Abstract
It is now well established that the peroxisomal membrane is not freely permeable to small molecules in vivo, which implies the existence of metabolite transporters in the peroxisomal membrane. A few putative peroxisomal metabolite transporters have indeed been identified, but the function of these proteins has remained largely unresolved so far. The only peroxisomal transporter characterized to a significant extent is the adenine nucleotide transporter, which is presumably required to sustain the activity of the intraperoxisomal very-long-chain-acyl-CoA synthetase. In addition to AMP, this acyl-CoA synthetase also produces pyrophosphate, which must be exported from the peroxisome. In the present study, we demonstrate that the peroxisomal membrane contains a transporter activity that facilitates the passage of phosphate and possibly pyrophosphate across the peroxisomal membrane. By reconstitution of peroxisomal membrane proteins in proteoliposomes, some kinetic parameters of the transporter could be established in vitro. The transporter can be distinguished from the mitochondrial phosphate transporter by its different sensitivity to inhibitors.
Keywords: mammalian peroxisome, peroxisomal membrane protein, phosphate, proteoliposome, pyrophosphate, transport
Abbreviations: NEM, N-ethylmaleimide; PHPG, p-hydroxyphenylglyoxal; PMP, peroxisomal membrane protein
INTRODUCTION
Peroxisomes are involved in a number of important metabolic pathways, including fatty acid α- and β-oxidation, biosynthesis of ether phospholipids and bile acids, and the degradation of purines, amino acids and polyamines [1]. Their importance is underscored by the existence of a number of severe human diseases (e.g. Zellweger syndrome, rhizomelic chondrodysplasia punctata, X-linked adrenoleucodystrophy and Refsum's disease), in which there is a defect in one or more peroxisomal functions [2,3].
In recent years, much has been learnt about the permeability properties of the peroxisomal membrane. Although the membrane of isolated peroxisomes obtained through cell fractionation appears to be freely permeable to small molecules [4–7], several lines of evidence indicate that, in vivo, the peroxisomal membrane is impermeable to a number of substrates and products of peroxisomal metabolism [8–15]. These findings strongly suggest the existence of specific transport systems. However, to date, only one peroxisomal transporter has been characterized to any significant extent, namely the adenine nucleotide transporter Ant1p/PMP34 (where PMP34 stands for 34 kDa peroxisomal membrane protein) [16,17].
In addition to PMP34, the peroxisomal membrane contains several other proteins that bear strong similarity to transporters. In mammalian peroxisomes four ATP-binding-cassette half-transporters have been identified: ALDP (adrenoleucodystrophy protein), ALDR (ALDP-related protein), PMP70 and P70R (PMP70-related protein) [18–21]. However, the exact function of these proteins remains unresolved at present.
Presumably, adenine nucleotide transport by PMP34 is required to sustain the activity of the peroxisomal matrix enzyme very-long-chain-acyl-CoA synthetase [22,23]. However, activity of this enzyme also produces pyrophosphate, which is not normally capable of traversing a lipid bilayer at a significant rate due to its polar nature. Therefore we anticipated that the peroxisomal membrane must also contain a pyrophosphate and/or phosphate transport system that is required to support intraperoxisomal fatty acid activation. In the present study, we demonstrate the presence of a specific (pyro)phosphate transporter activity in the membrane of Bos taurus kidney peroxisomes by reconstitution in proteoliposomes. We have determined several kinetic parameters and provide evidence that this transporter activity is distinct from the mitochondrial phosphate transporter.
EXPERIMENTAL
Materials
Egg-yolk phospholipids were obtained from Fluka (Zwijndrecht, The Netherlands). Nycodenz was purchased from Nycomed (Oslo, Norway), Bio-Beads SM-2 from Bio-Rad Laboratories (Veenendaal, The Netherlands) and Sephadex G-75 and [33P]-phosphate from Amersham Biosciences (Roosendael, The Netherlands). PMP70 antibody was purchased from Zymed (San Francisco, CA, U.S.A.). All other chemicals were of analytical grade and obtained from Sigma.
Preparation of highly purified peroxisomes
A fresh B. taurus kidney was obtained from a local abattoir and immediately immersed in ice-cold SEME buffer (250 mM sucrose, 1 mM EDTA, 50 mM Mops, pH 7.4, and 0.1%, v/v, ethanol). The cortex was manually separated from other tissues, and homogenized with a Potter–Elvehjem-type homogenizer (5 strokes at 500 rev./min). A post-nuclear supernatant was obtained by centrifugation for 5 min at 2400 g. From this post-nuclear supernatant, a crude organellar fraction was obtained by centrifugation for 30 min at 15000 g. This pellet was subjected to Nycodenz gradient centrifugation [24]. The gradients were harvested from the bottom, yielding 24 fractions/gradient. All fractions were diluted 4-fold with SEME buffer, and the organelles were then pelleted by centrifugation (30 min, 20000 g) for storage. The supernatants obtained after centrifugation were found not to contain activity of any of the marker enzymes tested (glutamate dehydrogenase, cytochrome c oxidase, catalase, esterase and alkaline phosphatase) and were subsequently discarded. The organelle pellets were stored at −80 °C until use. In some experiments, peroxisomal membrane preparations were used, which were prepared by sonicating an aliquot of the purified peroxisomal fraction in a buffer containing 1 M sodium chloride and 25 mM sodium phosphate (pH 7.4), followed by high-speed centrifugation (60 min, 100000 g). Sonication was performed on ice using a probe tip sonicator with a power output of 9 W (Model VC60; Sonics & Materials, Danbury, CT, U.S.A.), four times, each of 15 s duration, interrupted by intervals of 45 s.
Marker enzyme assays
Protein concentrations were determined by the bicinchoninic acid method [16]. Glutamate dehydrogenase was determined from the α-oxoglutarate-dependent rate of NADH oxidation (measured at A340) in a mixture containing 50 mM triethanolamine/HCl (pH 8.0), 2.5 mM EDTA, 100 mM NH4Cl, 1 mM ADP, 0.1% (v/v) Triton X-100, 0.3 mM NADH and 10 mM α-oxoglutarate. Cytochrome c oxidase activity was determined by the method of Cooperstein et al. [25]. Alkaline phosphatase activity was measured as described by Bowers et al. [26]. Esterase activity was determined by monitoring the p-nitrophenol formation (A405) in a mixture containing 50 μM EDTA, 50 mM potassium phosphate buffer (pH 6.6), 0.05% Triton X-100 and 2.5 mM p-nitrophenyl acetate. PMP70 was determined by SDS/PAGE followed by Western blotting using a commercial antibody directed against human PMP70, which was found to exhibit cross-reactivity with the B. taurus protein.
Proteoliposome assay
Reconstitution and transport assays were performed as described previously [17] but with minor modifications; 250 μg of protein was mixed with 112 μl of 10% (w/v) egg-yolk phospholipids in the form of sonicated liposomes, 0.4 mg of cardiolipin (sodium salt), 100 μl of 10% (w/v) Triton X-114, 20 mM sodium chloride, 10 μM sodium fluorescein, 50 mM potassium phosphate (pH 6.5), 20 mM Hepes (pH 6.5) and water to a final volume of 700 μl. This mixture was passed 14 times through an Amberlite column (5.0 cm×0.5 cm) pre-equilibrated with a buffer containing 20 mM sodium chloride, 10 μM sodium fluorescein, 50 mM potassium phosphate (pH 6.5) and 20 mM Hepes (pH 6.5). All steps were performed at 4 °C except for the passages through the Amberlite column, which were performed at room temperature (22 °C). The external substrate was removed from the proteoliposomes on a Sephadex G-75 column equilibrated with a buffer containing 70 mM NaCl and 20 mM Hepes (pH 6.5). The reaction was started by the addition of 3 mM 33P-labelled phosphate (15 MBq/mmol) to the proteoliposomes, followed by incubation at 30 °C for 10 min, except where otherwise indicated. The external radioactivity was removed by passing the reaction mixture through a Sephadex G-75 column, thereby stopping the assay, and the radioactivity associated with the proteoliposome fraction was measured by liquid-scintillation counting. The stop time was taken as the moment of addition to the Sephadex column.
Fluorescein was included in the vesicles to determine the internal volume. For this purpose, a 100 μl sample of the liposome suspension was suspended in a cuvette containing 900 μl of 0.1% Triton X-100 to obtain a clear solution and to release the fluorescein from the liposomes. Previous experiments showed that Triton X-100 has no effect on the fluorescence of sodium fluorescein up to a concentration of at least 0.5% (results not shown). The fluorescence was measured using an Aminco PA-256-E1 spectrofluorimeter (excitation at 494 nm and emission at 518 nm). A calibration curve ranging from 0 to 0.2 μM fluorescein was included in the experiment to allow calculations to be made.
RESULTS
Purification of B. taurus peroxisomes
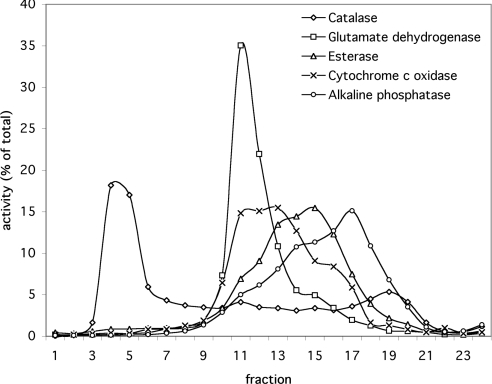
Highly purified peroxisomes were obtained by Nycodenz gradient centrifugation of a crude organellar fraction. Marker enzymes were measured in all fractions to determine the distribution of peroxisomes (catalase), mitochondrial matrix (glutamate dehydrogenase), mitochondrial inner membrane (cytochrome c oxidase), microsomes (esterase) and plasma membrane (alkaline phosphatase) in the gradient (Figure 1). As shown, peroxisomes are well separated from the other organelles.
Figure 1. Distribution of marker enzymes in the fractions of a B. taurus kidney Nycodenz gradient.
The marker enzymes measured indicate the distribution of peroxisomes (catalase), mitochondrial matrix (glutamate dehydrogenase), microsomes (esterase), mitochondrial inner membrane (cytochrome c oxidase) and plasma membrane (alkaline phosphatase). The activity is expressed as a percentage of the total activity of all fractions.
Phosphate uptake by proteoliposomes containing PMP
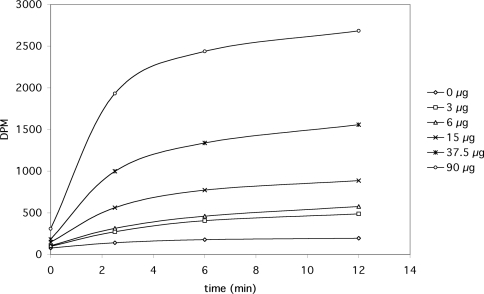
Increasing amounts of protein from highly purified peroxisomal fractions were reconstituted in proteoliposomes, and the time-dependent uptake of radiolabelled phosphate was monitored over time (Figure 2). As shown, a high rate of phosphate uptake was observed in proteoliposomes containing peroxisomal protein, whereas virtually no uptake was observed in liposomes without protein. The initial rate of phosphate uptake observed was approximately linear with the amount of protein. The phosphate uptake by proteoliposomes approached a final level that was also linear with protein. Liposomes prepared with protein but without internal phosphate (the difference in osmolarity was compensated for by the additional sucrose) exhibited a lower rate of phosphate uptake, amounting to approx. 70% of the activity in proteoliposomes containing phosphate. To investigate whether the transport of phosphate is influenced by a pH gradient across the liposomal membrane, as is the case with the mitochondrial phosphate transporter, we prepared proteoliposomes with an internal pH of either 7.5 or 5.5 and then assayed the uptake of phosphate at different external pH values of 5.5 and 7.5. Initial rates of uptake of 67±8% (pHin>pHout) and 63±5% (pHout>pHin) were observed (n=2) relative to the control situation (pHin=pHout=6.5), indicating that a pH gradient has virtually no influence on the initial rate of uptake.
Figure 2. Uptake of radiolabelled phosphate by proteoliposomes containing various amounts of highly purified (total) peroxisomal protein.
The results shown are from a representative experiment. Almost identical results were found in two additional experiments.
To test the possibility that the observed protein-dependent phosphate uptake is caused by an increase in the internal liposomal volume as a consequence of the increasing concentrations of protein, sodium fluorescein was included in the vesicles during the reconstitution step. The internal volume was determined by measuring the fluorescence of enclosed fluorescein (excitation at 494 nm and emission at 518 nm) for the proteoliposome preparations with variable protein content, and was found to be virtually constant. Small variations in the internal volume were not correlated to the protein content (Table 1). This indicates that the presence of protein does not alter the liposomal volume. The intravesicular volume was found to be approx. 1.5 μl/mg of lipid.
Table 1. Internal volume of proteoliposomal fractions containing variable amounts of protein, determined by measuring the fluorescence of enclosed sodium fluorescein.
As shown, the internal volume is independent of the amount of peroxisomal protein used in the reconstitution procedure and amounts to approx. 1.5 μl/mg of lipid.
| Protein (μg) | Internal volume (μl/mg of lipid) |
|---|---|
| 0 | 1.51 |
| 3 | 1.58 |
| 6 | 1.50 |
| 15 | 1.46 |
| 37.5 | 1.47 |
| 90 | 1.53 |
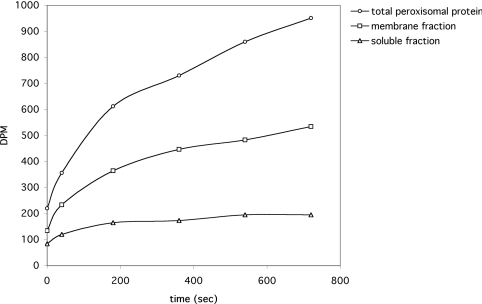
To determine whether the protein responsible for the observed uptake is a membrane-bound or soluble protein, 20 μg of peroxisomal protein was further fractionated by sonication in a buffer containing 1 M sodium chloride and 25 mM sodium phosphate (pH 7.4), followed by high-speed centrifugation (60 min at 100000 g). Markers for the peroxisomal matrix (3-hydroxyacyl-CoA dehydrogenase) and membrane (PMP70) were used to confirm the purity of both preparations (results not shown). Subsequently, the soluble, membrane-associated and total peroxisomal protein fractions were reconstituted in proteoliposomes, and the phosphate uptake was monitored over time. As shown in Figure 3, proteoliposomes containing PMP were found to take up phosphate at a much higher rate than proteoliposomes containing soluble protein.
Figure 3. The uptake of radiolabelled phosphate by proteoliposomes containing subfractionated peroxisomal protein.
Soluble and membrane proteins were separated by sonication in high saline (1 M NaCl), followed by high-speed centrifugation (60 min, 100000 g). The pellet and supernatant fractions were used to prepare proteoliposomes. In addition, proteoliposomes were prepared using total peroxisomal protein for comparison. The uptake of radiolabelled phosphate by each of these proteoliposomes is shown. The experiment was conducted in duplicate and the results shown are from a representative experiment.
Distribution of phosphate transport activity in different fractions obtained by Nycodenz gradient centrifugation
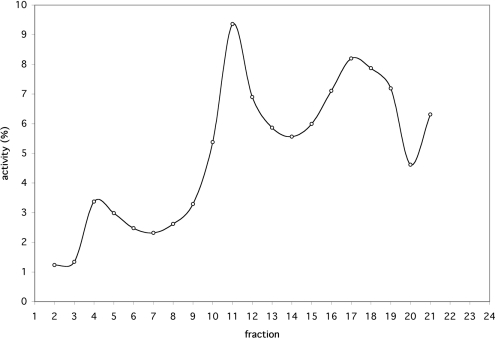
To determine the subcellular distribution of the phosphate transport activity observed in the proteoliposome assay, fractions 2–21 of the Nycodenz gradient shown in Figure 1 were tested using this assay. For this purpose, the organelle pellets of all fractions were resuspended in identical volumes of reconstitution buffer and reconstituted in proteoliposomes. Radiolabelled phosphate uptake was determined after 10 min. Activity appears to be associated mainly with the peroxisomal, mitochondrial and plasma-membrane fractions (Figure 4).
Figure 4. Activity of phosphate transport in different fractions obtained by Nycodenz gradient centrifugation of a light-chain mitochondrial fraction prepared from a fresh B. taurus kidney.
Activity was measured as described in the Materials and methods section using the proteoliposome assay. The activity is expressed as a percentage of the total activity of all fractions. The rate of phosphate uptake by proteopliposomes prepared with each of the fractions was assayed in duplicate. The results shown are from a representative set.
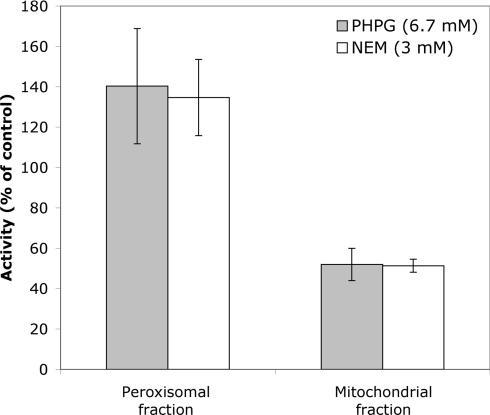
Differential sensitivity of phosphate transport activity in peroxisomal and mitochondrial fractions to PHPG (p-hydroxyphenylglyoxal) and NEM (N-ethylmaleimide)
In an attempt to differentiate between the activities found in peroxisomal and mitochondrial fractions, the effects of NEM (a thiol-group-modifying reagent) and PHPG (an arginine-modifying reagent) were tested. Proteoliposomes were preincubated with 6.7 mM PHPG or 3 mM NEM for 5 min at 35 °C before starting the assay with radiolabelled phosphate. The amount of phosphate taken up was determined after 10 min. As shown in Figure 5, the activity in the mitochondrial fraction (fraction 11 from the gradient in Figure 1) was sensitive to both PHPG and NEM and is decreased to approx. 50% by both inhibitors. In contrast, the activity associated with the peroxisomal fraction (fraction 4 from the gradient in Figure 1) was not inhibited by these compounds, but was somewhat increased, to approx. 135% of the control liposomes, by both inhibitors.
Figure 5. Effect of PHPG (6.7 mM) or NEM (3 mM) on the initial rate of uptake of radiolabelled phosphate by proteoliposomes containing peroxisomal protein (fraction 4 of the Nycodenz gradient in Figure 2) or mitochondrial protein (fraction 11).
Proteoliposomes were preincubated with either of the inhibitors for 3 min at 35 °C, after which the transport assay was initiated by the addition of radiolabelled phosphate. The rate of uptake is expressed as a percentage of the uptake observed using identical proteoliposomes that had not been incubated with inhibitor (control). The results shown are the averages for three experiments. The error bars indicate S.D.
Previously, a higher sensitivity of the bovine mitochondrial phosphate transporter to thiol reagents such as NEM has been reported [27]. Therefore higher concentrations of NEM were tested, but were found not to inhibit further the mitochondrial activity (results not shown). The mitochondrial peak fraction in the Nycodenz gradient also contains small amounts of other organelles, which might contribute to the remaining activity observed in the presence of inhibitors.
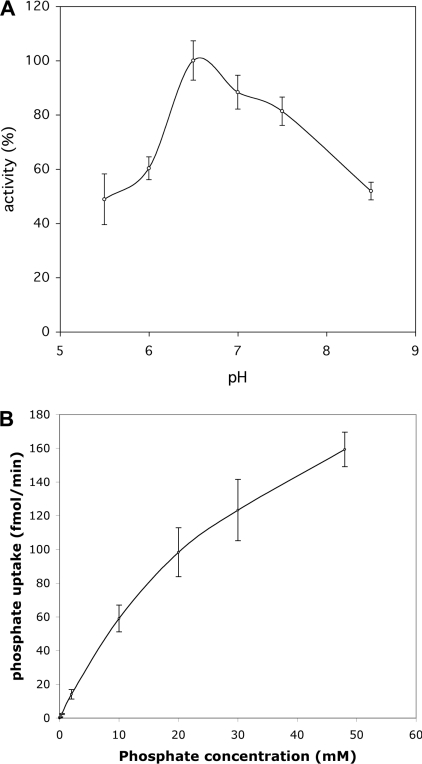
Characterization of phosphate transport activity in peroxisomal fractions
To establish some kinetic parameters of the peroxisomal phosphate transport activity, we determined the optimum pH and an approximate Km value. The proteoliposome assay was performed at various pH values (equal internal and external pH) and phosphate uptake was determined after 10 min (Figure 6A). The uptake was found to exhibit an optimum around pH 6.5. To determine a Km value for phosphate uptake, increasing concentrations of phosphate were added, and the uptake was monitored as a function of time. The rate of uptake as a function of the phosphate concentration is shown in Figure 6(B). However, Vmax was not reached, even at the highest concentration of phosphate, which prevented an accurate determination of the Km from these data. An approx. value of 39 mM was calculated by fitting the available data to the Michaelis–Menten equation. To test the possibility that the effect is caused by the increasing osmolarity of the external solution with phosphate concentration, a similar experiment was performed using sucrose solutions. Virtually no effect of sucrose was observed up to a concentration of 165 mM (results not shown), which is iso-osmotic with 75 mM potassium phosphate solution [28].
Figure 6. Initial rate of phosphate uptake by proteoliposomes containing peroxisomal protein (A) as a function of the pH (pHin=pHout) and (B) as a function of the external phosphate concentration.
Each data point is the mean for duplicate measurements. The error bars indicate S.D.
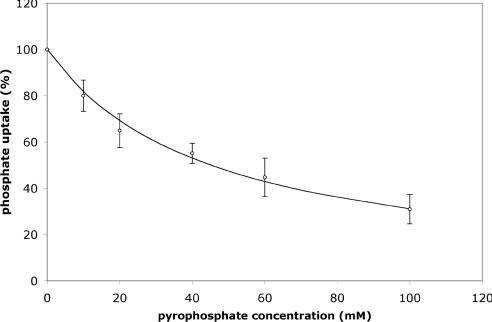
Direct measurement of the transport of pyrophosphate was not possible due to the unavailability of radiolabelled pyrophosphate. However, to obtain presumptive evidence that pyrophosphate is also a substrate for the observed phosphate transporter, increasing concentrations of unlabelled sodium pyrophosphate were added to the assay medium to compete with radiolabelled phosphate. The observed rate of phosphate uptake was decreased to approx. 50% of the maximal rate at a concentration of 42 mM pyrophosphate (Figure 7).
Figure 7. Effect of increasing concentrations of pyrophosphate on the rate of phosphate uptake by proteoliposomes containing peroxisomal protein.
Proteliposomes containing 20 μg of peroxisomal protein were used for this assay. Sodium pyrophosphate was added in the indicated amounts at the start of the assay. The results shown are the means±S.D. for two experiments.
DISCUSSION
Our results demonstrate for the first time that the membrane from B. taurus kidney peroxisomes contains a transporter that facilitates the translocation of phosphate. The peroxisomal fractions of a Nycodenz gradient contain highly purified peroxisomes with no significant contamination from the mitochondria or plasma membrane (Figure 1). These fractions displayed phosphate transport activity in the proteoliposome assay employed, which is linear with protein and saturable. We did not observe an ‘overshoot’ phenomenon in our experiments, but this phenomenon is not always observed in counterflow-type experiments since it is caused by the combined effects of antiport activity and diffusion across the liposomal membrane. The charged, hydrophilic phosphate ion is not able to diffuse across a lipid bilayer at a significant rate; hence the leakage of imported radiolabelled phosphate is expected to be very low within the timeframe of our experiments. Indeed, the uptake of phosphate by liposomes without protein proceeds only at a very low rate, as shown in Figure 2. Therefore the amount of radiolabelled substrate taken up by the proteoliposomes is expected to approach isotopic equilibrium with time if every liposome contains at least one transporter molecule. The total intraliposomal volume can be calculated from the amount of intraliposomal fluorescein, and was found to be approx. 1.5 μl/mg of lipid. From this, it may be estimated that, when isotopic equilibrium is reached, the liposomes contain approx. 2700 d.p.m. of radiolabelled phosphate in the experiment of Figure 2. This is in excellent agreement with the observed value at high protein concentrations, wherein the concentration of liposomes without transporter molecules is lowest. Studies by other researchers also have shown that the overshoot phenomenon is not always observed in proteoliposomal assays of phosphate transport [29,30].
The rate of uptake of radiolabelled phosphate was only slightly decreased in the absence of internal phosphate (Figure 2), amounting to 69% of the control. Although this may suggest that uptake of phosphate is not necessarily coupled with the counter-transport of another ion through the same carrier, future studies will have to reveal whether the transport of phosphate is truly unidirectional, especially since it is well known that a single carrier may catalyse both an exchange reaction as well as unidirectional transport. A good example in this respect is the mitochondrial carnitine/acylcarnitine carrier, which catalyses the exchange between intramitochondrial (acyl)carnitine but also catalyses the unidirectional uptake of carnitine [31].
The addition of pyrophosphate to the assay medium was found to decrease the uptake of phosphate. Although no direct evidence could be provided showing that pyrophosphate is transported due to the unavailability of radiolabelled substrate, the result does suggest that pyrophosphate is capable of competing with phosphate for uptake. An alternative explanation could be that the peroxisomal matrix contains a pyrophosphatase. However, no detectable pyrophosphatase activity was observed in the peroxisomal fractions of the Nycodenz gradient (results not shown).
The peroxisomal activity can be distinguished from the mitochondrial activity by its different sensitivity to the inhibitors NEM and PHPG. Whereas the activity in the mitochondrial fraction is decreased by these reagents, the activity in the peroxisomal fraction is not. It should be noted that the activities of the yeast and human peroxisomal adenine nucleotide transporters, Ant1p and PMP34 respectively, using ATP as substrate, were both found to be decreased by NEM ([16]; and W. F. Visser, unpublished work).
The assay method used in the present study, in which PMPs are reconstituted in liposomes, enables direct measurements of transport processes, which are not possible with isolated peroxisomes due to the permeability of the membrane of isolated peroxisomes. Generally, reconstitution requires relatively large amounts of protein, which is usually acquired by overexpression of the protein in a suitable host. However, to investigate transport processes mediated by proteins for which no gene has previously been identified, as is the case for the phosphate transporter described here, overexpression cannot be employed. In the present study, we show that overexpression is not strictly required to enable the measurement of peroxisomal transporters. This method may be employed to identify transporters for other peroxisomal metabolites in the future.
Acknowledgments
This study was supported by a grant from NWO-CW (Nederlandse Organisatie voor Wetenschappelijk Onderzoek-Chemische Wetenschappen) (reg. no. 99008).
References
- 1.van den Bosch H., Schutgens R. B., Wanders R. J., Tager J. M. Biochemistry of peroxisomes. Annu. Rev. Biochem. 1992;61:157–197. doi: 10.1146/annurev.bi.61.070192.001105. [DOI] [PubMed] [Google Scholar]
- 2.Wanders R. J., Schutgens R. B., Barth P. G. Peroxisomal disorders: a review. J. Neuropathol. Exp. Neurol. 1995;54:726–739. doi: 10.1097/00005072-199509000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Moser H. W. Peroxisomal disorders. Semin. Pediatr. Neurol. 1996;3:298–304. doi: 10.1016/s1071-9091(96)80033-7. [DOI] [PubMed] [Google Scholar]
- 4.Reumann S., Bettermann M., Benz R., Heldt H. W. Evidence for the presence of a porin in the membrane of glyoxysomes of castor bean. Plant Physiol. 1997;115:891–899. doi: 10.1104/pp.115.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reumann S., Maier E., Benz R., Heldt H. W. The membrane of leaf peroxisomes contains a porin-like channel. J. Biol. Chem. 1995;270:17559–17565. doi: 10.1074/jbc.270.29.17559. [DOI] [PubMed] [Google Scholar]
- 6.Reumann S., Maier E., Heldt H. W., Benz R. Permeability properties of the porin of spinach leaf peroxisomes. Eur. J. Biochem. 1998;251:359–366. doi: 10.1046/j.1432-1327.1998.2510359.x. [DOI] [PubMed] [Google Scholar]
- 7.van Veldhoven P. P., Just W. W., Mannaerts G. P. Permeability of the peroxisomal membrane to cofactors of beta-oxidation. Evidence for the presence of a pore-forming protein. J. Biol. Chem. 1987;262:4310–4318. [PubMed] [Google Scholar]
- 8.Dansen T. B., Wirtz K. W., Wanders R. J., Pap E. H. Peroxisomes in human fibroblasts have a basic pH. Nat. Cell Biol. 2000;2:51–53. doi: 10.1038/71375. [DOI] [PubMed] [Google Scholar]
- 9.Hettema E. H., van Roermund C. W., Distel B., van Den Berg M., Vilela C., Rodrigues-Pousada C., Wanders R. J. A., Tabak H. F. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty-acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 1996;15:3813–3822. [PMC free article] [PubMed] [Google Scholar]
- 10.Jankowski A., Kim J. H., Collins R. F., Daneman R., Walton P., Grinstein S. In situ measurements of the pH of mammalian peroxisomes using the fluorescent protein pHluorin. J. Biol. Chem. 2001;276:48748–48753. doi: 10.1074/jbc.M109003200. [DOI] [PubMed] [Google Scholar]
- 11.Nicolay K., Veenhuis M., Douma A. C., Harder W. A 31P-NMR study of the internal pH of yeast peroxisomes. Arch. Microbiol. 1987;147:37–41. doi: 10.1007/BF00492902. [DOI] [PubMed] [Google Scholar]
- 12.van Roermund C. W., Drissen R., van Den Berg M., Ijlst L., Hettema E. H., Tabak H. F., Waterham H. R., Wanders R. J. Identification of a peroxisomal ATP carrier required for medium-chain fatty acid beta-oxidation and normal peroxisome proliferation in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:4321–4329. doi: 10.1128/MCB.21.13.4321-4329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Roermund C. W., Elgersma Y., Singh N., Wanders R. J., Tabak H. F. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J. 1995;14:3480–3486. doi: 10.1002/j.1460-2075.1995.tb07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Roermund C. W., Hettema E. H., Kal A. J., van den Berg M., Tabak H. F., Wanders R. J. Peroxisomal beta-oxidation of polyunsaturated fatty acids in Saccharomyces cerevisiae: isocitrate dehydrogenase provides NADPH for reduction of double bonds at even positions. EMBO J. 1998;17:677–687. doi: 10.1093/emboj/17.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Roermund C. W., Hettema E. H., van den Berg M., Tabak H. F., Wanders R. J. Molecular characterization of carnitine-dependent transport of acetyl-CoA from peroxisomes to mitochondria in Saccharomyces cerevisiae and identification of a plasma membrane carnitine transporter, Agp2p. EMBO J. 1999;18:5843–5852. doi: 10.1093/emboj/18.21.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmieri L., Rottensteiner H., Girzalsky W., Scarcia P., Palmieri F., Erdmann R. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 2001;20:5049–5059. doi: 10.1093/emboj/20.18.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser W. F., van Roermund C. W., Waterham H. R., Wanders R. J. Identification of human PMP34 as a peroxisomal ATP transporter. Biochem. Biophys. Res. Commun. 2002;299:494–497. doi: 10.1016/s0006-291x(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 18.Holzinger A., Kammerer S., Roscher A. A. Primary structure of human PMP69, a putative peroxisomal ABC-transporter. Biochem. Biophys. Res. Commun. 1997;237:152–157. doi: 10.1006/bbrc.1997.7102. [DOI] [PubMed] [Google Scholar]
- 19.Kamijo K., Taketani S., Yokota S., Osumi T., Hashimoto T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J. Biol. Chem. 1990;265:4535–4540. [PubMed] [Google Scholar]
- 20.Lombard-Platet H., Savary S., Sarde C. O., Mandel J. L., Chimini G. A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1265–1269. doi: 10.1073/pnas.93.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moser J., Douar A. M., Sarde C. O., Kioschis P., Feil R., Moser H., Poustka A. M., Mandel J. L., Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature (London) 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg S. J., Wang S. J., Kim D. G., Mihalik S. J., Watkins P. A. Human very-long-chain acyl-CoA synthetase: cloning, topography, and relevance to branched-chain fatty acid metabolism. Biochem. Biophys. Res. Commun. 1999;257:615–621. doi: 10.1006/bbrc.1999.0510. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama A., Aoyama T., Kamijo K., Uchida Y., Kondo N., Orii T., Hashimoto T. Molecular cloning of cDNA encoding rat very long-chain acyl-CoA synthetase. J. Biol. Chem. 1996;271:30360–30365. doi: 10.1074/jbc.271.48.30360. [DOI] [PubMed] [Google Scholar]
- 24.Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J. Cell Biol. 1968;37:482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooperstein S. J., Lazarow A. A microspectrophotometric method for the determination of cytochrome oxidase. J. Biol. Chem. 1951;189:665–670. [PubMed] [Google Scholar]
- 26.Bowers G. N., McComb R. B. Measurement of total alkaline phosphatase activity in human serum. Clin. Chem. 1975;21:1988–1995. [PubMed] [Google Scholar]
- 27.Takabatake R., Hata S., Taniguchi M., Kouchi H. Isolation and characterisation of cDNAs encoding mitochondrial phosphate transporters in soybean, maize, rice and Arabidopsis. Plant Mol. Biol. 1999;40:479–486. doi: 10.1023/a:1006285009435. [DOI] [PubMed] [Google Scholar]
- 28.Budavari S., O'Neil M. J., Smith A., Heckelman P. R. H. Rahway, NJ: Merck & Co.; 1989. The Merck Index. [Google Scholar]
- 29.Kaplan R. S. Purification and characterisation of the reconstitutively active phosphate transporter from rat liver mitochondria. J. Biol. Chem. 1986;261:12767–12773. [PubMed] [Google Scholar]
- 30.McIntosh C. A., Oliver D. J. The phosphate transporter from pea mitochondria. Plant Physiol. 1994;105:47–52. doi: 10.1104/pp.105.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Indiveri C., Tonazzi A., Palmieri F. Characterization of the unidirectional transport of carnitine catalyzed by the reconstituted carnitine carrier from rat liver mitochondria. Biochim. Biophys. Acta. 1991;1069:110–116. doi: 10.1016/0005-2736(91)90110-t. [DOI] [PubMed] [Google Scholar]