Abstract
The process of surface adhesion and biofilm development is a survival strategy employed by virtually all bacteria and refined over millions of years. This process is designed to anchor microorganisms in a nutritionally advantageous environment and to permit their escape to greener pastures when essential growth factors have been exhausted. Bacterial attachment to a surface can be divided into several distinct phases, including primary and reversible adhesion, secondary and irreversible adhesion, and biofilm formation. Each of these phases is ultimately controlled by the expression of one or more gene products. Ultrastructurally, the mature bacterial biofilm resembles an underwater coral reef containing pyramidal or mushroom-shaped microcolonies of organisms embedded within an extracellular glycocalyx, with channels and cavities to allow the exchange of nutrients and waste. The biofilm protects its inhabitants from predators, dehydration, biocides, and other environmental extremes while regulating population growth and diversity through primitive cell signals. From a physiological standpoint, surface-bound bacteria behave quite differently from their planktonic counterparts. Recognizing that bacteria naturally occur as surface-bound and often polymicrobic communities, the practice of performing antimicrobial susceptibility tests using pure cultures and in a planktonic growth mode should be questioned. That this model does not reflect conditions found in nature might help explain the difficulties encountered in the management and treatment of biomedical implant infections.
INTRODUCTION
Given their druthers, bacteria prefer a community-based, surface-bound, sedentary lifestyle to a nomadic existence. ZoBell and others (45, 62, 89, 98, 100) recognized this tendency early in the twentieth century as a habitual characteristic of aquatic bacterial populations. In fact, these early observations provided tremendous insight into contemporary models of bacterial adhesion, given the unavailability of analytic and molecular tools at the time.
In natural aquatic ecosystems, surface-associated microorganisms vastly outnumber organisms in suspension (100). The propensity for bacteria to colonize surfaces is advantageous from an ecological standpoint because it preferentially targets specialized microorganisms to specific locations, encouraging symbiotic relationships. Examples of these relationships are abundant in nature and include the prokaryotic diazotrophs that colonize the roots of legumes (76) and the diverse residential microbial flora inhabiting the digestive tract of ruminants that promotes the degradation and recycling of insoluble materials (10).
The inclination for bacteria to become surface bound is so ubiquitous in diverse ecosystems that it suggests a strong survival and/or selective advantage for surface dwellers over their free-ranging counterparts (17, 20, 100). There may be an obvious explanation for bacterial adhesion, because nutrients in an aqueous environment tend to concentrate near a solid surface (99). That many specialized structures and complex ligand interactions have evolved in prokaryotes designed specifically for surface recognition and biofilm formation is another clue supporting the importance of microbial adhesion (2, 6). From an evolutionary standpoint, the selective advantage of bacterial adhesion has been postulated to favor the localization of surface-bound bacterial populations in nutritionally favorable, nonhostile environments and at the same time provide some level of protection from external predation (18). When the environment ceases to support the bacterial load, the equilibrium is shifted to favor dissociation of individual cells from the biofilm to seek more favorable habitats (i.e., to go where no bacterium has gone before).
The inclination for bacteria to colonize surfaces is a double-edged sword, however, that can prove either beneficial or potentially destructive. Whereas nitrogen fixation and bioremediation of wastewater are beneficial functions of microbial biofilms, biofouling, i.e., the obstruction of fluid flow through conduits, over surfaces, through filters, or across heat exchangers, and corrosion are major economic liabilities of the food, maritime, petroleum, and manufacturing industries (8, 17). In some instances, the corrosive damage caused by bacterial biofilms provides a never-ending source of economic benefit, as most of us have appreciated after a trip to the dentist.
Over the past century, the study of microbial adhesion has generated a language all its own. Since many of these terms are still in use, a brief discussion of their meaning with reference to biofilm development would be apropos. The terms sessile and planktonic have evolved to describe surface-bound and free-floating microorganisms, respectively. The surface of interest to which sessile organisms are attached can be either abiotic (inert materials) or biotic (living tissue or cells). A glycocalyx is the glue that holds the biofilm fast to the colonized surface and is a complex of exopolysaccharides of bacterial origin and trapped exogenous substances found in the local environment, including nucleic acids, proteins, minerals, nutrients, cell wall material, etc. (17). One of the more figurative descriptors to appear in the bacterial adhesion literature is slime, a term used by Heukelekian and Heller in 1940 (47) to describe a bacterial biofilm layer and resurrected by Christensen et al. in 1982 (11) to designate the glycocalyx produced by highly adherent strains of Staphylococcus epidermidis recovered from infected biomedical implants.
WHAT IS A BIOFILM?
Costerton et al. (20) define a biofilm as “a structured community of bacterial cells enclosed in a self-produced polymeric matrix and adherent to an inert or living surface.” Elder and colleagues (33) describe a biofilm in more cooperative terms as “a functional consortium of microorganisms organised within an extensive exopolymer matrix,” whereas Carpentier and Cerf (8) simplify the concept as “a community of microbes embedded in an organic polymer matrix, adhering to a surface.” Underlying each of these definitions are the three basic ingredients of a biofilm: microbes, glycocalyx, and surface. If one of these components is removed from the mix, a biofilm does not develop. Clearly, this is an oversimplification of a fairly complex process that does not take into account the type of microorganism, the composition of the surface, or the influences of environmental factors, but any of these definitions will serve as a starting point for the balance of this review. Indeed, it will be discussed later that a primitive yet functional degree of organization and cooperativity exists with biofilms to allow maximum interaction with the environment without compromising cell survival or exhausting available resources. While a diverse number of microorganisms are capable of generating biofilms, only bacteria will be considered throughout this discussion.
BIOFILMS ARE UNIVERSAL
Virtually any surface—animal, mineral, or vegetable (i.e., biotic or abiotic)—is fair game for bacterial colonization and biofilm formation, including contact lenses, ship hulls, dairy and petroleum pipelines, rocks in streams, and all varieties of biomedical implants and transcutaneous devices (for reviews, see references 8, 17, 19, 20, and 33). In many circumstances, the surface might also be a nutrient source, such as cellulose in the paper industry (19). One has only to experience the process of cleaning a J trap in a clogged sink drain to fully appreciate the potential magnitude of bacterial biofilms and the process of biofouling on a small scale. While surfaces or surface coatings that retard bacterial adhesion have been described (86), none have been developed that prevent it (18). Similarly, no bacterial species has been observed to exist in a fully planktonic state under all growth conditions (8, 12).
THE BIOFILM GLYCOCALYX: A BACTERIAL FORCE FIELD
Bacterial exopolysaccharides are the main component of the biofilm glycocalyx, which has also been coined the slime layer (7, 16). When fully hydrated, the glycocalyx is predominantly water (16). In most species, the glycocalyx is predominantly anionic and creates an efficient scavenging system for trapping and concentrating essential minerals and nutrients from the surrounding environment (8, 17). We take advantage of the scavenging ability of biofilms in the process of wastewater treatment. As an added advantage, the glycocalyx provides a certain degree of protection for its inhabitants against certain environmental threats, including biocides, antibiotics, antibody, surfactants, bacteriophages, and foraging predators such as free-living amoebae and white blood cells (reviewed in references 8, 17, 19, and 33). In essence, the glycocalyx creates a three-dimensional force field that surrounds, anchors, and protects surface-bound bacteria (Fig. 1).
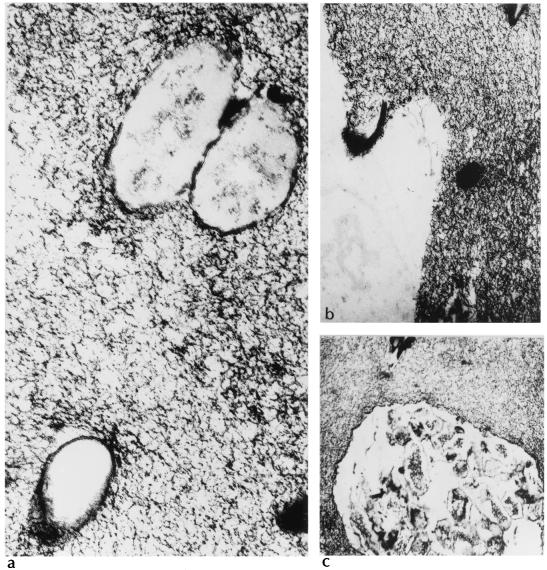
FIG. 1.
Thin-section electron micrographs of exopolysaccharide obtained from a liquid culture of a mucoid strain of Pseudomonas aeruginosa. The micrographs demonstrate individual cells (a and b) and microcolonies (c) entrapped in a matrix of alginic acid exopolysaccharide. Magnification, ×50,000 for panel a and ×30,000 for panels b and c.
BIOFILM DIVERSITY
When a biofilm is composed of heterogeneous species (which is more likely in nature than single species), the metabolic by-products of one organism might serve to support the growth of another, while the adhesion of one species might provide ligands allowing the attachment of others (17, 60, 66, 94). Conversely, the competition for nutrients and accumulation of toxic by-products generated by primary colonizers can limit the species diversity within a biofilm (66).
PROCESS OF BACTERIAL ADHESION
The process of bacterial attachment to an available surface (living or abiotic) and the subsequent development of a biofilm can be described in fairly simple or incredibly elaborate terms depending on the level of detail required or sought. Obviously, the process is dictated by a number of variables, including the species of bacteria, surface composition, environmental factors, and essential gene products. As an oversimplified rule of thumb, primary adhesion between bacteria and abiotic surfaces is generally mediated by nonspecific (e.g., hydrophobic) interactions, whereas adhesion to living or devitalized tissue is accomplished through specific molecular (lectin, ligand, or adhesin) docking mechanisms (8).
In its most basic form, bacterial adhesion (as a process distinct from but integral in biofilm formation) can be divided into two stages: the primary or docking stage and the secondary or locking phase (2, 76, 68). This process conjures up images of the Russian Soyuz shuttle docking with the Mir space station. Some authors will include an additional step in this process called surface conditioning to describe the interaction of the substratum with its environment (6, 41). Conditioning occurs, for example, when a foreign body is placed in the bloodstream and the native surface is modified by the adsorption of water, albumin, lipids, extracellular matrix molecules, complement, fibronectin, inorganic salts, etc. Once a surface has been conditioned, its properties are permanently altered, so that the affinity of an organism for a native or a conditioned surface can be quite different.
Docking: Primary Bacterial Adhesion
Primary adhesion constitutes the serendipitous meeting between a conditioned surface and a planktonic microorganism. This stage is reversible and is dictated by a number of physiochemical variables that define the interaction between the bacterial cell surface and the conditioned surface of interest (2, 67). First, the organism must be brought into close approximation of the surface, propelled either randomly (for example, by a stream of fluid flowing over a surface) or in a directed fashion via chemotaxis and motility. Once the organism reaches critical proximity to a surface (usually <1 nm), the final determination of adhesion depends on the net sum of attractive or repulsive forces generated between the two surfaces. These forces include electrostatic and hydrophobic interactions, steric hindrance, van der Waals forces, temperature, and hydrodynamic forces, to name a few (reviewed eloquently in references 2 and 8). Electrostatic interactions tend to favor repulsion, because most bacteria and inert surfaces are negatively charged (8, 54). Stenotrophomonas maltophilia is one exception to this rule, and the overall positive surface charge of this organism at physiological pH can promote primary adhesion to negatively charged materials such as Teflon (54). Hydrophobic interactions probably have greater influence on the outcome of primary adhesion (8).
Surface Conditioning
It is important to remember, however, that primary contact generally occurs between an organism and a conditioned surface and the hydrophobicity of the latter can vary greatly depending on the molecules in the conditioning film (2, 6). For example, Wang et al. demonstrated that primary adhesion of Staphylococcus epidermidis to polyethylene disks was enhanced in the presence of surface-activated platelets and reduced by adsorbed plasma proteins relative to uncoated polyethylene (90). Using polymethylmethacrylate as a substratum, Herrmann et al. (46) showed that the adhesion of coagulase-negative staphylococci was enhanced when the surface had been coated with various plasma proteins, including fibronectin. Dunne and Burd (30), however, provided evidence that fibronectin and proteolytic fragments of fibronectin produced a dose-dependent reduction in the adhesion of S. epidermidis to coated plastic surfaces, so there is plenty of room for further elucidation.
A net repulsion between two surfaces can be overcome by specific molecular interactions mediated by adhesins located on structures extending from the cell surface, such as pili (2, 6). The longevity of primary adhesion depends on the sum total of all these variables, but surface chemistry pushes the equilibrium in favor of adhesion by predicting that organic substances in solution will concentrate near a surface and that microorganisms tend to congregate in nutrient-rich environs (8, 19, 99).
Locking: Secondary Bacterial Adhesion
The second stage of adhesion is the anchoring or locking phase and employs molecularly mediated binding between specific adhesins and the surface (2). At this point, loosely bound organisms consolidate the adhesion process by producing exopolysaccharides that complex with surface materials and/or receptor-specific ligands located on pili, fimbriae, and fibrillae, or both. At the conclusion of the second stage, adhesion becomes irreversible in the absence of physical or chemical intervention, and the organism is attached firmly to the surface like a cocoon on a leaf. With certain organisms, several distinct adhesins might be used for surface attachment depending on the environment. In the case of Vibrio cholerae El Tor, a toxin-coregulated pilus is used to attach to and colonize intestinal epithelium during the process of human infection, whereas a mannose-sensitive hemagglutinin is the primary adhesin used to anchor to abiotic surfaces in an aquatic environment (91).
During this stage of adhesion, planktonic microorganisms can also stick to each other or different species of surface-bound organisms, forming aggregates on the substratum. Interestingly, the presence of one species of microorganism on a surface can promote the adhesion of another (60, 70). All bacteria produce multiple adhesins, and some are regulated at the transcriptional level, permitting organisms to switch from sessile to planktonic forms under different environmental influences (2, 96). Such is the case with S. epidermidis, which produces a polysaccharide intercellular adhesin (PIA) that is essential for cell-to-cell adhesion and subsequent biofilm formation (42, 43, 64, 65).
BIOFILM MATURATION
Once bacteria have irreversibly attached to a surface, the process of biofilm maturation begins. The overall density and complexity of the biofilm increase as surface-bound organisms begin to actively replicate (and die) and extracellular components generated by attached bacteria interact with organic and inorganic molecules in the immediate environment to create the glycocalyx. In the case of infected biomedical implants, this might include host-derived inflammatory response proteins or matrix proteins such as complement, fibrinogen, fibronectin, and glycosaminoglycans attached to the conditioned device.
The growth potential of any bacterial biofilm is limited by the availability of nutrients in the immediate environment, the perfusion of those nutrients to cells within the biofilm, and the removal of waste. In addition, there exists an optimum hydrodynamic flow across the biofilm that favors growth and perfusion rather than erosion of the outermost layers (8). Other factors that control biofilm maturation include internal pH, oxygen perfusion, carbon source, and osmolarity (8, 73). At some point, the biofilm reaches a critical mass, and a dynamic equilibrium is reached at which the outermost layer of growth (farthest from the surface) begins to generate planktonic organisms. These organisms are now free to escape the biofilm and colonize other surfaces. Cells nearest the surface become quiescent or die secondary to a lack of nutrients or perfusion, decreased pH, pO2, or an accumulation of toxic metabolic by-products (57).
Recent evidence suggests that the primary development, maturation, and breakdown of a biofilm might be regulated at the level of population density-dependent gene expression controlled by cell-to-cell signaling molecules such as acylated homoserine lactones (1, 25, 69, 87). Once fully matured, a biofilm generates altered patterns of bacterial growth, physiological cooperation, and metabolic efficiency, all of which provide a form of functional communal coordination that mimics primitive eukaryotic tissue (18, 19).
BIOFILM RESISTANCE TO ANTIMICROBIAL AGENTS
When dealing with infected biomedical implants, it is important to recognize that bacteria present in a mature biofilm behave quite differently from their planktonic counterparts. In particular, biofilm organisms are far more resistant to antimicrobial agents than are organisms in suspension. In some extreme cases, the concentrations of antibiotics required to achieve bactericidal activity against adherent organisms can be three to four orders of magnitude higher than for planktonic bacteria, depending on the species-drug combination (9, 85). At least three mechanisms have been proposed to account for the increased resistance of biofilms to antimicrobial agents.
Biofilm as a Molecular Filter
The first of these mechanisms suggests that the biofilm glycocalyx prevents the perfusion of biocides to cellular targets, while the second recognizes the nearly dormant growth pattern of bacterial populations within the biofilm that renders organisms indifferent to antibiotic activity. The third proposes that the microenvironment of the biofilm adversely affects the activity of the antimicrobials (4, 9, 40, 85).
In support of the first of these proposals, Farber et al. (35) found that cell extracts of the slime polysaccharide of S. epidermidis interfered with the antimicrobial activity of glycopeptide antibiotics. The addition of 0.5% slime extract to broth microdilution susceptibility plates increased the MIC of both vancomycin and teichoplanin approximately fivefold versus both slime-positive and -negative strains. Slime also negated the synergistic effects of both vancomycin and gentamicin while having no effect on the activity of clindamycin, rifampin, and cefazolin. The authors suggested that the slime either physically complexes with and inactivates glycopeptides or coats the cell wall to create a permeability barrier. In an in vitro model, however, Dunne et al. (31) showed that a slime-positive S. epidermidis biofilm did not prevent the perfusion of vancomycin or rifampin, nor could it be sterilized in the presence of either or both antibiotics at concentrations exceeding bactericidal levels (Fig. 2). Nickel and colleagues (72) created an artificial Pseudomonas aeruginosa biofilm on urinary catheter material using a modified Robbins device and showed that exposure to 1 g of tobramycin/ml for 12 h did not sterilize the biofilm. Interestingly, the MIC of tobramycin for the surviving organisms was not affected. In a related study, Nichols et al. (71) investigated the binding of [3H]tobramycin to the alginic acid exopolysaccharide produced by mucoid strains of Pseudomonas aeruginosa and to commercially prepared alginate. The authors were able to show concentration-dependent binding of tobramycin to both. As further evidence of this activity, the addition of 1% alginate to tobramycin in a well diffusion assay demonstrated reduced zones of inhibition versus Escherichia coli and Staphylococcus aureus, indicating that this exopolysaccharide interferes with either the antimicrobial action of the drug or the perfusion of tobramycin through the medium.
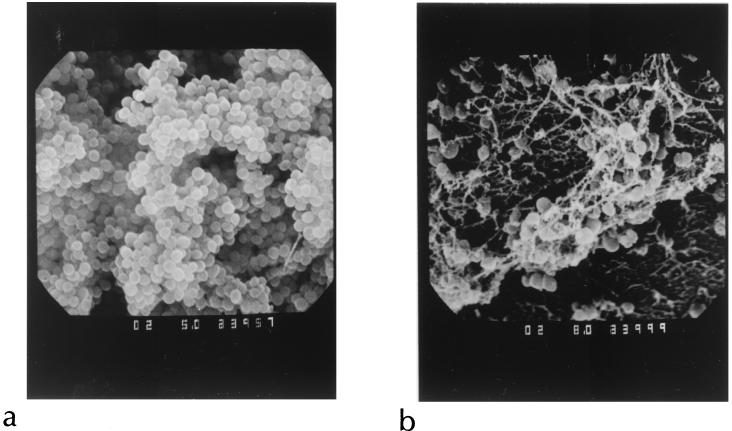
FIG. 2.
Scanning electron micrograph of an untreated biofilm of S. epidermidis (a) and an identical biofilm exposed to vancomycin and rifampin for 72 h at concentrations exceeding the MIC and MBC for the organism (b). Despite obvious changes in the treated biofilm, viable organisms were recovered for which the MIC and MBC of both agents were unaltered. Reprinted from reference 31 with permission of the American Society for Microbiology.
These observations were supported by the report of Coquet et al. (15), who exposed alginate-embedded biofilms of P. aeruginosa to 15 times the MIC of tobramycin, 20 times the MIC of imipenem, or both and compared the results to those for planktonic cultures of the same organism. While the planktonic cultures showed an approximately 100,000-fold reduction in the viable cell count after 6 h of exposure to either tobramycin or imipenem, neither drug produced more than a 1,000-fold reduction in the viable cell count of embedded organisms after 10 h of incubation. Furthermore, while the combination of tobramycin and imipenem demonstrated a synergistic effect against planktonic organisms, no such effect was observed with alginate-embedded bacteria. Hoyle et al. (49) showed that biofilms of P. aeruginosa established on dialysis membranes retarded the diffusion of piperacillin. In the presence of Ca2+, the diffusion of piperacillin was completely prevented, presumably by creating an alginic acid-Ca2+ barrier matrix.
Increased Resistance Reflects Altered Growth Rates
Eng et al. (34) provided evidence in support of the second theory of biofilm resistance, i.e., that altered rates of bacterial growth dictate the response to antimicrobial agents. By controlling the growth rate of bacteria through nutrient limitation, the authors were able to demonstrate that only fluoroquinolone antibiotics produced bactericidal effects against stationary-phase gram-negative organisms. Furthermore, no class of antimicrobial agent was bactericidal versus growth-limited S. aureus. An increase in the nutrient concentration and subsequent growth rate was followed by an increase in the activity of multiple classes of antimicrobial agents. Gander and Gilbert (37) used ciprofloxacin-treated E. coli biofilms to replicate these findings. Anwar and others (3) were able to demonstrate age-related differences in the response of S. aureus biofilms to antimicrobial therapy. Exposure of 4-day-old biofilms to tobramycin and/or cephalexin produced a rapid reduction in viable-cell counts, whereas biofilms developed over a 13-day period demonstrated marked resistance to either drug or a combination of both. The authors suggest that these findings are due, at least in part, to the reduced metabolic activity of cells embedded in the aged biofilm.
Does the Microenvironment Affect Antimicrobial Activity?
In terms of microenvironment, it is likely that the same factors that adversely influence antimicrobial activity in vitro, including pH, pCO2, pO2, divalent cation concentration, hydration level, and pyrimidine concentration, will also produce undesirable effects at the deepest layers of a bacterial biofilm (53), where acidic and anaerobic conditions persist. While detailed studies of these factors vis-à-vis antibiotic activity in biofilm environs are lacking, one could predict, based on disk diffusion and broth microdilution susceptibility testing, that the activity of aminoglycosides, macrolides, and tetracyclines would likely be compromised in an acidic milieu with increased pCO2. Also, the polyanionic nature of the alginic acid exopolysaccharide of P. aeruginosa (28, 61) would certainly tend to concentrate divalent cations. This, in turn, would also affect the activity of aminoglycosides and tetracyclines (53).
All told, the intractability of biomedical implant infections to successful antimicrobial therapy is likely a result of the combination of the perfusion barrier, reduced growth rate, and extreme microenvironmental conditions of the biofilm. The use of antibiotics in the treatment of infected implants, however, likely pushes the equilibrium in favor of the sessile rather than planktonic growth state.
BIOFILM ARCHITECTURE
Scanning the Surface
From a three-dimensional perspective, we tend to think of bacterial biofilms as a mass of organisms uniformly distributed throughout a polysaccharide matrix overlaying a surface. Nothing could be further from the truth. Biofilms have been visualized by a variety of means, including light microscopy with computer enhancement, transmission electron microscopy, and scanning electron microscopy (17, 55, 58, 81). Each of these methods, however, has limitations, either due to issues of resolution or by the creation of artifacts caused by dehydration or processing techniques. Alternatively, scanning confocal laser microscopy (SCLM) provides three-dimensional, noninvasive inspection and computer reconstruction of mature biofilms without appreciable distortion of architecture in a manner similar to computer-assisted tomography and magnetic resonance imaging methods.
Using SCLM and biofilms of P. aeruginosa, Pseudomonas fluorescens, and Vibrio parahaemolyticus, Lawrence et al. (59) were able to create an enhanced conceptual image of bacterial biofilm architecture as it exists in nature. For these studies, biofilms were generated with each of these organisms using continuous-flow slide culture chambers and examined at various time intervals. The results showed that V. parahaemolyticus and P. aeruginosa produced biofilms that were approximately two times the thickness of P. fluorescens biofilms. Each biofilm demonstrated variation in depth and in the ratio of cellular to noncellular material. All biofilms were highly hydrated, open structures composed of 73 to 98% noncellular material, including water channels and exopolysaccharide. The cells within Pseudomonas biofilms were more tightly packed at the surface and less dense near the periphery of the biofilm, i.e., pyramidal in shape. Conversely, biofilms produced by V. parahaemolyticus showed the opposite arrangement; cell density was greatest near the periphery. In addition, V. parahaemolyticus biofilms had extensive void spaces within the inner regions of the biofilm. The authors proposed that the difference in architecture demonstrated by V. parahaemolyticus biofilms might be due to the expression of lateral flagella stimulated by surface contact, as previously described (5).
The porosity and channels throughout Pseudomonas and Vibrio biofilms allow the free diffusion of low-molecular-weight compounds such as fluorescein, which indicates that the spatial arrangement of cells, pores, and water channels within the biofilm permits access to nutrients as well as antibiotics. This presents somewhat of a paradox, in that biofilms appear to be permeable to low-molecular-weight compounds and yet demonstrate grossly elevated MBCs of antimicrobial agents that show acceptable in vitro activity against planktonic cultures.
Three-Dimensional Perspective of Biofilms
Conceptually, if we were able to explore a bacterial biofilm at a microscopic level (something like Isaac Asimov's The Fantastic Journey or the movie Honey, I Shrunk the Kids), it might look like an underwater coral reef with pyramid or mushroom-shaped projections extending away from the surface and channels and caverns running throughout. Instead of the calcified exoskeleton, though, the viable organisms would be encased in a gelatinous glycocalyx, giving the visual impression of a lava lamp. As in a coral reef, the surface of a bacterial biofilm would be fertile ground for secondary colonization by additional organisms. The hydrodynamic flow of liquid over and through the biofilm would likely break fragments containing viable organisms away from the surface, which, in turn, would be carried with the current and deposited elsewhere for further colonization.
The maximum growth potential of the coral reef or the bacterial biofilm is ultimately limited by the availability of nutrients and waste removal. In the case of bacterial biofilms, growth is also limited by the expression of quorum-sensing molecules released in response to nutrient limitation, accumulation of toxic by-product, and possibly other factors (1, 25, 69, 73, 87).
PROTOTYPICAL BIOFILMS
Perhaps the two most intensely studied biofilm-producing microorganisms of medical importance are P. aeruginosa and S. epidermidis, the former because of its well-recognized ability to achieve chronic pulmonary colonization in patients with cystic fibrosis and the latter for its propensity to infect biomedical implants and transcutaneous devices. Rather than provide an historical overview of the investigations which have contributed to current concepts regarding the role that biofilms play in the diseases caused by each of these organisms, this section will concentrate primarily on the molecular genetics that determine the course of biofilm formation. Because the story is somewhat more complex and divergent, I will begin first with S. epidermidis.
THE CONS BIOFILM FESTIVAL
In 1982, Christensen et al. (11) observed that strains of S. epidermidis associated with an outbreak of catheter-related sepsis were phenotypically distinguished from nonoutbreak strains by their ability to form a dense, alcian blue-stained film or slime layer on the inner aspect of glass culture tubes containing Trypticase soy broth as a growth medium. Furthermore, scanning electron microscopic examination of slime-positive strains grown on intravascular catheter sections demonstrated dense growth of adherent organisms encased in extracellular material. The authors presumed that this material was polysaccharide in nature due to the staining properties of alcian blue. Strains of S. epidermidis such as these have since been shown to frequently produce biomedical implant infections of all kinds (82).
Since this report, several groups have attempted to chemically define the extracellular antigens of S. epidermidis associated with adhesion and/or slime production. For example, Peters et al. (77) characterized the extracellular slime substance of S. epidermidis as a mannose-rich glycocalyx that is reactive with concanavalin A. Tojo et al. (88) isolated a galactose-rich capsular polysaccharide (CPA or PS/A) that might have a role in the primary adhesion of S. epidermidis to smooth, abiotic surfaces. In 1990, Christensen et al. (13) purified a protease- and heat-stable, glucose-rich extracellular slime-associated antigen (SAA) that was antigenically distinct from CPA. Based on adhesion studies using CPA-positive, SAA-positive, and SAA-negative strains, the authors concluded that CPA mediated primary adhesion while SAA promoted surface accumulation (i.e., biofilm maturation) of S. epidermidis. This helped explain their observation that certain slime-negative strains were still capable of producing a biofilm. To make matters even more intriguing, Hussain et al. in 1993 (50) reported that the bulk of the slime glycocalyx of S. epidermidis was composed of teichoic acid fragments and protein.
Confused? Fortunately, the combined efforts of several independent laboratories began to put the pieces of the S. epidermidis adhesion puzzle together during the latter half of the "90s into a unified and logical process that involves several stages and functional gene products. A summary of these findings is most easily explained if the entire process is broken down into the components of primary adhesion, cellular accumulation, and glycocalyx production.
What Makes S. epidermidis Stick?
Primary or nonspecific adhesion is likely the end result of several variables, including cellular and surface hydrophobicity (48, 75), expression of adhesion-specific antigens such as the CPA-PS/A polysaccharide described by Tojo et al. (88), and environmental factors discussed earlier in this review. In 1996, however, Christine Heilmann and colleagues (42) found that a Tn917 insertion mutant of S. epidermidis had lost the ability to stick to a polystyrene surface. This mutant was significantly less hydrophobic than the wild-type strain and had concomitantly lost the expression of four cell surface proteins. Genetic reconstitution of one of those proteins (60 kDa) completely restored primary adhesion to plastic, giving rise to the theory that specific adhesins are required for the first stage of biofilm development. Further analysis showed that the 60-kDa adhesin protein appeared to be a proteolytic fragment of a much larger gene product that bears sequence homology to the major Alt autolysin of S. aureus (44).
Secondary Adhesion of S. epidermidis and PIA
The second stage of biofilm development is characterized by the surface accumulation of cellular aggregates (43). This stage appears to be mediated by a polysaccharide antigen that promotes intercellular adhesion (42, 63, 64). This polysaccharide intercellular adhesin (PIA) is a product of the icaADBC gene cluster (21, 43) and is a virulence factor in the pathogenesis of foreign-body infections (83, 84, 95). Chemically, PIA is a surface-associated, linear β1-6 N-acetyl-d-glucosaminylglycan (65) that is similar if not identical to the hemagglutinin of S. epidermidis (36).
The expression of icaA, icaD, and icaC is absolutely necessary for the synthesis of PIA (38), but a number of biofilm-negative, ica-positive phase variants of S. epidermidis have been described in which the icaA and icaC genes are inactivated by the insertion sequence IS 256 (95, 96). The process, however, is reversible, and the excision of IS 256 from these strains on subculture fully restores the ability to produce PIA and a biofilm (95, 96). In an icaprom::lacZ expression construct, Rachid et al. (80) found that subinhibitory concentrations of tetracycline and quinupristin-dalfopristin produced strong induction of the ica promoter, while penicillin, oxacillin, chloramphenicol, clindamycin, gentamicin, ofloxacin, and teichoplanin had no such effect. These findings were confirmed using a quantitative in vitro biofilm assay, indicating that certain treatment modalities could theoretically produce undesirable effects leading to enhanced biofilm synthesis.
It is possible that other intrinsic factors are involved in PIA-induced cellular aggregation. Dunne and Burd (29) demonstrated that increasing concentrations of Mg2+ enhanced biofilm production by S. epidermidis, while EDTA caused a dose-dependent decrease in the accumulation of cells on a plastic surface. Interestingly, Hussain et al. (51) identified a 140-kDa extracellular protein in a strain of S. epidermidis that appears to have the same function as PIA. The addition of protease or antiserum directed against this protein prevents the formation of cellular aggregates on surfaces. Given the relative importance of biofilm formation to bacterial survival, it is likely that a variety of secondary pathways have evolved in bacteria in order to preserve this function.
Biofilm Maturation of S. epidermidis and the Slime Layer
The final phase of biofilm maturation in S. epidermidis, generation of a slime glycocalyx, is not essential to the overall process of surface colonization (13). Furthermore, it is possible that the chemical composition of the slime material varies from strain to strain (13, 50, 77, 88). Irrespective of the chemical nature of the glycocalyx, it is likely that this material adds to the stability of the biofilm, making biomedical implants colonized with slime-positive strains even more difficult to sterilize. Clearly, additional studies are required to achieve some consensus as to the nature of the slime exopolysaccharide, but it is certain that the process of biofilm formation by S. epidermidis consists of multiple phases and might provide several attractive targets to prevent foreign-body infections through the development of vaccines. A schematic representation of this process is depicted in Fig. 3.
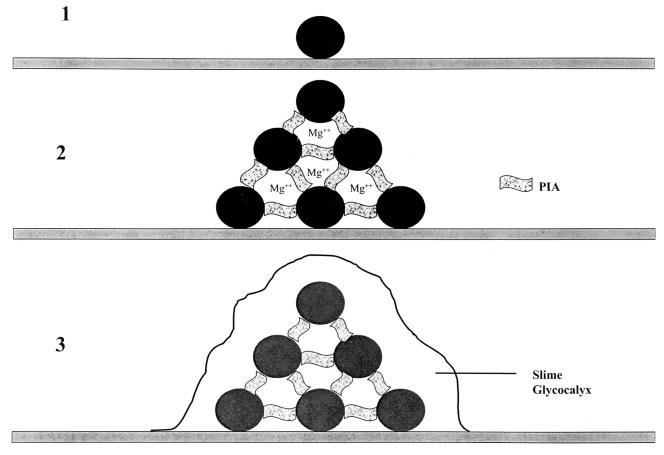
FIG. 3.
Schematic representation of biofilm formation by S. epidermidis. Primary adhesion (step 1) of individual cells to a surface is influenced by physical interactions (hydrophobic, electrostatic), which in turn might be influenced by cell surface adhesions. Cellular aggregation (step 2) is mediated by polysaccharide intercellular adhesin (PIA), the gene product of the icaADBC gene cluster, and (speculatively) other factors, such as divalent cations. The final phase (step 3) is characterized by the generation of a slime exopolysaccharide that encases surface-bound organisms in a gelatinous matrix but is not essential to biofilm development.
THE PSEUDOMONAS AERUGINOSA MODEL
Pseudomonas Adhesion: Motility and the Twitch
The development of surface-associated biofilms by P. aeruginosa is quite similar to that by S. epidermidis in that primary adhesion, cellular aggregation, and glycocalyx production are observed, but this organism adds an unusual twist to the process. O'Toole and Kolter (74) generated 13 transposon insertion mutants of wild-type P. aeruginosa strain PA14 that were surface attachment deficient (sad). Unlike the wild-type parent strain, sad mutants were unable to produce a substantial biofilm. Southern blot analysis detected only a single transposon insertion for each mutant. Three of the 13 mutants had become nonmotile, and the insertion sequence of one was located in a DNA sequence having 40% identity to the flgK gene sequence of Salmonella enterica serovar Typhimurium and Escherichia coli. The flgK gene of these organisms codes for the flagellum-associated hook protein 1, and its loss leads to the production of nonfunctional flagella and loss of motility.
A second class of sad mutants was identified in which the transposon insertion was located in genes (pilB and pilC and a pilY1 homologue) coding for the synthesis of type IV pili. These appendages are responsible for a peculiar surface-associated motion known as twitching motility, not to be confused with Elvis. Twitching motility is a creeping or walking-like movement across a surface, thought to result from the extension and contraction of type IV pili (22, 92), almost like actin-mediated pseudopod motility in eukaryotic cells. To place these observations in perspective, the wild-type parent strain first forms a surface monolayer of cells through primary adhesion. Monolayer cells then “walk” via twitching motility to form cellular aggregates on surfaces that eventually differentiate into microcolonies. The sad mutants defective in flagellar motility are unable to form a monolayer of cells on a surface, while the mutants deficient in type IV pili form monolayers but not cellular aggregates. Taken together, these findings indicate that flagellar motility is required for primary adhesion and type IV pili are essential for cellular aggregation. It has been suggested previously that flagellar motility might be necessary to bring cells into close proximity with a surface (58). The association between motility and biofilm development has been noted with other organisms, such as P. fluorescens and E. coli (56, 78).
Pseudomonas Biofilm Maturation
In P. aeruginosa, the exopolysaccharide glycocalyx is alginic acid, a linear polymer consisting of β-1,4-linked d-mannuronic acid and various amounts of its C-5 epimer l-guluronic acid (28, 61). The synthesis of alginic acid is under the control of the algACD gene cluster (23), similar to the ica gene locus of S. epidermidis. The production of alginic acid by P. aeruginosa (as determined by an increase in the activity of promoters that regulate the genes responsible for the biosynthesis of alginate) is upregulated in response to various environmental factors, including high osmolarity, high oxygen tension, ethanol exposure, and nitrogen limitation (26, 27, 97).
The algC gene codes for a phosphomannomutase that is essential for the production of alginic acid at a key point of regulation. Using a reporter gene construct (algCprom::lacZ), Davies et al. (23, 24) were able to demonstrate that an increased percentage of cells began to upregulate algC synthesis following attachment to a glass surface. This activation was initiated after 15 min of attachment, increased through 2 h of incubation, and decreased thereafter. The expression of the algC reporter gene by biofilm organisms was nearly 19 times greater than that of planktonic cells. Furthermore, biofilm organisms accumulated more than twice as much uronic acid (as a marker of alginic acid synthesis) as surface-free organisms. Cells that did not demonstrate increased expression of algC had a greater likelihood of detaching from the biofilm.
These results strongly support the idea of surface activation of glycocalyx production by P. aeruginosa, but since the algC gene is also required for lipopolysaccharide synthesis, the results must be interpreted with some caution. The authors go on to speculate that regulation of algC might also involve quorum-sensing molecules such as the homoserine lactone family of autoinducers. A schematic representation of the phases of biofilm production by P. aeruginosa is depicted in Fig. 4.
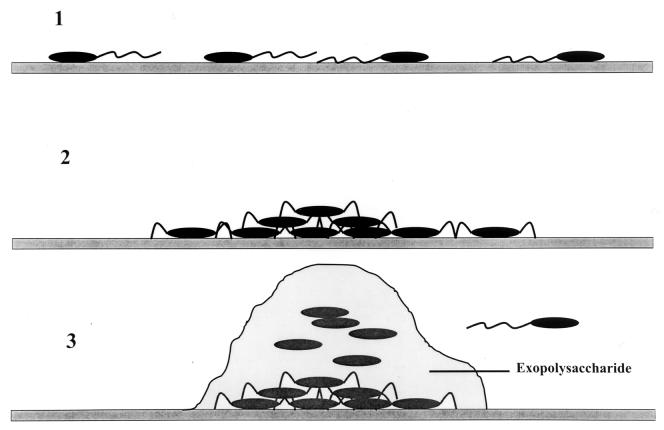
FIG. 4.
Schematic representation of biofilm formation by P. aeruginosa. Step 1 represents the primary adhesion of individual cells to a targeted surface that is dependent on motility, i.e., the production of functional flagella. The aggregation phase (step 2) of biofilm development requires the synthesis of type IV pili, which allow the cells to migrate across a surface and congregate in microcolonies. The final phase (step 3) of biofilm development by P. aeruginosa calls for the elaboration of an alginic acid-like exopolysaccharide by the algACD gene cluster. Cells near the outer surface can dislodge from the biofilm and escape to colonize new microenvironments.
PARTING OBSERVATIONS
Throughout the years, a multitude of methods have been examined for the removal and/or prevention of bacterial biofilms on surfaces, including the use of biocides and antibiotics, ultrasound, chelation, scraping (called pigging in the oil industry [17]), enzymatic digestion, and high-pressure spraying, to name a few (8, 17, 39, 52). All have had variable and usually temporary success. This is particularly true for infected biomedical devices, where the outcome of antimicrobial therapy is dependent on overcoming the inherent resistance of surface-bound organisms.
This finding highlights an interesting paradox that was discussed by Costerton et al. (17), i.e., susceptibility testing performed in clinical microbiology laboratories necessitates the use of pure cultures grown in nutritionally rich media and in a planktonic growth state. In reality, nothing could be further from the conditions found in nature or in diseased individuals. It is almost certain that the tendency for most, if not all, bacteria to preferentially attach to surfaces is a fundamental survival feature that evolved over millions of years to deal with tremendous fluctuations in environmental conditions. In the health care industry, we have rediscovered those tendencies through the extensive use of transcutaneous catheters and biomedical implants. So why are we still testing the susceptibility of microorganisms using planktonic growth conditions?
It might be time for clinical laboratories to explore new means of testing organisms isolated from infected implants in the growth mode that is most likely encountered in situ. Would it not be better to report test results that hint at potential treatment failures when dealing with bacteria in a biofilm than to naively generate results that apply only to planktonic growth? From studies of an animal model of S. epidermidis foreign-body infection and an in vitro susceptibility assay using biofilm organisms, Widmer et al. (93) astutely concluded “Drug efficacy on stationary and adherent microorganisms, but not minimum inhibiting concentrations [of planktonic organisms], predicted the outcome of device-related infections.” This conclusion was also reached by Anwar et al. (3), who further lobbied for the development and use of a biofilm eradicating concentration (BEC) result as a means of predicting therapeutic outcome for foreign-body infections.
A variety of in vitro models and devices have been specifically developed for research (3, 14, 32, 37, 57, 79, 93) and/or commercial (9) applications but would require substantial evaluation, standardization, and education prior to routine use in the clinical microbiology laboratory. Still, these efforts are certainly warranted if the ultimate goal is to better approximate natural conditions and provide an accurate estimate of therapeutic outcome.
REFERENCES
- 1.Allison, D. G., B. Ruiz, C. SanJose, A. Jaspe, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179-184. [DOI] [PubMed] [Google Scholar]
- 2.An, Y. H., R. B. Dickinson, and R. J. Doyle. 2000. Mechanisms of bacterial adhesion and pathogenesis of implant and tissue infections, p. 1-27. In Y. H. An and R. J. Friedman (ed.), Handbook of bacterial adhesion: principles, methods, and applications. Humana Press, Totowa, N.J.
- 3.Anwar, H., J. L. Strap, and J. W. Costerton. 1992. Eradication of biofilm cells of Staphylococcus aureus with tobramycin and cephalexin. Can. J. Microbiol. 38:618-625. [DOI] [PubMed] [Google Scholar]
- 4.Anwar, H., J. L. Strap, and J. W. Costerton. 1992. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob. Agents Chemother. 36:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belas, M. R., and R. R. Colwell. 1982. Adsorption kinetics of laterally and polarly flagellated Vibrio. J. Bacteriol. 151:1568-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boland, T., R. A. Latour, and F. J. Sutzenberger. 2000. Molecular basis of bacterial adhesion, p. 29-41. In Y. H. An and R. J. Friedman (ed.), Handbook of bacterial adhesion: principles, methods, and applications, 1st ed. Humana Press, Totowa, N.J.
- 7.Brading, M. G., J. Jass, and H. M. Lappin-Scott. 1995. Dynamics of bacterial biofilm formation, p. 46-63. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms. Cambridge University Press, New York, N.Y.
- 8.Carpentier, B., and O. Cerf. 1993. Biofilms and their consequences, with particular reference to hygiene in the food industry. J. Appl. Bacteriol. 75:499-511. [DOI] [PubMed] [Google Scholar]
- 9.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, K.-J., T. A. McAllister, and J. W. Costerton. 1995. Biofilms of the ruminant digestive tract, p. 221-232. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms, 1st ed. Cambridge University Press, New York, N.Y.
- 11.Christensen, G. D., W. A. Simpson, A. L. Bisno, and E. H. Beachey. 1982. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen, B. E. 1989. The role of extracellular polysaccharides in biofilms. J. Biotechnol. 10:181-202. [Google Scholar]
- 13.Christensen, G. D., L. P. Barker, T. P. Mawhinney, L. M. Baddour, and W. A. Simpson. 1990. Identification of an antigenic marker of slime production for Staphylococcus epidermidis. Infect. Immun. 58:2906-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen, G. D., W. A. Simpson, J. O. Anglen, and B. J. Gainor. 2000. Methods for evaluating attached bacteria and biofilms: an overview, p. 213-234. In Y. H. An and R. J. Friedman (ed.), Handbook of bacterial adhesion: principles, methods, and applications, 1st ed. Humana Press, Totowa, N.J.
- 15.Coquet, L., G.-A. Junter, and T. Jouenne. 1998. Resistance of artificial biofilms of Pseudomonas aeruginosa to imipenem and tobramycin. J. Antimicrob. Chemother. 42:755-760. [DOI] [PubMed] [Google Scholar]
- 16.Costerton, J. W., T. J. Marrie, and K.-J. Cheng. 1985. Phenomena of bacterial adhesion, p. 650-654. In D. C. Savage and M. Fletcher (ed.), Bacterial adhesion: mechanisms and physiological significance. Plenum Press, New York, N.Y.
- 17.Costerton, J. W., K.-J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 18.Costerton, J. W., and H. M. Lappin-Scott. 1995. Introduction to microbial biofilms, p. 1-11. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms, 1st ed. Cambridge University Press, New York, N.Y.
- 19.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 20.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 21.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darzins, A. L., and M. A. Russell. 1997. Molecular genetic analysis of type-4 pilus biogenesis and twitching motility using Pseudomonas aeruginosa as a model system—a review. Gene 192:109-115. [DOI] [PubMed] [Google Scholar]
- 23.Davies, D. G., A. M. Chakrabarty, and G. G. Geesey. 1993. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 59:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies, D. G., and G. G. Geesey. 1995. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61:860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 26.DeVault, J. D., A. Berry, T. K. Misra, and A. M. Chakrabarty. 1989. Environmental sensory signals and microbial pathogenesis: Pseudomonas aeruginosa infection in cystic fibrosis. Bio/Technology 7:352-357. [Google Scholar]
- 27.DeVault, J. D., K. Kimbara, and A. M. Chakrabarty. 1990. Pulmonary dehydration and infection in cystic fibrosis: evidence that ethanol activates alginate gene expression and induction of mucoidy in Pseudomonas aeruginosa. Mol. Microbiol. 4:737-745. [DOI] [PubMed] [Google Scholar]
- 28.Dunne, W. M., Jr., and F. L. A. Buckmire. 1985. Effects of divalent cations on the synthesis of alginic acid-like exopolysaccharide from mucoid Pseudomonas aeruginosa. Microbios 43:193-216. [PubMed] [Google Scholar]
- 29.Dunne, W. M., Jr., and E. M. Burd. 1992. The effects of magnesium, calcium, EDTA, and pH on the in vitro adhesion of Staphylococcus epidermidis to plastic. Microbiol. Immunol. 36:1019-1027. [DOI] [PubMed] [Google Scholar]
- 30.Dunne, W. M., and E. M. Burd. 1993. Fibronectin and proteolytic fragments of fibronectin interfere with the adhesion of Stahpylococcus epidermidis to plastic. J. Appl. Bacteriol. 74:411-416. [DOI] [PubMed] [Google Scholar]
- 31.Dunne, W. M., Jr., E. O. Mason, Jr., and S. L. Kaplan. 1993. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 37:2522-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunne, W. M., Jr. 2000. Evaluating adherent bacteria and biofilm using biochemical and immunochemical methods, p. 273-284. In Y. H. An and R. J. Friedman (ed.), Handbook of bacterial adhesion: principles, methods, and applications, 1st ed. Humana Press, Totowa, N.J.
- 33.Elder, M. J., F. Stapleton, E. Evans, and J. K. G. Dart. 1995. Biofilm-related infections in ophthalmology. Eye 9:102-109. [DOI] [PubMed] [Google Scholar]
- 34.Eng, R. H. K., F. T. Padberg, S. M. Smith, E. N. Tan, and C. E. Cherubin. 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob. Agents Chemother. 35:1824-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farber, B. F., M. H. Kaplan, and A. G. Clogston. 1990. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J. Infect. Dis. 161:37-40. [DOI] [PubMed] [Google Scholar]
- 36.Fey, P. D., J. S. Ulphani, F. Götz, C. Heilmann, D. Mack, and M. E. Rupp. 1999. Characterization of the relationship between polysaccharide intercellular adhesin and hemagglutination in Staphylococcus epidermidis. J. Infect. Dis. 179:1561-1564. [DOI] [PubMed] [Google Scholar]
- 37.Gander, S., and P. Gilbert. 1997. The development of a small-scale biofilm model suitable for studying the effects of antibiotics on biofilms of gram-negative bacteria. J. Antimicrob. Chemother. 40:329-334. [DOI] [PubMed] [Google Scholar]
- 38.Gerke, C., A. Kraft, R. Süssmuth, O. Schweitzer, and F. Götz. 1998. Characterization of the N-acetylglucosaminyltransferase activity in the biosynthesis of the Staphylococcus epidermidis intercellular adhesin. J. Biol. Chem. 273:18586-18593. [DOI] [PubMed] [Google Scholar]
- 39.Gibson, H., J. H. Taylor, K. E. Hall, and J. T. Holah. 1999. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J. Appl. Microbiol. 87:41-48. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert, P., and M. R. W. Brown. 1995. Mechanisms of the protection of bacterial biofilms from antimicrobial agents, p. 118-130. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms, 1st ed. Cambridge University Press, New York, N.Y.
- 41.Gristina, A. G. 1987. Biomaterial-centered infection, microbial vs tissue integration. Science 237:1588-1597. [DOI] [PubMed] [Google Scholar]
- 42.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Götz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 44.Heilmann, C., M. Hussain, G. Peters, and F. Götz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 25:1013-1024. [DOI] [PubMed] [Google Scholar]
- 45.Henrici, A. T. 1936. Studies of freshwater bacteria. III. Quantitative aspects of the direct microscopic method. J. Bacteriol. 30:61-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrmann, M., P. E. Baudaux, D. Pittet, R. Auckenthaler, P. D. Lew, F. Schumacher-Perdreau, G. Peters, and F. A. Waldvogel. 1988. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 158:693-701. [DOI] [PubMed] [Google Scholar]
- 47.Heukelekian, H., and A. Heller. 1940. Relation between food concentration and surface for bacterial growth. J. Bacteriol. 40:546-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogt, A. H., J. Dankert, C. E. Hylstaert, and J. Feijen. 1986. Cell surface characteristics of coagulase-negative staphylococci and their adherence to fluorinated poly (ethylenepropylene). Infect. Immun. 51:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoyle, B. D., J. Alcantara, and J. W. Costerton. 1992. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob. Agents Chemother. 36:2054-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussain, M., M. H. Wilcox, and P. J. White. 1993. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol. Rev. 104:191-208. [DOI] [PubMed] [Google Scholar]
- 51.Hussain, M., M. Herrman, C. von Eiff, F. Perdreau-Remington, and G. Peters. 1997. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect. Immun. 65:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johansen, C., P. Falholt, and L. Gram. 1997. Enzymatic removal and disinfection of bacterial biofilms. Appl. Environ. Microbiol. 63:3724-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jorgensen, J. H., J. D. Turnidge, and J. A. Washington. 1999. Antibacterial susceptibility tests: dilution and disk diffusion methods, p. 1526-1543. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 54.Jucker, B. A., H. Harms, and A. J. B. Zehnder. 1996. Adhesion of the positively charged bacterium Stenotrophomonas (Xanthomonas) maltophilia 70401 to glass and Teflon. J. Bacteriol. 178:5472-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kinner, N. E., D. L. Balkwill, and P. L. Bishop. 1983. Light and electron microscopic studies of microorganisms growing in rotating biological contactor biofilms. Appl. Environ. Microbiol. 45:1659-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korber, D. R., J. R. Lawrence, and D. E. Caldwell. 1994. Effect of motility on surface colonization and reproductive success of Pseudomonas fluorescens in dual-dilution continuous culture and batch culture systems. Appl. Environ. Microbiol. 60:1421-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.La Tourette Prosser, B., D. Taylor, B. A. Dix, and R. Cleeland. 1987. Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrob. Agents Chemother. 31:1502-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawrence, J. R., P. J. Delaquis, D. R. Korber, and D. E. Caldwell. 1987. Behavior of Pseudomonas fluorescens within the hydrodynamic boundary layers of surface microenvironments. Microb. Ecol. 14:1-14. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence, J. R., D. R. Korber, B. D. Hoyle, J. W. Costerton, and D. E. Caldwell. 1991. Optical sectioning of microbial biofilms. J. Bacteriol. 173:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leung, J. W., Y. L. Liu, T. Desta, E. Libby, J. F. Inciardi, and K. Lam. 1998. Is there a synergistic effect between mixed bacterial infection in biofilm formation on biliary stents? Gastrointest. Endosc. 48:250-257. [DOI] [PubMed] [Google Scholar]
- 61.Linker, A., and R. S. Jones. 1966. A new polysaccharide resembling alginic acid isolated from pseudomonads. J. Biol. Chem. 241:470-481. [PubMed] [Google Scholar]
- 62.Lloyd, B. 1930. Bacteria of the Clyde Sea area: a quantitative investigation. J. Marine Biol. Assoc. (United Kingdom) 16:879-907. [Google Scholar]
- 63.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 62:3244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1-6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marsh, P. D. 1995. Dental plaque, p. 282-300. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms. Cambridge University Press, New York, N.Y.
- 67.Marshall, K. C., R. Stout, and R. Mitchell. 1971. Mechanism of initial events in the sorption of marine bacteria to surfaces. J. Gen. Microbiol. 68:337-348. [Google Scholar]
- 68.Marshall, K. C. 1985. Mechanisms of bacterial adhesion at solid-water interfaces, p. 133-141. In D. C. Savage and M. Fletcher (ed.), Bacterial adhesion: mechanisms and physiological significance. Plenum Press, New York, N.Y.
- 69.McLean, J. J. C., M. Whitely, D. J. Strickler, and W. C. Fuqua. 1997. Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiol. Lett. 154:259-263. [DOI] [PubMed] [Google Scholar]
- 70.Merritt, K., and Y. H. An. 2000. Factors influencing bacterial adhesion, p. 53-72. In Y. H. An and R. J. Friedman (ed.), Handbook of bacterial adhesion: principles, methods, and applications, Humana Press, Totowa, N.J.
- 71.Nichols, W. W., S. M. Dorrington, M. P. E. Slack, and H. L. Walmsley. 1988. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob. Agents Chemother. 32:518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nickel, J. C., I. Ruseska, J. B. Wright, and J. W. Costerton. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent, signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 74.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 75.Pascual, A., A. Fleer, N. A. C. Westerdaal, and J. Verhoef. 1986. Modulation of adherence of coagulase-negative staphylococci to Teflon catheters in vitro. Eur. J. Clin. Microbiol. 5:518-522. [DOI] [PubMed] [Google Scholar]
- 76.Pearce, D., M. J. Bazin, and J. M. Lynch. 1995. The rhisosphere as a biofilm, p. 207-220. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms, 1st ed. Cambridge University Press, New York, N.Y.
- 77.Peters, G., F. Schumacher-Perdreau, B. Jansen, M. Bey, and G. Pulverer. 1987. Biology of S. epidermidis extracellular slime. Zentbl. Bakteriol. Suppl. Mikrobiol. Hyg. Abt. 1(Suppl. 16):15-32. [Google Scholar]
- 78.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: defining the roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 79.Pratten, J., and M. Wilson. 1999. Antimicrobial susceptibility and composition of microcosm dental plaques supplemented with sucrose. Antimicrob. Agents Chemother. 43:1595-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rachid, S., K. Ohlsen, W. Witte, J. Hacker, and W. Ziebuhr. 2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robinson, R. W., D. E. Akin, R. A. Nordstedt, M. V. Thomas, and H. C. Aldrich. 1984. Light and electron microscopic examinations of methane-producing biofilms from anaerobic fixed-bed reactors. Appl. Environ. Microbiol. 48:127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rupp, M. E., and G. L. Archer. 1994. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin. Infect. Dis. 19:231-245. [DOI] [PubMed] [Google Scholar]
- 83.Rupp, M. E., J. S. Ulphani, P. D. Fey, K. Bartscht, and D. Mack. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schierholz, J. M., J. Beuth, D. König, A. Nürnberger, and G. Pulverer. 1999. Antimicrobial substances and effects on sessile bacteria. Zentbl. Bakteriol. 289:165-177. [DOI] [PubMed] [Google Scholar]
- 86.Sheng, W.-H., W.-J. Ho, J.-T. Wang, S.-C. Chang, P.-R. Hsueh, and K.-T. Luh. 2000. Evaluation of antiseptic-impregnated central venous catheters for prevention of catheter-related infection in intensive care unit patients. Diagn. Microbiol. Infect. Dis. 38:1-5. [DOI] [PubMed] [Google Scholar]
- 87.Stickler, D. J., N. S. Morris, R. J. C. McLean, and C. Fuqua. 1998. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl. Environ. Microbiol. 64:3486-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tojo, M., N. Yamashita, D. Goldmann, and G. B. Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713-722. [DOI] [PubMed] [Google Scholar]
- 89.Waksman, S. A., H. W. Reuszer, C. L. Carey, M. Hotchkiss, and C. E. Renn. 1933. Studies on the biology and chemistry of the Gulf of Maine. III. Bacteriological investigations of the seawater and marine bottoms. Biol. Bull. 64:183-205. [Google Scholar]
- 90.Wang, I.-W., J. M. Anderson, and R. E. Marchant. 1993. Staphylococcus epidermidis adhesion to hydrophobic biomedical polymer is mediated by platelets. J. Infect. Dis. 167:329-336. [DOI] [PubMed] [Google Scholar]
- 91.Watnick, P. I., K. J. Fullner, and R. Kolter. 1999. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J. Bacteriol. 181:3606-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whitchurch, C. B., M. Hobbs, S. P. Livingston, V. Krishnapillai, and J. S. Mattick. 1990. Characterization of a Pseudomonas aeruginosa twitching motility gene and eveidence for a specialized protein export system widespread in eubacteria. Gene 101:33-44. [DOI] [PubMed] [Google Scholar]
- 93.Widmer, A. F., R. Frei, Z. Rajacic, and W. Zimmerli. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 162:96-102. [DOI] [PubMed] [Google Scholar]
- 94.Wolfaardt, G. M., J. R. Lawrence, R. D. Roberts, S. J. Caldwell, and D. E. Caldwell. 1994. Multicellular organization in a degradative biofilm community. Appl. Environ. Microbiol. 60:434-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ziebuhr, W., C. Heilmann, F. Götz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ziebuhr, W., V. Krimmer, S. Rachid, I. Löbner, F. Götz, and J. Hacker. 1999. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol. Microbiol. 32:345-356. [DOI] [PubMed] [Google Scholar]
- 97.Zielinski, N. A., R. Maharaj, S. Roychoudhury, C. E. Danganan, W. Hendrickson, and A. M. Chakrabarty. 1992. Alginate synthesis in Pseudomonas aeruginosa: environmental regulation of the algC promoter. J. Bacteriol. 174:7680-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.ZoBell, C. E., and E. C. Allen. 1935. The significance of marine bacteria in the fouling of submerged surfaces. J. Bacteriol. 29:239-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.ZoBell, C. E. 1937. The influence of solid surface on the physiological activities of bacteria in sea water. J. Bacteriol. 33:86. [Google Scholar]
- 100.ZoBell, C. E. 1943. The effect of solid surfaces on bacterial activity. J. Bacteriol. 46:39-56. [DOI] [PMC free article] [PubMed] [Google Scholar]