Abstract
The heterogeneous group of disorders known as oculocutaneous albinism (OCA) shares cutaneous and ocular hypopigmentation associated with common developmental abnormalities of the eye. Mutations of at least 11 loci produce this phenotype. The majority of affected individuals develop some cutaneous melanin; this is predominantly seen as yellow/blond hair, whereas fewer have brown hair. The OCA phenotype is dependent on the constitutional pigmentation background of the family, with more OCA pigmentation found in families with darker constitutional pigmentation, which indicates that other genes may modify the OCA phenotype. Sequence variation in the melanocortin-1 receptor (MC1R) gene is associated with red hair in the normal population, but red hair is unusual in OCA. We identified eight probands with OCA who had red hair at birth. Mutations in the P gene were responsible for classic phenotype of oculocutaneous albinism type 2 (OCA2) in all eight, and mutations in the MC1R gene were responsible for the red (rather than yellow/blond) hair in the six of eight who continued to have red hair after birth. This is the first demonstration of a gene modifying the OCA phenotype in humans.
Melanins are biological polymers that are synthesized by the melanocyte in the skin and hair follicle, the iris stroma and retinal pigment epithelium (RPE), and the inner ear. The cutaneous and iridial melanocytes can make two general types of melanin, brown/black eumelanin and yellow/red pheomelanin (Prota 1992). The type that is synthesized is dependent on protein and enzyme components of the melanin pathway that are delivered to the pigment granule (melanosome) during its development (Hearing 2000; Toyofuku et al. 2001). Tyrosinase is the major enzyme in melanin synthesis, and no melanin forms in the absence of activity of this key enzyme (Silvers 1979; Oetting and King 1999). Other proteins play a role in melanin formation, but the essential nature of their function in melanin synthesis has not been established (Hearing 2000). Melanin serves as a photoprotective pigment in the skin and eye and plays an unknown role in the developing mammalian eye, where it is found long before light exposure (Sturm et al. 2001; Sturm 2002).
The switch between the synthesis of eumelanin or pheomelanin is controlled, to a large extent, by the melanocortin-1 receptor (MC1R) on the melanocyte (Sturm et al. 2001). Stimulation of this receptor by the melanocyte-stimulating hormone (MSH) induces the cell to make black/brown eumelanin. The antagonist to the receptor is the agouti-signaling protein, which seems to block the effect of MSH and results in a cell that makes yellow/red pheomelanin. Recent interest in the MC1R gene has led to the identification of a number of common mutations or variants that are associated with red hair and light skin that tans poorly or not at all (Valverde et al. 1995; Box et al. 1997; Koppula et al. 1997; Schioth et al. 1999; Flanagan et al. 2000; Palmer et al. 2000; Grimes et al. 2001; Healy et al. 2001; Schaffer and Bolognia 2001; Smith et al. 2001; Sturm et al. 2001; Sturm 2002). Mutations in this gene have also been found in animals with red coats (Robbins et al. 1993; Newton et al. 2000). Although the association of MC1R and red hair has been intriguing to human geneticists, the potential association of MC1R mutations with susceptibility to melanoma and skin cancer has been important in increasing our understanding of the epidemiology of these cancers (Valverde et al. 1996; Palmer et al. 2000; Box et al. 2001a, 2001b; Kennedy et al. 2001). Of most importance, the development of red hair cannot be solely explained by variation in the MC1R gene, and other genes are likely to be involved in the regulation of the amount and type of melanins that are produced by the melanocyte (Box et al. 1997; Akey et al. 2001).
Oculocutaneous albinism (OCA) is the result of a reduction in the amount of melanin synthesized in the melanocyte. Cutaneous hypopigmentation produces light hair and sun-sensitive skin. Reduction of melanin in the RPE is associated with foveal hypoplasia (which results in reduced visual acuity) and abnormal projections of the optic nerves to the brain (which usually results in strabismus) (King et al. 2001). At least 11 genes have been associated with an oculocutaneous phenotype in humans. The two most common types are type 1 (OCA1 [MIM 203100]), which results from mutations in the tyrosinase gene on chromosome 11p, and type 2 (OCA2 [MIM 203200]), which results from mutations in the P gene on chromosome 15q. Type 3 (OCA3 [MIM 278400]) is described as “rufous or red OCA with brick-red bronze or mahogany skin and ginger or reddish hair” and is associated with mutations in the TYRP1 gene on chromosome 9p (Manga et al. 1997). This phenotype has been recognized only in the African population; the white equivalent is unknown. Type 4 (OCA4 [MIM 606574]) has been associated with the MATP gene on chromosome 5p, and one affected individual has been described with a phenotype similar to that of OCA2 (Newton et al. 2001). There are six types of Hermansky-Pudlak syndrome that have OCA associated with storage-pool–deficient platelets. (Shotelersuk and Gahl 1998; Dell’Angelica et al. 1999; Anikster et al. 2001; Huizing et al. 2001; Suzuki et al. 2002; Zhang et al. 2003). The final type of OCA is Chediak-Higashi syndrome (CHS [MIM 214500]), and albinism is a minor component of this complex phenotype (Introne et al. 1999).
The phenotype of OCA usually ranges from white hair and skin to various shades of light brown or light-to-dark-blond hair and minimal to moderate amounts of generalized skin pigmentation. Many individuals with OCA do form some cutaneous and iridial pigment, but this is usually yellow pheomelanin pigment or pigment that evolves, with time, through a yellow pheomelanin phase to a light-brown eumelanin phase. We now describe an unusual pigmentation phenotype that involves red rather than yellow hair in individuals with OCA2 rather than rufous “red” OCA. Our studies of the P gene (OCA2) and the MC1R gene (red hair) clearly identify this latter gene as a major modifier of the OCA2 phenotype.
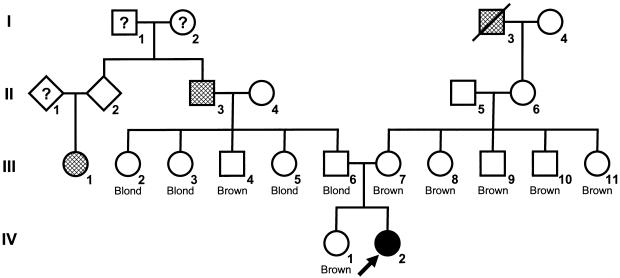
We identified eight probands who presented with OCA associated with red hair at birth and obtained clinical information and peripheral blood for DNA after obtaining informed consent (table 1). The initial proband (individual 1) was a female who had reddish-blond hair, white skin, blue irides, and nystagmus at birth. She was diagnosed with OCA at age 2 years because of nystagmus, blue irides that had minimal transillumination, reduced retinal pigment, foveal hypoplasia, and reduced visual acuity. Corrected visual acuity was measured at 20/100 at 3.5 years and 20/60 at age 10 years. Examination at age 18 years showed red scalp hair, yellow eyebrow and eyelash hair, white skin that did not tan, light freckles, and scattered amelanotic nevi (fig. 1). The irides were light blue and showed grade-1 transillumination (on a scale of 1 [minimal] to 4 [complete]) (Summers et al. 1988). Coarse gray-black melanin pigment was absent by ophthalmoscopy, and only a rudimentary reflex was noted in the hypoplastic but opaque macula. Corrected acuity was 20/60. The diagnosis was probable OCA2, on the basis of the presence of pigmented hair at birth, but the red color of the hair was unusual and had not been described in the literature associated with this type of OCA. There was no family history of OCA, but her mother and father had relatives with red hair (fig. 2).
Table 1.
Probands with OCA2 and Red Hair: Results of P and MC1R Gene Analysis
|
P Mutationa |
||||||||
| Individual | Age(years) | Sex | Ethnic Originb | Hair Color at Birth | Hair Color at Time of Study | Maternal | Paternal | MC1R Mutationsc |
| 1 | 18.0 | F | NE | Red | Red | W679C | N489D | R151C R160W |
| 2 | .33 | M | NE, AA, NA | Orange red | Orange red | V443I | P743L | R151C R160W |
| 3 | .75 | F | AA | Red | Red | delEx7 | V443I | 0 |
| 4 | .83 | F | Ash | Blond/red | Blond/red | V443I | 0 | V60L M92V T314T |
| 5 | 1.00 | M | Ash | Red | Red | 0 | V443I | D84E R151C |
| 6 | 2.00 | M | NE | Blond/red | Blond/red | R290G | 0 | V60L |
| 7 | 9.00 | M | AA, WI, PR | Red | Blond/brown | 0 | IVS12+5g→a | 0 |
| 8 | 17.0 | F | AA | Red | Dark Blond | NW273KV | 0 | 0 |
Maternal and paternal P gene mutations identified. 0 = no mutation identified. Analysis included the entire coding region and part of the flanking intron sequences for each of the 24 exons by use of the dideoxy method of Sanger with automated fluorescent DNA sequencing (Oetting et al. 1994).
Self-reported maternal and paternal ethnic background: AA = African American, Ash = Ashkenazi Jew, NA = Native American, NE = Northern European, PR = Puerto Rican, WI = West Indian.
MC1R mutations identified in proband. Sequence analysis included the entire coding region and part of the flanking sequences of the exon by use of the dideoxy method of Sanger with automated fluorescent DNA sequencing (Oetting et al. 1994).
Figure 1.
Proband at age 18 years, showing red hair and light skin
Figure 2.
Family pedigree. Proband marked with arrow. Hair color indicated for parental generation and sibling. Hatched family members have red hair.
We first analyzed the P gene to establish the cause of each proband’s OCA. Mutations of the 24-exon P gene, mapping to chromosome 15q11–13, are responsible for OCA2 (Gardner et al. 1992; Lee et al. 1994a; Spritz et al. 1997; Oetting and King 1999; Passmore et al. 1999). The gene is also highly polymorphic, with >30 nonpathologic sequence variants reported (Lee et al. 1995; Albinism Database). The P gene encodes a melanosomal protein with 12 membrane-spanning regions (Rosemblat et al. 1994). The precise function of the protein remains unknown, but studies suggest that it is involved in regulation of the intraorganelle pH or structure (Orlow and Brilliant 1999; Puri et al. 2000).
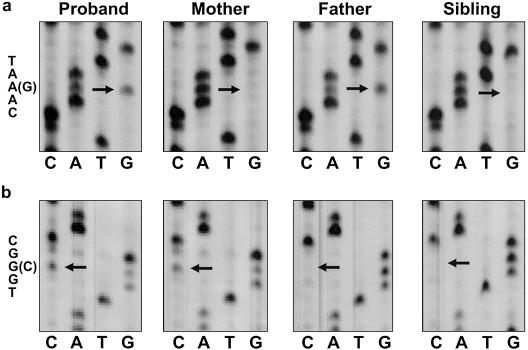
The sequence of the P gene was determined in DNA from each proband and the parents, by use of methods established in our laboratory (Oetting et al. 1998; Oetting and King 1999). All case subjects were compound heterozygotes with different maternal and paternal mutations (table 1). Both mutations were identified in individuals 1–3, and only one of the two mutations was identified in individuals 4–8. For individual 1, the paternal mutation (N489D) was a base change at base 1465 (A→G), which resulted in an asparagine→aspartic acid substitution at codon 489 in the third intracellular loop (fig. 3a). This mutation has been shown to produce OCA2 when homozygous or allelic with another P gene mutation (Spritz et al. 1997; Oetting and King 1999). The maternal mutation (W679C) was a base change at base 2037 (G→C), which resulted in a tryptophan→cysteine substitution at codon 679 (fig. 3b). A change at amino acid 679, near the transmembrane region of extracellular loop 4, was previously proven deleterious and associated with OCA2 (Lee et al. 1994b; Passmore et al. 1999). The mutations in individuals 2–8 are listed in table 1. The V443I is a common P gene mutation associated with the typical OCA2 phenotype, and the exon 7 deletion is the common P gene mutation in the sub-Saharan African population. R290G, NW273KV, and P743L have been reported with a typical OCA2 phenotype (Lee et al. 1994b; Passmore et al. 1999; Kerr et al. 2000), and IVS12+5g→a is novel and, to our knowledge, has not been reported in OCA2 to date.
Figure 3.
P gene sequencing results. a, Proband heterozygous at exon 14, codon 489, with a change from asparagine (AAT) to an aspartic acid (GAT). Mutation N489D is paternal in origin. b, Proband heterozygous at exon 19, codon 679, with a change from tryptophan (TGG) to cysteine (TGC). Mutation W679C is maternal in origin.
Once the diagnosis of OCA2 was established, the MC1R gene was analyzed to determine if mutations in this gene were modifying the typical OCA2 phenotype by the development of red rather than the typical yellow/blond hair. The MC1R gene encodes a 7-transmembrane G-protein-coupled receptor with two alternatively spliced variants, and, in melanocytes, the proportion of pheomelanin to eumelanin is regulated, in part, by the MSH through the MC1R (Mountjoy et al. 1992). The MC1R gene was analyzed in DNA from each proband, and six of the eight were found to have known MC1R variants (table 1) (Harding et al. 2000).
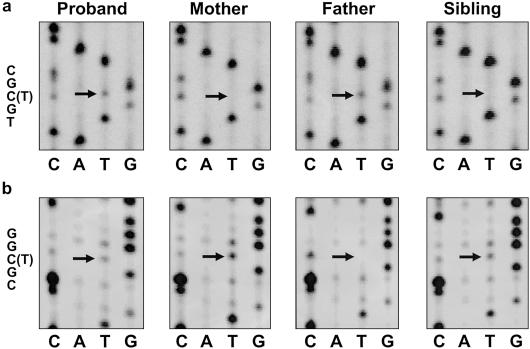
Individual 1 was a compound heterozygote: the paternal change (R151C) was a base change at base 451 (C→T) that resulted in an arginine→cysteine substitution at codon 151 in the second intracellular loop of the protein (fig. 4a), and the maternal change (R160W) was a base change at base 478 (C→T) that resulted in an arginine→tryptophan substitution at codon 160 in the transmembrane boundary of the second intracellular loop of the protein (fig. 4b). Loss-of-function variants of MC1R are unable to stimulate cAMP after stimulation with MSH, which results in a reduction of eumelanin synthesis in the melanocyte (Frandberg et al. 1998; Schioth et al. 1999; Healy et al. 2001). Furthermore, there is a strong statistical correlation between red hair and the mutations that have been described (Flanagan et al. 2000; Harding et al. 2000; Sturm et al. 2001). Individual 4 had three MC1R gene mutations, as has been reported elsewhere in studies of red hair (Valverde et al. 1995; Box et al. 1997; Palmer et al. 2000; Healy et al. 2001). Individuals 7 and 8 did not have MC1R gene mutations; both were reported to have red hair at birth that did not persist as they developed (table 1). This suggests that MC1R mutations may not be responsible for red hair that is present at birth but not later in life.
Figure 4.
MC1R gene sequencing results. a, Proband heterozygous at codon 151, with a change from arginine (CGC) to cysteine (TGC). Mutation R151C is paternal in origin. b, Proband heterozygous at codon 160, with a change from arginine (CGG) to tryptophan (TGG). Mutation R160W is maternal in origin. The sibling is also heterozygous for the maternal mutation.
The only other type of OCA that is associated with red hair is rufous or “red” albinism (OCA3), identified only in the African population and caused by mutations of the tyrosinase-related protein-1 (TYRP1) gene (Kromberg et al. 1990; Manga et al. 1997). Affected individuals have reddish or ginger-colored hair and skin and visual problems that are less severe than in other forms of OCA, but they do not have bright red hair.
One additional observation is suggested with these individuals. The albinism phenotype, by definition, includes abnormal ganglion cell development of the retina, clinically recognized as foveal hypoplasia, and reduced visual acuity. Visual acuity in OCA is usually in the range of 20/100–20/200, but a moderate number of affected individuals have visual acuity better than 20/100 (Summers et al. 1991, 1996; Summers 1996). This phenotype, common to all types of OCA, is produced by mutations in genes that affect the ability of melanocyte to synthesize melanin through different mechanisms, which suggests that the melanin itself plays an important role in retinal development (Schraermeyer 1990; Raymond and Jackson 1995; Ilia and Jeffery 1996; Schraermeyer and Heimann 1999; Grant et al. 2001; Donatien and Jeffery 2002). The RPE is thought to make predominantly eumelanin, but perhaps pheomelanin may also be made in the retina and have some role in retinal development. The visual acuity of individual 1 was 20/60, and this better acuity could possibly be explained by the presence of some pheomelanin in her RPE.
Our study characterizes the association of OCA2 with red hair in humans, which is the result of mutations in the P gene and in the MC1R gene. To our knowledge, this study is the first description of a gene that can modify the typical OCA2 phenotype in humans and demonstrates the complexity of pigment genetics through the action/interaction of two genes. This finding is not unexpected, however, as Silvers has reviewed the interaction of many loci on coat color in the mouse and has noted that the agouti and the extension (MC1R) loci can modify the phenotype produced by mutations in the murine p gene (Silvers 1979). Human OCA is a complex model system that can be used to reflect the many studies of coat color in the mouse, and it is expected that additional loci that modify the human phenotype will be described in future studies.
Acknowledgments
We thank the families for their willingness to participate in these studies, which were supported, in part, by National Institutes of Health grant AR44649 and the Bernard and Mary Ellen Black family.
Electronic-Database Information
URLs for data presented herein are as follows:
- Albinism Database, http://www.cbc.umn.edu/tad/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for OCA1, OCA2, OCA3, OCA4, and CHS)
References
- Akey JM, Wang H, Xiong M, Wu H, Liu W, Shriver MD, Jin L (2001) Interaction between the melanocortin-1 receptor and P genes contributes to inter-individual variation in skin pigmentation phenotypes in a Tibetan population. Hum Genet 108:516–520 [DOI] [PubMed] [Google Scholar]
- Anikster Y, Huizing M, White J, Shevchenko YO, Fitzpatrick DL, Touchman JW, Compton JG, Bale SJ, Swank RT, Gahl WA, Toro JR (2001) Mutation of a new gene causes a unique form of Hermansky-Pudlak syndrome in a genetic isolate of central Puerto Rico. Nat Genet 28:376–380 [DOI] [PubMed] [Google Scholar]
- Box NF, Duffy DL, Chen W, Stark M, Martin NG, Sturm RA, Hayward NK (2001a) MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am J Hum Genet 69:765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box NF, Duffy DL, Irving RE, Russell A, Chen W, Griffyths LR, Parsons PG, Green AC, Sturm RA (2001b) Melanocortin-1 receptor genotype is a risk factor for basal and squamous cell carcinoma. J Invest Dermatol 116:224–229 [DOI] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, O’Gorman LE, Martin NG, Sturm RA (1997) Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet 6:1891–1897 [DOI] [PubMed] [Google Scholar]
- Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS (1999) Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β 3A subunit of the AP-3 adaptor. Mol Cell 3:11–21 [DOI] [PubMed] [Google Scholar]
- Donatien P, Jeffery G (2002) Correlation between rod photoreceptor numbers and levels of ocular pigmentation. Invest Ophthalmol Vis Sci 43:1198–1203 [PubMed] [Google Scholar]
- Flanagan N, Healy E, Ray A, Philips S, Todd C, Jackson IJ, Birch-Machin MA, Rees JL (2000) Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum Mol Genet 9:2531–2537 [DOI] [PubMed] [Google Scholar]
- Frandberg PA, Doufexis M, Kapas S, Chhajlani V (1998) Human pigmentation phenotype: a point mutation generates nonfunctional MSH receptor. Biochem Biophys Res Commun 245:490–492 [DOI] [PubMed] [Google Scholar]
- Gardner JM, Nakatsu Y, Gondo Y, Lee S, Lyon MF, King RA, Brilliant MH (1992) The mouse pink-eyed dilution gene: association with human Prader-Willi and Angelman syndromes. Science 257:1121–1124 [DOI] [PubMed] [Google Scholar]
- Grant S, Patel NN, Philp AR, Grey CN, Lucas RD, Foster RG, Bowmaker JK, Jeffery G (2001) Rod photopigment deficits in albinos are specific to mammals and arise during retinal development. Vis Neurosci 18:245–251 [DOI] [PubMed] [Google Scholar]
- Grimes EA, Noake PJ, Dixon L, Urquhart A (2001) Sequence polymorphism in the human melanocortin 1 receptor gene as an indicator of the red hair phenotype. Forensic Science International 122:124–129 [DOI] [PubMed] [Google Scholar]
- Harding RM, Healy E, Ray AJ, Ellis NS, Flanagan N, Todd C, Dixon C, Sajantila A, Jackson IJ, Birch-Machin MA, Rees JL (2000) Evidence for variable selective pressures at MC1R. Am J Hum Genet 66:1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy E, Jordan SA, Budd PS, Suffolk R, Rees JL, Jackson IJ (2001) Functional variation of MC1R alleles from red-haired individuals. Hum Mol Genet 10:2397–2402 [DOI] [PubMed] [Google Scholar]
- Hearing VJ (2000) The melanosome: the perfect model for cellular responses to the environment. Pigment Cell Res 13:S23–S34 [DOI] [PubMed] [Google Scholar]
- Huizing M, Anikster Y, Fitzpatrick DL, Jeong AB, D’Souza M, Rausche M, Toro JR, Kaiser-Kupfer MI, White JG, Gahl WA (2001) Hermansky-Pudlak syndrome type 3 in Ashkenazi Jews and other non-Puerto Rican patients with hypopigmentation and platelet storage-pool deficiency. Am J Hum Genet 69:1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilia M, Jeffery G (1996) Delayed neurogenesis in the albino retina: evidence of a role for melanin in regulating the pace of cell generation. Brain Res Dev Brain Res 95:176–183 [DOI] [PubMed] [Google Scholar]
- Introne W, Boissy RE, Gahl WA (1999) Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab 68:283–303 [DOI] [PubMed] [Google Scholar]
- Kennedy C, ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bavinck JN (2001) Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol 117:294–300 [DOI] [PubMed] [Google Scholar]
- Kerr R, Stevens G, Manga P, Salm S, John P, Haw T, Ramsay M (2000) Identification of P gene mutations in individuals with oculocutaneous albinism in sub-Saharan Africa. Hum Mutat 15:166–172 [DOI] [PubMed] [Google Scholar]
- King RA, Hearing VJ, Creel DJ, Oetting (2001) Albinism. In Scriver CR, et al (eds) The metabolic & molecular bases of inherited disease, 8th ed. McGraw-Hill, New York, 5587–5627 [Google Scholar]
- Koppula SV, Robbins LS, Lu D, Baack E, White CR Jr, Swanson NA, Cone RD (1997) Identification of common polymorphisms in the coding sequence of the human MSH receptor (MC1R) with possible biological effects. Hum Mutat 9:30–36 [DOI] [PubMed] [Google Scholar]
- Kromberg JGR, Castle DJ, Zwane EM, Bothwell J, Kidson S, Bartel P, Phillips JI, Jenkins T (1990) Red or rufous albinism in southern Africa. Ophthalmic Paediatr Genet 11:229–235 [DOI] [PubMed] [Google Scholar]
- Lee S-T, Nicholls RD, Bundey S, Laxova R, Musarella M, Spritz RA (1994a) Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader-Willi syndrome plus albinism. N Engl J Med 330:529–534 [DOI] [PubMed] [Google Scholar]
- Lee S-T, Nicholls RD, Jong MTC, Spritz RA (1995) Organization and sequence of the human P gene and identification of a new family of transport pump proteins. Genomics 26:354–363 [DOI] [PubMed] [Google Scholar]
- Lee S-T, Nicholls RD, Schnur RE, Guida LC, Lu-Kuo J, Spinner NB, Zackai EH, Spritz RA (1994b) Diverse mutations of the P gene among African-Americans with type II (tyrosinase-positive) oculocutaneous albinism (OCA2). Hum Mol Genet 3:2047–2051 [PubMed] [Google Scholar]
- Manga P, Kromberg JGR, Box NF, Sturm RA, Jenkins T, Ramsay M (1997) Rufous oculocutaneous albinism in southern African blacks is caused by mutations in the TYRP1 gene. Am J Hum Genet 61:1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD (1992) The cloning of a family of genes that encode the melanocortin receptors. Science 257:1248–1251 [DOI] [PubMed] [Google Scholar]
- Newton JM, Cohen-Barak O, Hagiwara N, Gardner JM, Davisson MT, King RA, Brilliant MH (2001) Mutations in the human orthologue of the mouse underwhite gene (uw) underlie a new form of oculocutaneous albinism, OCA4. Am J Hum Genet 69:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton JM, Wilkie AL, He L, Jordan SA, Metallinos DL, Holmes NG, Jackson IJ, Barsh GS (2000) Melanocortin 1 receptor variation in the domestic dog. Mamm Genome 11:24–30 [DOI] [PubMed] [Google Scholar]
- Oetting WS, Fryer JP, Oofuji Y, Middendorf LR, Brumbaugh JA, Summers CG, King RA (1994) Analysis of tyrosinase gene mutations using direct automated infrared fluorescence DNA sequencing of amplified exons. Electrophoresis 15:159–164 [DOI] [PubMed] [Google Scholar]
- Oetting WS, Gardner JM, Fryer JP, Ching A, Durham-Pierre D, King RA, Brilliant MH (1998) Mutations of the human P gene associated with Type II oculocutaneous albinism (OCA2). Mutations in brief no. 205. Online. Hum Mutat 12:434 [DOI] [PubMed] [Google Scholar]
- Oetting WS, King RA (1999) Molecular basis of albinism: mutations and polymorphisms of pigment genes associated with albinism. Hum Mutat 13:99–115 [DOI] [PubMed] [Google Scholar]
- Orlow SJ, Brilliant MH (1999) The pink-eyed dilution locus controls the biogenesis of melanosomes and levels of melanosomal proteins in the eye. Exp Eye Res 68:147–154 [DOI] [PubMed] [Google Scholar]
- Palmer JS, Duffy DL, Box NF, Aitken JF, O’Gorman LE, Green AC, Hayward NK, Martin NG, Sturm RA (2000) Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet 66:176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore LA, Kaesmann-Kellner B, Weber BH (1999) Novel and recurrent mutations in the tyrosinase gene and the P gene in the German albino population. Hum Genet 105:200–210 [DOI] [PubMed] [Google Scholar]
- Prota G (1992) Melanins and melanogenesis. Academic Press, New York [Google Scholar]
- Puri N, Gardner JM, Brilliant MH (2000) Aberrant pH of melanosomes in pink-eyed dilution (p) mutant melanocytes. J Invest Dermatol 115:607–613 [DOI] [PubMed] [Google Scholar]
- Raymond SM, Jackson IJ (1995) The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr Biol 5:1286–1295 [DOI] [PubMed] [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD (1993) Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72:827–834 [DOI] [PubMed] [Google Scholar]
- Rosemblat S, Durham-Pierre D, Gardner JM, Nakatsu Y, Brilliant MH, Orlow SJ (1994) Identification of a melanosomal membrane protein encoded by the pink-eyed dilution (type II oculocutaneous albinism) gene. Proc Natl Acad Sci USA 91:12071–12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer JV, Bolognia JL (2001) The melanocortin-1 receptor: red hair and beyond. Arch Dermatol 137:1477–1485 [DOI] [PubMed] [Google Scholar]
- Schioth HB, Phillips SR, Rudzish R, Birch-Machin MA, Wikberg JE, Rees JL (1999) Loss of function mutations of the human melanocortin 1 receptor are common and are associated with red hair. Biochem Biophys Res Commun 260:488–491 [DOI] [PubMed] [Google Scholar]
- Schraermeyer U (1990) Further evidence for synthesis of screening pigment granules involved in the photosensory membrane turnover of the crayfish photoreceptor. Pigment Cell Res 3:279–289 [DOI] [PubMed] [Google Scholar]
- Schraermeyer U, Heimann K (1999) Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res 12:219–236 [DOI] [PubMed] [Google Scholar]
- Shotelersuk V, Gahl WA (1998) Hermansky-Pudlak syndrome: models for intracellular vesicle formation. Mol Genet Metab 65:85–96 [DOI] [PubMed] [Google Scholar]
- Silvers WK (1979) The coat colors of mice: a model for mammalian gene action and interaction. Springer-Verlag, New York [Google Scholar]
- Smith AG, Box NF, Marks LH, Chen W, Smit DJ, Wyeth JR, Huttley GA, Easteal S, Sturm RA (2001) The human melanocortin-1 receptor locus: analysis of transcription unit, locus polymorphism and haplotype evolution. Gene 281:81–94 [DOI] [PubMed] [Google Scholar]
- Spritz RA, Lee S-T, Fukai K, Brondum-Nielsen K, Chitayat D, Lipson MH, Musarella MA, Rosenmann A, Weleber RG (1997) Novel mutations of the P gene in type II oculocutaneous albinism (OCA2). Hum Mutat 10:175–177 [DOI] [PubMed] [Google Scholar]
- Sturm RA (2002) Skin colour and skin cancer: MC1R, the genetic link. Melanoma Res 12:405–416 [DOI] [PubMed] [Google Scholar]
- Sturm RA, Teasdale RD, Box NF (2001) Human pigmentation genes: identification, structure and consequences of polymorphic variation. Gene 277:49–62 [DOI] [PubMed] [Google Scholar]
- Summers CG (1996) Vision in albinism. Trans Am Ophthalmol Soc 94:1095–1155 [PMC free article] [PubMed] [Google Scholar]
- Summers CG, Creel DJ, Townsend D, King RA (1991) Variable expression of vision in sibs with albinism. Am J Med Genet 40:327–331 [DOI] [PubMed] [Google Scholar]
- Summers CG, Knobloch WH, Witkop CJ, King RA (1988) Hermansky-Pudlak syndrome: ophthalmic findings. Ophthalmology 95:545–554 [DOI] [PubMed] [Google Scholar]
- Summers CG, Oetting WS, King RA (1996) Diagnosis of oculocutaneous albinism with molecular analysis. Am J Ophthalmol 121:724–726 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Li W, Zhang Q, Karim A, Novak EK, Sviderskaya EV, Hill SP, Bennett DC, Levin AV, Nieuwenhuis HK, Fong CT, Castellan C, Miterski B, Swank RT, Spritz RA (2002) Hermansky-Pudlak syndrome is caused by mutations in HPS4, the human homolog of the mouse light-ear gene. Nat Genet 30:321–324 [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Wada I, Valencia JC, Kushimoto T, Ferrans VJ, Hearing VJ (2001) Oculocutaneous albinism types 1 and 3 are ER retention diseases: mutation of tyrosinase or Tyrp1 can affect the processing of both mutant and wild-type proteins. FASEB J 15:2149–2161 [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ (1995) Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet 11:328–330 [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Sikkink S, Haldane F, Thody AJ, Carothers A, Jackson IJ, Rees JL (1996) The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum Mol Genet 5:1663–1666 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao B, Li W, Oiso N, Novak RK, Rusiniak ME, Gautam R, Chintala S, O’Brien EP, Zhang Y, Roe BA, Elliott RW, Eicher EM, Liang P, Kratz C, Legius E, Spritz RA, O’Sullivan TN, Copeland NG, Jenkins NASRT (2003) Ru2 and Ru encode mouse orthologs of the genes mutated in human Hermansky-Pudlak syndrome types 5 and 6. Nat Genet 33:145–153 [DOI] [PubMed] [Google Scholar]