Abstract
The widespread adoption of preoperative embolization in highly vascularized brain tumors often involves the frequent use of Embosphere (Merit Medical Systems, South Jordan, Utah, USA). Nevertheless, inconsistency in size selection and dilution rates across different institutions requires comprehensive examination. This study explored the appropriate size and dilution rate of Embosphere microspheres. To assess catheter occlusion and Embosphere breakage, various dilutions (4-, 10-, 20-, 30-, and 60-fold) of Embosphere 300-500 and 500-700 μm were injected into the catheter in vitro. Results indicated that 20-fold or higher dilutions of Embosphere 300-500 μm and 30-fold or higher dilutions of Embosphere 500-700 μm showed no occlusion of the Excelsior SL-10 microcatheter (Stryker, Fremont, CA, USA) or Embosphere breakage. For embolization, to reduce the risk of Excelsior SL-10 occlusion further, a 30-fold dilution of Embosphere 300-500 μm and a 60-fold dilution of Embosphere 500-700 μm were employed. For 195 blood vessels in 107 patients (84 with meningioma and 23 with schwannoma), embolization was carried out using a 30-fold dilution of Embosphere 300-500 μm when the provocative test was negative and a 60-fold dilution of Embosphere 500-700 μm when the test was positive or when there was a risk of migration into neurotrophic vessels. Contrast-enhanced magnetic resonance imaging after embolization revealed a reduced enhancement effect in 69.1% of cases. Embolization using a 30-fold dilution of Embosphere 300-500 μm and a 60-fold dilution of Embosphere 500-700 μm with an Excelsior SL-10 catheter is safe and satisfactory, which minimizes microcatheter occlusion.
Keywords: embosphere, embolization, brain tumor, size, dilution rate
Introduction
Excision of highly vascularized brain tumors poses challenges in controlling intraoperative bleeding, which leads to increased blood loss and prolonged surgeries. For such tumors, preoperative embolization of the feeding artery of the tumor (tumor embolization) has become a crucial technique, which aims to reduce intraoperative bleeding and enhance surgical maneuverability.1,2)
When conducting tumor embolization, the Embosphere (Merit Medical Systems, South Jordan, Utah, USA) is utilized as the first-line agent. Embosphere microspheres comprise hydrophilic, nonabsorbable, and biocompatible acrylic-type copolymer coated with swine-derived gelatin. Embosphere microspheres with a particle volume of 2 mL are dispersed in physiological saline to obtain a total volume of 9 mL and are filled in a 20-mL syringe. The Embosphere consists of elastic microparticles3,4) that are relatively uniform in size5) and characterized by high periphery reachability.6,7) Five classes of particle size distributions are available: 100-300, 300-500, 500-700, 700-900, and 900-1200 μm. In Japan, Embosphere obtained pharmaceutical approval in June 2013 and was introduced in the market in January 2014, marking it as the first spherical embolic material approved for head, neck, and cerebral nerve regions in Japan, which subsequently became widely utilized.
To mitigate the risk of migration into neurotrophic vessels, a particle size of ≥300 μm is recommended for Embosphere use in the cerebral nerve region.8) For Embosphere 300-500 and 500-700 μm, microcatheter inner diameters of at least 0.018 and 0.021 inches are recommended, respectively.8) Nonetheless, larger external diameters of microcatheters with ≥0.018-inch inner diameters often lead to vessel wedging, hindering bloodstream transport. The use of a small-diameter microcatheter aims to prevent such failures, which ensures effective tumor embolization. There is, however, an issue of occlusion in the microcatheter.
In this study, we aimed to identify the appropriate size and dilution rate of the Embosphere to achieve effective embolization. Employing the small-diameter Excelsior SL-10 catheter (lumen 0.0165 inches; Stryker, Fremont, CA, USA), we assessed the efficacy of Embosphere for tumor embolization.
Materials and Methods
Ethics
This study was carried out based on the ethical guidelines for research that involves human subjects (provisional translation as of March 2015) and the principles of the Declaration of Helsinki and subsequent amendments. Moreover, the Ethics Review Committee of Tokyo Medical University approved this study (approval code: T2020-0030), and all patients who participated in the study provided written informed consent.
Embosphere size and dilution method
Considering the suitability of Embosphere with a size of 300 μm or more for cerebral nerve region use,8) we examined dilution rates for two size classes: 300-500 and 500-700 μm.
The dilution method was as follows: 1 mL was taken from a syringe with a total volume of 9 mL (Embosphere, 2 mL) while stirring, which was utilized as the undiluted solution. The solution was then diluted 4, 10, 20, 30, and 60 times with half-diluted contrast medium. For instance, 29 mL of semi-diluted contrast medium (15 mL of contrast medium + 14 mL of heparinized saline) was added to the collected 1 mL to make 30 mL, which was a 30-fold dilution. Subsequently, 2.0 mL of each dilution was placed in a 2.5-mL syringe. In a simulated brain tumor embolization setup, the syringe tip was shaken up and down, and the total volume was injected into an Excelsior SL-10 catheter. We repeated this procedure five times and observed the presence of microcatheter lumen occlusion (inability to push the syringe) and the Embosphere emerging from the tip of the microcatheter in the physiological saline pad and calculated the breakage rate (number of broken Embosphere/all Embosphere emerging from the microcatheter). Based on this observation, the Embosphere dilution rate was set, and embolization was performed.
Safety and efficacy evaluation of embolization
Tumor embolization was conducted prior to resection if the tumor was highly stained on cerebral angiography and it was deemed difficult to treat the feeding artery during the resection procedure. After tumor embolization, contrast-enhanced magnetic resonance imaging (MRI) was performed 3-5 days later to evaluate embolization status, and resection was carried out 5-7 days later. A total of 107 patients (84 meningiomas and 23 schwannomas; 32 males and 75 females; mean age 53.1 years) who underwent tumor embolization for the feeding artery from the external carotid artery system between January 2015 and November 2023, we assessed the safety and efficacy of the method. Evaluation criteria included the presence/absence of catheter occlusion and complications during embolization, development of new neurological symptoms in the perioperative period of embolization, and reduction of enhancement in contrast-enhanced MRI following embolization.
Technique of brain tumor embolization
(1) A 6 F sheath introducer was inserted into the femoral artery under local anesthesia. After confirming the absence of challenges in the puncture site, heparin was administered intravenously to keep the activated clotting time at 200-250 s.
(2) The 6 F guiding catheter was retained in the external carotid artery, and a microcatheter (Excelsior SL-10) was introduced to the periphery of the nutrient vessel as deeply as possible, ensuring to avoid wedging.
(3) Superselective angiography was performed to confirm the blood flow distribution to the tumor and examine the presence of dangerous anastomosis.
(4) From the planned occlusion site, 30 mg of 1% lidocaine was infused to conduct a provocative test (in cases with positive results, a subsequent provocative test was skipped because time was required for symptom improvement).
(5) Embolization was performed with Embosphere 300-500 μm in cases with negative provocative test results and with Embosphere 500-700 μm in cases with positive provocative test results, to prevent migration into neurotrophic vessels (in the absence of a provocative test, Embosphere 500-700 μm was used when there was an anatomical risk of migration into neurotrophic vessels). Dilution was conducted at the rate determined in vitro.
(6) A 2.5-mL syringe was utilized for injection. The Embosphere was injected while shaking the tip of the syringe vertically to stir the microspheres to avoid excessive emergence of microspheres, which may cause occlusion at the funnel-shaped hub of the microcatheter.
(7) Attention was paid to possible regurgitation in pulsatile under fluoroscopy. When the blood flow of the nutrient vessel became stagnant, tumor distribution was confirmed using digital subtraction angiography.
(8) In cases of no distribution in the tumor, embolization was performed on the proximal side of the nutrient vessel to prevent reopening, with the use of a platinum coil from that site.
Results
Results in vitro
Various dilution rates of Embosphere 300-500 μm were explored, revealing that 4-, 10-, 20-, 30-, and 60-fold dilutions resulted in no Embosphere breakage, except once of microcatheter occlusion at the 10-fold dilution. For Embosphere 500-700 μm, breakage occurred with the 4- and 10-fold dilutions in 0.89% and 1.25% of the samples, respectively, whereas no breakage with 20-fold or higher dilutions was found. Microcatheter occlusion occurred twice each with 4- and 10-fold dilutions. The 20-fold dilution caused no occlusion, but resistance was noted in one case. The 30-fold and higher dilutions were associated with no occlusion or increased resistance (Table 1).
Table 1.
Embosphere size, dilution rate, and breakage rate using an Excelsior SL-10 catheter
| [300-500 μm] | |||||
| Dilution rate (-fold) | 4 | 10 | 20 | 30 | 60 |
| Breakage rate (%) | 0 | 0 | 0 | 0 | 0 |
| Catheter occlusion | – | + (1/5) * | – | – | – |
| [500-700 μm] | |||||
| Dilution rate (-fold) | 4 | 10 | 20 | 30 | 60 |
| Breakage rate (%) | 0.89 | 1.25 | 0 | 0 | 0 |
| Catheter occlusion | + (2/5) * | + (2/5) * | ±** (1/5) * | – | – |
Two milliliters of the Embosphere was placed in a 2.5-mL syringe, and the whole volume was injected in quintuplicate.
*Frequency of occurrence in five repeated iterations.
**No occlusion but resistance appeared.
With microcatheter occlusion, pressing in with a 1-mL syringe successfully reopened occluded microcatheters, but the rate of Embosphere breakage was as high as 60-69% (Fig. 1). Embosphere 300-500 and 500-700 μm presented no complications with 20-fold or higher and 30-fold or higher dilutions, respectively. However, to further reduce the risk of microcatheter occlusion, we used one-degree higher dilutions, that is, 30-fold dilution for Embosphere 300-500 μm and 60-fold dilution for 500-700 μm.
Fig. 1.

Broken Embosphere particles.
A 10-fold dilution of Embosphere 500-700 μm was injected into an Excelsior SL-10 catheter. When the Excelsior SL-10 catheter was occluded, it was reopened using a press-in with a 1-mL syringe. Several Embosphere particles released at that time were broken (arrows).
The breakage rate was 60-69%.
Clinical results
In the cohort of 107 patients (32 men and 75 women; mean age of 53.1 years [range 18-83 years]), 84 had meningioma, 23 had schwannoma, and the mean tumor size was 40.0 mm (range, 15-77 mm). Overall, 91 (85.0%) patients had a skull base tumor. Embolization was carried out in 195 vessels, consisting the middle meningeal artery (MMA), 76 (39.0%) vessels (including the petrosal branch, 29 vessels); ascending pharyngeal artery (APhA), 53 (27.2%) vessels (including the neuromeningeal branch, 43 vessels); internal maxillary artery (IMA), 27 (13.8%) vessels; occipital artery, 17 (8.7%) vessels (including the mastoid branch, 12 vessels); accessory meningeal artery, 15 (7.7%) vessels; and posterior auricular artery, 7 (3.6%) vessels. Embosphere 300-500 μm was used in 20 (10.3%) vessels, and Embosphere 500-700 μm was utilized in 175 (89.7%) vessels (Table 2A).
Table 2A.
Patient characteristics
| Total number of patients | 107 |
| Age (years) | 53.1 (18-83) |
| Sex (male/female) | 32/75 |
| Meningioma/ Schwannoma | 84/23 |
| Tumor size (mm) | 40.0 (15-77) |
| Tumor location | |
| Skull base | 91 (85.0%) |
| Non-skull base | 16 (15.0%) |
| Embolized feeding artery | 195 |
| MMA | 76 (39.0%) (petrosal branch, n = 29) |
| APhA | 53 (27.2%) (neuromeningeal branch, n = 43) |
| IMA | 27 (13.8%) |
| OA | 17 (8.7%) (mastoid branch, n = 12) |
| AMA | 15 (7.7%) |
| PAA | 7 (3.6%) |
| Embosphere size (μm) | |
| 300-500 | 20 (10.3%) |
| 500-700 | 175 (89.7%) |
Microcatheter occlusion during embolization occurred in four vessels (one MMA petrosal branch, one APhA neuromeningeal branch, and two IMAs). A 60-fold dilution of Embosphere 500-700 μm was utilized in all these cases. When occlusion occurred, the basic procedure was removing the microcatheter and washing the lumen outside the body, which was performed for two vessels. In one case where re-introduction of the microcatheter was considered difficult, a micro guidewire was inserted to reopen the catheter. In one vessel (MMA petrosal branch), a press-in with a 1-mL syringe achieved reopening, but slight bleeding took place in the tumor (Fig. 2). The patient experienced no headache or neurological symptoms, indicating a favorable clinical course.
Fig. 2.
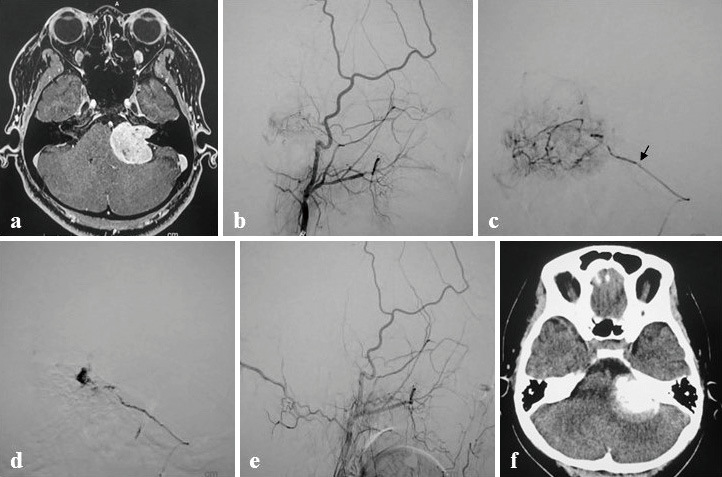
A 32-year-old woman with hearing loss in the left ear as the initial symptom.
a A contrast-enhanced magnetic resonance image showing a 34-mm-sized left acoustic schwannoma.
b A left ECAG lateral image showing tumor stain from the left MMA petrosal branch.
c Excelsior SL-10 was introduced in the left MMA petrosal branch, and imaging was performed. The tip of Excelsior SL-10 is shown (arrow). A provocative test showed symptoms of CNS VI. Embolization was performed with a 60-fold dilution of Embosphere 500-700 μm. Given that the microcatheter was clogged in the middle, a press-in with a 1-mL syringe was conducted to achieve recanalization.
d A microangiographic image after recanalization showing a finding suggestive of intratumoral hemorrhage. The lesion was closed with a coil on the near side.
e A left ECAG lateral image at the completion of embolization showing the disappearance of tumor stain. No headache or neurological symptoms developed.
f A head CT image immediately after embolization showing marked pooling of contrast medium in the tumor (tumor contrast retention).
ECAG, external carotid angiography; CT, computed tomography; MMA, middle meningeal artery
Worsening of neurological symptoms occurred in three (2.8%) cases after embolization (Table 2B). One had accompanying hydrocephalus before embolization, which became aggravated after embolization, which indicates the progression of impairment of consciousness. In the other two cases, mild cranial nerve disorder occurred after embolization but was improved in 6-12 months.
Table 2.
Worsening of neurological symptoms after embolization
Evaluation using contrast-enhanced MRI after embolization was conducted in 94 of 107 patients who underwent embolization, and a reduced enhancement effect was observed in 65 (69.1%) patients. In the group with reduced enhancement effect, the mean blood loss and operative time during removal were 267.5 ± 251 mL and 359.5 ± 89.5 minutes, respectively, and in the group without reduction, they were 460.3 ± 409.3 mL and 320.8 ± 143.1 minutes, respectively.
An illustrative case
A 39-year-old woman with hearing loss in the right ear as the initial symptom was diagnosed with a posterior petrous meningioma (measuring 30 mm with uniform enhancement) on contrast-enhanced MRI (Fig. 3). Right external carotid angiography revealed tumor stains from the neuromeningeal branch of the right APhA. An Excelsior SL-10 catheter was introduced into the right APhA, and microangiography exhibited tumor stains from the jugular artery neuromeningeal branch. When the provocative test with 30-mg 1% lidocaine was conducted, damage to CNS X occurred. After symptom improvement, embolization using a 60-fold dilution of Embosphere 500-700 μm was carried out, and the tumor stain disappeared. Then, the neuromeningeal branch was occluded with a platinum coil, which resulted in the disappearance of the feeding artery. No new neurological symptoms occurred after embolization, and enhancement of the tumor was markedly reduced on contrast-enhanced MRI 4 days after embolization.
Fig. 3.

An illustrative case.
a A contrast-enhanced magnetic resonance image showing a 30-mm-sized posterior petrous meningioma with uniform enhancement.
b A right ECAG lateral image showing tumor stain from the right ascending pharyngeal artery neuromeningeal branch.
c A microangiographic image from the right ascending pharyngeal artery showing a tumor stain from the jugular artery neuromeningeal branch.
d The tumor stain disappeared after embolization with Embosphere.
e, f Post-embolization final right ECAG lateral images showing the disappearance of the feeding artery.
g A contrast-enhanced magnetic resonance image 4 days post-embolization demonstrating a markedly reduced tumor contrast effect.
ECAG, external carotid angiography
Discussion
In this study, we explored different sizes and dilutions to determine the appropriate size and dilution rate of the Embosphere. The Excelsior SL-10 lumen we utilized in this study was 0.0165 inches (0.419 mm, 419 μm), and passing through it required a reduction of 16.2% for a particle size of 500 μm and 40.1% for a particle size of 700 μm. Laurent et al.4) revealed that the Embosphere was elastic, passed through the microcatheter as an ellipsoid even when the inner diameter was small, and passed through at 33-40% reduction without breakage, which was consistent with our in vitro experimental data that showed passage with low breakage rate.
In terms of microcatheter occlusion during the procedure, no occlusion was noted in 20 vessels embolized with a 30-fold dilution of Embosphere 300-500 μm, but occlusion occurred in four (2.3%) of the 175 vessels embolized with a 60-fold dilution of Embosphere 500-700 μm. Although Embosphere 500-700-μm microspheres had been confirmed in vitro to cause no microcatheter occlusion when 30-fold or higher dilutions were employed, occlusion occurred during embolization even with a 60-fold dilution. This discrepancy is due to the microcatheter's strong tortuosity, causing increased friction during the passage of ellipsoidal Embosphere microspheres and subsequent obstruction.
In principle, occlusion involves removing the microcatheter and washing the lumen outside the body. Nevertheless, reopening with a micro guidewire may be considered if re-introducing the microcatheter is challenging. In such cases, as the clogged Embosphere microspheres are pushed to the microcatheter tip, the procedure should be performed while ensuring caution due to potential occlusion at the site. Additionally, in vitro experiments have shown that a press-in with a 1-mL syringe results in recanalization, but rapid injection may lead to intense pressure on the feeding artery, which causes intratumoral hemorrhage. In this study, this event was noted in one blood vessel (Fig. 2). Thus, a press-in should not be implemented if a microcatheter is selectively inserted into a feeding artery. When an embolic material is injected in usual cases, excessive pressure may break the blood vessel in the tumor and induce intratumoral hemorrhage;9) therefore, caution is required.
When the particle size is 100-300 μm, 2 mL of Embosphere contains approximately 400,000 particles; therefore, a 30-fold dilution contains 1776 particles per milliliter. The number of particles is approximately 60,000, 18,000, 7,000, and 3,000, respectively, for each of the size classes of 300-500, 500-700, 700-900, and 900-1200 μm; for 30-fold dilutions, the number of particles per milliliter is 222, 66, 26, and 11, respectively. The smaller the number of particles injected, the lower the risk of catheter blockage but the longer it takes to embolize. In this study, Embosphere 300-500 and 500-700 μm were diluted 30-fold and 60-fold, respectively, for embolization. For the 60-fold dilution of Embosphere 500-700 μm, the incidence of occlusion was as low as 2.3%, and the dilution rate was considered appropriate.
Of the three patients with aggravated neurological symptoms after embolization, one had disturbed consciousness associated with aggravation of hydrocephalus and was diagnosed with post-embolization neurological syndrome,10) defined as a late-onset worsening of existing neurological symptoms along with systemic symptoms including inflammatory reaction. In the other two patients, the symptoms appeared a little longer after embolization and were judged to represent ischemia of neurotrophic vessels. Nevertheless, Embosphere 500-700 μm is large and did not cause complete obstruction of neurotrophic vessels. Therefore, presumably, symptoms that occurred after a lapse of time were mild and did not lead to permanent impairment.
Since the neurotrophic blood vessels were approximately 200-300 μm in diameter, Embosphere 500-700 μm would not reach the neurotrophic blood vessels. However, when pressurized from a microcatheter, the microspheres could reach the vicinity of the neurotrophic vessels because of the dilation of blood vessels and the elasticity of the Embosphere. To avoid pressing into the blood vessel with a positive provocative test result, care should be taken.
In this series, adverse events were observed in four (3.7%) patients, including one with asymptomatic tumor hemorrhage and three with worsening neurological symptoms, but there were no permanent complications. Complications of brain tumor embolization have been reported to range from 3.7% to 5.6%,11-13) and Rosen et al.14) reported permanent complications in 9% of 167 patients treated with embolization for skull base meningioma. Considering that 91 (85.0%) patients had skull base tumors, it can be said that embolization was obtained safely.
The loss of contrast on contrast-enhanced MRI after embolization indicates the presence of tumor necrosis and is a predictor of decreased blood loss during tumor resection.15-17) We explored the amount of blood loss for each surgical approach after embolization of a skull base meningioma and reported that in the lateral suboccipital approach, the amount of blood loss was significantly less when contrast-enhanced MRI showed a weakening of the contrast effect; the weaker the contrast effect, the less blood loss was observed.18) Thus, contrast-enhanced MRI is useful for the evaluation of embolization. In this study, contrast-enhanced MRI after embolization was conducted in 94 patients, and the enhancement effect was attenuated in 65 (69.1%) patients. Embolization was carried out using Embosphere of relatively large sizes, 300-500 μm in 20 (10.3%) vessels and 500-700 μm in 175 (89.7%) vessels, with satisfactory results. This suggests that the use of a microcatheter with a small diameter allowed the Embosphere microspheres to run with the bloodstream without wedging the blood vessel and reach a more distal site, exhibiting their characteristics.
Limitations
This study has some limitations. First, the number of participants was limited. Second, because the Embosphere was injected manually, the volume and pressure of injection were not constant. Whether differences in the volume and pressure of injection influence the risk of occlusion in a microcatheter is unclear. Additionally, a press-in may increase the accessibility of the Embosphere to distal areas under the influence of other factors, including increased pressure of injection and dilation of blood vessels. Although this remains unproven, further analysis is mandatory after the accumulation of relevant cases.
Conclusion
In conclusion, a 30-fold dilution of Embosphere 300-500 μm and a 60-fold dilution of Embosphere 500-700 μm with a small-diameter microcatheter (Excelsior SL-10) enabled safe and satisfactory embolization. The 30-fold dilution for Embosphere 300-500 μm and 60-fold dilution for Embosphere 500-700 μm are associated with only a few occlusions in small-diameter microcatheters and are considered appropriate dilution rates that help enhance the efficacy of embolization.
Abbreviations
APhA Ascending pharyngeal artery
IMA Internal maxillary artery
MMA Middle meningeal artery
MRI Magnetic resonance imaging
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of Data and Materials
Data can be acquired upon suitable request from the corresponding author.
Conflicts of Interest Disclosure
All authors have no conflict of interest.
References
- 1). Raper DMS, Starke RM, Henderson F Jr., et al. : Preoperative embolization of intracranial meningiomas: Efficacy, technical considerations, and complications. AJNR Am J Neuroradiol 2014: 35: 1798-1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Mine B, Delpierre I, Hassid S, De Witte O, Lubicz B: The role of interventional neuroradiology in the management of skull base tumours and related surgical complications. B-ENT 2011: 7: 61-66 [PubMed] [Google Scholar]
- 3). Rodiek SO, Stölzle A, Lumenta ChB: Preoperative embolization of intracranial meningiomas with Embosphere microspheres. Minim Invasive Neurosurg 2004: 47: 299-305 [DOI] [PubMed] [Google Scholar]
- 4). Laurent A, Beaujeux R, Wassef M, Rüfenacht D, Boschetti E, Merland JJ: Trisacryl gelatin microspheres for therapeutic embolization, I: development and in vitro evaluation. AJNR Am J Neuroradiol 1996: 17: 533-540 [PMC free article] [PubMed] [Google Scholar]
- 5). Verret V, Ghegediban SH, Wassef M, Pelage JP, Golzarian J, Laurent A: The arterial distribution of Embozene and Embosphere microspheres in sheep kidney and uterus embolization models. J Vasc Interv Radiol 2011: 22: 220-228 [DOI] [PubMed] [Google Scholar]
- 6). Bendszus M, Klein R, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L: Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. AJNR Am J Neuroradiol 2000: 21: 255-261 [PMC free article] [PubMed] [Google Scholar]
- 7). Beaujeux R, Laurent A, Wassef M, et al. : Trisacryl gelatin microspheres for therapeutic embolization, II: preliminary clinical evaluation in tumors and arteriovenous malformations. AJNR Am J Neuroradiol 1996: 17: 541-548 [PMC free article] [PubMed] [Google Scholar]
- 8). Requirements for systems related to proper use of medical devices “Embosphere” and “Hepasphere” in the neurology field. jsnet.website/documents.php?id=109 (Accessed Jan 10 2024)
- 9). Manaka H, Sakata K, Tatezuki J, Shinohara T, Shimohigashi W, Yamamoto T: Safety and efficacy of preoperative embolization in patients with meningioma. J Neurol Surg B Skull Base 2018: 79: S328-S333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Tanaka Y, Hashimoto T, Watanabe D, et al. : Post-embolization neurological syndrome after embolization for intracranial and skull 23 base tumors: transient exacerbation of neurological symptoms with inflammatory responses. Neuroradiology 2018: 60: 843-851 [DOI] [PubMed] [Google Scholar]
- 11). Carli DF, Sluzewski M, Beute GN, van Rooij WJ: Complications of particle embolization of meningiomas: frequency, risk factors, and outcome. AJNR Am J Neuroradiol 2010: 31: 152-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Shah AH, Patel N, Raper DMS, et al. : The role of preoperative embolization for intracranial meningiomas. J Neurosurg 2013: 119: 364-372 [DOI] [PubMed] [Google Scholar]
- 13). Sugiu K, Hishikawa T, Murai S, et al. : Treatment outcome of intracranial tumor embolization in Japan: Japanese Registry of NeuroEndovascular Therapy 3 (JR-NET3). Neurol Med Chir (Tokyo) 2019: 59: 41-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Rosen CL, Ammerman JM, Sekhar LN, Bank WO: Outcome analysis of preoperative embolization in cranial base surgery. Acta Neurochir (Wien) 2002: 144: 1157-1164 [DOI] [PubMed] [Google Scholar]
- 15). Nguyen HS, Janich K, Doan N, Patel M, Li L, Mueller W: Extent of T1+C intensity is a predictor of blood loss in resection of meningioma. World Neurosurg 2017: 101: 69-75 [DOI] [PubMed] [Google Scholar]
- 16). Ali R, Khan M, Chang V, et al. : MRI pre- and post-embolization enhancement patterns predict surgical outcomes in intracranial meningiomas. J Neuroimaging 2016: 26: 130-135 [DOI] [PubMed] [Google Scholar]
- 17). Bendszus M, Warmuth-Metz M, Klein R, et al. : Sequential MRI and MR spectroscopy in embolized meningiomas: correlation with surgical and histopathological findings. Neuroradiology 2002: 44: 77-82 [DOI] [PubMed] [Google Scholar]
- 18). Okada H, Hashimoto T, Tanaka Y, Sakamoto H, Kohno M: Embolization of skull base meningiomas with embosphere microspheres: Factors predicting treatment response and evaluation of complications. World Neurosurg 2022: 162: e178-e186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be acquired upon suitable request from the corresponding author.



