Abstract
Junctional Adhesion Molecule-A (JAM-A) is a transmembrane adhesive protein expressed at endothelial junctions and in leukocytes. Here we report that JAM-A is required for the correct infiltration of polymorphonuclear leukocytes (PMN) into an inflamed peritoneum or in the heart upon ischemia-reperfusion injury. The defect was not observed in mice with an endothelium-restricted deficiency of the protein but was still detectable in mice transplanted with bone marrow from JAM-A-/- donors. Microscopic examination of mesenteric and heart microvasculature of JAM-A-/- mice showed high numbers of PMN adherent on the endothelium or entrapped between endothelial cells and the basement membrane. In vitro, in the absence of JAM-A, PMN adhered more efficiently to endothelial cells and basement membrane proteins, and their polarized movement was strongly reduced. This paper describes a nonredundant role of JAM-A in controlling PMN diapedesis through the vessel wall.
Keywords: endothelial cell junctions, leukocyte transmigration, myocardial infarction, polymorphonuclear leukocytes
Acute inflammatory reactions or ischemia-reperfusion injury are accompanied by leukocyte adhesion to the blood vessel wall and subsequent infiltration into underlying tissues (1). Although the molecular mechanisms regulating leukocyte adhesion to inflamed endothelial cells have been elucidated to a large extent, we have only a partial picture of how leukocytes can open endothelial junctions and move to the underlying tissues in a polarized fashion. A hypothetical and general model is that proteins at endothelial junctions interact with leukocytes and actively direct their diapedesis through the cleft (1-4). In previous work (5), we identified Junctional Adhesion Molecule-A (JAM-A) as a member of the CD2 subgroup of the Ig superfamily (5). JAM-A is expressed in endothelial and epithelial cells, leukocytes, and platelets (4). JAM-A is localized at tight junctions, and some reports suggest that JAM-A at endothelial junctions may contribute to leukocyte diapedesis (2, 4-7). JAM-A is a ligand for integrin αL-β2 (8), but homophilic binding of endothelial and leukocyte JAM-A may also occur. In a recent paper (9), a soluble JAM-A/Fc fragment was found to inhibit mononuclear cell recruitment on inflamed endothelium. Furthermore, two additional members of the JAM family (JAM-B and -C) have been identified (10-12). JAM-B and -C are expressed by endothelial cells and are specifically enriched in some vessels, such as high endothelial venules and lymphatics. JAM-C was found in platelets and T, natural killer, and dendritic cells (13-15) and has been implicated in lymphocyte transmigration.
In the present paper, we used JAM-A-/- mice to directly address the problem of the role of JAM-A in leukocyte diapedesis. We found that the passage of polymorphonuclear leukocytes (PMN) through the vessel wall was substantially reduced in both inflammatory peritonitis and cardiac ischemia-reperfusion injury, a well accepted model of acute myocardial infarction. Genetic analysis revealed that the role of JAM-A in PMN trafficking in these models was independent of its expression in the endothelium. Further studies in vitro indicated that JAM-A is required for correct PMN adhesion and polarized movement. These data reveal a role for JAM-A in directing PMN extravasation.
Methods
Mice. Generation and genotyping of JAM-A+/+, JAM-A-/-, and Tie-2 Cre JAM-A-/- mice were developed as described (16). These mice were originally generated on a C57BL6/129/CD1 background, then they were backcrossed once to C57BL/6J.
Peritoneal Inflammation Model. JAM-A+/+, JAM-A-/-, and Tie-2 Cre JAM-A-/- mice (10-12 weeks old) were injected i.p. with 1 ml of a 3% thioglycollate medium solution (Sigma), as described (17). At various time points after stimulation, the peritoneal exudate was harvested by an i.p. lavage with sterile saline and total leukocytes counted.
Bone Marrow Transplantation. Bone marrow chimeric mice were produced as described (17, 18) by transplanting 17 × 106 whole bone marrow cells isolated from JAM-A-/- and JAM-A+/+ mice into lethally irradiated (9.5 Gy) JAM-A+/+ hosts. The blood leukocyte phenotype of recipients was determined after 8 weeks by FACS analysis (17).
In Vivo Cardiac Ischemia Reperfusion. Twelve-week-old JAM-A+/+, JAM-A-/-, and Tie-2 Cre JAM-A-/- mice underwent 30-min cardiac ischemia followed by reperfusion of 6 h and 25 days (19). Six hours after the onset of reperfusion, before death, the ligature was retied and Evans blue dye injected i.v. The area lacking blue stain was consider as the area at risk (AAR, i.e., made ischemic). Heart sections below the ligature were used for PMN count and for infarct size assessment by triphenyltetrazolium. In another group of animals, green and blue particles (Unisperse, CIBA Pharmaceutical) in suspension were injected into the vena cava, respectively before and after retying the ligature around the coronary artery, to assess extent of reperfusion. Perfused capillaries (capillaries containing green particles) within the AAR were counted and expressed per mm2 of AAR as an index of effectiveness of reperfusion. In animals killed at 25 days, infarct size was calculated by estimating the number of lost myocytes from the left ventricle by the histomorphometric technique (20), and echocardiography (SSD-5500, ALOKA, Tokyo) was performed (21). Systolic blood pressure and heart rate were measured in conscious mice by tail cuff.
In Vitro Assays. JAM-A+/+ and JAM-A-/- endothelial cells were isolated from the lungs and cultured as described (16). Intercellular adhesion molecule (ICAM)-1-/- and ICAM-1+/+ endothelial cells were kindly provided by B. Engelhardt, Theodor Kocher Institute, University of Bern, Bern, Switzerland. Bone marrow PMN cells were purified (90-95% purity), as described (22). PMN adhesion to endothelial cells grown in 96-well plates or to fibronectin (20 μg/ml) or laminin (20 mg/ml) was assessed by labeling PMN with the fluorochrome Cell Tracker Green (5 μM) (Molecular Probes) (23) and adding 105 cells per well. Fluorescence was measured by using a fluorimeter (Multilabel Counter, Wallac 1420, PerkinElmer). Cell-binding values were calculated as cells bound/mm2, as described (24).
For transmigration experiments, 24-well Transwell plates with a 5-μm pore size polycarbonate filter (Costar) were used to culture endothelial cell monolayers as described (5, 16). Briefly, 51Cr-labeled (Amersham Pharmacia) PMN (5 × 104/well in 0.1 ml) were seeded in the upper compartment, and chemotaxis was induced by the addition of the synthetic peptide WKYMVm (25) (kind gift of G. Berton and L. Fumagalli, University of Verona, Verona, Italy), 5 nM for 90 min, in the lower well of Transwell chambers.
Dunn Chamber. Dunn chambers (Weber Scientific, Hamilton, NJ) were set up as described (26) with 5 nM of the synthetic peptide WKYMVm in the outer well. For each experiment, two Dunn chambers were run in parallel. Cells were visualized, and images were acquired with an Olympus CellR̂ time lapse work-station inverted microscope and ×20 phase-contrast objectives. The camera was set up to acquire one frame every 30 sec over a 40-min period. The processed sequences were then replayed as a movie. Cell tracks were generated from the time-lapse images and analyzed by using image j, Version 1.34K, http://rsb.info.nih.gov/ij.
Histology, Immunohistochemistry, and Electron Microscopy. All methods for histology, immunohistochemistry, and electron microscopy are detailed in Supporting Text, which is published as supporting information on the PNAS web site (for antibody suppliers, see Supporting Text).
Results
PMN Influx into Inflammatory Sites Is Impaired in JAM-A-/-Mice. JAM-A-/- mice were generated by using a Cre-Lox strategy as described (16). Fig. 1a shows that thioglycollate injection increased PMN number in the peritoneum, peaking at 24 h and declining within 48-72 h. The number of infiltrated PMN was significantly lower in JAM-A-/- mice at 24 h (50% reduction). The expected increase in PMN mobilization in blood induced by thioglycollate was not statistically different in JAM-A+/+ and JAM-A-/- mice (data not shown). Microscopic examination of mesenteric postcapillary venules revealed that PMN were trapped on the surface or behind the endothelium (Fig. 1 b and c).
Fig. 1.
Reduced PMN infiltration in inflamed peritoneum in JAM-A-/- mice. (a) JAM-A+/+ (σ), JAM-A-/- (λ), and Tie-2 Cre JAM-A-/- (ν) mice were injected i.p. with thioglycollate. At the indicated times after thioglycollate injection, the peritoneal exudates were collected and total PMN counted. Data are means ± SEM of one representative experiment (at least eight mice per group) of three experiments performed. *, P < 0.01 vs. JAM-A+/+ and Tie-2 Cre JAM-A-/- mice by analysis of variance and Duncan's test. Light micrographs of mesenteric postcapillary venules stained with toluidine blue obtained from JAM-A-/- (b) and JAM-A+/+ (c) mice 24 h after thioglycollate injection. Note that a higher number of PMN adhere to the surface of the microvasculature (arrows) or remain underneath the endothelium (arrowheads) in JAM-A-/- mice. (Bar, 50 μm.) (d) JAM-A+/+ mice were lethally irradiated and then transplanted with bone marrow derived either from JAM-A-/- (dashed column) or JAM-A+/+ (black column) mice. Peritoneal PMN were counted 24 h after thioglycollate injection. Data are means ± SEM of five replicates from a typical experiment of the three performed. *, P < 0.01 vs. JAM-A+/+ mice (Student's t test).
As shown in Fig. 1a, when Tie-2 Cre JAM-A-/- mice were subjected to peritoneal injection of thioglycollate, neutrophil influx was comparable to control mice, indicating that their diapedesis could take place also in the absence of endothelial JAM-A.
To further prove this point, bone marrow cells obtained from JAM-A+/+ or JAM-A-/- donor mice were transplanted into lethally irradiated JAM-A+/+ mice. As reported in Fig. 1d, the animals transplanted with JAM-A-/- bone marrow presented significantly lower PMN infiltration into the inflamed peritoneal cavity. Reconstitution of peripheral blood cells was comparable in the two groups of animals (Table 1, which is published as supporting information on the PNAS web site).
PMN Infiltration in the Myocardium Is Reduced in JAM-A-/- Mice upon Ischemia-Reperfusion Injury. In this experimental system, the number of PMN infiltrating the AAR was reduced by >50% in JAM-A-/- as compared with JAM-A+/+ and Tie-2 Cre JAM-A-/- mice (Fig. 2a), whereas a higher number of JAM-A-/- PMN remained adherent to the vessel wall (Fig. 2b). JAM-A-/- PMN were frequently aggregated and partially or completely occluded the lumen of small vessels (Fig. 2c). In some cases, they seemed entrapped between endothelial cells and the basement membrane or even within the endothelium (Fig. 2d).
Fig. 2.
Reduced PMN infiltration in ischemic heart tissue in JAM-A-/- mice. (a) Number of PMN within the AAR or (b) within the lumen or the wall of coronary vessels of hearts from JAM-A-/- (n = 9), JAM-A+/+ (n = 7), and Tie-2 Cre JAM-A-/- mice (n = 8). Values are expressed as means ± SEM. *, P < 0.05 vs. JAM-A+/+ and Tie-2 Cre JAM-A-/- mice (Student's t test). (c) EM of aggregates of PMN (arrow) in coronary vessels of JAM-A-/- mice. (Bar, 2.5 μm.) (d) EM analysis of heart microvessels showing PMN (arrow) between endothelial cells (arrowhead) and the basal membrane (asterisk) of the vessel wall in the heart of JAM-A-/- mice. (Bar, 2.5 μm.) (e) Number of perfused capillaries within the AAR and interventricular septum from JAM-A-/- (n = 6) and JAM-A+/+ (n = 6) mice. JAM-A-/- mice show the same density of perfused capillaries in spared myocardium (septum), but a lower density in the AAR (*, P < 0.05 vs. JAM-A+/+).
After 6 h of reperfusion, the number of perfused capillaries (Fig. 2e) within the AAR was 45% less in JAM-A-/-, suggesting that PMN entrapment in the microcirculation affected reperfusion of AAR (27). Perfusion of the spared myocardium (i.e., interventricular septum) was comparable (Fig. 2e), indicating that the defect was limited to the ischemic area. Infarct size tended to be higher in JAM-A-/- (43.3 ± 7.8% and 35.1 ± 7.9% in JAM-A-/- and JAM-A+/+).
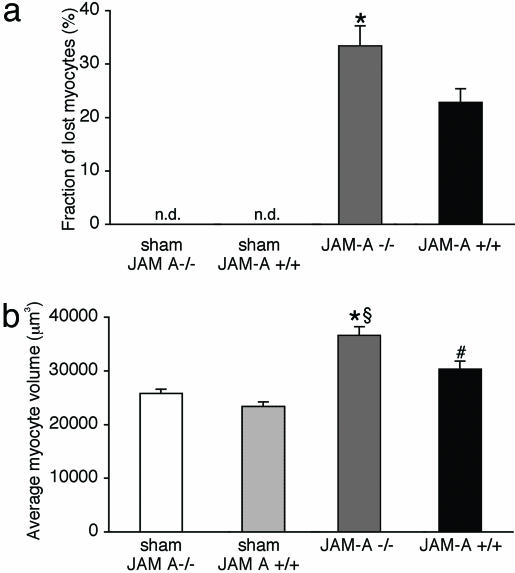
After 25 days of reperfusion, the left ventricle wall corresponding to the AAR was thinner in JAM-A-/- mice, and the fraction of myocytes lost from the left ventricle was higher in the absence of JAM-A (Fig. 3a), whereas myocyte volume was increased (Fig. 3b). Echocardiography showed that left-ventricle internal diameter in diastole tended to be larger and fractional shortening significantly lower in JAM-A-/- mice as compared with JAM-A+/+ (Tables 2 and 3, which are published as supporting information on the PNAS web site).
Fig. 3.
Effect of ischemia reperfusion injury. After 25 days reperfusion, the fraction of lost cardiac myocytes (a) appears larger in JAM-A-/- mice (*, P < 0.05 vs. JAM-A+/+) when compared with the respective sham-operated mice (JAM-A-/-, n = 10; JAM-A+/+, n = 6); n.d., not detectable. (b) Compensatory hypertrophy of cardiac myocytes surviving ischemia-reperfusion was significantly higher in JAM-A-/- than in JAM-A+/+ (*, P < 0.05 vs. JAM-A+/+;§, P < 0.05 vs. JAM-A-/- sham; #, P < 0.05 vs. JAM-A+/+ sham mice). Data are means ± SEM. Data were analyzed by using Student's t test.
JAM-A-/-PMN Adhere More Effectively to Endothelial Cells in Vitro, but Their Diapedesis Is Inhibited. In vitro experiments were designed to further distinguish between the role of JAM-A in PMN and in endothelial cells. As reported in Fig. 4a, JAM-A-/- PMN adhered more efficiently to the endothelium, but the percentage of adherent PMN that could transmigrate was significantly reduced (Fig. 4b). As a chemotactic stimulus, we used the peptide WKYMVm, which is known to interact more effectively with the mouse fMLP receptor (25). Similarly to in vivo conditions, the presence or absence of JAM-A in endothelial cells did not change these parameters. PMN adhesion was increased also by using other substrata such as ICAM-1+/+ and -1-/- endothelial cells or fibronectin and laminin (Fig. 4 c and d).
Fig. 4.
Altered adhesion and transmigration of JAM-A-/- PMN in vitro. (a) Adhesion of JAM-A-/- PMN (dashed columns) to endothelial cells expressing or not JAM-A (EC-JAM-A-/- and EC-JAM-A+/+, respectively) was significantly increased in comparison with JAM-A+/+ PMN (black columns). (b) Transmigration of JAM-A-/- PMN (dashed columns) through both JAM-A-positive and -null endothelial monolayers was decreased as compared with JAM-A+/+ PMN (black columns). Data represent the percentage of total cells migrated. (c) Adhesion of JAM-A-/- PMN (dashed columns) to endothelial cells expressing or not ICAM-1 (EC-ICAM-1-/- and EC-ICAM-1+/+, respectively) or (d) to fibronectin or laminin was significantly increased in comparison with JAM-A+/+ PMN (black columns). Results are means ± SEM of data obtained with cells derived from three to six mice. *, P < 0.01 vs. JAM-A+/+ PMN by Student's t test.
Because adhesion and diapedesis require cytoskeletal rearrangement and polarized movement of PMN, we investigated whether the absence of JAM-A could change actin organization. When seeded on fibronectin, JAM-A-/- PMN appeared more spread (Fig. 5A, compare a and d) and presented an altered morphology upon chemotactic stimulation (compare Fig. 5A b and c and e, f, and f′). In JAM-A+/+ PMN, actin was typically polarized at the leading front (Fig. 5A f and f′, arrowheads), whereas at the rear, a short uropod could be observed (Fig. 5A f and f′, arrows). In contrast, in JAM-A-/- PMN, actin did not concentrate at the leading edge (Fig. 5Ac, arrowheads) but was mostly localized at the basis of the uropods (Fig. 5Ac, arrow). In addition, JAM-A-/- cells presented remarkably long uropods (Fig. 5A b and c). The length of the tails ranged <2-5 μm in JAM-A+/+ cells and 5 to >8 μm in JAM-A-/- cells. Time-lapse video microscopy showed that JAM-A-/- PMN moved less effectively toward a chemotactic stimulus (Fig. 5B), and that they were unable to correctly detach and retract the uropod (Movies 1 and 2, which are published as supporting information on the PNAS web site). Overall, these data support observations made in vivo and indicate that JAM-A expressed by PMN is required for their correct adhesion and polarized migration.
Fig. 5.
Defective PMN polarization and motility in the absence of JAM-A. (A) PMN (0.4 × 106) were added to fibronectin- (20 μg/ml) coated coverslips for 10 min with (b and c and e, f, and f′) or without (a-d) WKYMVm 5 nM for 30 min. Cells were then analyzed for actin distribution by immunofluorescence. In the absence of stimuli, JAM-A-/- cells (a) were more spread out than JAM-A+/+ PMN (d). In the presence of WKYMVm, JAM-A-/- PMN presented a more elongated morphology and long tails (compare b and c and e, f, and f′). JAM-A+/+ PMN show actin clustering at the leading edge (f and f′, arrowheads) and a short uropod (f and f′, arrow); in contrast, JAM-A-/- PMN actin was absent or poorly organized at the leading edge (c, arrowheads), whereas it was concentrated at the opposite site at the basis of the uropod (c, arrows). [Bars (a and c), 12.5 μm; (b), 25 μm.] (B) JAM-A+/+ and JAM-A-/- PMN were exposed to WKYMVm 5 nM, and their migration was followed by time-lapse microscopy for 40 min. The migration tracks are shown as plots with the starting point for each cell at the intersection of the x and y axes. Cell tracks from a typical experiment of two performed are reported. A total of 20 cells were analyzed. The x and y axes are scaled in micrometers (μm).
The phenotype of PMN and endothelial cells, derived from JAM-A+/+ and JAM-A-/- mice, is reported in Figs. 6-8, which are published as supporting information on the PNAS web site.
Discussion
In this work, we report that tissue infiltration of PMN in peritonitis and heart ischemia and reperfusion is significantly reduced in JAM-A-/- mice. Genetic analysis, bone marrow transplantation, and in vitro adhesion and transmigration assays all revealed that the role of JAM-A in PMN trafficking is independent of endothelial JAM-A. This was a surprising finding, because previous studies in vitro and in vivo showed that endothelial JAM-A may facilitate leukocyte diapedesis through junctions (2, 4, 7-9, 28). It is possible that other members of the family, such as JAM-B and -C, may substitute for JAM-A in the endothelium, at least in the tissues considered here (29). This is supported by the observation that JAM-B and -C were detectable in endothelial cells of the mesenteric and cardiac microcirculation (data not shown), whereas both of them were barely detectable or absent in PMN (15, 29). Recently, in ischemia-reperfusion injury in the liver (28), it was possible to detect a role of endothelial JAM-A, likely because JAM-B and -C are poorly expressed in the vasculature of this organ.
Although thioglycollate-induced peritonitis is an acute inflammatory response, the cardiac ischemia-reperfusion model allows us to study chronic consequences of JAM-A inactivation. Although still debated, infiltration of PMN after reperfusion may be detrimental to cardiac recovery, because these cells release oxygen radicals and lytic enzymes, which may extend tissue damage (19, 30). We report here that structural and functional heart recovery under reperfusion was hampered by the lack of JAM-A after 25 days of reperfusion, although a lower number of PMN could infiltrate the AAR of JAM-A-/- mice. An explanation for this apparent discrepancy is that JAM-A-/- PMN, unable to correctly extravasate, remained entrapped within the microvasculature, causing impairment of blood flow during reperfusion of the ischemic area (AAR) (27).
An important issue is the mechanism of action of JAM-A. Consistent with in vivo models, in vitro experiments showed that JAM-A-/- PMN adhere more strongly and transmigrate less efficiently through both JAM-A-/- or JAM-A+/+ endothelial monolayers. PMN diapedesis requires the polarized movement of the cell toward a concentration gradient of a chemotactic stimulus. When exposed to an attractant, these cells orient themselves and move by forming pseudopods at the front and retracting the uropod at the rear (31-33). This typical organization is affected by the lack of JAM-A expression. Actin is strongly reduced at the leading edge, whereas it concentrates at the basis of the uropod. JAM-A-/- PMN adhere to and exhibit greater spreading on different substrata. Time-lapse video microscopy shows that in the absence of JAM-A, PMN cannot easily detach and move toward the chemokine gradient. Indeed, they seem to be unable to retract the uropod, which remains attached to the substratum and elongates while the cells are trying to move toward the chemotactic stimulus. These observations strongly suggest that the increase in adhesion observed in absence of JAM-A inhibits PMN movement.
Other authors (34) have found that antibodies to platelet endothelial cell adhesion molecule (PECAM)-1 induced PMN to remain attached to the subendothelial cell basement membrane. PECAM-1 gene inactivation leads to a defect in PMN infiltration in the inflamed peritoneum similar to that observed in JAM-A-null mice (35). Loike et al. (36) showed that activation of integrin α5β1 caused increase in PMN adhesion, which prevented their movement. Finally, other conditions causing elongation of leukocyte uropod were accompanied by reduction in directional cell motility (23, 37).
Although the molecular mechanism(s) through which JAM-A controls cell adhesion and movement remains to be clarified in more detail, the data reported here introduce concepts on the action of JAM-A in leukocytes. Consistently, a recent paper by Shaw et al. (38) reported in vitro data suggesting that endothelial JAM-A does not significantly contribute to PMN adhesion and transmigration.
In epithelial cells, the use of JAM-A small interference RNA reduces cell adhesion and integrin organization (39). None of these effects were observed in JAM-A-/- endothelial cells (not shown) and, as reported here, JAM-A-null PMN adhere even better to different substrata. The cell type and/or use of a different strategy to knockdown JAM-A may explain this discrepancy.
In previous work (16), we found that JAM-A-/- dendritic cells presented an increased random but not directional motility, thus suggesting, also in this case, a defect in cell polarization. In vivo, however, this resulted in increased recruitment of dendritic cells to lymph nodes and an increased delayed hypersensitivity reaction. It is therefore possible that the consequences of JAM-A targeting are versatile in different cell types and tissues, depending on the sites of cell diapedesis (lymphatics vs. blood vessels) and/or the coexistence of other members of JAM family.
Supplementary Material
Acknowledgments
We acknowledge M. Faretta, M. Garrè, and P. Transidico of the Fondazione Italiana per la Ricerca sul Cancro, Institute of Molecular Oncology, Istituto Europeo di Oncologia (IFOM-IEO) Campus facility for confocal microscopy analysis. Antonio Bai performed the in vivo blood pressure and echocardiographic measurements. This work was supported by the Associazione Italiana per la Ricerca sul Cancro; the European Community (QLRT-2001-02059, Integrated Project Contract No LSHG-CT-2004-503573, NoE MAIN 502935, and NoE EVGN LSHM-CT-2003-503254); Consiglio Nazionale delle Ricerche/Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) (CNR.02.731.DEJA); Italian Ministry of Health, MIUR/Fondo per gli Investimenti della Ricerca di Base (RBNE01MWA_009, RBNE01F8LT_007); Special Project Stem Cells (CS36 and CS39); and Cofin 2003 (2003058397_04), Human Frontier Science Foundation. M.R.C. was supported by a fellowship from the Fondazione Italiana per la Ricerca sul Cancro.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: JAM, Junctional Adhesion Molecule; PMN, polymorphonuclear leukocytes; AAR, area at risk; ICAM, intracellular adhesion molecule.
References
- 1.Muller, W. A. (2003) Trends Immunol. 24, 326-333. [Google Scholar]
- 2.Vestweber, D. (2002) Curr. Opin. Cell Biol. 14, 587-593. [DOI] [PubMed] [Google Scholar]
- 3.Mamdouh, Z., Chen, X., Pierini, L. M., Maxfield, F. R. & Muller, W. A. (2003) Nature 421, 748-753. [DOI] [PubMed] [Google Scholar]
- 4.Dejana, E. (2004) Nat. Rev. Mol. Cell. Biol. 5, 261-270. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Padura, I., Lostaglio, S., Schneemann, M., Williams, L., Romano, M., Fruscella, P., Panzeri, C., Stoppacciaro, A., Ruco, L., Villa, A., et al. (1998) J. Cell Biol. 142, 117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Maschio, A., De Luigi, A., Martin-Padura, I., Brockhaus, M., Bartfai, T., Fruscella, P., Adorini, L., Martino, G. V., Furlan, M., De Simoni, M. G., et al. (1999) J. Exp. Med. 190, 1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebnet, K., Suzuki, A., Ohno, S. & Vestweber, D. (2004) J. Cell Sci. 117, 19-29. [DOI] [PubMed] [Google Scholar]
- 8.Ostermann, G., Weber, K. S., Zernecke, A., Schroder, A. & Weber, C. (2002) Nat. Immunol. 3, 151-158. [DOI] [PubMed] [Google Scholar]
- 9.Ostermann, G., Fraemohs, L., Baltus, T., Schober, A., Lietz, M., Zernecke, A., Liehn, E. A. & Weber, C. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 729-735. [DOI] [PubMed] [Google Scholar]
- 10.Aurrand-Lions, M. A., Duncan, L., Du Pasquier, L. & Imhof, B. A. (2000) Curr. Topics Microbiol. Immunol. 251, 91-98. [DOI] [PubMed] [Google Scholar]
- 11.Aurrand-Lions, M., Duncan, L., Ballestrem, C. & Imhof, B. A. (2001) J. Biol. Chem. 276, 2733-2741. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham, S. A., Arrate, M. P., Rodriguez, J. M., Bjercke, R. J., Vanderslice, P., Morris, A. P. & Brock, T. A. (2000) J. Biol. Chem. 275, 34750-34756. [DOI] [PubMed] [Google Scholar]
- 13.Santoso, S., Sachs, U. J., Kroll, H., Linder, M., Ruf, A., Preissner, K. T. & Chavakis, T. (2002) J. Exp. Med. 196, 679-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang, T. W., Chiu, H. H., Gurney, A., Sidle, A., Tumas, D. B., Schow, P., Foster, J., Klassen, T., Dennis, K., DeMarco, R. A., et al. (2002) J. Immunol. 168, 1618-1626. [DOI] [PubMed] [Google Scholar]
- 15.Johnson-Leger, C. A., Aurrand-Lions, M., Beltraminelli, N., Fasel, N. & Imhof, B. A. (2002) Blood 100, 2479-2486. [DOI] [PubMed] [Google Scholar]
- 16.Cera, M. R., Del Prete, A., Vecchi, A., Corada, M., Martin-Padura, I., Motoike, T., Tonetti, P., Bazzoni, G., Vermi, W., Gentili, F., et al. (2004) J. Clin. Invest. 114, 729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dangerfield, J., Larbi, K. Y., Huang, M. T., Dewar, A. & Nourshargh, S. (2002) J. Exp. Med. 196, 1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harari, O. A., McHale, J. F., Marshall, D., Ahmed, S., Brown, D., Askenase, P. W. & Haskard, D. O. (1999) J. Immunol. 163, 6860-6866. [PubMed] [Google Scholar]
- 19.Frangogiannis, N. G., Smith, C. W. & Entman, M. L. (2002) Cardiovasc. Res. 53, 31-47. [DOI] [PubMed] [Google Scholar]
- 20.Anversa, P., Beghi, C., Kikkawa, Y. & Olivetti, G. (1985) Am. J. Pathol. 118, 484-492. [PMC free article] [PubMed] [Google Scholar]
- 21.Galli, D., Innocenzi, A., Staszewsky, L., Zanetta, L., Sampaolesi, M., Bai, A., Martinoli, E., Carlo, E., Balconi, G., Fiordaliso, F., et al. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 692-697. [DOI] [PubMed] [Google Scholar]
- 22.Lowell, C. A., Fumagalli, L. & Berton, G. (1996) J. Cell Biol. 133, 895-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worthylake, R. A., Lemoine, S., Watson, J. M. & Burridge, K. (2001) J. Cell Biol. 154, 147-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazzoni, G., Shih, D. T., Buck, C. A. & Hemler, M. E. (1995) J. Biol. Chem. 270, 25570-25577. [DOI] [PubMed] [Google Scholar]
- 25.He, R., Tan, L., Browning, D. D., Wang, J. M. & Ye, R. D. (2000) J. Immunol. 165, 4598-4605. [DOI] [PubMed] [Google Scholar]
- 26.Zicha, D., Allen, W. E., Brickell, P. M., Kinnon, C., Dunn, G. A., Jones, G. E. & Thrasher, A. J. (1998) Br. J. Haematol. 101, 659-665. [DOI] [PubMed] [Google Scholar]
- 27.Reffemann, T. & Kloner, R. A. (2004) J. Cardiovasc. Pharmacol. Ther. 9, 163-172. [DOI] [PubMed] [Google Scholar]
- 28.Khandoga, A., Kessler, J. S., Meissner, H., Hanschen, M., Corada, M., Motoike, T., Enders, G., Dejana, E. & Krombach, F. (2005) Blood 106, 725-733. [DOI] [PubMed] [Google Scholar]
- 29.Aurrand-Lions, M., Johnson-Leger, C., Wong, C., Du Pasquier, L. & Imhof, B. A. (2001) Blood 98, 3699-3707. [DOI] [PubMed] [Google Scholar]
- 30.Hansen, P. R. (1995) Circulation 91, 1872-1885. [DOI] [PubMed] [Google Scholar]
- 31.Van Haastert, P. J. & Devreotes, P. N. (2004) Nat. Rev. Mol. Cell. Biol. 5, 626-634. [DOI] [PubMed] [Google Scholar]
- 32.Weiner, O. D., Servant, G., Welch, M. D., Mitchison, T. J., Sedat, J. W. & Bourne, H. R. (1999) Nat. Cell Biol. 1, 75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T. & Horwitz, A. R. (2003) Science 302, 1704-1709. [DOI] [PubMed] [Google Scholar]
- 34.Bogen, S., Pak, J., Garifallou, M., Deng, X. & Muller, W. A. (1994) J. Exp. Med. 179, 1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan, G. S., Andrew, D. P., Takimoto, H., Kaufman, S. A., Yoshida, H., Spellberg, J., Luis de la Pompa, J., Elia, A., Wakeham, A., Karan-Tamir, B., et al. (1999) J. Immunol. 162, 3022-3030. [PubMed] [Google Scholar]
- 36.Loike, J. D., Cao, L., Budhu, S., Hoffman, S. & Silverstein, S. C. (2001) J. Immunol. 166, 7534-7542. [DOI] [PubMed] [Google Scholar]
- 37.Lawson, M. A. & Maxfield, F. R. (1995) Nature 377, 75-79. [DOI] [PubMed] [Google Scholar]
- 38.Shaw, S. K., Ma, S., Kim, M. B., Rao, R. M., Hartman, C. U., Froio, R. M., Yang, L., Jones, T., Liu, Y., Nusrat, A., et al. (2004) J. Exp. Med. 200, 1571-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandell, K. J., Babbin, B. A., Nusrat, A. & Parkos, C. A. (2005) J. Biol. Chem. 280, 11665-11674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.