Abstract
Cerebello-oculo-renal syndromes (CORSs) and Joubert syndrome (JS) are clinically and genetically heterogeneous autosomal recessive syndromes that share a complex neuroradiological malformation resembling a molar tooth on brain axial images, a condition referred to as “molar tooth on imaging” (MTI) or the “molar tooth sign.” The current literature on these syndromes is complex, with overlapping and incomplete phenotypes that complicate the selection of clinically homogeneous cases for genetic purposes. So far, only one locus (JBTS1 on 9q34) has been mapped, in two families with JS. Here, we describe a large consanguineous family with JS and nephronophthisis, representing a novel cerebello-renal phenotype. We have mapped this condition to the pericentromeric region of chromosome 11 and have named the locus “CORS2.” The acronym “CORS” is proposed for all loci associated with JS, CORSs, and related phenotypes sharing the MTI, because this neuroradiological sign seems to be the unifying feature of these clinically heterogeneous syndromes.
Cerebello-oculo-renal syndromes (CORSs) are a group of autosomal recessive syndromes characterized by the variable association of brain, eye, and kidney abnormalities, plus occasional malformations in other organs, such as the liver (Chance et al. 1999; Satran et al. 1999). Neuroradiologically, these syndromes share a complex brain stem malformation characterized by the appearance, on brain magnetic resonance imaging (MRI), of a form resembling a molar tooth, a condition referred to as “molar tooth on imaging” (MTI) or the “molar tooth sign.” The MTI consists of cerebellar vermian hypoplasia/aplasia, thickened and reoriented superior cerebellar peduncles, and an abnormally large interpeduncular fossa, giving the appearance of a molar tooth on transaxial slices (Maria et al. 1997). The phenotypic presentation of CORSs is highly heterogeneous. Neurological features can include ataxia, hypotonia, psychomotor developmental delay, oculomotor disorders (such as oculomotor apraxia and nystagmus), and changes in the respiratory rhythm that appear mainly in the neonatal period. Ocular abnormalities comprise Leber congenital amaurosis, other noncongenital and less specific retinopathies, and chorioretinal colobomas, with a clinical presentation ranging from blindness soon after birth to a mild reduction of visual acuity at a later age. Renal abnormalities consist of either cystic dysplastic kidneys or juvenile nephronophthisis and can be asymptomatic or mildly symptomatic until adolescence, when chronic renal failure may occur. Hepatic fibrosis is sometimes present, as are other distinctive malformations (skeletal abnormalities, congenital heart defects, tongue tumors, and various CNS malformations). CORSs are often considered as variants of Joubert syndrome (JS [MIM 213300]) (also known as “Joubert-Boltshauser syndrome”), which is characterized only by neurological signs plus the MTI (Saraiva and Baraitser 1992; Chance et al. 1999; Maria et al. 1999a; Satran et al. 1999). However, retinal involvement has been frequently described in patients with JS (type B), and a subgroup of these patients also manifested renal abnormalities (Saraiva and Baraitser 1992; Steinlin et al. 1997; Satran et al. 1999; Sztriha et al. 1999). Clinical diagnostic criteria for JS and CORSs have been established, but incomplete and overlapping phenotypes, difficult to frame within a specific syndrome, have often been described. Intrafamilial phenotypic variability is also present in JS and CORSs. Discordance between affected siblings was reported for several clinical features, such as retinopathy, oculomotor apraxia, and respiratory rhythm changes (Steinlin et al. 1997; Pellegrino et al. 1999; Satran et al. 1999; Sztriha et al. 1999). This wide clinical heterogeneity has led to considerable uncertainty in the nosologic delineation of CORSs, especially in relation to the JS phenotype (table 1).
Table 1.
Clinical and Genetic Features of JS and Most Frequent CORSs, Showing the High Degree of Clinical Overlap among Syndromes[Note]
| Type and Specifics of Anomaly | JS Type A | JS Type B | Senior-Löken | COACHa | Arima | COGAN | Cerebello-Renalb |
| Neuroimaging: | |||||||
| Vermian hypoplasia/aplasia | + | + | + | + | + | (+) | + |
| MTI | + | + | +/? | +/? | +/? | ? | + |
| Other CNS malformations | (+) | (+) | (+) | (+) | (+) | − | − |
| Brain: | |||||||
| Hypotonia | + | + | + | + | + | (+) | + |
| Psychomotor retardation | + | + | (+) | + | (+) | (+) | + |
| Ataxia | + | + | (+) | + | (+) | (+) | + |
| Oculomotor abnormalities | (+) | (+) | (+) | (+) | (+) | + | + |
| Episodic hyperpnea | (+) | (+) | (+) | (+) | (+) | − | − |
| Kidney: | |||||||
| Nephronophthisis | − | − | + | + | − | (+) | + |
| Cystic dysplastic kidney | − | (+) | − | − | + | − | − |
| Eye: | |||||||
| Leber amaurosis/other retinopathy | − | +/+ | +/+ | − | +/+ | − | − |
| Chorio-retinal colobomas | (+) | (+) | − | + | (+) | − | − |
| Other: | |||||||
| Hepatic fibrosis | − | − | (+) | + | (+) | − | − |
| Other malformations | (+) | (+) | (+) | (+) | (+) | − | − |
| Genetics | JBTS1,other loci | JBTS1, CORS2, other loci | NPHP1-3-4,other loci | ? | ? | NPHP1,other loci | CORS2,other loci? |
Note.— + = always present; (+) = not always present; − absent; ? = unknown; +/? = results of neuroradiological examination not described in some cases.
COACH = cerebellar vermis hypoplasia/aplasia, oligophrenia, atiaxia congenita, coloboma ocular, and hepatic fibrosis.
Present family.
The present classification of CORSs remains complex, making it difficult to pool families together for gene-mapping efforts. In fact, a genomewide search in 10 small families with JS failed to detect significant linkage. Families were not clinically homogeneous, because some patients had additional renal or ocular involvement (Chance et al. 1999). A different approach, based on direct mutation screening of candidate genes (such as genes involved in cerebellar development) in patients with sporadic JS and in small families with the syndrome, has not proved fruitful so far (Pellegrino et al. 1997; Blair et al. 2002). This could be due to the difficulty of selecting suitable candidate genes solely on the basis of their expected function and expression pattern, given the limited knowledge of the underlying pathogenetic mechanisms of these syndromes.
A powerful approach toward the identification of recessive disease genes is homozygosity mapping in large consanguineous families, but such families are rare. Since 1999, only one locus (JBTS1) has been mapped to chromosome 9q34.3, in two large inbred families with JS. All patients presented with ataxia, hypotonia, severe psychomotor delay, and oculomotor abnormalities. In one patient, a retinopathy was also present. Neuroimaging showed the typical MTI (Saar et al. 1999; Sztriha et al. 1999). Linkage to JBTS1 was excluded in several other families with JS, supporting genetic heterogeneity (Saar et al. 1999; Blair et al. 2002). Meanwhile, four genetic loci responsible for isolated nephronophthisis (the most frequent inherited cause of chronic renal failure in children) have been identified on chromosomes 2q13 (NPHP1), 9q22-31 (NPHP2), 3q22 (NPHP3), and 1p36 (NPHP4); and two genes (nephrocystin [NPHP1] and nephroretinin [NPHP4]) have been cloned (Hildebrandt et al. 1997; Haider et al. 1998; Omran et al. 2000; Mollet et al. 2002; Otto et al. 2002). Several families presenting with nephronophthisis associated with Leber congenital amaurosis (Senior-Löken syndrome [MIM 266900]) have shown linkage to the NPHP3 or NPHP4 loci (Omran et al. 2002; Schuermann et al. 2002). Moreover, deletions or point mutations in the NPHP1 gene have been reported in patients with sporadic nephronophthisis and a milder form of retinopathy (Caridi et al. 1998) and in patients with nephronophthisis and congenital oculomotor apraxia (COGAN syndrome [MIM 257550]) (Saunier et al. 1997; Betz et al. 2000). The latter phenotype was also described in a family carrying a heterozygous mutation in the NPHP4 gene (Mollet et al. 2002). Unfortunately, neurological and neuroradiological data are not reported for most of these patients.
A definite association of JS with proven MTI and nephronophthisis but without retinal involvement has never been reported within the spectrum of CORSs. Here, we describe a large consanguineous family from Sicily with a syndrome characterized by neurological involvement, juvenile nephronophthisis, and the typical MTI, and we report the mapping of a novel locus to the pericentromeric region of chromosome 11. Four affected individuals (three affected sibs and a maternal uncle) were identified. A genealogical study traced the origin of the family to a common ancestral couple six generations back. The pedigree is shown in figure 1.
Figure 1.
Pedigree of the family and haplotypes of marker loci spanning the CORS2 locus on chromosome 11p11.2-q12.3. Blackened symbols denote affected individuals. A thin horizontal bar above a symbols indicates a member of the family who was examined clinically and who provided a blood sample. The blackened bar denotes the disease-associated haplotype. Arrow indicates the proband.
All four patients presented with nystagmus and ocular motor apraxia, with impairment of smooth pursuit and saccades; one (patient VI:1) also had bilateral ptosis and strabismus. Neonatal irregular breathing was never reported. Head circumference was within normal values, and no dysmorphic features were present. All patients had developmental delay and moderate-to-severe psychomotor retardation, hypotonia with well-preserved deep tendon reflexes, and truncal ataxia. Brain MRI revealed cerebellar vermian aplasia and the MTI (figure 2).
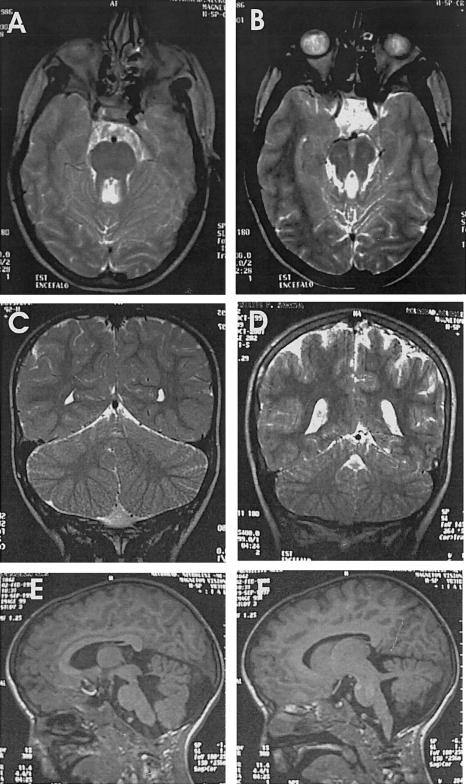
Figure 2.
Results of brain MRI in affected individuals. A, Patient VI:1. Axial T2-weighted image at the pontine level shows thickened superior cerebellar peduncles and umbrella-shaped fourth ventricle, giving the appearance of a molar tooth. B, Patient VI:1. Axial T2-weighted image at the mesencephalic level shows an abnormally deep interpeduncular fossa and thickened superior cerebellar peduncles. C, Patient VI:3. D, Patient VI:2. Coronal T2-weighted images in panels C and D show cerebellar hemispheres separated only by a thin sagittal cleft (buttock sign). E, Patient VI:3. Sagittal T1-weighted image demonstrates cerebellar vermian aplasia, a fourth ventricle enlarged and deformed. F, Patient VI:3. Sagittal T1-weighted image demonstrates prominent superior cerebellar peduncles running horizontally toward the brain stem.
All four patients had renal involvement. Patient V:7 (age 26 years) developed acute renal failure at the age of 17 years and underwent renal transplantation a few months later. Histological examination showed tubular basement membrane disintegration, tubular atrophy, and interstitial fibrosis; a diagnosis of juvenile nephronophthisis was made. Patient VI:1 (age 17 years) developed mild polyuria and polydipsia in late childhood. Renal ultrasonography showed kidneys of normal size, with increased echogenicity and normal corticomedullary differentiation. A urinary concentration test after intranasal administration of DDAVP (1-desamino-8-D-arginine vasopressin) showed a maximum osmolarity of 450 mOsm/kg H2O (normal values >850 mOsm/kg H2O), indicating defective urinary concentration capacity. At the age of 15 years, she developed chronic renal failure, but clinical symptoms did not worsen over time. The other two patients (VI:2 and VI:3), aged 12 and 8 years, are clinically asymptomatic, but the DDAVP concentration test revealed pathological values in both cases, the maximum osmolarity being 660 and 490 mOsm/kg H2O, respectively. In patient VI:3, renal ultrasonography showed increased kidney echogenicity. Renal cysts were not detected in any of the patients. Ocular examinations (electroretinogram, visual evoked potentials, and inspection of the fundus oculi) yielded normal results in all patients, ruling out a possible ocular involvement (such as a retinopathy or chorioretinal colobomas).
After informed consent was obtained, blood was sampled from the 4 patients and from 11 healthy family members. Linkage to JBTS1 and to all NPHP loci was excluded by genotyping microsatellite markers spanning the linked intervals; 400 microsatellite markers covering all autosomes (ABI PRISM Linkage Mapping Set, version 2) were then analyzed in the four patients. These individuals were homozygous for the same allele at 13 marker loci (6 contiguous markers on chromosome 11 and 7 isolated markers, 1 each on chromosomes 2, 4, 5, 7, 9, 13, and 15). The regions surrounding these loci were saturated with closely spaced microsatellite markers from the Marshfield genetic map. All available family members were genotyped, and haplotypes were constructed, assigning phase on the basis of the minimum number of recombinants. Two-point LOD scores were generated using the FASTLINK version of the MLINK program, assuming equal male-female recombination rate, autosomal recessive inheritance, a gene frequency of 0.001 with complete penetrance, and equal allele frequencies for each marker (Cottingham et al. 1993). A combination of negative LOD scores and the detection of different haplotypes carried by the affected individuals allowed exclusion of all the autosomes except the pericentromeric region of chromosome 11 (fig. 1). This region was further saturated with novel polymorphic dinucleotide repeats selected from the Human Genome Working Draft, using the tandem repeats finder software described by Benson (1999). The position of these novel markers on the physical map of human chromosome 11 is reported in table 2; primers and PCR conditions are available on request.
Table 2.
Pairwise LOD Scores between CORS2 and Markers on Chromosome 11p11.2-q12.3[Note]
|
LOD Score at θ = |
|||||||||
| Marker | cMa | Mbb | .0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D11S4083 | 47.06 | 37.171 | −∞ | 2.37 | 2.67 | 2.49 | 1.84 | 1.10 | .42 |
| J39003 | NA | 39.003 | −∞ | 1.64 | 1.98 | 1.83 | 1.22 | .58 | .18 |
| J42721 | NA | 42.721 | −∞ | 2.00 | 2.34 | 2.19 | 1.55 | .82 | .26 |
| D11S1993 | 54.09 | 44.293 | −∞ | 2.23 | 2.55 | 2.37 | 1.74 | 1.03 | .39 |
| D11S986 | 56.76 | 45.399 | 3.74 | 3.65 | 3.28 | 2.81 | 1.85 | .92 | .24 |
| D11S4109 | 58.40 | 48.479 | 1.63 | 1.58 | 1.37 | 1.12 | .64 | .29 | .10 |
| D11S1313 | 58.40 | 57.747 | 3.96 | 3.87 | 3.52 | 3.07 | 2.14 | 1.21 | .41 |
| J58329 | NA | 58.329 | 3.49 | 3.41 | 3.07 | 2.64 | 1.76 | .91 | .26 |
| J59897 | NA | 59.897 | 3.44 | 3.36 | 3.02 | 2.59 | 1.71 | .87 | .25 |
| D11S4191 | 60.09 | 61.513 | 2.84 | 2.79 | 2.55 | 2.24 | 1.59 | .92 | .33 |
| J61808 | NA | 61.808 | 2.80 | 2.74 | 2.51 | 2.21 | 1.56 | .89 | .30 |
| J61943 | NA | 61.943 | 1.57 | 1.53 | 1.38 | 1.18 | .78 | .40 | .11 |
| D11S1765 | 61.78 | 62.291 | 2.80 | 2.74 | 2.51 | 2.20 | 1.56 | .89 | .30 |
| D11S4076 | 62.62 | 62.938 | 2.80 | 2.74 | 2.51 | 2.21 | 1.56 | .89 | .30 |
| J63302 | NA | 63.302 | 1.12 | 1.09 | .99 | .85 | .59 | .35 | .15 |
| D11S1883 | 65.05 | 64.949 | −∞ | 1.11 | 1.55 | 1.52 | 1.13 | .64 | .24 |
Note.— Novel polymorphic dinucleotide repeats selected from the Human Genome Working Draft using the Tandem Repeat Finder software; names have been arbitrarily assigned.
Position of the microsatellite marker on the genetic map. NA = information not available because markers are newly generated.
Position of the microsatellite marker on the physical map.
All four affected individuals in the family shared a large region of homozygosity between markers D11S4083 and J63302, identifying a novel locus. To prevent possible confusion in the nomenclature of new genetic loci that could result from the significant clinical and genetic heterogeneity of these syndromes, we propose to adopt the acronym “CORS” for loci identified in all cerebello-oculo-renal syndromes sharing the pathognomonic MTI, including JS and incomplete phenotypes such as COGAN and cerebello-renal syndromes. So far, only JBTS1 has been clearly associated with the MTI (Saar et al. 1999; Sztriha et al. 1999). We therefore named the present locus “CORS2,” suggesting “CORS1” as an alternative symbol for the JBTS1 locus on chromosome 9q34.
The maximum LOD score obtained was 3.96, with a recombination fraction (θ) of 0.00, for marker D11S1313 (table 2). A substantial refinement of the linked region was obtained by considering haplotypes in individual V:9, who is the healthy sister of patient V:7 and shares with him a region of homozygosity at the upper end of the interval. A recombination event between markers D11S1993 and D11S986 on her maternal chromosome marks the upper boundary of the CORS2 locus, which maps to an 11-cM interval between flanking markers D11S1993 and D11S1883, spanning cytogenetic bands 11p11.2-q12.3. The linked region covers 20.6 Mb on the physical map of chromosome 11 and comprises ∼5 Mb of centromeric DNA.
The lower extent of the CORS2 locus could be further refined by analyzing haplotypes in unaffected family members V:3 and V:5. In fact, individual V:5 (father of the three affected sibs) and his brother (individual V:3) also share with the patients a region of homozygosity, from D11S4191 to J63302. The identification of a common ancestor in the family and the high degree of consanguineous marriage among family members strongly suggests that this region of homozygosity is also due to identity by descent, thereby identifying D11S4191 as the lower flanking marker of the linked interval and reducing the locus to 6 cM/17.2 Mb, still encompassing the centromere. However, in this case, identity by descent cannot be definitely proved, because of the lack of an affected individual in the sibship of V:3 and V:5, which accounts for the positive LOD scores obtained for markers D11S4191 to J63302 (table 2).
The coexistence of renal and cerebellar abnormalities in the present family could facilitate the selection of candidate genes, because the pathogenetic mechanism leading to nephronophthisis has been partly revealed by functional studies on nephrocystin and nephroretinin proteins. Nephrocystin, which is encoded by the NPHP1 gene, contains several domains of protein-protein interaction. It is thought to be involved in focal adhesion and/or adherence-junction signaling, through its interaction with the p130cas protein, a major mediator of focal adhesion assembly, and with E-cadherin, a constitutive protein of adherence junctions of epithelial cells (Hildebrandt and Otto 2000). Nephroretinin, a product of the NPHP4 gene, has a proline-rich domain, and it has been shown to interact with the SH3 domain of nephrocystin, suggesting a role as an additional partner of the nephrocystin multimolecular signaling complex that regulates cell-cell and cell-matrix adhesion (Mollet et al. 2002).
Candidate genes for CORS2 are CTNND1 (coding for p120ctn catenin, a protein that binds to E-cadherin and is implicated in cell-cell adhesion and ligand-induced receptor signaling) and LOC90139 (coding for a tetraspanin similar to uroplakin1, an integral protein playing a major role in urinary epithelium adhesion mechanisms). Also, other possible candidate genes within the CORS2 locus code for proteins expressed in the CNS and the kidney and putatively involved in neurite growth, focal adhesion processes, and signal transduction pathways (MDK, ARHGAP1, Pacsin 3, and MADD). Yet the CORS2 physical region is still large and contains >100 genes, several of which are of unknown function. The identification of other families linked to CORS2 would greatly help to refine this genetic locus, thereby reducing the number of possible candidate genes. Because large informative families are rare, linkage efforts often rely on pooling small families. Recently, a genomewide search in 20 small families with JS/CORSs and the MTI showed evidence of linkage to the same pericentromeric region of chromosome 11 (CORS2) in 3 consanguineous families (Keeler et al. 2003 [in this issue]). The independent identification of the same locus in four distinct families by use of different approaches strongly suggests that CORS2 could be a relevant locus for JS and CORSs. However, further work on additional families is needed to assess the prevalence of CORS2 and to establish genotype-phenotype correlations.
This is the first report of a pure cerebello-renal syndrome with a neuroradiologically proven MTI. Saraiva and Baraitser (1992) divided patients with JS into two groups, based on the absence (type A) or presence (type B) of retinopathy, and suggested that renal involvement in JS occurs only when retinal abnormalities are also present. Nevertheless, occasional descriptions of cerebello-renal phenotypes can be found in the literature, although neuroradiological images showing the MTI are never presented in these cases. Maria and colleagues (1999b) described six patients with JS who had renal, but not retinal or other ocular, abnormalities; a clinical description of the neurological and renal phenotype in these patients was not provided. The two siblings with JS and nephronophthisis described by Steinlin and coworkers (1997) did not have a retinopathy, but fundus oculi examination revealed a “fundus flavus,” representing a mild, clinically asymptomatic retinal abnormality. Electroretinogram performed in one sib at age 1 year showed normal results, and a follow-up examination at age 10 years still did not reveal signs of ocular involvement (Steinlin et al. 1997). In two of the four patients with COGAN syndrome and nephronophthisis who carried deletions in the NPHP1 gene, oculomotor abnormalities were associated with motor delay, cerebellar ataxia, and/or mild mental retardation, and, in two of them, vermian hypoplasia was described. Each of these patients had at least one sib with isolated nephronophthisis, suggesting that this phenotype could also be part of the spectrum of CORSs (Saunier et al. 1997; Satran et al. 1999).
The rare occurrence of pure cerebello-renal syndromes could result, in part, from the underdiagnosis of renal involvement in patients with JS. In the present family, the two youngest patients, ages 8 and 10 years, did not show any clinical sign of renal involvement, such as polyuria or polydipsia. Creatinine and other parameters of kidney function were within normal limits, and results of kidney ultrasound were normal, at least in patient VI:2. The presence of a positive family history for nephronophthisis prompted us to perform a DDAVP test, which showed an abnormal urinary concentration ability in both patients. This is in line with the natural history of isolated juvenile nephronophthisis, which can be asymptomatic or mildly symptomatic for years before leading to end-stage renal disease, usually in late childhood or adolescence (Hildebrandt and Otto 2000).
In agreement with the research directions recommended by the Joubert Syndrome Workshop in 1998 (Spinella 1999), we believe that the establishment of an internationally recognized diagnostic algorithm, to be followed in each patient who is suspected of having CORS or MTI, is a crucial step toward obtaining a precise phenotypic characterization of these syndromes, facilitating the selection of homogeneous families to be pooled for genetic studies. The application of a standardized diagnostic protocol would allow information about families with CORSs to be shared among different research groups, in an international effort aimed at collecting large numbers of informative families, thus optimizing linkage approaches. We also propose a specific genetic nomenclature (“CORS”) for all loci involved in CORSs, JS, and related syndromes sharing the MTI, because this peculiar neuroradiological sign appears to be the unifying and characteristic feature of these clinically heterogeneous disorders.
Acknowledgments
We thank Dr. Carmelo Fede, for performing DDAVP test in patients, and Dr. Daniela Zuccarello, for collecting blood samples of some family members. We are grateful to Dr. Joseph Gleeson and collaborators for exchanging manuscripts prior to submission. This work was partly supported by the Italian Ministry of Health (Ricerca Finalizzata 2002 n. ICS160.3/RF02.160; Ricerca Corrente 2003) and the Italian Ministry of University and Research (COFIN 2002).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics/ (for chromosome 11 genetic map)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for COGAN syndrome, JS, and Senior-Löken syndrome)
- UCSC Genome Bioinformatics, http://genome.cse.ucsc.edu/ (for chromosome 11 physical map; November 2002)
References
- Benson G (1999) Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res 27:573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz R, Rensing C, Otto E, Mincheva A, Zehnder D, Lichter P, Hildebrandt F (2000) Children with ocular motor apraxia type COGAN carry deletions in the gene (NPHP1) for juvenile nephronophthisis. J Pediatr 136:828–831 [PubMed] [Google Scholar]
- Blair IP, Gibson RR, Bennett CL, Chance PF (2002) Search for genes involved in Joubert syndrome: evidence that one or more major loci are yet to be identified and exclusion of candidate genes EN1, EN2, FGF8 and BARHL1. Am J Med Genet 107:190–196 [PubMed] [Google Scholar]
- Caridi G, Murer L, Bellantuono R, Sorino P, Caringella DA, Gusmano R, Ghiggeri GM (1998) Renal-retinal syndromes: association of retinal anomalies and recessive nephronophthisis in patients with homozygous deletions of the NPH1 locus. Am J Kidney Dis 32:1059–1062 [DOI] [PubMed] [Google Scholar]
- Chance PF, Cavalier L, Satran D, Pellegrino JE, Koenig M, Dobyns WB (1999) Clinical nosologic and genetic aspects of Joubert and related syndromes. J Child Neurol 14:660–666 [DOI] [PubMed] [Google Scholar]
- Cottingham RW Jr, Idury RM, Schäffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- Haider NB, Carmi R, Shalev H, Sheffield VC, Landau D (1998) A Bedouin kindred with infantile nephronophthisis demonstrates linkage to chromosome 9 by homozygosity mapping. Am J Hum Genet 63:1404–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J, Hanusch H, Brandis M (1997) A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet 17:149–153 [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Otto E (2000) Molecular genetics of nephronophthisis and medullary cystic kidney disease. J Am Soc Nephrol 11:1753–1761 [DOI] [PubMed] [Google Scholar]
- Keeler LC, Marsh SE, Leeflang EP, Woods CG, Sztriha L (2003) Linkage analysis in families with Joubert syndrome plus oculo-renal involvement identifies the CORS2 locus on chromosome 11p12-q13.3. Am J Hum Genet 73:656–662 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria BL, Boltshauser E, Palmer SC, Tran TX (1999a) Clinical features and revised diagnostic criteria in Joubert syndrome. J Child Neurol 14:583–591 [DOI] [PubMed] [Google Scholar]
- Maria BL, Hoang KB, Tusa RJ, Mancuso AA, Hamed LM, Quisling RG, Hove MT, Fennell EB, Booth-Jones M, Ringdahl DM, Yachnis AT, Creel G, Frerking B (1997) “Joubert syndrome” revisited: key ocular motor signs with magnetic resonance imaging correlation. J Child Neurol 12:423–430 [DOI] [PubMed] [Google Scholar]
- Maria BL, Quisling RG, Rosainz LC, Yachnis AT, Gitten J, Dede D, Fennell E (1999b) Molar tooth sign in Joubert syndrome: clinical, radiological, and pathologic significance. J Child Neurol 14:368–376 [DOI] [PubMed] [Google Scholar]
- Mollet G, Salomon R, Gribouval O, Silbermann F, Bacq D, Landthaler G, Milford D, Nayir A, Rizzoni G, Antignac C, Saunier S (2002) The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nature Genet 32:300–305 [DOI] [PubMed] [Google Scholar]
- Omran H, Fernandez C, Jung M, Häffner K, Fargier B, Villaquiran A, Waldherr R, Gretz N, Brandis M, Ruschendorf F, Reis A, Hildebrandt F (2000) Identification of a new gene locus for adolescent nephronophthisis, on chromosome 3q22 in a large Venezuelan pedigree. Am J Hum Genet 66:118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E, Hoefele J, Ruf R, Mueller AM, Hillet KS, Wolf MTF, Schuermann MJ, Becker A, Birkenhäger R, Sudbrak R, Hennies HC, Nürnberg P, Hildebrandt F (2002) A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet 71:1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino JE, Lensch MW, Muenke M, Chance PF (1997) Clinical and molecular analysis in Joubert syndrome. Am J Med Genet 72:59–62 [DOI] [PubMed] [Google Scholar]
- Saar K, Al-Gazali L, Sztriha L, Rüeschendorf F, Nur-E-Kamal M, Reis A, Bayoumi R (1999) Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am J Hum Genet 65:1666–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva JM, Baraitser M (1992) Joubert syndrome: a review. Am J Med Genet 43:726–731 [DOI] [PubMed] [Google Scholar]
- Satran D, Pierpoint MEM, Dobyns WB (1999) Cerebello-oculo-renal syndromes including Arima, Senior-Löken and COACH syndromes: more than just variants of Joubert syndrome. Am J Med Genet 86:459–469 [PubMed] [Google Scholar]
- Saunier S, Morin G, Calado J, Bennessy F, Silbermann F, Antignac C (1997) Large deletions of the NPH1 region in COGAN syndrome (CS) associated with familial juvenile nephronophthisis (NPH). Am J Hum Genet Suppl 61:A346 [Google Scholar]
- Schuermann MJ, Otto E, Becker A, Saar K, Rüschendorf F, Polak BC, Ala-Mello S, Hoefele J, Wiedensohler A, Haller M, Omran H, Nürnberg P, Hildebrandt F (2002) Mapping of gene loci for nephronophthisis type 4 and Senior-Löken syndrome, to chromosome 1p36. Am J Hum Genet 70:1240–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella GM (1999) Research directions: follow-up of the Joubert Syndrome Workshop, October 21, 1998. J Child Neurol 14:667–668 [DOI] [PubMed] [Google Scholar]
- Steinlin M, Schmid M, Landau K, Boltshauser E (1997) Follow-up in children with Joubert syndrome. Neuropediatrics 28:204–211 [DOI] [PubMed] [Google Scholar]
- Sztriha L, Al-Gazali LI, Aithala GR, Nork M (1999) Joubert’s syndrome: new cases and review of clinicopathologic correlation. Pediatr Neurol 20:274–281 [DOI] [PubMed] [Google Scholar]