Efficacy of silver-coating central venous catheters in reducing bacterial colonization. Crit. Care Med. 27:515-521. [DOI] [PubMed] [Google Scholar]
- 11.Baillie, G. S., and J. Douglas. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42:1900-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bank, H. L., and H. O. Williamson. 1983. Scanning electron microscopy of Dalkon Shield tails. Fertil. Steril. 40:334-339. [DOI] [PubMed] [Google Scholar]
- 13.Barbeau, J., R. Tanguay, E. Faucher, C. Avezard, L. Trudel, L. Cote, and A. P. Prevost. 1996. Multiparametric analysis of waterline contamination in dental units. Appl. Environ. Microbiol. 62:3954-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbeau, J., L. ten Bokum, C. Gauthier, and A. P. Prevost. 1998. Cross contamination potential of saliva ejectors used in dentistry. J. Hosp. Infect. 40:303-311. [DOI] [PubMed] [Google Scholar]
- 15.Berger, M., P. E. Gallerstein, P. Benhuri, R. Balla, and E. Goldberg. 1981. Evaluation of aortic valve endocarditis by two-dimensional echocardiography. Chest 80:61-67. [PubMed] [Google Scholar]
- 16.Biedlingmaier, J., R. Samaranayake, and P. Whelan. 1998. Resistance to biofilm formation on otologic implant materials. Otolaryngol. Head Neck Surg. 118:444-451. [DOI] [PubMed] [Google Scholar]
- 17.Blenkinsopp, S. A., A. E. Khoury, and J. W. Costerton. 1992. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 58:3770-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloemberg, G. V., G. A. O'Toole, B. J. J. Lugtenberg, and R. Kolter. 1997. Green fluorescent protein as a marker for Pseudomonas spp. Appl. Environ. Microbiol. 63:4543-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boswald, M., S. Lugauer, A. Regenfus, G. G. Braun, P. Martus, C. Geis, J. Scharf, T. Bechert, J. Griel, and J.-P. Guggenbichler. 1999. Reduced rates of catheter-associated infection by use of a new silver-impregnated central venous catheter. Infection 27:S56-S60. [DOI] [PubMed] [Google Scholar]
- 20.Bradshaw, D. J., P. D. Marsh, C. Allison, and K. M. Schilling. 1996. Effect of oxygen, inoculum composition and flow rate on development of mixed-culture oral biofilms. Microbiology 142:623-629. [DOI] [PubMed] [Google Scholar]
- 21.Braunwald, E. 1997. Valvular heart disease, p. 1007-1076. In E. Braunwald (ed.), Heart disease, 5th ed., vol. 2. W. B. Saunders Co., Philadelphia, Pa. [Google Scholar]
- 22.Buda, A. J., R. J. Zotz, M. S. LeMire, and D. S. Bach. 1986. Prognostic significance of vegetations detected by two-dimensional echocardiography in infective endocarditis. Am. Heart J. 112:1291-1296. [DOI] [PubMed] [Google Scholar]
- 23.Callacombe, S. J., and L. L. Fernandes. 1995. Detecting Legionella pneumophila in water systems: a comparison of various dental units. JADA 126:603-608. [DOI] [PubMed] [Google Scholar]
- 24.Carrel, T., T. Nguyen, B. Kipfer, and U. Althaus. 1998. Definitive cure of recurrent prosthetic endocarditis using silver-coated St. Jude Medical Heart Valves: a preliminary case report. J. Heart Valve Dis. 7:531-533. [PubMed] [Google Scholar]
- 25.Cercenado, E., J. Ena, M. Rodriguez-Creixems, I. Romero, and E. Bouza. 1990. A conservative procedure for the diagnosis of catheter-related infections. Arch. Intern. Med. 150:1417-1420. [PubMed] [Google Scholar]
- 26.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Characklis, W. G. 1990. Biofilm processes, p. 195-232. In W. G. Characklis and K. C. Marshall (ed.), Biofilms. John Wiley & Sons, New York, N.Y.
- 28.Characklis, W. G., and K. C. Marshall. 1990. Biofilms: a basis for an interdisciplinary approach, p. 3-15. In W. G. Characklis and K. C. Marshall (ed.), Biofilms. John Wiley & Sons, New York, N.Y.
- 29.Characklis, W. G., G. A. McFeters, and K. C. Marshall. 1990. Physiological ecology in biofilm systems, p. 341-394. In W. G. Characklis and K. C. Marshall (ed.), Biofilms. John Wiley and Sons, New York, N.Y.
- 30.Characklis, W. G., and K. C. Marshall (ed.). 1990. Biofilms. John Wiley & Sons, Inc., New York, N.Y.
- 31.Chesney, P. J. 1994. Infections of the female genital tract, p. 347-374. In A. L. Bisno and F. A. Waldovogel (ed.), Infections associated with indwelling medical devices, 2nd ed. American Society for Microbiology, Washington, D.C.
- 32.Chuard, C., P. Vaudaux, F. A. Waldovogel, and D. P. Lew. 1993. Susceptibility of Staphylococcus aureus growing on fibronectin-coated surfaces to bactericidal antibiotics. Antimicrob. Agents Chemother. 37:625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cochrane, D. M. G., M. R. W. Brown, H. Anwar, P. H. Weller, K. Lam, and J. W. Costerton. 1988. Antibody response to Pseudomonas aeruginosa surface protein antigens in a rat model of chronic lung infection. J. Med. Microbiol. 27:255-261. [DOI] [PubMed] [Google Scholar]
- 34.Cook, G., J. W. Costerton, and R. O. Darouiche. 2000. Direct confocal microscopy studies of the bacterial colonization in vitro of a silver-coated heart valve sewing cuff. Int. J. Antimicrob. Agents 13:169-173. [DOI] [PubMed] [Google Scholar]
- 35.Corbet, E. F., and W. I. R. Davies. 1993. The role of supragingival plaque in the control of progressive periodontal disease. J. Clin. Periodontol. 20:307-313. [DOI] [PubMed] [Google Scholar]
- 36.Corona, M. L., S. G. Peters, B. J. Narr, and R. L. Thompson. 1990. Subspecialty clinics: critical care medicine. Infections related to central venous catheters. Mayo Clin. Proc. 65:979-986. [DOI] [PubMed] [Google Scholar]
- 37.Costerton, J. W., G. G. Geesey, and G. K. Cheng. 1978. How bacteria stick. Sci. Am. 238:86-95. [DOI] [PubMed] [Google Scholar]
- 38.Costerton, J. W., and H. M. Lappin-Scott. 1995. Introduction to microbial biofilms, p. 1-11. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms. Cambridge University Press, Cambridge, United Kingdom.
- 39.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 40.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 41.Costerton, J. W., K.-J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 42.Dagostino, L., A. E. Goodman, and K. C. Marshall. 1991. Physiological responses induced in bacteria adhering to surfaces. Biofouling 4:113-119. [Google Scholar]
- 43.Dall, L., W. G. Barnes, J. W. Lane, and J. Mills. 1987. Enzymatic modification of glycocalyx in the treatment of experimental endocarditis due to viridins streptococci. J. Infect. Dis. 156:736-740. [DOI] [PubMed] [Google Scholar]
- 44.Darouiche, R. O. 1999. Anti-infective efficacy of silver-coated medical prostheses. Clin. Infect. Dis. 29:1371-1377. [DOI] [PubMed] [Google Scholar]
- 45.Darouiche, R. O., I. I. Raad, S. O. Heard, J. I. Thornby, O. C. Wenker, A. Gabrielli, J. Berg, N. Khardori, H. Hanna, R. Hachem, R. Harris, and G. Mayhall. 1999. A comparison of two antimicrobial-impregnated central venous catheters. N. Engl. J. Med. 340:1-8. [DOI] [PubMed] [Google Scholar]
- 46.Dart, J. K. G. 1996. Contact lens and prosthesis infections, p. 1-30. In W. Tasman and E. A. Jaeger (ed.), Duane's foundations of clinical ophthalmology. Lippincott-Raven, Philadelphia, Pa.
- 47.Davies, D. G., and G. G. Geesey. 1995. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61:860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 49.Davis, B. D. 1980. Evolution of microbiology and of microbes, p. 7. In B. D. Davis, R. Dulbecco, H. N. Eisen, and H. S. Ginsberg (ed.), Microbiology, 3rd ed. Harper and Row, Philadelphia, Pa.
- 50.de Beer, D., P. Stoodley, and Z. Lewandowski. 1994. Liquid flow in heterogeneous biofilms. Biotechnol. Bioeng. 44:636-641. [DOI] [PubMed] [Google Scholar]
- 51.Desai, M., T. Buhler, P. H. Weller, M. R. W. Brown. 1998. Increasing resistance of planktonic and biofilm cultures of Burkholderia cepacia to ciprofloxacin and ceftazidime during exponential growth. J. Antimicrob. Chemother. 42:153-160. [DOI] [PubMed] [Google Scholar]
- 52.Domingue, G. J., and W. J. G. Hellstrom. 1998. Prostatitis. Clin. Microbiol. Rev. 11:604-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donlan, R. M., R. Murga, M. Bell, C. M. Toscano, J. H. Carr, T. J. Novicki, C. Zuckerman, L. C. Corey, and J. M. Miller. 2001. Protocol for the detection of biofilms on needleless connectors attached to central venous catheters. J. Clin. Microbiol. 39:750-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donlan, R. M., R. Murga, and L. Carson. 1999. Growing biofilms in intravenous fluids, p. 23-29. In J. Wimpenny, P. Gilbert, J. Walker, M. Brading, and R. Bayston (ed.), Biofilms: the good, the bad, and the ugly. Bioline, Cardiff, Wales.
- 55.Douglas, J. L., and C. G. Cobbs. 1992. Prosthetic valve endocarditis, p. 375-396. In D. Kaye (ed.), Infective endocarditis, 2nd ed. Raven Press Ltd., New York, N.Y.
- 56.DuGuid, I. G., E. Evans, M. R. W. Brown, and P. Gilbert. 1990. Growth-rate-dependent killing by ciprofloxacin of biofilm-derived Staphylococcus epidermidis; evidence for cell-cycle dependency. J. Antimicrob. Chemother. 30:791-802. [DOI] [PubMed] [Google Scholar]
- 57.DuGuid, I. G., E. Evans, M. R. W. Brown, and P. Gilbert. 1992. Effect of biofilm culture on the susceptibility of Staphylococcus epidermidis to tobramycin. J. Antimicrob. Chemother. 30:803-810. [DOI] [PubMed] [Google Scholar]
- 58.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis II. Survival of bacteria in endocardial vegetations. Br. J. Pathol. 53:50-53. [PMC free article] [PubMed] [Google Scholar]
- 59.Durack, D. T. 1975. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J. Pathol. 115:81-89. [DOI] [PubMed] [Google Scholar]
- 60.Dzink, J. L., S. S. Socransky, and A. D. Haffajee. 1988. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J. Clin. Periodontol. 15:316-323. [DOI] [PubMed] [Google Scholar]
- 61.Ehlers, L. J., and E. J. Bouwer. 1999. RP4 plasmid transfer among species of Pseudomonas in a biofilm reactor. Water Sci. Technol. 7:163-171. [Google Scholar]
- 62.Elliott, T. S. J., H. A. Moss, S. E. Tebbs, I. C. Wilson, R. S. Bonser, T. R. Graham, L. P. Burke, and M. H. Faroqui. 1997. Novel approach to investigate a source of microbial contamination of central venous catheters. Eur. J. Clin. Microbiol. Infect. Dis. 16:210-213. [DOI] [PubMed] [Google Scholar]
- 63.Evans, D. J., D. G. Allison, M. R. W. Brown, and P. Gilbert. 1990. Effect of growth-rate on resistance of gram-negative biofilms to cetrimide. J. Antimicrob. Chemother. 26:473-478. [DOI] [PubMed] [Google Scholar]
- 64.Farber, B. F., H. Hsi-Chia, E. D. Donnenfield, H. D. Perry, A. Epstein, and A. Wolff. 1995. A novel antibiofilm technology for contact lens solutions. Ophthalmology 102:831-836. [DOI] [PubMed] [Google Scholar]
- 65.Feigin, R. D., M. W. Kline, S. R. Hyatt, and K. L. Ford III. 1992. Otitis media, p. 174-189. In R. D. Feigin and J. D. Cherry (ed.), Textbook of pediatric infectious diseases, 3rd ed., vol. 1. W. B. Saunders Co., Philadelphia, Pa. [Google Scholar]
- 66.Ferguson, D. J. P., A. A. McColm, D. M. Ryan, and P. Acred. 1986. A morphological study of experimental staphylococcal endocarditis and aortitis. II. Interrelationship of bacteria, vegetation and cardiovasculature in established infections. Br. J. Exp. Pathol. 67:679-686. [PMC free article] [PubMed] [Google Scholar]
- 67.Fiehn, N.-E., and K. Henriksen. 1988. Methods of disinfection of the water system of dental units by water chlorination. J. Dent. Res. 67:1499-1504. [DOI] [PubMed] [Google Scholar]
- 68.Flowers, R. H., K. J. Schwenzer, R. F. Kopel, M. J. Fisch, S. I. Tucker, and B. M. Farr. 1989. Efficacy of an attachable subcutaneous cuff for the prevention of intravascular catheter-related infection. JAMA 261:878-883. [PubMed] [Google Scholar]
- 69.Freeman, R., and F. K. Gould. 1985. Infection and intravenous catheters. J. Antimicrob. Chemother. 15:258.. [DOI] [PubMed] [Google Scholar]
- 70.Furuhashi, M., and T. Miyamae. 1985. Prevention of bacterial contamination of water in dental units. J. Hosp. Infect. 6:81-88. [DOI] [PubMed] [Google Scholar]
- 71.Ganderton, L., J. Chawla, C. Winters, J. Wimpenny, and D. Stickler. 1992. Scanning electron microscopy of bacterial biofilms on indwelling bladder catheters. Eur. J. Clin. Microbiol. Infect. Dis. 11:789-796. [DOI] [PubMed] [Google Scholar]
- 72.Gandhi, P. A., A. D. Sawant, L. A. Wilson, and D. G. Ahearn. 1993. Adaptation and growth of Serratia marcescens in contact lens disinfectant solutions containing chlorhexidine gluconate. Appl. Environ. Microbiol. 59:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giebink, G. S., S. K. Juhn, M. L. Weber, and C. T. Le. 1982. The bacteriology and cytology of chronic otitis media with effusion. Pediatric Infect. Dis. 1:98-103. [DOI] [PubMed] [Google Scholar]
- 74.Gordon, C. A., N. A. Hodges, and C. Marriott. 1988. Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J. Antimicrob. Chemother. 22:667-674. [DOI] [PubMed] [Google Scholar]
- 75.Gourin, C. G., and R. N. Hubbell. 1999. Otorrhea after insertion of silver oxide-impregnated silastic tympanostomy tubes. Arch. Otolaryngol. Head Neck Surg. 125:446-450. [PubMed] [Google Scholar]
- 76.Govan, J. R. W., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hancock, E. W. 1994. Artificial valve disease, p. 1539-1545. In R. C. Schlant, R. W. Alexander, R. A. O'Rourke, R. Roberts, and E. H. Sonnenblick (ed.), The heart arteries and veins, 8th ed., vol. 2. McGraw-Hill, Inc., New York, N.Y. [Google Scholar]
- 78.Hartman, G., and R. Wise. 1998. Quorum sensing: potential means of treating gram-negative infections? Lancet 351:848-849. [DOI] [PubMed] [Google Scholar]
- 79.Hatch, R. A., and N. L. Schiller. 1998. Alginate lyase promotes diffusion of aminoglycosides through the extracellular polysaccharide of mucoid Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:974-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hausner, M., and S. Wuertz. 1999. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl. Environ. Microbiol. 65:3710-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reference deleted.
- 82.Holland, S. P., R. G. Mathias, D. W. Morck, J. Chiu, and S. G. Slade. 2000. Diffuse lamellar keratitis related to endotoxins released from sterilizer reservoir biofilms. Ophthalmology 107:1227-1234. [DOI] [PubMed] [Google Scholar]
- 83.Hoyle, B. D., C. K. W. Wong, and J. W. Costerton. 1992. Disparate efficacy of tobramycin on Ca+2-, Mg+2-, and HEPES-treated Pseudomonas aeruginosa biofilms. Can. J. Microbiol. 38:1214-1218. [DOI] [PubMed] [Google Scholar]
- 84.Hoyle, B. D., L. J. Williams, and J. W. Costerton. 1993. Production of mucoid exopolysaccharide during development of Pseudomonas aeruginosa biofilms. Infect. Immun. 61:777-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang, C.-T., G. James, W. G. Pitt, and P. S. Stewart. 1996. Effects of ultrasonic treatment on the efficacy of gentamicin against established Pseudomonas aeruginosa biofilms. Colloids Surfaces B Biointerfaces 6:235-242. [Google Scholar]
- 86.Hughes, C. F., and N. Noble. 1988. Vegetectomy: an alternative surgical treatment for infective endocarditis of the atrioventricular valves in drug addicts. J. Thorac. Cardiovasc. Surg. 95:857-861. [PubMed] [Google Scholar]
- 87.Illingworth, B. L., K. Twenden, R. F. Schroeder, and J. D. Cameron. 1998. In vivo efficacy of silver-coated (silzone) infection-resistant polyester fabric against a biofilm-producing bacteria, Staphylococcus epidermidis. J. Heart Valve Dis. 7:524-530. [PubMed] [Google Scholar]
- 88.Jacobs, L., E. E. DeBruyn, and T. E. Cloete. 1996. Spectrophotometric monitoring of biofouling. Water Sci. Technol. 34:533-540. [Google Scholar]
- 89.Jaques, M., M. E. Olson, and J. W. Costerton. 1986. Microbial colonization of tailed and tailless intrauterine contraceptive devices: influence of the mode of insertion in the rabbit. Am. J. Obstet. Gynecol. 154:648-655. [DOI] [PubMed] [Google Scholar]
- 90.Jensen, E. T., A. Kharazmi, K. Lam, J. W. Costerton, and N. Hoiby. 1990. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect. Immun. 58:2383-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen, E. T., A. Kharazmi, N. Hoiby, and J. W. Costerton. 1992. Some bacterial parameters influencing the neutrophil oxidative burst to Pseudomonas aeruginosa biofilms. APMIS 100:727-733. [PubMed] [Google Scholar]
- 92.Jeong, S.-N., S.-B. Han, D.-W. Lee, and I. Magnusson. 1994. Effects of tetracycline-containing gel and a mixture of tetracycline and citric acid-containing gel on non-surgical periodontal therapy. J. Periodontol. 65:840-847. [DOI] [PubMed] [Google Scholar]
- 93.Johansen, C., P. Falholt, and L. Gram. 1997. Enzymatic removal and disinfection of bacterial biofilm. Appl. Environ. Microbiol. 63:3724-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnson, J. R., P. Delavari, and M. Azar. 1999. Activities of a nitrofurazone-containing urinary catheter and a silver hydrogel catheter against multidrug-resistant bacteria characteristic of catheter-associated urinary tract infection. Antimicrob. Agents Chemother. 43:2990-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson, J. R., T. Berggren, and A. J. Conway. 1993. Activity of a nitrofurazone matrix urinary catheter against catheter-associated uropathogens. Antimicrob. Agents Chemother. 37:2033-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joly, V., B. Pangon, J.-M. Vallois, L. Abel, N. Brion, A. Bure, N. P. Chau, A. Contrepois, and C. Carbon. 1987. Value of antibiotic levels in serum and cardiac vegetations for predicting antibacterial effect of ceftriaxone in experimental Escherichia coli endocarditis. Antimicrob. Agents Chemother. 31:1632-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamal, G. D., M. A. Pfaller, L. E. Rempe, and P. J. R. Jebson. 1991. Reduced intravascular catheter infection by antibiotic bonding. A prospective, randomized, controlled trial. JAMA 265:2364-2368. [PubMed] [Google Scholar]
- 98.Karchmer, A. W., and G. W. Gibbons. 1994. Infections of prosthetic heart valves and vascular grafts, p. 213-249. In A. L. Bisno and F. A. Waldvogel (ed.), Infections associated with indwelling medical devices, 2nd ed. American Society for Microbiology, Washington, D.C.
- 99.Kaye, D., and M. T. Hessen. 1994. Infections associated with foreign bodies in the urinary tract, p. 291-307. In A. L. Bisno and F. A. Waldovogel (ed.), Infections associated with indwelling medical devices, 2nd ed. American Society for Microbiology, Washington, D.C.
- 100.Kharazmi, A., B. Giwercman, and N. Hoiby. 1999. Robbins Device in biofilm research. Methods Enzymol. 310:207-215. [DOI] [PubMed] [Google Scholar]
- 101.Kinniment, S. L., J. W. T. Wimpenny, D. Adams, and P. D. Marsh. 1996. The effect of chlorhexidine on defined, mixed culture oral biofilms grown in a novel model system. J. Appl. Bacteriol. 81:120-125. [DOI] [PubMed] [Google Scholar]
- 102.Kite, P., B. M. Dobbins, M. H. Wilcox, W. N. Fawley, A. J. L. Kindon, D. Thomas, M. J. Tighe, and M. J. McMahon. 1997. Evaluation of a novel endoluminal brush method for in situ diagnosis of catheter related sepsis. J. Clin. Pathol. 50:270-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kjaergard, H. K., J. Tingleff, U. Abildgaard, and G. Pettersson. 1999. Recurrent endocarditis in silver-coated heart valve prosthesis. J. Heart Valve Dis. 8:140-142. [PubMed] [Google Scholar]
- 104.Koch, C., and N. Hoiby. 1993. Pathogenesis of cystic fibrosis. Lancet 341:1065-1069. [DOI] [PubMed] [Google Scholar]
- 105.Kolenbrander, P. E., and J. London. 1993. Adhere today, here tomorrow: oral bacterial adherence. J. Bacteriol. 175:3247-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kondoh, K., and M. Hashiba. 1998. Inhibitory effect of macrolide antibiotics on biofilm formation by Pseudomonas aeruginosa. Nippon Jibiinkoka Gakkai Kaiho 101:25-36. [DOI] [PubMed] [Google Scholar]
- 107.Krause, P. J., N. J. Owens, C. H. Nightingale, J. J. Klimek, W. B. Lehmann, and R. Quintiliani. 1982. Penetration of amoxicillin, cefaclor, erythromycin-sulfisoxazole, and trimethoprim-sulfamethoxazole into the middle ear fluid of patients with chronic serous otitis media. J. Infect. Dis. 145:815-821. [DOI] [PubMed] [Google Scholar]
- 108.Reference deleted.
- 109.Ladd, T. I., D. Schmiel, J. C. Nickel, and J. W. Costerton. 1985. Rapid method for detection of adherent bacteria on Foley urinary catheters. J. Clin. Microbiol. 21:1004-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lam, J., R. Chan, and J. W. Costerton. 1980. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 28:546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lamont, R. J., A. Chan, C. M. Belton, K. T. Izutsu, D. Vasel, and A. Weinberg. 1995. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 63:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lamont, R. J., and H. F. Jenkinson. 1998. Life below gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lappin-Scott, H. M., and J. W. Costerton (ed.). 1995. Microbial biofilms. Cambridge University Press, Cambridge, England.
- 114.Larsen, T., and N.-E. Fiehn. 1996. Resistance of Streptococcus sanguis biofilms to antimicrobial agents. APMIS 104:280-284. [PubMed] [Google Scholar]
- 115.Lawrence, J. R., D. R. Korber, B. D. Hoyle, and J. W. Costerton. 1991. Optical sectioning of microbial biofilms. J. Bacteriol. 173:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewandowski, Z. 2000. Structure and function of biofilms, p. 1-17. In L. V. Evans (ed.), Biofilms: recent advances in their study and control. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 117.Lewis, R. 1998. A review of bacteriological culture of removed intrauterine contraceptive devices. Br. J. Fam. Plan. 24:95-97. [PubMed] [Google Scholar]
- 118.Livornese, L. L., and O. M. Korzeniowski. 1992. Pathogenesis of infective endocarditis, p. 19-35. In D. Kaye (ed.), Infective endocarditis, 2nd ed. Raven Press, New York, N.Y.
- 119.Lowrance, J. H., L. M. Baddour, and W. A. Simpson. 1990. The role of fibronectin binding on the rate model of experimental endocarditis caused by Streptococcus sanguis. J. Clin. Investig. 86:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lowrance, J. H., D. L. Hasty, and W. A. Simpson. 1988. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect. Immun. 56:2279-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lutas, E. M., R. B. Roberts, R. B. Devereux, and L. M. Prieto. 1986. Relation between the presence of echocardiographic vegetations and the complication rate in infective endocarditis. Am. Heart J. 112:107-113. [DOI] [PubMed] [Google Scholar]
- 122.Maki, D. G., and J. D. Band. 1981. A comparative study of polyantibiotic and iodophor ointments in prevention of vascular catheter-related infection. Am. J. Med. 70:739-744. [DOI] [PubMed] [Google Scholar]
- 123.Maki, D. G. 1994. Infections caused by intravascular devices used for infusion therapy: pathogenesis, prevention, and management, p. 155-212. In A. L. Bisno and F. A. Waldovogel (ed.), Infections associated with indwelling medical devices, 2nd ed. American Society for Microbiology, Washington, D.C.
- 124.Maki, D. G., S. M. Stolz, S. Wheeler, and L. A. Mermel. 1997. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann. Intern. Med. 127:257-266. [DOI] [PubMed] [Google Scholar]
- 125.Maki, D., L. Cobb, J. K. Garman, J. M. Shapiro, M. Ringer, and R. B. Helgerson. 1988. An attachable silver-impregnated cuff for prevention of infection with central venous catheters: a prospective randomized multicenter trial. Am. J. Med. 85:307-314. [DOI] [PubMed] [Google Scholar]
- 126.Maki, D. G., C. E. Weise, and H. W. Sarafin. 1977. A semiquantitative culture method for identifying intravenous-catheter-related infection. New Engl. J. Med. 296:1305-1309. [DOI] [PubMed] [Google Scholar]
- 127.Marrie, T. J., and J. W. Costerton. 1983. A scanning and transmission electron microscopic study of the surfaces of intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 146:384-394. [DOI] [PubMed] [Google Scholar]
- 128.Marsh, P. D. 1995. Dental plaque, p. 282-300. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms. Cambridge University Press, Cambridge, United Kingdom,
- 129.Marshall, K. C. 1976. Interfaces in microbial ecology, p. 44-47. Harvard University Press, Cambridge, Mass.
- 130.Mathee, K., O. Ciofu, C. Sternberg, P. W. Lindum, J. I. A. Campbell, P. Jensen, M. Givskov, D. E. Ohman, S. Molin, N. Hoiby, and A. Kharazmi. 1999. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 145:1349-1357. [DOI] [PubMed] [Google Scholar]
- 131.May, T. B., D. Shinabarger, R. Maharaj, J. Kato, L. Chu, J. D. DeVault, S. Roychoudhury, N. A. Zielinski, A. Berry, R. K. Rothmel, T. K. Misra, and A. M. Chakrabarty. 1991. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin. Microbiol. Rev. 4:191-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mayo, J. A., K. M. Oertling, and S. C. Andrieu. 1990. Bacterial biofilm: a source of contamination in dental air-water-syringes. Clin. Prev. Dent. 12:13-20. [PubMed] [Google Scholar]
- 133.McLaughlin-Borlace, L., F. Stapleton, M. Matheson, and J. K. G. Dart. 1998. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J. Appl. Microbiol. 84:827-838. [DOI] [PubMed] [Google Scholar]
- 134.McLean, R. J. C., J. C. Nickel, and M. E. Olson. 1995. Biofilm associated urinary tract infections, p. 261-273. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms. Cambridge University Press, Cambridge, United Kingdom.
- 135.Meluleni, G. J., M. Grout, D. J. Evans, and G. B. Pier. 1995. Mucoid Pseudomonas aeruginosa growing in a biofilm in vitro are killed by opsonic antibodies to the mucoid exopolysaccharide capsule but not by antibodies produced during chronic lung infection in cystic fibrosis patients. J. Immunol. 155:2029-2038. [PubMed] [Google Scholar]
- 136.Miller, M. J., L. A. Wilson, and D. J. Ahearn. 1988. Effects of protein, mucin, and human tears on adherence of Pseudomonas aeruginosa to hydrophilic contact lenses. J. Clin. Microbiol. 26:513-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miller, M. J., and D. G. Ahearn. 1987. Adherence of Pseudomonas aeruginosa to hydrophilic contact lenses and other substrata. J. Clin. Microbiol. 25:1392-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mills, S. E., P. W. Lauderdale, and R. B. Mayhew. 1986. Reduction of microbial contamination in dental units with povidone-iodine 10%. JADA 113:280-284. [DOI] [PubMed] [Google Scholar]
- 139.Mittelman, M. W., L. L. Kohring, and D. C. White. 1992. Multipurpose laminar-flow adhesion cells for the study of bacterial colonization and biofilm formation. Biofouling 6:39-51. [Google Scholar]
- 140.Moore, W. E. C., L. V. Holdeman, E. P. Cato, R. M. Smibert, J. A. Burmeister, and R. R. Ranney. 1983. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect. Immun. 42:510-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morris, N. S., and D. J. Stickler. 1998. The effect of urease inhibitors on the encrustation of urethral catheters. Urol. Res. 26:275-279. [DOI] [PubMed] [Google Scholar]
- 142.Morris, N. S., D. J. Stickler, and C. Winters. 1997. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms. Br. J. Urol. 80:58-63. [DOI] [PubMed] [Google Scholar]
- 143.Murdoch-Kinch, C. A., N. A. Andrews, S. Atwan, R. Jude, M. J. Gleason, and J. A. Molinari. 1997. Comparison of dental water quality management procedures. JADA 128:1235-1243. [DOI] [PubMed] [Google Scholar]
- 144.Murga, R., J. M. Miller, and R. M. Donlan. 2001. Biofilm formation by gram-negative bacteria on central venous catheter connectors: effect of conditioning films in a laboratory model. J. Clin. Microbiol. 39:2294-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nickel, J. C., J. Downey, and J. W. Costerton. 1992. Movement of Pseudomonas aeruginosa along catheter surfaces. Urology 34:93-98. [DOI] [PubMed] [Google Scholar]
- 146.Nickel, J. C., J. W. Costerton, R. J. C. McLean, and M. Olson. 1994. Bacterial biofilms: influence on the pathogenesis, diagnosis, and treatment of urinary tract infections. J. Antimicrob. Chemother. 33(Suppl. A):31-41. [DOI] [PubMed] [Google Scholar]
- 147.Nickel, J. C., and J. W. Costerton. 1992. Coagulase-negative staphylococcus in chronic prostatitis. J. Urol. 147:398-401. [DOI] [PubMed] [Google Scholar]
- 148.Nickel, J. C., M. E. Olson, and J. W. Costerton. 1991. Rat model of experimental bacterial prostatitis. Infection 19(Suppl. 3):S126-S128. [DOI] [PubMed] [Google Scholar]
- 149.Nickel, J. C., I. Ruseska, J. B. Wright, and J. W. Costerton. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nickel, J. C., M. E. Olson, A. Barabas, H. Benediktsson, M. K. Dasgupta, and J. W. Costerton. 1990. Pathogenesis of chronic bacterial prostatitis in an animal model. Br J. Urol. 66:47-54. [DOI] [PubMed] [Google Scholar]
- 151.Nickel, J. C., and J. W. Costerton. 1993. Bacterial localization in antibiotic-refractory chronic bacterial prostatitis. Prostate 23:107-114. [DOI] [PubMed] [Google Scholar]
- 152.Nickel, J. C., J. A. Downey, and J. W. Costerton. 1989. Ultrastructural study of microbiologic colonization of urinary catheters. Urology 34:284-291. [DOI] [PubMed] [Google Scholar]
- 153.Olson, M. E., J. C. Nickel, A. E. Khoury, D. W. Morck, R. Cleeland, and J. W. Costerton. 1989. Amdinocillin treatment of catheter-associated bacteriuria in rabbits. J. Infect. Dis. 159:1065-1072. [DOI] [PubMed] [Google Scholar]
- 154.Omar, A. A., H. N. Newman, and J. Osborn. 1990. Darkground microscopy of subgingival plaque from the top to the bottom of the periodontal pocket. J. Clin. Periodontol. 17:364-370. [DOI] [PubMed] [Google Scholar]
- 155.Pankhurst, C. L., J. N. Philpott-Howard, J. H. Hewitt, and M. W. Casewell. 1990. The efficacy of chlorination and filtration in the control and eradication of Legionella from dental chair water systems. J. Hosp. Infect. 16:9-18. [DOI] [PubMed] [Google Scholar]
- 156.Perrotta, E., and M. Fiore. 1997. Native valve infective endocarditis caused by Streptococcus bovis. Efficacy of short-term antibiotic therapy and usefulness of serial echocardiographic evaluation. Recent Prog. Med. 88:521-525. [PubMed] [Google Scholar]
- 157.Pugach, J. L., V. Ditizio, M. W. Mittelman, A. W. Bruce, F. DiCosmo, and A. E. Khoury. 1999. Antibiotic hydrogel coated Foley catheters for prevention of urinary tract infection in a rabbit model. J. Urol. 162:883-887. [DOI] [PubMed] [Google Scholar]
- 158.Reference deleted.
- 159.Quirynen, M., C. M. L. Bollen, B. N. A. Vandekerckhove, C. Dekeyser, W. Papaioannou, and H. Eyssen. 1995. Full- vs. partial-mouth disinfection in the treatment of periodontal infections: short-term clinical and microbiological observations. J. Dent. Res. 74:1459-1467. [DOI] [PubMed] [Google Scholar]
- 160.Raad, I., W. Costerton, U. Sabharwal, M. Sacilowski, W. Anaissie, and G. P. Bodey. 1993. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 168:400-407. [DOI] [PubMed] [Google Scholar]
- 161.Raad, I. I., M. F. Sabbagh, K. H. Rand, and R. J. Sherertz. 1992. Quantitative tip culture methods and the diagnosis of central venous catheter-related infections. Diagn. Microbiol. Infect. Dis. 15:13-20. [DOI] [PubMed] [Google Scholar]
- 162.Raad, I. 1998. Intravascular-catheter-related infections. Lancet 351:893-898. [DOI] [PubMed] [Google Scholar]
- 163.Reynolds, M. A., C. K. Lavigne, G. E. Minah, and J. B. Suzuki. 1992. Clinical effects of simultaneous ultrasonic scaling and subgingival scaling and subgingival irrigation with chlorhexidine. Mediating influence of periodontal probing depth. J. Clin. Periodontol. 19:595-600. [DOI] [PubMed] [Google Scholar]
- 164.Rioufol, C., C. Devys, G. Meunier, M. Perraud, and D. Goullet. 1999. Quantitative determination of endotoxins released by bacterial biofilms. J. Hosp. Infect. 43:203-209. [DOI] [PubMed] [Google Scholar]
- 165.Roberts, A. P., J. Pratten, M. Wilson, and P. Mullany. 1999. Transfer of a conjugative transposon, Tn 5397, in a model oral biofilm. FEMS Microbiol. Lett. 177:63-66. [DOI] [PubMed] [Google Scholar]
- 166.Roberts, R. B. 1992. Streptococcal endocarditis: the viridins and beta hemolytic streptococci, p. 191-208. In D. Kaye (ed.), Infective endocarditis, 2nd ed. Raven Press, New York, N.Y.
- 167.Rogers, J., D. I. Norkett, P. Bracegirdle, A. B. Dowsett, J. T. Walker, T. Brooks, and C. W. Keevil. 1996. Examination of biofilm formation and risk of infection associated with the use of urinary catheters with leg bags. J. Hosp. Infect. 32:105-115. [DOI] [PubMed] [Google Scholar]
- 168.Rohmann, S., R. Erhel, H. Darius, T. Makowski, and J. Meyer. 1997. Effect of antibiotic treatment on vegetation size and complication rate in infective endocarditis. Clin. Cardiol. 20:132-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Saidi, I. S., J. F. Biedlingmaier, and P. Whelan. 1999. In vivo resistance to bacterial biofilm formation on tympanostomy tubes as a function of tube material. Otolaryngol. Head Neck Surg. 120:621-627. [DOI] [PubMed] [Google Scholar]
- 171.Saint, S., and B. A. Lipsky. 1999. Preventing catheter-related bacteriuria: Should we? Can we? How? Arch. Intern. Med. 159:800-808. [DOI] [PubMed] [Google Scholar]
- 172.Sandoe, J. A., K. G. Kerr, G. W. Reynolds, and S. Jain. 1999. Staphylococcus capitus endocarditis: two cases and review of the literature. Heart Sep. 82:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Santiago, J. I., M. K. Huntington, A. M. Johnston, R. S. Quinn, and J. F. Williams. 1994. Microbial contamination of dental unit waterlines: short-and long-term effects of flushing. Gen. Dent. 42:528-535. [PubMed] [Google Scholar]
- 174.Schierholz, J. M., J. Beuth, G. Pulverer, and D.-P. Konig. 1999. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infection. Am. J. Med. 107:534-535. [DOI] [PubMed] [Google Scholar]
- 175.Sedor, J., and S. G. Mulholland. 1999. Hospital-acquired urinary tract infections associated with the indwelling catheter. Urol. Clin. N. Am. 26:821-828. [DOI] [PubMed] [Google Scholar]
- 176.Shapiro, L., and R. E. Stallard. 1977. Etiology of periodontal disease, p. 74-80. In R. C. Caldwell and R. E. Stallard (ed.), A textbook of preventive dentistry. W. B. Saunders, Philadelphia, Pa.
- 177.Shearer, B. G. 1996. Biofilm and the dental office. J. Am. Dental Assoc. 127:181-189. [DOI] [PubMed] [Google Scholar]
- 178.Sherertz, R. J., I. I. Raad, A. Belani, L. C. Koo, K. H. Rand, D. L. Pickett, S. A. Straub, and L. L. Fauerbach. 1990. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J. Clin. Microbiol. 28:76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shiau, A.-L., and C.-L. Wu. 1998. The inhibitory effect of Staphylococcus epidermidis slime on the phagocytosis of murine peritoneal macrophages is interferon-independent. Microbiol. Immunol. 42:33-40. [DOI] [PubMed] [Google Scholar]
- 180.Socransky, S. S., and A. D. Haffajee. 1992. The bacteriology of destructive periodontal disease: current concepts. J. Periodontol. 63:322-331. [DOI] [PubMed] [Google Scholar]
- 181.Souli, M., and H. Giamarellou. 1998. Effects of slime produced by clinical isolates of coagulase-negative staphylococci on activities of various antimicrobial agents. Antimicrob. Agents Chemother. 42:939-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stapleton, F., and J. Dart. 1995. Pseudomonas keratitis associated with biofilm formation on a disposable soft contact lens. Br. J. Ophthalmol. 79:864-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stapleton, F., J. K. Dart, M. Matheson, and E. G. Woodward. 1993. Bacterial adherence and glycocalyx formation on unworn hydrogel lenses. J. Br. Contact Lens Assoc. 16:113-117. [Google Scholar]
- 184.Stenfors, L. E., and S. Raisanen. 1988. Quantification of bacteria in middle ear effusions. Acta Otolaryngol. (Stockholm) 106:435-440. [DOI] [PubMed] [Google Scholar]
- 185.Stewart, J. A., D. Silimperi, P. Harris, N. K. Wise, T. D. Fraker, and J. A. Kisslo. 1980. Echocardiographic documentation of vegetative lesions in infective endocarditis: clinical implications. Circulation 61:374-380. [DOI] [PubMed] [Google Scholar]
- 186.Stewart, P. S. 1996. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 40:2517-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stickler, D., N. Morris, M.-C. Moreno, and N. Sabbuba. 1998. Studies on the formation of crystalline bacterial biofilms on urethral catheters. Eur. J. Clin. Microbiol. Infect. Dis. 17:649-652. [DOI] [PubMed] [Google Scholar]
- 188.Stickler, D., and P. Hewett. 1991. Activity of antiseptics against biofilms of mixed bacterial species growing on silicone surfaces. Eur. J. Clin. Microbiol. Infect. Dis. 10:416-421. [DOI] [PubMed] [Google Scholar]
- 189.Stickler, D. J. 1996. Bacterial biofilms and the encrustation of urethral catheters. Biofouling 94:293-305. [Google Scholar]
- 190.Stickler, D. J., J. B. King, C. Winters, and N. S. Morris. 1993. Blockage of urethral catheters by bacterial biofilms. J. Infect. 27:133-135. [DOI] [PubMed] [Google Scholar]
- 191.Stickler, D., and G. Hughes. 1999. Ability of Proteus mirabilis to swarm over urethral catheters. Eur J. Clin. Microbiol. Dis. 18:206-208. [DOI] [PubMed] [Google Scholar]
- 192.Stickler, D., L. Ganderton, J. King, J. Nettleton, and C. Winters. 1993. Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol. Res. 21:407-411. [DOI] [PubMed] [Google Scholar]
- 193.Stickler, D. J., N. S. Morris, R. J. C. McLean, and C. Fuqua. 1998. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl. Environ. Microbiol. 64:3486-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stickler, D. J., J. King, J. Nettleton, and C. Winters. 1993. The structure of urinary catheter encrusting bacterial biofilms. Cells Mater. 3:315-319. [Google Scholar]
- 195.Stickler, D. J., N. S. Morris, and C. Winters. 1999. Simple physical model to study formation and physiology of biofilms on urethral catheters. Methods Enzymol. 310:494-501. [DOI] [PubMed] [Google Scholar]
- 196.Stiles, G. L., and G. C. Friesinger. 1980. Bacterial endocarditis with aortic regurgitation: implications of embolism. South. Med. J. 73:582-586. [DOI] [PubMed] [Google Scholar]
- 197.Stoodley, P., Z. Lewandowski, J. D. Boyle, and H. M. Lappin-Scott. 1998. Oscillation characteristics of biofilm streamers in turbulent flowing water as related to drag and pressure drop. Biotechnol. Bioeng. 57:536-544. [DOI] [PubMed] [Google Scholar]
- 198.Suci, P. A., M. W. Mittelman, F. P. Yu, and G. G. Geesey. 1994. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 38:2125-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tall, B. D., H. N. Williams, K. S. George, R. T. Gray, and M. Walch. 1995. Bacterial succession within a biofilm in water supply lines of dental air-water syringes. Can. J. Microbiol. 41:647-654. [DOI] [PubMed] [Google Scholar]
- 200.Tatum, H. J., F. H. Schmidt, D. Phillips, M. McCarty, and W. M. O'Leary. 1975. The Dalkon Shield controversy. JAMA 231:711-717. [DOI] [PubMed] [Google Scholar]
- 201.Taylor, R. J. 1996. Efficacy of industrial biocides against bacterial biofilms. Ph.D. thesis. University of Birmingham, Birmingham, United Kingdom.
- 202.Tenney, J. H., M. R. Moody, K. A. Newman, S. C. Schimpff, J. C. Wade, J. W. Costerton, and W. P. Reed. 1986. Adherent microorganisms on lumenal surfaces of long-term intravenous catheters. Arch. Intern. Med. 146:1949-1954. [PubMed] [Google Scholar]
- 203.Tresse, O., T. Jouenne, and G.-A. Junter. 1995. The role of oxygen limitation in the resistance of agar-entrapped, sessile-like Escherichia coli to aminoglycoside and β-lactam antibiotics. J. Antimicrob. Chemother. 36:521-526. [DOI] [PubMed] [Google Scholar]
- 204.Tunkel, A. R., and G. L. Mandell. 1992. Infecting microorganisms, p. 85-97. In D. Kaye (ed.), Infective endocarditis, 2nd ed. Raven Press, New York, N.Y.
- 205.Tunney, M. M., D. S. Jones, and S. P. Gorman. 1999. Biofilm and biofilm-related encrustations of urinary tract devices. Methods Enzymol. 310:558-566. [DOI] [PubMed] [Google Scholar]
- 206.Veenstra, D. L., S. Saint, S. Saha, T. Lumley, and S. D. Sullivan. 1999. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection. JAMA 281:261-267. [DOI] [PubMed] [Google Scholar]
- 207.Vincent, F. C., A. R. Tibi, and J. C. Darbord. 1989. A bacterial biofilm in a hemodialysis system. Assessment of disinfection and crossing of endotoxin. ASAIO Trans. 35:310-313. [DOI] [PubMed] [Google Scholar]
- 208.Vorachit, M., K. Lam, P. Jayanetra, and J. W. Costerton. 1993. Resistance of Pseudomonas pseudomallei growing as a biofilm on silastic disks to ceftazidime and cotrimoxazole. Antimicrob. Agents Chemother. 37:2000-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Walker, J. T., A. D. Roberts, V. J. Lucas, M. A. Roper, and R. G. Brown. 1999. Quantitative assessment of biocide control of biofilms including Legionella pneumophila using total viable counts, fluorescence microscopy, and image analysis. Methods Enzymol. 310:629-637. [DOI] [PubMed] [Google Scholar]
- 210.Reference deleted.
- 211.Ward, K. H., M. E. Olson, K. Lam, and J. W. Costerton. 1992. Mechanism of persistent infection associated with peritoneal implants. J. Med. Microbiol. 36:406-413. [DOI] [PubMed] [Google Scholar]
- 212.Wells, C. J., G. J. Leech, A. M. Lever, and M. H. Wansbrough-Jones. 1995. Treatment of native valve Candida endocarditis with flucanazole. J. Infect. Dis. 31:233-235. [DOI] [PubMed] [Google Scholar]
- 213.Whitehouse, R. L. S., E. Peters, J. Lizotte, and C. Lilge. 1991. Influence of biofilms on microbial contamination in dental unit water. J. Dent. 19:290-295. [DOI] [PubMed] [Google Scholar]
- 214.Wiernikowski, J. T., D. Elder-Thornley, S. Dawson, M. Rothney, and S. Smith. 1991. Bacterial colonization of tunneled right atrial catheters in pediatric oncology: a comparison of sterile saline and bacteriostatic saline flush solutions. Am. J. Pediatr. Hematol. Oncol. 13:137-140. [DOI] [PubMed] [Google Scholar]
- 215.Williams, I., W. A. Venables, D. Lloyd, F. Paul, and I. Critchley. 1997. The effects of adherence to silicone surfaces on antibiotic susceptibility in Staphylococcus aureus. Microbiology 143:2407-2413. [DOI] [PubMed] [Google Scholar]
- 216.Williams, H. N., H. Quinby, and E. Romberg. 1994. Evaluation and use of a low nutrient medium and reduced incubation temperature to study bacterial contamination in the water supply of dental units. Can. J. Microbiol. 40:127-131. [DOI] [PubMed] [Google Scholar]
- 217.Williams, H. N., J. Kelley, D. Folineo, G. C. Williams, C. L. Hawley, and J. Sibiski. 1994. Assessing microbial contamination in clean water dental units and compliance with disinfection protocol. JADA 125:1205-1211. [DOI] [PubMed] [Google Scholar]
- 218.Wilson, L. A., A. D. Sawant, and D. G. Ahearn. 1991. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch. Ophthalmol. 109:1155-1157. [DOI] [PubMed] [Google Scholar]
- 219.Wolf, A. S., and D. Kreiger. 1986. Bacterial colonization of intrauterine devices (IUDs). Arch. Gynecol. 239:31-37. [DOI] [PubMed] [Google Scholar]
- 220.Yasuda, H., Y. Ajiki, J. Aoyama, and T. Yokota. 1994. Interaction between human polymorphonuclear leucocytes and bacteria released from in-vitro bacterial biofilm models. J. Med. Microbiol. 41:359-367. [DOI] [PubMed] [Google Scholar]
- 221.Zelver, N., M. Hamilton, B. Pitts, D. Goeres, D. Walker, P. Sturman, and J. Heersink. 1999. Measuring antimicrobial effects on biofilm bacteria: from laboratory to field. Methods Enzymol. 310:629-637. [DOI] [PubMed] [Google Scholar]
- 222.Zimakoff, J., B. Pontoppidan, S. O. Larsen, and D. J. Stickler. 1993. Management of urinary bladder function in Danish hospitals, nursing homes and home care. J. Hosp. Infect. 24:183-199. [DOI] [PubMed] [Google Scholar]
- 223.Zips, A., G. Schaule, and H. C. Flemming. 1990. Ultrasound as a means of detaching biofilms. Biofouling 2:323-333. [Google Scholar]
- 224.Zufferey, J., R. Rime, P. Francioli, and J. Bille. 1988. Simple method for rapid diagnosis of catheter-associated infection by direct acridine orange staining of catheter tips. J. Clin. Microbiol. 26:175-177. [DOI] [PMC free article] [PubMed] [Google Scholar]