Abstract
Introduction
The use of addictive substances remains a major health problem in the U.S. An increase in drug overdose and mortality was observed during the COVID-19 pandemic, especially in underserved populations. This surge also impacted pregnant women especially with the use of marijuana (THC) and opioids.
Methods
Retrospective study analyzing mother-infant dyads with reported Meconium Drug Screen (MDS) results, from January 2018 through April 2023 at a reference hospital serving an urban and rural area of Arkansas. Due to the absence of universal screening, the prevalence of drug use was variable during the study period. An adjusted monthly positive rate (AMPR) was calculated by considering the expected number of positive MDS screenings per month and adjusting it based on the screening rate per month and the monthly birth count.
Results
Among 8,030 live births, 957 dyads were included in the analysis, with 47% (N = 450) of infants testing positive for at least one substance. Of these, 64.2%, 11.1%, and 6.7% were positive for THC, amphetamines, and opioids, respectively; and 17.8% tested positive for more than one substance. Infants with a positive MDS (MDS+) had significantly lower weight, height and head circumference with higher preterm rates and longer hospital stays. Mothers who smoked during pregnancy were more than twice as likely to have an MDS + result than those who did not (OR 2.39 (95% CI: 1.34–3.02), and most were from metropolitan areas (73%) or white (67%). The adjusted MDS + rate or AMPR significantly increased over the study period from 6.8% (January 2018) to 7.4% (April 2023). However, the COVID-19 pandemic did not significantly impact these rates. Compared to amphetamines and opioids, THC usage significantly increased during the study period and this increase was more pronounced after the state’s legalization of THC.
Conclusion
Maternal substance use, predominantly THC, continues to increase, particularly following its legalization. Infants who were MDS + presented adverse neonatal outcomes, with the majority of the mothers being white and from urban settings. Maternal self-reported smoking was associated with increased usage of other substances. Racial disparities were observed during the study, underscoring the need for universal drug testing and targeted interventions.
Keywords: Perinatal drug use, Maternal substance use, Maternal-child health, Meconium drug screening, Infant health
Introduction
Substance use during pregnancy remains a pervasive public health issue, with implications for healthcare systems and societal well-being [1]. Tobacco, alcohol, marijuana, and illicit drugs such as amphetamines and opioids have been associated with negative pregnancy and adverse neonatal outcomes [2–7]. Despite public health efforts, the prevalence of prenatal substance use has shown concerning trends, particularly during periods of heightened societal stress, such as the COVID-19 pandemic [8].
The health risks associated with prenatal substance exposure are well-documented. For instance, maternal smoking is linked to intrauterine growth restriction, preterm birth, and sudden infant death syndrome [9]. Prenatal alcohol exposure can result in fetal alcohol spectrum disorders, characterized by cognitive, behavioral, and physical impairments. Marijuana use during pregnancy is associated with lower birth weight and developmental delays [10]. The use of amphetamines and opioids is particularly concerning because of their association with neonatal abstinence syndrome (NAS) [11, 12].
Previous studies have identified several risk factors, including younger maternal age, lower socioeconomic status, and higher rates of tobacco use among substance-using mothers [13, 14]. It has also been shown that the COVID-19 pandemic has had widespread effects on public health, including changes in substance use behaviors [15, 16]. However, many of these data are derived from self-reports or specific small-scale studies, raising concerns about underreporting [17, 18]. Meconium drug screening (MDS) is an essential tool for detecting in utero exposure to various substances, providing a comprehensive view of maternal drug use during pregnancy, typically from the second trimester onward [19–21].
This study aims to provide a detailed analysis of maternal and infant characteristics associated with MDS outcomes in a regional hospital in northeast Arkansas, covering pre, during and post COVID-19 pandemic periods. By examining demographic, behavioral, and health-related variables, we seek to identify key risk factors, disparities and temporal trends in substance exposure. Additionally, we explore the specific impacts of different substances on neonatal health and the variations in screening and detection rates before, during, and after the COVID-19 pandemic, covering a five-year period.
By addressing these objectives, our overarching goal is to deepen our understanding of prenatal substance exposure and to inform targeted public health interventions that can effectively mitigate its adverse effects.
Methods
Data collection
This retrospective study utilized electronic medical records (EMR) data from a birth hospital in Northeast Arkansas (NEA), which serves as a referral center for 23 urban and rural counties in Arkansas and Missouri. This study was approved by the Institutional Review Board. The inclusion criterion was neonates who had a MDS reported from January 2018 through April 2023 (the study period). At this institution, drug screening is performed prenatally and MDS is ordered for neonates with risk factors for prenatal drug exposure, i.e. born to drug-dependent mothers or mothers with a history of drug use or a positive drug test; infants presenting withdrawal symptoms and; infants with maternal risk factors for drug abuse (no prenatal care, precipitous vaginal delivery, unexplained abruption, altered mental status, and outward clinical signs such as track marks, multiple skin infections, and tooth decay).
Demographic information, including neonatal birth information such as date, birth weight (BW), height, head circumference (HC), gestational age (GA), and neonatal length of stay (LOS), was collected for all enrolled infants. Demographic maternal information included age, race, smoking status, number of cigarettes per day, number of gestations, number of living children, race, and residential 5-digit zip codes. Meconium drug screens reported positive or negative results for 7 drugs: amphetamines, benzodiazepines, tetrahydrocannabinol (THC), cocaine, opioids, phencyclidine, and methadone. The opioids included in the MDS panel include morphine, hydromorphone, and oxycodone.
Data processing
We defined ‘teen pregnancies’ as a maternal age < 20 years, ‘preterm infant’ as a GA < 37 weeks, and ‘low birth weight’ as a BW < 2500gm. The MDS data was stratified by three 2-year time periods related to the COVID-19 pandemic: ‘pre-pandemic’ (01/2018–02/2020), ‘pandemic’ (03/2020–03/2022), and ‘post-pandemic’ (04/2022–04/2023). Maternal residential zip codes were classified as either ‘metropolitan’ or ‘non-metropolitan’ on the basis of the RUCA 4–10 classification [22]. The ‘race’ variable was collapsed into 3 categories: White (607, 63.4%), African American (274, 28.6%), and Other, which consisted of 31 ‘Unable to obtain’, 21 Native Hawaiian/Pacific Island, 16 ‘More than one race’, 4 Asian, and 3 others.
Although 7 drugs have been reported in the MDS, the results for 4 drugs were not considered for analysis due to the small sample size: benzodiazepines (n = 4), cocaine (n = 12), phencyclidine (n = 0), and methadone (n = 11). A monthly ‘screening rate’ variable was created, which is the count of MDS performed divided by the total birth count for that month. A monthly ‘positive rate’ was created which is the count of positive MDS results divided by the total birth count for that month. Because not every neonate had an MDS, this positive rate is not reflective of the population, and as such, was adjusted on the basis of the monthly screening rate (see below for details). Additionally, positive rates were calculated for each of the three drugs considered in the study (THC, opioids, and amphetamines).
Data analysis
Demographic data are presented for both maternal and infant characteristics as medians and interquartile ranges (IQRs) for continuous variables, and frequencies and as percentages for categorical variables. Neonatal outcomes and maternal factors were compared between those who tested positive for each type of drug in the MDS and those who tested negative for all drugs via the Mann-Whitney U test for continuous variables and the chi-square test for categorical variables. Differences between groups were considered significant if the p-value was < 0.05.
Trends in the positive MDS rates over time were examined to identify changes in slope (rate of change of the trend line) over the study period and in the three defined time periods related to the pandemic. Temporal trends were analyzed via OLS regression where the time of the study was the independent variable. The estimated slopes were considered increasing or decreasing if they differed from 0. Rates were considered significantly increasing or decreasing if the p-value from the linear regression was less than 0.05. The MDS positive rate for the 12-month period following the study period (May 2023-May 2024) was forecasted via exponential smoothing.
Adjusting the MDS positive rate
The outcome of interest in this study was the prevalence of drug use during the study period. However, due to a lack of universal screening, screening rates vary by month. Neonates were selected to have an MDS on the basis of risk factors described earlier. Additionally, the positive rate is strongly correlated with the screening rate; as more individuals are screened in a given month, there will be more positive results for that month. Therefore, to capture the true positive detection rates, the MDS positive rates were adjusted according to the screening rate via the following formula.
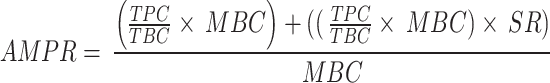 |
where AMPR represents the adjusted monthly positive rate, TPC represents the total positive count, TBC represents the total birth count, MBC represents the monthly birth count, and SR represents the screening rate. In this formula, the first term (TPC/TBC×MBC) represents the expected count of positive MDS screenings per month. The second term ((TPCTBC×MBC) ×SR) adjusts this expected count on the basis of the screening rate per month. These two components are then added together and divided by the monthly birth count to yield the adjusted monthly positive rate (AMPR).
By adjusting for the screening rate, we effectively compensate for the main limitation of traditional positive rate calculations, which might overlook the impact of variable screening rates on the perceived prevalence of a condition. The proposed method acknowledges that the raw number of positives detected is influenced by how many individuals were tested and how many were born, providing a more accurate picture of the positive rate among newborns. This can be especially important for tracking trends over time, evaluating the effectiveness of interventions, and ensuring that resources are allocated efficiently.
Calculating Growth Rates: To measure and compare the changes in positive MDS rates over time, we calculated growth rates for four different time periods: overall (spanning the entire study period), pre, during- and post-pandemic. For each time period, the growth rate was calculated as follows:
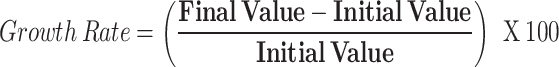 |
where the ‘initial value’ represents the MDS positive rate at the beginning of the time period, and the ‘final value’ is the rate at the end of the time period. This formula provides a simple yet effective measure of how rapidly the positivity rate changes over time and allows us to assess the impact of the pandemic on drug use patterns.
There are limitations in terms of growth rates, including the ability to handle high volatility in data. If there is an abnormally high positive rate at one time, it could lead to a strong positive growth rate, even if there is an overall decline. This could be misleading if not balanced with a broader analysis. To address this, we recommend interpreting these findings alongside the slope and coefficient from a regression analysis. This offers a more comprehensive and nuanced picture of changes over time.
Logistic regression analysis was performed using maternal characteristics, including maternal age, smoking during pregnancy, geographic residential location, maternal race, and time period (pre-, during, and post-COVID-19 pandemic) as predictors and, MDS positive or negative results as the outcome, where positive results represent being positive for at least one drug. Odds ratios (ORs) and 95% CIs were estimated to compare the odds of having a positive MDS screening for different age, racial, geographic, and other groups.
All analysis were performed via pandas, statsmodels, and scikit-learn libraries in Python 3.9.
Results
Overall characteristics of the mother/infant dyads (Table 1)
Table 1.
Maternal and newborn characteristics overall and grouped by Meconium Drug Screen result
| Characteristic | Overall | Negative for all substances | Positive for at least one substance |
|---|---|---|---|
| Total n (%) | 957 | 507 / 957 (53.0%) | 450 / 957 (47.0%) |
| Mother characteristics | |||
| Age (years) median, IQR | 27 (23–32) | 27 (22–32) | 28 (24–32)* |
|
Smoking during pregnancy indicated n (%) |
|||
| Yes | 251 (29.5%) | 90 (19.9%) | 161 (40.5%)* |
| No | 599 (70.5%) | 362 (80.1%) | 237 (59.5%)* |
| Number of cigarettes median, IQR | 0 (0–0) | 0 (0–0) | 0 (0-3.75)* |
| Number of pregnancies median, IQR | 3 (2-4.25) | 3 (2–4) | 3 (2–5)* |
| Number of live children median, IQR | 2 (1–3) | 1 (0–3) | 2 (1–3)* |
| Geographical location n (%) | |||
| Metropolitan | 647 (67.6%) | 316 (62.3%) | 331 (73.6%)* |
| Non-metropolitan | 310 (32.4%) | 191 (37.7%) | 119 (26.4%)* |
| Race n (%) | |||
| White | 607 (63.4%) | 307 (60.6%) | 300 (66.7%)* |
| African American | 274 (28.6%) | 143 (28.2%) | 131 (29.1%)* |
| Other | 76 (7.9%) | 57 (11.2%) | 19 (4.2%)* |
| Teenage mother (< 20 years) n (%) | 53 (5.5%) | 38 (7.5%) | 15 (3.3%)* |
| Infant characteristics | |||
| Length of stay (days) median, IQR | 2 (2–3) | 2 (2–3) | 2 (2–4) |
| Weight (g) median, IQR | 3100 (2700–3500) | 3200 (2800–3500) | 3000 (2700–3400)* |
| Height (cm) median, IQR | 50 (48.3–52.1) | 50.8 (48.3–52.2) | 49.5 (48.0-51.5)* |
| Head circumference (cm) median, IQR | 33.5 (32.0-33.5) | 33.7 (32.4–34.9) | 33.5 (31.8–34.3)* |
| Gestational age (weeks) median, IQR | 39 (37–39) | 39 (38–40) | 38 (37–39)* |
| Preterm birth (< 37 weeks) n (%) | 152 (15.9%) | 71 (14.0%) | 81 (18.0%) |
| Low birth weight (< 2500 g) n (%) | 180 (18.8%) | 91 (17.9%) | 89 (19.8%) |
| Time period n (%) | |||
| Pre-pandemic | 243 (25.4%) | 127 (25.0%) | 116 (25.8%) |
| Pandemic | 421 (44.0%) | 213 (42.0%) | 208 (46.2%) |
| Post-pandemic | 293 (30.6%) | 167 (32.9%) | 126 (28.0%) |
Continuous variables are presented as medians and IQRs, and categorical variables as frequencies and percentages
IQR: interquartile range, n: number
*: significant difference compared to the MDS- group based on p < 0.05 using Mann-Whitney U and X2 tests
Mothers
There were 8,030 live births during the study period and 957 neonates had an MDS. Thus, a total of 957 infant/mother dyads were included in the final analysis. Table 1 presents the maternal and infant characteristics grouped by the MDS results. Approximately 29.5% of the mothers reported smoking during pregnancy and ~ 40.5% of mothers with MDS + infants smoked during pregnancy. The number of cigarettes smoked by mothers with MDS + babies was greater than that smoked by those with MDS- babies. Among the MDS + mothers, more than 73% came from metropolitan areas, and most (~ 67% of MDS+) were white, whereas black mothers constituted ~ 29% of the racial distribution. Interestingly, only 3.3% of the MDS + mothers were teenagers, whereas 7.5% of the MDS- mothers were teenagers.
Infants
A total of 47% (N = 450) of the infants tested positive for at least one substance. Among the MDS + infants, nearly 18% had preterm birth, whereas 14% had a negative MDS. Compared with those with negative MDS- results, infants with MDS + results were also slightly but significantly lower in weight, height, head circumference and gestational age (Table 1, lower panel). Interestingly, screening rates increased significantly during the pandemic period (by > 73%) compared with those during the pre-pandemic period, as the detection rate of MDS + babies (by > 79%). The rates, however, decreased during the post-pandemic period.
Characteristics of MDS + Mother/Infant dyads exposed to different substances of abuse (Table 2)
Table 2.
Maternal and newborn characteristics grouped by Meconium Drug Screen + result
| Characteristic | THC positive | Amphetamines positive | Opioids positive | Positive for more than one substance |
|---|---|---|---|---|
| Total n (%) | 289 / 450 (64.2%) | 50 / 450 (11.1%) | 30 / 450 (6.7%) | 80 / 450 (17.8%) |
| Mother characteristics | ||||
| Age (years) median, IQR | 26 (23–30) | 31 (28–34)* | 31 (27–35)* | 30 (28 − 24)* |
| Smoking during pregnancy indicated n (%) | ||||
| Yes | 76 (29.3%)* | 31 (72.1%)* | 12 (44.4%)* | 41 (60.3%)* |
| No | 183 (70.7%)* | 12 (27.9%)* | 15 (55.6%)* | 27 (39.7%)* |
| Number of cigarettes median, IQR | 0 (0–0) | 4 (0–10)* | 0 (0-4.3)* | 0.5 (0-9.5)* |
| Number of pregnancies median, IQR | 3 (2–4) | 4 (3–7)* | 4 (2–5) | 4 (3–6)* |
| Number of live children median, IQR | 1 (0–2) | 2 (1.5-4)* | 2 (1-3.3) | 2 (1–4)* |
| Geographical location n (%) | ||||
| Metropolitan | 222 (76.8%)* | 29 (58.0%) | 21 (70.0%) | 58 (72.5%) |
| Non-metropolitan | 67 (23.2%)* | 21 (42.0%) | 9 (30.0%) | 22 (27.5%) |
| Race n (%) | ||||
| White | 173 (59.9%)* | 44 (88.0%)* | 24 (80.0%) | 58 (72.5%) |
| African American | 106 (36.7%)* | 4 (8.0%)* | 4 (13.3%) | 17 (21.2%) |
| Other | 10 (3.5%)* | 2 (4.0%) | 2 (6.7%) | 5 (6.2%) |
| Teenage mother (< 20 years) n (%) | 13 (4.5%)* | 1 (2.0%) | 1 (3.3%) | 0 (0%) |
| Infant characteristics | ||||
| Length of stay (days) median, IQR | 2 (2–3)* | 4 (2-9.75)* | 3.5 (2-11.8)* | 3 (2–6)* |
| Weight (g) median, IQR | 3000 (2700–3400)* | 3000 (2500–3400) | 3100 (2700–3500) | 3000 (2700–3400) |
| Height (cm) median, IQR | 50 (48.3–51.4)* | 49.5 (46.1–50.8)* | 49.5 (47.6–52.7) | 49.5 (47–52) |
| Head circumference (cm) median, IQR | 33.5 (31.8–34.5)* | 33 (31.0-33.7)* | 33.5 (31.9–35.0) | 33.5 (32.4–34.3) |
| Gestational age (weeks) median, IQR | 39 (37–39) | 38 (36–39)* | 38 (36–39)* | 38 (37–39) |
| Preterm birth (< 37 weeks) n (%) | 42 (14.5%) | 14 (28.0%)* | 10 (33.3%)* | 15 (18.8%) |
| Low birth weight (< 2500 g) n (%) | 51 (17.6%) | 16 (32.0%) | 7 (23.3%) | 15 (18.8%) |
| Time period n (%) | ||||
| Pre-pandemic | 60 (20.8%) | 17 (34.0%)* | 12 (40.0%) | 26 (32.5%) |
| Pandemic | 131 (45.3%) | 26 (52.0%)* | 14 (46.7%) | 37 (46.2%) |
| Post-pandemic | 98 (33.9%) | 7 (14.0%)* | 4 (13.3%) | 17 (21.2%) |
Continuous variables are presented as medians and IQRs, and categorical variables as frequencies and percentages
IQR: interquartile range, n: number
*: significant difference compared to the MDS- group based on p < 0.05 using Mann-Whitney U and X2 tests
Mothers. Among the MDS + babies (and therefore their mothers), 64.2%, 11.1% and 6.7% were positive for THC, amphetamines and opioids, respectively (Table 2, upper panel). Nearly 18% were positive for a combination of more than one substance. In terms of age distribution, THC + mothers were significantly younger (median = 26), whereas Opioids + and Amphetamine + mothers were significantly older (median = 31) than the MDS- mothers were (median = 27). Notably, the smoking rate was the highest among amphetamine + mothers, nearly 72% of whom smoked during pregnancy at a median rate of 4 cigarettes per day. THC was the most common drug found in MDS + babies in both metropolitan and nonmetropolitan areas and among all races. However, among the amphetamine + and opioid + mothers, nearly 88% and 80% were white, respectively.
Infants. The LOS was significantly longer in all MDS + infants but was especially longer in amphetamine+ (median = 4 days) and opioid+ (median = 3.5 days) babies compared to that in MDS- babies (median = 2 days). The median BW, height and HC were significantly lower in THC+ (median = 3000 gm, 50 cm and 33.5 cm respectively) and amphetamine+ (median = 3000 gm, 49.5 cm and 33 cm respectively) babies compared to that in the MDS- group (median = 3200 gm, 50.8 cm and 33.7 cm respectively). The preterm birth rate of the amphetamine+ (28%) and opioid+ (33.3%) babies was nearly double that of the MDS- group (14%). Gestational age was also slightly but significantly lower in the amphetamine+ (median = 38 weeks) and opioid+ (median = 38 weeks) babies that in the MDS- (median = 39 weeks) babies. Amphetamine + MDS screening rates were significantly higher in the pre-pandemic (34%) and pandemic (52%) period but significantly lower in the post-pandemic period (14%), that in the MDS- group (25%, 42% and 32.9% for pre-, pandemic and post-pandemic periods, respectively).
Changes in meconium drug screening rates and outcomes during the study period
We also assessed the changes in screening rates (# of screenings/# of births), MDS positive rates (# of positives/# of births) and adjusted MDS positive rates (adjusted # of positives/# of births) over the entire study period as well as over the pre-, during- and post- pandemic periods. All three rates increased throughout the entire study period (Fig. 1) with rates of change of + 0.002%, + 0.001% and + 0.0001% per month, respectively. The adjusted MDS positive rates (AMPRs) increased significantly over the study period (p-value < 0.001) from 6.8% in January 2018 to 7.4% in April 2023. AMPRs increased steadily during the pre-pandemic and pandemic periods with overall growth rates of 5.32% and 8.31%, respectively (Table 3). However, in the post-pandemic period, the growth rate decreased to -1.55%.
Fig. 1.
Monthly meconium drug screening (MDS) rates (# of screenings/# of births), MDS positive rates (# of positives/# of births), and adjusted MDS positive rates (AMPRs) (adjusted # of positives/# of births) over the entire study period colored by pre-, during, and post COVID 19 pandemic time periods. The trend lines and 95% confidence bands show an overall increasing trend in all three measures over the study period with rates of + 0.002%, + 0.001%, and + 0.0001% per month, respectively. The AMPR had an increasing rate of change during the pre-pandemic period and pandemic periods (rates of + 0.0001% and + 0.0002% per month, respectively), which then decreased to + 0.0001% rate during the post pandemic period
Table 3.
Growth rates and rates of change (slope) of the adjusted monthly positive rate for different substances across the study period
| Substance | Time period | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Pre-pandemic | Pandemic | Post-pandemic | |||||
| Growth rate | Rate of change | Growth rate | Rate of change | Growth rate | Rate of change | Growth rate | Rate of change | |
| Any | 0.1267 | + 0.0001%* | 0.0532 | + 0.0002%* | 0.0851 | + 0.0001%* | -0.0155 | + 0.0001%* |
| THC | 4.7413 | + 0.0922%* | 1.1361 | + 0.0406%* | 0.5697 | + 0.0304%* | 0.0172 | + 0.1178%* |
| Amphetamines | -0.0664 | -0.0069%* | 0.0155 | + 0.0046% | 0.1464 | + 0.0217%* | -0.5038 | -0.0663%* |
| Opioids | 0.0813 | -0.0055%* | -0.2736 | -0.0127% | 0.2207 | + 0.0022% | -0.1401 | -0.0046% |
*: significant rate of change according to the OLS regression results using p < 0.05
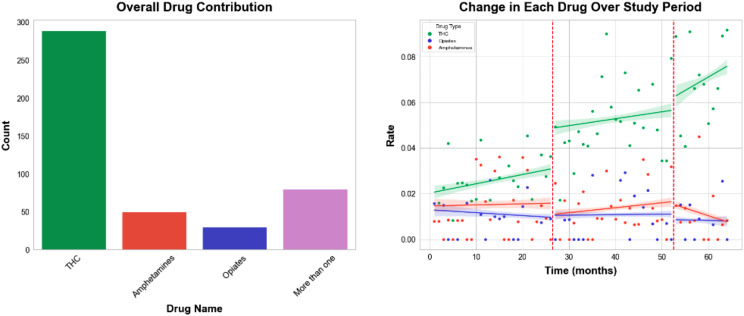
The rates of THC + increased considerably throughout the pre-pandemic, pandemic, and post-pandemic periods, with the highest rate of change observed during the post-pandemic period (Fig. 2 and Table 3). Amphetamines + had a relatively modest increasing rate during the pre-pandemic and pandemic periods but a decreasing rate in the post-pandemic period. Opioids + rates, on the contrary, had modestly decreasing trends during the pre-pandemic and post-pandemic periods, but increased slightly during the pandemic.
Fig. 2.
(left) Bar chart of the drug contributions to the Meconium Drug Screen (MDS) + tests. (right) Monthly positive rates for the three MDS drug results of interest: THC, amphetamines, and opioids. The vertical red lines indicate the start of the pandemic period and the post pandemic period. The trend lines and 95% confidence bands show an increasing trend in THC + rates over the entire study period (rate + 0.092% per month, p-value < 0.001, growth rate 473.13%), and overall decreasing rates for amphetamines and opioids (rates − 0.007%, -0.006% per month; p-values 0.002, and 0.002; growth rates − 6.64%, and 8.13%, respectively)
Interestingly, when we examined at the rate of THC usage through the lens of medical marijuana dispensaries, we observed that the rate of change for THC was not significant before the first dispensary opened in Arkansas, but then steadily increased at a rate of + 0.1% per month after the first dispensary was opened in May of 2019 (Fig. 3).
Fig. 3.
The THC + rate before the first medical marijuana dispensary opened in Arkansas (green) and after the opening of the first dispensary in May 2019 (blue). Trend lines and 95% confidence bands show a constant THC + rate pre-dispensary (p-value 0.91) and a significant increase after the first dispensary opened (rate + 0.100% per month, p-value < 0.001)
Maternal characteristics as risk factors for MDS + babies
Logistic regression analysis with “Positive for at least one substance” as the outcome, revealed that neonates whose mothers indicated smoking cigarettes during pregnancy were more than twice as likely to have a positive MDS than those who did not smoke (OR 2.39 (95% CI: 1.34–3.02)) (Table 4 and 5). In our data, 251 mothers (64%) indicated smoking cigarettes during pregnancy, and more than half of those mothers tested positive for at least one substance in the MDS. Those in the ‘Other’ race category, which included Asians and native Americans/Pacific Islanders, had nearly 66% lower odds of a positive MDS screening test than African Americans did (OR 0.33 (95% CI: 0.14–0.77)), whereas there was no significant racial difference between Whites and African Americans. Neonates whose mothers came from a metropolitan area were twice as likely to have a positive MDS screening than those whose mothers from a non-metropolitan area were (OR 2.01 (1.34–3.02)). We, however, did not have access to variables such as socioeconomic status, prenatal care utilization, or maternal health history in our data which would have made our model more robust.
Table 4.
OLS model results for predicting adjusted MDS positive rates (AMPR) using time as the sole predictor are presented, with coefficients and their corresponding standard errors expressed in scientific notation. The p-value indicates the statistical significance of the predictor, while the R² value represents the proportion of variance in AMPR explained by the model
| Time period | Coefficient | Standard Error | p-value | R 2 |
|---|---|---|---|---|
| Overall | 1.41 × 10− 4 | 3.74 × 10− 6 | < 0.001 | 0.596 |
| Pre-pandemic | 1.51 × 10− 4 | 1.47 × 10− 5 | < 0.001 | 0.307 |
| Pandemic | 1.36 × 10− 4 | 1.49 × 10− 5 | < 0.001 | 0.165 |
| Post-pandemic | 7.78 × 10− 5 | 2.99 × 10− 5 | 0.01 | 0.023 |
Table 5.
Logistic regression results for maternal characteristics with Meconium Drug screen + or meconium drug screen - being the outcome and maternal age, smoking indicated, geographic location, race, and time period being the predictors
| Variable | Odds Ratio (OR) | Lower bound of the 95% CI | Upper bound of the 95% CI |
|---|---|---|---|
| Maternal age | 1.01 | 0.98 | 1.04 |
| Smoking indicated | |||
| Yes | 2.39 | 1.61 | 3.55 |
| Geographic location | |||
| Metropolitan | 2.01 | 1.34 | 3.02 |
| Race | |||
| White | 1.2 | 0.79 | 1.84 |
| Other | 0.33 | 0.14 | 0.77 |
| Time | |||
| Pandemic | 1.2 | 0.79 | 1.84 |
| Post-pandemic | 0.91 | 0.57 | 1.45 |
*95% CI: 95% confidence interval of the odds ratio estimate
Discussion
This study provides a comprehensive analysis of mother/infant dyads who underwent meconium drug screening (MDS) over a five-year period, highlighting the characteristics and outcomes of the MDS-positive (MDS+) and MDS-negative (MDS-) groups. The study period covers the pre-, during and post- COVID-19 pandemic periods. Our findings revealed significant differences in maternal behaviors, demographic factors, and infant outcomes associated with prenatal substance exposure.
Our study included 8,030 live births, with 957 infants receiving MDS during the study period. Of these, nearly half tested positive for at least one substance used. The prevalence of neonatal exposure to maternal substance use significantly increased during the study period and was not affected by the COVID-19 pandemic. Among these, the most prevalent substance detected was THC, accounting for 68.5% of the total screenings. Newborns positive for any drug had a significantly lower birth weight and were born earlier than those with a negative screen. Notably, maternal smoking during pregnancy was greater among MDS + mothers, indicating a strong association between tobacco use and exposure to other substances. MDS + mothers smoked more cigarettes on average, reinforcing the need for targeted smoking cessation interventions in this population.
In this study, the racial composition of mothers whose babies received a MDS, was distributed as follows: 63.4% identified as White and 28.6% as African Americans. This contrasts with the broader demographic trends in Arkansas, as reported by a 2022 study from the Kaiser Family Foundation, which indicates that within the state, 67% of women are White and 16% are African American [23]. These figures suggest a notable disparity between the racial distribution of mothers whose infants had an MDS and the general female population in Arkansas. Within Craighead County, where the majority of the mothers in this study reside, 77.9% of the residents are White and 18% are African American. Research shows that Black, Hispanic, and other people of color are considerably less likely to receive and complete treatment for substance use disorders during pregnancy compared to White women, even when experiencing similar rates of substance use; this disparity is often linked to factors like access to healthcare, socioeconomic status, and potential implicit bias within the medical system [24]. The racial disparities observed in our study may stem from intergenerational trauma [25] and systemic discrimination which have contributed to socioeconomic disadvantage [26] and stress in marginalized communities. Implicit bias and unequal access to healthcare [27] further exacerbate these disparities which underscore the need for culturally competent care and equitable maternal health services.
The current recommendation is to screen all pregnant women for substance use universally via approved questionnaires [28]. However, many medical facilities employ targeted drug testing for women with specific risk factors, leading to unreliable testing, bias, and racial inequities [29–31]. Additionally, a survey of OBGYNs revealed that 79% of respondents frequently screen for substance use, but only 11% use a validated instrument [28, 32]. Therefore, universal drug testing should be considered to obtain accurate data and enable earlier identification of affected mother/infant dyads, resulting in more effective interventions and improved outcomes. However, this practice can pose legal and ethical challenges [28, 31, 33, 34]. Currently, 37 states and the District of Columbia require clinicians to report suspected prenatal drug use [30], and 25 states consider drug use during pregnancy to be child abuse or even assault [34, 35]. These legal implications create ethical dilemmas, as maternal drug screening requires informed consent owing to the potential legal and social consequences of a positive test. This can lead to decreased prenatal care or changes in behavior around the time of delivery, which can affect the outcomes for the dyad [34, 35]. Conversely, Faherty et al. reported that NAS rates were higher in states with punitive policies than in those without [36]. Nonetheless, universal drug screening has been implemented in some areas, resulting in better identification of infants at risk for NAS, polysubstance use, and improved analysis of trends in drug use [37–39].
Infants born to MDS + mothers demonstrated poorer health outcomes, including higher rates of preterm birth; lower measurements of weight, height, head circumference, and gestational age; and longer LOS. These findings align with the literature linking prenatal substance exposure to adverse neonatal outcomes [12, 40, 41]. The increase in screening and positive detection rates during the pandemic highlights the impact of stress and lifestyle changes on substance use behaviors [8]. Infant health outcomes vary by substance. Amphetamine + and opioid + infants had longer hospital stays and higher preterm birth rates. The growth measurements were notably lower in the THC + and amphetamine + infants than in the control infants, suggesting specific developmental impacts of these substances.
Among the commonly detected substances used, THC was the most prevalent, followed by amphetamines and opioids in much smaller proportions as has also been reported in previous literature [42]. A notable proportion, approximately 18%, tested positive for multiple substances. Age variations were evident, with younger mothers predominantly using THC and older mothers using amphetamines and opioids. While this has not been explicitly reported in the published literature, a recent NIDA report may provide insights into the reason for this age variation [43]. According to the NIDA report, marijuana use among young adults reached an all-time high in 2021, reflecting a broader trend of increasing cannabis use among younger populations. Older adults, including older mothers, might be more likely to use prescription drugs, including opioids and amphetamines, which are often related to chronic pain or other medical conditions [44]. In contrast to expectations, teen pregnancy was less common among MDS + mothers than among MDS- mothers.
Many pregnant mothers consider THC, the psychoactive component of cannabis, to be safe and use it to alleviate symptoms such as nausea and anxiety [45]. This perception of safety often stems from anecdotal evidence and the growing acceptance of cannabis in various regions including Arkansas [45]. However, scientific research indicates that exposure to THC in utero can have detrimental effects on infants, leading to developmental issues, cognitive impairments, and an increased risk of behavioral problems as the child grows [7, 46–49]. Medical marijuana was legalized in Arkansas in November 2016 and the first dispensary opened in May 2019, resulting in a significant increase in its prevalence, which is consistent with other reports showing that legalization increases the prevalence of THC usage [50–52]. Despite its perceived benefits for maternal comfort, the potential risks to fetal development highlight the need for caution and more comprehensive education on the use of THC during pregnancy [1].
Our analysis revealed a steady increase in MDS rates and adjusted MDS positive rates (AMPRs) over the study period. Importantly, our data revealed that the AMPR increased during the pre-pandemic, pandemic and post-pandemic periods. However, in the post-pandemic period, although the relationship between time and positive rates persisted as significant, the magnitude of this effect appeared to have diminished. This suggests that while there is an ongoing increase in MDS positive rates, the rate of this increase has slowed down in the post-pandemic landscape. This trend underscores the need for continuous monitoring and intervention strategies during such periods of stress in society. THC + rates increased after the pandemic, potentially influenced by the opening of medical marijuana dispensaries, indicating a need for policy considerations regarding marijuana accessibility and education.
Logistic regression analysis revealed key maternal characteristics associated with positive MDS outcomes. Maternal smoking during pregnancy more than doubled the likelihood of a positive MDS, highlighting the importance of smoking cessation programs. Metropolitan residency also doubled the degree of risk (OR 2.01), suggesting that urban-focused preventive measures may be needed. Racial disparities were evident, with those in the ‘Other’ category having significantly lower odds of positive MDS than African Americans did.
The strengths of our study include the large number of mother/infant dyads included and the use of an AMPR, which allowed us to adjust for the lack of universal drug testing and the monthly variations in screening rates.
However, our study had several limitations. The retrospective design of our study inherently limited our ability to establish causal relationships between maternal substance use and neonatal outcomes. The absence of universal drug screening at the hospital may have introduced potential selection bias which, in turn, may have underestimated the true prevalence of substance use and neonatal exposure, as it excluded asymptomatic or undiagnosed cases. Additionally, the lack of testing for fentanyl—a substance increasingly implicated in the opioid epidemic—represents a critical gap in our analysis. Fentanyl exposure is associated with significant neonatal morbidity, including increased risks of neonatal abstinence syndrome and respiratory complications [8, 53, 54]. It’s omission in our study precluded an understanding of its impact within our cohort. Data on vaping and the differentiation between smoking and vaping usage are also lacking in this study. Further limitations of our study include the absence of critical confounding factors such as socioeconomic status (SES), prenatal care utilization, maternal health history, and environmental exposures, which can significantly influence both maternal substance use and neonatal outcomes. For example, low SES is associated with higher substance use and adverse neonatal outcomes due to financial stress and limited healthcare access [1]. Inadequate prenatal care can exacerbate risks like preterm birth and low birth weight [8], while maternal health history and environmental exposures may contribute to neonatal complications through inflammatory and epigenetic pathways [9, 19]. Future research incorporating these factors is vital for improving predictive models and interventions.
Conclusion
Maternal substance use has increased in NEA over the last 5 years and has been driven mostly by THC usage. This study underscores the critical need for targeted interventions addressing maternal substance use, especially tobacco, and emphasizes the importance of tailored healthcare strategies in metropolitan areas. The significant health disparities observed in MDS + infants call for enhanced prenatal care and postnatal support systems. Continuous surveillance of substance use trends and proactive policy measures are essential to mitigate adverse effects on neonatal health. Further research is warranted to explore the long-term impacts of prenatal substance exposure and to develop comprehensive intervention frameworks.
Author contributions
SB and EG: Conceptualization, methodology, interpretation and writing. JB: Data aggregation, management, statistical analysis and interpretation. All authors reviewed the manuscript.
Funding
This study was not supported by any sponsor or funder.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This investigation was carried out in strict accordance with the Declaration of Helsinki, and it was reviewed and approved by the St. Bernards Institutional Review Board Office which waived the informed consent requirement (Approval number: 221202).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gunn JK, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C, Ehiri JE. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones HE, Fielder A. Neonatal abstinence syndrome: historical perspective, current focus, future directions. Prev Med. 2015;80:12–7. [DOI] [PubMed] [Google Scholar]
- 3.Slotkin T. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285(3):931–45. [PubMed] [Google Scholar]
- 4.Behnke M, Smith VC, Committee on Substance A. Committee on F, Newborn: prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131(3):e1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers JM. Tobacco and pregnancy: overview of exposures and effects. Birth Defects Res C Embryo Today. 2008;84(1):1–15. [DOI] [PubMed] [Google Scholar]
- 6.Varner MW, Silver RM, Rowland Hogue CJ, Willinger M, Parker CB, Thorsten VR, Goldenberg RL, Saade GR, Dudley DJ, Coustan D, et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet Gynecol. 2014;123(1):113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young-Wolff KC, Adams SR, Alexeeff SE, Zhu Y, Chojolan E, Slama NE, Does MB, Silver LD, Ansley D, Castellanos CL et al. Prenatal Cannabis Use and maternal pregnancy outcomes. JAMA Intern Med 2024. [DOI] [PMC free article] [PubMed]
- 8.Lien J, Hayes T, Liu-Smith F, Rana D. Comparing maternal substance use and perinatal outcomes before and during the COVID-19 pandemic. J Perinatology: Official J Calif Perinat Association. 2023;43(5):664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, Krewski D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11(5–6):373–517. [DOI] [PubMed] [Google Scholar]
- 10.Kharbanda EO, Vazquez-Benitez G, Kunin-Batson A, Nordin JD, Olsen A, Romitti PA. Birth and early developmental screening outcomes associated with cannabis exposure during pregnancy. J Perinatology: Official J Calif Perinat Association. 2020;40(3):473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells CLH. Treatment of neonatal abstinence syndrome due to Crystal Methamphetamine: a review of clinical effectiveness and guidelines [Internet]. In. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019. [PubMed] [Google Scholar]
- 12.Patrick SW, Barfield WD, Poindexter BB, Committee On F, Newborn COSU. Prevention: neonatal opioid withdrawal syndrome. Pediatrics 2020, 146(5). [DOI] [PubMed]
- 13.Louw KA. Substance use in pregnancy: the medical challenge. Obstet Med. 2018;11(2):54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horan H, Thompson A, Willard K, Mobley E, McDaniel J, Robertson E, McIntosh S, Albright DL. Social determinants Associated with Substance Use and Treatment seeking in females of Reproductive Age in the United States. J Womens Health (Larchmt). 2024;33(5):584–93. [DOI] [PubMed] [Google Scholar]
- 15.Chacon NC, Walia N, Allen A, Sciancalepore A, Tiong J, Quick R, Mada S, Diaz MA, Rodriguez I. Substance use during COVID-19 pandemic: impact on the underserved communities. Discoveries (Craiova). 2021;9(4):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolinski RS, Walters S, Salisbury-Afshar E, Ouellet LJ, Jenkins WD, Almirol E, Van Ham B, Fletcher S, Johnson C, Schneider JA et al. The impact of the COVID-19 pandemic on Drug Use behaviors, Fentanyl exposure, and Harm Reduction Service support among people who use drugs in rural settings. Int J Environ Res Public Health 2022, 19(4). [DOI] [PMC free article] [PubMed]
- 17.Young-Wolff KC, Ray GT, Alexeeff SE, Adams SR, Does MB, Ansley D, Avalos LA. Rates of prenatal Cannabis Use among pregnant women before and during the COVID-19 pandemic. JAMA. 2021;326(17):1745–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kar P, Tomfohr-Madsen L, Giesbrecht G, Bagshawe M, Lebel C. Alcohol and substance use in pregnancy during the COVID-19 pandemic. Drug Alcohol Depend. 2021;225:108760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson RE, Metz TD, Ward RM, McKnite AM, Enioutina EY, Sherwin CM, Watt KM, Job KM. Drug exposure during pregnancy: current understanding and approaches to measure maternal-fetal drug exposure. Front Pharmacol. 2023;14:1111601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wabuyele SL, Colby JM, McMillin GA. Detection of drug-exposed newborns. Ther Drug Monit. 2018;40(2):166–85. [DOI] [PubMed] [Google Scholar]
- 21.Bar-Oz B, Klein J, Karaskov T, Koren G. Comparison of meconium and neonatal hair analysis for detection of gestational exposure to drugs of abuse. Archives Disease Child Fetal Neonatal Ed. 2003;88(2):F98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rural-Urban Commuting Area Codes. [https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
- 23.Arkansas Women’s Demographic Data. [https://www.kff.org/interactive/womens-health-profiles/arkansas/demographics/
- 24.Gao YA, Drake C, Krans EE, Chen Q, Jarlenski MP. Explaining racial-ethnic disparities in the receipt of medication for opioid use disorder during pregnancy. J Addict Med. 2022;16(6):e356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotero M. A conceptual model of historical trauma: Implications for Public Health Practice and Research. J Health Disparities Res Pract. 2009;1(1):93–108. [Google Scholar]
- 26.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32(1):20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, Eng E, Day SH, Coyne-Beasley T. Implicit Racial/Ethnic Bias among Health Care Professionals and its influence on Health Care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ecker J, Abuhamad A, Hill W, Bailit J, Bateman BT, Berghella V, Blake-Lamb T, Guille C, Landau R, Minkoff H, et al. Substance use disorders in pregnancy: clinical, ethical, and research imperatives of the opioid epidemic: a report of a joint workshop of the Society for Maternal-Fetal Medicine, American College of Obstetricians and gynecologists, and American Society of Addiction Medicine. Am J Obstet Gynecol. 2019;221(1):B5–28. [DOI] [PubMed] [Google Scholar]
- 29.Schultz A, Nandi P, Williamson C, Kowalski L, Chen Y, Breeze J, Evans M. Racial difference in urine drug screening on a labor and delivery unit. Am J Obstet Gynecol 2022, 226(1).
- 30.Schoneich S, Plegue M, Waidley V, McCabe K, Wu J, Chandanabhumma PP, Shetty C, Frank CJ, Oshman L. Incidence of Newborn Drug Testing and variations by birthing parent race and ethnicity before and after recreational Cannabis legalization. JAMA Netw Open. 2023;6(3):e232058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.As Substance Abuse Rises. Hospitals Drug Test Mothers, Newborns [https://www.aacc.org/cln/articles/2016/march/as-substance-abuse-rises-hospitals-drug-test-mothers-newborns
- 32.Ko JY, Tong VT, Haight SC, Terplan M, Stark L, Snead C, Schulkin J. Obstetrician-gynecologists’ practices and attitudes on substance use screening during pregnancy. J Perinatology: Official J Calif Perinat Association. 2020;40(3):422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price HR, Collier AC, Wright TE. Screening pregnant women and their neonates for Illicit Drug Use: consideration of the Integrated Technical, Medical, ethical, legal, and Social issues. Front Pharmacol. 2018;9:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.House SJ, Coker JL, Stowe ZN. Perinatal substance abuse: at the clinical crossroads of policy and practice. Am J Psychiatry. 2016;173(11):1077–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCabe K. Criminalization of care: drug testing pregnant patients. J Health Soc Behav. 2022;63(2):162–76. [DOI] [PubMed] [Google Scholar]
- 36.Faherty LJ, Kranz AM, Russell-Fritch J, Patrick SW, Cantor J, Stein BD. Association of Punitive and Reporting State policies related to substance use in pregnancy with rates of neonatal abstinence syndrome. JAMA Netw Open. 2019;2(11):e1914078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wexelblatt SL, Ward LP, Torok K, Tisdale E, Meinzen-Derr JK, Greenberg JM. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582–6. [DOI] [PubMed] [Google Scholar]
- 38.Yossuck P, Tacker DH. Drug positivity findings from a Universal Umbilical Cord Tissue Drug Analysis Program in Appalachia. J Appl Lab Med. 2021;6(1):285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith BL, Hall ES, McAllister JM, Marcotte MP, Setchell KDR, Megaraj V, Jimenez KL, John Winhusen T, Wexelblatt SL. Rates of substance and polysubstance use through universal maternal testing at the time of delivery. J Perinatology: Official J Calif Perinat Association. 2022;42(8):1026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oei JL, Kingsbury A, Dhawan A, Burns L, Feller JM, Clews S, Falconer J, Abdel-Latif ME. Amphetamines, the pregnant woman and her children: a review. J Perinatology: Official J Calif Perinat Association. 2012;32(10):737–47. [DOI] [PubMed] [Google Scholar]
- 41.Marchand G, Masoud AT, Govindan M, Ware K, King A, Ruther S, Brazil G, Ulibarri H, Parise J, Arroyo A, et al. Birth outcomes of neonates exposed to Marijuana in Utero: a systematic review and Meta-analysis. JAMA Netw Open. 2022;5(1):e2145653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engelgardt P, Krzyzanowski M, Borkowska-Sztachanska M, Wasilewska A, Ciucias M. The impact of lifetime substance use on psychiatric comorbidities and treatment seeking in patients with alcohol use disorders. Sci Rep. 2024;14(1):14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marijuana. and hallucinogen use among young adults reached all time-high in 2021 [https://nida.nih.gov/news-events/news-releases/2022/08/marijuana-and-hallucinogen-use-among-young-adults-reached-all-time-high-in-2021
- 44.Women and drugs: health and social responses [https://www.emcdda.europa.eu/publications/mini-guides/women-and-drugs-h
- 45.Satti MA, Reed EG, Wenker ES, Mitchell SL, Schulkin J, Power ML, Mackeen AD. Factors that shape pregnant women’s perceptions regarding the safety of cannabis use during pregnancy. J Cannabis Res. 2022;4(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tadesse AW, Ayano G, Dachew BA, Betts K, Alati R. Exposure to maternal cannabis use disorder and risk of autism spectrum disorder in offspring: a data linkage cohort study. Psychiatry Res. 2024;337:115971. [DOI] [PubMed] [Google Scholar]
- 47.Ryan KS, Karpf JA, Chan CN, Hagen OL, McFarland TJ, Urian JW, Wang X, Boniface ER, Hakar MH, Terrobias JJD, et al. Prenatal delta-9-tetrahydrocannabinol exposure alters fetal neurodevelopment in rhesus macaques. Sci Rep. 2024;14(1):5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo JO, Shaw B, Robalino S, Ayers CK, Durbin S, Rushkin MC, Olyaei A, Kansagara D, Harrod CS. Cannabis use in pregnancy and neonatal outcomes: a systematic review and Meta-analysis. Cannabis Cannabinoid Res. 2024;9(2):470–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castelli V, Lavanco G, Feo S, D’Amico C, Micale V, Kuchar M, Plescia F, Brancato A, Cannizzaro C. Prenatal exposure to Delta9-Tetrahydrocannabinol affects Hippocampus-related cognitive functions in the adolescent rat offspring: focus on specific markers of neuroplasticity. Pharmaceutics 2023, 15(2). [DOI] [PMC free article] [PubMed]
- 50.Gnofam M, Allshouse AA, Stickrath EH, Metz TD. Impact of Marijuana legalization on prevalence of maternal Marijuana Use and Perinatal outcomes. Am J Perinatol. 2020;37(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navarrete F, Garcia-Gutierrez MS, Gasparyan A, Austrich-Olivares A, Femenia T, Manzanares J. Cannabis Use in pregnant and Breastfeeding women: behavioral and neurobiological consequences. Front Psychiatry. 2020;11:586447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres J, Miller C, Apostol M, Gross J, Maxwell JR. The impact of recreational cannabinoid legalization on utilization in a pregnant population. Front Public Health. 2024;12:1278834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.[Internet] MTBFS. Fentanyl. In. Brentwood (TN). Organization of Teratology Information Specialists (OTIS); 2023.
- 54.Alipio JB, Haga C, Fox ME, Arakawa K, Balaji R, Cramer N, Lobo MK, Keller A. Perinatal fentanyl exposure leads to long-lasting impairments in Somatosensory Circuit function and behavior. J Neurosci. 2021;41(15):3400–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.