Abstract
The thymus harbors an organ-typical dense network of branching and anastomosing blood vessels. To address the molecular basis for morphogenesis of this thymus-specific vascular pattern, we have inactivated a key vascular growth factor, VEGF-A, in thymus epithelial cells (TECs). Both Vegf-A alleles were deleted in TECs by a complementation strategy termed nude mouse [mutated in the transcription factor Foxn1 (forkhead box N1)] blastocyst complementation. Injection of Foxn1+/+ ES cells into Foxn1nu/nu blastocysts reconstituted a functional thymus. By dissecting thymus stromal cell subsets, we have defined, in addition to medullary TECs (mTECs) and cortical TECs (cTECs), another prominent stromal cell subset designated cortical mesenchymal cells (cMes). In chimeric thymi, mTECs and cTECs but not cMes were exclusively ES cell-derived. According to this distinct origin, the Vegf-A gene was deleted in mTECs and cTECs, whereas cMes still expressed Vegf-A. This genetic mosaic was associated with hypovascularization and disruption of the organ-typical network of vascular arcades. Thus, vascular growth factor production by TECs is required for normal thymus vascular architecture. These experiments provide insights into Foxn1-dependent and Foxn1-independent stromal cell development and demonstrate the value of this chimeric approach to analyzing gene function in thymus epithelium.
Keywords: mesenchyme, nude mice, thymus development, vascular endothelial growth factor, nude mouse blastocyst complementation
The thymus arises from an endodermal epithelial anlage (1), and contact of this epithelium with mesenchyme is essential for its development. T lymphocytes develop in the thymus in close contact with thymus epithelial cells (TECs), which provide MHC, antigenic peptides, and growth factors (2–5). However, TECs may not only constitute the “structure” for T cell development but may also contribute to organ-specific tissue organization. Thymus blood vessels show a typical architecture characterized by large vessels localized at the cortico-medullary junction and a fine network of branching and anastomosing arcades extending far into the cortex (6–8). The molecular basis for formation of this thymus-typical vasculature is unknown. We have detected expression of a key vascular growth factor gene, Vegf-A (referred to below as Vegf), in fetal and adult TECs. Mice lacking both or even a single allele of Vegf die in midgestation (refs. 9 and 10 and reviewed in refs. 11–16). Embryonic development is also disrupted in mice expressing hypomorphic Vegf alleles (17) or increased levels of VEGF (18), pointing to a requirement for a tight regulation of VEGF levels during development.
Strategies to specifically target mutations into the thymic epithelium have not been developed. To determine the role of TEC-derived VEGF for thymus vascularization, we have devised a general method to target mutations into TECs, termed “nude mouse blastocyst complementation.” Nude (nu) mice bear an inactivating mutation in the transcription factor forkhead box N1 (Foxn1), formerly termed winged-helix-nude (Whn) gene (19–21). Foxn1 is essential for thymus development by acting cell-autonomously in the epithelial thymus anlage (3). We show here that Foxn1nu TECs were replaced by Foxn1+ (+ indicates WT) TECs in chimeras obtained by injection of Foxn1+/+ ES cells → Foxn1nu/nu blastocysts. Consequently, ES cells bearing homozygous null mutations in a gene of interest target those mutations to TECs. We have generated “Vegf-/- chimeras” (Vegf-/- ES cells → Foxn1nu/nu blastocysts) and “Vegf+/+ chimeras” (Vegf+/+ ES cells → Foxn1nu/nu blastocysts). In Vegf-/- chimeras, Vegf expression was completely abrogated in medullary TECs (mTECs) and in cortical TECs (cTECs), both of which were Foxn1-dependent. The development of cortical mesenchymal cells (cMes), which we identified as a major constituent of the cortex, was Foxn1-independent. In this study, we describe the role of epithelium for thymus-typical vascular patterning.
Materials and Methods
ES Cells. Vegf-null mutant (D3- and R1-based) ES cell lines are described in ref. 9. The genomic region spanning exon 2 to exon 4 of the Vegf gene was deleted by means of homologous recombination, rendering the gene nonfunctional. Several independent ES clones derived from either D3 or R1 ES cell lines were used throughout the experiments. For each ES cell line used, genomic deletions at the Vegf loci were verified by long-range PCR (Fig. 6A, which is published as supporting information on the PNAS web site).
Generation and Genomic Typing of Chimeras. Nude mice were Swiss nudes (Iffa Credo), NMRI nudes (Taconic Farms), or Black nudes (B6;Cg/JBomTac-Foxn1nuN3, Taconic Farms). Mice were timed-mated to generate blastocysts, which were injected with 10–15 ES cells. The oligonucleotide pair D16mit19 (Map-Pairs, Research Genetics, Huntsville, AL) revealed a polymorphic size difference comparing 129/Sv ES cell (130 bp) and Swiss nude blastocyst (120 bp). DNA chimerism was quantified by comparison of the two bands diagnostic for each of the two strains. This analysis was linear in the tested range (Fig. 6B). In individual mice, the chimerism was determined in several tissues (skin, lung, thymus, eye, brain, heart, kidney, muscle, lymph node, liver, tail, spleen, and, in some cases, ear). Chimerism was determined for a total of 48 organs from four Vegf+/+ chimeras. Of these, 24 organs showed comparable ES cell and blastocyst composition, 2 organs were dominated by ES cell DNA, and 22 organs were dominated by blastocyst DNA. We analyzed a total of 87 organs from seven Vegf -/- chimeras. Thirty-nine organs showed comparable amounts of ES cell and blastocyst DNA, 18 organs contained mostly ES cell DNA, and 29 organs contained mostly blastocyst DNA. Examples of these analyses are shown in Fig. 6B.
Purification of Thymus Stromal Cell Populations. Stromal cells were purified essentially as described in ref. 22 by cell sorting according to their cell surface phenotypes. Thymus tissue was enzymatically digested, and the stroma fractions were enriched by density gradient centrifugation. Details of this purification of stromal cell populations and flow cytometric analysis of peripheral blood T cells are given in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
RT-PCR. RT-PCR was performed as described in ref. 23. PCR conditions, oligonucleotides, and annealing temperatures are described in Supporting Materials and Methods.
Immunohistology. Tissue processing, cutting, staining for immunohistology, and photographic image processing were performed as described in ref. 24. The antibodies used, fluorochrome labelings, and concentrations are stated in Supporting Materials and Methods.
Electron Microscopy. Tissue samples were fixed in phosphate-buffered 2.5% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated in a graded series of ethanol, and embedded in epon. Sections of 0.5 μm were cut and stained with azure-II methylen blue for light microscopic evaluation. Ultrathin sections (50–80 nm) were cut, collected on copper grids, and automatically stained with uranyl acetate and lead citrate for observation with a Zeiss EM 10 electron microscope.
Angiography. To visualize blood vessels by whole-mount fluorescence microscopy, mice were injected i.v. with 200 μg of FITC-labeled BS-1 (Bandeiraea simplicifolia, Sigma) in 300 μl of PBS. Two-and-a-half minutes after injection, mice were killed, and liver, muscle, and thymus tissues were removed; tissue fragments, placed onto glass slides and softly flattened by coverslips, were examined under a fluorescence microscope. Digital images were processed with openlab software.
Results
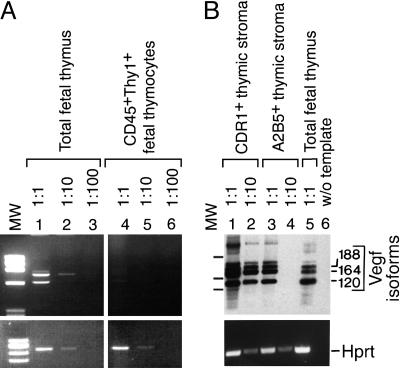
Vegf Expression in Thymic Stromal Cells. Thymic expression of the vasculo/angiogenic factors VEGF, Ang-1, and Ang-2 (reviewed in refs. 25 and 26) was analyzed by RT-PCR. Several VEGF isoforms were expressed at day 15.5 in total fetal thymus but not in thymocytes (Fig. 1A). Thymi were further fractionated by using markers known to separate fetal cortical (CDR1) and fetal medullary (A2B5) epithelium (2). RNAs encoding VEGF120, VEGF164, and VEGF188 were expressed in both types of epithelia. Template titrations suggested that expression of Vegf was ≈10-fold enriched in cTECs when compared with mTECs (Fig. 1B). In addition to Vegf, Ang-1 and Ang-2 RNAs were expressed in the fetal thymus. In contrast to Vegf, however, mTECs were strongly positive but cTECs were only weakly positive for Ang-2 expression (data not shown). We focused our analysis on the role of Vegf.
Fig. 1.
Vegf expression in the developing thymus. (A) Vegf isoforms (Upper) and Hprt (Lower) were amplified by PCR from serial dilutions of cDNAs from total day-15.5 fetal thymus, which includes thymocytes and thymic stroma (lanes 1–3), and from cell sorter-purified CD45+Thy1+ thymocytes (lanes 4–6). PCR products in ethidium bromide-stained agarose gels are shown. (B) Thymus epithelium expresses Vegf. Thymus epithelium was sorted into cortical (CDR1+, lanes 1 and 2) and medullary (A2B5+, lanes 3 and 4) TECs. Total thymus (lane 5) and template omission (lane 6) served as positive and negative controls, respectively. Vegf PCR products were positively identified by Southern blotting, using an internal oligonucleotide probe. Thymus-expressed Vegf RNAs include isoforms VEGF120 (544-bp PCR product), VEGF164 (748-bp PCR product), and VEGF188 (820-bp PCR product). Molecular weight (MW) markers are 1,033, 653, and 517 bp.
Nude Mouse Blastocyst Complementation. To inactivate both Vegf alleles in thymus epithelium, we devised an ES cell-mediated complementation strategy (Fig. 2A). Mice were constructed by transfer of Foxn1+/+ ES cells into Foxn1nu/nu blastocysts. Because Foxn1 acts in cis but not in trans (27, 28), we reasoned that this strategy should yield Foxn1-expressing TECs of ES cell origin but not of blastocyst origin. In addition, we speculated that chimeric mice derived from Vegf-/- ES cells together with Vegf+/+ blastocysts might overcome the lethality of Vegf+/- or Vegf-/- mutations. Indeed, complementation of Foxn1nu/nu blastocysts with Vegf-/- ES cells proved successful in generating viable offspring, demonstrating the feasibility of this strategy to study thymus epithelium in lethal genotypes. Thymus-positive chimeras were identified by the presence of T cells in peripheral blood (Fig. 2B).
Fig. 2.
Gene targeting of thymus epithelium by means of ES cell-mediated nude mouse blastocyst complementation. (A) Injection of Foxn1+/+ ES cells into Foxn1nu/nu blastocysts leads to the development of chimeras in which cells are of either ES cell origin or blastocyst origin. The Foxn1-expressing thymic epithelium is of ES cell origin but not of blastocyst origin because the Foxn1 gene acts in cis but not in trans (27, 28). (B) Screening for thymus formation in chimeric mice. Peripheral blood leukocytes from a WT control mouse (a) and from three chimeras (b–d) were analyzed for the presence of T cells.
Origin of TECs in Nude Blastocyst Chimeras. The origin of Foxn1-expressing thymic epithelium in ES cell → nude blastocyst chimeras was determined in fetal and adult chimeras (Fig. 7A, which is published as supporting information on the PNAS web site). RNA was isolated from chimeric and control thymi and was used to amplify the Foxn1 cDNA by RT-PCR using primers flanking the Foxn1nu single nucleotide deletion (19). Digestion of PCR products released fragments of 45 bp, indicative of Foxn1+, or 44 bp, indicative of Foxn1nu mRNAs. In fetal chimeras, thymic Foxn1 expression was predominantly ES cell-derived, and in adult chimeras, the Foxn1-expressing epithelium was exclusively ES cell-derived. This preeminence of Foxn1+ epithelium over Foxn1nu epithelium was observed for both Vegf+/+ and Vegf-/- adult chimeras (Fig. 7A).
To determine the TEC origin by histology, MHC-mismatched chimeras were generated (24). Foxn1+/+ ES cells [129/ola (MHC class II: I-Ab)] were injected into Foxn1nu/nu blastocysts [NMRI (I-Aq)]. Three-color analyses for expression of I-Ab vs. I-Aq vs. cytokeratin as a panthymic epithelial marker (24, 29) showed that both cortical and medullary cytokeratin+ cells were ES cell-derived (Fig. 7B). Collectively, Foxn1 RNA expression and histological marker studies demonstrated the ES cell origin of TECs.
In contrast to the selective origin of TECs, other organs showed variable contribution of ES cell or blastocyst origin, and there was no obvious bias for or against a Vegf-/- ES cell contribution. In fact, Vegf-deficient ES-derived tissues could strongly contribute to all analyzed tissues, and, remarkably, mice with a dose of >50% of Vegf-/- tissues were viable (see Materials and Methods and Fig. 6B).
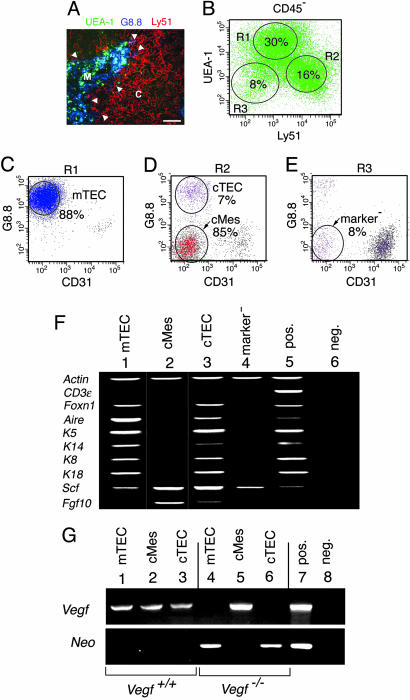
Loss of Vegf Expression in Medullary and Cortical Epithelium but Not in Cortical Mesenchyme. The nonlymphoid (CD45-) stromal component of the thymus was dissected into phenotypically defined subsets (22, 36). Stromal cell populations were purified by cell sorting. The specific expression pattern of the established markers UEA-1 (medullary), G8.8 (medullary and cortical), and Ly51 (cortical) was confirmed on thymus sections (Fig. 3A). CD45- cells were resolved into UEA-1+Ly51- (region R1 in Fig. 3B), UEA-1-Ly51+ (region R2 in Fig. 3B), and UEA-1-Ly51- (region R3 in Fig. 3B) cells. All subsets were further analyzed for expression of G8.8 and, to exclude endothelial cells, CD31 (Fig. 3 C–E). Medullary TECs were sorted as CD45-UEA-1+Ly51-G8.8+CD31- (Fig. 3C). CD45-UEA-1-Ly51+ cells were sorted into cTECs (G8.8+CD31-) and a G8.8-CD31- population (Fig. 3D). The latter population was a major cell type in the cortex (Fig. 3A) and exceeded numbers of cTECs 10-fold (Fig. 3D). These cells lacked epithelial marker gene expression, and we refer to the CD45-UEA-1-Ly51+G8.8-CD31- population as cMes (Fig. 3D). UEA-1-Ly51- stromal cells lacking expression of G8.8 and CD31 were designated marker- (Fig. 3E).
Fig. 3.
Loss of Vegf expression in TECs but not in thymic cMes. (A) Expression of the stromal cell markers UEA-1 (green), G8.8 (blue), and Ly51 (red) on thymus sections. UEA-1+ cells were located in the medulla (M). Most G8.8+ cells were found in the medulla; few were found at the corticomedullary junction (arrow-heads). Ly51+ cells were located in the cortex (C). (Scale bar, 120 μm.) (B–E) Flow cytometry on total thymus cell suspensions. CD45- cells were resolved by UEA-1 vs. Ly51 staining (B). Subpopulations gated according to regions R1, R2, and R3 were analyzed for expression of G8.8 and CD31 (C–E). Medullary TECs were sorted as CD45-UEA-1+Ly51- G8.8+CD31- cells (C). CD45-UEA-1-Ly51+ stromal cells (region R2 in B) were separated into cTECs (G8.8+CD31-) and cMes (G8.8-CD31-) (D). UEA-1-Ly51- stromal cells (R3 in B) lacked expression of G8.8 and CD31 and were designated marker- (E). (F) Stromal cells were analyzed for expression of lineage hallmark genes by RT-PCR using comparable amounts of cDNAs. All populations expressed the positive control gene Actin (lanes 1–5). Stromal cells were free of thymocyte contamination, as shown by lack of CD3ε expression (lanes 1–4). TEC subsets (mTECs, lane 1; cTECs, lane 3) expressed typical TEC genes: i.e., Foxn1, Aire, Keratins 5, 14, 8, and 18, and thymus growth factors (Scf and Fgf10). In contrast, cMes (lane 2) and the marker- population (lane 4) lacked TEC gene expression. (G) TEC subsets but not cMes were Vegf-deficient in Vegf-/- chimeras. Stromal cell subsets defined as in A–F were sorted from WT thymus (lanes 1–3) and from Vegf-/- chimeras (lanes 4–6) and were analyzed for expression of Vegf (Upper) and for expression of the ES cell marker Neo (Lower). Vegf was expressed in all subsets in the normal thymus; it was absent from both TEC subsets (lanes 4 and 6) but not from cMes (lane 5) in Vegf-/- chimeras. Expression of Neo RNA in both TEC subsets (lanes 4 and 6) correlates with the ES cell origin of TECs. Lack of Neo RNA in cMes (lane 5) indicates their nude origin. Total WT thymus (lane 7) and template omission (lane 8) served as positive and negative controls.
Each population was analyzed by RT-PCR for expression of hallmark genes indicative of epithelial vs. mesenchymal lineages (Fig. 3F). All stromal cell preparations were free of thymocyte contamination, as shown by lack of CD3ε expression. Both mTECs and cTECs expressed genes typical for thymus epithelium: i.e., Foxn1, Aire, Keratins 5, 8, 14, and 18, and thymic growth factors Scf (mTECs and cTECs) and Fgf10 (cTECs). In contrast, cMes and the marker- population lacked epithelial gene expression. These experiments demonstrated the purity of the sorted subsets and established membership of each nonhematopoietic population to epithelial and nonepithelial lineages.
Stromal cell subsets were sorted from WT mice and from a pool of seven Vegf-/- chimeras and were analyzed for Vegf expression (Fig. 3G Upper). In the normal thymus, Vegf was expressed in all subsets. This expression pattern was markedly altered in Vegf-/- chimeras. Vegf expression was absent from mTECs and cTECs (Fig. 3G, lanes 4 and 6) but not from cMes (Fig. 3G, lane 5). Because ES cell-derived tissues bear the neomycin resistance gene (Neo) (9), the same cDNA templates were analyzed for Neo expression (Fig. 3G Lower). As expected, Neo mRNA was absent from normal stroma (Fig. 3G, lanes 1–3). In Vegf-/- chimeras, cell types lacking Vegf expression (i.e., mTECs and cTECs) expressed Neo, indicating their ES cell origin (Fig. 3G, lanes 4 and 6). In contrast, cMes expressed Vegf but not Neo, indicative of the blastocyst origin of these cells. Thus, chimeric thymi were composed of Vegf-deficient mTEC and cTEC populations and of Vegf-expressing cMes. This expression pattern correlated well with the distinct origin of the respective cell types.
Thymi in Vegf-/- Chimeras Support T Cell Development. Thymus-positive chimeras were obtained from both Vegf+/+ ES cell and Vegf-/- ES cell → nude blastocyst injections. There was no significant difference in thymus size comparing Vegf+/+ [3.2 × 107 thymocytes (mean from four mice; range 3 × 106-8.5 × 107)] and Vegf-/- [1.6 × 107 thymocytes (mean from six mice; range 4 × 106-3.2 × 107)] chimeras. This reduction in thymus size compared with the normal thymus was probably due to limiting numbers of Foxn1+/+ TECs in many chimeras regardless of the ES cell genotype. Thymi in both Vegf+/+ and Vegf-/- chimeras harbored all major subpopulations characterized by expression of CD4 and CD8. In the peripheral blood, CD4:CD8 ratios were similar in Vegf+/+ [3.6 ± 1.4 (mean ± standard deviation from 9 mice)] and Vegf-/- [2.7 ± 1.2 (mean ± standard deviation from 12 mice)] chimeras. Hence, expression of Vegf by thymic epithelium had no role for T cell development, and the peripheral T cell pool behaved comparably in both types of chimeras.
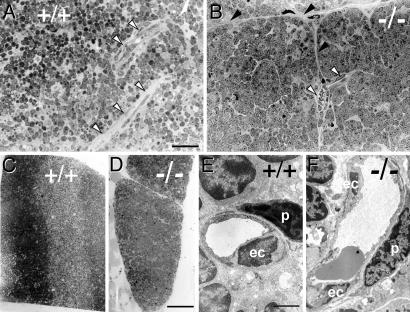
Altered Blood Vessel Architecture in Vegf-/- Chimeras. Thymic blood vessels were compared in Vegf+/+ and Vegf-/- chimeras by light and by electron microscopy (Fig. 4). Thymi of Vegf+/+ chimeras showed a dense network of capillaries within the thymic parenchyme (Fig. 4A). In contrast, thymi from Vegf-/- chimeras harbored multiple intrathymic septae with blood vessels aligned along these structures (Fig. 4B). Moreover, thymic epithelium in Vegf-/- chimeras showed lobulation and “island” formation (Fig. 4 A vs. B). This lobulation was also evident at lower magnification (Fig. 4 C vs. D). The effect of Vegf-deficient TECs on intrathymic endothelium was examined at the ultra-structural level. Microvessels were lined by a continuous layer of capillary endothelial cells, which was covered by surrounding pericytes in both types of chimeras (Fig. 4 E vs. F). Collectively, lack of Vegf in TECs resulted in altered blood vessel topology (mostly septum-associated vessels). Nevertheless, vessels were present, and their endothelium appeared normal.
Fig. 4.
Morphological and electron microscope analysis of chimeric thymi. (A–D) Morphological analysis of thymi in Vegf+/+ (A and C) and Vegf-/- (B and D) chimeras. Thymi were sectioned at 0.5 μm. In the Vegf+/+ thymus, blood vessels were embedded in the thymic stroma (A, open arrows). In contrast, vessels in Vegf-/- thymus (B, open arrows) were septum-associated (B, solid arrows). In addition, the thymus in the Vegf-/- chimera was segmented into multiple small lobe-like structures (pronounced on the left side in B and apparent in D as an overview). (E and F) Electron microscopic analysis of Vegf+/+ and Vegf-/- thymi identified normal microvessels with intact endothelial cell (ec) lining and surrounding pericytes (p) in both types of chimeras (E vs. F). (Scale bar in A, which applies to A and B:50 μm; scale bar in D, which applies to C and D: 300 μm; scale bar in E, which applies to E and F: 6 μm.)
To assess the overall vascular architecture, blood vessels were visualized by angiography. Chimeras were injected intravenously with FITC-labeled Bandeiraea simplicifolia (BS-1) lectin, which binds to carbohydrate moieties on endothelial cells (30, 31). The vessel pattern was visualized by whole-mount fluorescence microscopy. Angiography was performed on thymus, muscle, and liver comparing Vegf+/+ and Vegf-/- chimeras (Fig. 5). Liver and muscle blood vessels were characteristic for each organ, and no differences were observed between Vegf+/+ and Vegf-/- chimeras.
Fig. 5.
Angiography of Vegf+/+ and Vegf-/- chimeras. (A–F) Vegf+/+ (A, C, and E) and Vegf-/- (B, D, and F) chimeras were injected intravenously with the FITC-labeled lectin BS-1. Whole-mount preparations of thymus (A and B), muscle (C and D), and liver (E and F) were examined by fluorescence microscopy (fluorescence in black). Longitudinal blood vessels along muscle fibers (C and D) and fine vascular networks in the liver (E and F) were comparable in Vegf+/+ and Vegf-/- chimeras. Morphometric analyses revealed 41 and 40 vessel branches per mm2 in muscles from Vegf+/+ (C) and Vegf-/- (D) chimeras, respectively, and 183 and 162 branches in the livers from Vegf+/+ (E) and Vegf-/- (F) chimeras, respectively. In the thymus in Vegf+/+ chimeras, blood vessel architecture was characterized by a dense and regular vascular network with frequent branches and loop structures. The Vegf+/+ chimeric thymus contained 123 vessel branches per mm2 of thymus cortex (A). Thymus vasculature in Vegf-/- chimeras showed reduced vessel density and branching frequencies. The Vegf-/- chimera thymus contained 39 vessel branches per mm2 of thymus cortex (B). In addition, the diameter of vessels in Vegf-/- chimeras was larger compared with Vegf+/+ chimeras. (G–J) Angiography is shown at higher magnification for three Vegf-/- chimeras (G–I) compared with WT thymus (J). Morphometric analyses of Vegf-/- chimeras revealed 52, 85, and 40 vessel branches per mm2 of thymus in G, H, and I, respectively. The control WT thymus harbored 190 branches per mm2 of thymus (J).
Thymus blood vessels formed a dense and regular network in Vegf+/+ chimeras. These vessels showed frequent branches that formed loop-like structures (Fig. 5A). In contrast, thymus vasculature was altered in Vegf-/- chimeras. Here, vessel densities and branching frequencies were markedly reduced (numbers are shown in the legend of Fig. 5B). Moreover, vessels in Vegf-/- chimeras were larger when compared with Vegf+/+ chimeras. At higher magnification, the virtual absence of the regular loop-like network and the increased vessel size were evident (Fig. 5 G–I) when compared with the WT (Fig. 5J). The mutant thymus displayed reduced vessel density, increased vessel size, reduced vessel branching, limited vascular loop formation, and vessel septum association.
Discussion
Origin of Thymus Stromal Cells in ES Cell → Nude Blastocyst Chimeras. Injection of Foxn1+/+ ES cells into Foxn1nu/nu blastocysts rescued thymus development in vivo. Chimeric thymi expressed Foxn1+ but not Foxn1nu mRNA, indicating that Foxn1nu/nu blastocysts did not contribute to the Foxn1-expressing stromal cell population. Histological analysis of cortex and medulla showed that MHC class II+ keratin+ cells were only ES cell-derived. These experiments were complemented by “retrospective analysis” of the origin of stromal cell subsets, which was facilitated by stromal cell retrieval from chimeric thymi. Cell fractionation experiments have not only confirmed Foxn1 expression in mTECs and cTECs (21, 22) but also have shown directly that the development of both TEC subsets is Foxn1-dependent. The origin of mTECs and cTECs from Vegf-/- Foxn1+/+ ES cells implied that these populations were Vegf-deficient, which was indeed the case. In addition to mTECs and cTECs, we have purified yet another stromal cell subset (CD45-G8.8-UEA-1-Ly51+). These cells were Foxn1-, expressed none of the analyzed keratin genes, and lacked the endothelial marker CD31, which excludes membership of these cells to hematopoietic, epithelial, or endothelial lineages. Therefore, we consider these cells as mesenchymal, and, based on their histological location, refer to them as cortical mesenchyme. Unexpectedly, both by histology and quantitative retrieval experiments, cMes constituted the major component of the thymus cortex. In Vegf-/- ES cell → Vegf+/+ nude blastocyst chimeras, cMes were from nude origin. There is no obvious reason for this bias toward a Foxn1nu origin of cMes, and it remains to be determined whether this bias also applies to other ES cell → nude blastocyst combinations or whether this skewing is related to the lack of Vegf expression in ES cell-derived cells.
Vascular Phenotype in Chimeric Mice. In the normal thymus, blood vessels showed frequent branches and loop formation. This network of “branching and anastomosing arcades” (6) protruded densely into the cortex. In Vegf-/- chimeras, blood vessels were not completely absent, but they were mostly confined to intrathymic septae whose structures are dominated by mesenchymal cells emanating from the capsule (Fig. 4). Mesenchymal association of blood vessels, together with the fact that cortical mesenchyme was the major source of VEGF in the chimeric thymus, strongly suggests that vasculogenesis was partially rescued by the mesenchymal component. Nevertheless, size, density, and branching pattern of blood vessels were clearly altered. Hence, genetic ablation of Vegf expression in TECs disrupted the typical vascular arcades, most of all in the cortex. This phenotype was thymus-specific because vascular architecture was normal in other organs.
Abundant levels of hypoxia inducible factor (Hif)1α RNA were detected in the developing thymus (data not shown). Thymocyte cellularity increases ≈5-fold per day from days 14 to 18 of development. This burst may contribute to hypoxic conditions within the thymus. Hypoxia can induce Vegf transcription (34). As a result, Vegf can be expressed by epithelium and mesenchyme. Our approach cannot discriminate the contribution of the two epithelial subsets to blood vessel formation. Perhaps only one or both of these two TEC subsets, as well as the cMes, are important. In addition, there is yet another nonhematopoietic, nonendothelial, nonepithelial subset. This rare marker- cell population can also produce VEGF and thus could potentially contribute to the formation of blood vessels in the chimeras. Nevertheless, ablation of Vegf in TECs alone was sufficient to disrupt the network of thymic blood vessels. This requirement for spatially correct Vegf expression is reminiscent of recent data from the brain where neuroectoderm-derived Vegf acts as a key regulator of brain angiogenesis and provides instructive cues for the correct spatial organization of the vasculature (35).
Potential and Limitations of Nude Blastocyst Complementation. T cell development has been analyzed by “RAG (recombination activating gene) blastocyst complementation” (32) or constitutive or conditional gene targeting (33). By comparison, data on the genetic requirements for the development and function of thymic epithelium are rare. We have presented an innovative approach to introduce mutations into TECs. In fetal chimeras, thymus development was functional, as shown by the presence of Vγ3+ T cells, a strictly fetal stage-dependent and thymus-dependent lineage (not shown). Similar to RAG complementation, chimeric thymi did not always reach WT size, even when WT ES cells were used. In addition, the approach does not abrogate gene expression in all cellular components of the thymus, notably the mesenchyme. Nevertheless, nude complementation can be useful to identify the specific impact of epithelial mutations on the thymus. In the future, an alternative to the approach presented here might be conditional gene targeting using thymus stromal cell-specific Cre recombinase-expressing mice. Owing to the lack of known TEC-specific genes, such mice are not yet available. However, even TEC-specific Cre-deleter mice may meet similar limitations inherent to genetically mosaic thymi with specific deletion in TECs but not in mesenchyme. Given that many genes of putative importance for T cell development (e.g., growth factors or Notch ligands) are expressed side by side in TECs and mesenchyme, it will be a challenging task in thymus biology to achieve selective genetic manipulation in all stromal components of the thymus.
Supplementary Material
Acknowledgments
We thank Drs. B. Kyewski and L. Klein for advice on cell isolation, H. J. Fehling for discussions, U. Müller and K. Westfal for blastocyst injections, and M. Dessing for help with microscopy. This work was initiated at the former Basel Institute for Immunology and was supported by Deutsche Forschungsgemeinschaft Grants SFB 497-B5 (to H.-R.R.), MU 1607/1-1 (to S.M.M.), and AU 83/3-3 (to H.G.A.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TEC, thymus epithelial cell; mTEC, medullary TEC; cTEC, cortical TEC; cMes, cortical mesenchymal cells.
References
- 1.Gordon, J., Wilson, V. A., Blair, N. F., Sheridan, J., Farley, A., Wilson, L., Manley, N. R. & Blackburn, C. C. (2004) Nat. Immunol. 5, 546-553. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, G. & Jenkinson, E. J. (2001) Nat. Rev. Immunol. 1, 31-40. [DOI] [PubMed] [Google Scholar]
- 3.Boehm, T., Bleul, C. C. & Schorpp, M. (2003) Immunol. Rev. 195, 15-27. [DOI] [PubMed] [Google Scholar]
- 4.Manley, N. R. & Blackburn, C. C. (2004) in Handbook of Stem Cells, ed. Lanza, R. (Elsevier, Amsterdam), Vol. 1, pp. 391-406. [Google Scholar]
- 5.Rodewald, H. R. (2004) in Adult Stem Cells, ed. Turksen, K. (Humana, Totowa, NJ), pp. 83-100.
- 6.Raviola, E. & Karnovsky, M. J. (1972) J. Exp. Med. 136, 466-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato, S. (1997) Microsc. Res. Tech. 38, 287-299. [DOI] [PubMed] [Google Scholar]
- 8.Anderson, M., Anderson, S. K. & Farr, A. G. (2000) Int. Immunol. 12, 1105-1110. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet, P., Ferreira, V., Breier, G., Pollefeyt, S., Kieckens, L., Gertsenstein, M., Fahrig, M., Vandenhoeck, A., Harpal, K., Eberhardt, C., et al. (1996) Nature 380, 435-439. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara, N., Carver-Moore, K., Chen, H., Dowd, M., Lu, L., O'Shea, K. S., Powell-Braxton, L., Hillan, K. J. & Moore, M. W. (1996) Nature 380, 439-442. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet, P. & Collen, D. (1999) Curr. Top. Microbiol. Immunol. 237, 133-158. [DOI] [PubMed] [Google Scholar]
- 12.Yancopoulos, G. D., Davis, S., Gale, N. W., Rudge, J. S., Wiegand, S. J. & Holash, J. (2000) Nature 407, 242-248. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara, N., Gerber, H. P. & LeCouter, J. (2003) Nat. Med. 9, 669-676. [DOI] [PubMed] [Google Scholar]
- 14.Nagy, J. A., Dvorak, A. M. & Dvorak, H. F. (2003) Trends Cardiovasc. Med. 13, 169-175. [DOI] [PubMed] [Google Scholar]
- 15.Tjwa, M., Luttun, A., Autiero, M. & Carmeliet, P. (2003) Cell Tissue Res. 314, 5-14. [DOI] [PubMed] [Google Scholar]
- 16.Tammela, T., Enholm, B., Alitalo, K. & Paavonen, K. (2005) Cardiovasc. Res. 65, 550-563. [DOI] [PubMed] [Google Scholar]
- 17.Damert, A., Miquerol, L., Gertsenstein, M., Risau, W. & Nagy, A. (2002) Development (Cambridge, U.K.) 129, 1881-1892. [DOI] [PubMed] [Google Scholar]
- 18.Miquerol, L., Langille, B. L. & Nagy, A. (2000) Development (Cambridge, U.K.) 127, 3941-3946. [DOI] [PubMed] [Google Scholar]
- 19.Nehls, M., Pfeifer, D., Schorpp, M., Hedrich, H. & Boehm, T. (1994) Nature 372, 103-107. [DOI] [PubMed] [Google Scholar]
- 20.Kaestner, K. H., Knochel, W. & Martinez, D. E. (2000) Genes Dev. 14, 142-146. [PubMed] [Google Scholar]
- 21.Nehls, M., Kyewski, B., Messerle, M., Waldschutz, R., Schuddekopf, K., Smith, A. J. & Boehm, T. (1996) Science 272, 886-889. [DOI] [PubMed] [Google Scholar]
- 22.Derbinski, J., Schulte, A., Kyewski, B. & Klein, L. (2001) Nat. Immunol. 2, 1032-1039. [DOI] [PubMed] [Google Scholar]
- 23.Waskow, C., Paul, S., Haller, C., Gassmann, M. & Rodewald, H. R. (2002) Immunity 17, 277-288. [DOI] [PubMed] [Google Scholar]
- 24.Rodewald, H. R., Paul, S., Haller, C., Bluethmann, H. & Blum, C. (2001) Nature 414, 763-768. [DOI] [PubMed] [Google Scholar]
- 25.Davis, S. & Yancopoulos, G. D. (1999) Curr. Top. Microbiol. Immunol. 237, 173-185. [DOI] [PubMed] [Google Scholar]
- 26.Rossant, J. & Hirashima, M. (2003) Curr. Opin. Genet. Dev. 13, 408-412. [DOI] [PubMed] [Google Scholar]
- 27.Blackburn, C. C., Augustine, C. L., Li, R., Harvey, R. P., Malin, M. A., Boyd, R. L., Miller, J. F. & Morahan, G. (1996) Proc. Natl. Acad. Sci. USA 93, 5742-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinic, M. M., Rulicke, T., Althage, A., Odermatt, B., Hochli, M., Lamarre, A., Dumrese, T., Speiser, D. E., Kyburz, D., Hengartner, H. & Zinkernagel, R. M. (2003) Proc. Natl. Acad. Sci. USA 100, 1861-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suniara, R. K., Jenkinson, E. J. & Owen, J. J. (1999) Eur. J. Immunol. 29, 75-80. [DOI] [PubMed] [Google Scholar]
- 30.Augustin, H. G., Braun, K., Telemenakis, I., Modlich, U. & Kuhn, W. (1995) Am. J. Pathol. 147, 339-351. [PMC free article] [PubMed] [Google Scholar]
- 31.Hashizume, H., Baluk, P., Morikawa, S., McLean, J. W., Thurston, G., Roberge, S., Jain, R. K. & McDonald, D. M. (2000) Am. J. Pathol. 156, 1363-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen, J., Lansford, R., Stewart, V., Young, F. & Alt, F. W. (1993) Proc. Natl. Acad. Sci. USA 90, 4528-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajewsky, K., Gu, H., Kuhn, R., Betz, U. A., Muller, W., Roes, J. & Schwenk, F. (1996) J. Clin. Invest. 98, 600-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pages, G. & Pouyssegur, J. (2005) Cardiovasc. Res. 65, 564-573. [DOI] [PubMed] [Google Scholar]
- 35.Raab, S., Beck, H., Gaumann, A., Yuce, A., Gerber, H. P., Plate, K., Hammes, H. P., Ferrara, N. & Breier, G. (2004) Thromb. Haemost. 91, 595-605. [DOI] [PubMed] [Google Scholar]
- 36.Gray, D. H., Chidgey, A. P. & Boyd, R. L. (2002) J. Immunol. Methods 260, 15-28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.