Abstract
Spectral and catalytic properties of the flavoenzyme AAO (aryl-alcohol oxidase) from Pleurotus eryngii were investigated using recombinant enzyme. Unlike most flavoprotein oxidases, AAO does not thermodynamically stabilize a flavin semiquinone radical and forms no sulphite adduct. AAO catalyses the oxidative dehydrogenation of a wide range of unsaturated primary alcohols with hydrogen peroxide production. This differentiates the enzyme from VAO (vanillyl-alcohol oxidase), which is specific for phenolic compounds. Moreover, AAO is optimally active in the pH range of 5–6, whereas VAO has an optimum at pH 10. Kinetic studies showed that AAO is most active with p-anisyl alcohol and 2,4-hexadien-1-ol. AAO converts m- and p-chlorinated benzyl alcohols at a similar rate as it does benzyl alcohol, but introduction of a p-methoxy substituent in benzyl alcohol increases the reaction rate approx. 5-fold. AAO also exhibits low activity on aromatic aldehydes. 19F NMR analysis showed that fluorinated benzaldehydes are converted into the corresponding benzoic acids. Inhibition studies revealed that the AAO active site can bind a wide range of aromatic ligands, chavicol (4-allylphenol) and p-anisic (4-methoxybenzoic) acid being the best competitive inhibitors. Uncompetitive inhibition was observed with 4-methoxybenzylamine. The properties described above render AAO a unique oxidase. The possible mechanism of AAO binding and oxidation of substrates is discussed in the light of the results of the inhibition and kinetic studies.
Keywords: aryl-alcohol oxidase, conjugated double bond, flavoenzyme, reaction mechanism, substrate binding
Abbreviations: AAO, aryl-alcohol oxidase; GMC, glucose/methanol/choline (oxidoreductase family); VAO, vanillyl-alcohol oxidase
INTRODUCTION
Aryl-alcohol oxidase (AAO; EC 1.1.3.7) activity was described for the first time in the fungus Polystictus versicolor (a synonym of Trametes versicolor) in 1960 [1]. Since then, AAO has been detected and characterized in other white-rot basidiomycetes, including four Pleurotus species [2–5], Bjerkandera adusta [6], and some ascomycetous fungi [7–9]. White-rot fungi are responsible for lignin degradation, and AAO participates in this process by generating hydrogen peroxide in the redox cycling of aromatic fungal metabolites [10], also involving mycelial dehydrogenases [11,12].
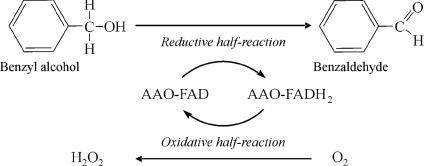
AAO, a monomeric glycosylated protein of 73 kDa [13], is one of the many flavoenzymes that contain a non-covalently bound FAD cofactor [14]. The enzyme from Pleurotus eryngii has a wide substrate specificity, oxidizing primary, polyunsaturated alcohols [13]. The reaction of AAO (Scheme 1) is initiated by the oxidative dehydrogenation of the substrate (reductive half-reaction), and is completed by flavin reoxidation by molecular oxygen with the production of hydrogen peroxide (oxidative half-reaction).
Scheme 1. Redox reaction catalysed by AAO.
Both AAO reductive and oxidative half-reactions, by alcohol and O2 respectively, are shown. Benzyl alcohol is included as an example of AAO substrate.
The AAO genes from P. eryngii [15] and Pleurotus pulmonarius [4] have been cloned and sequenced. These genes are very similar, with 95% identity at the amino acid sequence level. Primary structure analysis revealed that AAO belongs to the GMC (glucose/methanol/choline) oxidoreductase family of flavoenzymes [16]. In contrast with the related vanillyl-alcohol oxidase (VAO; EC 1.1.3.38) family [17], these enzymes share a conserved ADP-binding motif (βαβ-fold) involved in FAD binding, which includes a Gly-Xaa-Gly-Xaa-Xaa-Xaa-Gly fingerprint sequence near the N-terminus [18]. A molecular model of AAO from P. eryngii was built using the Aspergillus niger glucose oxidase crystal structure as template (Protein Data Bank entry 1QJN) [19]. The cDNA from P. eryngii has been expressed in Emericella nidulans (conidial state Aspergillus nidulans) and Escherichia coli, showing the recombinant AAO has catalytic properties similar to those of the wild-type enzyme [20]. Recombinant AAO production represents an improvement in terms of enzyme yield and production time [13], and facilitates characterization of the enzyme in more detail [21]. In the present paper, we report on the spectral and catalytic properties of AAO after its heterologous expression in E. nidulans.
EXPERIMENTAL
Chemicals
o-Fluorobenzyl, m-fluorobenzyl and p-fluorobenzyl alcohols, and p-fluorobenzaldehyde were purchased from Fluorochem (Old Glossop, Derbyshire, U.K.). m-Anisyl (3-methoxybenzyl), p-anisyl (4-methoxybenzyl), benzyl, m-chlorobenzyl, p-chlorobenzyl, cinnamyl (3-phenyl-2-propen-1-ol), isovanillyl (3-hydroxy-4-methoxybenzyl), vanillyl (4-hydroxy-3-methoxybenzyl) and veratryl (3,4-dimethoxybenzyl) alcohols, p-anisic (4-methoxybenzoic) acid, p-(hydroxymethyl)benzoic acid, 2,4-hexadien-1-ol, toluene, benzylmethyl ether and 4-methoxybenzylamine were obtained from Sigma–Aldrich (St Louis, MO, U.S.A.). Chavicol (4-allylphenol) was from Quest (Houston, TX, U.S.A.). 3-Chloro-p-anisyl alcohol was a gift from Dr E. de Jong (Wageningen, The Netherlands). 5-Deazariboflavin was a gift from Dr G. Tollin (Department of Biochemistry and Molecular Physics, University of Arizona, AZ, U.S.A.).
Organisms and vectors
E. nidulans biA1, metG1, argB2 (IJFM A729) was used as a heterologous expression host [22]. pALAAO vector containing the P. eryngii AAO cDNA was used to transform the above E. nidulans strain [20].
Expression and purification of recombinant enzyme
cDNA encoding P. eryngii AAO with its own signal peptide was expressed in E. nidulans (argB− strain) under the alcA promoter of the same fungus. Recombinant AAO was obtained from cultures grown at 28 °C (with shaking at 180 rev./min) in a minimal medium that, after 24 h, was replaced with the induction medium containing threonine [20]. After 48 h induction, the recombinant enzyme was purified using Sephacryl S-200 and Mono-Q chromatography, according to the procedure developed for wild-type AAO from P. eryngii [13]. Protein bands, after SDS/PAGE, were stained with Coomassie Brilliant Blue.
Enzyme activity and steady-state kinetics
The standard AAO activity was measured spectrophotometrically by monitoring the oxidation of veratryl alcohol to veratraldehyde at 310 nm. The reaction mixture contained 8 mM substrate in air-saturated 100 mM sodium phosphate buffer, pH 6. One unit of AAO activity is defined as the amount of enzyme that converts 1 μmol of alcohol to aldehyde per min at 24 °C.
AAO activity at different pH values was estimated in 100 mM citrate–phosphate–borate buffer, pH 2–11, and the relative activity values obtained were fitted to a three-state model (with two pKa values) using the following equation:
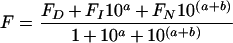 |
where FD, FI and FN are the relative activities (%) at acidic, neutral and basic pH values respectively; a=m(pH−pK1) and b=n(pH−pK2); and m and n are the number of protons in the two transitions (between the three pH states).
Steady-state kinetics were performed at 24 °C in the above buffer, pH 6 (and also at pH 9 in the case of isovanillyl and vanillyl alcohols). AAO activity was determined spectrophotometrically by measuring the rate of oxidation of different alcohols to the corresponding aldehydes. The following molar absorption coefficients were calculated under assay conditions (pH 6) using commercial standards: m-chlorobenzaldehyde (ϵ240 5923 M−1·cm−1), p-chlorobenzaldehyde (ϵ260 15862 M−1·cm−1), cinnamaldehyde (ϵ310 15600 M−1·cm−1), m-fluorobenzaldehyde (ϵ246 10280 M−1·cm−1), p-fluorobenzaldehyde (ϵ252 13700 M−1·cm−1), 2,4-hexadien-1-al (ϵ280 30140 M−1·cm−1), isovanillin (ϵ307 7383 M−1·cm−1) and vanillin (ϵ309 8332 M−1·cm−1). The molar absorption coefficients of isovanillin (ϵ360 2234 M−1·cm−1) and vanillin (ϵ347 22410 M−1·cm−1) were estimated also at pH 9. Molar absorption coefficients of benzaldehyde (ϵ250 13800 M−1·cm−1), veratraldehyde (ϵ310 9300 M−1·cm−1), m-anisaldehyde (ϵ314 2540 M−1·cm−1) and p-anisaldehyde (ϵ285 16950 M−1·cm−1) were from Guillén et al. [13], and that of 3-chloro-p-anisaldehyde (ϵ295 15000 M−1·cm−1) from de Jong et al. [23]. After non-linear fitting of data (three replicates) using SigmaPlot, mean and standard errors were obtained from the normalized Michaelis–Menten equation.
Inhibition studies were performed in the above buffer at pH 6 (and pH 8 in the case of 4-methoxybenzylamine), with veratryl alcohol as the varying substrate.
19F NMR analysis
AAO-mediated conversion of fluorinated substrates was studied by 19F NMR. The enzymatic reactions contained 1 mM fluorinated aromatic substrate, and 1 mM sodium ascorbate in 2 ml of 100 mM sodium phosphate buffer, pH 6. Reactions were started by addition of 100 m-units of AAO (500 m-units in the case of p-fluorobenzaldehyde), and were allowed to proceed at 24 °C under agitation. After 1 h incubation, the reactions were stopped by freezing in liquid nitrogen, and samples were stored at −20 °C until analysis. 19F NMR measurements were performed on a Bruker DPX 400 NMR spectrometer, as described previously [24].
Spectroscopic studies
AAO absorption spectra were recorded at room temperature on a Hewlett–Packard 8453 diode-array spectrophotometer. Enzyme concentration was determined using the AAO molar absorption coefficient, which was estimated in 100 mM sodium phosphate, pH 6, after thermal denaturation, enabling estimation of enzyme concentration from the amount of FAD released (ϵ450 12250 M−1·cm−1) [25].
Enzyme reduction experiments were performed in 100 mM sodium phosphate buffer, pH 6, under anaerobic conditions, which were reached by several cycles of atmosphere evacuation and flushing with argon, and followed by monitoring changes in the AAO absorption spectrum. In these experiments, 24 μM AAO was reduced with different concentrations of a stock solution of 16 mM p-anisyl alcohol in the presence of glucose oxidase (10 units/ml) and glucose (310 mM), which were added to both substrate and enzyme solution to remove trace amounts of oxygen. Dithionite reduction of AAO was investigated by titrating 26 μM enzyme with a stock solution of 250 mM sodium dithionite under an argon atmosphere.
AAO (17 μM) reaction with sulphite was studied in 100 mM sodium phosphate buffer, pH 7, using different sulphite concentrations (1–20 mM), and final changes in the absorption spectrum were recorded.
Photoreduction of AAO (11 μM) was carried out in an anaerobic cuvette. The reaction was performed in 100 mM sodium phosphate buffer, pH 6 (or pH 9), containing 1 mM EDTA and 5 μM 5-deazariboflavin. Solutions were made anaerobic by several cycles of evacuation and flushing with argon. Absorption spectra were recorded after different periods of irradiation with a 150-W light source [25].
Stopped-flow measurements
Stopped-flow measurements were made using an Applied Photophysics SX17.MV spectrophotometer, interfaced with an Acorn 5000 computer using the SX18.MV software from Applied Photophysics. Samples were made anaerobic before introducing them into the stopped-flow syringes. Reactions were allowed to proceed in 100 mM sodium phosphate buffer, pH 6, at 20 °C, and kinetics traces were followed at 460 nm, the wavelength at which oxidized AAO has an absorbance maximum.
RESULTS
Enzyme production
Recombinant AAO was expressed in E. nidulans and purified in two chromatographic steps, with a final yield up to 3 mg of pure AAO per litre of fungal culture. Its electrophoretic homogeneity was confirmed by SDS/PAGE.
Spectral properties
Purified AAO showed absorption maxima in the visible region at 385 nm and 463 nm (with troughs at 414 nm and 317 nm; Figure 1) and an A280/A463 ratio of 10. After heat treatment (70 °C for 10 min), the AAO protein was precipitated, and the cofactor absorption spectrum lost the 463 nm maximum, which was replaced by the 450 nm maximum of free flavin, indicating that the flavin was not covalently bound to the protein. From these spectral changes, the molar absorption coefficient of the recombinant enzyme (ϵ463 10280 M−1·cm−1) was estimated.
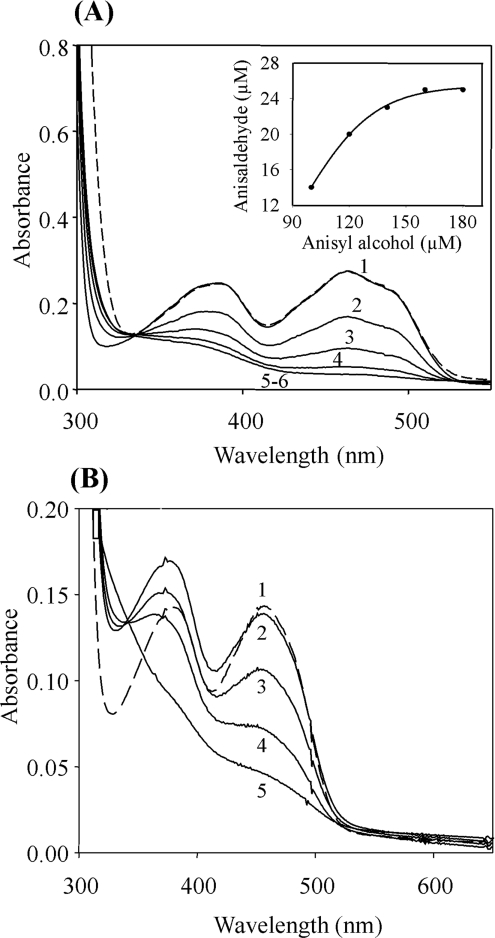
Figure 1. Anaerobic reduction of AAO.
(A) To investigate AAO reduction by substrate, an anaerobic solution of AAO (24 μM) in 100 mM sodium phosphate buffer, pH 6, containing 10 units/ml of glucose oxidase and 310 mM glucose (added anaerobically) was titrated with the following concentrations of p-anisyl alcohol, in 100 mM sodium phosphate buffer, pH 6 (prepared also under anaerobic conditions): 0 μM (trace 1), 100 μM (trace 2), 120 μM (trace 3), 140 μM (trace 4), 160 μM (trace 5) and 180 μM (trace 6). The spectra were corrected for dilution. The initial spectrum of oxidized AAO (trace 1) was recovered after reoxidation with O2 (---). The inset shows the p-anisaldehyde produced during AAO reduction. The aldehyde concentrations (being respectively 14, 20, 23 and 25 μM) were calculated from the increase of absorbance at 298 nm. All spectra were recorded at 24 °C. (B) To investigate AAO photoreduction, an anaerobic cuvette containing an anaerobic solution of AAO (12 μM), EDTA (1 mM) and 5-deazariboflavin (5 μM) in 100 mM sodium phosphate buffer, pH 6 (prepared anaerobically) was irradiated with a 150 W light source at 24 °C. Spectra were recorded before (trace 1, dashed line) and after (trace 2) adding 5-deazariboflavin and EDTA, and after 10 min (trace 3), 20 min (trace 4) and 40 min (trace 5) of subsequent illumination.
AAO does not react with sulphite, since no spectral changes were observed in the presence of up to 20 mM sodium sulphite. Anaerobic reduction of the enzyme by substrate was studied using p-anisyl alcohol. A decrease in the 463 nm absorbance was observed during p-anisyl alcohol oxidation, revealing the participation of the flavin cofactor in the catalytic activity of AAO (Figure 1A). In these experiments, an isosbestic point was observed at 330 nm, indicative of a two-electron reduction of the cofactor [25]. Excess of p-anisyl alcohol (5–6-fold) was required for complete reduction of AAO. During AAO reduction, stoichiometric amounts of p-anisaldehyde (calculated from its absorbance at 298 nm) were formed (Figure 1A, inset). The anaerobic reduction of AAO by p-anisyl alcohol was also followed using the stopped-flow equipment coupled to a photodiode array detector. The spectral changes observed in the 350–650 nm range showed the reduction of the oxidized flavin to the hydroquinone form, without formation of an observable semiquinone intermediate.
Anaerobic titration of AAO with dithionite gave similar spectra, where only two chemical species (corresponding to reduced and oxidized enzyme) could be identified. Dithionite reduction appeared as a monophasic process when analysed using the stopped-flow equipment. In both anaerobic reduction experiments (using p-anisyl alcohol and dithionite), reoxidation with air restored the spectral properties of the oxidized form of AAO.
Photoreduction at pH 6 (Figure 1B) and pH 9 did not show semiquinone formation, and only fully oxidized and reduced species were observed. Reoxidation completely restored the original spectrum.
Catalytic properties
The catalytic versatility of AAO was investigated in the present study using the recombinant enzyme from E. nidulans and a wide range of possible electron donors. First, the ability of AAO to catalyse reactions other than unsaturated alcohol oxidation, such as oxidative hydroxylation, demethylation and deamination, was evaluated using benzylmethyl ether, toluene and 4-methoxybenzylamine as possible substrates. However, the enzyme showed no activity on these compounds, which acted as AAO inhibitors (see below).
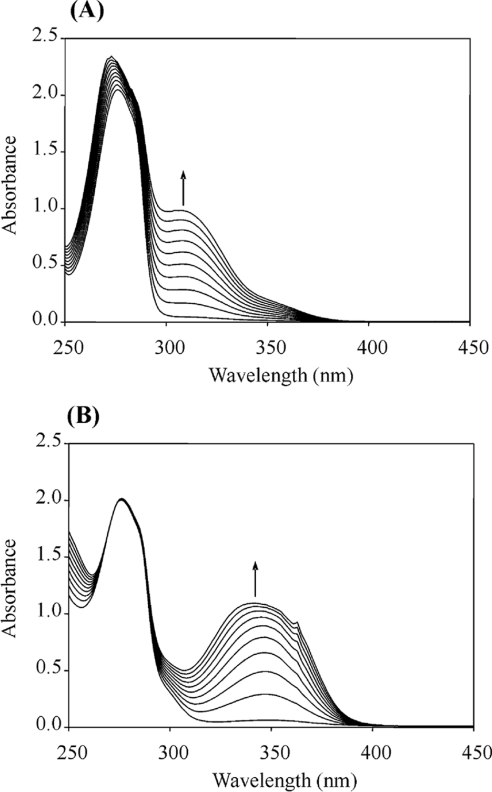
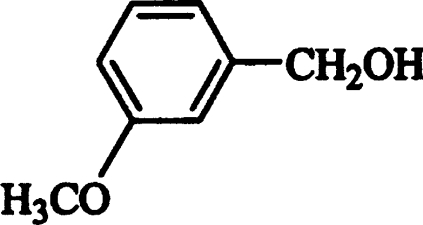
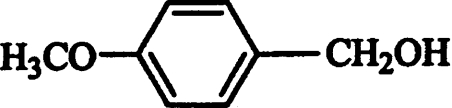
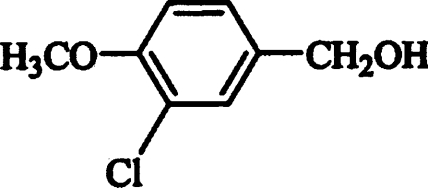
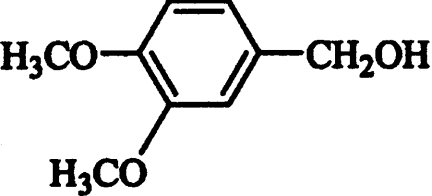
Then, the effect of different substituents in the benzyl alcohol ring (chlorine, fluorine, methoxyl, hydroxyl and carboxyl) on AAO activity was evaluated. Oxidation of the different alcohols resulted in formation of the corresponding aldehydes that were detected by characteristic absorption maxima (Figure 2). Novel types of P. eryngii AAO substrates were found, such as halogenated benzyl alcohols. The steady-state kinetic parameters of different p- and m-substituted benzyl alcohols were determined. Table 1 shows the Km, kcat and catalytic efficiency (kcat/Km) values, together with those of benzyl alcohol, cinnamyl alcohol, and 2,4-hexadien-1-ol. Considering the efficiency values, p-anisyl alcohol was the best AAO substrate, whereas p-(hydroxymethyl)benzoic acid was the worst substrate (owing to the low AAO activity on this compound, its kinetic constants in Table 1 are only estimates). 3-Chloro-p-anisyl and 2,4-hexadien-1-ol also appeared to be very good AAO substrates.
Figure 2. Spectral changes during AAO oxidation of vanillyl alcohol at two different pH values.
The reaction mixtures contained 2 mM vanillyl alcohol in 100 mM sodium phosphate buffer at pH 6 (A) or pH 9 (B). The spectra were recorded every 60 min during 10 h, at 24 °C, after addition of AAO (50 m-units/ml) and increases (arrows) were observed at the absorbance maxima of the aldehydes.
Table 1. Steady-state kinetic constants for AAO oxidation of different substrates.
AAO oxidation assays on a variety of substrates were performed in 100 mM sodium phosphate buffer, pH 6, at 24 °C. After non-linear fitting of data, mean and standard errors were obtained from the normalized Michaelis-Menten equation.
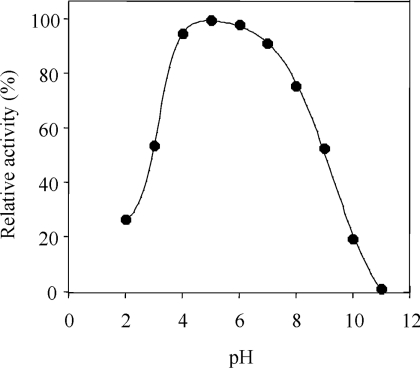
The rates of AAO oxidation of the phenolic vanillyl and isovanillyl alcohols were very different. As shown in Table 1, isovanillyl alcohol is a good AAO substrate, with a kcat value similar to that of veratryl alcohol. By contrast, AAO activity on vanillyl alcohol (2 mM, at pH 6) was very low (0.59 s−1) compared with isovanillyl alcohol (42.6 s−1), and its kinetic parameters were not determined. AAO activity showed a broad pH optimum, with more than 90% maximal activity on veratryl alcohol in the range of pH 4–7, and a significant decrease at pH 9 (Figure 3), although the enzyme was very stable at pH 6–9. An increase of pH over the optimum (pH 6) produced opposite effects on the AAO affinity and kcat. At pH 9, the affinity for veratryl (Km 347±38 μM) and isovanillyl (Km 418±65 μM) alcohols was increased by over 30% with respect to pH 6 (Table 1), whereas the kcat value for these two alcohols (37±1 and 49±2 s−1 respectively) was decreased by nearly 60%. Despite low AAO activity on vanillyl alcohol, spectral changes were observed during long-term enzymatic conversion at pH 6 and 9 (Figure 2), and comparison with spectra of authentic standards showed formation of vanillin. The effect of pH on this reaction was similar to that found with isovanillyl alcohol, i.e. higher oxidation rate at pH 6 (0.59 s−1) than at pH 9 (0.48 s−1).
Figure 3. Influence of pH on AAO activity.
AAO activity at different pH values was estimated by adding 20 μl of a concentrate AAO solution (730 m-units/ml) previously dialysed against 10 mM sodium citrate–phosphate–borate buffer, pH 6, to 1 ml of 100 mM sodium citrate–phosphate–borate buffer of different pH values (pH 2–10). Activity was determined spectrophotometrically by oxidation of 8 mM veratryl alcohol to veratraldehyde at 310 nm, at 24 °C. The three-state equation indicated in the Experimental section was adjusted, and two pKa values (3.1±0.1 and 9.0±0.2) were calculated.
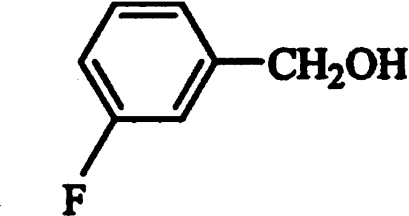
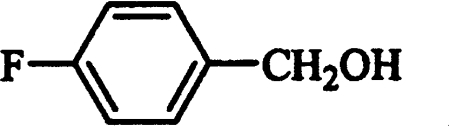
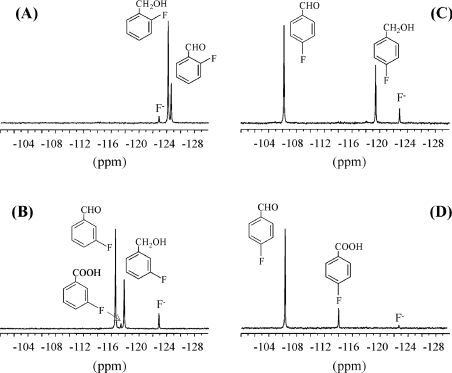
Conversion of fluorinated benzyl compounds by AAO was also investigated by 19F NMR. After 1 h incubation of o-fluorobenzyl, m-fluorobenzyl and p-fluorobenzyl alcohols with the enzyme at pH 6, the corresponding aldehydes were identified as main reaction products (Figures 4A–4C and Table 2). From Figures 4(A)–(C), it can be seen that p-fluorobenzyl alcohol is a better AAO substrate than m-fluorobenzyl alcohol, and that the latter compound is a better substrate than o-fluorobenzyl alcohol. Kinetic parameters for o-fluorobenzyl alcohol were not determined due to low AAO activity (0.83 s−1 at 1.25 mM substrate concentration, pH 6) compared with benzyl alcohol (3.74 s−1). AAO oxidation of m-fluorobenzyl alcohol (Figure 4B and Table 3) showed a major m-fluorobenzaldehyde signal at −116.6 p.p.m., and an additional lower signal at −117.4 p.p.m. corresponding to m-fluorobenzoic acid, and indicative of aldehyde oxidase activity [13]. The aldehyde activity of AAO was investigated further using p-fluorobenzaldehyde as a substrate (Figure 4D and Table 3). Although the aldehyde oxidase activity only represented 4% of the p-fluorobenzyl alcohol activity, it could be demonstrated easily by 19F NMR, and the reaction product was identified as p-fluorobenzoic acid (−114.2 p.p.m.). During the AAO reaction with fluorinated substrates, some release of fluoride anion, with a 19F NMR resonance at −122.6 p.p.m., was observed. This release was significantly higher than that found in the corresponding controls, but represented less than 10% of the total conversion of fluorinated benzyl alcohols by AAO.
Figure 4. 19F NMR analysis of AAO oxidation of fluoroaromatic compounds.
19F NMR spectra during o-fluorobenzyl alcohol (A), m-fluorobenzyl alcohol (B), p-fluorobenzyl alcohol (C) and p-fluorobenzaldehyde (D) incubation (1 h) with AAO. The enzymatic mixtures (2 ml) contained 1 mM fluorinated substrate, 1 mM sodium ascorbate and 100 m-units AAO (500 m-units in the case of p-fluorobenzaldehyde) in 100 mM sodium phosphate buffer, pH 6, at 24 °C.
Table 2. 19F NMR chemical shift values of fluorinated aromatic compounds.
Chemical shift values of the different substrates and products identified during 19F NMR analysis (Figure 4) of AAO oxidation of fluorinated compounds were obtained in 100 mM sodium phosphate buffer, pH 6.
| Compound | Produced from… | Chemical shift (p.p.m.) |
|---|---|---|
| p-Fluorobenzaldehyde | p-Fluorobenzyl alcohol | 106.4 |
| p-Fluorobenzoic acid | p-Fluorobenzaldehyde | 114.2 |
| m-Fluorobenzaldehyde | m-Fluorobenzyl alcohol | 116.6 |
| m-Fluorobenzoic acid | m-Fluorobenzyl alcohol | 117.4 |
| m-Fluorobenzyl alcohol | − | 117.8 |
| p-Fluorobenzyl alcohol | − | 119.3 |
| Fluoride anion | − | 122.6 |
| o-Fluorobenzyl alcohol | − | 124.1 |
| o-Fluorobenzaldehyde | o-Fluorobenzyl alcohol | 124.6 |
Table 3. Inhibition constants for different AAO inhibitors.
Inhibition of veratryl alcohol oxidation by different aromatic compounds was estimated in 100 mM sodium phosphate buffer, pH 6 (or pH 8 in the case of 4-methoxybenzylamine). Results are presented as means±S.E.M.
| Inhibitor | Structure | Type of inhibition | Ki (mM) |
|---|---|---|---|
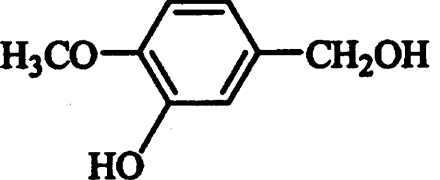
| Phenol* |  |
Competitive | 1.92 |
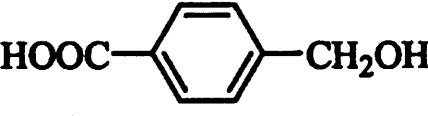
| 3-Phenyl-1-propanol* | 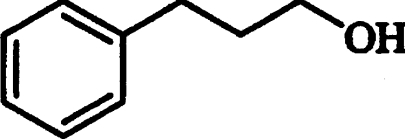 |
Competitive | 4.48 |
| Chavicol | 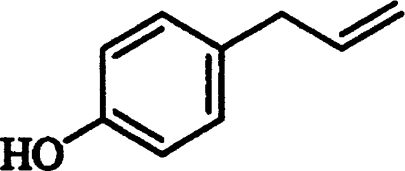 |
Competitive | 0.11±0.02 |
| Toluene | 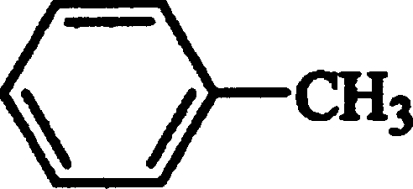 |
Competitive | 0.75±0.05 |
| Benzylmethyl ether | 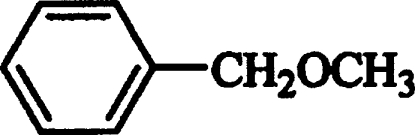 |
Competitive | 1.35±0.80 |
| p-Anisic acid | 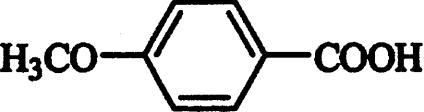 |
Competitive | 0.08±0.02 |
| 4-Methoxybenzylamine | 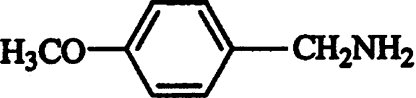 |
Uncompetitive | 0.25±0.02 |
* Data are from Guillén [40].
Inhibition studies
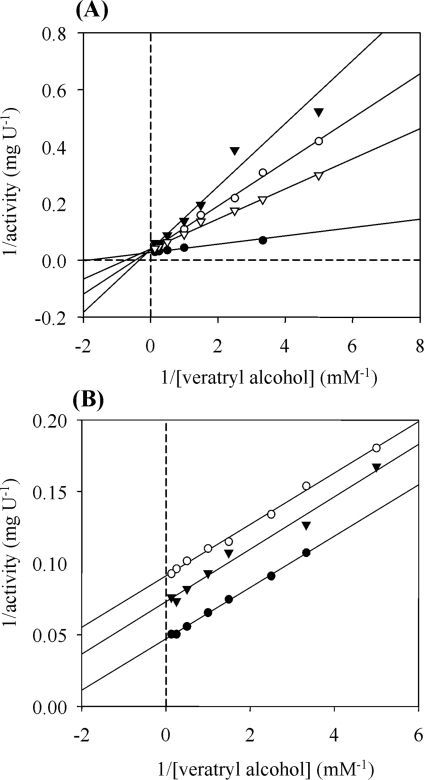
Inhibition studies were performed to obtain additional information on the binding mechanism of AAO substrates. The inhibitory effect of different aromatic compounds on veratryl alcohol oxidation by recombinant AAO is shown in Table 3. In addition to phenol, already known from previous studies [13], a second phenolic competitive inhibitor, chavicol, was found with a Ki of 0.11 mM (Figure 5A). Moreover, several non-phenolic aromatic compounds, such as p-anisic acid, toluene and benzylmethyl ether, were found to be competitive inhibitors of AAO. Among them, p-anisic acid showed a strong interaction with AAO, similar to that found for chavicol. Enzyme complexes with the two latter compounds were reversible, as the inhibitors could be removed by dialysis. Finally, 4-methoxybenzylamine was found to be an uncompetitive inhibitor with high affinity for AAO. This inhibition was pH-dependent, being optimal at pH 8 (Ki 0.25 mM; Figure 5B).
Figure 5. Competitive and uncompetitive inhibition of AAO by chavicol (A) and 4-methoxybenzylamine (B) respectively.
Inhibition of AAO activity was investigated in 100 mM sodium phosphate buffer, pH 6 (A) or pH 8 (B), at 24 °C, using veratryl alcohol as substrate (absorbance increase at 310 nm). (A) The different lines correspond to reactions allowed to proceed without inhibitor (●), or with 0.2 mM (▽), 0.3 mM (○) or 0.5 mM chavicol (▼). (B) The different lines correspond to reactions allowed to proceed without inhibitor (●), or with 0.15 mM (▼) or 0.3 mM (○) 4-methoxybenzylamine. All fits have r values >0.995.
DISCUSSION
Flavoprotein oxidases share some properties, such as stabilization of the flavin semiquinone anion radical and formation of flavin N(5)-sulphite adducts [26]. These properties, together with the formation of benzoquinoid anionic forms of artificial flavins in reconstituted proteins, point to the existence of a positively charged protein locus (due to a basic residue or α-helix dipole) interacting with the pyrimidine ring of the flavin [27]. Flavin radical stabilization does not necessarily imply that the anionic radical is involved in catalysis, but the possibility to stabilize it under certain conditions provides some useful structural information. The absorption spectrum of recombinant AAO was very similar to that of wild-type enzyme from P. eryngii [13], and included the optical maxima of flavin-containing proteins. Photoreduction (in the presence of EDTA and 5-deazariboflavin) directly gave a fully reduced FAD product, and the same two-electron reduction process was observed during dithionite and substrate reduction. Moreover, AAO did not show any reactivity with sulphite. These spectral properties are described here for the first time, and show that AAO is an atypical flavoprotein oxidase. There are other flavoprotein oxidases that do not form sulphite complexes, as described also for putrescine oxidase, VAO and monoamine oxidase [28–30]. VAO has an aspartate residue (Asp170) near flavin N-5 that would hamper adduct formation by electrostatic repulsion [28]. However, the molecular model of AAO [31] shows a histidine residue at this position and an α-helix near the N(1)–C(2)=O locus of the flavin that should stabilize the anionic form of the reduced flavin, as found in glucose oxidase [32].
Previous substrate specificity studies have shown that P. eryngii AAO catalyses the conversion of primary alcohols of very different chemical structures [13]. The results obtained with the recombinant AAO confirm the previous findings, and show that AAO is able to oxidize unsaturated alcohols and aldehydes, but does not catalyse reactions of oxidative deamination, hydroxylation and demethylation described for other oxidases, such as VAO [33]. Dual alcohol and aldehyde oxidase activity has been mentioned for other flavoprotein alcohol oxidases, such as methanol oxidase, but was not investigated in detail [34]. Aldehyde oxidase activity has been described also in xanthine oxidase. This iron–molybdenum flavoprotein catalyses the oxidation of a wide range of N-heterocycles and aldehydes, including substituted benzaldehydes [35]. Here, we demonstrated for the first time using 19F NMR spectroscopy the formation of an aromatic acid as a result of the aldehyde oxidase activity of AAO. On the other hand, the kinetic data presented for 2,4-hexadien-1-ol show that AAO is able to efficiently oxidize aliphatic unsaturated alcohols in addition to the aromatic ones.
In the present paper we have shown for the first time that halogenated benzyl alcohols are substrates of P. eryngii AAO. The enzymatic reaction rate with these compounds was similar to the rate observed with the parent benzyl alcohol, but the substrate affinity increased with the size of the substituent. Especially, the presence of a chlorine substituent in 3-chloro-p-anisyl alcohol increased AAO binding with respect to p-anisyl alcohol (these two alcohols showing the highest affinity values). Structure–activity relationship studies on monoamine oxidase A oxidation of different p-substituted benzylamine analogues showed similar binding effects [36]. 3-Chloro-p-anisyl alcohol was reported to be a good substrate of Bjerkandera sp. AAO, and it has been postulated that this white-rot fungus secretes chlorinated anisyl metabolites for hydrogen peroxide production through a redox cycle [23], analogous to that described previously in P. eryngii on the basis of non-chlorinated aromatic metabolites [11,12].
p-Hydroxybenzyl alcohols are very poor AAO substrates, which is in agreement with earlier studies by Guillén et al. [13]. The importance of the substituent position for AAO activity is dramatically illustrated by the comparison between isovanillyl alcohol, a good substrate, and vanillyl alcohol, with less than 1.5% the activity of the above isomer. On the other hand, the presence of a p-carboxy substituent caused a 1000-fold decrease in the efficiency of AAO oxidation with respect to benzyl alcohol.
Inhibition studies showed that the AAO active site can bind not only benzyl alcohol substrates, but also different aromatic compounds without an alcohol group. Inhibitors included aromatic compounds bearing side chains of variable size, ranging from a methyl group to an aliphatic chain of up to four carbon atoms. Several phenolic (chavicol) and non-phenolic (toluene, benzylmethyl ether and anisic acid) competitive inhibitors of AAO are described here for the first time. The results indicate that a strict requirement for efficient AAO binding is the presence of at least two conjugated double bonds, as shown in 2,4-hexadien-1-ol. This non-aromatic compound was shown to be one of the best AAO substrates. Moreover, the alcohol function must be located at Cα of the conjugated double bond system in order to be oxidized by AAO [13]. In fact, cinnamyl alcohol is a good substrate, but 3-phenyl-1-propanol is a competitive inhibitor of the enzyme. On the other hand, the results obtained with 4-methoxybenzylamine suggest a more complex inhibition mechanism by this substrate analogue that would involve its binding to the AAO–substrate complex. The fact that inhibition was observed only at pH 8 suggests that the amine group needs to be positively charged to interact with the enzyme.
The best competitive inhibitor of AAO, chavicol, is a good substrate of VAO, and the opposite holds true for cinnamyl alcohol, being an AAO substrate and VAO inhibitor [37]. Both oxidases recognize aromatic compounds of similar structures, but VAO is active with 4-hydroxybenzyl alcohols, whereas AAO oxidizes ‘non-activated’ aryl alcohols. VAO has an optimal turnover rate around pH 10. This high pH facilitates the deprotonation of the phenolic substrate and, via hydride transfer to the flavin, the formation of a p-quinone methide product intermediate [33]. Such an intermediate is not produced during oxidation of non-activated alcohols by AAO and other oxidases (e.g. glucose oxidase). By contrast, AAO turnover is optimal around pH 5–6, although substrate binding is slightly better at pH 9, as shown for both veratryl and isovanillyl alcohols. The very low AAO activity with 4-hydroxybenzyl alcohols suggests that the presence of a hydroxy group at the p-position interferes with the mechanism of benzyl alcohol oxidation. Moreover, with vanillyl alcohol as a substrate, the reaction rate of AAO is faster at neutral than at alkaline pH. Assuming that the reductive half-reaction is rate-limiting, this supports the notion that a quinone methide intermediate is not produced in AAO catalysis.
The molecular model of AAO suggests that AAO may have catalytic features in common with glucose oxidase [31]. Two possible mechanisms for glucose oxidation by glucose oxidase have been suggested: (i) hydride transfer from substrate C1 to flavin N-5; or (ii) nucleophilic addition by the 1-hydroxy group of β-D-glucose to the C-4a position of the flavin, followed by proton abstraction from C-1 of the substrate [38]. Both mechanisms are expected to be assisted by general base catalysis, with the His516 and His559 residues of glucose oxidase acting as proton acceptors in the reductive half-reaction [39]. These residues are conserved in AAO (His502 and His546), and it has been proposed by Varela et al. [31] that they would also act as general bases in the reductive half-reaction and as general acids in the oxidative half-reaction. The role of these two histidine residues is currently under investigation using site-directed mutagenesis. In any case, the high activity of AAO with p-methoxybenzyl alcohols, compared with the activity with benzyl alcohols bearing electron-withdrawing substituents, is compatible with an electrophilic attack on the aromatic alcohols, which would probably be produced via hydride transfer in a base-assisted mechanism involving His502 and/or His546. An opposite effect of p-substituents was observed in monoamine oxidase A oxidation of benzylamines [36]. Electron-withdrawing substituents increased the reaction rate, supporting a nucleophilic attack resulting in Cα–H bond cleavage by proton abstraction. The monoamine oxidase B crystal structure supported this mechanism with flavin adduct formation [30]. Finally, it is important to mention that the low aromatic aldehyde oxidase activity of AAO seems to be produced by a mechanism different from that responsible for aromatic alcohol oxidation, probably involving nucleophilic attack on aromatic aldehydes by AAO. This is supported by the results of Guillén et al. [13], showing the highest AAO aldehyde oxidase activity on aromatic aldehydes with electron-withdrawing substituents, such as p-nitrobenzaldehyde, and low activity on p-anisaldehyde.
Concluding remarks
AAO is a unique enzyme due to a combination of several spectral and catalytic properties described here. Unlike other flavoenzyme oxidases, it does not stabilize a semiquinone anionic radical during light reduction. This is even more remarkable, taking into account that the AAO molecular model available shows the presence of both a histidine residue and an α-helix near the flavin ring, which should provide a positively charged environment stabilizing the flavin anionic radical. Moreover, AAO does not react with sulphite, as reported for most flavoenzymes using oxygen as electron acceptor (oxidases).
A second oxidase acting on aromatic alcohols, VAO, has been described. However, AAO differs from VAO by its ability to oxidize ‘non-activated’ alcohols (lacking a phenolic group). Moreover, the effect of pH on AAO oxidation of some phenolic alcohols suggests that a substrate quinone methide intermediate is not produced, as found for VAO. Substrate specificity and inhibition studies showed that AAO binds (aliphatic or aromatic) conjugated double bond systems, and is able to oxidize aliphatic polyunsaturated alcohols efficiently (a kcat of 119 s−1 for 2,4-hexadien-1-ol) in addition to aromatic primary alcohols (in Cα), and also slowly oxidizes aromatic aldehydes to acids. Moreover, the kinetic study of AAO oxidation of substituted benzyl alcohols showed the highest activity in the presence of electron donor substituents (a kcat of 142 s−1 for p-anisyl alcohol compared with 30 s−1 for benzyl alcohol). This suggests an electrophilic attack mechanism assisted by the presence of two histidine residues localized near N-5 of the flavin ring, which could act as general bases (for hydride transfer) in the reductive half-reaction, as described for glucose oxidase.
Acknowledgments
This work has been supported by the Spanish Biotechnology Programme (BIO 2002-1166) and the EU project QLK3-99-590. We thank Ed de Jong (Wageningen, UR, The Netherlands) for a sample of 3-chloro-p-anisyl alcohol, and Mariëlle Moonen (Wageningen, UR, The Netherlands) for assistance in NMR experiments. P. F. acknowledges a Fellowship of the Spanish Ministry of Science and Technology.
References
- 1.Farmer V. C., Henderson M. E. K., Russell J. D. Aromatic-alcohol-oxidase activity in the growth medium of Polystictus versicolor. Biochem. J. 1960;74:257–262. doi: 10.1042/bj0740257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourbonnais R., Paice M. G. Veratryl alcohol oxidases from the lignin degrading basidiomycete Pleurotus sajor-caju. Biochem. J. 1988;255:445–450. doi: 10.1042/bj2550445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sannia G., Limongi P., Cocca E., Buonocore F., Nitti G., Giardina P. Purification and characterization of a veratryl alcohol oxidase enzyme from the lignin degrading basidiomycete Pleurotus ostreatus. Biochim. Biophys. Acta. 1991;1073:114–119. doi: 10.1016/0304-4165(91)90190-r. [DOI] [PubMed] [Google Scholar]
- 4.Varela E., Böckle B., Romero A., Martínez A. T., Martínez M. J. Biochemical characterization, cDNA cloning and protein crystallization of aryl-alcohol oxidase from Pleurotus pulmonarius. BBA Protein Struct. Mol. Enzym. 2000;1476:129–138. doi: 10.1016/s0167-4838(99)00227-7. [DOI] [PubMed] [Google Scholar]
- 5.Guillén F., Martínez A. T., Martínez M. J. Production of hydrogen peroxide by aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Appl. Microbiol. Biotechnol. 1990;32:465–469. [Google Scholar]
- 6.Muheim A., Waldner R., Leisola M. S. A., Fiechter A. An extracellular aryl-alcohol oxidase from the white-rot fungus Bjerkandera adusta. Enzyme Microb. Technol. 1990;12:204–209. [Google Scholar]
- 7.Goetghebeur M., Nicolas M., Brun S., Galzy P. Production and excretion of benzyl alcohol oxidase in Botrytis cinerea. Phytochemistry. 1992;31:413–416. [Google Scholar]
- 8.Regalado V., Perestelo F., Rodríguez A., Carnicero A., Sosa F. J., de la Fuente G., Falcón M. A. Activated oxygen species and two extracellular enzymes: laccase and aryl-alcohol oxidase, novel for the lignin-degrading fungus Fusarium proliferatum. Appl. Microbiol. Biotechnol. 1999;51:388–390. [Google Scholar]
- 9.Kim S. J., Suzuki N., Uematsu Y., Shoda M. Characterization of aryl alcohol oxidase produced by dye-decolorizing fungus, Geotrichum candidum Dec1. J. Biosci. Bioeng. 2001;91:166–172. doi: 10.1263/jbb.91.166. [DOI] [PubMed] [Google Scholar]
- 10.Gutiérrez A., Caramelo L., Prieto A., Martínez M. J., Martínez A. T. Anisaldehyde production and aryl-alcohol oxidase and dehydrogenase activities in ligninolytic fungi from the genus Pleurotus. Appl. Environ. Microbiol. 1994;60:1783–1788. doi: 10.1128/aem.60.6.1783-1788.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillén F., Evans C. S. Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl. Environ. Microbiol. 1994;60:2811–2817. doi: 10.1128/aem.60.8.2811-2817.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillén F., Martínez A. T., Martínez M. J., Evans C. S. Hydrogen peroxide-producing system of Pleurotus eryngii involving the extracellular enzyme aryl-alcohol oxidase. Appl. Microbiol. Biotechnol. 1994;41:465–470. [Google Scholar]
- 13.Guillén F., Martínez A. T., Martínez M. J. Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur. J. Biochem. 1992;209:603–611. doi: 10.1111/j.1432-1033.1992.tb17326.x. [DOI] [PubMed] [Google Scholar]
- 14.Hefti M. H., Vervoort J., van Berkel W. J. H. Deflavination and reconstitution of flavoproteins – tackling fold and function. Eur. J. Biochem. 2003;270:4227–4242. doi: 10.1046/j.1432-1033.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- 15.Varela E., Martínez A. T., Martínez M. J. Molecular cloning of aryl-alcohol oxidase from Pleurotus eryngii, an enzyme involved in lignin degradation. Biochem. J. 1999;341:113–117. [PMC free article] [PubMed] [Google Scholar]
- 16.Zámocký M., Hallberg M., Ludwig R., Divne C., Haltrich D. Ancestral gene fusion in cellobiose dehydrogenases reflects a specific evolution of GMC oxidoreductases in fungi. Gene. 2004;338:1–14. doi: 10.1016/j.gene.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Fraaije M. W., van Berkel W. J. H., Benen J. A. E., Visser J., Mattevi A. A novel oxidoreductase family sharing a conserved FAD-binding domain. Trends Biochem. Sci. 1998;23:206–207. doi: 10.1016/s0968-0004(98)01210-9. [DOI] [PubMed] [Google Scholar]
- 18.Wierenga R. K., Terpstra P., Hol W. G. L. Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J. Mol. Biol. 1986;187:101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- 19.Hecht H. J., Kalisz H. M., Hendle J., Schmid R. D., Schomburg D. Crystal structure of glucose oxidase from Aspergillus niger refined at 2.3 Å resolution. J. Mol. Biol. 1993;229:153–172. doi: 10.1006/jmbi.1993.1015. [DOI] [PubMed] [Google Scholar]
- 20.Varela E., Guillén F., Martínez A. T., Martínez M. J. Expression of Pleurotus eryngii aryl-alcohol oxidase in Aspergillus nidulans: purification and characterization of the recombinant enzyme. Biochim. Biophys. Acta. 2001;1546:107–113. doi: 10.1016/s0167-4838(00)00301-0. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira P., Guillén F., Ruiz-Dueñas F. J., García de Lacoba M., Martínez M. J., van Berkel W. J. H., Martínez A. T. Structure–function studies on fungal aryl-alcohol oxidase. In: Chapman S. K., Perham R. N., Scrutton N. S., editors. Flavins and Flavoproteins. Berlin: Rudolf Weber; 2002. pp. 155–160. [Google Scholar]
- 22.Fernández-Cañón J. M., Peñalva M. A. Overexpression of two penicillin structural genes in Aspergillus nidulans. Mol. Gen. Genet. 1995;246:110–118. doi: 10.1007/BF00290139. [DOI] [PubMed] [Google Scholar]
- 23.de Jong E., Cazemier A. E., Field J. A., de Bont J. A. M. Physiological role of chlorinated aryl alcohols biosynthesized de novo by the white rot fungus Bjerkandera sp strain BOS55. Appl. Environ. Microbiol. 1994;60:271–277. doi: 10.1128/aem.60.1.271-277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moonen M. J. H., Rietjens I. M. C. M., van Berkel W. J. 19F NMR study on the biological Baeyer-Villiger oxidation of acetophenones. J. Ind. Microbiol. Biotechnol. 2001;26:35–42. doi: 10.1038/sj/jim/7000071. [DOI] [PubMed] [Google Scholar]
- 25.Macheroux P. UV–visible spectroscopy as a tool to study flavoproteins. In: Chapman S. K., Reid G. A., editors. Flavoprotein Protocols. Totowa: Humana Press; 1999. pp. 1–7. [DOI] [PubMed] [Google Scholar]
- 26.Massey V. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans. 2000;28:283–296. [PubMed] [Google Scholar]
- 27.Fraaije M. W., Mattevi A. Flavoenzymes: diverse catalysts with recurrent features. Trends Biochem. Sci. 2000;25:126–132. doi: 10.1016/s0968-0004(99)01533-9. [DOI] [PubMed] [Google Scholar]
- 28.Mattevi A., Fraaije M. W., Mozzarelli A., Olivi L., Coda A., van Berkel W. J. H. Crystal structures and inhibitor binding in the octameric flavoenzyme vanillyl-alcohol oxidase: the shape of the active-site cavity controls substrate specificity. Structure. 1997;5:907–920. doi: 10.1016/s0969-2126(97)00245-1. [DOI] [PubMed] [Google Scholar]
- 29.De Sa R. J. Putrescine oxidase from Micrococcus rubens. Purification and properties of the enzyme. J. Biol. Chem. 1972;247:5527–5534. [PubMed] [Google Scholar]
- 30.Binda C., Newton-Vinson P., Hubalek F., Edmondson D. E., Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002;9:22–26. doi: 10.1038/nsb732. [DOI] [PubMed] [Google Scholar]
- 31.Varela E., Martínez M. J., Martínez A. T. Aryl-alcohol oxidase protein sequence: a comparison with glucose oxidase and other FAD oxidoreductases. Biochim. Biophys. Acta. 2000;1481:202–208. doi: 10.1016/s0167-4838(00)00127-8. [DOI] [PubMed] [Google Scholar]
- 32.Wohlfahrt G., Witt S., Hendle J., Schomburg D., Kalisz H. M., Hecht H.-J. 1.8 and 1.9 Å resolution structures of the Penicillium amagasekiense and Aspergillus niger glucose oxidase as a basis for modelling substrate complexes. Acta Crystallogr. Sect. D Biol. Crystallogr. 1999;55:969–977. doi: 10.1107/s0907444999003431. [DOI] [PubMed] [Google Scholar]
- 33.Fraaije M. W., van Berkel W. J. H. Catalytic mechanisms of the oxidative demethoxylation of 4-(methoxymethyl)phenol by vanillyl-alcohol oxidase. Evidence for formation of a p-quinone methide intermediate. J. Biol. Chem. 1997;272:18111–18116. doi: 10.1074/jbc.272.29.18111. [DOI] [PubMed] [Google Scholar]
- 34.Hopkins T. R. A multipurpose enzyme sensor based on alcohol oxidase. Intern. Biotechnol. Lab. December. 1985:20–25. [Google Scholar]
- 35.Panoutsopoulos G. I., Beedham C. Kinetics and specificity of guinea pig liver aldehyde oxidase and bovine milk xanthine oxidase towards substituted benzaldehydes. Acta Biochim. Pol. 2004;51:649–663. [PubMed] [Google Scholar]
- 36.Miller J. R., Edmondson D. E. Structure–activity relationships in the oxidation of para-substituted benzylamine analogues by recombinant human liver monoamine oxidase A. Biochemistry. 1999;38:13670–13683. doi: 10.1021/bi990920y. [DOI] [PubMed] [Google Scholar]
- 37.Fraaije M. W., Veeger C., van Berkel W. J. H. Substrate specificity of flavin-dependent vanillyl-alcohol oxidase from Penicillium simplicissimum. Evidence for the production of 4-hydroxycinnamyl alcohols from allylphenols. Eur. J. Biochem. 1995;234:271–277. doi: 10.1111/j.1432-1033.1995.271_c.x. [DOI] [PubMed] [Google Scholar]
- 38.Weibel M. K., Bright H. J. The glucose oxidase mechanism. Interpretation of the pH dependence. J. Biol. Chem. 1971;246:2734–2744. [PubMed] [Google Scholar]
- 39.Witt S., Wohlfahrt G., Schomburg D., Hecht H. J., Kalisz H. M. Conserved arginine-516 of Penicillium amagasakiense glucose oxidase is essential for the efficient binding of β-D-glucose. Biochem. J. 2000;347:553–559. doi: 10.1042/0264-6021:3470553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guillén F. PhD. Thesis. Madrid: Universidad de Alcalá de Henares; 1991. Aril-Alcohol Oxidasa de Pleurotus eryngii. Estudio en Relación con la Degradación de la Lignina. [Google Scholar]