Abstract
Joubert syndrome (JS) is an autosomal recessive developmental brain condition characterized by hypoplasia/dysplasia of the cerebellar vermis and by ataxia, hypotonia, oculomotor apraxia, and neonatal breathing dysregulation. A form of JS that includes retinal dysplasia and cystic dysplastic kidneys has been differentiated from other forms of JS, called either “JS type B” or “cerebello-oculo-renal syndrome” (CORS), but the genetic basis of this condition is unknown. Here, we describe three consanguineous families that display CORS. Linkage analysis defines a novel locus on chromosome 11p12-q13.3, with a maximum two-point LOD score of Z=5.2 at the marker D11S1915. Therefore, the cerebello-oculo-renal form of JS is a distinct genetic entity from the Joubert syndrome 1 (JBTS1) locus described elsewhere, in which there is minimal involvement of retina or kidney. We suggest the term “CORS2” for this new locus.
Joubert syndrome (JS) (also known as “Joubert-Boltshauser syndrome”) is a rare autosomal recessive syndrome characterized by hypoplasia/dysplasia of the cerebellar vermis. It was first described in 1968 by Maria Joubert and colleagues (Joubert et al. 1968) as “a syndrome of familial dysgenesis of the vermis accompanied by hyperventilation, abnormal eye movements, and retardation.” Genetic mapping studies in two Middle Eastern families have identified one locus for classical JS on chromosome 9q34.3, named “JBTS1” (MIM 213300), defining a candidate interval of 13 cM (Saar et al. 1999). Two other families with JS presented in this study and four additional families ascertained by our group (Keeler et al. 2001) showed recombinations through the candidate interval, which suggests that JS is likely to be genetically heterogeneous. No causative genes for JS or related conditions have been identified to date.
Ocular and renal features are often seen in conjunction with JS that together constitute the cerebello-oculo-renal syndromes (Satran et al. 1999) and are likely genetically distinct from classical JS. Ocular involvement includes Leber congenital amaurosis (LCA [congenital rod-cone dystrophy]) and coloboma (a fissure of the eye epithelium). Renal involvement includes cystic dysplastic kidney disease (CDK) and nephronophthisis (NPHP). The form of JS with ocular or renal involvement has been called “type B” (Hildebrandt et al. 1992) or “type II” (King and Stephenson 1984; King et al. 1984). There is significant overlap of these syndromes with both Dekaban-Arima (DAS [incorporating LCA plus CDK] [MIM 243910]) and Senior-Löken syndromes (SLS [incorporating LCA plus NPHP] [MIM 266900]). In fact, several patients with DAS or SLS display the radiographic hallmarks of JS, including cerebellar vermis hypoplasia (Satran et al. 1999), which further suggests overlap in these syndromes. However, in a study of 11 patients with JS type B, none displayed mutations in the NPHP1 gene that are identified in some patients with SLS (Hildebrandt et al. 1998). These data suggest that JS type B may be genetically distinct from classical JS, as well as from DAS and SLS.
The “molar-tooth” sign on brain imaging (MTI) is an additional radiographic feature that may help narrow the phenotypic spectrum of the disorder. It is found in the majority of patients with clinical and radiographic features of JS (Maria et al. 1999b) and is one of the diagnostic hallmarks. The patients originally described by Dr. Joubert also display this feature (Andermann et al. 1999), and it was present in at least one of the families mapping to chromosome 9q34.3. The MTI represents a constellation of anatomic brain malformations that together result in the brainstem isthmus and upper pons taking on the appearance of a “molar tooth” on axial brain MRI. These malformations include a deep interpeduncular fossa at the level of the isthmus and upper pons; elongated, thick, and maloriented superior cerebellar peduncles; and hypoplasia/aplasia/dysplasia of the superior cerebellar vermis (Maria et al. 1997).
We ascertained DNA samples from affected and unaffected individuals from 20 families—16 of which have a history of consanguinity—that met diagnostic criteria for JS (Maria et al. 1999a). The first 13 families enrolled in our study were analyzed by genome scan, two of which were consanguineous and displayed JS plus oculo-renal involvement (families 002 and 006). Eighty-seven samples and controls were analyzed for 374 polymorphic markers (Marshfield Center for Medical Genetics Screening Set 11), with an average spacing of 10 cM, which gave a total of ∼32,500 genotypes. The results from families 002 and 006 were analyzed separately from those of the remaining families without evidence of extra-CNS involvement. Two-point linkage analysis was performed using the MLINK component of the LINKAGE computer program (Lathrop et al. 1985), under the assumption of an autosomal recessive inheritance pattern, disease allele frequency set at 0.001 with full penetrance, and equal allele frequencies. LOD scores were calculated at θ=0 and 0.1 for each marker.
Only one locus was identified from the analysis of families 002 and 006, with a combined peak two-point LOD score Z=3.4 for marker D11S1985, at 11p12-q13.3. At this locus, Z was positive in five sequential markers from the screening set in these two families. No other loci were identified with Z>2 for families 002 and 006, and the 11p12-q13 locus was excluded in the remaining informative families without extra-CNS involvement. Analysis of these five markers in five additional consanguineous multiplex families with JS plus clear evidence of oculo-renal involvement, which were ascertained subsequent to the genome scan, identified one additional family (family 129) mapping to 11p12-q13.
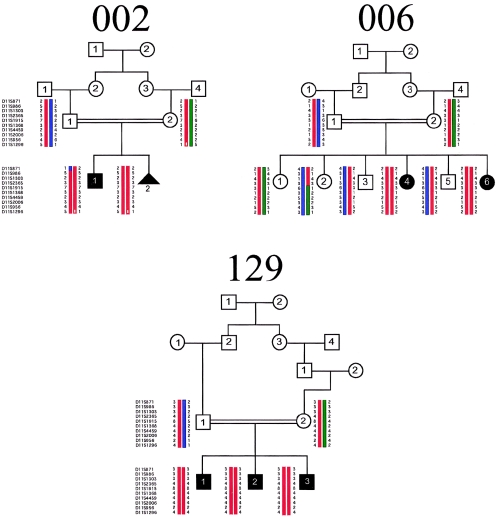
Additional markers in this region were selected at 1–3 cM intervals, on the basis of the Genome Database genetic map and the Human Genome Browser physical map. Genotypes were analyzed by denaturing gel electrophoresis. All seven affected children from the three families were homozygous for 12 sequential markers in this interval. In contrast, none of the parents or unaffected siblings were homozygous for these haplotypes (fig. 1). The diseased haplotypes do not appear to be shared among the three families, at least at this resolution, as there is no evidence of allele sharing among the affected members at this locus. A maximum two-point LOD score of Z=5.23 at θ=0 was identified for marker D11S1915, with all other markers in the surrounding region producing positive scores as high as 5.03 (table 1; fig. 2).
Figure 1.
Pedigree and haplotype analysis for markers on chromosome 11p12-q13.3 for families 002, 006, and 129. Blackened symbols represent affected individuals; the triangle represents a deceased fetus.
Table 1.
Two-Point LOD Scores on Chromosome 11p12-11q13.3 for Families 002, 006, and 129 at θ=0.0–0.4
| LOD Score at θ = |
||||||
| Marker | 0 | .05 | .1 | .2 | .3 | .4 |
| D11S1993 | −4.3419 | 3.146 | 2.883 | 2.071 | 1.205 | .463 |
| D11S871 | −4.3419 | 2.605 | 2.379 | 1.646 | .872 | .265 |
| D11S986 | 3.151 | 2.71 | 2.276 | 1.449 | .737 | .235 |
| D11S1303 | 2.831 | 2.485 | 2.138 | 1.453 | .817 | .299 |
| D11S2365 | 3.186 | 2.785 | 2.381 | 1.581 | .838 | .261 |
| D11S1915 | 5.238 | 4.653 | 4.058 | 2.856 | 1.693 | .683 |
| D11S1326 | 3.831 | 3.356 | 2.88 | 1.95 | 1.101 | .422 |
| D11S2363 | 4.272 | 3.789 | 3.296 | 2.295 | 1.326 | .502 |
| D11S1368 | 4.434 | 3.879 | 3.319 | 2.202 | 1.159 | .355 |
| D11S4459 | 2.971 | 2.613 | 2.253 | 1.539 | .872 | .324 |
| D11S2005 | 3.186 | 2.785 | 2.381 | 1.581 | .838 | .261 |
| D11S2006 | 4.434 | 3.879 | 3.319 | 2.202 | 1.159 | .355 |
| D11S956 | 5.034 | 4.459 | 3.876 | 2.707 | 1.586 | .626 |
| D11S1286 | .823 | .692 | .567 | .348 | .179 | .064 |
| D11S1296 | 1.757 | 2.664 | 2.498 | 1.852 | 1.102 | .427 |
| D11S971 | −.72 | .349 | .444 | .367 | .208 | .066 |
| D11S1369 | 1.757 | 2.644 | 2.462 | 1.795 | 1.046 | .398 |
| D11S2371 | −1.184 | −.111 | .025 | .045 | −.002 | −.027 |
| D11S2002 | −1.544 | 1.04 | 1.153 | .83 | .386 | .067 |
| D11S2000 | −1.3020 | −1.606 | −.506 | .18 | .246 | .123 |
Figure 2.
Two-point LOD scores performed with MLINK for families 002, 006, and 129 across the genetic interval. Markers are listed according their genetic position in cM.
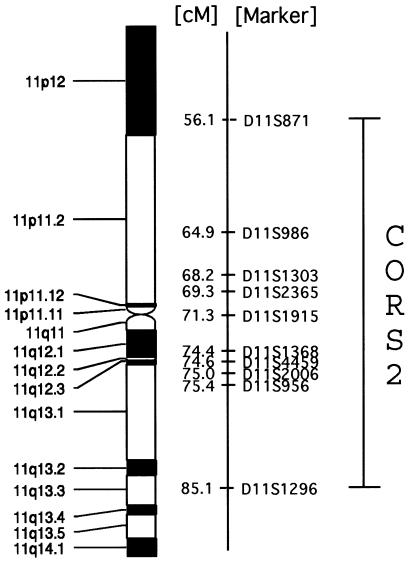
Recombinations were identified in D11S871 and D11S1296, which produced a minimal candidate interval of ∼29 cM encompassing ∼30 Mbp (fig. 3), including a 5-Mbp, currently unsequenced gap at the centromere. The candidate interval contains ∼500 known or predicted genes, many of which are expressed in the developing human brain. The MIM gene list includes the developing brain-expressed genes EHF, MDK, and CTNND1, which are thought to play diverse roles. ALX4, SCA5, NNO1, SLO, SPG17, and BBS1 are disease loci on chromosome 11 that at least partially overlap this interval and that involve a brain phenotype, but insufficient clinical overlap is present to suggest allelic variation with this locus. These data suggest that identification of the causative gene will require further refinement of the candidate interval and analysis of multiple candidate genes.
Figure 3.
Genetic map across the CORS2 candidate interval 11p12-q13.3.
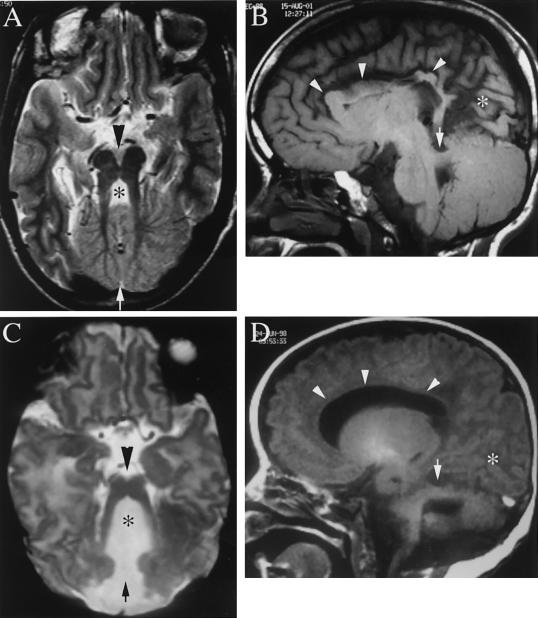
The affected members of these three families display the oculo-renal form of JS (JS type B). Family 002 is from northern Pakistan, and the parents are first cousins. The living child displays the characteristic breathing abnormality, hypotonia, ataxia, and oculomotor apraxia, the hallmarks of JS. Brain images are not available. He has moderate visual impairment but no obvious retinopathy, although an electroretinogram has not been performed. A renal ultrasound showed generalized increased echogenicity, with multiple small cysts throughout, and loss of the normal cortico-medullary differentiation bilaterally. There were no liver, digit, tongue, cardiac, or hormonal abnormalities. A deceased fetus showed a posterior encephalocele with a cerebellar malformation, not otherwise specified. The kidneys were abnormal, with cystic dilatation of the collecting ducts, but no further information is available. Family 006 is from the United Arab Emirates, and the parents are first cousins. The mother had six pregnancies with two affected children. The 15-year-old affected child had hydrocephalus at birth, requiring a ventriculoperitoneal shunt. Facial dysmorphic features included a depressed nasal bridge with hypertelorism, high-arched palate, and low-set ears. There were no abnormal eye movements or panting respirations in the neonatal period, although she displayed severe hypotonia and developmental delay. Brain MRI showed the MTI, absence of the cerebellar vermis, and abnormal kinked corpus callosum (fig. 4). The occipital cortex appeared malformed, with possible polymicrogyria. Ophthalmological examination showed double contoured coloboma of the retina and choroid below the optic disk, as well as optic atrophy and impaired vision, although an electroretinogram was not performed. The 5-year-old affected child had an enlarged head circumference at birth and required a ventriculoperitoneal shunt for hydrocephalus at age 14 mo. She had similar facial dysmorphisms, as well as panting respirations, jerky eye movements, hypotonia, and mental retardation. Brain MRI showed the MTI, thin corpus callosum, and possibly malformed occipital cortex. Ophthalmological examination showed bilateral coloboma of the optic-nerve head. Abdominal ultrasound in both affected children showed normal-sized liver and kidneys. In the younger affected child, there was evidence of calcification at the renal pyramid, suggestive of nephrocalcinosis, but no renal cysts were identified that would be suggestive of NPHP or CDK. A karyotype in both children was normal. Family 129 is from the United Arab Emirates and displays consanguinity, with three affected children. This family was reported elsewhere as family B (Saar et al. 1999). Breathing was abnormal, with panting respirations, in the neonatal period in all three affected children. Hypotonia, ataxia, and abnormal eye movement were seen in all. Brain MRI showed absence of the cerebellar vermis, but images are not available. All three had retinal dystrophy and moderate visual impairment. A renal ultrasound was normal in all three, and there were no renal symptoms. Tongue tumors, hydrocephalus, polydactyly, pituitary abnormalities, seizures, diabetes, and cardiac abnormalities were absent.
Figure 4.
Brain MRI from family 006, demonstrating the key radiographic findings in JS in the older (A and B) and younger (C and D) affected children. A and C, T2-weighted axial images, demonstrating the MTI, with a deep interpeduncular fossa (arrowhead), absence of the cerebellar vermis (arrow), and enlarged 4th ventricle (asterisk). B and D, T1-weighted saggital images, demonstrating a maloriented superior cerebellar peduncle (arrow). Additional abnormalities in these children include kinked (B) or thin (D) corpus callosum (arrowheads) and a possible occipital gyral malformation (asterisk).
The three families reported here display the oculo-renal form of JS, with kidney cysts in family 002, coloboma in family 006, and retinopathy in family 129. The children from all three families had evidence of visual impairment, although electroretinograms were not performed, and, thus, the diagnosis of LCA is not certain. Coloboma has been reported in several patients with JS (Kher et al. 1994; Dahlstrom et al. 2000) and can be seen as part of DAS and COACH (hypoplasia of cerebellar vermis, oligophrenia, congenital ataxia, coloboma, and hepatic fibrosis) syndromes (Gentile et al. 1996), as well as other JS variants (Gleeson et al., in press). The phenotypic variability between these two families suggests that there may be genetic modifiers that influence both the disease severity and feature expressivity.
We suggest the name “CORS2” for this new locus because of the apparently frequent association of oculo-renal involvement in affected individuals. We infer that a gene mutation within this locus is responsible for at least some of the cases of JS type B. The affected members from these families display the JS spectrum of disorders but with a phenotype that is largely distinct from that seen in patients mapping to the JBTS1 locus. Only one member of one of the two families linked to 9q34.3 displayed retinopathy (Sztriha et al. 1999), and none of the patients displayed coloboma or renal abnormalities. Therefore, although there may be some overlap in the phenotypic spectrum of these conditions, they appear to be largely distinct, and, thus, we prefer distinct nomenclature. We anticipate that there might be some overlap in the phenotypic spectrum of JBTS1 with CORS, and, thus, we have reserved the alternative term “CORS1” for JBTS1.
While this manuscript was in preparation, we learned that a second group had independently identified the CORS2 locus in a large inbred European pedigree with JS type B (Valente et al. 2003 [in this issue]), which provides independent verification of the relevance of this CORS2 locus in the CORS phenotype. The full phenotypic spectrum of disease that relates to CORS2 will need to be further examined, as the relatively small number of patients represented in these studies likely do not encompass the full range of clinical variability associated with this locus.
Acknowledgments
The authors thank James L. Weber and the National Heart, Lung, and Blood Institute Mammalian Genotyping Service, for running the original genome scan, and the patients and their families, for participation. We thank Enza Maria Valente et al. for exchanging manuscripts prior to publication and for agreeing on the “CORS” nomenclature. This work was supported by a grant from the March of Dimes. L.C.K. is a member of the Genetics Training Program, and E.P.L. received funding from the Neuroplasticity of Aging Training Grant at the University of California San Diego.
Electronic-Database Information
URLs for data presented herein are as follows:
- Genome Database, http://www.gdb.org/
- Human Genome Browser, http://www.genome.ucsc.edu/
- Marshfield Center for Medical Genetics, http://research.marshfieldclinic.org/genetics/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
References
- Andermann F, Andermann E, Ptito A, Fontaine S, Joubert M (1999) History of Joubert syndrome and a 30-year follow-up of the original proband. J Child Neurol 14:565–569 [DOI] [PubMed] [Google Scholar]
- Dahlstrom JE, Cookman J, Jain S (2000) Joubert syndrome: an affected female with bilateral colobomata. Pathology 32:283–285 [PubMed] [Google Scholar]
- Gentile M, Di Carlo A, Susca F, Gambotto A, Caruso ML, Panella C, Vajro P, Guanti G (1996) COACH syndrome: report of two brothers with congenital hepatic fibrosis, cerebellar vermis hypoplasia, oligophrenia, ataxia, and mental retardation. Am J Med Genet 64:514–520 [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Keeler LC, Parisi MA, Marsh SE, Chance PF, Glass IA, Graham JM Jr, Maria BL, Zackai EH, Barkovich AJ, Dobyns WB. The molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet (in press) [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Nothwang HG, Vossmerbaumer U, Springer C, Strahm B, Hoppe B, Keuth B, Fuchshuber A, Querfeld U, Neuhaus TJ, Brandis M (1998) Lack of large, homozygous deletions of the nephronophthisis 1 region in Joubert syndrome type B. APN Study Group. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Pediatr Nephrol 12:16–19 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Waldherr R, Kutt R, Brandis M (1992) The nephronophthisis complex: clinical and genetic aspects. Clin Investig 70:802–808 [DOI] [PubMed] [Google Scholar]
- Joubert M, Eisenring JJ, Andermann F (1968) Familial dysgenesis of the vermis: a syndrome of hyperventilation, abnormal eye movements and retardation. Neurology 18:302–303 [PubMed] [Google Scholar]
- Keeler LC, Leeflang EP, Sztriha L, Al-Gazali L, Nour-E-Kamal M, Frossard PM, Bayoumi R, Saar K, Rueschendorf F, Reis M, Ben-Zeev B, Chance PF, Dobyns WB, Barkovich AJ, Gleeson JG (2001) Joubert syndrome: Exclusion of linkage in additional families to 9q34.3. Am Soc Hum Genet Abst 2021 [Google Scholar]
- Kher AS, Chattopadhyay A, Divekar A, Khambekar K, Bharucha BA (1994) Joubert syndrome with polydactyly and optic coloboma in two sibs. Indian J Pediatr 61:729–732 [DOI] [PubMed] [Google Scholar]
- King MD, Dudgeon J, Stephenson JB (1984) Joubert’s syndrome with retinal dysplasia: neonatal tachypnoea as the clue to a genetic brain-eye malformation. Arch Dis Child 59:709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MD, Stephenson JB (1984) Association of Joubert’s syndrome with Leber’s congenital amaurosis. Arch Neurol 41:1235 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1985) Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet 37:482–498 [PMC free article] [PubMed] [Google Scholar]
- Maria BL, Boltshauser E, Palmer SC, Tran TX (1999a) Clinical features and revised diagnostic criteria in Joubert syndrome. J Child Neurol 14:583–590; discussion 590–591 [DOI] [PubMed] [Google Scholar]
- Maria BL, Hoang KB, Tusa RJ, Mancuso AA, Hamed LM, Quisling RG, Hove MT, Fennell EB, Booth-Jones M, Ringdahl DM, Yachnis AT, Creel G, Frerking B (1997) “Joubert syndrome” revisited: key ocular motor signs with magnetic resonance imaging correlation. J Child Neurol 12:423–430 [DOI] [PubMed] [Google Scholar]
- Maria BL, Quisling RG, Rosainz LC, Yachnis AT, Gitten J, Dede D, Fennell E (1999b) Molar tooth sign in Joubert syndrome: clinical, radiologic, and pathologic significance. J Child Neurol 14:368–376 [DOI] [PubMed] [Google Scholar]
- Saar K, Al-Gazali L, Sztriha L, Rueschendorf F, Nur-E-Kamal M, Reis A, Bayoumi R (1999) Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am J Hum Genet 65:1666–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satran D, Pierpont ME, Dobyns WB (1999) Cerebello-oculo-renal syndromes including Arima, Senior-Loken and COACH syndromes: more than just variants of Joubert syndrome. Am J Med Genet 86:459–469 [PubMed] [Google Scholar]
- Sztriha L, Al-Gazali LI, Aithala GR, Nork M (1999) Joubert’s syndrome: new cases and review of clinicopathologic correlation. Pediatr Neurol 20:274–281 [DOI] [PubMed] [Google Scholar]
- Valente EM, Salpietro DC, Brancati F, Bertini E, Galluccio T, Tortorella G, Briuglia S, Dallapiccola B (2003) Description, nomenclature, and mapping of a novel cerebello-renal syndrome with the molar tooth malformation. Am J Hum Genet 73:663–670 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]