Abstract
Dracunculiasis, also known as guinea worm disease, is caused by the large female of the nematode Dracunculus medinensis, which emerges painfully and slowly from the skin, usually on the lower limbs. The disease can infect animals, and sustainable animal cycles occur in North America and Central Asia but do not act as reservoirs of human infection. The disease is endemic across the Sahel belt of Africa from Mauritania to Ethiopia, having been eliminated from Asia and some African countries. It has a significant socioeconomic impact because of the temporary disability that it causes. Dracunculiasis is exclusively caught from drinking water, usually from ponds. A campaign to eradicate the disease was launched in the 1980s and has made significant progress. The strategy of the campaign is discussed, including water supply, health education, case management, and vector control. Current issues including the integration of the campaign into primary health care and the mapping of cases by using geographic information systems are also considered. Finally, some lessons for other disease control and eradication programs are outlined.
INTRODUCTION
Guinea worm disease, also known as dracunculiasis (or dracunculosis), is a long-established human infection which was clearly referred to by various authors from India, Greece, and the Middle East in antiquity; female worms have been seen in Egyptian mummies (2, 150). A curious monograph by Velschius (159) discussed real and imagined references in ancient writings and sculptures, including a supposed relationship with the caduceus motif. James Africanus Horton, the first West African to be trained in Europe as a medical doctor, wrote a book about the disease (87), mistakenly supposing that it was transmitted through the soles of the feet.
Connection of infection with water sources was recognized early, and it is probable that, if the prepatent period were not so long, the mode of infection would have been obvious many centuries earlier. As it was, this was determined in 1870 by a Russian naturalist, Alexei Fedchenko (67), who found that larvae expelled from emerging female worms in the limbs of sufferers developed in freshwater microcrustaceans (cyclops) living in ponds, which were then ingested in drinking water.
In historical times infection occurred in Algeria, Egypt (162), Gambia, Guinea Conakry, Iraq, Brazil, and the West Indies (163) but died out spontaneously in those countries and was eliminated from Uzbekistan in 1932 and from southern Iran in 1972.
BIOLOGY OF THE DISEASE
Morphology
The single species causing the disease in humans, Dracunculus medinensis (Linnaeus, 1758; Gallandant, 1773), belongs to the nematode superfamily Dracunculoidea of the order Spirurida. Most spirurids are tissue parasites and produce eggs containing larvae or free larvae which require arthropod intermediate hosts. The best-known examples of this order are the filariae (superfamily Filarioidea), including the important human parasites Wuchereria bancrofti and Brugia malayi (against both of which a global campaign is just beginning), Onchocerca volvulus, and Loa loa. For many years Dracunculus was included among the filariae, but it differs from them, mainly in the disparate sizes of the sexes and life history. The validity of the relationships among the various groups of spirurids has been supported by recent analyses of small-subunit rRNA sequences (16).
The same or similar species of Dracunculus have been reported sporadically for mammals and reptiles (snakes and turtles) in many parts of the world (116); species from birds have been separated into a different genus, Avioserpens.
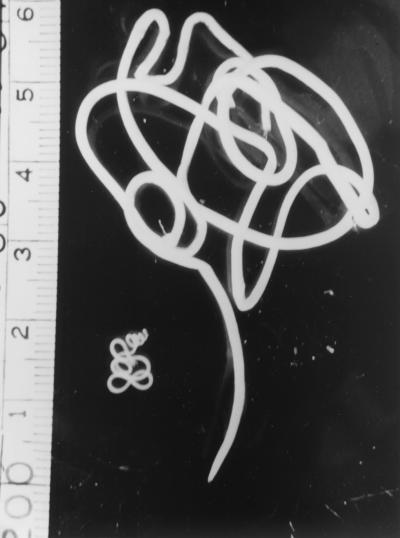
A mature female D. medinensis is one of the longest nematodes, measuring up to 100 cm, but is only 1 to 2 mm wide (Fig. 1). The vulva is halfway down the body. In filarial females the microfilarial prelarvae emerge from this opening throughout the life of the worm. In Dracunculus, however, it is closed with a plug, and the whole body cavity is filled with the uterus, which extends anteriorly and posteriorly and contains from 1 to 3 million first-stage larvae. The gut is also completely flattened and is nonfunctional. Bright red worms have been reported from Pakistan (56), but their significance is not known. Males have been recovered only doubtfully from humans, but those from experimental animals measure 15 to 40 by 0.4 mm; the tail has three to six preanal and four to six pairs of postanal papillae, with subequal spicules (490 to 750 μm long) and an accessory organ, the gubernaculum (about 117 μm).
FIG. 1.
Male and female D. medinensis worms. The female worm is the larger of the two. The ruler at the left is in centimeters.
Life Cycle
The mature female worm lives in the subcutaneous connective tissues, moves to the surface of the skin, and provokes the formation of a blister, which bursts, causing the anterior end of the worm to be exposed. If the affected portion of the body (usually the foot or leg) is cooled by immersion in water, first-stage larvae are expelled in large numbers from the ruptured uterus. These measure 643 (490 to 737) by 23 (18 to 24) μm with a pointed tail and a fully formed gut, although they do not feed. They move actively in the water, resembling a free-living nematode, and can live for a few days in water.
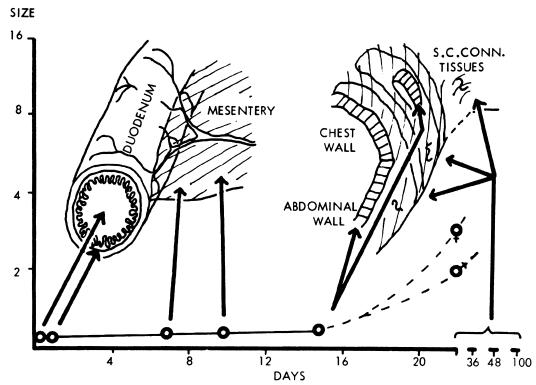
For further development, the larvae need to be ingested by suitable predatory species of copepods, measuring 1 to 2 mm. Until a few years ago, these were all included in the single genus Cyclops, but this has now been subdivided, and the most important intermediate hosts belong to the genera Mesocyclops (M. aequatorialis and M. kieferi), Metacyclops (M. margaretae), and Thermocyclops (T. crassus, T. incisus, T. inopinus, and T. oblongatus) (119). Larvae develop to the infective third stage in the body cavity in 14 days at 26°C. For further development of the larva, the host copepod needs to be ingested in drinking water obtained from ponds or open wells. Once in the human body, larvae are released in the stomach and migrate through the intestinal wall, across the peritoneal cavity, and into the wall of the abdomen and thorax by 15 days (Fig. 2). After two molts the sexually mature males and females meet and mate in about 100 days, while they are both of comparable size. The males remain in the tissues and in a few months become encapsulated and die. In experimental infections by Dracunculus insignis in ferrets, males can live for at least 330 days (19) and therefore are potentially able to fertilize females for another year. The females move down the muscle planes and by 10 months have grown greatly, with the uterus being filled with larvae. They emerge about 1 year after infection, usually from the feet and lower legs (Fig. 3) but occasionally from any other part of the body.
FIG. 2.
Development of Dracunculus in the mammalian host. S.C.CONN., subcutaneous connective tissues. Sizes at left are given in centimeters.
FIG. 3.
A human case of guinea worm disease, with an emerging worm. The worm is often wound on a stick, a practice which is believed to have given rise to the caduceus symbol of medicine. (Copyright A. Tayeh.)
The process from ingestion of a larva to emergence of an adult worm typically takes about a year, enabling transmission to occur annually at the most favorable season. However, in individual cases it can last from 10 to 14 months.
Zoonotic Aspects
The eradication of any human disease becomes a vastly more difficult enterprise if there are animal reservoirs of infection. There is no evidence that animals act or have ever acted as reservoir hosts of human guinea worm infection, but the theoretical possibility that they could do so has not been conclusively disproved. Studies of animal infections in areas where dracunculiasis has been recently eliminated but without much improvement in drinking water sources might provide useful information regarding this question.
Table 1 lists the species of Dracunculus which have been reported. Emerging female worms are recovered sporadically from a wide range of mammals both from parts of the world where dracunculiasis is endemic and from parts where it is not (12, 49, 69, 71, 89, 90, 103, 106, 110, 135, 140; review in reference 116). Unfortunately, in almost all cases only a portion of a female worm is recovered, usually in a flaccid state after the enclosed larvae have been ejected, and identification with regard to species is impossible. In general, worms in mammals are regarded as D. medinensis in the Old World and South America and as D. insignis in North America. Dracunculus has a very short patent period, at the most a few weeks, and an emerging worm is not easy to see in fur-bearing carnivores (Fig. 4), so it is likely that infection is much more common in many countries than is currently recognized. This is well illustrated by the situation in North America, where there are one or two reports of infection in wild carnivores and domestic dogs each year, mostly from states in the southeastern United States and from southern Ontario, Canada (e.g., see reference 14). However, in studies where large numbers of raccoons were dissected and immature worms were found, prevalence rates of 13.8% (144) to about 50% (47) were recorded.
TABLE 1.
Reported species of Dracunculusa
| Species | Host | Geographical distribution |
|---|---|---|
| D. alii | Snakes | India |
| D. coluberensis | Snakes | India |
| D. dahomensis | Snakes | West and Central Africa |
| D. doi | Snakes | India |
| D. houdemeri | Snakes | Vietnam |
| D. ophidensis | Snakes | Italy, USAb |
| D. oesophageus | Snakes | Italy, Madagascar |
| Dracunculus species | Snakes | England, Brazil, Trinidad, USA |
| D. globocephalus | Turtles | USA |
| D. fuellebornius | Opossums | Brazil |
| D. insignis | Dogs, wild carnivores | Canada, USA |
| D. lutrae | Otters | Canada |
| D. medinensis | Many mammals | Africa, Americas, Asia |
Note that some authorities have recognized only two species: D. medinensis in mammals and D. oesophageus in reptiles (116).
USA, United States of America.
FIG. 4.
Guinea worm in a laboratory cat infected with cyclops from a pond in Nigeria.
The taxonomic status of this North American parasite is also worthy of investigation. It was described by Leidy in a short account in 1858 and confirmed as a different species (D. insignis) by Chitwood (43) in 1950, because of differences in the length of the gubernaculum and number of preanal papillae from males of D. medinensis. However, when more males of the latter species were described (116), these differences were not found to be valid, and the species was never accepted by all authorities: e.g., Faust et al. in a standard textbook (66) stated, “In reservoir hosts D. medinensis is found in fur-bearing mammals in North America.” Species of copepods which transmit D. medinensis are also susceptible to larvae of D. insignis (149). If these parasites are all of the human-infecting species, then this is encouraging for world elimination of human infections, since almost no autochthonous infections have been reported from North America (see reference 146 for one such case; Spearman et al. [145] reported on imported cases), although calcified worms have been seen on roentgenograms (E. H. Michelson, personal communication).
An isolated autochthonous human case of dracunculiasis has been reported from Japan (105), and similar older cases occurred in Korea (76) and Indonesia (157). In all of these presumably zoonotic cases, there was the possibility that infection was contracted from ingesting raw freshwater fishes as paratenic hosts (in which the immature parasites ingested in cyclops can survive but do not develop) rather than from drinking water: certainly Dracunculus in North America can be transmitted experimentally by amphibians (47, 54). Experimentally, rodents can be infected with larvae of the human parasite, which can live for months in the peritoneal cavity without development, and these could act as paratenic hosts for infections in carnivores (R. Muller, unpublished data). Infections in reptiles appear to be due to separate species (see reference 116).
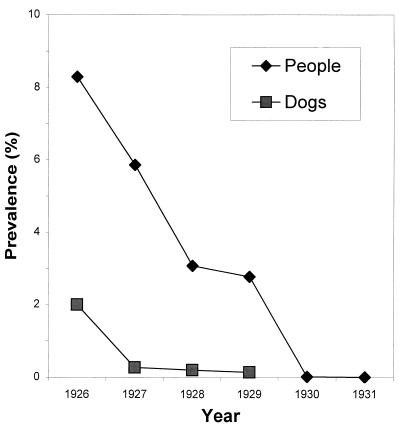
The infections in domestic animals reported from areas of endemicity are possibly of human origin. This supposition is supported by the higher prevalence found by surveys of the human population than by the dissection of dogs culled during the 1920s in Bukhara, Uzbekistan (Fig. 5). However, worms are still found in dogs in the former areas of endemicity of Uzbekistan (99) and Tamil Nadu (106) and also in dogs and cats in the areas of Azerbaijan, Kazakhstan, and possibly Turkmenia where the disease is not endemic (44, 45, 71, 110, 158). It is very likely that in many parts of the world there are widespread but underreported animal cycles completely independent of human infection: for example, there was a recent report from China of infection with D. medinensis in a cat (69). It is significant that the disease has not returned to Uzbekistan in the 70 years since it was eliminated there, in spite of the fact that about 40% of the rural population still does not have piped water and only 33% in the area surrounding Bukhara, the historic focus (171), have piped water. Nor has it returned to Egypt, Iran, Gambia, or Guinea, where it disappeared decades ago.
FIG. 5.
Prevalence of dracunculiasis in people and dogs in Old Bukhara, Uzbekistan, 1926 to 1931. Data are from reference 171.
The question of whether animal reservoirs of D. medinensis exist should be clarified by projected DNA sequence studies, but in any event animal infections are unlikely to prove a human public health problem once complete eradication of the human disease has been achieved, particularly if safe water sources are provided.
CLINICAL ASPECTS
Clinical Manifestations and Pathogenesis
Preemergent female worms can move easily through the connective tissues, but when they are about to emerge to the surface, a few larvae are released into the subdermis through a rupture at the anterior end. The host reaction results in the formation of a burning, painful blister, which bursts in a few days to give a shallow ulcer, and there is then a marked inflammatory response against the cuticle of the entire worm, preventing its removal. The bacteriologically sterile blister fluid contains larvae surrounded principally by polymorphonuclear neutrophils with macrophages, lymphocytes, and eosinophils (117). After expulsion of thousands of larvae, the end of the worm dries up, and this process is repeated a few times, with the complete worm being extruded in a few weeks. The lesion then resolves quickly. Unfortunately, the track of the worm becomes secondarily infected in about half of all cases, and patients become severely incapacitated. In one study in an area of Nigeria, 58% of patients, mostly in the 15- to 49-year-old working-age group or of school age, were disabled for an average of 12.7 weeks during the yam and rice harvest time (143). In a study in Ghana, 28% of patients had continuing pain 12 to 18 months after emergence of worms and 0.5% (as in many studies) had permanent physical impairment, in the form of “locked” knees or other joints (88). In another study in Benin there was 0.3% mortality from tetanus and septicemia (42). Female worms sometimes burst in the tissues, resulting in a very large pus-filled abscess and severe cellulitis. Infertile females or males elicit a slight inflammatory reaction and sometimes calcify, showing up on a roentgenogram.
Dracunculiasis is unusual among parasitic infections in that there is little evidence of acquired immunity and the same individual can be reinfected many times. The response to the extrusion of larvae is indicative of an Arthus reaction followed by a delayed hypersensitivity response (117).
Diagnosis
Patients in an area of endemicity have no doubt about the diagnosis when, or just before, the blister forms from the local itching and then sharp pain and often general allergic symptoms including urticaria follow. Once the blister has burst, cold water will encourage the release of larvae, which can be seen microscopically under low power. Immunodiagnostic methods are not useful in practice because it has not been proved that they can detect prepatent infections, mainly because of the lack of prepatent serum samples. However, antibodies can be detected in patent infections by enzyme-linked immunosorbent assay or dot-enzyme-linked immunosorbent assay, using whole-worm antigens. The most specific reaction appears to be for detection of immunoglobulin G4 (17). This test might be able to detect prepatent infections up to 6 months before emergence (11), in which case it could have practical importance. No evidence was found for the presence of circulating antigen (18).
Treatment
Treatment by winding worms out on a stick, a few centimeters a day, has been practiced since antiquity and is still useful, particularly when combined with a clean dressing and antibiotic ointment to prevent secondary bacterial infection (115). There is no evidence that any chemotherapeutic agent has a direct action against guinea worms, although various benzimidazoles may have an anti-inflammatory action, aiding elimination (123), and aspirin was found elsewhere to be equally effective (Muller, unpublished). Ivermectin is effective against many other nematodes but had no action in one trial, nor in experimental infections (55, 96). Chippaux (37) found that treatment with mebendazole was associated with aberrant migration of the worms, which were more likely than usual to emerge at places other than the lower limbs.
SOCIOECONOMIC IMPACT
In recent years, the understanding has grown that biological and technical feasibility is not the only criterion to consider before launching an eradication program. Costs and benefits are no less important (52). The benefits of dracunculiasis eradication, in contrast to those of smallpox and polio, will accrue almost exclusively to the population in which the disease is endemic (10).
In the past, most cases of dracunculiasis went unreported for a number of reasons: most health centers had little to offer the patient besides palliative treatment; most patients live in poor, remote rural areas and are hindered by their disease from walking to a health facility; and most recover spontaneously after expulsion of the worm. For example, a large teaching hospital in Nigeria never saw dracunculiasis cases in the Casualty Department, although the disease was endemic in villages a few kilometers away. Because few cases were reported, the disease was often considered an exotic curiosity rather than a major public health problem. However, in areas of endemicity its social, economic, nutritional, and educational consequences, and the costs incurred by the individuals, households, and communities which suffer from it, can be substantial.
Disability
Dracunculiasis is rarely fatal; studies in India based on medical records suggest a case fatality rate of 0.1% or less, and this is probably a generous estimate, because only persons with severe complications usually seek treatment from health facilities (4). The proportion of patients permanently disabled by the disease is also small; a number of studies have found it to be less than 1% (94, 132, 142).
The social impact of guinea worm disease is mainly attributable to the temporary disability suffered by the patient. Two longitudinal studies in Nigeria (5, 143) found that 58 to 76% of patients were unable to leave their beds for approximately a month during and after emergence of the worm. The more severe and protracted disability is associated with secondary infection of the lesion; this occurs in roughly half the cases (124, 184).
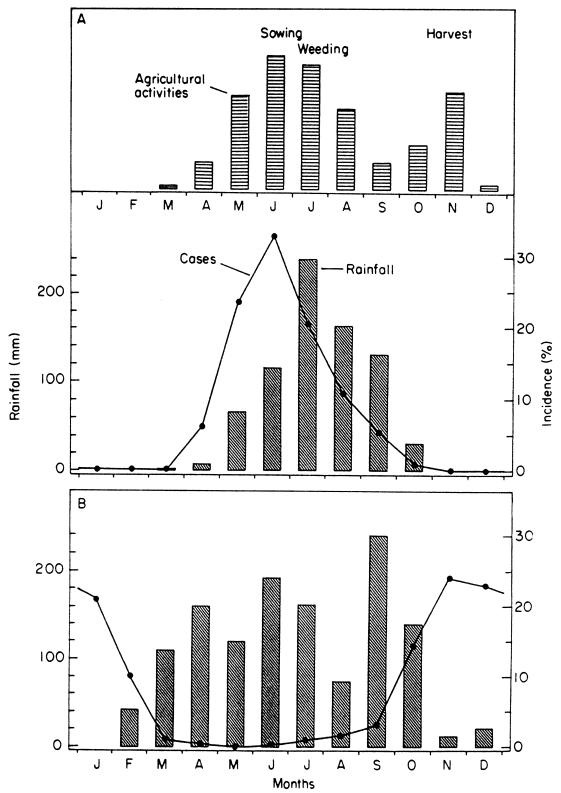
The impact of this temporary disability is reinforced by the seasonal pattern of worm emergence, often peaking at stages of the agricultural year when labor is in maximum demand. This is illustrated in Fig. 6, where the topmost section shows the seasonal variation in agricultural activity in northern Burkina Faso, peaking with the sowing season in June. The middle section shows the corresponding variation in cases of dracunculiasis, also peaking in June. The pattern typical of the forest zone to the south is shown in the bottom section of Fig. 6; here cases peak in the dry season, but this is the time for yam and rice harvesting (143). This seasonality means that a whole community can be laid prostrate simultaneously and household members can be prevented from substituting for one another in agricultural and other tasks (13). Indeed, it has been claimed that the effect of the disease on agricultural productivity can be detected in satellite photographs (6). The Dogon people of Mali refer to the infection as “the disease of the empty granary” (172).
FIG. 6.
Seasonality of dracunculiasis in the Sahel (A) and the forest zone of West Africa (B), compared with the rainfall and the Sahel's seasonal pattern of agricultural labor demand. Reproduced from reference 147 with permission of Taylor & Francis Ltd. Months are January, February, March, April, May, June, July, August, September, October, November, and December, from left to right, respectively.
The impact of guinea worm disease does not end when the worm is out and the sufferer returns to work. A study in Ghana (88) found that, between 12 and 18 months after emergence of a worm, 34% of patients still had some difficulty performing everyday activities, usually due to pain attributable by its location and the date of onset to the episode of dracunculiasis. While this disability is not necessarily permanent, it extends beyond the incapacity occurring during worm emergence.
Economic Impact
Some attempts to estimate the economic impact of dracunculiasis have simply multiplied the number of days of labor lost by the mean value of production per day or by the wage rate. From such a simplification, it is a small step to multiply the loss per household to derive an estimated cost for a whole region. One such study (50), based on a survey of 87 households, estimated that the rice-growing areas in three states of southern Nigeria sustained an annual loss of $20 million due to guinea worm disease. In spite of its simplistic argument, this study was extremely effective in mobilizing the support of senior politicians in Nigeria for the eradication of the disease (64).
It has been argued previously that this method of calculation uses an oversimplified approach and is likely to overestimate the cost (75, 128), as it does not allow for the various coping strategies by which households respond to illness (such as abandoning other tasks and using additional labor), which qualitative studies have found to be common in peasant farming (23, 38). A more sophisticated approach is to examine the impact on actual production (24) or even to include the incidence and duration of dracunculiasis-induced disability as predictive variables in an agricultural production function. Audibert (9) used this approach, in a setting in northeastern Mali where the incidence of guinea worm disease was “relatively low” (between 3 and 33% in the villages studied), to show that temporary disability accounted for a reduction of 5% in the overall production of two important subsistence crops: sorghum, mainly grown by men, and peanuts, cultivated by women.
However, there is also a cost to the coping strategies, which cannot be measured using this approach. Mutual assistance (164) simply transfers the cost of the disease to other households and is of little help to wage laborers (36). The simplistic approach used for the Nigerian study mentioned above may be more accurate than it seems.
Nutrition, Education, and Perpetual Benefits
Such economic calculations do not take into account the unequal distribution of costs within the family and the way in which disease, by impacting more on the production of some crops than others (24), can have a disproportionate effect on nutritional status. A survey in South Kordofan, Sudan (151), found that, in households where more than half the adult members had suffered from dracunculiasis in the previous year, the children under 6 years old were nearly three times as likely to be malnourished, as indicated by wasting.
Children also suffer in other ways from guinea worm disease in their families. Children miss school when they have guinea worm (in rural Africa, school is usually a long walk away) and also when they have to substitute for their ill parents in doing agricultural work and other household tasks. As a result, school attendance suffers during the peak season (22, 60, 62, 92, 124), and schools in areas of endemicity often have to close for 1 month in each year as a result.
Guinea worm disease is of special interest to economists not only because of its direct and measurable impact on production but also because of the singular ability of disease eradication to produce a perpetual stream of benefits at no ongoing cost. It is, therefore, surprising that a recent cost-benefit analysis of the eradication effort by the World Bank (104) considers only the benefits from incidence reduction during the campaign; their estimate of a 29% economic rate of return for the global campaign, which they estimate to have cost some $90 million to date, is certainly pessimistic.
EPIDEMIOLOGY
Water Sources
Because Dracunculus larvae need a period of 12 to 14 days to develop in the cyclops and become infective, dracunculiasis is not normally caught from flowing water sources such as rivers and streams. Deep wells are rarely implicated in transmission (31, 118); few cyclops are found in them, probably because the lack of light at the bottom constrains the population of zooplankton, which are the cyclops' natural diet. Thus, ponds and sometimes shallow or step wells are the main sources of the disease, and the epidemiology of dracunculiasis is chiefly determined by the use of such sources for drinking water.
Numerous studies have illustrated the predominant role of ponds in dracunculiasis transmission in various parts of Nigeria (57, 58, 59, 60, 61, 100, 127), Ghana (112, 138), Burkina Faso (70), Togo (129), Uganda (77), Pakistan (86), India (98), and what is now Uzbekistan (171).
Many (probably most) of the ponds involved in transmission are human-made. Table 2 shows the findings of Steib and Mayer (147) for a village in Northeast Burkina Faso, which illustrate the large number of ponds which can be found in a single village, even in a semiarid, Sahelian setting, and the degree to which relatively few human-made ponds, with specific characteristics, play a significant role in transmission.
TABLE 2.
Types of water sources and their relative importance in guinea worm transmission in Dara village, Burkina Fasoa
| Characteristic | Draw wells | Periodic streams | Large natural ponds | Cattle waterings | Small natural ponds | Small human-made ponds |
|---|---|---|---|---|---|---|
| Diam (m) | 2 | 10 | 50-200 | 17-45 | 7-15 | 4-9 |
| Maximum depth (cm) | Up to 500 | 250 | 100-130 | 45-80 | 25-30 | 30-55 |
| Potential focus? | No | No | Yes | Yes | Yes | Yes |
| No. found and studied | 4 | 4 | 4 | 8 | ||
| No. of guinea worm patients regularly using them | 9 | 2 | 5 | 90 | ||
| % | 8.5 | 1.9 | 4.7 | 84.9 |
Adapted from reference 147 with permission of Taylor & Francis Ltd.
Other types of human-made ponds, sometimes larger than those identified by Steib and Mayer, have been implicated elsewhere. These have included “boullies,” which are large dew ponds excavated for community water storage on the Mossi plateau of central Burkina Faso (101); small dams in northern Ghana (152); “ataparas” (also known as valley tanks), which are similar reservoirs in northern Uganda (77); hundreds of drinking-water ponds recently built in Anambra State, Nigeria; “hafirs,” built to store water from ephemeral streams in Sudan (31); and municipal ponds in Old Bukhara, Uzbekistan.
Outbreaks of guinea worm disease have occasionally been blamed on the construction of large dams (3, 59). However, their role in transmission has usually resulted from the use of ponds left by the receding water during drawdown of the water level.
Various types of wells and storage tanks can become sources of the disease. Rectangular masonry-lined step wells were the principal sources of infection in Rajasthan, India (142), and shallow wells have been implicated in Mali (130). Scoop wells dug in sandy riverbeds can also be a source, although if these water holes are exhausted by the drawing of water each day, no cyclops population can be sustained in them, and so transmission cannot occur (31). In Iran, the “berkeh” (traditional covered water storage cisterns with a diameter of over 10 m) were implicated (137).
Transmission has also occurred from rainwater storage reservoirs used by individual households, such as the “karkour” of the Nuba Mountains of Sudan (31) and in an isolated incident in 1993 in El Rohaibat, Libya, from a buried reservoir filled from a farm tank which an infected migrant worker had contaminated (102). Relatively few cases are caused in this way, because there must be an index case in the household to contaminate the reservoir, but when they do occur, transmission is more intense than usual because the infected cyclops are contained in a smaller volume of water, and this is reflected in a higher average number of worms per patient (32).
Villages of Endemicity
The fact that dracunculiasis transmission depends on the characteristics of the water source has an important consequence for the design of eradication programs. It occurs only in a limited number of so-called “villages of endemicity,” on which eradication activities can focus. For example, in 1990 the national case search conducted in all 8,068 villages of Burkina Faso found cases in only 2,621 (101). Local health staff in Bam Province found that villages known to have ponds were twice as likely as others to be among these villages of endemicity. There has been a tendency in some countries for the list of villages of endemicity to “drift” by some 30% per year and sometimes even twice this, but the appearance of a large proportion of new villages seems to be largely the consequence of cases reappearing in villages removed prematurely from the “endemicity” list (111), rather than a change in the set of villages susceptible to the disease. A period of 1 year without cases is insufficient evidence that transmission has been interrupted, as the full cycle from emergence of a worm in the index case to detection of a secondary case can take up to 16 months.
Seasonality
Dracunculus takes roughly a year from ingestion of an infected cyclops by the human host to emergence of an adult worm. This makes it well suited for environments in which transmission can occur only at a particular time of year. As a result, there is a strong seasonal peak in incidence rates in most communities of endemicity.
Two broad patterns of seasonality are found in the African areas of endemicity, depending on climatic factors. In some countries, both patterns occur, each in a different climatic zone (Table 3). To the north in the Sahelian zone, transmission of dracunculiasis is generally limited to the rainy season from May to August with a peak in June and July (73). Steib and Mayer (147) attributed this pattern to the presence of T. inopinus in the surface and shallow water used for drinking; however, others have found it more difficult to correlate occurrence of cyclopoids in the local water sources with the prevalence of infection among the people using them (185). More fundamentally, many water sources involved dry up and hand pumps are repaired in the dry season, so that the population turns to safer groundwater sources (48).
TABLE 3.
Peak transmission seasons for dracunculiasis in 14 African countries
| Country | Occurrence in montha:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan | Feb | Mar | Apr | May | June | July | Aug | Sept | Oct | Nov | Dec | |
| Mauritania | X | X | X | X | ||||||||
| Chad | X | X | X | X | X | |||||||
| Mali | X | X | X | X | X | |||||||
| Niger | X | X | X | X | X | |||||||
| Burkina Faso | X | X | X | X | X | X | ||||||
| Cameroon | X | X | X | X | X | X | ||||||
| Sudan | X | X | X | X | X | X | ||||||
| Uganda | X | X | X | X | X | |||||||
| Ethiopia | X | X | X | X | X | |||||||
| Ivory Coast | X | X | X | X | X | X | X | |||||
| Ghana | X | X | X | X | X | X | X | |||||
| Benin | X | X | X | X | X | X | ||||||
| Togo | X | X | X | X | X | |||||||
| Nigeria | X | X | X | X | X | X | X | X | ||||
Abbreviations: Jan, January; Feb, February; Mar, March; Apr, April; Aug, August; Sept, September; Oct, October; Nov, November; Dec, December.
Further south in the humid savanna and forest zone, the opposite pattern is found, with a peak in the dry season. This may be the early dry season (September to January), as in some parts of Oyo State, Nigeria (61, 100); Danfa in Ghana (13); and southern Togo (129), particularly in villages with shallow ponds which usually dry up by January. The disease often occurs or continues towards the end of the dry season (January to May) in Ghana (13, 25, 112, 138), southern Benin (41), Kwara and Anambra states in Nigeria (1, 58, 60, 124), and Uganda (77). This dry season transmission is often associated with the consumption of water from ponds or water holes formed (or dug) in the beds of seasonal rivers when flow has ceased (38, 114). It has also been suggested (41) that transmission does not occur when there is less than one susceptible cyclopoid per liter in the pond and that this accounts for seasonal variations in incidence.
There are local variations in these patterns. The duration and intensity of transmission in particular villages often depend on whether and when the local dams or ponds dried up the previous year (152), and some villages have very different seasonal peaks from those of the surrounding area because of local circumstances. For example, in some villages along the banks of the Niger and Volta rivers the incidence peaks when the river level falls and water is taken from holes dug in the riverbed.
Individual Risk Factors
In the same way as dracunculiasis exhibits a variety of seasonal patterns, which can be explained in terms of seasonal variations in water source use, the incidence of the disease has been found to vary with age and sex in different ways, but these can generally be understood from the way that people of different ages and genders behave with regard to their sources of drinking water.
For example, a significantly higher prevalence has been found in women in Ethiopia (97) and in men in India (98) and sometimes in West Africa (3, 39, 123); however, when behavioral risk factors (such as work in the fields or collection of water) are taken into account, the difference between the sexes is not significant (153).
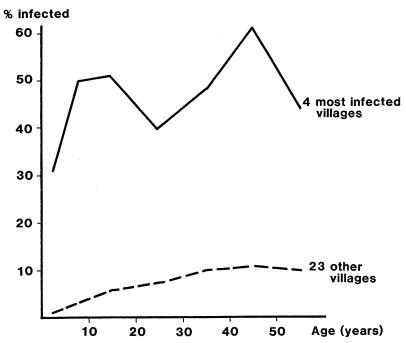
Two possible age prevalence profiles are illustrated in Fig. 7. The graph for four high-prevalence villages, each with an infected water source, shows similar prevalences in children and in adults. The remaining 23 villages in the study area show a lower prevalence in all ages but significantly less in children than in adults. The former is characteristic of communities where the water carried home is infected, while the latter is indicative of an association with mobility, where infection is acquired from water sources outside the community.
FIG. 7.
Age prevalence curves for 4 high-endemicity and 23 lower-endemicity villages in South Kordofan, Sudan. The curves were plotted from data in reference 31.
Other individual risk factors are important, particularly those associated with mobility (160); however, the strongest of all is infection in the previous year. A minority of people suffer recurrent infection in spite of drinking from the same water sources as the rest of the population, reflecting the variability in individuals' susceptibility to the disease (39, 112, 153).
THE ERADICATION INITIATIVE
As one of us first pointed out (118), guinea worm disease is a promising candidate for successful eradication. The cyclops is not a mobile vector like a mosquito, and the carrier state in both the cyclops and human hosts is of limited duration. Diagnosis is easy and unambiguous; cheap and effective measures are available to prevent transmission. The disease has a limited geographical distribution, and even within this area it is found only in certain communities of endemicity. Its markedly seasonal distribution in time also permits a more intensive focus on its prevention in seasonal campaigns. Lastly, as discussed above, transmission from animals to people is practically unknown.
The suggestion that dracunculiasis might be eradicable fell on fertile ground. Smallpox had been eradicated in 1977, and some of those associated with that achievement were looking for a suitable candidate to adopt for the sequel (7, 78, 141), while taking care to avoid a mistake like the targeting of malaria in 1955 (109). Another possible candidate was poliomyelitis, and indeed some of those involved in guinea worm eradication have seen it as a rehearsal for the eradication of polio, particularly in the difficult African terrain where the last cases of both diseases will probably occur. Table 4 compares the prospects of all four diseases in the eradication stakes.
TABLE 4.
Characteristics of past and present eradication candidatesa
| Characteristic | Presence of characteristic for diseaseb:
|
|||
|---|---|---|---|---|
| Malaria | Small- pox | Polio | Dracun- culiasis | |
| Agent | ||||
| No animal reservoir | + | + | + | + |
| No mobile vector | − | + | + | + |
| Host | ||||
| Limited carrier state | − | + | + | + |
| Easy diagnosis | − | + | − | + |
| Distribution | ||||
| Limited area of endemicity | + | + | − | ++ |
| Seasonal transmission | + | + | +/− | + |
| Threat to Western countries and visitors | + | + | + | − |
| Intervention | ||||
| Existence of effective drugs | + | − | − | − |
| Cheapness and completeness of prevention | − | + | + | + |
| No behavioral change needed for prevention | − | + | + | − |
Adapted from reference 79 with permission of the publisher.
++, highly present and favorable for eradication; +, present;+/−, somewhat present; −, absent.
Choosing a target is easy, however, compared with the task of mobilizing the resources for the battle. Much of the credit for that achievement goes to members and former members of the staff of the Centers for Disease Control and Prevention, who through an advocacy campaign beginning in 1980 and sustained over more than a decade (79, 80, 82, 83) succeeded in convincing former U.S. president Jimmy Carter, the United Nations Children's Fund (UNICEF) Executive Board, the 1989 African Regional Committee of the World Health Organization (WHO), and the 1990 World Summit for Children to take up the challenge. In 1991 the World Health Assembly declared “its commitment to the goal of eradicating dracunculiasis by the end of 1995, this date being technically feasible given appropriate political, social, and economic support.” The target date was set in order to enhance the advocacy effort at the international level and in the countries of endemicity as well.
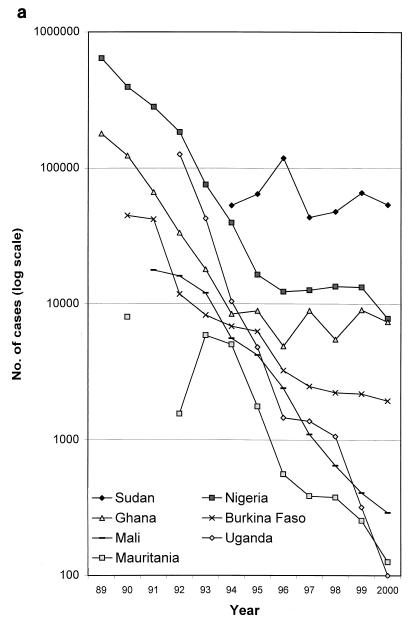
This advocacy effort needed to be replicated in each country to get a national program established (64). In 1982, India was the first to initiate a national eradication campaign. By 1990, four other countries had followed: Pakistan, Ghana, Nigeria, and Cameroon. In the following 5 years, all the other known countries of endemicity also established national eradication programs, and substantial and progressive reductions in disease incidence were recorded each year, particularly at the beginning of the campaign (Fig. 8a and b).
FIG. 8.
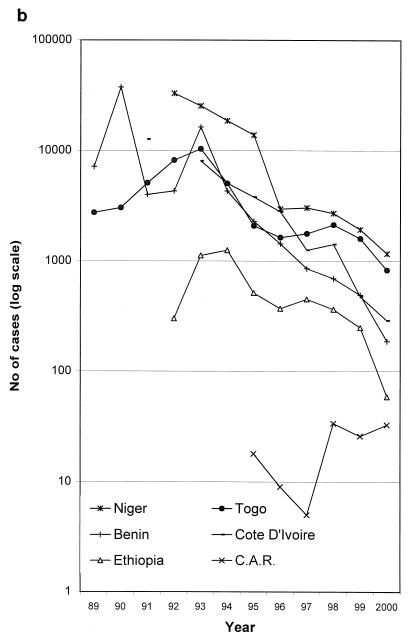
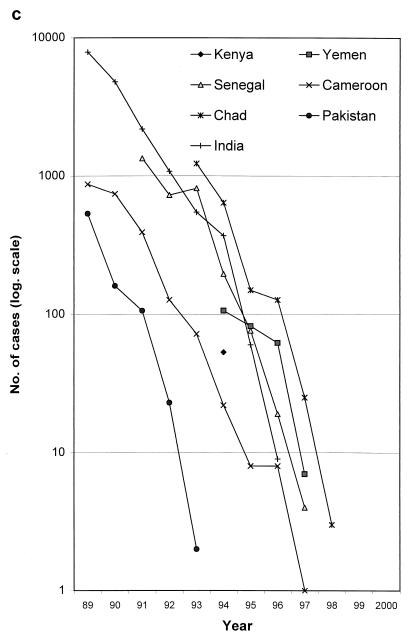
The decline in cases of guinea worm disease by country and by year, 1989 to 2000. Note the logarithmic vertical scale. (a) Selected countries of endemicity. The poor performances of Sudan, due to the civil war, and of Ghana and Nigeria in the late 1990s can be seen. (b) The remaining countries of endemicity. The rise in cases in the initial years of some national programs (e.g., Togo and Ethiopia) is attributable to gradual improvements in case detection. C.A.R., Central African Republic. (c) The countries which have interrupted transmission; the last point shown for each country is for the last year in which autochthonous cases were reported. Data are courtesy of WHO.
The advocacy effort needed to be maintained as the initiative advanced. On one hand, this was to kindle and sustain the interest of donor agencies which supported water supply programs, technical assistance, vehicles, filter cloth, temephos, training of staff and volunteers, and field allowances. On the other, it aimed to keep up the commitment of governments of countries of endemicity and the enthusiasm of program staff, an essential function since the bulk of the staff engaged in the initiative were health workers whose salaries were supported from the budgets of national ministries of health.
An important means to this end has been the series of regional conferences and meetings of national program coordinators, together with program review meetings at which each national coordinator would give a presentation on her or his program and then answer a barrage of penetrating questions from her or his skeptical colleagues from neighboring countries. Donor involvement and coordination were also supported by regular interagency meetings, usually held in the United States or Africa and attended by representatives of the Carter Center, UNICEF, the U.S. Agency for International Development, the World Bank, WHO, and other agencies. High-level advocacy was also supported by the continuing involvement of Jimmy Carter and two African former heads of state whom he persuaded to play a similar role, A. T. Touré of Mali and Yakubu Gowon of Nigeria.
The principal international stakeholders in the program have been relatively few in number: the Carter Center, UNICEF, the World Bank, and WHO. Other major supporters, such as the Bill and Melinda Gates Foundation and the British and Japanese bilateral aid programs, have usually channeled their funding through these agencies. The major players liaise periodically to identify major gaps in the funding of national programs, and in most countries of endemicity, one or another of them has, by consensus, taken the leading role. From 1992 to 1996, when national eradication programs were being established, UNICEF and WHO maintained a joint technical team based in Ouagadougou, Burkina Faso, to provide technical support to national program coordinators in the region and to external support agencies; the Carter Center also seconded one of its staff to this team in 1994.
The outcome of all this effort has been a remarkable 98% reduction in the number of cases, from an estimated 3.3 million worldwide in 1986 (161) to only 75,223 cases reported in 2000. Only 14 countries, all in Africa, reported indigenous cases in 2000 (180).
INTERVENTIONS
The development and selection of the most effective interventions for dracunculiasis prevention illustrate some general points about the design of programs to combat infectious diseases: field experience is more reliable than theory or basic biology, but successful programs are often based on remarkably little systematic evidence from the field. As a result, mistakes are made along the way, and there is a need to learn from experience (108).
Given the transmission cycle of the parasite and the absence of an effective vaccine, a number of interventions would seem a priori to be worth considering: (i) provision of a safe water supply, (ii) filtration of one's drinking water to remove cyclops, (iii) searching for patients with active cases and proper management of cases, (iv) ensuring that patients avoid contact with ponds, and (v) killing or removing cyclops in ponds. These are considered in turn.
Safe Water Supply
In the early discussion of strategies for eradication of dracunculiasis, provision of a safe water supply was generally seen as the intervention of choice (121), although water supplies are built and maintained for many other reasons besides the prevention of guinea worm disease. The eradication goal was originally proposed as a target for the International Water Decade (79, 80). Early eradication efforts in Nigeria (61, 62, 91) and Benin (139) invested the major part of their budgets in water supply construction.
There is plenty of evidence for the impact of water supplies on dracunculiasis (15, 31, 62, 77, 98, 112, 133, 156). Table 5 provides a summary; all the impacts reported there were assessed after at least a year and in one case (62) after 3 years. India's rural water supply program gave priority to villages of endemicity and, by the time that the national eradication program was concluded, had provided a supply to every village of endemicity in the country. This was an important contribution to that country's successful elimination of the disease in 1997. However, there are also some important limitations to the effectiveness of water supply as a preventive intervention.
TABLE 5.
Studies of the impact of water supply on dracunculiasisa
| Country and reference | % Reduction in annual incidenceb | Comment |
|---|---|---|
| Ghana (112) | 37% reduction for boreholes, deep wells, and dams, compared to ponds, rivers, and shallow wells. | Less risky sources were generally not used exclusively; often they were used only when ponds and pools dried up. |
| India (133) | 78% reduction for village with decreased use of unprotected step wells. | Prevalence in 4 villages increased with proportion of the population using step wells. |
| India (15) | 84% of affected families used pond water, 65% used well water, and 38% used tube wells. | Majority of families using wells and tube wells switched to pond water in summer when improved supplies became more salty and inadequate |
| India (98) | 98% reduction associated with draw well use as opposed to pond use. | 93% of those surveyed used pond water sources. |
| Nigeria (62) | 81% reduction in 20 villages provided with boreholes and pumps, along with health education. | Less reduction in villages where boreholes were not convenient or had unpalatable water; no change in control villages. |
| Nigeria (156) | 72% reduction 18 mo after provision of water supply. | Not clear how postprevalence was determined; prevalence higher in those >20 yr old; reductions greater in those <20 yr old. |
| Uganda (77) | ±90% reduction for borehole use in rainy season; ±60% reduction in dry season. | Boreholes, on average, were closer than other sources; exact figures for percent reduction not given. |
| Sudan (31) | 63% reduction for well or borehole use compared to surface sources | Many hand pump users sometimes used other sources because of breakdown, queuing, and inadequate yield. |
Adapted from reference 65.
All reductions were assessed at least 1 year after intervention.
First, water supplies cannot be expected to function without maintenance. Many water systems in Africa have fallen into disuse for this reason within a few years of construction, and in some cases the resulting reversion to unprotected water sources has allowed the disease to persist (15, 147) or, in periurban settings, to develop in epidemic form (21, 57). The experience of the Ivory Coast is a salutary reminder of this.
In the 1970s, the Ivory Coast was in the vanguard of African countries providing rural water supplies. Between 1973 and 1985, a total of 12,500 new boreholes were installed in rural areas, at a cost of many millions of dollars. As a result, the annual number of cases of guinea worm disease fell from 67,123 in 1966 to only 1,889 in 1985 (165). In 1991, however the national case search found an estimated 12,690 cases (134). The case enumeration methods may not have been exactly comparable, but the main reason behind this recrudescence of cases became clear in 1992 when UNICEF carried out a survey of hand pumps in three of the subprefectures of highest endemicity of the country and found that more than half of them were out of order, in spite of a recently concluded hand pump rehabilitation project in the area. Since then, hand pump maintenance has much improved, and the incidence of dracunculiasis has fallen sharply, with only 467 cases reported during 1999.
The second limitation is that provision of water supply to every village and hamlet is not always feasible. In the Benin project mentioned above, the prevalence of guinea worm disease in villages of endemicity with fewer than 150 inhabitants was four times that in the largest villages (154), but they were specifically excluded from the borehole program as they were considered too small to justify the cost of drilling and to maintain a hand pump sustainably (186).
Third, a functioning water supply will still be ineffective if it is not used. The most common cause of nonuse is that the supply is not close enough to people's homes. In the countries of endemicity of the Sahel, a hand pump may be the only source of water for miles around in the dry season; however, guinea worm transmission peaks during the rains, when people are often infected from the many ephemeral ponds which are within a few hundred yards of their houses. In southern Sanmatenga Province, one of the major foci of guinea worm disease in Burkina Faso, there is a working hand pump for every 600 people, but for more than 1 household in 10 that pump is more than 1 km away.
Fourth, much of the population in the rural Sahel migrates. In addition to the movement of the nomadic pastoral population, it is common practice in countries such as Burkina Faso and Niger for a village to disperse during the growing season (which is also the peak dracunculiasis transmission season) to a number of small and seasonally occupied hamlets, some of which may be in other districts, or even to sow their crops in several different areas and tend those where the region's unpredictable rainfall turns out to be most plentiful. When a borehole can cost as much as $10,000, it is not a cost-effective option to provide one for every such hamlet.
Fifth, water supplies alone cannot eliminate dracunculiasis if they are not used exclusively. Guinea worm infection is often acquired through casual use of unprotected sources when people are away from home, especially when they are working in the fields. This is confirmed by the common finding that adults, particularly farmers, are more commonly infected than are children (13, 31) and that people traveling away from their village are at greater risk (153).
Finally, water supplies are expensive. Typical rural water supplies in sub-Saharan Africa have a median capital cost of over $40 per person served (167), with an additional recurrent maintenance cost; while the maintenance costs are probably comparable with the recurrent costs of delivering other interventions, the capital cost is an order of magnitude greater.
This does not mean that water supply has no role to play; in some cases, particularly in the few urban foci of dracunculiasis, it has been decisive. However, its impact must be assessed realistically. It can be seen as transforming a high-prevalence community where all water is contaminated (like the four villages in Fig. 7) to a low-prevalence community where only those who use unprotected sources are at risk (like the 23 villages in Fig. 7).
Filtration of Drinking Water
Since an adult cyclopoid is over 1 mm long, it can easily be removed by filtering the water through an ordinary cloth. The filtration may be easy, but that does not mean people will do it. For millions of poor and mostly illiterate villagers, living in thousands of remote and frequently inaccessible communities and speaking hundreds of different languages, to change their behavior in this way is by any standard a major challenge to health education planning. It is remarkable that this has been achieved at all in practice, bearing in mind the low level of involvement of health education professionals in most national programs; with a few laudable exceptions, the approach adopted has been what Brieger (20) describes as “the behavioristic mode utilizing simplistic, professionally determined messages,” and in many countries of endemicity the involvement of health educators has been largely confined to the production of visual aids and a walk-on part in the training program.
As the national eradication programs took shape during the early 1990s, it became clear that they would rely largely on health education to promote the use of cloth filters. The initial evidence base for this emphasis was little more than the experience of three villages in Burkina Faso (70). However, the strategies of a number of successful public health initiatives in the past have been based on debatable evidence or worked out only during implementation; for example, there were cogent arguments at the outset of the Onchocerciasis Control Program that it would fail (H. Disney, personal communication), and the “search and contain” strategy which successfully eradicated smallpox was discovered only when the campaign was already under way (68). Perhaps the decisive argument in favor of health education was the fact that this was something which ministries of health could do without having to mobilize the support of other ministries (such as water) or hefty financial resources.
Fortunately, the filtering of water builds on existing practices in the region of endemicity, as cloth or sieves are widely used in Africa to filter various liquids. Early eradication programs distributed cotton cloth, but this was sometimes used as clothing or for decoration, and homemakers also complained that it soon became clogged with the sediment in the water so that too much time was needed to do the family's filtering.
An important additional step to successfully inducing behavior change was, therefore, the introduction of the right type of filter cloth. In the early 1990s, the cotton cloth was replaced by a monofilament nylon cloth, which was donated in huge quantities by Precision Fabrics Group through the Carter Center and which is less susceptible to clogging (53). More recently, a somewhat cheaper polyester fabric has been found to be equally effective and acceptable (125). These filters have proved so popular that many households have bought them and filtered their water with them whether or not they have heard that it can prevent guinea worm disease.
Several hundred thousand square meters of this cloth were donated during the 1990s; at the sale price of the fabric, this represents a donation of over $14 million (33). A study in Pakistan (93) found the filters in satisfactory condition after 12 to 15 months of use. Unfortunately, it gradually became clear that in Africa the cloth could not be expected to last for more than a year, particularly when people washed it regularly, with the vigor that they customarily used on other items of domestic laundry. This meant that filters had to be replaced regularly. Since the ending of the fabric donation program in 1998, the national programs have had to distribute cotton cloth or to find the funding to purchase monofilament fabric at some $4 per square meter. The cost of the cloth has encouraged national programs to find more economical ways of using it, such as stitching a patch of it into a hole in a larger piece of ordinary cotton baft (35).
One particularly cost-effective use of the material is to fix it over the end of a piece of 10- to 20-mm-diameter plastic pipe, 100 to 200 mm long. This “straw” filter can then be taken on journeys or to the fields and used to drink from ponds. A hole can be drilled through the pipe so that it can hang around the neck from a string. First introduced with success by the Mauritanian program, it has also been used in Niger and in southern Sudan.
When the nylon cloth was being donated, some national programs, such as those of Burkina Faso and Togo, sold the filters for a nominal sum. This was to help pay for the cost of making up the cloth into a handy form and also seen as ensuring that those who acquired the filters would value them. During Togo's general strike in the mid-1990s, the revenue from sale of filters kept health workers motivated and active in the eradication program when no other government programs were functioning. However, not every household chose to buy a filter. An evaluation of Burkina Faso's program in 1994 found that only 59% of households in villages of endemicity had acquired filters; the constraint was not in the distribution system, as some filters had reached all but 3 of the 31 villages surveyed (S. Cairncross, unpublished data). Ironically, now that the national programs have to purchase the filter cloth, all of them have decided to distribute filters free of charge in communities of endemicity, with a view to ensuring complete coverage.
Case Management
Surgical extraction of the worm was first recommended by Avicenna (980 to 1037) and was practiced by traditional healers in Iran (168) and in what is now Uzbekistan (171). Great skill is required to avoid breaking the worm, which is sometimes caught around joints or tendons. Extraction before emergence avoids the pain and suffering caused as the worm emerges and also contains the case by preventing contamination of water sources. When the technique was refined and applied in India by B. L. Sharma, an ayurvedic practitioner supported by UNICEF, a further advantage was apparent: people would come from far and wide to have their worms extracted, greatly improving the effectiveness of case detection and hence of case containment (136).
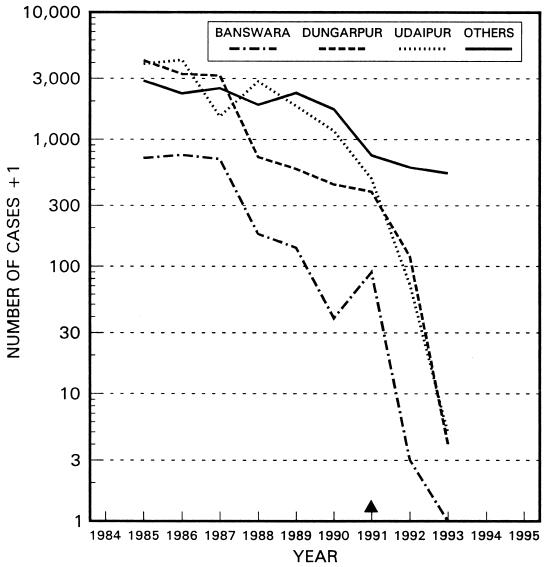
Evidence of the effectiveness of this treatment, particularly in the final stages of a local campaign to eliminate dracunculiasis, is provided in Fig. 9, showing the decline in cases in the three districts where Sharma practiced, by comparison with the other districts of endemicity of Rajasthan, India.
FIG. 9.
Decline in guinea worm cases from 1985 to 1993 in three districts of Rajasthan State, India, where surgical extraction was practiced from 1991 onward. The total of cases for all other districts in the state is also shown. Information provided by UNICEF.
However, there has also been some unease at the ethics of using an invasive treatment in an age of human immunodeficiency virus and about the difficulty of maintaining quality standards in the rough conditions of the field. Even in Rajasthan, where the technique was an important component of the eradication program, the extractions were performed by only two people (mainly by Sharma himself), and the measure was never accepted by the Indian medical profession or adopted by the national eradication program. Ghana, the only African country to include this intervention in its dracunculiasis eradication program, abandoned it in early 2000, considering that it distracted the attention of health workers (who received a reward for each worm extracted) from the implementation and supervision of the basic community-based interventions described above.
A more widely used form of case management is to apply an occlusive bandage to the lesion where the worm is emerging. This does not prevent the release of larvae into the environment, but it does discourage the patient from immersing the affected part in a pond; immersion transforms a neat bandage into a soggy mass of wet cotton wool, which is likely to fall off. Coupled with other palliative treatments by the village health worker, such as “controlled immersion” of the lesion in a bucket of water, disinfection of the lesion, topical application of emollient creams, and even antibiotic ointment (166), bandaging by a village health worker has been used to promote early self-reporting of cases.
Preventing Patients' Contact with Ponds
Preventing patient contact with ponds was an important component of the world's first endeavor at dracunculiasis elimination, conducted in the Old City of Bukhara, Uzbekistan, in the 1920s (171). Efforts were made to identify patients early and keep them under observation during the 2 weeks following initial emergence of their worms, and Muslim worshippers were prevented from approaching the main ponds in the town to perform their ablutions.
In the early stages of the worldwide eradication campaign, however, such measures were ruled out as impractical in a rural context where case detection within even a month of emergence could be considered an impressive achievement. Instead, the message that patients should not contaminate ponds was usually a secondary message in the general health education materials. As a result, people were far more aware of how one catches the disease than of how one passes it on. For example, an evaluation survey of 26 villages in Niger in 1994 showed that, while 54% of householders knew that dracunculiasis is transmitted in drinking water, only 13% could say how ponds become infected with it (26).
More recently, it has become clear that people sometimes respond so well to this message that it can have a significant effect on transmission in the complete absence of filter cloth. For example, in an area of some 160 villages in Adior District in Bahr el Ghazal Province, Sudan, in 1999, not enough cloth filters were available, so that only 44% of households had one. Nevertheless, a reduction of 88% in the number of cases was achieved, with only 515 cases reported in the first 10 months of 2000 compared with 4,177 in the same period in 1999. The prevention of pond contamination by patients was a major feature of the 4,304 health education sessions performed by village volunteers during 1999, following a training course developed by WHO and the Carter Center in collaboration with the nongovernmental organizations (NGOs) operating locally and the Sudan Ministry of Health.
Killing or Removing Cyclops
The intervention of killing or removing cyclops has also been tried since the earliest efforts to control transmission of the disease: Leiper (107) used steam to kill cyclops in Indian step wells, and Turkhud (155) used potassium permanganate. Ten years later, when Isaev tried to apply their methods to the ponds of Old Bukhara, he found them impractical; an average pond required 300 kg of disinfectant, and the water became undrinkable (95).
In more recent times, temephos, an organophosphate insecticide safe for use in drinking water sources, has been used in many countries of endemicity, and $2 million worth has been donated to the campaign by its manufacturers (33). Cyclopicide played a prominent role in the eradication programs launched in the 1980s in India, Pakistan, and Cameroon. All these programs have successfully achieved elimination, but this took many years (Fig. 8c). Did vector control help?
In Africa, vector control has not proved as easy as initially anticipated, in spite of there being fewer ponds per person than there were step wells in India. Chemical treatment of African ponds, even by highly qualified research teams, has been found on a number of occasions to be of questionable effectiveness 74, 148; O. Doumbo, personal communication). When the treatment is not fully effective, there is an increased risk of cyclops developing resistance to the cyclopicide. Even treatment which successfully removes the cyclops from the pond does not always eliminate guinea worm disease, as other contaminated water sources are often in use (40, 113).
Transmission is most intense in ponds which are in the final stages of drying up (13, 152) and which therefore may be missed by the treatment team. This is because the infected cyclops, which tend to sink to the bottom (122, 126), are increasingly likely to be scooped up as the pond becomes a shallow puddle.
Treatment of ponds can also consume substantial resources, particularly in terms of trained staff. It is harder to calculate the volume of an irregular pond than of a rectangular Indian step well, although this is essential in order to estimate the dose of insecticide required (34). Given the difficulty, it is impressive that local health technicians have demonstrated on training courses that they can measure pond volumes to within ±10% (A. Maïiga, personal communication). However, it usually takes them the best part of half a day to do so.
Moreover, the pond volume varies with time; if rainfall after the treatment does not dilute the insecticide to harmless levels, the pond may still need to be remeasured the following month, although a solution used in India to avoid continual remeasurement of the volume is to insert a calibrated scale in the pond. Treatment has to be applied at least monthly to be effective, and there is evidence to suggest that an even shorter treatment interval is required (40). With as many as 10 ponds to treat per village (Table 2), it becomes a very labor-intensive activity. There is some evidence that it has diverted staff from other, more important activities (26).
As always, there are exceptions. In 1997, the eradication program staff in northern Ghana found that most of their cases came from only four district towns, each of which used water from a dam. The dams were nearly 10 times the maximum size recommended for treatment (34). Nevertheless, after some experimentation, they found that cyclopicide could be applied very effectively to these (28)
Case Containment
By the middle 1990s, as case numbers began to come down, there was increased enthusiasm to step up the level of intervention and move towards “case containment.” Essentially, this involves a shift of emphasis from helping individuals to protect their own health by avoidance or filtration of infected water towards the protection of ponds and the community at large from contamination by infected people.
If this is to be effective, it requires detection of each case before or immediately after the emergence of the worm and measures to ensure that it could give rise to no subsequent case. Controlled immersion and bandaging, as described above, help to encourage self-reporting of cases, essential if the cases are to be detected in time, and also discourage patients from immersing their lesions in water. Patients and their families are urged to keep out of water sources until the worms have completely emerged and are also interviewed to ascertain whether they have already contaminated any ponds. If so, remedial measures are taken, such as alerting the community to this possibility, treatment of the affected ponds with cyclopicide, and checking that every household in the village has a cloth filter in good condition and knows how to use it. To ensure the effective implementation of case containment, each case should be reported to the supervisor of the village health worker within 7 days, and the supervisor should visit to verify the diagnosis and ensure that all necessary measures have been taken.
These measures require substantial additional resources (72): the cost per village is probably at least double the cost of the conventional approach (29). Some of the principal international agencies involved expressed concern that these resources should be deployed effectively, so in 1994 a meeting was organized in Nairobi, Kenya, at which technical standards for effective case containment were defined and agreed on (8). According to these criteria, a case is considered to have been successfully contained only if (i) it was detected before or within 24 h of worm emergence; (ii) the patient has not entered any water source since the worm emerged, or if so, the source has been treated in time to prevent transmission; (iii) the case has been properly managed by cleaning and bandaging until the worm(s) is fully removed, and the patient has been discouraged from contaminating any water source; and (iv) the diagnosis and the containment of the case have been verified by a supervisor within 7 days of worm emergence.
It followed from this that eradication programs where the resources did not permit a supervisor to visit each village of endemicity more than once a month could not be considered to be implementing case containment, even if the village health worker did carry out some containment activities; the Nairobi participants referred to this as “intensified case management.” Table 6 shows the conditions required for effective implementation of eradication activities at these two levels of intensity. It has taken several years for all the countries of endemicity to adopt these standards; for example, Niger maintained that a case had been contained if the case was detected in a week, coming into line with other countries only in 2000. Even now, a number of countries do not confirm the diagnosis and containment within a week.
TABLE 6.
Conditions required and guideline values for effective implementation of case containment activities at each level of intensitya
| Required condition or guideline | Maximum value for level of intensity:
|
|
|---|---|---|
| Intensified case management | Case containment | |
| Conditions | ||
| Population/VHWb | 500 | 500 |
| No. of VHWs/supervisor | 20 (preferably 10) | 4 |
| No. of villages of endemicity/district | 30 | 10 |
| No. of cases/village/mo (peak month; village of highest endemicity) | 30 | 12 |
| No. of cases/village/yr (village of highest endemicity) | 100 | 35 |
| Guidelines or standards | ||
| Time for detection of case | 24 h | 24 h |
| Time for report of case to supervisor | 30 days | 7 days |
| Case confirmation | Sample of cases | All cases, by VHW's supervisor |
| Frequency (or maximum response time) of visits by supervisor to villages of endemicity | 30 days | 7 days |
| Frequency of district coordinator visits to each: | ||
| Supervisor | 30 days | 30 days |
| Village of endemicity | 180 days | 60 days |
Adapted from reference 8.
VHW, village health worker.
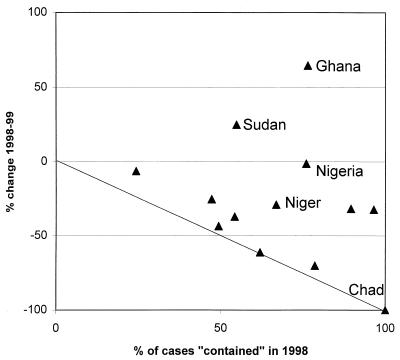
To some extent the concern with standards has proved justified, as shown by Fig. 10, which compares the proportion of cases “contained” in 1998 with the percent reduction in incidence from 1998 to 1999 for each country of endemicity. One would expect those countries where a higher proportion of cases was contained to experience the greatest reduction. The line on the graph shows the expected relationship, on the assumptions that (i) containment of cases is completely effective but (ii) that each “uncontained” case gives rise to one other in the subsequent year. Seven countries lie close to the line, but they should have been below it; during the early 1990s, before they implemented case containment, most of the countries of endemicity already experienced an annual drop of 30 to 50% in the number of cases.
FIG. 10.
Case containment: proportion of cases contained in 1998 and year-on-year national case reductions from 1998 to 1999 for countries of endemicity in Africa. Each point on the graph represents one country's performance.
Figure 10 illustrates the problems of the countries with the most cases, particularly Sudan, Nigeria, and Ghana. The relative ease with which Chad, with only two cases to contain, achieved a 100% reduction proved to be illusory; three cases were subsequently found to have occurred in Chad in 2000 (182). Indeed, the impact of the case containment strategy is hard to discern in the national surveillance data.
Intervention Conclusions
The intervention which has so far had the greatest impact is health education, initially to promote the use of cloth filters to remove cyclops from drinking water and latterly also to prevent the contamination of ponds by patients. It has proved much more effective than many health education professionals would expect. Water supply and vector control have proved more expensive in financial or human resources and have been most effective when deployed in specific settings where they have an advantage. Case containment is a measure whose time must come, but it appears that it may have been applied too early in some countries, resulting in a loss in program quality and hence in effectiveness.
CURRENT ISSUES
The Integration Debate
The strategy followed by most countries of endemicity as they set up eradication programs during the early 1990s was set out in a seminal paper which built on the experiences of Pakistan, Ghana, and Nigeria (85). Volunteer village health workers, providing health education, distributing cloth filters, and carrying out surveillance, were central to the strategy. It was foreseen that they would cover more villages in less time than the provision of safe water supplies required. While the initial search for cases should be a vertically organized operation, the authors proposed that “where they exist already, primary health care (PHC) workers in endemic villages should be used; where they do not yet exist, an appropriate villager should be designated (who may later be incorporated into the country's PHC programme).“
In the ensuing years, the networks of village volunteers which were being established proved remarkably effective in carrying out surveillance and health education (29). However, the degree of their integration into the primary health care program was a subject of intense debate. Their success led some stakeholders in the global campaign to advocate that they should be used to monitor conditions other than dracunculiasis, such as immunization status and acute flaccid paralysis as a marker for possible polio epidemics. As they saw it, the cost of establishing the network was an investment which should serve other health objectives (131). The cost of setting up such a network is little more than the annual cost of its maintenance, but the main item is the salaries of those who supervise the volunteers, and in most countries these are met from the general health budget and not from funds earmarked for guinea worm eradication. The other major items are the cost of annual retraining for the volunteers and occasional gifts in kind to motivate them, such as T-shirts or the salt and soap given to the volunteers in South Sudan; other health programs, particularly those with external support, have been ready to contribute toward these.
Others doubted the wisdom of integration, wary of putting the eradication effort at the mercy of an often weak primary health care system and dissipating eradication resources on other activities (84). Prior experience of the inappropriate “integration” of smallpox eradication with measles control, and of yaws control in Ghana (81), influenced their thinking. There were other concerns, too. One was the danger of overburdening the volunteers, though a recent field assessment of integrated surveillance in northern Ghana found no objective evidence that the surveillance activities were interfering with health workers' ability to carry out the eradication work effectively (187). Another source of unease, with some justification, was that the collection of surveillance data is useless if, as has occurred in a number of countries of endemicity, it is not used for any action. Without a system to make use of it, the data would be collected for their own sake. Moreover, most of the national programs operate in only a limited number of villages of endemicity, whereas some of the wider objectives would require complete coverage.
The integration debate led to a joint statement in favor of integrated surveillance by the four principal agencies involved in the eradication effort (183), but of course a question such as “to integrate, or not to integrate?” does not have a universal answer; the answer can only be worked out on the ground in each specific context. Moreover, surveillance is only one component of a public health system, and others may benefit from a degree of integration. Certainly, there have been substantial synergies from combining dracunculiasis eradication activities with others in a number of cases. Both dracunculiasis eradication and the wider health agenda can benefit, as long as the combination is opportune and has been made judiciously. Examples include (i) the integrated surveillance for guinea worm, immunization, yellow fever, and cerebrospinal meningitis in the 3,743 villages of Northern Region, Ghana (187), where initial difficulties in harmonizing case definitions were ironed out during the first year of operation; (ii) the mobilization of underused government field staff to supervise the village health workers in Mopti Region, Mali (30), while also supporting their work to promote latrine construction, better agriculture, etc.; (iii) use of the guinea worm surveillance system for implementation and monitoring of trachoma elimination activities in Zinder Region, Niger; (iv) the use of the 6-month “guinea worm cease-fire” in southern Sudan in 1995 to treat people at risk for onchocerciasis; treat children with vitamin A; and vaccinate cattle against rinderpest and children against measles, polio, and tuberculosis (84); (v) also in southern Sudan, the use of the guinea worm program's training and supervision system for a pilot program in which village health volunteers in remote villages were trained to identify and treat fever and malaria, cough and difficult breathing, and dehydration due to diarrhea; and (vi) the use of the polio national immunization days to detect 400 new villages of endemicity in southern Sudan during the first campaign in this area in 1998 (174), where the same process in the following 2 years led to the detection of a further 350 villages of endemicity.
Geographic Information Systems (GIS)
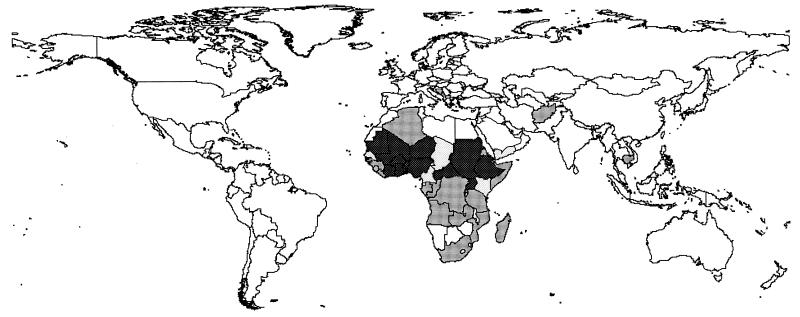
The use of GIS is another area where dracunculiasis eradication has interacted positively with other public health activities. The dracunculiasis eradication campaign was the first global public health initiative to pioneer the use of GIS as a planning tool. Figure 11 shows a spot map of the villages where the disease is still endemic.
FIG. 11.
Spot map of Africa showing villages of endemicity in 1999. Note that for Nigeria, Sudan, and Ghana, for which full village mapping data do not exist, villages are distributed randomly according to the administrative level and not their exact positions. Data courtesy of WHO Healthmap.
This application of GIS had its origins in the link between the water and health sectors from the beginning of the campaign. Rural water supply programs in the countries of endemicity of West Africa are largely based on boreholes with hand pumps; rural water projects in a number of countries supported by the Coopération Française and United Nations Development Program were developing computer databases of the boreholes drilled, for purposes of asset management and geohydrological analysis. These databases naturally included data on the borehole positions, and maps could be drawn from them by using GIS software. From this it was a small step to using this software to map other items of rural infrastructure (schools, health posts, and the presence of village health workers) and also guinea worm surveillance data. Thus, villages of endemicity and safe water points were plotted on digitized maps early in the 1990s by the UNICEF water sector in most of the countries of endemicity of francophone Africa (46); in the anglophone countries of endemicity such as Ghana, Nigeria, and Uganda a specific mapping exercise was required. GIS was not applied to guinea worm data in India or Pakistan before the disease was eliminated from those countries in the middle 1990s, although other computer databases were set up to manage the surveillance data.
In 1993 the WHO-UNICEF Joint Program on Data Management and Mapping, called HealthMap, was established at WHO headquarters, in order to implement and maintain a GIS for the guinea worm eradication program at global, national, and local levels. Commercial software was used at international and national levels to enter, store, and maintain data on over 80,000 georeferenced villages in sub-Saharan Africa, such as village populations and school, health, and water supply infrastructure, in addition to the epidemiological information on guinea worm disease.
In 1997 it was felt necessary to develop dedicated software, adapted to the specific needs of dracunculiasis eradication as well as other public health initiatives and which can be easily used by technical staff at national and subnational levels in the countries of endemicity. The HealthMapper software was therefore developed by the HealthMap unit; it contains a standardized georeferenced database of country, regional, district, and subdistrict boundaries, with rivers, roads, villages, and water, health, and social infrastructure. The system also comprises a user-friendly mapping interface and a database management facility (175). The main users have been involved in the refinement of this software and in several workshops held to ensure that they can use it to enter, update, and analyze their epidemiological and program data and to show the data and analytical results in map form.
A number of the GIS databases currently used by countries of endemicity include community-based surveillance data on conditions other than dracunculiasis. Out of 11 francophone African countries of endemicity, births are recorded in 5, some categories of deaths are recorded in 7, and measles cases are recorded in 5. All include a number of operational parameters for monitoring the implementation of the eradication program, such as the availability of cloth filters and the treatment of ponds (176).
The cost of developing these databases and the software to manage them has largely been borne by international agencies. The national staff who maintain them would in any case be required to collate and analyze surveillance data, so that the opportunity cost to the countries concerned is very small.
The databases have only recently begun to prove their worth. For example, the requirement to maintain them has proved a powerful means of encouraging program staff to continue monitoring the status of villages which, for lack of dracunculiasis cases, would otherwise be dropped from surveillance. The software has also proved invaluable in a number of unexpected ways, often by bringing ambiguities to light and requiring that those who use it make consistent decisions. For example, plotting the areas of activity of NGOs on a map of southern Sudan helped to identify the overlaps and lacunae in NGO support for eradication activities. In Benin, efforts by UNICEF to map the areas of responsibility of village health workers' supervisors showed that commune boundaries had not been clearly defined--a fact not widely recognized until then. Discussions at a regional training workshop on the use of the software revealed inconsistencies between countries in the definition of a village of endemicity (173) and other indicators used by the national eradication programs. The effort to achieve a consensus on appropriate definitions for the most critical indicators was a very useful exercise for the local as well as the international staff involved.
It is not only spatial analysis which is facilitated by such databases. For example, they show that, in Sudan in 1999, at least 9 of the 12 monthly reports due were received from 48% of over 3,800 accessible villages of endemicity in the country which had cases, an impressive achievement in a country at war. Only 32% of the villages with no cases in 1999 submitted nine or more reports; the difference may be due to health worker apathy in the absence of cases, but poor reporting could also explain the lack of cases, so it highlights a problem needing investigation.
These databases will also prove their worth in establishing the completeness of the surveillance arrangements for the certification of eradication. The International Commission for the Certification of Dracunculiasis Eradication (ICCDE) has determined that at least 9 out of 12 monthly reports must be received from all villages of endemicity (“acceptable” surveillance) or all 12 from at least 85% of villages of endemicity (“effective” surveillance) for the surveillance results to be used for certification purposes (170). Functions have been added to the GIS software to permit these indicators to be shown by village or by administrative division.
The positive experience of using GIS for dracunculiasis eradication formed the basis on which the same team has subsequently developed GIS software to support elimination control programs for other diseases, particularly leprosy, lymphatic filariasis, and onchocerciasis.
The Certification of Dracunculiasis Eradication
In 1991 the World Health Assembly urged the WHO Director General to: “immediately initiate country by country certification of elimination so that the certification process can be completed by end of the 1990s.”
The criteria for the certification of dracunculiasis eradication (169) were developed by WHO in consultation with the main partners and were given their final revision and endorsement at the first meeting of the ICCDE held in Geneva, Switzerland, in March 1996. They are complemented by a number of explanatory documents and guidelines (170, 177).
All countries with dracunculiasis transmission after 1980 are required to maintain active surveillance for at least 3 years after achieving interruption of transmission. In addition, a Country Report needs to be submitted to the ICCDE through WHO, describing the procedures undertaken and providing evidence of the reliability of the surveillance system and its ability to detect any case of dracunculiasis, even in the most remote areas of the country. A panel of specialists has been created, and the members have been assigned to the International Certification Teams (ICTs). The visits of the ICTs are necessary in all countries of previous endemicity, in order to assess the reliability of the surveillance systems in the field and to review and document the overall process which led to the interruption of transmission.
After its first meeting, the ICCDE met in January 1997, February 1998, and February 2000. ICTs have visited Iran, Pakistan, Egypt, India, and Libya. On the basis of the recommendations produced by the ICCDE, 151 countries and territories have so far been certified as free of dracunculiasis transmission by the WHO (178) (Fig. 12). Among these countries, Pakistan and India achieved interruption of transmission after the beginning of the global eradication campaign in the 1980s.
FIG. 12.
Global status of certification of Dracunculus eradication as of the fourth meeting of the ICCDE, in February 2000. Countries and territories marked by white space are certified to be free of transmission, those with pale gray shading are under precertification surveillance, those with medium gray shading are not yet certified, and those with dark shading are countries of endemicity (reporting indigenous cases in 1999).
Kenya, Cameroon, and Senegal have also achieved interruption of transmission, reporting zero cases for more than 1 year. They are now in the precertification phase, during which active surveillance needs to be maintained for at least 3 consecutive years with no cases reported. Countries bordering countries of high endemicity like Sudan cannot be certified, unless interruption of transmission is also achieved in the cross-border areas. This means that the precertification phase will last longer than 3 years for Kenya and probably Uganda, Ethiopia, and the Central African Republic, despite the fact that interruption of transmission may have been or may be achieved in these last three countries during 2001 or 2002.
The ICCDE has been useful not only for the certification process. Some of its recommendations have focused on critical aspects of the eradication campaign, like the surveillance systems, and their implications for the certification criteria or the revision of guidelines and tools for the production of country reports and the ICT visits. An important recommendation of the fourth ICCDE meeting was the sequencing of the genome of D. medinensis while suitable genetic material can still be obtained (179).
A dramatic illustration of the practical value of the ICCDE was the recent visit by a member of the ICCDE to Yemen, 3 years after the last reported case. This member and another epidemiologist found reports of cases apparently continuing to occur undetected in Lahj, Al-Hodeidah, and Al-Mahweet governorates, areas not hitherto suspected and therefore not covered by the surveillance system (181). There are obvious lessons for surveillance in other countries approaching the target of elimination at the national level. Vigilance must be maintained and not limited to the formerly known areas of endemicity. Several types of surveillance activity, such as the collection and prompt investigation of rumors and the offering of rewards for confirmed indigenous cases, are needed to complement official notifiability and specific community-based surveillance and to give wider geographical coverage.
In addition, as the final phase of the campaign approaches, it is becoming more important to link the work of the ICCDE to the context of the countries where dracunculiasis is still endemic, which represent the most difficult areas in which to interrupt transmission. For example, most of the cases now remaining in the world are in Sudan, largely in the zones affected by that country's civil war. Other areas of endemicity, such as parts of Mali, Niger, and Uganda, have also been affected by conflict, and security concerns prevented the Egyptian ICT from visiting the south of that country (nearest to Sudan, where the disease is endemic). Nomadism and transhumance are also common in areas of endemicity. There is a need for innovative strategies to conduct preventive interventions, as well as to maintain active surveillance in these very difficult areas. The contribution of the ICCDE on these issues may be crucial.
Concluding Remarks
What lessons can be drawn from the experience of guinea worm eradication efforts which are applicable to the control or elimination of other diseases?
The first lesson (a valuable one at a time when a number of infections are emerging or reemerging in the developing world) is that it is possible, at affordable expense, to control and even eliminate a disease at a national level, even in the remotest and most neglected areas of some of the poorest countries of the world and in spite of the fact that the key interventions involve substantial and sustained changes in behavior. In particular, the evidence suggests that the eradication of human dracunculiasis will be sustainable, in spite of the documented examples of animal cycles of the parasite.
The second is that the fundamental ingredient of success in an endeavor of this kind is political support (from politicians and from society at large), without which it will be starved of the resources that it requires. Mobilizing and maintaining this support become harder as the campaign advances, leaving a dwindling exposed population as the residual beneficiaries. In the case of guinea worm, the last areas of endemicity will be among the most remote and neglected in the world, and many of them (such as the war zone in southern Sudan) are under only tenuous control by the national governments concerned. This is not a new lesson, but it is underlined by the way in which a number of national programs have faltered when starved of funds or shown renewed vigor when advocacy was strengthened.
Ghana provides an object lesson in the need for timely funding. In 1997-1998 the Sector-Wide Approach was introduced by the major external donors to Ghana's health sector. One donor agency made its contribution to the common pot only after a delay of several months, and this affected the funding of health activities across the board. The Ministry of Health saw little option but to maintain curative health services by cutting the funding of preventive activities, and the guinea worm eradication program, previously supported by earmarked funds, lost out. By the time the money came through it was too late, as the peak transmission season had passed. As a result, the number of cases nearly doubled in 1999. This setback was only partially reversed in 2000.
The example of Nigeria illustrates the potential of high-level advocacy. In the late 1980s, an intensive advocacy effort led to the initial launch of Nigeria's eradication program (63, 64), but by 1996 the number of cases was stable. In 1998, Yakubu Gowon, former head of state, became “guinea worm eradication ambassador,” and during 1999 he worked hard at this task, traveling across the country, visiting political leaders in the states of highest endemicity, and returning a few months later to monitor their progress. After 4 years of stability, the number of cases reported fell by nearly half in 2000.
It is important, however, not to confuse advocacy goals with planning targets. Some countries pushed forward to implement case containment, or began to offer cash rewards for reported cases, when there were still such large numbers of cases that the quality of the case containment system could not be guaranteed or when the reliability, transparency, and even the funding of the reward system were not secure. The resources invested in these premature additions might have been better spent on the basic strategy.
There have been times when the international agencies involved in the campaign have failed to maintain the collegial dialogue required to build and maintain consensus about strategy as the situation evolves. This was most evident in the debate about integration but has also affected some more detailed questions. It is not clear to what extent, if any, such polemics have harmed the eradication effort; they have at least wasted a substantial amount of energy. What is clearer is that interagency collaboration and consensus will become more important, not less, if innovative strategies are to be identified and adopted to respond to the new challenges of the final phases of the eradication campaign. When such consensus has been achieved, the eradication effort has always benefited. It has been noted that “a sense of common purpose, dedication and commitment, and a collaborative mode of behaviour” is a hallmark of successful disease control programs (108); the guinea worm eradication effort needs these more than ever.
The fear has sometimes been expressed that the resources allocated for guinea worm eradication will divert the energy of health workers from other essential tasks, and on the other hand some have hoped that the eradication campaign will blaze a trail where other health programs can follow. In practice, there is little evidence that the functioning of government health systems has been greatly affected, for good or ill, by dracunculiasis eradication. However, in the many communities where the disease has disappeared in recent years, thanks to the eradication initiative, this has surely helped to build public confidence in health workers and the health system and increased people's readiness for change. Future health interventions, especially those requiring behavioral change, will fall on more fertile ground as a result.
Whatever the degree of support afforded to guinea worm eradication by politicians, international agencies, and health staff, much of the success achieved so far is attributable to the remarkable support by simple villagers, who have demonstrated commitment, skill, and resourcefulness beyond what many would have imagined. Not least among the unsung heroes of the campaign are the thousands of volunteer village health workers who have spread the word about prevention, conscientiously detected and reported cases of disease, and increasingly taken steps to ensure that those cases were contained. They and the population have often surprised the program managers with their willingness and ability to respond to challenges. For example, early skepticism that health education would have much impact on the contamination of ponds by infected cases has been shown in recent years to be unfounded.
Above all, the guinea worm eradication campaign has shown that even in poor, small, and remote villages in developing countries, health interventions based in the community and implemented by the villagers are feasible and can have a significant impact on the burden of disease. The challenge is to mobilize the proven capacity of the population and of local volunteers to improve the health of their communities and to fight not only guinea worm disease but also the other diseases of poverty.
Acknowledgments
The eradication of guinea worm disease is a collaborative endeavor towards which many people have contributed their efforts and ideas, in addition to those cited above. The help of Ahmed Tayeh and the comments of Philippe Ranque on a draft of the manuscript were particularly valuable.
REFERENCES
- 1.Abolarin, M. O. 1981. Guinea worm infection in a Nigerian village. Trop. Geogr. Med 33:83-88. [PubMed] [Google Scholar]
- 2.Adamson, P. 1988. Dracontiasis in antiquity. Med. Hist 32:204-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adekolu-John, E. A. 1983. The impact of lake creation on guinea-worm transmission in Nigeria on the Eastern side of Kainji Lake. Int. J. Parasitol 13:427-432. [DOI] [PubMed] [Google Scholar]
- 4.Adeyeba, O. A. 1985. Secondary infection in dracunculiasis: bacteria and morbidity. Int. J. Zoonoses 12:147-149. [PubMed] [Google Scholar]
- 5.Adeyeba, O. A., and O. O. Kale. 1991. Epidemiology of dracunculiasis and its socio-economic impact in a village in south-west Nigeria. West Afr. J. Med 10:208-215. [PubMed] [Google Scholar]
- 6.Ahearn, S. C., and C. de Rooy. 1996. Monitoring the effects of dracunculiasis remediation on agricultural productivity using satellite data. Remote Sens 17:917-929. [Google Scholar]
- 7.Anonymous. 1983. After smallpox, guinea worm? Lancet i:161-162. [Google Scholar]
- 8.Anonymous. 1994. Case containment strategy for eradication of dracunculiasis in Africa. Centers for Disease Control with Global 2000, United Nations Children's Fund, and World Health Organization, Atlanta, Ga.
- 9.Audibert, M. 1993. Invalidité temporaire et production agricole: les effets de la dracunculose dans une agriculture de subsistance. Rev. Écon. Dév 1:23-36. [Google Scholar]
- 10.Aylward, B., K. A. Hennessey, N. Zagaria, J.-M. Olivé, and S. Cochi. 2000. When is a disease eradicable? 100 years of lessons learned. Am. J. Public Health 90(10):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bapna, S., and D. M. Renapurkar. 1996. Immunodiagnosis of early dracunculiasis. J. Commun. Dis 28:33-37. [PubMed] [Google Scholar]
- 12.Batliwala, J. C. 1983. Guinea-worm in the horse. Vet. J 36:412-413. [Google Scholar]
- 13.Belcher, D. W., F. K. Wurapa, W. B. Ward, and I. M. Lourie. 1975. Guinea worm in southern Ghana; its epidemiology and impact on agricultural productivity. Am. J. Trop. Med. Hyg 24:243-249. [DOI] [PubMed] [Google Scholar]
- 14.Beyer, T. A., J. P. Tao, Z. X. Wang, et al. 1999. Massive Dracunculus insignis in a dog. Am. Vet. Med. Assoc 214:366-368. [PubMed] [Google Scholar]
- 15.Bhatt, A. N., and K. H. Palan. 1978. Guinea-worm infection in Banaskantha District of Gujarat—some important epidemiological aspects. Indian J. Med. Sci 32:1-4. [PubMed] [Google Scholar]
- 16.Blaxter, M., M. Aslett, D. Guitiano, J. Daub, et al. 1999. Parasitic helminth genomes. Parasitology 118(Suppl.):S39-S61. [DOI] [PubMed] [Google Scholar]
- 17.Bloch, P., P. E. Simonsen, and B. J. Vennervald. 1993. The antibody response to Dracunculus medinensis in an endemic human population of northern Ghana. J. Helminthol 67:37-48. [DOI] [PubMed] [Google Scholar]
- 18.Bloch, P., B. J. Vennervald, and P. E. Simonsen. 1998. Studies on immunodiagnosis of dracunculiasis. II. Search for circulating antigens. Acta Trop 70:303-315. [DOI] [PubMed] [Google Scholar]
- 19.Brandt, F. H., and M. L. Eberhard. 1990. Distribution, behavior, and course of patency of Dracunculus insignis in experimentally infected ferrets. J. Parasitol 76:515-518. [PubMed] [Google Scholar]
- 20.Brieger, W. R. 1996. Health education to promote community involvement in the control of tropical diseases. Acta Trop 61:93-106. [DOI] [PubMed] [Google Scholar]
- 21.Brieger, W. R., J. D. Adeniyi, and S. U. Akpovi. 1982. Complexities of guinea worm disease. World Health Forum 3:216-217. [Google Scholar]
- 22.Brieger, W. R., E. A. Olumide, and O. O. Kale. 1983. Effect of guinea worm on schoolchildren. World Health Forum 4:324-325. [Google Scholar]
- 23.Brieger, W. R., S. Watts, and M. Yacoob. 1989. Guinea worm, maternal morbidity and child health. J. Trop. Paediatr 35:285-288. [DOI] [PubMed] [Google Scholar]
- 24.Brieger, W. R., and J. Guyer. 1990. Farmers' loss due to guinea worm disease: a pilot study. J. Trop. Med. Hyg 93:106-111. [PubMed] [Google Scholar]
- 25.Bugri, S. Z. 1981. Guinea worm: an indicator of the quality and quantity of rural water supply in Northern Ghana. M.S. dissertation. London School of Hygiene and Tropical Medicine, London, United Kingdom.
- 26.Cairncross, S. 1995. Evaluation du PNEVG du Niger, 6-15 décembre de 1994. UNICEF Mission Report. United Nations Children's Fund, New York, N.Y.
- 27.Reference deleted.
- 28.Cairncross, S., S. D. Anemana, and A. Olsen. 1999. Towards the eradication of guinea worm; a Danish-Ghanaian collaboration. Parasitol. Today 15:127-129. [DOI] [PubMed] [Google Scholar]
- 29.Cairncross, S., E. I. Braide, and S. Z. Bugri. 1996. Community participation in the eradication of guinea worm disease. Acta Trop 61:121-136. [DOI] [PubMed] [Google Scholar]
- 30.Cairncross, S., F. T. Cutts, and H. Periès. 1997. Vertical programmes; what are they good for? Lancet 349:SIII20-SIII22. [Google Scholar]
- 31.Cairncross, S., and A. Tayeh. 1988. Guinea worm and water supply in Kordofan, Sudan. J. Inst. Water Environ. Management 2:268-274. [Google Scholar]
- 32.Cairncross, S., and A. Tayeh. 1989. Aggregation of Dracunculus medinensis in communities using different types of water source in Sudan. Trans. R. Soc. Trop. Med. Hyg 83:431. [Google Scholar]
- 33.Carter, J. 1999. The power of partnership; the eradication of guinea worm disease, p. 141-147. In Cooperation South no. 2. United Nations Development Program, New York, N.Y.
- 34.Centers for Disease Control. 1989. Guidelines for chemical control of copepod populations in dracunculiasis eradication programs. WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis at Centers for Disease Control, Atlanta, Ga.
- 35.Centers for Disease Control and Prevention. 1999. Guinea worm wrap-up no. 92. WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis, Atlanta, Ga.
- 36.Chippaux, J. P. 1992. The impact of dracunculiasis in a sugar-cane plantation. Trans. R. Soc. Trop. Med. Hyg 86:72.. [DOI] [PubMed] [Google Scholar]
- 37.Chippaux, J. P. 1991. Mebendazole treatment of dracunculiasis. Trans. R. Soc. Trop. Med. Hyg 85:280.. [DOI] [PubMed] [Google Scholar]
- 38.Chippaux, J. P., A. Banzou, and K. Agbede. 1992. Social and economic impact of dracunculosis; a longitudinal study carried out in 2 villages in Benin. Bull. W. H. O 70:73-78. [PMC free article] [PubMed] [Google Scholar]
- 39.Chippaux, J. P., L. de Souza, and A. Massougbodji. 1991. Aspects epidémiologiques de la dracunculose au Bénin. 1. Incidences, localisation des emergences et fréquence de réinfestations. Bull. Soc. Pathol. Exot 84:345-350. [PubMed] [Google Scholar]
- 40.Chippaux, J. P., I. Laniyan, and M. Akogbeto. 1991. Evaluation de l'efficacité du Témephos dans la lutte contre la dracunculose. Ann. Soc. Belg. Méd. Trop 71:279-285. [PubMed] [Google Scholar]
- 41.Chippaux, J. P., and A. Massougbodji. 1991. Aspects épidémiologiques de la dracunculose au Bénin 2. Relations entre la périodicité des émergences et l'origine de l'eau de boisson. Bull. Soc. Pathol. Exot 84:351-357. [PubMed] [Google Scholar]
- 42.Chippaux, J. P., and A. Massougbodji. 1991. Évaluation clinique et épidémiologique de la dracunculose au Bénin. Méd. Trop 51:269-274. [PubMed] [Google Scholar]
- 43.Chitwood, B. G. 1950. The male of Dracunculus insignis (Leidy, 1858) Chandler, 1942. Proc. Helminthol. Soc. Wash 17:14-15. [Google Scholar]
- 44.Chun Sun, F. 1958. Dracunculus infection in dogs in Kazakhstan. Med. Parazitol 27:219. (In Russian.) [Google Scholar]
- 45.Chun Sun, F. 1966. A case of Dracunculus infection in a domestic cat in the Kazakh SSR. Med. Parazitol 35:374-375. (In Russian.) [PubMed] [Google Scholar]
- 46.Clarke, K. C., J. P. Osleeb, J. M. Sherry, J.-P. Meert, and R. W. Larsson. 1991. The use of remote sensing and geographic information systems in UNICEF's dracunculiasis (Guinea worm) eradication effort. Prev. Vet. Med 11:229-235. [Google Scholar]
- 47.Crichton, V. F. J., and M. Beverley-Burton. 1977. Observations on the seasonal prevalence, pathology and transmission of Dracunculus insignis (Nematodoidea: Dracunculoidea) in the raccoon (Procyon lotor (L.)) in Ontario. J. Wildl. Dis 13:273-280. [DOI] [PubMed] [Google Scholar]
- 48.Curtis, V., I. Diallo, J. Konaté, and T. Skitt. 1993. The hand-pump maintenance project in Seno and Oudalan, Burkina Faso; evaluation report. Save the Children Fund, London, United Kingdom.
- 49.Davidson, W. R., V. F. Nettles, L. F. Hayes, E. W. Howerth, and C. E. Coavillion. 1992. Disease diagnosed in gray foxes (Urocyon cinereoargeneus) from the southeast United States. J. Wildl. Dis 28:28-33. [DOI] [PubMed] [Google Scholar]
- 50.de Rooy, C., and L. D. Edungbola. 1988. Guinea worm control as a major contributor to self-sufficiency in rice production in Nigeria. UNICEF working document. United Nations Children's Fund, New York, N.Y.
- 51.Reference deleted.
- 52.Dowdle, W. R., and D. R. Hopkins. ed. 1998. Report of the Dahlem Workshop on the Eradication of Infectious Diseases. John Wiley & Sons, Chichester, England.
- 53.Duke, B. L. 1984. Filtering out the guinea worm. World Health March 1984:29. [Google Scholar]
- 54.Eberhard, M. L., and F. H. Brandt. 1995. The role of tadpoles and frogs as paratenic hosts in the life cycle of Dracunculus insignis (Nematoidea: Dracunculoidea). J. Parasitol 81:792-793. [PubMed] [Google Scholar]
- 55.Eberhard, M. L., F. H. Brandt, and A. Hightower. 1990. Chemoprophylactic drug trials for treatment of dracunculiasis using the Dracunculus insignis ferret model. J. Helminthol 64:79-86. [DOI] [PubMed] [Google Scholar]
- 56.Eberhard, M. L., M. A. Rab, and M. N. Dilshad. 1989. Red Dracunculus medinensis. Am. J. Trop. Med. Hyg 41:479-481. [DOI] [PubMed] [Google Scholar]
- 57.Edungbola, L. D. 1980. Water utilization and its health implications in Ilorin, Kwara State, Nigeria. Acta Trop 37:73.. [PubMed] [Google Scholar]
- 58.Edungbola, L. D. 1983. Babana Parasitic Disease Project. II. Prevalence and impact of dracontiasis in Babana District. Trans. R. Soc. Trop. Med. Hyg 77:310-315. [DOI] [PubMed] [Google Scholar]
- 59.Edungbola, L. D., and S. J. Watts. 1984. An outbreak of dracunculiasis in a peri-urban community of Ilorin, Kwara State, Nigeria. Acta Trop 41:155-163. [PubMed] [Google Scholar]
- 60.Edungbola, L. D., and S. J. Watts. 1985. Epidemiological assessment of the distribution and endemicity of guinea worm infection in Asa, Kwara Sate, Nigeria. Trop. Geogr. Med 37:22-28. [PubMed] [Google Scholar]
- 61.Edungbola, L. D., and S. J. Watts. 1990. The elimination of dracunculiasis in Igbon, Oyo State, Nigeria: the success of self-help activities. J. Trop. Med. Hyg 93:1-6. [PubMed] [Google Scholar]
- 62.Edungbola, L. D., S. J. Watts, T. O. Alabi, and A. B. Bello. 1988. The impact of a UNICEF-assisted rural water project on the prevalence of guinea worm disease in Asa, Kwara State, Nigeria. Am. J. Trop. Med. Hyg 39:79-85. [DOI] [PubMed] [Google Scholar]
- 63.Edungbola, L. D., S. J. Watts, O. O. Kale, G. S. Smith, and D. R. Hopkins. 1986. A method of rapid assessment of the distribution and endemicity of dracunculiasis in Nigeria. Soc. Sci. Med 23:550-558. [DOI] [PubMed] [Google Scholar]
- 64.Edungbola, L. D., P. C. Withers, Jr., E. I. Braide, O. O. Kale, L. O. Sadiq, B. C. Nwobi, T. Alakija, P. McConnon, and D. R. Hopkins. 1992. Mobilization strategy for guinea worm eradication in Nigeria. Am. J. Trop. Med. Hyg 47:529-538. [DOI] [PubMed] [Google Scholar]
- 65.Esrey, S. A., J. B. Potash, L. Roberts, and C. Shiff. 1990. Health benefits from improvements in water supply and sanitation; survey and analysis of the literature on selected diseases. WASH technical report no. 66. WASH for U.S. Agency for International Development, Arlington, Va.
- 66.Faust, E. C., P. C. Beaver, and R. C. Jung. 1968. Animal agents and vectors of human disease, 3rd ed., p. 297. Lea & Febiger, Philadelphia, Pa.
- 67.Fedchenko, A. P. 1971. Concerning the structure and reproduction of the guinea-worm (Filaria medinensis L.). Am. J. Trop. Med. Hyg 20:511-523. (Translation of 1870 original.) [PubMed] [Google Scholar]
- 68.Foege, W. H. 1998. Smallpox eradication in West and Central Africa revisited. Bull. W. H. O 76:233-235. [PMC free article] [PubMed] [Google Scholar]
- 69.Fu, A. Q., J. P. Tao, Z. X. Wang, B. L. Jiang, Y. X. Liu, and H. H. Qui. 1999. Observations on the morphology of Dracunculus medinensis from a cat in China. Chin. J. Zoonoses 15:35-38. (In Chinese with English summary.) [Google Scholar]
- 70.Gbary, A. R., T. R. Guiguémdé, and J.-B. Ouédraogo. 1987. La dracunculose, un fléau éradiqué dans trois villages du Burkina Faso par l'éducation sanitaire. Bull. Soc. Pathol. Exot 80:390-395. [PubMed] [Google Scholar]
- 71.Ghenis, D. E. 1972. New cases of Dracunculus medinensis L., 1758 detected in domestic cats and dogs in Kazakhstan. Med. Parazitol 41:365.. [PubMed] [Google Scholar]
- 72.Greer, G., M. Dama, S. Graham, R. Migliani, M. Alami, and A. Sam-Abbenyi. 1994. Cameroon: an African model for final stages of guinea worm eradication. Am. J. Trop. Med. Hyg 50:393-400. [DOI] [PubMed] [Google Scholar]
- 73.Guiguémdé, T. R. 1985. Climatic characteristics of endemic zones and epidemiologic modalities of drancunculosis in Africa. Bull. Soc. Pathol. Exot 79:89-95. [PubMed] [Google Scholar]
- 74.Guiguémdé, T. R., A. R. Gbary, and J.-B. Ouédraogo. 1990. Lutte contre la dracunculose: problematique du traitement chimique des points d'eau au Temephos (Abate) en Afrique. Pub. Méd. Afr 110:19-22. [Google Scholar]
- 75.Guiguemdé, T. R., F. Orivel, B. Millot, A. R. Gbary, and J.-B. Ouédraogo. 1986. Le poids socio-économique de la dracunculose; méthode de calcul du coût économique chiffré de la maladie. Étud. Méd 3:113-124. [Google Scholar]
- 76.Hashikura, T. 1927. A case of Filaria medinensis in Chosen (Korea). Jpn. Med. World 7:145-146. (In Japanese.) [Google Scholar]
- 77.Henderson, P. L., R. E. Fontaine, and G. Kyeyune. 1988. Guinea worm disease in northern Uganda: a major public health problem controllable through an effective water programme. Int. J. Epidemiol 17:434-440. [DOI] [PubMed] [Google Scholar]
- 78.Hopkins, D. R. 1976. After smallpox eradication: yaws? Am. J. Trop. Med. Hyg 25:860-865. [DOI] [PubMed] [Google Scholar]
- 79.Hopkins, D. R. 1983. Dracunculiasis: an eradicable scourge. Epidemiol. Rev 5:208-219. [DOI] [PubMed] [Google Scholar]
- 80.Hopkins, D. R. 1984. Guinea worm and the decade. World Health November 1984:22-23. [Google Scholar]
- 81.Hopkins, D. R. 1985. Control of yaws and other endemic treponematoses: implementation of vertical and/or integrated programs. Rev. Infect. Dis 7(Suppl. 2):S338-S342. [DOI] [PubMed] [Google Scholar]
- 82.Hopkins, D. R. 1987. Dracunculiasis eradication: a mid-decade status report. Am. J. Trop. Med. Hyg 37:115-118. [DOI] [PubMed] [Google Scholar]
- 83.Hopkins, D. R. 1990. We can get rid of guinea worm. World Health January-February 1990:26-27. [Google Scholar]
- 84.Hopkins, D. R. 1998. Perspectives from the dracunculiasis eradication programme. Bull. W. H. O 76(Suppl. 2):38-41. [PMC free article] [PubMed] [Google Scholar]
- 85.Hopkins, D. R., and E. Ruiz-Tiben. 1991. Strategies for dracunculiasis eradication. Bull. W. H. O 69:533-540. [PMC free article] [PubMed] [Google Scholar]
- 86.Hopkins, D. R., M. Azam, E. Ruiz-Tiben, and K. D. Kappus. 1995. Eradication of dracunculiasis from Pakistan. Lancet 346:621-624. [DOI] [PubMed] [Google Scholar]
- 87.Horton, J. A. 1868. Guinea worm, or dracunculus, its symptoms and progress, causes, pathological anatomy, results and radical cure. W. J. Johnson, London, United Kingdom.
- 88.Hours, M., and S. Cairncross. 1994. Long-term disability due to guinea worm disease. Trans. R. Soc. Trop. Med. Hyg 88:559-560. [DOI] [PubMed] [Google Scholar]
- 89.Hoyos, C. B., G. A. Jara, and C. M. Monzon. 1995. Reporte de un caso de dracunculosis en un cano en la Provincia de Formosa, Argentina. Rev. Inst. Med. Trop. Sao Paulo 37:273-275. [PubMed] [Google Scholar]
- 90.Hsu, P. K., and D. N. Li. 1981. Dracunculus medinensis (Linnaeus, 1788) from a cat in Guangdong. Ann. Bull. Soc. Parasitol. Guangdong Province 3:92. [Google Scholar]
- 91.Huttly, S. R. A., D. Blum, B. R. Kirkwood, R. N. Emeh, N. Okeke, M. Ajala, G. S. Smith, D. C. Carson, O. Dosunmu-Ogunbi, and R. G. Feachem. 1990. The Imo State (Nigeria) Drinking Water Supply and Sanitation Project 2. Impact on dracunculiasis, diarrhoea and nutritional status. Trans. R. Soc. Trop. Med. Hyg 84:316-321. [DOI] [PubMed] [Google Scholar]
- 92.Ilegbodu, V. A., O. O. Kale, R. A. Wise, B. L. Christensen, J. H. Steele, Jr., and L. A. Chambers. 1986. Impact of guinea worm disease on children in Nigeria. Am. J. Trop. Med. Hyg 35:962-964. [DOI] [PubMed] [Google Scholar]
- 93.Imtiaz, R., J. D. Anderson, V. Long, J. J. Sullivan, and B. L. Cline. 1990. Monofilament nylon filters for preventing dracunculiasis: durability and copepod retention after long term field use in Pakistan. Trop. Med. Parasitol 41:251-253. [PubMed] [Google Scholar]
- 94.Imtiaz, R., D. R. Hopkins, and E. Ruiz-Tiben. 1990. Permanent disability from dracunculiasis. Lancet 336:630.. [DOI] [PubMed] [Google Scholar]
- 95.Isaev, L. M. 1956. Guinea worm and its eradication from Uzbekistan. Proc. Uzbekistan Inst. Malaria Med. Parasitol. (Samarkand) II:3-14. [Google Scholar]
- 96.Issaka-Tinorgah, A., P. Magnussen, P. Bloch, and A. Yakubu. 1994. Lack of effect of ivermectin on prepatent guinea-worm: a single-blind, placebo-controlled trial. Trans. R. Soc. Trop. Med. Hyg 88:346-348. [DOI] [PubMed] [Google Scholar]
- 97.Jemaneh, L., and S. Taticheff. 1993. Dracunculiasis (guinea worm disease) in the Bume (Nyangaton) people of South Omo, Ethiopia. Ethiop. Med. J 31:209-222. [PubMed] [Google Scholar]
- 98.Johnson, S., and V. Joshi. 1982. Dracontiasis in Rajasthan. VI. Epidemiology of dracontiasis in Barmer District, Western Rajasthan, India. Int. J. Epidemiol 11:26-30. [DOI] [PubMed] [Google Scholar]
- 99.Kairov, I. K. 1973. Helminthiases with a natural focal occurrence in the Karakalpak area. Akad. Nauk. Kazakhskoi SSR 6:136-138. [Google Scholar]
- 100.Kale, O. O. 1977. The clinico-epidemiological profile of guinea worm in the Ibadan District of Nigeria. Am. J. Trop. Med. Hyg 26:208-214. [DOI] [PubMed] [Google Scholar]
- 101.Kambiré, S. R., L. T. Kangoye, R. Hien, G. Yameogo, Y. Hutin, J.-B. Ouédraogo, J.-P. Meert, and T. R. Guiguémdé. 1993. Dracunculiasis in Burkina Faso: results of a national survey. J. Trop. Med. Hyg 96:357-362. [PubMed] [Google Scholar]
- 102.Karam, M., and A. Tayeh. 1999. Eradication of Dracunculiasis in the Libyan Arab Jamahiriya; report of the International Certification Team. WHO/CDS/CEE/DRA.99.7. World Health Organization, Geneva, Switzerland.
- 103.Keeling, N. G., M. E. Roelke, and D. J. Forrester. 1993. Subcutaneous helminths of the raccoon (Procyon lotor) in southern Florida. J. Helminthol. Soc. Wash 60:115-117. [Google Scholar]
- 104.Kim, A., A. Tandon, and E. Ruiz-Tiben. 1997. Cost-benefit analysis of the global dracunculiasis eradication campaign. Policy working paper no. 1835. Africa Human Development Department, The World Bank, Washington, D.C.
- 105.Kobayashi, A., A. Katatura, A. Hamada, T. Suzuki, et al. 1986. Human case of dracunculiasis in Japan. Am. J. Trop. Med. Hyg 35:159-161. [DOI] [PubMed] [Google Scholar]
- 106.Lalitha, C. M., and R. Anandan. 1980. Guinea worm infection in dogs. Cheiron 9:198-199. [Google Scholar]
- 107.Leiper, R. T. 1911. A method for dealing with town wells infected with guinea worm. J. Lond. School Trop. Med 1:28-30. [Google Scholar]
- 108.Liese, B. H., P. S. Sachdeva, and D. G. Cochrane. 1991. Organizing and managing tropical disease control programs: lessons of success. World Bank technical paper no. 159. The World Bank, Washington, D.C. [DOI] [PubMed]
- 109.Litsios, S. 1996. The tomorrow of malaria. Pacific Press, Wellington, New Zealand.
- 110.Litvinov, V. F., and V. P. Vitvinov. 1981. Helminths of predatory mammals from eastern Azerbaijan SSR, USSR. Parazitologiya 15:219-223. (In Russian.) [Google Scholar]
- 111.Lucas, P., J.-P. Chippaux, N. Zagaria, J.-P. Meert, A. Maiga, and D. Yameogo. 1999. Les nouveaux villages d'endémie de la dracunculose: reemergence de la maladie ou dysfonctionnement du système de surveillance. Méd. Trop 59:141-145. [PubMed] [Google Scholar]
- 112.Lyons, G. R. L. 1972. Guinea-worm infection in the Wa District of north-western Ghana. Bull. W. H. O 47:601-610. [PMC free article] [PubMed] [Google Scholar]
- 113.Lyons, G. R. L. 1973. The control of guineaworm with Abate: a trial in a village of North-West Ghana. Bull. W. H. O 49:215-216. [PMC free article] [PubMed] [Google Scholar]
- 114.Macpherson, C. N. 1981. The existence of Dracunculus medinensis (Linnaeus, 1758) in Turkana, Kenya. Trans R. Soc. Trop. Med Hyg 75:680-681. [DOI] [PubMed] [Google Scholar]
- 115.Magnussen, P., A. Yakubu, and P. Bloch. 1994. The effect of antibiotic- and hydrocortisone-containing ointments in preventing secondary infection in guinea worm disease. Am. J. Trop. Med. Hyg 51:797-799. [DOI] [PubMed] [Google Scholar]
- 116.Muller, R. 1971. Dracunculus and dracunculiasis. Adv. Parasitol 9:73-151. [DOI] [PubMed] [Google Scholar]
- 117.Muller, R. 1976. The pathology of experimental Dracunculus infection and its relevance to chemotherapy, p. 133-148. In E. J. L. Soulsby (ed.), Pathophysiology of parasitic infection. Academic Press, New York, N.Y.
- 118.Muller, R. 1979. Guinea worm disease; epidemiology, control and treatment. Bull. W. H. O 57:683-689. [PMC free article] [PubMed] [Google Scholar]
- 119.Muller, R. 1991. Dracunculus in Africa, p. 204-223. In C. N. L. Macpherson and P. S. Craig (ed.), Parasitic helminths and zoonoses in Africa. Unwin Hyman, London, United Kingdom.
- 120.Reference deleted.
- 121.National Research Council. 1983. Opportunities for control of dracunculiasis; report of a workshop. National Academy Press, Washington, D.C.
- 122.Nugent, D. A. W., D. Scott, and B. B. Waddy. 1955. Effect of water-point treatment with DDT on the incidence of guinea worm infection. Trans. R. Soc. Trop. Med. Hyg 49:476-477. [DOI] [PubMed] [Google Scholar]
- 123.Nwoke, B. E. 1992. Behavioural aspects and their possible uses in the control of dracontiasis (guinea-worm) in Igwun river basin area of Imo State, Nigeria. Angew. Parasitol 33:205-210. [PubMed] [Google Scholar]
- 124.Nwosu, A. B. C., E. O. Ifezulike, and A. O. Anya. 1982. Endemic dracontiasis in Anambra State of Nigeria; geographic distribution, clinical features, epidemiology and socio-economic impact of the disease. Ann. Trop. Med. Parasitol 76:187-200. [DOI] [PubMed] [Google Scholar]
- 125.Olsen, A., P. Magnussen, and S. Anemana. 1997. The acceptability and effectiveness of a polyester drinking-water filter in a dracunculiasis-endemic village in Northern Region, Ghana. Bull. W. H. O 75:449-452. [PMC free article] [PubMed] [Google Scholar]
- 126.Onabamiro, S. D. 1954. The diurnal migration of cyclops infected with the larvae of Dracunculus medinensis (Linnaeus) with some observations on the development of the larval worms. W. Afr. Med. J 3:189-194. [PubMed] [Google Scholar]
- 127.Osisanya, J. O. S., E. I. Elueze, and F. I. Okoro. 1986. Dracontiasis: pattern of morbidity in a north-western village in Sokoto State, Nigeria. Trans. R. Soc. Trop. Med. Hyg 80:293-294. [DOI] [PubMed] [Google Scholar]
- 128.Paul, J. E. 1988. A field test report of implementation planning and a cost-benefit model for Guinea worm eradication in Pakistan. WASH field report no. 231, March 1988. WASH Project for U.S. Agency for International Development, Arlington, Va.
- 129.Petit, M. M., M. Denia'u, C. Tourte-Schaefer, and K. Amegbo. 1989. Étude épidémiologique longitudinale de la dracunculose dans le Sud du Togo. Bull. Soc. Pathol. Exot 82:520-530. [PubMed] [Google Scholar]
- 130.Ranque, P., I. S. Degoga, A. Tounkara, H. Balique, and M. Quilici. 1979. Répartition de la dracunculose au Mali; étude des biotopes à Cyclops. Méd. Trop 39:545-548. [PubMed] [Google Scholar]
- 131.Ranque, P., H. Periès, J.-P. Meert, and K. O'Neill. 1996. Situation actuelle de la campagne d'éradication de la dracunculose. Méd. Trop 56:289-296. [PubMed] [Google Scholar]
- 132.Rao, C. K., and G. V. M. Reddy. 1965. Dracontiasis in West Godavari and Kurnool Districts, Andhra Pradesh. Bull. Indian Soc. Malaria Commun. Dis 2:275-295. [Google Scholar]
- 133.Reddy, C. R. R. M., I. L. Narasaiah, and G. Parvathi. 1969. Epidemiological studies on guinea-worm infection. Bull. W. H. O 40:521-529. [PMC free article] [PubMed] [Google Scholar]
- 134.République du Côte d'Ivoire. 1992. Situation Epidémiologique du Ver de Guinée en Côte d'Ivoire. Ministère de la Santé et de la Protection Sociale, Abidjan, Ivory Coast.
- 135.Richardson, O. J., W. B. Owen, and D. E. Snyder. 1992. Helminth parasites of the raccoon (Procyon lotor) from north-central Arkansas. J. Parasitol 78:163-166. [PubMed] [Google Scholar]
- 136.Rohde, J. E., B. L. Sharma, H. Patton, C. Deegan, and J. M. Sherry. 1993. Surgical extraction of guinea worm: disability reduction and contribution to disease control. Am. J. Trop. Med. Hyg 48:71-76. [DOI] [PubMed] [Google Scholar]
- 137.Sahba, G. H., F. Arfaa, A. Fardin, and A. Ardalan. 1973. Studies on dracunculiasis in Iran. Am. J. Trop. Med. Hyg 22:343-347. [DOI] [PubMed] [Google Scholar]
- 138.Scott, D. 1960. An epidemiological note on guinea-worm infection in north-west Ashanti, Ghana. Ann. Trop. Med. Parasitol 54:32-43. [DOI] [PubMed] [Google Scholar]
- 139.Sebastian, H., J. Collbran, and M. Saint-Lot. 1991. Assessment of interagency collaboration in the Bénin Rural Water and Sanitation Project and its guinea worm reduction component. VBC report no. 81152. Vector Biology and Control Project for U.S. Agency for International Development, Arlington, Va.
- 140.Seville, R. S., and E. M. Addison. 1995. Nongastrointestinal helminths in marten (Martes americana) from Ontario, Canada. J. Wildl. Dis 31:529-533. [DOI] [PubMed] [Google Scholar]
- 141.Sharma, M. I. D. 1980. Lessons learnt from the intensified campaign against smallpox in India and their possible applicability to other health programmes, with particular reference to eradication of dracunculiasis. J. Commun. Dis 12:59-64. [PubMed] [Google Scholar]
- 142.Singh, J., and N. G. S. Raghavan. 1957. Dracontiasis in India, its public health importance and its control. Bull. Natl. Soc. India Malaria Mosquito-Borne Dis 5:141-158. [Google Scholar]
- 143.Smith, G. S., D. Blum, S. R. A. Huttly, N. Okeke, B. R. Kirkwood, and R. G. Feachem. 1989. Disability from dracunculiasis: effect on mobility. Ann. Trop. Med. Parasitol 83:151-158. [DOI] [PubMed] [Google Scholar]
- 144.Smith, R. A., M. L. Kennedy, and W. E. Wilhelm. 1985. Helminth parasites of the raccoon (Procyon lotor) from Tennessee and Kentucky. J. Parasitol 71:599-603. [Google Scholar]
- 145.Spearman, P., M. Spring, W. Moore, Jr., M. Barry, T. Minichiello, and J. Hadler. 1998. Imported dracunculiasis—United States 1995 and 1997. Morb. Mortal. Wkly. Rep 47:209-211. [PubMed] [Google Scholar]
- 146.Spiers, R. E., and A. J. Baum. 1953. Dracunculosis. J. Kans. Med. Soc 54:553-554. [PubMed] [Google Scholar]
- 147.Steib, K., and P. Mayer. 1988. Epidemiology and vectors of Dracunculus medinensis in northwest Burkina Faso, West Africa. Ann. Trop. Med. Parasitol 82:189-199. [DOI] [PubMed] [Google Scholar]
- 148.Sullivan, J. J. 1991. Field evaluation of slow-release temephos formulations for control of copepods in dracunculiasis elimination programs: update September 1991. Centers for Disease Control, Atlanta, Ga.
- 149.Sullivan, J. J., H. S. Bishop, and A. W. Hightower. 1991. Susceptibility of four species of copepods from areas of endemic Dracunculus medinensis, to the North American Dracunculus insignis. Ann. Trop. Med. Parasitol 85:637-643. [DOI] [PubMed] [Google Scholar]
- 150.Tayeh, A. 1996. Dracunculiasis, p. 286-293. In F. E. G. Cox (ed.), The Wellcome illustrated history of tropical diseases. The Wellcome Trust, London, United Kingdom.
- 151.Tayeh, A., and S. Cairncross. 1996. The impact of dracunculiasis on the nutritional status of children in South Kordofan, Sudan. Ann. Trop. Paediatr 16:221-226. [DOI] [PubMed] [Google Scholar]
- 152.Tayeh, A., and S. Cairncross. 1998. The effect of size of surface drinking water sources on dracunculiasis prevalence in the Northern Region of Ghana. Int. J. Environ. Health Res 8:285-292. [Google Scholar]
- 153.Tayeh, A., S. Cairncross, and G. H. Maude. 1993. Water sources and other determinants of dracunculiasis in the Northern Region of Ghana. J. Helminthol 67:213-225. [DOI] [PubMed] [Google Scholar]
- 154.Tempalski, B. 1991. The spatial distribution of dracunculiasis in Northern Zou Province, Bénin. M.A. thesis. City University of New York, New York, N.Y.
- 155.Turkhud, D. A. 1914. Report of the Bombay bacteriological laboratory for the year 1913, p. 14-16. Government Central Press, Bombay, India.
- 156.Udonsi, J. K. 1987. Control of endemic dracontiasis by provision of water supply in rural communities of Imo State, Nigeria. Public Health 101:63-70. [DOI] [PubMed] [Google Scholar]
- 157.van Heutsz. 1926. One indigenous and two imported cases of Dracunculus in Java. Geneeskd. Tijdschr. Ned.-Indie 66:25. (In Dutch.) [Google Scholar]
- 158.Velikanov, B. P. 1984. A case of Dracunculus medinensis infection in a dog in Turkmenia. Izv. Akad. Nauk Turkm. SSR Biol. Nauk 1:64-65. (In Russian.) [Google Scholar]
- 159.Velschius, G. H. 1674. Exercitationes de Vena medinensis et de vermiculis capillaribus infantium. Th. Goeblius, Augsburg, Germany.
- 160.Watts, S. J. 1984. Population mobility, development and dracunculiasis in Kwara State, Nigeria. Soc. Sci. Med 19:471-473. [DOI] [PubMed] [Google Scholar]
- 161.Watts, S. J. 1987. Dracunculiasis in Africa in 1986: its geographic extent, incidence, and at-risk population. Am. J. Trop. Med. Hyg 37:119-125. [DOI] [PubMed] [Google Scholar]
- 162.Watts, S. J. 1998. An ancient scourge: the end of dracunculiasis in Egypt. Soc. Sci. Med 46:811-819. [DOI] [PubMed] [Google Scholar]
- 163.Watts, S. J. 2000. Dracunculiasis in the Caribbean and South America: a contribution to the history of dracunculiasis eradication. Med. Hist 45:227-250. [PMC free article] [PubMed] [Google Scholar]
- 164.Watts, S. J., W. R. Brieger, and M. Yacoob. 1989. Guinea worm: an in-depth study of what happens to mothers, families and communities. Soc. Sci. Med 29:1043-1049. [DOI] [PubMed] [Google Scholar]
- 165.World Health Organization. 1987. Dracunculiasis: Ivory Coast. Wkly. Epidemiol. Rec 62:169-170. [Google Scholar]
- 166.World Health Organization. 1990. Press release WHO/45, 31 August 1990.
- 167.World Health Organization. 1992. The International Drinking Water Supply and Sanitation Decade; end of decade review (as of December 1990). WHO/CWS/92.12. World Health Organization, Geneva, Switzerland.
- 168.World Health Organization. 1992. Study mission to the Islamic Republic of Iran, 7 to 20 October 1992, by P. Ranque, Division of Tropical Diseases. World Health Organization, Geneva, Switzerland.
- 169.World Health Organization. 1996. Certification of dracunculiasis eradication; criteria, strategies, procedures—a practical guide. WHO/FIL/96.188 REV.1. World Health Organization, Geneva, Switzerland.
- 170.World Health Organization. 1996. Criteria for the certification of dracunculiasis eradication. WHO/FIL/96.187 Rev. 1. World Health Organization, Geneva, Switzerland.
- 171.World Health Organization. 1998. Dracunculiasis eradication in Uzbekistan: country report. WHO/CDS/CEE/DRA/99.9. World Health Organization, Geneva, Switzerland.
- 172.World Health Organization. 1998. Yoro, the empty granary. Film (35 mm) and videotape. Coproduction by WHO/ORSTOM/ENMP, Geneva, Switzerland.
- 173.World Health Organization. 1998. Dracunculiasis surveillance. Wkly. Epidemiol. Rec 73(45):345-346. [PubMed] [Google Scholar]
- 174.World Health Organization. 1999. Dracunculiasis surveillance, 1998. Wkly. Epidemiol. Rec 74(19):146-152. [PubMed] [Google Scholar]
- 175.World Health Organization. 1999. Geographical information systems (GIS); mapping for epidemiological surveillance. Wkly. Epidemiol. Rec 74(34):281-284. [PubMed] [Google Scholar]
- 176.World Health Organization. 1999. Gestion des données épidémiologiques et systèmes d'information géographique appliquées au programme d'éradication de la dracunculose. Rapport de l'atelier. WHO/CDS/CEE/DRA/99.4. World Health Organization, Geneva, Switzerland.
- 177.World Health Organization. 2000. Eradication of dracunculiasis; guidelines for international certification teams. WHO/CDS/CEE/DRA/2000.12. World Health Organization, Geneva, Switzerland.
- 178.World Health Organization. 2000. Dracunculiasis; certification of absence of transmission. Wkly. Epidemiol. Rec 75(10):77-79. [PubMed] [Google Scholar]
- 179.World Health Organization. 2000. International Commission for the Certification of Dracunculiasis Eradication; 4th Meeting, report and recommendations. WHO/CDS/CPE/2000.6. World Health Organization, Geneva, Switzerland.
- 180.World Health Organization. 2001. Dracunculiasis—global surveillance summary, 2000. Wkly. Epidemiol. Rec 76(18):133-139. [PubMed] [Google Scholar]
- 181.World Health Organization. 2001. Dracunculiasis, Yemen. Wkly. Epidemiol. Rec 76(3):22-23. [PubMed] [Google Scholar]
- 182.World Health Organization. 2001. Dracunculiasis surveillance, Chad. Wkly. Epidemiol. Rec 76(17):131-132. [PubMed] [Google Scholar]
- 183.World Health Organization-United Nations Children's Fund. 1993. Integrated surveillance in dracunculiasis eradication programmes; joint statement by WHO, UNICEF, Global 2000 and the WHO Collaborating Center at CDC. WHO-UNICEF Joint Committee on Health Policy, JCHPSS/94/2.9, Annex 5.
- 184.Wurapa, F. K., D. W. Belcher, and W. B. Ward. 1975. A clinical picture of guinea worm disease in southern Ghana. Ghana Med. J 14:10-15. [Google Scholar]
- 185.Yelifari, L., E. Frempong, and A. Olsen. 1997. The intermediate hosts of Dracunculus medinensis in Northern Region, Ghana. Ann. Trop. Med. Parasitol 91:403-409. [DOI] [PubMed] [Google Scholar]
- 186.Yellott, H. L. 1990. Enquête sur l'incidence de la dracunculose dans six districts du Zou Nord de la Républiue du Bénin. The Pragma Corporation, Cotonou, Benin.
- 187.Zucker, J. 1998. Assessment of community-based surveillance: Northern Region, Ghana. UNICEF mission report. United Nations Children's Fund, New York, N.Y.