Abstract
Objective
Previous studies have emphasized the independent effects of anthropometric indices—including body mass index (BMI), A Body Shape Index (ABSI), waist-to-height ratio (WHtR), body roundness index (BRI), and Conicity Index—on mortality. However, their combined impact, especially in diabetic populations with distinct obesity patterns, has been less frequently explored. This study investigates both the independent and combined effects of these anthropometric indices on mortality in diabetic Americans and compares their individual and combined diagnostic value.
Methods
A nationally representative cohort study was conducted using NHANES data (2005–2018), including 6,572 diabetic adults. Weighted Cox proportional hazards models and restricted cubic splines were applied to evaluate the independent and combined associations of anthropometric indices (BMI, ABSI, WHtR, BRI, and Conicity Index) with all-cause mortality. The weighted receiver operating characteristic (ROC) curve was used to assess the diagnostic value of individual anthropometric indices and their combinations in predicting mortality.
Results
Among all the anthropometric indices, ABSI exhibited the strongest independent association with all-cause mortality, outperforming other measures such as BMI, WHtR, BRI, and Conicity Index. A clear linear relationship was identified, with higher ABSI tertiles consistently linked to an increased risk of mortality. Notably, within each BMI tertile, ABSI effectively differentiated mortality risk, particularly in the highest tertile. Furthermore, ABSI demonstrated the highest predictive performance among individual metrics (weighted AUC = 0.653) and showed further improvement when combined with BMI (weighted AUC = 0.669).
Conclusion
BMI and ABSI collectively provide a comprehensive evaluation of mortality risk in diabetic populations, capturing the synergistic effects of general and central obesity. These findings highlight the importance of integrating BMI and ABSI into risk assessments to identify high-risk individuals and guide targeted interventions for reducing mortality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-025-01614-x.
Keywords: Diabetes, All-cause mortality, Central obesity, A body shape index, Body mass index
Introduction
In 2021, an estimated 529 million people worldwide were living with diabetes, including approximately 485 million individuals aged 20 to 79. Diabetes contributed to 37.8 million years of life lost (YLLs) due to premature mortality and 41.4 million years lost to disability (YLDs), resulting in a total of 79.2 million disability-adjusted life years (DALYs). By 2050, the global population with diabetes is projected to reach 1.31 billion, with roughly 49.6% of this increase attributed to rising obesity trends [1]. Although being overweight or obese is a major risk factor for the development of diabetes, emerging evidence suggests that overweight or obese individuals may have a lower mortality rate compared to those of normal weight. This counterintuitive phenomenon is known as the “obesity paradox.” When body mass index (BMI) is used as an indicator of obesity, the relationship between obesity and mortality among individuals with diabetes remains debated. Some studies have shown a positive linear association between BMI and mortality [2–3], while others have reported negative associations [4–9] or even U-shaped relationships [10–11]. Given the “obesity paradox” observed in patients with diabetes, relying solely on traditional indicators like BMI may not provide a comprehensive assessment of their prognosis.
Recently, additional anthropometric indices related to central obesity have been proposed to improve the assessment of the relationship between obesity and the prognosis of chronic diseases. These indices include A Body Shape Index (ABSI), waist-to-height ratio (WHtR), body roundness index (BRI), and Conicity Index, which provide simple and non-invasive assessment methods that can be easily implemented in settings with limited access to advanced technology. This approach facilitates early screening and monitoring, especially in resource-limited communities or primary healthcare settings, enabling the timely identification of high-risk individuals to help prevent the onset and progression of disease. Studies have confirmed associations between ABSI [12–15], WHtR [12–13], and BRI [12] with all-cause mortality in individuals with diabetes, while links between the Conicity Index and all-cause mortality have been identified in other populations [16–17]. ABSI, developed by Krakauer et al., is a body shape metric that adjusts waist circumference (WC) for height and weight [9]. It is positively correlated with visceral obesity and is independent of BMI in patients with diabetes [18]. Previous studies have shown that, among individuals with diabetes, ABSI has a stronger association with mortality risk compared to other central obesity measures, potentially resolving the “obesity paradox” [12–15]. The synergistic effects of ABSI and BMI have also been preliminarily explored. In the general population, the combination of ABSI and BMI provides better mortality risk stratification than the combination of BMI with other abdominal obesity metrics [9, 19]. Similarly, this combined advantage of BMI and ABSI has also been preliminarily observed in diabetic populations [12].
Previous studies have examined the effects of anthropometric indices on mortality, focusing primarily on their independent impacts while often overlooking their combined effects. BMI, a commonly used measure of overall obesity, fails to account for body fat distribution—a critical factor in obesity-related health risks. Indicators of central obesity, such as ABSI, which reflect abdominal fat accumulation, provide complementary insights. Despite their importance, the combined effect of BMI with ABSI and other central obesity indices, particularly in diabetic populations with distinct obesity patterns, remains underexplored. This study aims to address this gap by investigating both the independent associations of various anthropometric indices (ABSI, BMI, WHtR, BRI, and Conicity Index) with all-cause mortality and the combined impact of BMI with these central obesity indices in a cohort of American adults with diabetes. Furthermore, it compares their individual and combined diagnostic value for predicting mortality risk. This research provides new insights into obesity-related mortality risks, contributing to a deeper understanding of diabetes epidemiology.
Methods
Study design and participants
The National Health and Nutrition Examination Survey (NHANES) is a comprehensive research initiative aimed at assessing the health and nutritional status of adults and children in the United States. The study protocols were approved by the Research Ethics Review Board of the National Center for Health Statistics, and written informed consent was obtained from all participants to ensure their rights were protected. Data were gathered from seven survey cycles conducted between 2005 and 2018, accessible through the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). The survey initially included 39,749 participants aged 20 years or older. For this study, the target population was individuals diagnosed with diabetes, totaling 7,445 individuals, who were identified based on the diagnostic criteria established by the American Diabetes Association (ADA). Specifically, diabetes was defined by the presence of any of the following: a self-reported diagnosis of diabetes, the use of insulin or oral hypoglycemic medications, fasting blood glucose (FBG) levels of ≥ 126 mg/dL, or HbA1c levels of ≥ 6.5%. From this target group, individuals with missing data on key variables—WC, height, weight, or survival status—were excluded to ensure data completeness and reliability. After these exclusions, a total of 6,572 participants were retained for the final analysis. The selection process was summarized in the flowchart provided in Supplementary Material Figure S1.
Evaluation of variables
In this study, the selected anthropometric indicators and the calculation formula are as follows: BMI = weight (kg) / height (cm) 2. WHtR = WC (cm) / height (cm). BRI = 364.2-365.5×√1-[(WC (cm)/2Π)/(0.5×height (cm))]2 [20]. Conicity Index = WC (cm) / (0.109×√weight (kg)/height (m)) [21]. ABSI = WC (cm)/(BMI (kg/m2)2/3×height (cm)1/2 [9]. The methods for measuring weight, height, and WC are detailed on the NHANES website.
Evaluation of covariates
Based on previous studies [14, 22–23], we selected mortality risk factors and variables suspected to be confounders as covariates. Demographic and health-related information—including gender, age, education level, marital status, income-to-poverty ratio, systolic blood pressure (SBP), smoking habits, alcohol use, drug use, and disease status—was collected through household interviews conducted by NHANES. Current smoking was defined as having smoked at least 100 cigarettes in a lifetime and being a current smoker [24], while current drinking was classified as alcohol consumption more than once per month in the past year [24]. Hypertension was identified by a self-reported diagnosis, the use of antihypertensive medication, or measured SBP ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg. Chronic kidney disease (CKD) was diagnosed through self-reported physician assessments, an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m², or a urinary albumin-to-creatinine ratio (UACR) ≥ 30 mg/g [25]. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [26]. Chronic heart failure (CHF), coronary heart disease (CHD), stroke, and cancer were based on self-reported diagnoses. Clinical indicators—including white blood cell (WBC) count, red blood cell (RBC) count, platelet (PLT) count, hemoglobin, serum albumin, alanine aminotransferase (ALT), aspartate transaminase (AST), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and HbA1c—were measured in the NHANES laboratory. To improve the accuracy of the results, missing data were imputed using both the template method (R package ‘VIM’) and multiple imputation (R package ‘mice’).
Mortality assessment
Mortality data were sourced from the National Death Index (NDI) records maintained by the National Center for Health Statistics, with updates available through December 31, 2019. The primary outcome of this study was all-cause mortality, with causes of death categorized based on the International Statistical Classification of Diseases, 10th Revision (ICD-10). All-cause mortality included deaths from various causes such as heart disease (codes 054–068), malignant neoplasms (codes 019–043), accidents (codes 112–123), cerebrovascular diseases (code 070), diabetes mellitus (code 046), and other causes. The follow-up period was calculated from the date of the initial interview to the date of death or December 31, 2019, whichever occurred first.
Statistical analysis
The analysis was performed using RStudio, with statistical significance set at a two-sided P-value of < 0.05. To account for NHANES’ complex sampling design, sample weights were applied, with adjustments made for clustering and stratification. Continuous variables were expressed as means (standard error (SE)), while categorical variables were presented as frequencies (percentages). We calculated the mortality rates for each group in terms of deaths per 1,000 person-years and provided 95% confidence intervals (CI) to assess the precision of the estimates. Poisson regression analysis was used to evaluate the association between mortality risk and the various anthropometric indices, adjusting for potential confounders including gender, age, education level, marital status, income-to-poverty ratio, alcohol consumption, smoking status, WBC, RBC, PLT, hemoglobin, serum albumin, ALT, AST, TC, HDL-C, LDL-C, HbA1c, SBP, use of diabetic medications, insulin use, hypertension, CKD, CHF, CHD, stroke, and cancer. Kaplan-Meier survival curves were used to assess the time to the first death event, with comparisons made using the log-rank test. To assess the relationship between anthropometric indices and all-cause mortality, weighted Cox proportional hazards regression models were used, with hazard ratios (HR) and 95% CI reported. Three models were constructed. Model 0 did not adjust for any covariate. Model 1 adjusted for age and gender. Model 2 additionally adjusted for education status, marital status, income-to-poverty ratio, alcohol consumption, smoking status, WBC, RBC, PLT, hemoglobin, serum albumin, ALT, AST, TC, HDL-C, LDL-C, HbA1c, SBP, use of diabetic medications, insulin use, as well as hypertension, CKD, CHF, CHD, stroke, and cancer. We conducted a multicollinearity diagnostic analysis to ensure the robustness of our model adjustments. Variance Inflation Factors (VIF) were calculated for all covariates included in the model. A commonly accepted threshold of 5 was used to assess potential multicollinearity. The results showed that all VIF values were below this threshold, indicating that no significant multicollinearity was present in the model. The median of the tertiles of each anthropometric indices was used as a continuous variable to test P for trend. Restricted cubic spline (RCS) analysis was used to investigate the dose-response relationships between anthropometric indices and all-cause mortality. We evaluated the predictive performance of individual and combined indices for all-cause mortality using weighted receiver operating characteristic (ROC) curves. For the combined indices, we utilized logistic regression to generate a composite predictive score, which was analyzed using the pROC package in R to compute the corresponding weighted area under the curve (AUC), as well as to determine optimal thresholds. Differences in weighted AUCs between indices were statistically tested using the roc.test function based on DeLong’s test. To examine the robustness of our study, we performed a sensitivity analysis: participants who died from accidental causes were excluded.
Results
Table 1 presents the characteristics of participants stratified by BMI tertiles (Tertile 1: BMI < 28.28, Tertile 2: BMI 28.28–34.10, Tertile 3: BMI ≥ 34.10) and survival status (Non-death vs. Death). A higher proportion of females was observed in higher BMI categories, and the proportion of females was lower among deceased participants compared to survivors. Older participants were more prevalent among deceased individuals across all BMI tertiles, with mean age decreasing as BMI tertile increased. Notably, the mean age was significantly higher among those who died. Lower educational attainment and widowed/divorced/separated marital status were more common among deceased participants across all BMI tertiles. Higher BMI tertiles were associated with increased weight, WC, BMI, WHtR, BRI, and ABSI. Among deceased participants, WC, WHtR, BRI, Conicity Index, and ABSI were higher compared to survivors within the same BMI tertile, whereas weight and BMI were lower. Deceased participants exhibited lower RBC and PLT counts, while higher BMI tertiles were associated with increased WBC, RBC, and PLT counts. Hemoglobin and serum albumin levels were consistently lower among deceased participants, with serum albumin levels gradually decreasing across BMI tertiles. ALT and AST levels peaked in BMI Tertile 3, with AST levels significantly higher in deceased participants compared to survivors. HDL-C levels declined as BMI tertile increased, whereas LDL-C and HbA1c levels rose with higher BMI tertiles. TC and LDL-C levels were generally lower among deceased participants across all BMI tertiles. Conversely, SBP was consistently higher among deceased individuals in all BMI tertiles. The prevalence of hypertension, CKD, CHD, and cancer was markedly higher among deceased individuals across all BMI tertiles. Stroke and CHF were also more common among deceased participants, particularly in the highest BMI tertile.
Table 1.
Participant characteristics stratified by BMI tertiles and survival status
| Characteristics | BMI Tertile 1 (< 28.28) | BMI Tertile 2 (28.28 ~ 34.10) | BMI Tertile 3 (≥ 34.10) | |||
|---|---|---|---|---|---|---|
| Non-death | Death | Non-death | Death | Non-death | Death | |
| Gender, % | ||||||
| Male, % | 958 (57.6%) | 310 (58.9%) | 963 (53.5%) | 234 (61.1%) | 735 (39.2%) | 174 (54.0%)* |
| Female, % | 705 (42.4%) | 216 (41.1%) | 838 (46.5%) | 149 (38.9%) | 1142 (60.8%) | 148 (46.0%)* |
| Age, years | 58.00 (0.54) | 71.83 (0.60)* | 57.92 (0.40) | 70.88 (0.66)* | 53.13 (0.36) | 62.84 (0.767)* |
| Education | ||||||
| Under vocational school, % | 931 (56.0%) | 360 (68.4%)* | 989 (54.9%) | 253 (66.1%)* | 998 (53.2%) | 207 (64.3%) |
| Vocational schools and above, % | 732 (44.0%) | 166 (31.6%)* | 812 (45.1%) | 130 (33.9%)* | 879 (46.8%) | 115 (35.7%) |
| Marital status | ||||||
| Married or living with partner, % | 1089 (65.5%) | 244 (46.4%)* | 1143 (63.5%) | 215 (56.1%)* | 1094 (58.3%) | 164 (50.9%)* |
| Widowed, divorced, separated, or single, % | 574 (34.5%) | 282 (53.6%)* | 658 (36.5%) | 168 (43.9%)* | 783 (41.7%) | 158 (49.1%)* |
| Income-to-poverty ratio | 2.90 (0.06) | 2.26 (0.078)* | 2.93 (0.07) | 2.19 (0.10)* | 2.81 (0.05) | 2.56 (0.14)* |
| Weight, kg | 70.66 (0.43) | 67.93 (0.58)* | 88.22 (0.39) | 87.32 (0.78)* | 113.09 (0.65) | 112.98 (1.23)* |
| Height, cm | 167.30 (0.41) | 165.49 (0.47)* | 168.15 (0.35) | 167.67 (0.70)* | 167.04 (0.35) | 167.18 (0.73)* |
| WC, cm | 93.15 (0.31) | 94.40 (0.47) * | 107.71 (0.27) | 110.49 (0.58)* | 125.40 (0.40) | 127.43 (0.77)* |
| BMI, kg/m2 | 25.11 (0.08) | 24.70 (0.15)* | 31.09 (0.07) | 30.95 (0.08)* | 40.39 (0.16) | 40.37 (0.43)* |
| WHtR | 0.558 (0.002) | 0.571 (0.003)* | 0.642 (0.002) | 0.660 (0.003)* | 0.752 (0.002) | 0.763 (0.005)* |
| BRI | 4.56 (0.04) | 4.85 (0.06)* | 6.44 (0.04) | 6.89 (0.08)* | 9.41 (0.06) | 9.74 (0.14)* |
| Conicity Index | 131.91 (0.27) | 135.54 (0.37)* | 136.74 (0.28) | 140.80 (0.61)* | 140.32 (0.24) | 142.72 (0.51)* |
| ABSI | 0.841 (0.002) | 0.866 (0.002)* | 0.841 (0.002) | 0.866 (0.004)* | 0.827 (0.001) | 0.841 (0.003)* |
| WBC, 1000 cells/uL | 7.19 (0.08) | 7.83 (0.16)* | 7.72 (0.08) | 7.66 (0.14)* | 8.41 (0.07) | 8.65 (0.27)* |
| RBC, million cells/uL | 4.62 (0.02) | 4.36 (0.03)* | 4.73 (0.02) | 4.46 (0.03)* | 4.77 (0.02) | 4.62 (0.04)* |
| PLT, 1000 cells/uL | 236.98 (2.19) | 225.29 (4.09)* | 240.19 (2.38) | 228.42 (4.34)* | 258.59 (3.03) | 247.51 (7.13)* |
| Hemoglobin, g/dL | 14.04 (0.06) | 13.44 (0.09)* | 14.20 (0.05) | 13.11 (0.66)* | 14.07 (0.05) | 13.94 (0.12)* |
| Serium albumin, g/L | 42.44 (0.12) | 41.23 (0.20)* | 42.03 (0.12) | 40.48 (0.20)* | 40.45 (0.12) | 39.44 (0.27)* |
| ALT, U/L | 25.01 (0.53) | 23.17 (0.70)* | 27.95 (0.57) | 22.92 (0.72)* | 30.77 (0.76) | 30.09 (4.08)* |
| AST, U/L | 25.27 (0.33) | 27.57 (0.72)* | 26.13 (0.43) | 25.58 (0.70)* | 27.58 (0.66) | 29.81 (1.91)* |
| TC, mmol/L | 4.87 (0.04) | 4.60 (0.06)* | 4.88 (0.04) | 4.61 (0.08)* | 4.84 (0.04) | 4.74 (0.09)* |
| HDL-C, mmol/L | 1.36 (0.01) | 1.35 (0.02)* | 1.19 (0.01) | 1.20 (0.03)* | 1.17 (0.01) | 1.16 (0.03)* |
| LDL-C, mmol/L | 2.76 (0.03) | 2.53 (0.05)* | 2.79 (0.03) | 2.62 (0.09)* | 2.83 (0.03) | 2.77 (0.10)* |
| HbA1c, % | 6.82 (0.05) | 6.90 (0.07)* | 7.09 (0.05) | 7.01 (0.09)* | 7.12 (0.04) | 7.24 (0.13)* |
| SBP, mmHg | 123.67 (0.85) | 127.60 (2.05)* | 123.74 (0.83) | 126.04 (1.74)* | 122.25 (0.84) | 125.47 (1.94)* |
| Current drinking, % | 119 (7.2%) | 18 (3.4%)* | 128 (7.1%) | 10 (2.6%)* | 149 (7.9%) | 6 (1.9%)* |
| Current smoking, % | 306 (18.4%) | 112 (21.3%) | 284 (15.8%) | 56 (14.6%) | 290 (15.5%) | 63 (19.6%)* |
| Using diabetic pills, % | 1120 (67.3%) | 350 (66.5%)* | 1241 (68.9%) | 273 (71.3%) | 1324 (70.5%) | 231 (71.7%) |
| Using insulin, % | 237 (14.3%) | 109 (20.7%)* | 303 (16.8%) | 100 (26.1%)* | 364 (19.4%) | 112 (34.8%)* |
| Hypertension, % | 933 (56.1%) | 389 (74.0%)* | 1207 (67.0%) | 315 (82.2%)* | 1343 (71.6%) | 265 (82.3%)* |
| CKD, % | 548 (33.0%) | 341 (64.8%)* | 616 (34.2%) | 270 (70.5%)* | 677 (36.1%) | 207 (64.3%)* |
| CHF, % | 61 (3.7%) | 75 (14.3%)* | 88 (4.9%) | 79 (20.6%)* | 143 (7.6%) | 74 (23.0%)* |
| CHD, % | 97 (5.8%) | 88 (16.7%)* | 143 (7.9%) | 94 (24.5%)* | 144 (7.7%) | 54 (16.8%)* |
| Stroke, % | 91 (5.5%) | 67 (12.7%)* | 117 (6.5%) | 64 (16.7%)* | 116 (6.2%) | 52 (16.1%)* |
| Cancer, % | 184 (11.1%) | 127 (24.1%)* | 234 (13.0%) | 88 (23.0%)* | 189 (10.1%) | 70 (21.7%)* |
*Compared to Non-death within the same BMI tertile group, the Death group exhibits a statistically significant difference (P < 0.05), with adjustments made for sample weights. WC Waist circumference, BMI Body mass index, WHtR Waist-to-height ratio, BRI Body roundness index, ABSI A body shape index, WBC White blood cell, RBC Red blood cell, PLT Platelet, ALT Alanine aminotransferase, AST Aspartate transaminase, TC Total cholesterol, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, SBP Systolic blood pressure, CKD Chronic kidney disease, CHF Chronic heart failure, CHD Coronary heart disease
Table 2 presents the mortality rates for different variables, including BMI, WHtR, BRI, Conicity Index, and ABSI, along with death rates (per 1,000 person-years) and their corresponding 95% CI. The analysis was adjusted for potential confounders, including gender, age, education level, marital status, income-to-poverty ratio, alcohol consumption, smoking status, blood parameters (such as WBC, RBC, PLT, hemoglobin), serum albumin, ALT, AST, TC, HDL-C, LDL-C, HbA1c, SBP, use of diabetic medications, insulin use, as well as the presence of hypertension, CKD, CHF, CHD, stroke, and cancer. Mortality rates decreased progressively across BMI tertiles. The highest mortality rate was observed in the first tertile (BMI < 28.28) at 3.1 per 1,000 person-years (95% CI: 2.8–3.3), while the lowest was in the third tertile (BMI ≥ 34.10) at 1.7 per 1,000 person-years (95% CI: 1.6–1.9). The differences were statistically significant (P < 0.001). The highest mortality rate for WHtR was in the first tertile (WHtR < 0.61) at 2.5 per 1,000 person-years (95% CI: 2.2–2.7), while the second and third tertiles showed identical mortality rates of 2.2 per 1,000 person-years (95% CI: 2.0–2.4). The differences were statistically significant (P = 0.013). Similar to WHtR, the first tertile (BRI < 5.65) had the highest mortality rate at 2.5 per 1,000 person-years (95% CI: 2.2–2.7), while the second and third tertiles both had mortality rates of 2.2 per 1,000 person-years (95% CI: 2.0–2.4). The differences were statistically significant (P = 0.013). The first tertile (Conicity Index < 133.54) had the lowest mortality rate at 1.6 per 1,000 person-years (95% CI: 1.4–1.8), while the third tertile (≥ 140.50) had the highest rate at 3.2 per 1,000 person-years (95% CI: 2.9–3.5). However, the differences were not statistically significant (P = 0.103). Mortality rates showed a clear upward trend across ABSI tertiles. The lowest mortality rate was in the first tertile (ABSI < 0.82) at 1.2 per 1,000 person-years (95% CI: 1.0–1.4), while the highest was in the third tertile (≥ 0.86) at 4.0 per 1,000 person-years (95% CI: 3.7–4.3). The differences were highly statistically significant (P < 0.001). Supplementary Material Table S1 presents mortality rates stratified by BMI tertiles and further subdivided by tertiles of other anthropometric indices (WHtR, BRI, Conicity Index, and ABSI). In BMI Tertile 3 (≥ 34.10), the first ABSI tertile (< 0.82) had the lowest mortality rate at 1.0 per 1,000 person-years (95% CI: 0.8–1.3), while the third ABSI tertile (≥ 0.86) had the highest at 2.9 per 1,000 person-years (95% CI: 2.5–3.3), showing a significant upward trend in mortality with increasing ABSI tertiles (P = 0.004).
Table 2.
Mortality Rates Stratified by Anthropometric Indices (BMI, WHtR, BRI, Conicity Index, ABSI) and Tertiles
| Anthropometric Indices | Total Number | Number of deaths | Death rate per 1000 person years | Death rate per 1000 person years, 95%CI | P value |
|---|---|---|---|---|---|
| BMI | |||||
| Tertile 1 (< 28.28) | 172,069 | 526 | 0.0031 | 0.0028–0.0033 | < 0.001 |
| Tertile 2 (28.28 ~ 34.10) | 183,446 | 383 | 0.0021 | 0.0019–0.0023 | |
| Tertile 3 (≥ 34.10) | 184,010 | 322 | 0.0017 | 0.0016–0.0019 | |
| WHtR | |||||
| Tertile 1 (< 0.61) | 178,906 | 440 | 0.0025 | 0.0022–0.0027 | 0.013 |
| Tertile 2 (0.61 ~ 0.69) | 182,532 | 406 | 0.0022 | 0.0020–0.0024 | |
| Tertile 3 (≥ 0.69) | 178,087 | 385 | 0.0022 | 0.0019–0.0024 | |
| BRI | |||||
| Tertile 1 (< 5.65) | 178,873 | 440 | 0.0025 | 0.0022–0.0027 | 0.013 |
| Tertile 2 (5.65 ~ 7.56) | 182,372 | 406 | 0.0022 | 0.0020–0.0024 | |
| Tertile 3 (≥ 7.56) | 178,280 | 385 | 0.0022 | 0.0019–0.0024 | |
| Conicity index | |||||
| Tertile 1 (< 133.54) | 190,408 | 300 | 0.0016 | 0.0014–0.0018 | 0.103 |
| Tertile 2 (133.54 ~ 140.50) | 179,980 | 390 | 0.0022 | 0.0020–0.0024 | |
| Tertile 3 (≥ 140.50) | 169,137 | 541 | 0.0032 | 0.0029–0.0035 | |
| ABSI | |||||
| Tertile 1 (< 0.82) | 196,027 | 235 | 0.0012 | 0.0010–0.0014 | < 0.001 |
| Tertile 2 (0.82 ~ 0.86) | 181,232 | 343 | 0.0019 | 0.0017–0.0021 | |
| Tertile 3 (≥ 0.86) | 162,266 | 653 | 0.0040 | 0.0037–0.0043 |
BMI Body mass index, WHtR Waist-to-height ratio, BRI Body roundness index, ABSI A body shape index
Adjusted for gender, age, education status, marital status, income-to-poverty ratio, alcohol consumption, smoking status, WBC, RBC, PLT, hemoglobin, serium albumin, ALT, AST, TC, HDL-C, LDL-C, HbA1c, SBP, diabetic pills using, insulin using, hypertension, CKD, CHF, CHD, stroke and cancer
The Kaplan-Meier survival curves illustrate differences in survival probabilities across tertiles for various metrics, including BMI, WHtR, BRI, Conicity Index, and ABSI, as shown in Fig. 1. BMI shows a clear and significant difference (p < 0.0001), with Tertile 1 (lowest BMI) having the poorest survival and Tertile 3 (highest BMI) the best. WHtR and BRI demonstrate no significant differences between tertiles (p = 0.13). The Conicity Index reveals significant differences (p < 0.0001), with Tertile 3 showing the poorest survival. Similarly, ABSI has significant differences (p < 0.0001), where Tertile 3 (highest ABSI) has the lowest survival probability. The number-at-risk tables provide additional insight into participant distribution and follow-up duration for each tertile. Supplementary Material Figure S2 presents Kaplan-Meier survival curves stratified by BMI tertiles and further grouped by tertiles of other anthropometric indices (WHtR, BRI, Conicity Index, and ABSI). Across all BMI tertiles, ABSI tertiles showed significant differences in survival probabilities (P = 0.0001 for BMI Tertile 1, P < 0.0001 for Tertiles 2 and 3). Survival probabilities declined markedly with increasing ABSI tertiles, with the lowest survival observed in Tertile 3.
Fig. 1.
Kaplan-Meier Survival Analysis of All-Cause Mortality BMI Body mass index, WHtR Waist-to-height ratio, BRI Body roundness index, ABSI A body shape index
Figure 2 illustrates the HR 95% CI and trend tests (P for trend) for BMI, WHtR, BRI, Conicity Index, and ABSI across tertiles under three statistical models (Model 0, Model 1, and Model 2). In the fully adjusted model, participants in the higher tertile (Tertile 2) for BMI (HR = 0.70, 95% CI: 0.59–0.83), WHtR (HR = 0.84, 95% CI: 0.70–0.99), BRI (HR = 0 0.84, 95% CI: 0.70–0.99) were negatively associated with all = cause mortality compared to those in the lowest tertile (Tertile 1). Conversely, in the fully adjusted model, participants in the highest tertile (Tertile 3) for ABSI (HR = 1.55, 95% CI: 1.24–1.93) was positively associated with all-cause mortality compared to those in the lowest tertile (Tertile 1). Additionally, the association between Conicity Index and all-cause mortality was not statistically significant in the fully adjusted model. Supplementary Material Table S2 presents weighted Cox proportional hazards regression analysis of mortality stratified by BMI tertiles and further grouped by tertiles of other anthropometric indices (WHtR, BRI, Conicity Index, ABSI). ABSI is significantly associated with all-cause mortality across all BMI tertiles, particularly in the unadjusted and partially adjusted models, with ABSI Tertile 3 in BMI Tertile 3 showing a significant risk increase even in the fully adjusted model.
Fig. 2.
Weighted Cox Proportional Hazards Regression Analysis of Anthropometric Indicators and Their Relationship with All-Cause Mortality BMI Body mass index, WHtR Waist-to-height ratio, BRI Body roundness index, ABSI A body shape index Model 0 did not adjust for any covariate Model 1 adjusted for gender, age Model 2 adjusted for gender, age, education status, marital status, income-to-poverty ratio, alcohol consumption, smoking status, WBC, RBC, PLT, hemoglobin, serium albumin, ALT, AST, TC, HDL-C, LDL-C, HbA1c, SBP, diabetic pills using, insulin using, hypertension, CKD, CHF, CHD, stroke and cancer
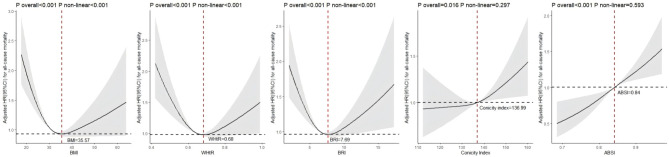
After adjusting for confounding factors such as gender, age, education level, marital status, income-to-poverty ratio, alcohol consumption, smoking status, WBC, RBC, PLT, hemoglobin, serum albumin, ALT, AST, TC, HDL-C, LDL-C, HbA1c, SBP, use of diabetes medications, insulin use, and histories of hypertension, CKD, CHF, CHD, stroke, and cancer, Fig. 3 shows the dose-response relationships between BMI, WHtR, BRI, Conicity Index, and ABSI with all-cause mortality. BMI, WHtR, and BRI exhibit significant U-shaped curves, with the lowest mortality risks observed at BMI = 35.57, WHtR = 0.68, and BRI = 7.69, respectively. Deviations from these optimal values on either side are associated with increased mortality risk (P overall < 0.001, P non-linear < 0.001). The relationship between the Conicity Index and all-cause mortality is overall significant (P overall = 0.016) but shows no significant non-linear trend (P non-linear = 0.297). Mortality risk remains relatively stable at lower Conicity Index values but begins to increase as the Conicity Index exceeds approximately 136.99, suggesting a threshold effect. The association between ABSI and all-cause mortality is highly significant (P overall < 0.001), with no evidence of a non-linear trend (P non-linear = 0.593). A clear dose-response relationship is observed, with mortality risk progressively increasing as ABSI values rise. The reference point for ABSI is approximately 0.84, where the HR equals 1. Supplementary Material Figure S3 illustrates RCS curves for the association between anthropometric indices (WHtR, BRI, Conicity Index, ABSI) and mortality risk, stratified by BMI tertiles. In the third BMI tertile, ABSI demonstrates a significant nonlinear relationship with mortality risk (P non-linear = 0.047), where mortality risk increased sharply at higher ABSI values.
Fig. 3.
RCS Analysis of Anthropometric Indicators and Their Relationship with All-Cause Mortality BMI Body mass index, WHtR Waist-to-height ratio, BRI Body roundness index, ABSI A body shape index, HR Hazard ratios, CI Confidence intervals Adjusted for gender, age, education status, marital status, income-to-poverty ratio, alcohol consumption, smoking status, WBC, RBC, PLT, hemoglobin, serium albumin, ALT, AST, TC, HDL-C, LDL-C, HbA1c, SBP, diabetic pills using, insulin using, hypertension, CKD, CHF, CHD, stroke and cancer
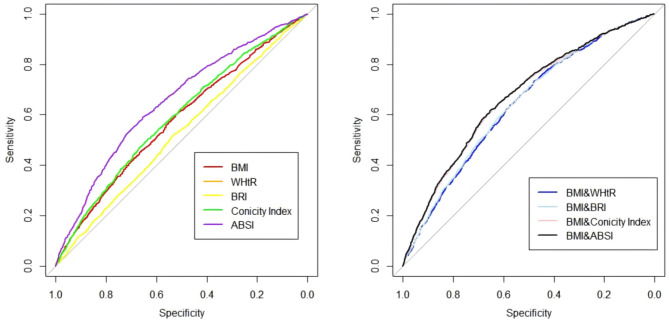
Based on the analysis of Table 3; Fig. 4, ABSI showed the best diagnostic performance among single metrics, with an weighted AUC of 0.653 (95% CI: 0.635–0.670), significantly outperforming other single metrics (BMI, WHtR, BRI, and Conicity Index). Its optimal cutoff value was 0.853, with a sensitivity of 0.591 and a specificity of 0.653. BMI and Conicity Index achieved weighted AUCs of 0.578 and 0.590, respectively, slightly lower than ABSI, while WHtR and BRI had the lowest performance with weighted AUCs of 0.526. Combining BMI with other metrics further improved diagnostic performance, with BMI & ABSI achieving the highest weighted AUC of 0.669 (95% CI: 0.653–0.686), significantly better than other combinations. BMI & Conicity Index followed with an weighted AUC of 0.666, while BMI & WHtR and BMI & BRI achieved weighted AUCs of 0.637 and 0.640, respectively.
Table 3.
Weighted ROC curves for Predicting all-cause Mortality using Anthropometric indicators and their combinations
| Anthropometric Indices | Weighted AUC | Weighted AUC 95% CI | Cutoff value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| BMI | 0.578 | 0.560–0.596 | 31.195 | 0.611 | 0.512 |
| WHtR | 0.526a | 0.508–0.544 | 0.642 | 0.521 | 0.532 |
| BRI | 0.526a | 0.508–0.544 | 6.407 | 0.521 | 0.532 |
| Conicity Index | 0.590bc | 0.573–0.608 | 139.822 | 0.475 | 0.663 |
| ABSI | 0.653abcd | 0.635–0.670 | 0.853 | 0.591 | 0.653 |
| BMI&WHtR | 0.637abcde | 0.620–0.654 | 15.91–51.3 & 0.40–1.01 | 0.732 | 0.480 |
| BMI&BRI | 0.640abcdf | 0.624–0.657 | 15.91–63.4 & 1.49–19.13 | 0.661 | 0.553 |
| BMI&Conicity Index | 0.666abcdefg | 0.650–0.683 | 15.91–48.8 & 122.55-170.41 | 0.657 | 0.609 |
| BMI&ABSI | 0.669abcdefgh | 0.653–0.686 | 15.91–51.3 & 0.81–1.06 | 0.616 | 0.654 |
BMI Body mass index, WHtR Waist-to-height ratio, BRI Body roundness index, ABSI A body shape index, HR Hazard ratios, CI Confidence intervals
a: Significantly different compared to BMI (P < 0.05), b: Significantly different compared to WHtR (P < 0.05), c: Significantly different compared to BRI (P < 0.05), d: Significantly different compared to Conicity Index (P < 0.05), e: Significantly different compared to ABSI (P < 0.05), f: Significantly different compared to the combination of BMI and WHtR (P < 0.05), g: Significantly different compared to the combination of BMI and BRI (P < 0.05), h: Significantly different compared to the combination of BMI and Conicity Index (P < 0.05)
Fig. 4.
Weighted ROC Curves for Predicting All-Cause Mortality Using Anthropometric Indicators and their combinations BMI Body mass index, WHtR Waist-to-height ratio, BRI Body roundness index, ABSI A body shape index
Supplementary Material Table S3 presents the results of the sensitivity analysis conducted after excluding individuals who died from accidental causes (n = 315). ABSI demonstrated a significant association with higher mortality risk, particularly in the third tertile (HR = 1.60, 95% CI: 1.26–2.03, P < 0.001). In the BMI-stratified analysis, individuals with high BMI (≥ 34.20) showed a significant increase in mortality risk associated with the third tertile of ABSI (HR = 2.03, 95% CI: 1.33–3.11, P = 0.001).
Discussion
Using data from NHANES database (2005–2018), we conducted a nationally representative longitudinal cohort study involving 6,572 diabetic adults. This study investigated the independent associations of various indices (BMI, ABSI, WHtR, BRI, Conicity Index) with all-cause mortality and the combined impact of BMI with ABSI and other central obesity indices in a cohort of American adults with diabetes. These results suggest that anthropometric indices are strongly associated with all-cause mortality in diabetes, with ABSI exhibiting a closer correlation than other individual metrics. Combining BMI with ABSI and other central obesity measures further improves diagnostic performance, with the combination of BMI and ABSI showing the highest effectiveness. This study highlights the superior diagnostic value of ABSI and its combination with BMI in predicting all-cause mortality in diabetes, offering valuable insights for improving risk assessment and clinical decision-making.
Previous research has extensively examined the effects of various anthropometric indices on mortality in diabetic populations, yielding inconsistent conclusions and primarily focusing on their independent impacts while overlooking their combined effects. For BMI, some studies have reported a linear positive correlation with mortality [2–3], while others have found negative correlations [4–9] or non-linear (U-shaped) associations [10–11]. Our findings show that BMI exhibited a U-shaped relationship with mortality, with the lowest risk observed in the highest tertile (Tertile 3), supporting the “obesity paradox.” Unlike BMI, ABSI, WHtR, and BRI are surrogate markers of central obesity. In one study with an average follow-up period of 10.2 years, all-cause mortality rates for ABSI, WHtR, and BRI were significantly higher in the fourth quartile compared to the second, and the combination of ABSI and BMI was associated with a higher all-cause mortality risk compared to combinations of BMI with other body metrics [12]. A prospective cohort study conducted in Italy found no evidence of an obesity paradox with WHtR or ABSI and notably identified ABSI as a better predictor of mortality risk associated with central adiposity than WC [13]. Research using the NHANES database revealed a linear positive correlation between ABSI and all-cause mortality [14], a finding similarly observed in the Australian population with diabetes [15]. Among these studies, ABSI stands out as having a particularly strong association with diabetes-related all-cause mortality compared to other central obesity surrogate markers and has been established as a risk factor for mortality in general populations across the U.S., Europe, and Asia [9, 27–29]. The Conicity Index, although not yet studied in diabetic populations, has been identified as an independent risk factor for all-cause mortality among older, non-cancer patients in China and as a predictor of all-cause mortality in patients with CKD [16–17]. To date, no research has compared these indices within the same diabetic cohort. More importantly, no studies have investigated the combined effects of BMI with ABSI and the other three central obesity surrogate markers on all-cause mortality in diabetic patients. Notably, the synergistic effects of ABSI and BMI have been preliminarily explored in the general population, with findings showing that the combination of ABSI and BMI provides better mortality risk stratification than combinations of BMI with other abdominal obesity metrics [9, 19].
We conducted a comprehensive evaluation of multiple anthropometric indices, including BMI, ABSI, WHtR, BRI, and Conicity Index, within the same diabetic cohort to assess their predictive value. Our findings revealed distinct patterns among these indices. As shown in Figs. 2 and 3, WHtR and BRI exhibited slight decreasing trends in mortality rates across tertiles; however, Fig. 4 demonstrated their limited predictive value. The Conicity Index showed a modest increase in mortality risk at higher tertiles, but this association became non-significant after adjusting for potential confounders, as illustrated in Figs. 2 and 3. In contrast, ABSI consistently demonstrated the strongest and most significant linear association with mortality risk. It maintained its significance across all statistical models, even after adjusting for confounders such as demographic factors, clinical parameters, and comorbidities. With a weighted AUC of 0.653, ABSI outperformed all other indices in diagnostic performance, as depicted in Fig. 4. These results align with previous studies, highlighting ABSI as the most prominent anthropometric predictor of mortality, without evidence of the “obesity paradox.” More importantly, our study provides novel insights into the combined effects of BMI with ABSI and the other three central obesity markers—WHtR, BRI, and Conicity Index—on mortality risk, emphasizing their interplay and collective impact. In Figure S2, we observed that ABSI’s third tertile had the lowest survival probabilities across all BMI categories. Consistently, in Table S2, the third tertile of ABSI had the highest mortality risk compared to the lowest tertile across all BMI categories. Sensitivity analyses, excluding accidental deaths (as shown in Table S3), confirmed the robustness of these findings. The highest ABSI tertile was significantly associated with increased mortality risk, and this association was particularly pronounced in individuals with higher BMI. Differences in weighted AUCs between indices were statistically tested in Table 3. For the combined indices, we utilized logistic regression to generate a composite predictive score and analyzed its corresponding weighted AUC using the weighted ROC curve. The results indicated that combining ABSI with BMI further enhanced diagnostic performance, achieving the highest weighted AUC (0.669), which was significantly superior to both individual indices and other combined metrics. This underscores the complementary value of these two measures.Overall, these findings highlight ABSI’s critical role as a robust and reliable predictor of mortality. Whether used independently or in combination with BMI, ABSI offers substantial clinical value in mortality risk assessment, underscoring the need to integrate it with traditional metrics in future research and clinical practice.
The mechanisms underlying the association between the combination of BMI and ABSI and all-cause mortality in individuals with diabetes are complex and multifaceted. BMI is a commonly used metric for assessing general obesity, but it does not differentiate between fat and lean mass or provide insights into fat distribution [30]. ABSI, on the other hand, adjusts waist circumference for height and BMI, offering a more refined assessment of central obesity and visceral fat accumulation, independent of overall body mass [31]. In diabetic populations, the combination of high BMI and high ABSI may signify a synergistic effect of excessive overall fat and central fat accumulation, exacerbating metabolic dysfunction more than either metric alone [32–33]. Visceral fat, a metabolically active tissue, secretes pro-inflammatory cytokines such as interleukin-6 and tumor necrosis factor-alpha, driving systemic inflammation [34]. Chronic inflammation is a critical driver of insulin resistance, a hallmark of diabetes, and accelerates atherosclerosis, increasing cardiovascular risk [35]. Elevated WBC levels observed in the third BMI tertile, particularly among deceased patients compared to survivors, underscore the inflammatory burden. While high BMI reflects greater overall energy reserves, it can mask the detrimental effects of central obesity if fat distribution is not considered [36]. High BMI combined with high ABSI suggests significant abdominal fat concentration, signaling excessive visceral fat. This is particularly hazardous in diabetic patients, where insulin sensitivity is already compromised. Excess visceral fat worsens insulin resistance, impairs glycemic control, and increases the risk of vascular complications such as nephropathy, retinopathy, and macroangiopathy [37]. As demonstrated in our baseline characteristics table (Table 1), the prevalence of hypertension, CKD, and CHD was significantly higher among deceased individuals across all BMI tertiles. Stroke and CHF were also more common among deceased participants, particularly in the highest BMI, with SBP consistently elevated in deceased individuals within each BMI tertile. This underscores the relationship between central obesity and vascular dysfunction, which may contribute to increased mortality.
Furthermore, the prothrombotic state induced by central obesity heightens the risk of stroke and myocardial infarction [38]. Excess visceral fat also impairs liver function, promoting NAFLD, which can progress to steatohepatitis and cirrhosis, further increasing morbidity and mortality [39]. Consistent with our study findings, ALT and AST levels peaked in BMI Tertile 3, with AST levels being significantly higher in deceased participants compared to survivors. High BMI and elevated ABSI may also indicate sarcopenic obesity—a condition characterized by increased fat mass and reduced muscle mass. In older diabetic patients, sarcopenic obesity can result in diminished physical function, greater frailty, and heightened vulnerability to adverse outcomes [40]. In our study, hemoglobin and serum albumin levels were consistently lower among deceased participants, with serum albumin levels gradually declining across BMI tertiles, particularly in individuals with higher BMI. These findings suggest the combined effects of sarcopenia and central obesity. Sarcopenic obesity exacerbates insulin resistance and inflammation, creating a vicious cycle that significantly increases mortality risk. In summary, the combined assessment of BMI and ABSI offers a more comprehensive evaluation of an individual’s obesity profile [41]. While BMI captures overall fat, ABSI provides critical insights into fat distribution and central obesity. The synergistic effect of these metrics likely represents the combined impact of general and central obesity on metabolic dysfunction, chronic inflammation, and increased cardiovascular and microvascular complications in diabetic patients. This integrated approach highlights the importance of considering both general and central obesity in health risk assessments. It emphasizes that fat distribution is as important as fat quantity, particularly in high-risk populations such as those with diabetes.
This study is a prospective analysis based on the NHANES database, with data collection conducted in strict adherence to standard operating procedures (SOPs) to ensure accuracy and consistency. Regular quality control measures and reviews were implemented to uphold high data quality standards. However, several limitations should be acknowledged. First, due to the inherent constraints of observational study designs, it is impossible to completely rule out reverse causality. Second, the data collected through interview surveys or questionnaires may be subject to recall bias, as participants are required to rely on their memory to provide information about their medical history, lifestyle habits, and other key variables. Third, as study variables were collected at a single time point, we could not assess the impact of changes over time in these variables on mortality risk. Lastly, we cannot entirely exclude the possibility that other unknown confounding factors may have influenced the results.
Conclusion
This study highlights the significant association between the combination of BMI and ABSI and all-cause mortality among American adults with diabetes. High BMI and ABSI jointly represent the compounding effects of general and central obesity, amplifying risks through metabolic dysfunction, systemic inflammation, and vascular complications. ABSI demonstrated superior predictive value, especially when combined with BMI. These findings underscore the importance of incorporating both metrics into mortality risk assessments to identify high-risk individuals and guide targeted interventions for improved metabolic health and reduced mortality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors extend their heartfelt gratitude to all the participants and staff of NHANES for their invaluable contributions to this study.
Author contributions
Shuwu Wei and Weimin Jiang conceived the study, participated in its design and coordination, analyzed the data and drafted the manuscript. Huijuan Zheng and Yaoxian Wang helped in screening clinical samples and statistical analysis. Jiale Zhang, Jie Yang, Yang Liu, Liqiao Sun and Xinrong Li, recruited patients and collected data. Junping Wei and Weiwei Sun participated in its design and coordination, and were responsible for project administration and visualization. All authors read and approved the final version of manuscript.
Funding
This project was funded by Natural Science Foundation of Beijing Municipality (No.7242255), the Traditional Chinese Medicine Evidence-Based Capacity Building Project(No.60104), the High Level Chinese Medical Hospital Promotion Project (No. HLCMHPP2023084), the Technology Innovation Project of Major Key Projects at the China Academy of Chinese Medical Sciences (No.C12021A01617), and the Beijing University of Traditional Chinese Medicine Qi-huang famous doctor training program (No.Y2023B08).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
The NHANES protocols were approved by the National Center for Health Statistics and the Ethics Review Board, with written informed consent obtained from all participants.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuwu Wei and Weimin Jiang have contributed equally to this work.
Contributor Information
Junping Wei, Email: weijunping@126.com.
Weiwei Sun, Email: sunweitcm@163.com.
References
- 1.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of Disease Study 2021. Lancet. 2023;402(10397):203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. 2005;28(4):799–805. [DOI] [PubMed] [Google Scholar]
- 3.Katzmarzyk PT, Hu G, Cefalu WT, Mire E, Bouchard C. The importance of waist circumference and BMI for mortality risk in diabetic adults. Diabetes Care. 2013;36(10):3128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kokkinos P, Myers J, Faselis C, Doumas M, Kheirbek R, Nylen E. BMI-mortality paradox and fitness in African American and cweighted AUCasian men with type 2 diabetes. Diabetes Care. 2012;35(5):1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, Anker SD. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012;162(1):20–6. [DOI] [PubMed] [Google Scholar]
- 6.Cho GJ, Yoo HJ, Hwang SY, Choi J, Lee KM, Choi KM, Baik SH, Han SW, Kim T. Differential relationship between waist circumference and mortality according to age, sex, and body mass index in Korean with age of 30–90 years; a nationwide health insurance database study. BMC Med. 2018;16(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou X, Chen S, Hu G, Chen P, Wu J, Ma X, Yang Z, Yang W, Jia W, China National Diabetes, Metabolic Disorders Study Group. Stronger associations of waist circumference and waist-to-height ratio with diabetes than BMI in Chinese adults. Diabetes Res Clin Pract. 2019;147:9–18. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81(3):555–63. [DOI] [PubMed] [Google Scholar]
- 9.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):e39504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalangot M, Tronko M, Kravchenko V, Kulchinska J, Hu G. Body mass index and the risk of total and cardiovascular mortality among patients with type 2 diabetes: a large prospective study in Ukraine. Heart. 2009;95(6):454–60. [DOI] [PubMed] [Google Scholar]
- 11.Zaccardi F, Dhalwani NN, Papamargaritis D, Webb DR, Murphy GJ, Davies MJ, Khunti K. Nonlinear association of BMI with all-cause and cardiovascular mortality in type 2 diabetes mellitus: a systematic review and meta-analysis of 414,587 participants in prospective studies. Diabetologia. 2017;60(2):240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li CI, Liu CS, Lin CH, Yang SY, Li TC, Lin CC. Association of body indices and risk of mortality in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2023;11(4):e003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orsi E, Solini A, Penno G, Bonora E, Fondelli C, Trevisan R, Vedovato M, Cavalot F, Lamacchia O, Haxhi J, Nicolucci A, Pugliese G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group. Body mass index versus surrogate measures of central adiposity as independent predictors of mortality in type 2 diabetes. Cardiovasc Diabetol. 2022;21(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X, Cao L, Liu Y, Huang W, Pei C, Wang X, Feng S, Song B. Sex- and age-specific differences in associations of a body shape index with all-cause and cardiovascular death risks among US adults with diabetes. Nutr Metab Cardiovasc Dis. 2023;33(3):551–9. [DOI] [PubMed] [Google Scholar]
- 15.Tate J, Knuiman M, Davis WA, Davis TME, Bruce DG. A comparison of obesity indices in relation to mortality in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2020;63(3):528–36. [DOI] [PubMed] [Google Scholar]
- 16.Zhang A, Li Y, Ma S, Bao Q, Sun J, Cai S, Li M, Su Y, Cheng B, Dong J, Zhang Y, Wang S, Zhu P. Conicity-index predicts all-cause mortality in Chinese older people: a 10-year community follow-up. BMC Geriatr. 2022;22(1):971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu K, Suliman ME, Qureshi AR, Chen Z, Avesani CM, Brismar TB, Ripsweden J, Barany P, Heimbürger O, Stenvinkel P, Lindholm B. Central obesity as assessed by conicity index and a-body shape index associates with cardiovascular risk factors and mortality in kidney failure patients. Front Nutr. 2023;10:1035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchi R, Asakawa M, Ohara N, Nakano Y, Takeuchi T, Murakami M, Sasahara Y, Numasawa M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. Indirect measure of visceral adiposity ‘A body shape index’ (ABSI) is associated with arterial stiffness in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2016;4(1):e000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christakoudi S, Tsilidis KK, Muller DC, Freisling H, Weiderpass E, Overvad K, Söderberg S, Häggström C, Pischon T, Dahm CC, Zhang J, Tjønneland A, Halkjær J, MacDonald C, Boutron-Ruault MC, Mancini FR, Kühn T, Kaaks R, Schulze MB, Trichopoulou A, Karakatsani A, Peppa E, Masala G, Pala V, Panico S, Tumino R, Sacerdote C, Quirós JR, Agudo A, Sánchez MJ, Cirera L, Barricarte-Gurrea A, Amiano P, Memarian E, Sonestedt E, Bueno-de-Mesquita B, May AM, Khaw KT, Wareham NJ, Tong TYN, Huybrechts I, Noh H, Aglago EK, Ellingjord-Dale M, Ward HA, Aune D, Riboli E. A body shape index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: results from a large European cohort. Sci Rep. 2020;10(1):14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, Maeda Y, McDougall A, Peterson CM, Ravussin E, Heymsfield SB. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obes (Silver Spring). 2013;21(11):2264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rato Q. Conicity index: An anthropometric measure to be evaluated. Rev Port Cardiol. 2017;36(5):365–366. English, Portuguese. [DOI] [PubMed]
- 22.Purdy JC, Shatzel JJ. The hematologic consequences of obesity. Eur J Haematol. 2021;106(3):306–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagomi A, Saito M, Ojima T, Ueno T, Hanazato M, Kondo K. Sociodemographic Heterogeneity in the associations of social isolation with mortality. JAMA Netw Open. 2024;7(5):e2413132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Wang L, He J, et al. 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–59. [DOI] [PubMed] [Google Scholar]
- 25.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63(5):820–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ofstad AP, Sommer C, Birkeland KI, Bjørgaas MR, Gran JM, Gulseth HL, Johansen OE. Comparison of the associations between non-traditional and traditional indices of adiposity and cardiovascular mortality: an observational study of one million person-years of follow-up. Int J Obes (Lond). 2019;43(5):1082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song X, Jousilahti P, Stehouwer CD, Söderberg S, Onat A, Laatikainen T, Yudkin JS, Dankner R, Morris R, Tuomilehto J, Qiao Q, DECODE Study Group. Cardiovascular and all-cause mortality in relation to various anthropometric measures of obesity in europeans. Nutr Metab Cardiovasc Dis. 2015;25(3):295–304. [DOI] [PubMed] [Google Scholar]
- 29.Sato Y, Fujimoto S, Konta T, Iseki K, Moriyama T, Yamagata K, Tsuruya K, Narita I, Kondo M, Kasahara M, Shibagaki Y, Asahi K, Watanabe T. Body shape index: sex-specific differences in predictive power for all-cause mortality in the Japanese population. PLoS ONE. 2017;12(5):e0177779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claudel SE, Verma A. Association between adipose deposition and mortality among adults without major cardiovascular risk factors. Diabetes Metab 2024 Nov 27:101595. [DOI] [PMC free article] [PubMed]
- 31.Kobayashi G, Shinozaki T, Okada H, Nakajima H, Hashimoto Y, Hamaguchi M, Kurogi K, Murata H, Yoshida N, Ito M, Ohkuma T, Horiguchi G, Teramukai S, Fukui M. Associations between anthropometric indices as complementary predictors and incidence of type 2 diabetes; Panasonic Cohort Study 21. Diabetes Res Clin Pract. 2024;217:111888. [DOI] [PubMed] [Google Scholar]
- 32.Yang D, Ma L, Cheng Y, Shi H, Liu Y, Shi C. Utility of anthropometric indexes for detecting metabolic syndrome in Resource-Limited regions of Northwestern China: cross-sectional study. JMIR Public Health Surveill. 2024;10:e57799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao T, Luo T, Pei H, Yimingniyazi B, Aili D, Aimudula A, Zhao H, Zhang H, Dai J, Wang D. Association between abdominal obesity indices and risk of cardiovascular events in Chinese populations with type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandsdal RM, Juhl CR, Jensen SBK, Lundgren JR, Janus C, Blond MB, Rosenkilde M, Bogh AF, Gliemann L, Jensen JB, Antoniades C, Stallknecht BM, Holst JJ, Madsbad S, Torekov SS. Combination of exercise and GLP-1 receptor agonist treatment reduces severity of metabolic syndrome, abdominal obesity, and inflammation: a randomized controlled trial. Cardiovasc Diabetol. 2023;22(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu X, Xie Q, Pan X, Zhang R, Zhang X, Peng G, Zhang Y, Shen S, Tong N. Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal Transduct Target Ther. 2024;9(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, Coutinho T, Jensen MD, Roger VL, Singh P, Lopez-Jimenez F. Normal-weight central obesity: implications for Total and Cardiovascular Mortality. Ann Intern Med. 2015;163(11):827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Chen Z, Pan L, Ma ZM, Zhang H, Li XJ, Li X. Effect of Moderate and Vigorous Aerobic Exercise on Incident diabetes in adults with obesity: a 10-Year follow-up of a Randomized Clinical Trial. JAMA Intern Med. 2023;183(3):272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koenen M, Hill MA, Cohen P, Sowers JR, Obesity. Adipose tissue and vascular dysfunction. Circ Res. 2021;128(7):951–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z, Zhang Y, Graham S, Wang X, Cai D, Huang M, Pique-Regi R, Dong XC, Chen YE, Willer C, Liu W. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol. 2020;73(2):263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polyzos SA, Vachliotis ID, Mantzoros CS. Sarcopenia, sarcopenic obesity and nonalcoholic fatty liver disease. Metabolism. 2023;147:155676. [DOI] [PubMed] [Google Scholar]
- 41.Benz E, Pinel A, Guillet C, Capel F, Pereira B, De Antonio M, Pouget M, Cruz-Jentoft AJ, Eglseer D, Topinkova E, Barazzoni R, Rivadeneira F, Ikram MA, Steur M, Voortman T, Schoufour JD, Weijs PJM, Boirie Y. Sarcopenia and Sarcopenic Obesity and mortality among older people. JAMA Netw Open. 2024;7(3):e243604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.