Abstract
Although attempts to develop methods for the in vitro cultivation of microsporidia began as early as 1937, the interest in the culture of these organisms was confined mostly to microsporidia that infect insects. The successful cultivation in 1969 of Encephalitozoon cuniculi, a microsporidium of mammalian origin, and the subsequent identification of these organisms as agents of human disease heightened interest in the cultivation of microsporidia. I describe the methodology as well as the cell lines, the culture media, and culture conditions used in the in vitro culture of microsporidia such as Brachiola (Nosema) algerae, Encephalitozoon cuniculi, E. hellem, E. intestinalis, Enterocytozoon bieneusi, Trachipleistophora hominis, and Vittaforma corneae that cause human disease.
INTRODUCTION
Background
The phylum Microsporidia (41) comprises obligate, amitochondriate eukaryotic protistan parasites. More than 1,200 species belonging to 143 genera have been described. The microsporidia are intracellular parasites that infect members of virtually every phylum of the animal kingdom. They produce environmentally resistant spores that are characterized by the presence of a unique apparatus, the polar tubule, which is found tightly coiled within the spore. The polar tubule, also called the polar filament, is extruded with great force upon contact with a suitable host cell, and the spore contents are injected into the new host. Although the first case of human microsporidiosis was reported in 1959 by Matsubayashi et al. (26) in a Japanese boy, it was only in the last decade that interest in the microsporidia heightened because of their association with diarrheal and disseminated diseases in humans with the human immunodeficiency virus (HIV) and AIDS. Therefore, microsporidia have also been identified as emerging opportunistic parasites.
In Vitro Culture before HIV and AIDS
Before the advent of HIV and AIDS, interest in the in vitro culture of microsporidia was confined to species of economic importance (Table 1). These included (i) Nosema apis and N. bombycis, which infect honey bees and silk worms, respectively; (ii) Ameson michaelis and Glugea stephani, which infect blue crabs and the winter flounder, respectively; (iii) Nosema locustae and Vairimorpha necatrix, both biological control agents of agricultural pests; (iv) Brachiola (Nosema) algerae, which parasitizes Anopheles spp., which vector malaria; and (v) Vavraia culiceis, which parasitizes Culex spp., which vector filarial parasites (22). However, interest in the in vitro culture of certain microsporidia that cause human disease intensified in recent years because several genera (e.g., Brachiola, Encephalitozoon, Enterocytozoon, Microsporidium, Nosema, Pleistophora, Trachipleistophora, and Vittaforma) have been identified as opportunistic pathogens of humans, especially in AIDS patients (36, 54) (Tables 2 and 3). Hence, a number of isolates of Encephalitozoon cuniculi, E. hellem, and E. intestinalis, two isolates each of Vittaforma corneae and Brachiola (Nosema) algerae, and one isolate of Trachipleistophora hominis have been established in culture (1, 3-17, 19-21, 23, 27, 30, 33-35, 38, 44-47, 49, 52, 56; M. Scaglia, personal communication; G. S. Visvesvara, G. J. Leitch, D. A. Schwartz, A. J. Da Silva, S. Wallace, H. Moura, N. J. Pieniazek, and R. T. Bryan, unpublished data; G. S. Visvesvara, H. Moura, G. J. Leitch, and S. Wallace, Program Abstr. 48th Annu. Meet. Am. Soc. Trop. Med. Hyg., Washington, D.C., 28 November-2 December 1999, abstr. 823). However, Enterocytozoon bieneusi, the most frequently identified microsporidian that causes gastrointestinal illness in patients with AIDS (36, 54), has not yet been established in continuous culture (48, 51). The genus Microsporidium is a catchall genus and was established to include several poorly described species with indefinite taxonomic status (2).
TABLE 1.
In vitro culture of microsporidia that infect economically important insects and fish
| Microsporidian | Host | Cell culture system | Reference |
|---|---|---|---|
| Nosema bombycis | Silk worm (Bombyx mori) | B. mori ovarian tissue | 41 |
| Nosema apis | Honey bee (Apis mellifera) | Aedes mellifera midgut explants | 22 |
| Ameson michaelis | Blue crab (Cancer sapidus) | Blue crab hemocytes, mouse macrophages, and others | 22 |
| Brachiola (Nosema) algerae | Mosquitoes | Wide variety of cells, including pig kidney, rat brain, and insect cell lines such as TN368, ATC15, DMI, and MOS55 | 22 |
| Vavraia culiceis | Mosquitoes | Insect cell line IZD-Mb-0503 | 22 |
TABLE 2.
Genera and species of microsporidia known to infect immunodeficient humans
| Species | Clinical manifestation(s) in immunocompromised host | In vitro culture achieved | Other animals infected |
|---|---|---|---|
| Brachiola (Nosema) algerae | Skin infection | + | Mosquitoes |
| Encephalitozoon cuniculi | Keratoconjunctivitis, sinusitis, bronchitis, nephritis, peritonitis, hepatitis, intestinal infection, encephalitis | + | Rabbits, rodents, dogs, foxes, pigs, cats, leopards, monkeys |
| Encephalitozoon hellem | Keratoconjunctivitis, sinusitis, bronchitis, nephritis, cystitis, prostatitis, urethritis | + | Parrots, budgerigars, lovebirds |
| Encephalitozoon intestinalis | Keratoconjunctivitis, sinusitis, bronchitis, nephritis, chronic diarrhea, cholangitis | + | Goats, cows, dogs, donkeys, pigs |
| Enterocytozoon bieneusi | Chronic diarrhea, cholangitis, sinusitis | − | Pigs, monkeys, rabbits |
| Nosema connori | Disseminated infection | − | Not described |
| Nosema-like | Myositis | − | Not described |
| Pleistophora spp.a | Myositis | − | |
| Trachipleistophora hominis | Myositis, keratoconjunctivitis, sinusitis | + | Not described |
| Trachipleistophora anthropophthera | Disseminated infection, including encephalitis | − | Not described |
| Vittaforma corneae | Disseminated infection | + | Not described |
Several different species of Pleistophora have been described from insects, fish, amphibians, reptiles, etc. However, microsporidial species that are uninucleated throughout their life cycle and produce large numbers of spores contained within polysporophorous vesicles have also been included in the genus Pleistophora even though clear-cut taxonomic affinities of these microsporidia have not been established.
TABLE 3.
Species of microsporidia known to infect immunocompetent humans
| Species | Clinical manifestation in immunocompetent host | In vitro culture achieved |
|---|---|---|
| Brachiola (Nosema) algerae | Keratoconjunctivitis | + |
| Encephalitozoon intestinalis | Self-limiting diarrhea | − |
| Enterocytozoon bieneusi | Self-limiting diarrhea | − |
| Nosema ocularum | Keratitis | − |
| Vittaforma corneae | Keratitis | + |
| Microsporidium ceylonensisa | Keratitis | − |
| Microsporidium africanuma | Keratitis | − |
These are poorly described species and have been arbitrarily assigned to the genus Microsporidium, and the true taxonomic affinities of these two species have not been established.
IN VITRO CULTURE OF MICROSPORIDIA OF MAMMALIAN ORIGIN—EARLY YEARS
Although Trager in 1934 (42) first attempted to obtain in vitro cultures of the insect microsporidian Nosema bombycis, it took nearly 22 years to establish cultures of a microsporidian of mammalian origin. Morris, McCown, and Blount in 1956 (22) grew Encephalitozoon cuniculi of mouse origin, for a short time, in a mouse lymphosarcoma MB III cell line. However, interest in the cultivation of these parasites increased only after Shadduck in 1969 (37) succeeded in the continuous cultivation of E. cuniculi of rabbit origin in RK cells (2, 22, 52). Until 1990, E. cuniculi was the only microsporidian from mammalian hosts that had been cultivated in vitro, either for short periods or continuously, on a variety of cell lines by a number of researchers (2, 22, 52). Although a number of isolates originating from different mammalian hosts have been established in culture, only those isolated from humans will be discussed here. In Table 4 are listed the various microsporidia belonging to different genera and species, the different types of cell lines in which they are grown, and the media, supplements, and culture conditions used.
TABLE 4.
Cell lines, media, and supplements that have been used to cultivate human-infecting opportunistic microsporidia
| Species | Cell linesa | Medium | Supplements and culture conditionsb |
|---|---|---|---|
| Encephalitozoon cuniculi | E6, HLF, MDCK, MRC-5 | EMEM + 2 mM glutamine | 5 or 10% FBS or FCS and antibiotics (penicillin and streptomycin or gentamicin and amphotericin B), with or without 5% CO2 |
| Encephalitozoon hellem | MDCK, E6, HLF, MRC-5, RK-13, FBF | EMEM + 2 mM glutamine, RPMI | 5 or 10% FBS or FCS and antibiotics (penicillin and streptomycin or gentamicin, amphotericin B), with or without 5% CO2 |
| Encephalitozoon intestinalis | E6, HLF, MDCK, HEL, MDM, RK-13, I047, HT-29, Caco-2 | EMEM + 2 mM glutamine, RPMI 1640, DMEM + 2 mM glutamine | 5 or 10% FBS, 5 or 10% FBS or FCS, antibiotics (penicillin and streptomycin or gentamicin or a cocktail of amoxicillin, vancomycin, gentamicin, and flucytosine, amphotericin B), with or without 5% CO2 |
| Enterocytozoon bieneusi | E6, HLF, HT-29, Caco-2, MDCK, RK-13 | EMEM + 2 mM glutamine | 5 or 10% FBS, 5% or 10% FBS, EGF, transferrin, insulin, HC, selenium, HEPES |
| Vittaforma corneae | SIRC, MDCK, E6, HLF, MRC-5 | EMEM + 2 mM glutamine | 5% or 10% FBS, gentamicin, with or without 5% CO2 |
| Trachipleistophora hominis | MDCK, RK-13, COS-1, L6-C10, MM | DMEM + 2 mM glutamine | 10% FBS, 10% HS, gentamicin, with or without 5% CO2 |
| Brachiola algerae | E6, HLF | EMEM + 2 mM glutamine | 5 or 10% FBS, gentamicin and amphotericin B, without 5% CO2 |
E6, monkey kidney; HLF, human lung fibroblasts; MDCK, Madin-Darby canine kidney; MRC-5, lung fibroblasts; RK-13, rabbit kidney; FBF, fetal bovine lung fibroblasts; HEL, human embryonic lung; MDM, monocytes; I 047, intestinal; HT-29, human adenocarcinoma; Caco-2, human colon carcinoma; SIRC, rabbit corneal epithelium; COS-1, green monkey kidney; L6-C10, human cell line; MM, mouse myoblasts.
FCS, fetal calf serum; EGF, epidermal growth factor; HC, hydrocortisone; HS, human serum.
IN VITRO CULTURE OF HUMAN-INFECTING MICROSPORIDIA
Although it may be possible to identify the various microsporidia that infect humans from clinical specimens using serologic and/or molecular methods, none of these methods are commercially available. Further, in some cases microscopic examination of biopsy specimens may not yield conclusive results. Additionally, it is possible that microsporidial organisms may be present in very small numbers and can be easily missed during histologic examination. Some microsporidia such as Encephalitozoon species and Brachiola, even when they are present in small numbers, have the potential to become established in cell cultures, thus facilitating their easy identification at a later time. Therefore, attempts at culturing these organisms should be made, especially since many clinical laboratory personnel are familiar with cell culture methodology.
Encephalitozoon cuniculi
Currently, 13 isolates of E. cuniculi originating from different human specimens, including urine, bronchoalveolar lavage (BAL), sputum, and brain, have been established in culture. Of these, one isolate each was established from the United Kingdom (19) and Italy (31); two each from the United States (5) and Spain (6); and seven originated from Switzerland (9, 10, 25, 56; P. DePlazes, 1999, personal communication). The methods used to establish cultures of these microsporidia are relatively simple. For example, De Groote et al. (5) established cultures of E. cuniculi from the urine and sputum samples of an AIDS patient using the following procedure (Fig. 1 and 2).
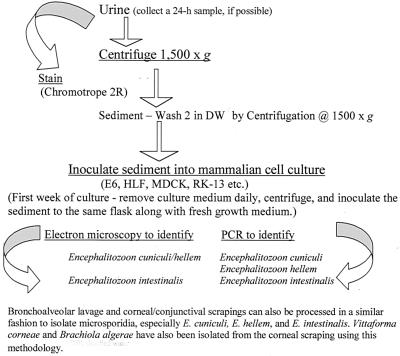
FIG. 1.
Flow chart to illustrate the isolation and continuous cultivation of microsporidia, especially Encephalitozoon species, from bronchoalveolar lavage samples, corneal/conjunctival scrapings, and urine samples. DW, distilled water.
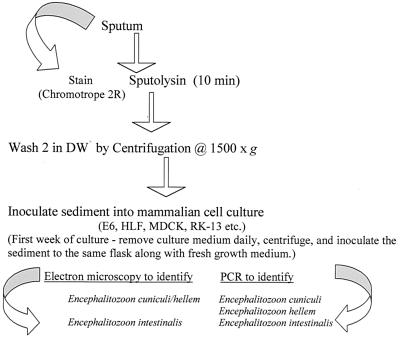
FIG. 2.
Flow chart to illustrate the isolation and continuous cultivation of microsporidia, especially Encephalitozoon species, from sputum. DW, distilled water.
To initiate cultures, the urine sample was centrifuged at 1,500 × g and the supernatant was aspirated. The sediment was washed twice by centrifugation as above in distilled water, and the sediment was inoculated into a monkey kidney (E6) cell culture that was maintained on Eagle's minimal essential medium (EMEM) fortified with 2 mM glutamine and 10% fetal bovine serum (FBS). The medium also contained 50 μg of gentamicin, 1,000 μg of piperacillin, and 5 μg of Fungizone per ml to prevent bacterial and fungal overgrowth. The sputum sample was treated with Sputolysin (Cal biochem, La Jolla, Calif.) to break up the mucus and washed twice in 50 ml of distilled water, and the supernatant was aspirated. The sediment was suspended in 1 ml of distilled water and then inoculated into a human lung fibroblast (HLF) monolayer along with the antibiotics as above. The culture medium from each flask was removed daily for the first week and twice weekly thereafter and replaced with fresh medium containing the antibiotics. The cultures were incubated at 37°C. After 4 to 6 weeks of such manipulations, many spores were seen in the culture supernatants and many E6 and HLF cells had become enlarged and distended with developing stages and spores (52). The presence of spores in smears made from centrifuged sediments from cultures was confirmed by staining with either the chromotrope 2R (55) or the Gram-chromotrope techniques (28) as well as the indirect immunofluorescence assay (Fig. 3A to E) (52).
FIG. 3.
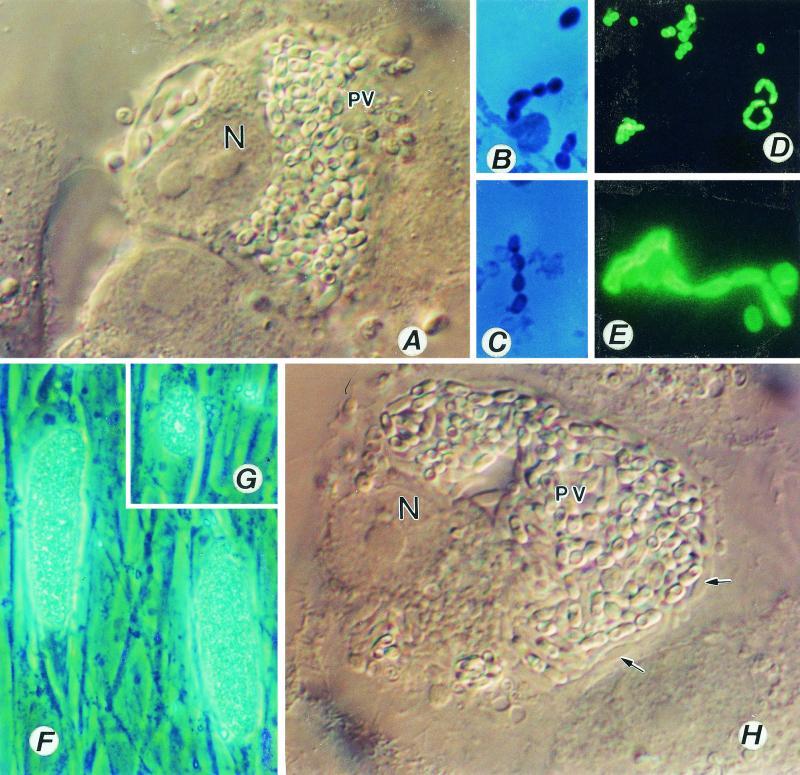
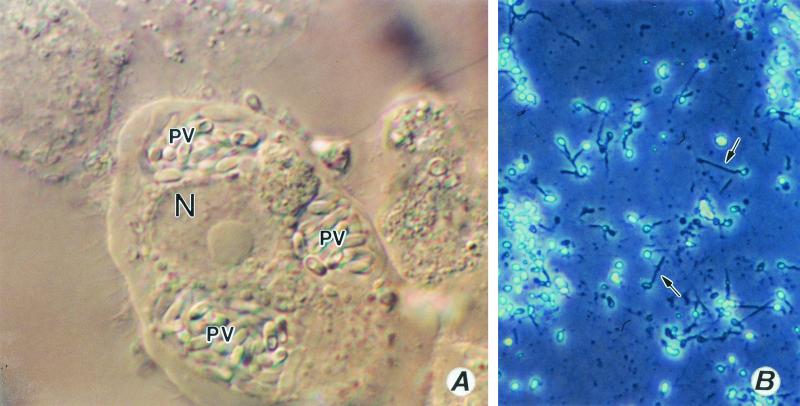
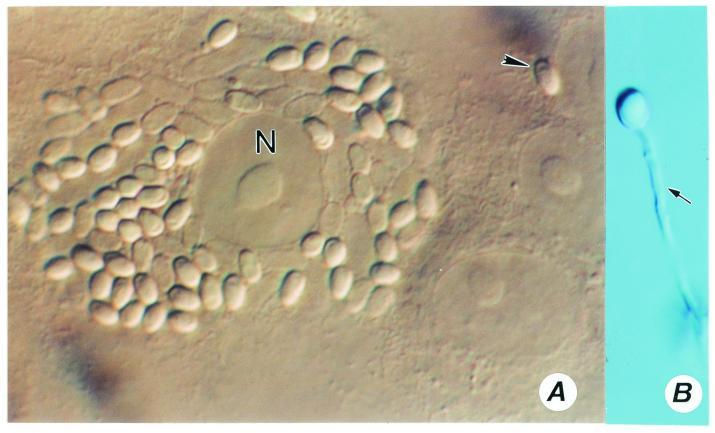
(A to E) Encephalitozoon cuniculi. (A) A monkey kidney (E6) cell distended with spores of E. cuniculi growing inside a parasitophorous vacuole (PV). Magnification, ×1,200. N, host cell nucleus. (B and C) Gram-chromotrope-stained preparations exhibiting chains of spores with four spores. Magnification, ×1,200. (D and E) Immunofluorescence patterns of spores. A centrifuged pellet obtained from an infected flask reacted first with a 1:1,000 dilution of a rabbit anti-E cuniculi serum and subsequently with a 1:100 dilution of a fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G. Note spore chains of two, three, and five spores (D). Magnification, ×250. Note chains of two and four spores (E). Magnification, ×1,200. Such chains of spores are also seen in cultures of E. hellem and E. intestinalis, illustrating polysporous sporogony, which is commonly seen in cultures infected with Encephalitozoon species. (F to H) Cell cultures infected with Encephalitozoon hellem. Note two HLF cells distended with spores and developing stages of the parasite, giving the appearance of corn on the cob (F). Magnification, ×600. (G) Early stages depicting the process of invasion and the formation of corn on the cob architecture, Magnification, ×600. (H) An E6 cell distended with spores and developing stages (small arrows) of E. hellem. Magnification, ×1,200.
Transmission electron microscopy (TEM) of the infected monolayers revealed the presence of an unseptated parasitophorous vacuole containing various developmental stages. The microsporidian was identified as E. cuniculi, based on PCR analysis of the DNA extracted from the infected cultures (5). Also, in 1995, Hollister et al. (19) from the United Kingdom established an isolate of E. cuniculi in culture by inoculating Madin-Darby canine kidney (MDCK) cells with spores isolated from the urine of an AIDS patient. They centrifuged the urine sample at 1,500 × g for 30 min and suspended the sediment with spores in EMEM containing 200 U of penicillin, 200 μg of streptomycin, and 2.5 μg of Fungizone per ml; the sample was incubated at 37°C for 4 h. Thereafter, the spore suspension was centrifuged, and the spores were suspended in EMEM with 10% FBS and inoculated into monolayers of MDCK cells growing on glass cover slips placed in the wells of 24-well plates. The cultures were then incubated at 37°C in the presence of a gas mixture of 5% CO2 in air. When growth was established, the cover slips containing the infected monolayers were transferred to 25-cm2 tissue culture flasks. The isolate was identified as E. cuniculi based on electron microscopy and DNA analysis.
Subsequently, many other E. cuniculi isolates have been established by a number of others. Notably, seven isolates from Switzerland were established in human diploid embryonic lung fibroblasts (MRC-5) (9, 10, 25, 56). These isolates were obtained from urine, BAL fluid, nasal or sinus aspirates, and cerebrospinal fluid. To establish cultures of these isolates, washed pellets from different clinical specimens were used. The pellets were suspended in 10 ml of 5 mM HCl, incubated for 10 min at room temperature, and washed again twice with Hanks' balanced salt solution. The pellets were inoculated into a monolayer of MRC-5 lung fibroblasts growing in 50-ml plastic tissue culture flasks. The medium used was EMEM supplemented with 10% heat-inactivated FBS, 100 U of penicillin, 100 μg of streptomycin, and 0.25 μg of Fungizone per ml. Cultures were incubated at 37°C in a CO2 incubator, and the culture medium was replaced weekly (P. DePlazes, 1999, personal communication). Del Aguila et al. (6) established two isolates, one from urine and the other from sputum, in E6 and HLF cell cultures, respectively. Recently, Rossi et al. from Italy established a culture of E. cuniculi in rabbit kidney cell culture from spores isolated from the nasal epithelium of an Italian man (31). Although most isolates will grow with the use of various methods described as above, some fail to grow in spite of repeated efforts.
Encephalitozoon hellem
Didier et al. (12) established three isolates of a microsporidian parasite in in vitro cultures. One of these isolates was obtained from the corneal tissue of an AIDS patient, and the other two were from conjunctival scrapings of two other AIDS patients (12). The patients' tissue samples were minced, inoculated separately into nearly confluent monolayers of MDCK cell cultures, and incubated at 37°C. The growth medium was RPMI containing 5% FBS. Although these isolates were identical morphologically to E. cuniculi, they differed from E. cuniculi in their antigenic make-up. These authors therefore identified the isolates as Encephalitozoon hellem.
Currently, more than 30 isolates of E. hellem have been established in culture. These isolates have originated from different parts of the world, including one each from the United Kingdom (20) and Spain (30), six from Switzerland (9, 10; P. DePlazes, 1999, personal communication), 11 from Italy (17, 33-35; M. Scaglia, personal communication), and 16 from the United States (4, 12, 13, 47, 48, 52). These isolates originated from different clinical specimens, including corneal or conjunctival scrapings (12, 13), urine (4, 9, 10, 13, 47, 49; P. DePlazes, 1999, personal communication; G. S. Visvesvara, G. J. Leitch, D. A. Schwartz, A. J. da Silva, S. Wallace, H. Moura, N. J. Pieniazek, and R. T. Bryan, unpublished data), BAL fluid (4, 17, 33-35; M. Scaglia, personal communication), sputum (G. S. Visvesvara, G. J. Leitch, D. A. Schwartz, A. J. da Silva, S. Wallace, H. Moura, N. J. Pieniazek, and R. T. Bryan, unpublished data), throat washes (17, 33-35; M. Scaglia, personal communication), and nasal mucosa (4, 20; G. S. Visvesvara, G. J. Leitch, D. A. Schwartz, A. J. da Silva, S. Wallace, H. Moura, N. J. Pieniazek, and R. T. Bryan, unpublished data). The cell lines used to culture these isolates included E6, HLF, MRC-5, MDCK, rabbit kidney (RK-13), and fetal bovine lung fibroblasts. The cell cultures were initially grown in tissue culture flasks or on cover slips placed in 24-well Nunc plates. The media used were EMEM or RPMI 1400, supplemented with FBS. The cultures were incubated at 37°C in either 5% CO2 or air.
In my laboratory, I and my colleagues processed urine, BAL, and sputum specimens as described above for E. cuniculi and inoculated them into E6 or HLF cell cultures. Nasal mucosa specimens were triturated before inoculation into HLF cell culture. The samples were then washed and inoculated into cell cultures as described above. The cell supernatant was removed at least once in 24 h or earlier, if necessary, i.e., if the monolayers appeared to flake off, and replenished with fresh medium. The supernatant was centrifuged, and the sediment was inoculated into the original flasks. In this manner, we established 11 isolates in continuous culture.
However, in other laboratories, different methods have been used to treat the samples before inoculation into cell cultures. For example, Scaglia et al. (34) suspended the samples in a solution (VIB) containing glutamine, 0.5% FBS, 1.5% NaHCO3, 500 U of penicillin, 500 μg of streptomycin, 100 μg of gentamicin, 50 μg of neomycin, and 25 μg of amphotericin B per ml for 2 h at 37°C before inoculation into cell cultures. In any case, after a few days or weeks of culture manipulations, spores of the isolate appeared in large numbers in the culture medium, indicating successful establishment of the cultures. Infected host cells at this time are usually distended and have the “corn on the cob” appearance (Fig. 3F and G). As development proceeds, these foci of infection increase in number, and within a few weeks about 70 to 80% of the monolayers will be infected and the host cells will be completely filled with spores (Fig. 3H) (51), and TEM will reveal parasitophorous vacuoles filled with developing stages and mature spores (52). It is not possible to distinguish E. hellem from E. cuniculi based on TEM micrographs (Fig. 4); additional studies using Western blot or PCR techniques are necessary to distinguish these two species.
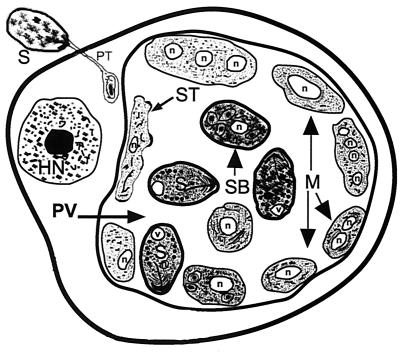
FIG. 4.
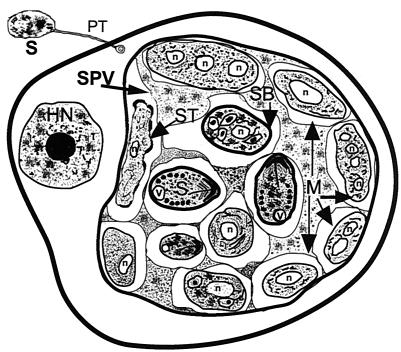
Diagrammatic representation of the characteristic features of a host cell after infection with E. cuniculi and/or E. hellem as seen with the transmission electron microscope. A spore discharges its polar tubule, and the infectious sporoplasm travels down the length of the tubule and infects the host cell. The microsporidian undergoes further development within a parasitophorous vacuole (PV). The developmental stages consist of meronts (M), sporonts (ST), sporoblasts (SB), and spores (S). Meronts are usually attached to the parasitophorous vacuole membrane and may contain one to four nuclei (n), indicating several cycles of merogonous development. Meronts develop into sporonts. During the transformation from meront to sporont, the cell membranes of meronts initially become thickened in a patchy fashion, appearing scalloped. Eventually the entire cell membrane becomes uniformly thickened. During sporogony, the sporont undergoes a division, usually one, forming two sporoblasts (disporous). Sometimes, two or three divisions may take place, resulting in four or eight spores (polysporous). The sporoblast undergoes further development and becomes a spore. Note the parasitophorous vacuole is not septated. HN, host cell nucleus.
Encephalitozoon intestinalis
Encephalitozoon intestinalis, originally described as Septata intestinalis, was recently reclassified as E. intestinalis because of its close antigenic and molecular relationship to other species of Encephalitozoon (18). To date, as many as 27 isolates have been established in culture. Of these, 10 were isolated from urine (7, 10, 27, 44, 46; P. DePlazes, 1999, personal communication), 8 from stool (44), 4 from sputum (7), 3 from nasal mucosa (9, 10, 14, 15; P. DePlazes, 1999, personal communication), and 1 each from BAL fluid (14) and a duodenal aspirate and biopsy (7). The cell lines used to propagate these microsporidia included E6 and HLF (7, 46), human embryonic lung (HEL) (15), monocytes (MDM) (15), MRC-5 (27), and RK-13, MDCK, intestinal (I 047), HT-29, and Caco-2 cell lines (14). The media used consisted of Dulbecco's modified Eagle's medium (DMEM) (7, 46), RPMI 1640 supplemented with heat-inactivated 10% FBS, 2 mM glutamine, penicillin, and streptomycin (14), and EMEM supplemented with 5% FBS, 100 U of penicillin, and 100 μg of streptomycin per ml (15).
Patient specimens (urine, BAL fluid, nasal epithelium, and sputum) were processed as described earlier for E. cuniculi and E. hellem. Duodenal aspirate and biopsy specimens were washed in saline, and the biopsy sample was triturated and washed before inoculation (7). Stool samples were processed as described by van Gool et al. (44), as follows: stool samples containing large numbers of spores were concentrated by a water-ether sedimentation method. These samples were obtained from several AIDS patients who were biopsy positive for E. bieneusi. The concentrated fecal samples were then suspended in an antibiotic cocktail containing 100 μg each of amoxicillin, vancomycin, and gentamicin per ml and 50 μg of flucytosine per ml, placed on a shaker, and incubated at 37°C for 18 h. The feces-spore-antibiotic mixture was centrifuged at 1,550 × g for 10 min, and the sediment was washed twice with phosphate-buffered saline (PBS, pH 7.2). About 400 μl of the antibiotic-treated stool mix was inoculated into monolayers of RK-13 cells established on collagen-treated 24.5-mm Transwell membranes (Costar) with 0.4-μm pore size, placed in a six-well microtiter plate containing DMEM (Gibco no. 041-01095), supplemented with 10% heat-inactivated FBS and 2.5 μg of erythromycin per ml. The culture dishes were centrifuged at 1,070 × g for 30 min in a microtiter plate centrifuge. The pH of the medium changed from 7.0 to 8.0. After the centrifugation, the Transwell membranes were gently washed twice with the culture medium, and the culture dishes containing the Transwell membranes were incubated for 2 days at 37°C in a CO2 incubator. The Transwell membranes were again inoculated with the stool-antibiotic mix as before. The medium in the dishes below the Transwell membranes was replaced every 2 days with fresh medium. Using this method, van Gool et al (44) hoped to establish cultures of E. bieneusi but instead obtained cultures of E. intestinalis.
In my laboratory, using methods of inoculation and manipulation as described for E. cuniculi and E. hellem, I have established several isolates of E. intestinalis in culture. Usually, foci of infection appeared in the inoculated monolayers after about 2 weeks, and within a few weeks of incubation, greater than 85% of the cell culture was infected. At this time, a large number of host cells were distended with spores (Fig. 5A), and clusters of spores were also seen in the culture medium. Smears stained with the Quick-hot Gram-chromotrope also revealed chains of spores, each chain with two, three, four, or even eight spores (as in Fig. 3B to E). After several months of culture, all three species completely destroyed the host cell culture. At this stage the surface of the culture flasks contained cytoskeletal elements of the host cells and spores, some of which had already discharged their polar tubules (Fig. 5B). TEM revealed the development of the parasites within a parasitophorous vacuole that appeared honeycomb-shaped, with septa comprised of fine mesh-like fibrous materials (Fig. 6).
FIG. 5.
(A) An E6 cell infected with E. intestinalis. Magnification, ×1,200. Note the well-defined multiple parasitophorous vacuoles (PV); N, host cell nucleus. (B) An HLF cell culture completely destroyed by E. hellem. Note the spores with everted polar tubules (at arrows), Magnification, ×600. All three species of Encephalitozoon destroy the cell culture, and often the cell cultures are completely covered by spores that either are intact or have discharged their polar tubules.
FIG. 6.
Diagrammatic representation of the characteristic features of a host cell infected with E. intestinalis as seen with the transmission electron microscope. The characteristic features are identical to those of E. cuniculi and E. hellem except for the presence of a septated parasitophorous vacuole (SPV). M, meront; n, parasite nucleus; SB, sporoblast; ST, sporont; v, posterior vacuole; HN, host cell nucleus.
Encephalitozoon sp.
Bocket et al. (1) isolated a microsporidian parasite in an MRC-5 cell line from a urine sample of an AIDS patient. The urine sample was centrifuged at 200 × g for 10 min, and 0.2 ml of the sediment was suspended in EMEM containing 10% FBS, 100 U of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B per ml and stored at 4°C. The sediment was later inoculated into MRC-5, human larynx (Hep2), and African green monkey kidney (Vero) cell lines and incubated at 37°C in a humidified atmosphere in a 5% CO2 incubator. The culture medium was removed weekly and exchanged with fresh medium. Microscopic examination revealed that 20% of the cells were rounded and enlarged and exhibited cytopathic effects in the intracytoplasmic vesicles. Hematoxylin and eosin and Giemsa staining of the cells revealed the presence of small nucleated basophilic bodies that were later identified as microsporidia by TEM. Although they did not identify these agents as Encephalitozoon spp., it is apparent from their published electron micrographs that the parasite definitely belongs to the genus Encephalitozoon because of the presence of parasitophorous vacuole-containing developmental stages. Since no clear-cut septation of the parasitophorous vacuoles is seen, it was most likely E. cuniculi or E. hellem.
Desser et al. (11) isolated a microsporidian identified as Encephalitozoon sp. by inoculating MRC-5 cell lines with corneal scrapings obtained from an AIDS patient. The MRC-5 cell line was grown on cover slips placed in plastic Leighton tubes containing EMEM supplemented with 10% FBS. Since the authors described the development of the parasites within a parasitophorous vacuole that was not septated, it is quite likely that the parasite described was either E. cuniculi or E. hellem.
Furuya et al. (16) isolated Encephalitozoon-like organisms from a human liver lesion produced by larval Echinococcus multilocularis. The liver lesion was minced and digested with 0.25% trypsin (Difco) at room temperature for 30 min. The trypsin digests were filtered through a stainless steel mesh (no. 200) to remove cellular debris, and the filtrate was washed twice in RPMI 1640 medium by centrifugation at 500 × g for 5 min. The sediment was dispersed in RPMI medium containing 500 U of penicillin per ml, 500 μg of streptomycin per ml, 2 mM l-glutamine, 1 mM sodium pyruvate, and 10% FBS, plated on collagen-coated plastic dishes, and incubated at 37°C in a CO2 incubator. After the 10th passage, some of the cells were found to be infected with Encephalitozoon-like parasites which, after repeated subcultures, took over the cell culture. Electron microscopy revealed that the parasite developed within an unseptated parasitophorous vacuole.
Enterocytozoon bieneusi
Enterocytozoon bieneusi is the most frequently identified microsporidian in AIDS patients with diarrhea. Nevertheless, it has eluded all efforts at continuous in vitro culture. In an attempt to establish cultures of this parasite, van Gool et al. (44) inoculated cell cultures with stool samples from several patients who had been biopsy confirmed for E. bieneusi. However, they were unable to culture E. bieneusi but instead established cultures of E. intestinalis. This is probably because the fecal specimens contained both E. bieneusi and E. intestinalis, as it is known that some patients are concurrently infected with both E. bieneusi and E. intestinalis. In such cases, E. intestinalis, which is relatively easy to culture, probably grew quickly and established itself, whereas E. bieneusi failed to grow. All stages of E. intestinalis grow within a septated parasitophorous vacuole (Fig. 6), whereas all stages of Enterocytozoon bieneusi grow within the host cell cytoplasm without any septation between the stages and no parasitophorous vacuoles (Fig. 7).
FIG. 7.
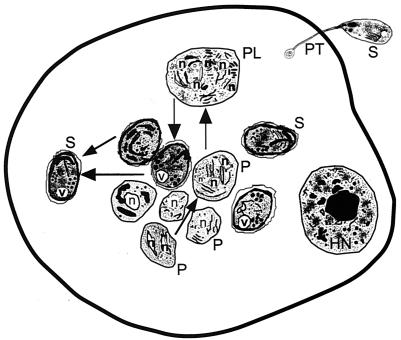
Diagrammatic representation of the characteristic features of a host cell infected with Enterocytozoon bieneusi as seen with the transmission electron microscope. Note that the development takes place in the absence of a parasitophorous vacuole. All stages of the parasite develop within the host cell cytoplasm. The structure formed at early stages of development during the course of infection is called a plasmodium (P) and may contain one or more nuclei. The cell surface of the plasmodium thickens during further development, leading to the formation of sporogonial plasmodium (PL), which divides to form several sporoblast cells. The sporoblasts develop into spores (S). V, posterior vacuole; HN, host cell nucleus.
A short-term culture of several isolates of E. bieneusi was, however, achieved by Visvesvara et al. (48, 51) by inoculating duodenal aspirate and biopsy specimens into E6 and HLF monolayers. The cultures were, however, destroyed by coinfection with an adenovirus that was also present in the patients' samples (51). The short-term cultures lasted anywhere from 6 weeks to 6 months. Both centrifuged duodenal aspirate and macerated biopsy specimens originating from the same patient were inoculated into the same culture flasks. After several weeks of culture, gram-positive spore-like structures measuring 1 to 1.2 μm long were observed (48). TEM revealed various stages of the parasites to be in clusters and in close proximity to one another without any intervening spaces (48). The proliferating stages were characterized by clear spaces, interpreted as electron-lucent inclusions. The proliferative stages appeared in close association with the host endoplasmic reticulum (ER) and mitochondria. Mature spores and sporoblasts with double rows of polar tubule coils were seen. However, the cultures gradually deteriorated and eventually were lost.
Whether EMEM was supplemented with 5% FBS and antibiotics or with nonessential amino acids, 10 mM HEPES, 2 mM l-glutamine, 10% FBS, 0.005 μg of epidermal growth factor per ml, 5 μg of transferrin per ml, 5 μg of insulin per ml, 0.005 μg of selenium, 0.0072 μg of hydrocortisone per ml, 50 μg of gentamicin per ml, and 5 μg of amphotericin B per ml did not make any difference. In addition, it made no difference whether the inoculated cell cultures were incubated in an ordinary laboratory incubator without any special gases, in a 5% CO2 incubator, or in a candle jar. Since many of these clinical specimens were obtained from AIDS patients, they may have contained, in addition to the microsporidians, other microorganisms and viruses. While appropriate antibiotics can eliminate bacterial and fungal contaminants, if the specimens contain adenovirus, the E. bieneusi-inoculated cell culture will be destroyed by viral growth, as has been described previously by Visvesvara et al. (51). Whether fecal samples containing E. bieneusi can be treated with appropriate drugs that will neutralize the DNA-containing adenoviruses without killing E. bieneusi is not known.
Vittaforma corneae (Nosema corneum)
The first successful cultivation of a human microsporidian was achieved by Shadduck et al. (38). The microsporidian, identified as Nosema corneum, was isolated from the corneal biopsy of a 45-year-old immunocompetent male. They used several methods to isolate the organism. Portions of the cornea were minced and inoculated into three different cell lines: rabbit corneal epithelium (SIRC; ATCC 60), MDCK cells, and primary rabbit embryo fibroblasts obtained from 14- to 16-day-old rabbit fetuses. One portion was inoculated into partially confluent cell lines after digestion with 0.1% trypsin and 0.25% collagenase. A portion was explanted directly into 24-well plates. The cell cultures were maintained in EMEM supplemented with 5% FBS and 0.1% gentamicin and incubated in a 5% CO2 incubator. Thirty days postinoculation, foci of infection were seen in SIRC and MDCK cell lines inoculated with trypsin- and collagenase-treated corneal tissue. No growth was seen in rabbit embryo cells or corneal tissue directly explanted into culture wells.
Ultrastructurally, the organisms exhibited a diplokaryon resembling those of the genus Nosema, and therefore the authors classified them as a new species, N. corneum. Based on further study, Silveira and Canning (39, 40) concluded that this parasite was substantially different from Nosema and placed it in the genus Vittaforma under the new combination V. corneae (39). This organism can be grown in E6 and HLF also, in addition to MDCK and RK-13 cells. It grows very differently from Encephalitozoon species. It grows in a centrifugal formation, and the spores line up in rows, sometimes completely surrounding the host cell nucleus (Fig. 8A to E). Recently, Deplazes et al. (10) established a culture of V. corneae by inoculating a centrifuged urine sample into an MRC-5 cell line.
FIG. 8.
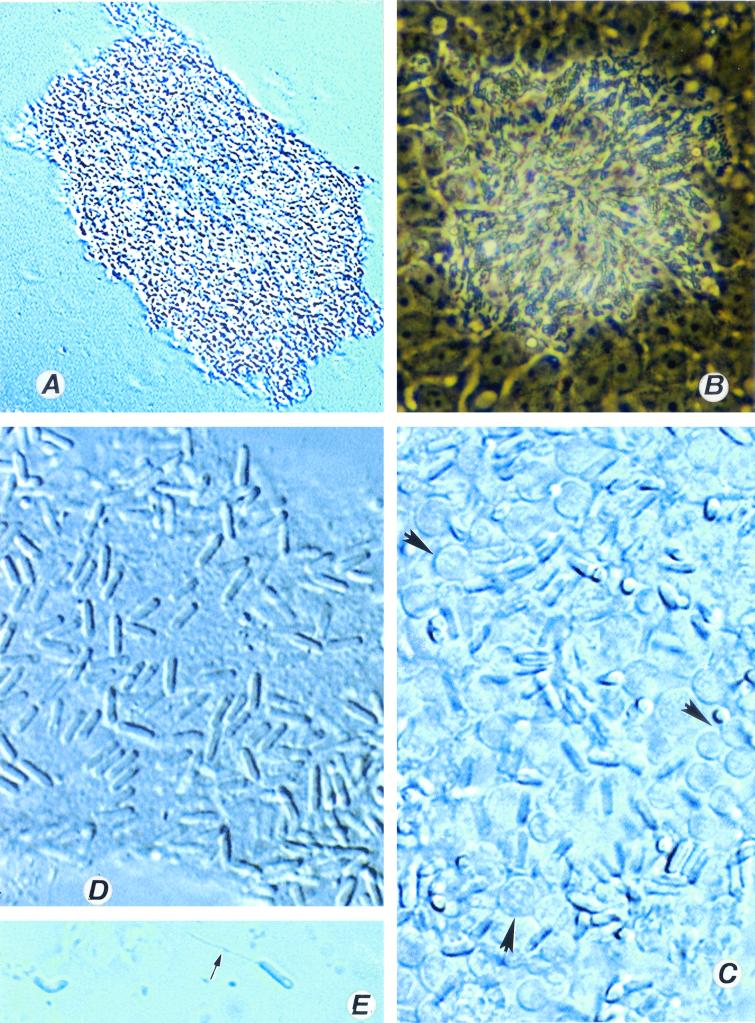
Vittaforma corneae. (A) Low-power view of a heavily infected culture. Magnification, ×120. (B) The same culture viewed with a high dry lens. Magnification, ×600. (C) A heavily infected cell culture with spores and developing stages (arrowhead) of V. corneae. Magnification, ×1,200. Note the absence of the normal architecture of the cell culture. (D) Fully formed spores are interspersed with the host cell debris. Magnification, ×1,200. (E) A spore with everted polar tubule (arrow) and a posterior vacuole. Magnification, ×1,200.
Trachipleistophora hominis
Hollister et al. (21) were successful in infecting cell cultures as well as mice even when material was received 9 days after being obtained from a patient. They purified spores from corneal scrapings and muscle biopsies and inoculated them into several cell lines, including MDCK, RK-13, green monkey kidney (COS-1), a human cell line (L6-C10), and mouse myoblasts. The cultures were established on cover slips in 24-well tissue culture plates containing MEM supplemented with 10% FBS (MDCK and RK-13), in DMEM with 2 mM glutamine and 10% FBS (COS-1 and L6-C10), or in DMEM with 10% FBS and 10% horse serum (mouse myoblasts). In order to collect spores, the muscle biopsy samples were teased apart and incubated with 0.25% trypsin for 15 min at 37°C. The suspension was washed twice by centrifugation, and the sediment was layered onto 50% Percoll in PBS. After washing the spores, they were suspended in PBS, their numbers were estimated, and 105 spores were inoculated into several wells of a 24-well culture plate or 106 spores were inoculated into a petri dish. Although all cell lines supported the growth of the parasites, COS-1 supported the best growth, followed by RK-13 cells. All stages of the parasite were obtained, and based on TEM analysis, it was concluded that the isolate was different from Pleistophora sp., and therefore a new genus and species, Trachipleistophora hominis, was established (21).
Brachiola (Nosema) algerae
Many different species of Nosema (e.g., N. algerae, N. bombycis, N. furnacalis, and N. pyrausta) that infect insects have been established in culture since Trager's initial efforts in 1926 (51). Mosquito-derived N. algerae (now reclassified as Brachiola algerae [24]) has been cultivated in pig kidney cells at 26 and 35°C, and the spores obtained from in vitro culture were shown to infect mosquitoes (43). However, when the cultures were incubated at 37 and 38°C, the parasites invaded the cell line at 37°C but died within 3 days and produced no spores, whereas no development was seen at 38°C. Recently, however, B. algerae isolated from mosquitoes has been shown to grow and produce viable spores at 37°C (29).
Although a few cases of ocular as well as disseminated infections by organisms classified as Nosema spp. in immunocompetent persons have been published (36, 54), it was only recently that a human-infecting B. algerae was established in culture (45). The cultures were established initially in HLF cells by inoculating a corneal scraping along with antibiotics as described for E. cuniculi (5). Subsequently the isolate was also grown in an E6 cell line. A second isolate of N. algerae was established in HLF and E6 cell lines by inoculating skin scrapings from an immunodeficient child suffering from acute lymphocytic leukemia (G. S. Visvesvara, H. Moura, G. J. Leitch, and S. Wallace, Program Abstr. 48th Annu. Meet. Am. Soc. Trop. Med. Hyg., Washington, D.C., 28 November-2 December 1999, abstr. 823). Spores and all developmental stages of B. algerae grow within the cytoplasm of the host cell, and in a heavily infected cell, the spores as well as the developing stages appear to surround the host cell nucleus (Fig. 9A and B).
FIG. 9.
(A) A host cell infected with Brachiola algerae. Note the arrangement of spores around the host (E6 cell) nucleus (N). A single spore is probably in the process of infecting an adjacent cell (arrowhead). Magnification, ×1,200. (B) A spore with an everted polar tubule. Magnification, ×1,200.
MAINTAINING AND PRESERVING INOCULATED CELL CULTURES
Routine Maintenance
Maintenance of established microsporidian cultures is relatively simple. The cultures should be examined microscopically before replenishing the medium or subculturing them. In well-established cultures, the medium may often look cloudy and appear contaminated. Microscopic examination, preferably with the use of an inverted microscope equipped with differential interference contrast or phase optics, will reveal whether the cultures are contaminated or just filled with spores that were expelled from the host cells. If no bacterial contamination is observed, the culture medium should be transferred to a centrifuge tube and fresh medium added to the culture flask. It is preferred that the medium be replaced twice a week for optimum growth of the parasites as well as the host cells. In this manner, the microsporidian-infected cell cultures can then be maintained for several months to a year or even longer (52). New host cells will take the place of dead cells that have flaked off, and the microsporidia, especially the encephalitozoa, will infect new host cells and grow within them. If the cultures need to be expanded rapidly to obtain large numbers of organisms for biochemical and molecular biologic work, it is suggested that the infected cell cultures be scraped and this material be inoculated into one or several fresh uninoculated cell lines. The infected cell cultures may also be trypsinized and the entire contents inoculated into several flasks.
In this laboratory, we routinely split the infected cell cultures into three flasks after trypsin treatment. For trypsinization, the medium is poured off and the infected culture is rinsed with about 1 ml (for 25-cm2 flasks) of a trypsin solution containing 0.05% trypsin and 0.53 mM EDTA in Ca2+- and Mg2+-free Hanks' balanced salt solution. About 1 ml of fresh trypsin solution is then added to barely cover the cell surface. The flask is then incubated for 1 to 3 min at 37°C and gently tapped to detach the cell. The cell suspension is next vigorously pipetted several times, followed by addition of 30 ml of fresh medium. Finally, the cell suspension is mixed thoroughly and aliquoted in 10-ml amounts to each of three 25-cm2 flasks (52). Within 3 to 4 days, monolayers of infected cell cultures are established, and the infected host cells have a corn on the cob appearance. In flasks maintained for several months, it is usual to see patches of broken cells and aggregates of cell debris and spores still attached to the flasks. In such cases, the spores have often extruded their polar tubules and resemble spermatozoa.
Cryopreservation
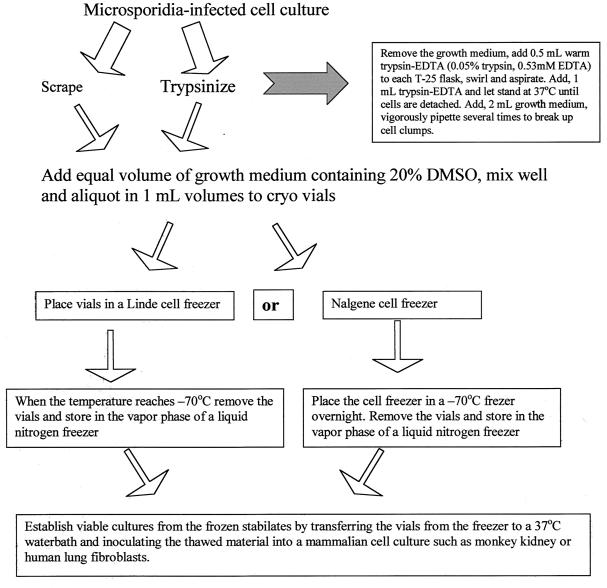
In my laboratory, microsporidium-infected cell cultures are cryopreserved immediately after being established. The ideal method is to scrape or trypsinize the microsporidium-infected cell culture and mix it with an equal volume of the growth medium containing 20% dimethyl sulfoxide. The mixture is then aliquoted in 1.0-ml amounts into plastic cryovials. The vials are placed in a controlled-rate cooling apparatus such as the Linde cell freezer and cooled at the rate of 1°C/min to a temperature of −60°C, at which point the vials are placed in the vapor phase of a liquid nitrogen freezer. However, such equipment is expensive and may not be available in many laboratories.
In this laboratory, we use a relatively simple and inexpensive apparatus, the Cell Freezer (NalgeneCryo 1°C Freezing Container, catalog no. 5100-0001; Nalgene Nunc International, Rochester, N.Y.). It consists of two components, a cylindrical plastic shell with a screw-cap closure and a molded plastic insert containing 18 wells (tube or vial holders) that accept 1-ml cryovials. Approximately 50 ml of either isopropanol or ethylene glycol is introduced into the plastic shell, and the filled cryovials are placed in the molded plastic insert. The Cell Freezer is then placed in a −70°C freezer overnight. The vials are then removed and stored in the vapor phase of a liquid nitrogen freezer. The frozen cultures, now referred to as stabilates, can be stored indefinitely in liquid nitrogen vapor (approximately −150°C). Viable cultures are established from frozen stabilates by immediately transferring the vials from the freezer to a 37°C waterbath and inoculating the thawed material into E6 or HLF cell cultures (Fig. 10). We have used this method to cryopreserve isolates of E. cuniculi, E. hellem, E. intestinalis, V. corneae, and Brachiola algerae (52).
FIG. 10.
Flow chart to illustrate cryopreservation of microsporidia. DMSO, dimethyl sulfoxide.
REFERENCES
- 1.Bocket, L., C. H. Marquette, A. Dewilde, D. Hober, and P. Wattre. 1992. Isolation and replication in human fibroblast cell (MRC-5) of a microsporidian from an AIDS patient. Microb. Pathog. 12:187-191. [DOI] [PubMed] [Google Scholar]
- 2.Canning, E. U., and J. Lom. 1986. The microsporidia of vertebrates. Academic Press, Inc., New York, N.Y.
- 3.Croppo, G. P., G. S. Visvesvara, G. J. Leitch, S. Wallace, and M. A. De Groote. 1997. Western blot and immunofluorescence analysis of a human isolate of Encephalitozoon cuniculi established in culture from the urine of a patient with AIDS. J. Parasitol. 83:66-69. [PubMed] [Google Scholar]
- 4.Croppo, G. P., G. S. Visvesvara, G. J. Leitch, S. Wallace, and D. A. Schwartz. 1998. Western blot identification of the microsporidian Encephalitozoon hellem using immunoglobulin G monoclonal antibodies. Arch. Pathol. Lab. Med. 122:182-186. [PubMed] [Google Scholar]
- 5.De Groote, M. A., G. S. Visvesvara, M. L. Wilson, N. J. Pieniazek, S. B. Slemenda, A. J. da Silva, G. J. Leitch, R. T. Bryan, and R. Reves. 1995. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J. Infect. Dis. 171:1375-1378. [DOI] [PubMed] [Google Scholar]
- 6.Del Aguila, C., H. Moura, S. Fenoy, R. Navajas, R. Lopez-Velez, L. Li, L. Xiao, G. J. Leitch, A. J. da Silva, N. J. Pieniazek, A. A. Lal, and G. S. Visvesvara. 2001. In vitro culture, ultrastructure, antigenic, and molecular characterization of Encephalitozoon cuniculi isolated from urine and sputum samples from a Spanish patient with AIDS. J. Clin. Microbiol. 39:1105-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Aguila, C., G. P. Croppo, H. Moura, A. J. da Silva, G. J. Leitch, D. M. Moss, S. Wallace, S. B. Slemenda, N. J. Pieniazek, and G. S. Visvesvara. 1998. Ultrastructure, immunofluorescence, Western blot, and PCR analysis of eight isolates of Encephalitozoon (Septata) intestinalis established in culture from sputum, urine samples and duodenal aspirates of five patients with AIDS. J. Clin. Microbiol. 36:1201-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Deplazes, P., A. Mathis, R. Baumgartner, I. Tanner, and R. Weber. 1996. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin. Infect. Dis. 22:557-559. [DOI] [PubMed] [Google Scholar]
- 10.Deplazes, P., A. Mathis, M. van Saanen, A. Iten, R. Keller, I. Tanner, M. P. Glauser, R. Weber, and E. U. Canning. 1998. Dual microsporidial infection due to Vittaforma corneae and Encephalitozoon hellem in a patient with AIDS. Clin. Infect. Dis. 27:1521-1524. [DOI] [PubMed] [Google Scholar]
- 11.Desser, S. S., H. Hong, and Y. J. Yang. 1992. Ultrastructure of the development of a species of Encephalitozoon cultured from the eye of an AIDS patient. Parasitol. Res. 78:677-683. [DOI] [PubMed] [Google Scholar]
- 12.Didier, E. S., P. J. Didier, D. N. Friedberg, S. M. Stenson, J. M. Orenstein, R. W. Yee, F. O. Tio, R. M. Davis, C. Vossbrinck., N. Millichamp, and J. S. Shadduck. 1991. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitis. J. Infect. Dis. 163:617-621. [DOI] [PubMed] [Google Scholar]
- 13.Didier, E. S., L. B. Rogers, A. D. Brush, S. Wong, V. Traina-Dorge, and D. Bertucci. 1996. Diagnosis of disseminated microsporidian Encephalitozoon hellem infection by PCR-Southern analysis and successful treatment with albendazole and fumagillin. J. Clin. Microbiol. 34:947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didier, E. S., L. B. Rogers, J. M. Orenstein, M. D. Baker, C. R. Vossbrinck, T. Van Gool, R. Hartskeerl, R. Soave, and L. M. Beaudet. 1996. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J. Eukaryot. Microbiol. 43:34-43. [DOI] [PubMed] [Google Scholar]
- 15.Doultree, J. V., A. L., Maerz, N. J. Ryan, R. W. Baird, E. Wright, S. M. Crowe, and J. A. Marshall. 1995. In vitro growth of the microsporidian Septata intestinalis from an AIDS patient with disseminated illness. J. Clin. Microbiol. 33:463-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuya, K., C. Sato, H. Nagano, N. Sato, and J. Uchino. 1995. Encephalitozoon-like organisms in patients with alveolar hydatid disease: cell culture, ultrastructure, histoimmunochemical localization and seroprevalence. J. Eukaryot. Microbiol. 42:518-525. [DOI] [PubMed] [Google Scholar]
- 17.Gatti, S., L. Sacchi, S. Novati, S. Corona, A. M. Bernuzzi, H. Moura, N. J. Pieniazek, G. S. Visvesvara, and M. Scaglia. 1997. Extraintestinal microsporidiosis in AIDS patients: clinical features and advanced protocols for diagnosis and characterization of the isolates. J. Eukaryot. Microbiol. 44:79S. [DOI] [PubMed] [Google Scholar]
- 18.Hartskeerl, R. A., T. van Gool, A. R. J. Schuitema, E. S. Didier, and W. J. Terpstra. 1995. Genetic and immunological characterization of the microsporidian Septata intestinalis Cali, Kotler and Orenstein, 1993: reclassification to Encephalitozoon intestinalis. Parasitology 110:277-285. [DOI] [PubMed] [Google Scholar]
- 19.Hollister, W. S., E. U. Canning, N. I. Colbourn, and E. J. Aarons. 1995. Encephalitozoon cuniculi isolated from the urine of an AIDS patient, which differs from canine and murine isolates. J. Eukaryot. Microbiol. 42:367-372. [DOI] [PubMed] [Google Scholar]
- 20.Hollister, W. S., E. U. Canning, N. I. Colbourn, A. Curry, and C. J. N. Lacey. 1993. Characterization of Encephalitozoon hellem (Microspora) isolated from the nasal mucosa of a patient with AIDS. Parasitology 107:351-358. [DOI] [PubMed] [Google Scholar]
- 21.Hollister, W. S., E. U. Canning, E. Weidner, A. S. Field, J. Kench, and D. J. Marriott. 1996. Development and ultrastructure of Trachipleistophora hominis n. g., n. sp. after in vitro isolation from an AIDS patient and inoculation into athymic mice. Parasitology 112:143-154. [DOI] [PubMed] [Google Scholar]
- 22.Jaronski, S. T. 1984. Microsporidia in cell culture. Adv. Cell Cult. 18:183-229. [Google Scholar]
- 23.Lowder, C. Y., J. T. McMahon, D. M. Meisler, E. M. Dodds, L. H. Calabrese, E. S. Didier, and A. Cali. 1996. Microsporidial keratoconjunctivitis caused by Septata intestinalis in a patient with acquired immunodeficiency syndrome. Am. J. Ophthalmol. 121:715-717. [DOI] [PubMed] [Google Scholar]
- 24.Lowman, P.M., P. M. Takvorian, and A. Cali. 2000. The effects of elevated temperatures and various time-temperature combinations on the development of Brachiola (Nosema) algerae n. comb. in mammalian cell culture. J. Eukaryot. Microbiol. 47:227-234. [DOI] [PubMed] [Google Scholar]
- 25.Mathis, A., M. Michel, H. Kuster, C. Muller, R. Weber, and P. DePlazes. 1997. Two Encephalitozoon cuniculi subtypes of human origin are infectious to rabbits. Parasitology 114:29-35. [DOI] [PubMed] [Google Scholar]
- 26.Matsubayashi, H., T. Koike, I. Mikata, H. Takei, and S. Hagiwara. 1959. A case of Encephalitozoon-like infection in man. Arch. Pathol. 67:181-187. [PubMed] [Google Scholar]
- 27.Molina, J. M., E. Oksenhendler, B. Beauvais, C. Sarfati, A. Jaccard, F. Derouin, and J. Modai. 1995. Disseminated microsporidiosis due to Septata intestinalis in a patient with AIDS: clinical features and response to albendazole therapy. J. Infect. Dis. 171:245-249. [DOI] [PubMed] [Google Scholar]
- 28.Moura, H., D. A. Schwartz, F. Bornay-Linares, F. C. Sodré, S. Wallace, and G. S. Visvesvara. 1997. A new and improved “quick-hot Gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch. Pathol. Lab. Med. 121:888-893. [PubMed] [Google Scholar]
- 29.Moura, H., A. J. da Silva, I. N. S. Moura, D. A. Schwartz, G. Leitch, S. Wallace, N. J. Pieniazek, R. A. Wirtz, and G. S. Visvesvara. 1999. Characterization of Nosema algerae isolates after continuous cultivation in mammalian cells at 37°C. J. Eukaryot. Microbiol. 46:14S-16S. [PubMed] [Google Scholar]
- 30.Peman, J., F. J. Bornay-Llinares, B. Acosta, J. Lopez-Aldeguer, I. Meseguer, M. J. Figueras, A. Hernandez, V. Peset, M. Gobernado, and G. S. Visvesvara. 1997. First report of case of Encephalitozoon sp. microsporidiosis in a Spanish AIDS patient. Res. Rev. Parasitol. 57:131-134. [Google Scholar]
- 31.Rossi, P., G. la Rosa, A. Ludovisi, A. Tamburrini, M. A. Gomez Morales, and E. Pozzio. 1998. Identification of a human isolate of Encephalitozoon cuniculi type I from Italy. Int. J. Parasitol. 28:1361-1366. [DOI] [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Scaglia, M., S. Gatti, L. Sacchi, S. Corona, G. Chichino, A. M. Bernuzzi, G. Barbarini, G. P. Croppo, A. J. Da Silva, N. J. Pieniazek, and G. S. Visvesvara. 1998. Asymptomatic respiratory tract microsporidiosis due to Encephalitozoon hellem in three patients with AIDS. Clin. Infect. Dis. 26:174-176. [DOI] [PubMed] [Google Scholar]
- 34.Scaglia, M., L. Sacchi, S. Gatti, A. M. Bernuzzi, P. D. P. Polver, I. Piacentini, E. Concia, G. P. Croppo, A. J. Da Silva, N. J. Pieniazek, S. B. Slemenda, S. Wallace, G. J. Leitch, and G. S. Visvesvara. 1994. Isolation and identification of Encephalitozoon hellem from an Italian AIDS patient with disseminated microsporidiosis. APMIS 102:817-827. [PubMed] [Google Scholar]
- 35.Scaglia, M., L. Sacchi, G. P. Croppo, A. da Silva, S. Gatti, S. Corona, A. Orani, A. M. Bernuzzi, N. J. Pieniazek, S. B. Slemenda, S. Wallace, and G. S. Visvesvara. 1997. Pulmonary microsporidiosis due to Encephalitozoon hellem in a patient with AIDS. J. Infect. 34:119-126. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz, D. A., and R. T. Bryan. 1997. Microsporidia, p. 61-94. In C.R. Horsburgh, Jr., and A. M. Nelson (ed.), Pathology of emerging infections. ASM Press, Washington, D.C.
- 37.Shadduck, J. A. 1969. Nosema cuniculi: in vitro isolation. Science 166:516-517. [DOI] [PubMed] [Google Scholar]
- 38.Shadduck, J. A., R. A. Meccoli, R. Davis, and R. L. Font. 1990. First isolation of a microisporidian from a human patient. J. Infect. Dis. 162:773-776. [DOI] [PubMed] [Google Scholar]
- 39.Silveira, H., and E. U. Canning. 1995. Vittaforma corneae N. Comb. for the human microsporidium Nosema corneum Shadduck, Meccoli, Davis and Font, 1990, based on its ultrastructure in the liver of experimentally infected athymic mice. J. Eukaryot. Microbiol. 42:158-165. [DOI] [PubMed] [Google Scholar]
- 40.Silveira, H., and E. U. Canning. 1995. In vitro cultivation of the human microsporidium Vittaforma corneae: development and effect of albendazole. Folia Parasitol. 42:241-250. [PubMed] [Google Scholar]
- 41.Sprague, V., and J. J. Bucknell. 1998. Note on the name-author-date combination for the taxon Microsporidies Balbiani, 1882, when ranked as a phylum. J. Invertebr. Pathol. 71:91. [DOI] [PubMed] [Google Scholar]
- 42.Trager, W. 1935. The hatching of spores of Nosema bombycis Nägeli and the partial development of the organism in tissue cultures. J. Parasitol. 23:226-227. [Google Scholar]
- 43.Undeen, A. H. 1975. Growth of Nosema algerae in pig kidney cell cultures. J. Protozool. 22:107-110. [DOI] [PubMed] [Google Scholar]
- 44.Van Gool, T., E. U. Canning, H. Gilis, M. A. van den Bergh Weerman, J. K. M. Eeftinck Schattenkerk, and J. Dankert. 1994. Septata intestinalis frequently isolated from stool of AIDS patients with a new cultivation method. Parasitology 109:281-289. [DOI] [PubMed] [Google Scholar]
- 45.Visvesvara, G. S., M. Belloso, H. Moura, A. J. da Silva, I. N. S. Moura, G. J. Leitch, D. A. Schwartz, P. Chavez-Barrios, S. Wallace, N. J. Pieniazek, and J. D. Goosey. 1999. Isolation of Nosema algerae from the cornea of an immunocompetent patient. J. Eukaryot. Microbiol. 46:10S. [PubMed] [Google Scholar]
- 46.Visvesvara, G. S., A. J. da Silva, G. P. Croppo, N. J. Pieniazek, G. J. Leitch, D. Ferguson, H. de Moura, S. Wallace, S. B. Slemenda, I. Tyrrel, D. F. Moore, and J. Meador. 1995. In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J. Clin. Microbiol. 33:930-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Visvesvara, G. S., G. J. Leitch, A. J. Da Silva, G. P. Croppo, H. Moura, S. Wallace, S. B. Slemenda, D. A. Schwartz, D. Moss, R. T. Bryan, and N. J. Pieniazek. 1994. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J. Clin. Microbiol. 32:2760-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visvesvara, G. S., G. J. Leitch, L. Gorelkin, M. C. Wilcox, R. Weber, and R. T. Bryan. 1995. Short term in vitro culture of Enterocytozoon bieneusi from four different patients with AIDS. J. Eukaryot. Microbiol. 42:506-510. [DOI] [PubMed] [Google Scholar]
- 49.Visvesvara, G. S., G. J. Leitch, H. Moura, S. Wallace, R. Weber, and R. T. Bryan. 1991. Culture, electron microscopy, and immunoblot studies on a microsporidian isolated from the urine of a patient with AIDS. J. Protozool. 38:105S-111S. [PubMed] [Google Scholar]
- 50.Reference deleted.
- 51.Visvesvara, G. S., G. J. Leitch, S. Wallace, C. Seaba, D. Erdman, and E. P. Ewing, Jr. 1996. Adenovirus masquerading as microsporidia. J. Parasitol. 82:316-319. [PubMed] [Google Scholar]
- 52.Visvesvara, G. S., H. Moura, G. J. Leitch, and D. A. Schwartz. 1999. Culture and propagation of microsporidia, p. 363-392. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 53.Reference deleted.
- 54.Weber, R., R. T. Bryan, D. A. Schwartz, and R. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weber, R., R. T. Bryan, R. L. Owen, C. Mel Wilcox, L. Gorelkin, and G. S. Visvesvara. 1992. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N. Engl. J. Med. 326:161-166. [DOI] [PubMed] [Google Scholar]
- 56.Weber, R., P. DePlazes, M. Flepp, A. Mathis, R. Baumann, B. Sauer, H. Kuster, and R. Luthy. 1997. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection. N. Engl. J. Med. 336:474-478. [DOI] [PubMed] [Google Scholar]