Abstract
The in vitro cultivation of protozoan parasites of the genus Cryptosporidium has advanced significantly in recent years. These obligate, intracellular parasites colonize the epithelium of the digestive and respiratory tracts, are often difficult to obtain in significant numbers, produce durable oocysts that defy conventional chemical disinfection methods, and are persistently infectious when stored at refrigerated temperatures (4 to 8°C). While continuous culture and efficient life cycle completion (oocyst production) have not yet been achieved in vitro, routine methods for parasite preparation and cell culture infection and assays for parasite life cycle development have been established. Parasite yields may be limited, but in vitro growth is sufficient to support a variety of research studies, including assessing potential drug therapies, evaluating oocyst disinfection methods, and characterizing life cycle stage development and differentiation.
INTRODUCTION
The protozoan organisms of the genus Cryptosporidium are obligate, intracellular parasites that infect the epithelial cells lining the luminal surfaces of the digestive and respiratory tracts of a wide variety of hosts. Like related coccidian parasites, Cryptosporidium spp. feature asexual and sexual components in their life cycles and produce thick-walled, environmentally hardy oocysts. Unique features include the absence of a sporulation period outside the host (oocysts are infectious as they are shed in the stool), the lack of sporocysts within the oocyst (i.e., sporozoites are “naked” within the oocyst), and autoinfection (the life cycle recurs within the same host, a consequence of thin-walled oocyst production). Cryptosporidial oocysts are also small: those of Cryptosporidium parvum are approximately 4.5 to 5 μm in diameter. Although at least eight recognized species of Cryptosporidium are considered valid, human cryptosporidiosis is primarily attributed to C. parvum. Small numbers of infections with other Cryptosporidium spp. in humans have been reported, essentially restricted to immunocompromised persons (e.g., human immunodeficiency virus-infected patients with AIDS) (35, 42, 63).
Rapid advances in molecular biological and biochemical techniques and information make inevitable the recognition of new species, subspecies, and strains. Accordingly, biological and molecular differences noted among isolates of C. parvum infecting various mammals led to the recognition of two major groupings that infect humans: a zoonotic or genotype 2 (strain) capable of infecting a wide range of mammals, especially livestock, and an anthroponotic or genotype 1 strain that has been detected only in humans and has not been successfully (or at least consistently) propagated in other mammalian hosts (8, 41, 59).
First described in mice by Tyzzer in 1907 (51), Cryptosporidium species were not recognized as important etiologic agents of human disease until the early 1980s. Previous studies documented their occurrence in livestock, but even veterinary interest was not piqued until cryptosporidiosis was recognized as a significant cause of morbidity and even mortality in AIDS patients. Cryptosporidiosis occurs worldwide and affects a wide range of mammals, avians, reptiles, and fish. Many outbreaks of human cryptosporidiosis have been attributed to contaminated drinking water, recreational water, and food. Environmentally hardy cryptosporidial oocysts are remarkably resistant to chemical disinfection (e.g., drinking water chlorination) but are susceptible to temperature extremes of freezing and heat (pasteurization). Infections in immunocompetent persons are generally short-lived, while immunocompromised (especially those with AIDS) persons may develop chronic, life-threatening conditions (20, 23, 39).
HISTORICAL BACKGROUND
The following section will summarize key publications introducing particular cell lines or models and modifications that encouraged the adoption of useful Cryptosporidium culture systems (Tables 1 and 2). In 1983, Woodmansee and Pohlenz described the first successful in vitro culture of Cryptosporidium sp. asexual life cycle stages (61). The authors employed a bovine isolate collected from the feces of experimentally infected calves (stored in 2% aqueous potassium dichromate [K2Cr2O7]). Oocysts purified by repeated sucrose flotation (1.18 g/cm3, 1,200 × g, 5 min) were preincubated in a reducing environment (0.02 M cysteine hydrochloride in 0.85% sodium chloride and 50% CO2 in air) and suspended in an excystation fluid (0.75% sodium taurocholate and 0.25% trypsin in phosphate-buffered saline [PBS, 0.01 M, pH 7.2]) for 30 min before being inoculated onto host cells. Human rectal tumor (HRT) cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 37°C and 5% CO2. Cells were passaged into Leighton tubes and incubated for 48 h before oocyst/sporozoite inoculation. Bright-field and electron microscopy revealed asexual stage development, but no evidence of sexual stages through 3 days postinoculation. Indirect immunofluorescence with immune human sera revealed distinctly reactive life cycle stages.
TABLE 1.
Cell culture abbreviations, acronyms, descriptions, and definitions
| Abbreviation | Name definition or use |
|---|---|
| BFTE | Bovine fallopian tube epithelial cells |
| BHK | Baby hamster kidney cells |
| Caco-2 | Human colonic adenocarcinoma cells |
| CAM | Chorioallantoic membrane |
| HCT-8 | Human colonic tumor (human ileocecal adenocarcinoma) cells |
| HFL | Human fetal lung cells |
| HRT | Human rectal tumor cells |
| HT29.74 | Human colon adenocarcinoma cells |
| Intestine 407 | Human embryonic intestine cells |
| L929 | Mouse fibroblast cells |
| LGA | Lewis rat small intestine carcinoma cells |
| MDBK | Madin-Darby bovine kidney cells |
| MDCK | Madin-Darby canine kidney cells |
| PCK | Primary chicken kidney cells |
| PK-10 | Porcine kidney cells |
| RL95-2 | Human endometrial carcinoma cells |
| T84 | Human colon carcinoma cells |
| Ficoll | Neutral, highly branched, hydrophilic polymer of sucrose used in gradient purification methods |
| K2Cr2O7 | Potassium dichromate (2.5% aqueous working concentration for storage of oocysts at 4°C) |
| Leighton tube | Glass culture tube with a flattened side in which a cover glass can be positioned to support cell growth |
| Percoll | Polyvinylpyrrolidone-coated silica particles (15-30 nm in diameter) used in gradient purification methods for cells |
TABLE 2.
Selected chronology of efforts to cultivate Cryptosporidium spp. in vitroa
| Cell line (ATCC no.) | Oocyst source(s) |
Cryptosporidium life cycle stage developmentb
|
Reference(s) | ||
|---|---|---|---|---|---|
| Asexual | Sexual | Oocysts | |||
| HRT | Bovine | Yes | ND | ND | 61 |
| Chicken CAM | Human, bovine | Yes | Yes | Yes | 16 |
| Human, passaged in a goat | Yes | NR | NR | 36 | |
| Chicken (C. baileyi) | Yes | Yes | Yes | 31 | |
| HFL | Human, passaged in a goat | Yes | Yes | Yes | 15 |
| PCK, PK-10 | Human, passaged in a goat | Yes | Yes | Yes | 15 |
| BHK (CRL-1632) | Human, passaged in a goat | Yes | NR | NR | 36 |
| MDCK (CCL-34) | Caprine | Yes | NR | NR | 32 |
| Bovine | Yes | Yes | Yes* | 25 | |
| Bovine | Yes | Yes | Yes | 47 | |
| Bovine | Yes | Yes | Yes* | 6 | |
| Caco-2 (HTB-37) | Human | ? | ? | Yes | 17 |
| Human, bovine | Yes | NR | NR | 24 | |
| Primary rat hepatocyte | Human | Yes | Yes | Yes | 17 |
| L929 (CCL-1) | Human, bovine | Yes | Yes | NR | 34 |
| LGA | Human, bovine | Yes | NR | NR | 10 |
| HT29.74 (HTB-38) | Human | Yes | ? | NR | 22 |
| Intestine 407 (CCL-6) | Bovine | Yes | NR | NR | 30 |
| RL95-2 (CRL-1671) | Bovine | Yes | Yes | ? | 43 |
| T84 (CCL-248) | Bovine | Yes | Yes | NR | 1 |
| BFTE | Bovine | Yes | Yes | Yes | 64 |
| MDBK (CCL-22) | Bovine | Yes | Yes | Yes | 25, 57 |
| HCT-8 (CCL-244) | Bovine | Yes | Yes | Yes | 38, 52-55 |
See Table 1 for descriptions of cell lines and abbreviations. See text for culture details.
NR, not reported; ND, not detected; ?, not clear from description; Yes*, immature and possible mature forms.
Current and Long described the development of Cryptosporidium spp. from humans and calves in the endoderm cells of the chorioallantoic membrane (CAM) of chicken embryos maintained at 37°C (16). There were no morphological differences in the developmental stages of the human and calf isolates grown in chicken embryos, and oocysts collected from the CAM were infectious for suckling mice. Despite this initial report of success in cultivating human and calf Cryptosporidium isolates, no reports completely replicated the results.
In 1984, Current and Haynes described the successful cultivation of a human Cryptosporidium isolate in human fetal lung (HFL), primary chicken kidney (PCK), and porcine kidney (PK-10) cells (15). While all three cell lines supported the development of the entire life cycle, the HFL cells gave the greatest yield. Oocysts were derived from an AIDS patient or from goats inoculated with the same isolate. The fact that the goat became infected suggests that this isolate was zoonotic, as opposed to isolates that are human specific (anthroponotic). Oocysts were stored in 2.5% K2Cr2O7 at 4°C, purified by Sheather's flotation, washed with PBS, incubated with antibiotics at 37°C (penicillin, streptomycin, and amphotericin B, to kill microbial contaminants), and excysted for 30 to 60 min at 37°C in PBS containing 0.75% sodium taurocholate and 0.25% trypsin. The excysted mixture of oocysts, oocyst walls, and free sporozoites was washed with PBS, suspended in growth medium, and inoculated onto cells in Leighton tubes at approximately 1.5 × 104 sporozoites/tube (5 cm2). Culture medium consisted of minimal essential medium (MEM) with Earle's salts, 10% FBS, l-glutamine, penicillin (100 IU/ml), streptomycin (100 μg/ml), and amphotericin B (0.25 μg/ml). Preparations were maintained at 37°C in 5% CO2. The authors reported the presence of type I meronts at 96 h, which they interpreted as evidence for asexual stage recycling in culture. Although this report inspired a number of laboratories to pursue HFL cells as a model for Cryptosporidium cultivation, anecdotal reports suggest that the attempts to replicate the success of the original study were disappointing.
Naciri and colleagues reported the successful growth of a human Cryptosporidium isolate in baby hamster kidney (BHK) cells and chicken embryos (36). Like the isolate used in the previous report, this isolate was originally obtained from an immunocompromised patient and propagated in goats, hence a zoonotic strain. Unlike the previous report of cultivation in chicken embryos, the authors reported the serial transfer of the isolate at weekly intervals. After 45 passages, there were mostly free sporozoites and merozoites in the chorioallantoic fluid. The report identified the isolate as Cryptosporidium muris (51), but the parasite described is morphologically consistent with C. parvum.
The cultivation of Cryptosporidium baileyi (AU-B1 isolate) in chicken embryos was reported by Lindsay and colleagues in 1988 (31). Oocyst-laden chicken feces were sieved and stored in 2.5% K2Cr2O7 at 4°C. Subsequently, oocysts were purified by Sheather's flotation, washed with distilled water, and stored in Hanks' balanced salts solution (HBSS) at 4°C until sanitized by suspension in 0.26% sodium hypochlorite. Residual hypochlorite was removed by washing with HBSS before oocyst inoculation into chicken embryos (4 × 105 oocysts/embryo). Embryos were incubated at 40°C up to 6 days (for de novo oocyst recovery). Embryo-generated oocysts were treated with sodium hypochlorite (0.5%, 4°C, 5 min), stored in HBSS (supplemented with 100 IU of penicillin and 100 μg of streptomycin per ml) at 4°C, and used to serially propagate the isolate through 20 embryo passages. Efforts to cultivate the C. baileyi isolate in vitro failed in multiple avian and mammalian primary and continuously growing cell lines (16 total).
Cryptosporidial sporozoite attachment to and invasion of MDCK cells were reported by Lumb and colleagues in 1988 (32). The authors used a Cryptosporidium isolate obtained from a naturally infected goat kid. Diarrheic goat feces were stored in 1% K2Cr2O7 at 4°C, treated with Sputolysin (Boehringer Ingelheim, Ridgefield, Conn.), subjected to ether sedimentation, and further purified with discontinuous Ficoll (Pharmacia, Piscataway, N.J.)-sodium diatrizoate gradients. MDCK cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and HEPES. Electron microscopy revealed numerous asexual stages (meronts) at 48 h postinfection.
Datry and colleagues reported the complete life cycle development of a human (AIDS-related) Cryptosporidium isolate in human colonic adenocarcinoma (Caco-2) cells and primary rat hepatocyte cells (17). Human stool was stored in 2.5% K2Cr2O7. Oocysts were purified via Sheather's flotation, washed with distilled water, sanitized with 1.75% sodium hypochlorite for 1 h, excysted in trypsin-EDTA-taurocholate at 37°C for 1 h, washed, suspended in DMEM or RPMI, and inoculated onto host cells (105 sporozoites/ml). Caco-2 cells (DMEM, 20% FBS) and primary rat hepatocytes (MEM, 10% FBS) performed best for parasite development. Asexual, sexual, and oocyst stages were observed in hepatocyte cultures. Only oocysts were clearly identified in Caco-2 cultures, but other unidentified developing stages were observed.
Mouse L929 fibroblasts were used to cultivate bovine and human isolates of Cryptosporidium spp. for the purpose of testing antimicrobial agents for activity against the in vitro development of the parasite (34). McDonald and colleagues (34) diluted stool specimens with water, sieved, and, when necessary, defatted the samples by ether sedimentation. The sediment was processed through saturated sodium chloride or Sheather's flotation, and the recovered oocysts were washed with water and stored in 2.5% K2Cr2O7 at 4°C. Purified oocyst preparations were washed with HBSS, treated with 0.5% trypsin (in HBSS) at 37°C for 30 min, excysted in 0.4% sodium taurocholate at 37°C for 45 min, washed with culture medium (RPMI 1640), and inoculated onto cells at 105 oocysts/culture chamber (24-well plate well or well of eight-chambered slide). Cells were incubated for 24 h at 37°C in candle jars, washed with HBSS, fixed with methanol, and stained with Giemsa stain. The authors noted mostly asexual stages, with modest numbers (less than 10%) of sexual stages at 72 h. Parasite numbers peaked between 48 and 72 h, and no discernible evidence of developmental stage recycling was apparent.
Bonnin and colleagues described the development of asexual stages of Cryptosporidium spp. of bovine and human origin in LGA cells (small intestine carcinoma from Lewis rats) (10). Human and bovine feces were stored in 2.5% K2Cr2O7 at 4°C. Oocysts were purified via flotation on saturated sodium chloride, sanitized by treatment with sodium hypochlorite, excysted in 0.04% sodium taurocholate (in Ham's F10 medium), washed, and inoculated onto cells at 2 × 104 sporozoites/0.5 cm2. Developing stages were visualized after methanol fixation and Giemsa staining or fixation for transmission electron microscopy.
Differentiated and undifferentiated HT29.74 human colon adenocarcinoma cells were compared for their potential support of C. parvum growth (22). The human (AIDS-related) oocyst sources that Flanigan and colleagues (22) used for their studies were stored in 2.5% K2Cr2O7 at 4°C, sieved (40-gauge stainless steel), purified over saturated sodium chloride shelf gradients, sieved through nylon mesh (30 nm), sanitized with 0.05% sodium hypochlorite (10 min), suspended in an antibiotic mixture (100 IU of penicillin, 0.1 mg of streptomycin, and 0.2 mg of neomycin per ml), and stored at 4°C before inoculation onto HT29.74 cells (maintained at 37°C and 5% CO2). Undifferentiated HT29.74 cells were maintained in RPMI 1640 containing 10% dialyzed FBS, 24 mM sodium bicarbonate, 1 mM sodium pyruvate, 20 mM HEPES, 2 mM l-glutamine, penicillin, and streptomycin, while differentiated HT29.74 cells were maintained in Leibovitz L-15 medium with 10% FBS, 5 mM galactose, 6 mM pyruvate, 1 mM l-glutamine, 20 mM HEPES, penicillin, and streptomycin. Oocysts were inoculated at 105 to 106/cm2 onto host cells. Slide chamber-grown cultures were fixed and stained with hematoxylin and eosin and examined by bright-field microscopy. Differentiated HT29.74 cells supported fivefold-higher levels of parasite development compared to undifferentiated cells. The reported data suggest that the infection efficiency was low and life cycle development was essentially restricted to asexual stages through 72 h postinfection. The model was later used by Wiest and colleagues to show the importance of microtubules in host cell invasion and parasite development (60). Treatment in vitro with either colchicine or vinblastine significantly reduced parasite numbers in a dose-dependent manner.
Gut and colleagues compared 15 cell lines for their potential support of C. parvum growth in vitro at 37°C and 6% CO2 (25). Although complete data were not presented, no growth was observed in 3 cell lines and 11 cell lines supported poor development (less than 0.1% infection), but MDCK and MDBK cells became heavily infected (up to 90%). The authors used a bovine oocyst source (AUCP-1) that was sieved, purified by sucrose density gradient centrifugation, sanitized with undiluted commercial bleach (5% sodium hypochlorite) for 5 min at 4°C, washed with RPMI 1640 medium, and stored at 4°C until used. Sporozoites were excysted by suspending oocysts in 0.75% sodium taurocholate under reduced oxygen, passed through 3-μm polycarbonate filters, suspended in culture medium, and inoculated onto host cells. The optimal culture medium reported for MDCK cells was composed of RPMI 1640, 10% FBS, 2.5 g of sodium bicarbonate per liter, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, 25 mM HEPES, nonessential amino acids, 0.24 U of insulin per ml, 13.6 mg of hypoxanthine per liter, 2.9 mg of thymidine per liter, 50 μM 2-mercaptoethanol, 0.1% yeast extract, 1% Ficoll 400, 4.5 g of glucose per liter, FBS (reduced to 1% during parasite development), and 0.1% rabbit bile. Parasite development through sexual stages, including immature oocysts (partial oocyst wall formation) was observed.
While other publications done around the time of the study by Gut and colleagues (25) were encouraging, the success reported in this study provided the impetus for several other laboratories to pursue the in vitro culture of Cryptosporidium spp. more aggressively. The utility of this model for evaluating antibody-mediated life cycle modulation was reported by Doyle and colleagues (18).
Kuhls and colleagues reported the utility of human embryonic intestine 407 cells (ATCC CCL-6) in studies of the attachment and invasion of sporozoites from a bovine C. parvum isolate (IOWA), but only reported data from 12 to 18 h postinfection (30). Clear development of asexual stages (meronts or schizonts) was apparent, and treatment with carbohydrates and/or lectins influenced the outcome of infection.
Rasmussen and colleagues described the development of a bovine C. parvum isolate in the human endometrial carcinoma cell line RL95-2 (43). Optimal parasite development was observed in RL95-2 cells cultured for 7 days before parasite inoculation on cover glass disks in a 1:1 mixture of DMEM and Ham's F12 medium supplemented with 10% FBS, 2 g of sodium bicarbonate per liter, 10 mM HEPES, 5 μg of bovine insulin, 100 IU of penicillin G per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphotericin B per liter. Oocysts were treated with 2% sodium hypochlorite (bleach), washed five times with PBS, excysted at 37°C in 0.75% sodium taurocholate and 0.25% trypsin (in PBS), washed with PBS, suspended in culture medium, filtered through 3-μm polycarbonate filters (not indicated whether these were absolute or nominal porosity filters), and inoculated onto host cells. After variable culture periods, cells on cover glass disks were washed in phosphate buffer, fixed with Bouin's fixative for 2 h, washed with ethanol (five changes in 1 h), and stained with Giemsa stain. The authors described apparent asexual and sexual stages under bright-field microscopy and possible oocysts via scanning electron microscopy.
While no comparative data were presented, Rosales and colleagues concluded that MDCK cells supported C. parvum development better than Vero, HeLa, and McCoy cells (47). The authors used oocysts derived from infected calves. MDCK cells were cultured under 5% CO2 at 37°C in MEM supplemented with 10% FBS. Excysted sporozoites were inoculated onto MDCK cells (12- to 24-h nonconfluent cultures grown on glass cover slips) in MEM without FBS. The inoculum was replaced with MEM plus FBS for periods of up to 120 h. The MDCK cells grew naturally polarized directly on cover glass disks in culture plates or on permeable membrane supports. Cultures on glass disks were removed, fixed with acetone (−20°C), and stained in order with Alcian blue and Giemsa stains. The authors described all life cycle stages under bright-field microscopy, including asexual stages persisting through 120 h postinoculation, perhaps indicating asexual stage recycling. Researchers from the same laboratory also described the development of a bovine isolate of C. parvum in unsensitized mouse peritoneal macrophages, further emphasizing the ability of this parasite to infect nonepithelial cells (33). The persistence of the life cycle stages in macrophages was interpreted as evidence of parasite escape from lysosomal degradation.
Arrowood and colleagues introduced a modified MDCK culture system for use in evaluating potential anticryptosporidial agents and to study C. parvum life cycle stage development (6). With a bovine-derived isolate of C. parvum (IOWA), MDCK and Caco-2 cells were cultivated under 5% CO2 at 37°C in conventional FBS-supplemented high-glucose DMEM culture medium and compared with MDCK cells cultivated in a synthetic, serum-free culture medium (Ultraculture; BioWhittaker, Walkersville, Md.). The MDCK/Ultraculture-based model outperformed the MDCK (two- to fivefold) and Caco-2 (2- to 20-fold) models with serum-supplemented DMEM in cryptosporidial development. Detailed culture conditions are presented below. The model has been used to evaluate potential anticryptosporidial agents (4, 6, 65-67).
Adams and colleagues used colonic epithelial (T84) cells to examine the role of bovine C. parvum infection on host cell membrane integrity and the modulation of macromolecular flow (1). Mature, polarized T84 cells were cultivated under 5% CO2 at 37°C (preincubated for 7 days to facilitate polarization and differentiation) in a 1:1 mixture of DMEM and Ham's F12 medium supplemented with 5% FCS, 14 mM sodium bicarbonate, 15 mM HEPES, penicillin, and streptomycin. Successful infection was achieved with 105 oocysts, and the alteration of epithelial barrier function was correlated with oocyst dose. The authors concluded that C. parvum was responsible for disrupting the epithelial cell barrier, not simply opening transcellular channels for flow. Asexual and sexual life cycle stages were identified in cultures by light and electron microscopy.
In a related study, Griffiths and colleagues examined the impact of cryptosporidial infection on Caco-2 cells grown on permeable membrane supports (24). Two isolates of C. parvum (human and bovine) were inoculated onto Caco-2 and Caco-2a cells grown under 5% CO2 at 37°C in DMEM supplemented with 20% FBS and 3.5 g of glucose per liter. Transmonolayer resistance (as a measure of membrane integrity and permeability) declined in a dose- and time-dependent manner following parasite inoculation. Molecules smaller than 1 kDa leaked through the monolayers, while proteins of 1.8 kDa or larger were excluded. Cellular lactate dehydrogenase was released into the culture medium on the apical side of the monolayer (where the parasites are located), but not on the basal side. Propidium iodide uptake by infected cells was interpreted as evidence that cryptosporidial infection killed host cells. Results did not achieve significance until data for both human and bovine isolates were pooled; either data set alone was not significant.
Primary bovine fallopian tube epithelial cells were described by Yang and colleagues as efficient hosts for a bovine C. parvum isolate (64). The authors described the examination of cells grown on cover slips following Giemsa staining and examination by scanning and transmission electron microscopy. Mice inoculated with infected monolayers developed patent cryptosporidial infections. The authors concluded that the monolayers contained de novo mature, infectious oocysts, because ethanol treatment of the monolayer did not prevent infection (life cycle stages other than oocysts were presumably killed by the ethanol treatment). The lengthy description of host cell isolation, establishment in culture, and electron microscopy methods is not summarized here, and the reader is directed to the original publication for complete details.
Upton and colleagues have published several studies examining the role of atmospheric conditions and medium composition on the performance of various cell lines supporting C. parvum in vitro development (19, 38, 52-57, 62). Ultimately, human colonic tumor (HCT-8) cells have performed best for these investigators when cultivated under 5% CO2 at 37°C in RPMI 1640 basal medium supplemented with 2 mM l-glutamine, 15 mM HEPES buffer, 50 mM glucose, 35 μg of ascorbic acid per ml, 4.0 μg of para-aminobenzoic acid per ml, 2.0 μg of calcium pantothenate per ml, and 1.0 μg of folic acid per ml. The medium was filter sterilized after adjusting the pH to 7.4, and 10% FBS was added. When needed, recommended antibiotic supplements were 100 U of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B per ml.
CRYOPRESERVATION
Despite promising studies, no validated methods exist for cryopreserving C. parvum oocysts or sporozoites (21, 48). A variety of standard conditions have been tried, including the use of dimethyl sulfoxide, glycerol, and an extracellular matrix (hydroxyethyl starch) previously reported to be useful for cryopreserving Plasmodium spp. (29), without success. Attempts to cryopreserve infected cell cultures have similarly failed (data not shown). This methodologic deficiency continues to impede the establishment of standardized cryptosporidial isolates or cloned stocks.
OOCYST SOURCES FOR IN VITRO CULTIVATION STUDIES
Although no cloned stocks of C. parvum isolates are yet available, oocysts can be obtained from several research or commercial sources. Given the typical C. parvum-like morphology, bovine origin, or livestock passage, it is probably safe to say that most of the in vitro cultivation studies used zoonotic strains of C. parvum. The use of other strains or species has been limited, but in general studies employed oocysts derived from clinical isolates. Workers in my laboratory have inoculated MDCK, Caco-2, and HCT-8 cells with both anthroponotic and zoonotic strains of C. parvum and observed successful development through to sexual stages (6). While partial to complete oocyst wall formation was evident in these cultures, significant development of mature oocysts has not been seen. Short- and long-term growth of zoonotic and anthroponotic isolates has been reported in HCT-8 cells (28).
STOOL COLLECTION AND PROCESSING FOR OOCYST RECOVERY
Cryptosporidium oocysts for in vitro cultivation studies have primarily come from clinical human or veterinary stool samples or from experimentally infected laboratory animals or livestock. Zoonotic C. parvum oocysts can be generated in small to moderate numbers in rodent animal models, but large-scale production is generally accomplished in neonatal livestock, especially bovine calves and goat kids (3, 5, 14, 52). Workers in my laboratory have introduced a number of improvements in the production and purification process for calf-derived oocysts to minimize labor, consumption of materials, and generation of hazardous waste (3). Stool specimens may be collected fresh into tap water or saline or suspended in a storage medium composed of aqueous K2Cr2O7 (2.5% [wt/vol] final concentration), often accomplished by mixing equal volumes of liquid stool with 5% (wt/vol) aqueous K2Cr2O7. The latter approach is attractive in that oocysts remain infectious for extended periods (>6 months) as long as the stool is stored at 4 to 8°C. An additional advantage is that the K2Cr2O7 effectively kills most viruses, vegetative bacteria, and fungi and significantly reduces offensive fecal odors.
Because oocysts are resistant to routine disinfection and remain infectious for extended periods, laboratory manipulation of these agents should be conducted under level 2 biological hazard conditions (2, 9, 11, 40). Human stool specimens as sources of oocysts are often defatted with organic solvents (especially diethyl ether or ethyl acetate) before further processing in order to enhance oocyst recovery and purity (14). Stools containing high levels of mucus may benefit from treatment with reducing agents (e.g., Sputolysin) or potassium hydroxide (0.1%) treatment at room temperature for 10 min, followed by centrifugation (1,500 × g, 10 min). Otherwise, oocysts may not adequately sediment from the stool suspension. Fat removal and mucus dissolution may shorten the shelf life (viability or infectivity) of the oocysts (data not shown). Symptomatic cryptosporidial infections in humans are usually associated with substantial oocyst numbers in stool samples (13). However, intermittent and variable oocyst excretion patterns were reported among symptomatic and asymptomatic individuals experimentally infected with C. parvum (12). The latter finding illustrates the difficulty often encountered when trying to obtain substantial quantities of human-derived oocysts for experimental study.
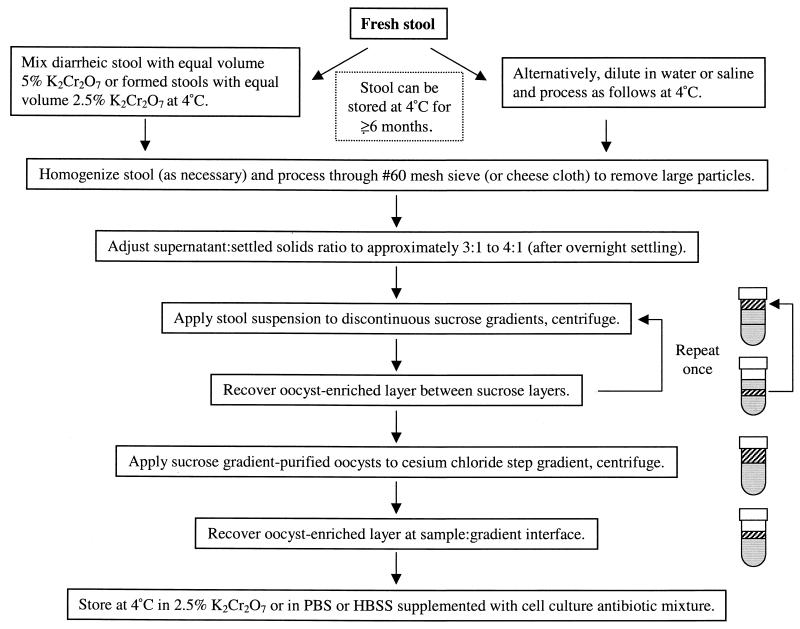
This laboratory routinely process K2Cr2O7-stabilized stool through 20- and 60-mesh stainless steel sieves to remove large debris. The sieved stool sample is applied to discontinuous sucrose gradients (scaled according to the available volume of sieved stool sample) essentially as described previously (3, 5). Large-scale gradients are prepared in 200-ml polycarbonate bottles by layering 80 ml of a 1.103-specific gravity sucrose solution beneath 80 ml of a 1.064-specific gravity sucrose solution. The respective solutions are prepared by diluting Sheather's solution (500 g of sucrose, 320 ml of H2O, 9 ml of aqueous phenol [85%]) into PBS and adding Tween 80. The 1.103-specific gravity sucrose solution contains 300 ml of Sheather's solution, 600 ml of PBS, and 9 ml of Tween 80; the 1.064-specific gravity sucrose solution contains 200 ml of Sheather's solution, 800 ml of PBS, and 9 ml of Tween 80. Forty milliliters of the sieved stool sample are overlaid onto the sucrose gradients, and the bottles are centrifuged at 1,000 × g for 25 min. The top layer is discarded (approximately 60 ml), and the oocyst-enriched layer occupying the interface between the sucrose layers (approximately 100 ml) is transferred to conical 175-ml polycarbonate or polypropylene centrifuge bottles (Nalgene). The oocyst-containing fraction is diluted to 175 ml with saline, and the bottles are centrifuged at 1,500 × g for 10 min. Two pellets (bottles) are pooled, washed with saline (1,500 × g, 10 min), and resuspended in 40 ml of K2Cr2O7. Secondary gradients are prepared and processed as described above, except the sample overlaid is the 40-ml primary gradient-purified oocyst fraction. The pellets collected from the secondary gradients are resuspended to 40 ml in K2Cr2O7 and stored at 4°C.
Final oocyst purification is accomplished with a simple, microscale cesium chloride (CsCl) gradient technique (3). The secondary sucrose gradient-purified oocyst fractions are centrifuged (1,500 × g, 10 min) and resuspended in 3 ml of saline. Siliconized 1.7-ml polypropylene microcentrifuge tubes are filled with 750 μl of cesium chloride (specific gravity 1.15 = 21.8 g of CsCl, 103.3 ml of deionized H2O). Oocyst suspension aliquots (500 μl) are carefully layered over the CsCl solution and centrifuged at 16,000 × g (approximately 13,200 rpm) for 3 min in a standard microcentrifuge. The upper 1-ml layers are collected, transferred to clean, siliconized 1.7-ml microcentrifuge tubes, diluted to 1.5 ml with saline, and centrifuged (16,000 × g, 3 min). Pellets from six tubes, corresponding to the original secondary oocyst fraction, are pooled, washed a second time with saline, resuspended in 1 ml of 2.5% K2Cr2O7, and stored at 4°C. At this point, oocysts are essentially free of gross debris and contaminating microorganisms. The few remaining bacteria are usually nonviable carcasses. Figure 1 summarizes the oocyst purification process.
FIG. 1.
Flow chart summarizing the purification and preparation of Cryptosporidium oocysts for use in cell culture studies.
Purified sporozoites can be obtained by Percoll gradient separation (5, 37, 52), cellulose ion exchange column chromatography (37, 44, 52), or simple filtration through 2- or 3-μm polycarbonate track etch membranes (14, 25, 66). A caveat regarding purifying cryptosporidial sporozoites is that they are somewhat fragile and perform best when handled least. Indeed, in my laboratory, excystation in advance of purification is best accomplished with synthetic sodium taurocholate, as crude taurocholate preparations quickly cause sporozoites to “round up” or appear “stumpy” and presumably interfere with invasion potential.
ROUTINE IN VITRO CULTIVATION
Oocyst and Sporozoite Preparation for Inoculation onto Cell Cultures
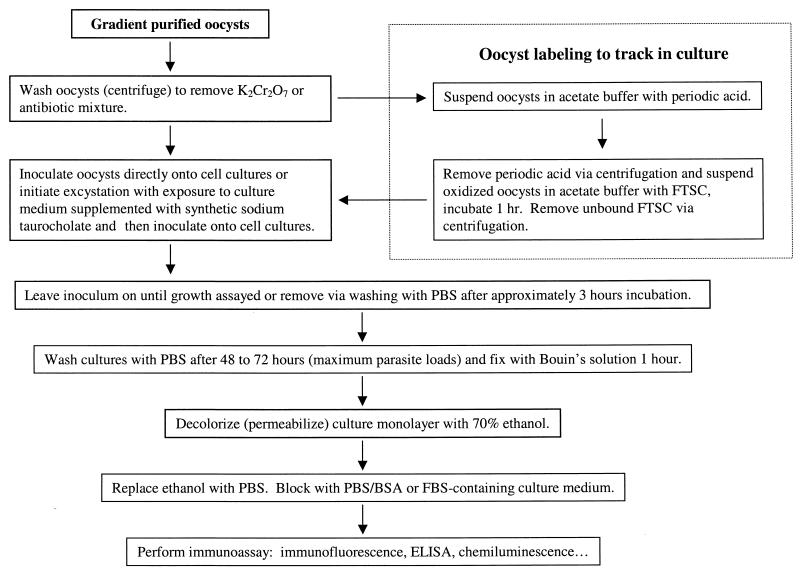
Figure 2 summarizes the process of establishing cryptosporidial infections in cell cultures with cryptosporidial oocysts (or purified sporozoites). In vitro culture is established by inoculating whole oocysts, mixtures of intact oocysts, excysted oocysts, and free sporozoites, or purified sporozoites. Each approach can achieve infection, but the efficiency of infection may be negatively impacted by oocyst age, excystation efficiency, or sporozoite integrity following purification. Excystation will often proceed in the cell culture medium soon after inoculation onto host cells, even without specific treatment to trigger it. Traditionally, oocysts have been suspended in a trypsin and sodium taurocholate solution (0.25% and 0.75%, respectively) prepared in a buffered salt solution (e.g., PBS or HBSS), sometimes simultaneously or in ordered sequence. Unless the oocyst source is old (>6 months), I prefer to use 0.75% synthetic sodium taurocholate alone in DMEM basal medium, incubating at 37°C for 10 to 15 min (as a trigger) or for 45 to 60 min for total sporozoite release.
FIG. 2.
Flow chart summarizing the in vitro culture of Cryptosporidium spp. and preparation of infected cell monolayers for parasite life cycle stage analysis and quantification.
Some investigators treat purified oocysts with sodium hypochlorite (dilute commercial bleach) at a 1:10 dilution (or even undiluted) for approximately 10 min at 4°C. The bleach is washed out (or neutralized with 0.1% sodium thiosulfate), and the oocysts are inoculated onto host cells. The bleach treatment triggers excystation (or at least may enhance overall excystation rates) and can provide additional sanitization of the oocyst sample in order to prevent unwanted microbial contamination of the host cells. I have not found the bleach step necessary in my studies and actively avoid it when performing disinfection or survival studies, as the bleach treatment may impact (damage) the disinfectant-weakened oocysts. Most oocyst preparations processed and stored in the presence of 2.5% K2Cr2O7 rarely have viable microbial contaminants if aseptic techniques are used during the postpurification handling of the samples.
Oocyst Labeling To Differentiate Inocula from Developing Parasites
A very simple technique for labeling inoculated oocysts facilitates differentiating the inoculated oocysts, oocyst walls, and contaminating organic debris (e.g., microbial carcasses) from de novo parasite life cycle stages (3). To minimize oocyst losses, I use siliconized microcentrifuge tubes throughout the method. Briefly, sucrose and cesium chloride gradient-purified oocysts are washed with acetate buffer (0.1 M acetate [pH 5.5] supplemented with 0.85% NaCl), suspended in acetate buffer supplemented with sodium periodate (10 mM, prepared fresh) for 20 min on ice, microcentrifuge washed (3 min, as above) with PBS-bovine serum albumin (BSA) (0.1 M PBS supplemented with 0.1% BSA), and suspended in 1 ml of acetate buffer supplemented with 150 μg of fluorescein thiosemicarbazide (FTSC; catalog no. 46985; Sigma Chemical Company, St. Louis, Mo.; 10× concentrate prepared fresh in dimethyl sulfoxide) per ml for 1 h at room temperature, protected from light. Unbound FTSC is removed by washing twice in PBS-BSA in the microcentrifuge. FTSC is bound to the periodic acid-oxidized carbohydrates, yielding highly fluorescent oocyst walls, bacteria, and other carbohydrate-rich organic debris. Sporozoites within oocysts remain unlabeled. By selecting appropriate detecting reagents and fluorochromes, the labeled inocula are easily distinguished from developing parasites by fluorescence microscopy.
Host Cell Preparation and Recommended Parasite Inoculum Densities
MDCK or HCT-8 cells are split and inoculated into appropriate culture chambers at ratios that will yield confluent monolayers within 48 to 96 h (I routinely use the latter time frame). Oocysts are generally inoculated at a ratio of 500 to 1,000 per cm2 of host cell monolayer (see Table 3 for ratios used with a variety of culture containers). Inoculum densities much higher than these promote monolayer damage and sloughing. I routinely observe an infection efficiency of approximately 5 to 10%, based on the numbers of oocysts inoculated, the excystation rate, the typical yield of sporozoites, and the common observation that not all sporozoites will attach and invade host cells (some attach and detach without invading). Parasite numbers reach a maximum approximately 48 to 72 h after inoculation. A general rule of thumb is that developing stage numbers will equal (or roughly double) the number of original oocysts or sporozoites in the inoculum. Some investigators observe increased infection efficiencies if the original inocula are gently centrifuged onto the host cell monolayers (58).
TABLE 3.
Recommended oocyst and sporozoite inoculum densities for various culture chambers and flasks
| Container | Unit | Dimensions (cm) | Area (cm2) | Medium vol (ml) | No. of sporozoites or oocysts |
|---|---|---|---|---|---|
| 1-well slide | Well | 2.0 × 4.3 | 8.6 | 4-5 | 1.5 × 106 |
| 2-well slide | Well | 2.1 × 2.1 | 4.4 | 1.0-1.5 | 0.5 × 106-1.0 × 106 |
| 4-well slide | Well | 0.9 × 2.1 | 1.9 | 0.4-0.5 | 1.5 × 106-2.0 × 105 |
| 8-well slide | Well | 0.9 × 0.9 | 0.8 | 0.3-0.4 | 1.5 × 105 |
| Leighton tube | Tube | 0.9 × 5.5 | 5.0 | 2-3 | 1.0 × 106 |
| 24-well plate | Well | 1.5 (diam) | 1.8 | 1-2 | 3.5 × 105 |
| 6-well plate | Well | 3.6 (diam) | 10.2 | 3-4 | 2 × 106-4 × 106 |
| Costar insert | Well | 2.4 (diam) | 4.5 | 1.0-1.5 | 0.5 × 106-1.0 × 106 |
| Small flask | Flask | 5 × 5 | 25 | 5-6 | 3.0 × 106-5.0 × 106 |
| Large flask | Flask | 7.5 × 10 | 75 | 15-20 | 1.0 × 107-1.5 × 107 |
Parasite Enumeration in Culture
Parasite development can be assessed with a variety of tools, including direct and immunofluorescence microscopy (4, 6, 49, 50), enzyme immunoassay (57, 62, 65, 67), and PCR assays (45, 46). Incorporation of exogenous uracil as a measure of parasite development was reported (56) but has not been successfully implemented by independent laboratories (unpublished data). I routinely use MDCK cells for immunofluorescence in cover glass-bottomed culture chambers (Nunc Lab-Tek, Rochester, N.Y.) because the cells grow flat and the developing parasites are found in a narrow focal plane, facilitating image capture for subsequent software-assisted analysis. HCT-8 cells, however, grow much more three-dimensionally (hills and valleys), complicating image capture-based microscopic analyses. However, both cell lines are well suited for real-time microscopy and for nonmicroscopic assays.
Immunofluorescence Identification of Parasites in Cell Cultures
A variety of antibody reagents (both polyclonal and monoclonal) have been used for immunofluorescence visualization of life cycle stages. I accomplish immunofluorescence imaging by rinsing inoculated cultures with PBS, fixing with Bouin's solution for 1 h, and decolorizing with 70% ethanol (five changes) for 1 h. Bouin's fixation enhances monolayer adhesion to culture chamber surfaces, especially glass. Monolayers are subsequently washed with PBS, and labeled (60 min at room temperature) with neat hybridoma culture supernatant containing a Cryptosporidium-specific monoclonal antibody (C3C3) or with C3C3 directly labeled with a fluorescent dye (fluorescein or indocarbocyanine [Research Organics, Cleveland, Ohio]). If nuclear staining is desired, primary antibody labeling solutions are supplemented with DAPI (4′,6′-diamidino-2-phenylindole, 0.25 μg/ml). The cultures with unlabeled primary antibody are subsequently labeled (60 min at room temperature) with an appropriate secondary reagent (e.g., indocarbocyanine-labeled goat anti-mouse immunoglobulin). Labeled cell cultures are washed with PBS-BSA, cover-slipped with mounting medium (supplemented with an antiquenching agent), and observed with a UV microscope. A suitable, long-term mounting medium can be prepared with 2.4 g of polyvinyl alcohol (Sigma P8136), 6 g of glycerol, 6 ml of H2O, 12 ml of 0.2 M Tris buffer (pH 8.5), and 2.5% (wt/vol) of the antiquenching agent DABCO (1,4-diazabicyclo-[2.2.2]-octane) (dissolve in the order listed and warm briefly to 50°C to dissolve completely) (26). The unused mounting medium can be stored at −20°C for several months.
Life cycle stages are identified by reactivity with monoclonal antibody, by size, and, if DAPI is employed, by distinctive nuclear staining. Only meronts and gamonts (≈3 μm) are enumerated to avoid counting nonviable but adherent sporozoites or merozoites. Developing stages (meronts and gamonts) are quantified as number per square millimeter with a variation of the formula reported by Augustine (7): stages per square millimeter = [(total number of stages in 30 fields)/(K × PC)], where PC is the average percent confluence for each field and the constant K represents the area (in square millimeters) of 30 microscopic fields (4, 6).
Alternatively, the entire monolayer is scanned at ×200 magnification and scored per field as containing or not containing life cycle stages in order to calculate the percentage of positive fields. Several publications give detailed descriptions of parasite enumeration techniques (4, 6, 50; H. L. Heindel, S. A. Hardy, A. Amirtharajah, and M. J. Arrowood, paper presented at the Proc. Am. Water Works Assoc. Water Quality Technol. Conf., Tampa, Fla., 31 October-3 November 1999). An advantage of the microscopic methods is the ability to identify very small numbers of developing parasites (even single meronts and gamonts). Trophozoites at an early stage of development are indistinguishable from newly invaded sporozoites or merozoites. Some sporozoites and merozoites never invade host cells but may attach to cell surfaces and round-up and appear to have entered the host cells, while others invade host cells and fail to develop further.
To avoid confusion, developing stages are discounted if smaller than 3 to 4 μm in diameter, thus restricting quantification to maturing meronts and gamonts. Nonmicroscopic methods often have a higher threshold of detection or may risk detecting antigens or nucleic acids that are part of the parasite inoculum. Figure 3 presents several microscopic views of C. parvum in culture, including differential interference contrast views of invading sporozoites, asexual and sexual stages, and DAPI-labeled parasite nuclei. Differentiation of the inoculum (oocysts and oocyst walls) from de novo parasites with the FTSC labeling technique described above is illustrated. When combined with contrasting antibody-fluorescent dye labels, differentiation of life cycle stages, including culture-derived oocysts, is readily accomplished.
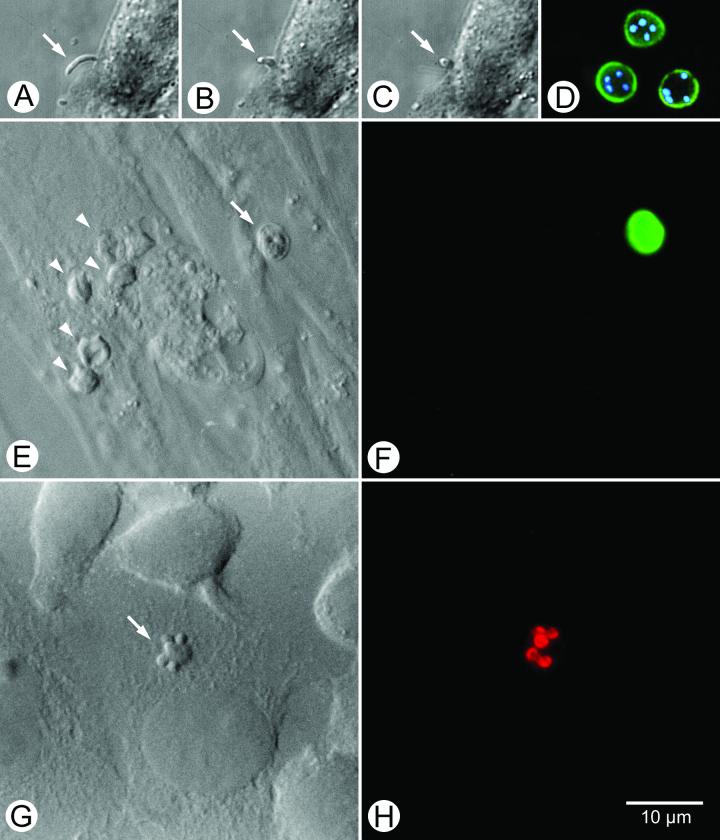
FIG. 3.
Panels A, B, and C show the progressive invasion of a host cell by a sporozoite of C. parvum. Panel D shows fluorescein-conjugated monoclonal antibody-labeled oocysts with internal sporozoite nuclei stained with the dye DAPI. Panel E shows the differential interference contrast bright-field view of host cells with developing asexual parasite life cycle stages (arrowheads) and an oocyst (arrow). The latter is part of the inoculum, as revealed by the bright green label evident in panel F, a result of the FTSC labeling process. Panel G shows a differential interference contrast view of a type I meront with immature (“budding”) merozoites (arrow) labeled with a monoclonal antibody conjugated with the fluorescent dye indocarbocyanine.
CONCLUDING REMARKS
Truly ideal and routine methods that support cryptosporidial development in vitro are not yet available. Methods that permit continuous development (as is routinely used with Toxoplasma spp.), efficient production of mature, infectious oocysts, and cryopreservation methods that would allow cloned stocks to be developed are missing. Consequently, primary isolates from clinical specimens are quite valuable for ongoing research studies. This is particularly true of the anthroponotic isolates, which are difficult, if not impossible, to propagate in animal models. If specimens are collected for studies requiring viable (nonfixed) oocysts, they should be stored unpreserved or in aqueous K2Cr2O7 at 4°C.
Until effective therapeutic agents are available and when questions of variable susceptibility arise, cryptosporidial cell culture will be of limited value in the clinical diagnostic microbiology laboratory and remain primarily a research tool. Despite limitations in the in vitro cultivation of Cryptosporidium spp., current methods are useful for assessing potential drug therapies, oocyst disinfection methods, gene expression, and a variety of biologic characteristics of this difficult but intriguing parasite.
REFERENCES
- 1.Adams, R. B., R. L. Guerrant, S. X. Zu, G. D. Fang, and J. K. Roche. 1994. Cryptosporidium parvum infection of intestinal epithelium: morphologic and functional studies in an in vitro model. J. Infect. Dis. 169:170-177. [DOI] [PubMed] [Google Scholar]
- 2.Angus, K. W., D. Sherwood, G. Hutchison, and I. Campbell. 1982. Evaluation of the effect of two aldehyde-based disinfectants on the infectivity of faecal cryptosporidia for mice. Res. Vet. Sci. 33:379-381. [PubMed] [Google Scholar]
- 3.Arrowood, M. J., and K. Donaldson. 1996. Improved purification methods for calf-derived Cryptosporidium parvum oocysts with discontinuous sucrose and cesium chloride gradients. J. Eukaryot. Microbiol. 43:S89. [DOI] [PubMed] [Google Scholar]
- 4.Arrowood, M. J., J. R. Mead, L. Xie, and X. You. 1996. In vitro anticryptosporidial activity of dinitroaniline herbicides. FEMS Microbiol. Lett. 136:245-249. [DOI] [PubMed] [Google Scholar]
- 5.Arrowood, M. J., and C. R. Sterling. 1987. Isolation of Cryptosporidium oocysts and sporozoites with discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol. 73:314-319. [PubMed] [Google Scholar]
- 6.Arrowood, M. J., L.-T. Xie, and M. R. Hurd. 1994. In vitro assays of maduramicin activity against Cryptosporidium parvum. J. Eukaryot. Microbiol. 41:23S. [PubMed] [Google Scholar]
- 7.Augustine, P. C. 1980. Effects of polyions, Ca++, and enzymes on penetration of cultured cells by Eimeria meleagridis sporozoites. J. Parasitol. 66:498-501. [PubMed] [Google Scholar]
- 8.Awad-el-Kariem, F. M., H. A. Robinson, D. A. Dyson, D. Evans, S. Wright, M. T. Fox, and V. McDonald. 1995. Differentiation between human and animal strains of Cryptosporidium parvum with isoenzyme typing. Parasitology 110:129-132. [DOI] [PubMed] [Google Scholar]
- 9.Blewett, D. A. 1989. Disinfection and oocysts, p. 107-115. In K. W. Angus and D. A. Blewett (ed.), Cryptosporidiosis. Proceedings of the First International Workshop. The Animal Diseases Research Association, Edinburgh, Scotland.
- 10.Bonnin, A., I. Salimbeni, J. F. Dubremetz, G. Harly, P. Chavanet, and P. Camerlynck. 1990. Mise au point d'un modèle expérimental de culture in vitro des stades asexués de Cryptosporidium sp. Ann. Parasitol. Hum. Comp. 65:41-43. [Google Scholar]
- 11.Campbell, I., S. Tzipori, G. Hutchison, and K. W. Angus. 1982. Effect of disinfectants on survival of Cryptosporidium oocysts. Vet. Rec. 111:414-415. [DOI] [PubMed] [Google Scholar]
- 12.Chappell, C. L., P. C. Okhuysen, C. R. Sterling, and H. L. DuPont. 1996. Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J. Infect. Dis. 173:232-236. [DOI] [PubMed] [Google Scholar]
- 13.Crawford, F. G., and S. H. Vermund. 1988. Human cryptosporidiosis. Crit. Rev. Microbiol. 16:113-159. [DOI] [PubMed] [Google Scholar]
- 14.Current, W. L. 1990. Techniques and laboratory maintenance of Cryptosporidium, p. 44-77. In J. P. Dubey, C. A. Speer, and R. Fayer (ed.), Cryptosporidiosis of man and animals. CRC Press, Boca Raton, Fla.
- 15.Current, W. L., and T. B. Haynes. 1984. Complete development of Cryptosporidium in cell culture. Science 224:603-605. [DOI] [PubMed] [Google Scholar]
- 16.Current, W. L., and P. L. Long. 1983. Development of human and calf Cryptosporidium in chicken embryos. J. Infect. Dis. 148:1108-1113. [DOI] [PubMed] [Google Scholar]
- 17.Datry, A., M. Danis, and M. Gentilini. 1989. Développement complet de Cryptosporidium en culture cellulaire: applications. Méd. Sci. 5:762-766. [Google Scholar]
- 18.Doyle, P. S., J. Crabb, and C. Petersen. 1993. Anti-Cryptosporidium parvum antibodies inhibit infectivity in vitro and in vivo. Infect. Immun. 61:4079-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggleston, M. T., M. Tilley, and S. J. Upton. 1994. Enhanced development of Cryptosporidium parvum in vitro by removal of oocyst toxins from infected cell monolayers. J. Helminthol. Soc. Wash. 61:122-125. [Google Scholar]
- 20.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30:1305-1322. [DOI] [PubMed] [Google Scholar]
- 21.Fayer, R., T. Nerad, W. Rall, D. S. Lindsay, and B. L. Blagburn. 1991. Studies on cryopreservation of Cryptosporidium parvum. J. Parasitol. 77:357-361. [PubMed] [Google Scholar]
- 22.Flanigan, T. P., T. Aji, R. Marshall, R. Soave, M. Aikawa, and C. Kaetzel. 1991. Asexual development of Cryptosporidium parvum within a differentiated human enterocyte cell line. Infect. Immun. 59:234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths, J. K. 1998. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv. Parasitol. 40:37-85. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths, J. K., R. Moore, S. Dooley, G. T. Keusch, and S. Tzipori. 1994. Cryptosporidium parvum infection of Caco-2 cell monolayers induces an apical monolayer defect, selectively increases transmonolayer permeability, and causes epithelial cell death. Infect. Immun. 62:4506-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gut, J., C. Petersen, R. Nelson, and J. Leech. 1991. Cryptosporidium parvum: in vitro cultivation in Madin-Darby canine kidney cells. J. Protozool. 38:S72-S73. [PubMed] [Google Scholar]
- 26.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual, p. 418. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Reference deleted.
- 28.Hijjawi, N. S., B. P. Meloni, U. M. Morgan, and R. C. Thompson. 2001. Complete development and long-term maintenance of Cryptosporidium parvum human and cattle genotypes in cell culture. Int. J. Parasitol. 31:1048-1055. [DOI] [PubMed] [Google Scholar]
- 29.Hollingdale, M. R., P. Leland, C. I. Sigler, and J. L. Leef. 1985. In vitro infectivity of cryopreserved Plasmodium berghei sporozoites to cultured cells. Trans. R. Soc. Med. Hyg. 79:206-208. [DOI] [PubMed] [Google Scholar]
- 30.Kuhls, T. L., D. A. Mosier, and D. L. Crawford. 1991. Effects of carbohydrates and lectins on cryptosporidial sporozoite penetration of cultured cell monolayers. J. Protozool. 38:S74-S76. [PubMed] [Google Scholar]
- 31.Lindsay, D. S., C. A. Sundermann, and B. L. Blagburn. 1988. Cultivation of Cryptosporidium baileyi: studies with cell cultures, avian embryos, and pathogenicity of chicken embryo-passaged oocysts. J. Parasitol. 74:288-293. [PubMed] [Google Scholar]
- 32.Lumb, J., K. Smith, P. J. O'Donoghue, and J. A. Lanser. 1988. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue culture cells. Parasitol. Res. 74:531-536. [DOI] [PubMed] [Google Scholar]
- 33.Martinez, F., C. Mascaro, M. J. Rosales, J. Diaz, J. Cifuentes, and A. Osuna. 1992. In vitro multiplication of Cryptosporidium parvum in mouse peritoneal macrophages. Vet. Parasitol. 42:27-31. [DOI] [PubMed] [Google Scholar]
- 34.McDonald, V., R. Stables, D. C. Warhurst, M. R. Barer, D. A. Blewett, H. D. Chapman, G. M. Connolly, P. L. Chiodini, and K. P. W. J. McAdam. 1990. In vitro cultivation of Cryptosporidium parvum and screening for anticryptosporidial drugs. Antimicrob. Agents Chemother. 34:1498-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan, U., R. Weber, L. Xiao, I. Sulaiman, R. C. Thompson, W. Ndiritu, A. Lal, A. Moore, and P. Deplazes. 2000. Molecular characterization of Cryptosporidium isolates obtained from human immunodeficiency virus-infected individuals living in Switzerland, Kenya, and the United States. J. Clin. Microbiol. 38:1180-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naciri, M., P. Yvore, C. de Boissieu, and E. Esnault. 1986. Multiplication de Cryptosporidium muris (Tyzzer 1907) in vitro entretien d'une souche sur oeufs embryonnes. Rec. Med. Vet. 162:51-56. [Google Scholar]
- 37.Nesterenko, M. V., and S. J. Upton. 1996. A rapid microcentrifuge procedure for purification of Cryptosporidium sporozoites. J. Microbiol. Methods 25:87-89. [Google Scholar]
- 38.Nesterenko, M. V., K. M. Woods, and S. J. Upton. 1997. Effects of manganese salts on the AIDS-related pathogen. Cryptosporidium parvum in vitro and in vivo. Biol. Trace Elem. Res. 56:243-253. [DOI] [PubMed] [Google Scholar]
- 39.O'Donoghue, P. J. 1995. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 25:139-195. [DOI] [PubMed] [Google Scholar]
- 40.Pavlasek, I. 1984. Effect of disinfectants in infectiousness of oocysts of Cryptosporidium sp. Cesk. Epidemiol. Mikrobiol. Imunol. 33:97-101. [PubMed] [Google Scholar]
- 41.Peng, M. M., L. Xiao, A. R. Freeman, M. J. Arrowood, A. A. Escalante, A. C. Weltman, C. S. Ong, W. R. MacKenzie, A. A. Lal, and C. B. Beard. 1997. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg. Infect. Dis. 3:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pieniazek, N. J., F. J. Bornay-Llinares, S. B. Slemenda, A. J. da Silva, I. N. Moura, M. J. Arrowood, O. Ditrich, and D. G. Addiss. 1999. New Cryptosporidium genotypes in HIV-infected persons. Emerg. Infect. Dis. 5:444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasmussen, K. R., N. C. Larsen, and M. C. Healey. 1993. Complete development of Cryptosporidium parvum in a human endometrial carcinoma cell line. Infect. Immun. 61:1482-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riggs, M. W., and L. E. Perryman. 1987. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect. Immun. 55:2081-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rochelle, P. A., D. M. Ferguson, T. J. Handojo, R. De Leon, M. H. Stewart, and R. L. Wolfe. 1997. An assay combining cell culture with reverse transcriptase PCR to detect and determine the infectivity of waterborne Cryptosporidium parvum. Appl. Environ. Microbiol. 63:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rochelle, P. A., D. M. Ferguson, T. J. Handojo, R. De Leon, M. H. Stewart, and R. L. Wolfe. 1996. Development of a rapid detection procedure for Cryptosporidium, with in vitro cell culture combined with PCR. J. Eukaryot. Microbiol. 43:72.S. [DOI] [PubMed] [Google Scholar]
- 47.Rosales, M. J., J. Cifuentes, and C. Mascaro. 1993. Cryptosporidium parvum: culture in MDCK cells. Exp. Parasitol. 76:209-212. [DOI] [PubMed] [Google Scholar]
- 48.Rossi, P., E. Pozio, and M. G. Besse. 1990. Cryopreservation of Cryptosporidium sp. oocysts. Trans. R. Soc. Trop. Med. Hyg. 84:68. [DOI] [PubMed] [Google Scholar]
- 49.Slifko, T. R., D. Friedman, J. B. Rose, and W. Jakubowski. 1997. An in vitro method for detecting infectious Cryptosporidium oocysts with cell culture. Appl. Environ. Microbiol. 63:3669-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slifko, T. R., D. E. Huffman, and J. B. Rose. 1999. A most-probable-number assay for enumeration of infectious Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 65:3936-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tyzzer, E. E. 1907. A sporozoan found in the peptic glands of the common mouse. Proc. Soc. Exp. Biol. Med. 5:12-13. [Google Scholar]
- 52.Upton, S. J. 1997. In vitro culture, p. 43-64. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fla.
- 53.Upton, S. J., M. Tilley, and D. B. Brillhart. 1994. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol. Lett. 118:233-236. [DOI] [PubMed] [Google Scholar]
- 54.Upton, S. J., M. Tilley, and D. B. Brillhart. 1994. Comparative development of Cryptosporidium parvum in MDBK and HCT-8 cells under select atmospheres. Biomed. Lett. 49:265-271. [Google Scholar]
- 55.Upton, S. J., M. Tilley, and D. B. Brillhart. 1995. Effects of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J. Clin. Microbiol. 33:371-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Upton, S. J., M. Tilley, R. R. Mitschler, and B. S. Oppert. 1991. Incorporation of exogenous uracil by Cryptosporidium parvum in vitro. J. Clin. Microbiol. 29:1062-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Upton, S. J., M. Tilley, M. V. Nesterenko, and D. B. Brillhart. 1994. A simple and reliable method of producing in vitro infections of Cryptosporidium parvum (Apicomplexa). FEMS Microbiol. Lett. 118:45-49. [DOI] [PubMed] [Google Scholar]
- 58.Weir, S. C., N. J. Pokorny, R. A. Carreno, J. T. Trevors, and H. Lee. 2001. Improving the rate of infectivity of Cryptosporidium parvum oocysts in cell culture with centrifugation. J. Parasitol. 87:1502-1504. [DOI] [PubMed] [Google Scholar]
- 59.Widmer, G., D. Akiyoshi, M. A. Buckholt, X. Feng, S. M. Rich, K. M. Deary, C. A. Bowman, P. Xu, Y. Wang, X. Wang, G. A. Buck, and S. Tzipori. 2000. Animal propagation and genomic survey of a genotype 1 isolate of Cryptosporidium parvum. Mol. Biochem. Parasitol. 108:187-197. [DOI] [PubMed] [Google Scholar]
- 60.Wiest, P. M., J. H. Johnson, and T. P. Flanigan. 1993. Microtubule inhibitors block Cryptosporidium parvum infection of a human enterocyte cell line. Infect. Immun. 61:4888-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodmansee, D. B., and J. F. L. Pohlenz. 1983. Development of Cryptosporidium sp. in a human rectal tumor cell line, p. 306-319. In Proceedings of the Fourth International Symposium on Neonatal Diarrhea. Veterinary Infectious Disease Organization, University of Saskatchewan, Saskatoon, Canada.
- 62.Woods, K. M., M. V. Nesterenko, and S. J. Upton. 1995. Development of a microtitre ELISA to quantify development of Cryptosporidium parvum in vitro. FEMS Microbiol. Lett. 128:89-94. [DOI] [PubMed] [Google Scholar]
- 63.Xiao, L., C. Bern, J. Limor, I. Sulaiman, J. Roberts, W. Checkley, L. Cabrera, R. H. Gilman, and A. A. Lal. 2001. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 183:492-497. [DOI] [PubMed] [Google Scholar]
- 64.Yang, S., M. C. Healey, C. Du, and J. Zhang. 1996. Complete development of Cryptosporidium parvum in bovine fallopian tube epithelial cells. Infect. Immun. 64:349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.You, X., M. J. Arrowood, M. Lejkowski, L. Xie, R. F. Schinazi, and J. R. Mead. 1996. A chemiluminescence immunoassay for evaluation of Cryptosporidium parvum growth in vitro. FEMS Microbiol. Lett. 136:251-256. [DOI] [PubMed] [Google Scholar]
- 66.You, X., R. F. Schinazi, M. J. Arrowood, M. Lejkowski, A. S. Juodawlkis, and J. R. Mead. 1998. In-vitro activities of paromomycin and lasalocid evaluated in combination against Cryptosporidium parvum. J. Antimicrob. Chemother. 41:293-296. [DOI] [PubMed] [Google Scholar]
- 67.You, X. D., M. J. Arrowood, M. Lejkowski, L. T. Xie, R. F. Schinazi, and J. R. Mead. 1996. In vitro evaluation of anticryptosporidial agents with MDCK cell culture and chemiluminescence immunoassay. J. Eukaryot. Microbiol. 43:87S. [DOI] [PubMed] [Google Scholar]