Abstract
Coastal marine ecosystems provide important ecosystem services to human populations worldwide. Understanding the contexts in which a species has markedly higher reproductive output is vital for effective management and conservation of these valuable and highly impacted systems. We documented reproductive hotspots along the Oregon coast for an ecologically significant marine invertebrate, the intertidal barnacle Balanus glandula. Greater larval production in both natural and experimental populations was associated with higher primary productivity in the adjacent nearshore ocean, providing strong evidence for bottom-up forcing. Mean cumulative larval production per 100 cm2 in natural barnacle populations in the region of higher primary productivity was almost 5× that of populations in the less productive region. Mean estimated larval production per individual in experimental populations in the region of higher primary productivity was >2× that of populations in the region of lower productivity, and mean larval production per 100 cm2 was >120× greater in the region of higher productivity. Our results highlight the importance of spatial heterogeneity in reproduction and other ecological processes in the marine environment and provide a mechanistic basis for evaluating the relative contributions of different sites when designing marine reserves and other protected areas. Our findings also advance the understanding of the role of bottom-up influences on population and community dynamics and contribute data for the next generation of models of marine community dynamics.
Keywords: Balanus glandula, bottom-up effects, intertidal ecosystems, marine ecology, marine reserves
Documenting spatial variation in reproductive output and understanding the ecological and biogeophysical processes underlying spatial variation in reproduction are critically important and sorely understudied aspects of coastal conservation science. To fulfill biodiversity conservation and fisheries goals, marine reserves and other marine protected areas often are expected to act as “sources” (1), providing offspring to seed populations in less protected areas of the ocean (2). Yet it has been very difficult to identify marine population sources, in large part because many marine organisms have microscopic larval stages and can disperse widely during this early life-history phase.
Identifying spatial variation in reproduction and linking it to underlying mechanisms is a necessary precursor to understanding source-sink dynamics in the marine environment. First principles suggest why organisms in some places should produce more offspring than those in other places, e.g., due to genetic or environmental variation. Yet few empirical examples (3-8) exist from marine populations of spatial variation in reproductive output at scales relevant to management (10s to 100s of kilometers). Moreover, marine population models for reserve design often include the assumption that the coastal ocean is a well-mixed and fairly uniform environment, where potential recruits to the adult populations are part of a global “pool” (9). This view persists despite considerable evidence to the contrary from studies of recruitment (10, 11), larval dispersal (12), species interactions (13, 14), coastal oceanography (15, 16), community assembly (17), and other ecological processes at multiple spatial scales.
After decades of focusing on top-down (i.e., consumer-driven) effects on population and community dynamics, ecologists recognize that top-down and bottom-up (i.e., variation in nutrients and productivity) mechanisms act in concert to regulate ecological systems (18-21). Nonetheless, empirical examples of bottom-up effects on marine populations and communities are relatively rare, in part because of the difficulty of manipulating potential factors on the appropriate spatial and temporal scales (3, 20). Here we test whether bottom-up ecological processes, specifically variation in nearshore primary productivity on the scale of 10s of kilometers, influences reproductive output in an ecologically and scientifically important primary consumer, the intertidal barnacle Balanus glandula. B. glandula plays a crucial role in rocky shore community dynamics as prey for numerous important predators, provides habitat for many organisms, and facilitates the establishment of later successional species such as mussels (22-24). Also, it can be easily observed and manipulated in the field, due to its sessile adult form, abundance, and small size. This species is reproductively active <1 year after settlement in this region (25) and releases larvae into the plankton after brooding them within its carapace for several weeks (26). B. glandula has served as an important model species for marine ecologists for >100 years, in part because its life history is similar to that of most marine species (27). Consequently, the documentation of B. glandula reproductive hotspots is relevant to broader issues in marine conservation and management.
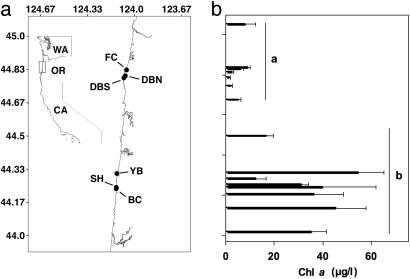
Previous investigations on the Oregon coast indicated that the rocky intertidal ecosystems that were adjacent to nearshore ocean areas with higher levels of phytoplankton differ markedly in community structure and rates of key ecological processes from those sites with consistently lower phytoplankton levels (28, 29). Phytoplankton abundance was measured as chlorophyll a concentration, because it is a strong proxy for primary productivity in the study system (28). These persistent differences in primary productivity have been attributed to the generation of a recurrent eddy by the wider continental shelf and more complex bottom topography at Cape Perpetua (the higher productivity region) relative to Cape Foulweather (the lower productivity region) (30, 31) (Fig. 1). Because phytoplankton is known to be a major food resource for barnacles (32), we predicted that Cape Perpetua barnacle populations would have greater reproductive output than those at Cape Foulweather. To evaluate this hypothesis, we conducted concurrent experimental and observational studies of barnacle populations that spanned the regions of varying primary productivity.
Fig. 1.
Map of the Oregon coast. (a) The main study sites nested within each cape (n = 3 per cape). (b) Long-term chlorophyll a values (μg/liter) from May to September (1998-2003) at latitudinally matched sites (n = 7 per cape). Arithmetic means + standard errors are shown. Means with dissimilar letters were different (t test by cape: t = -6.917, P < 0.0001, df = 12). Sites north of 44.6° N were classified as Cape Foulweather sites, and those south of 44.6° N were classified as Cape Perpetua sites.
Methods
Study Sites. The two capes are ≈80 km apart on the Oregon coast. Representative wave-exposed intertidal sites were selected within each cape. The lower-productivity sites within the Cape Foulweather region were Fogarty Creek (FC); Depoe Bay North (DBN), 3 km south of FC; and Depoe Bay South (DBS), 1 km south of DBN (Fig. 1). The higher-productivity sites within the Cape Perpetua region were Yachats Beach (YB); Strawberry Hill (SH), 6 km south of YB; and Bob Creek (BC), 1 km south of SH. B. glandula ranges from the low to the high intertidal zones in this region, from ≈0 m to + 3.5 m above mean lower low water (H.M.L., unpublished work). It occupies both primary space (i.e., bare substrata) and secondary space (e.g., shells of the mussel Mytilus californianus) in the mid zone and primary space in the low and high zones. Because its highest densities and most persistent populations occur in the mid and high zones, we focused our studies on these portions of the intertidal zone.
Plate Experiment. Using standardized artificial surfaces (i.e., settlement plates), we outplanted barnacles of similar age and origin to three sites nested within each cape (Fig. 1). This approach allowed us to independently evaluate the effects of regional productivity differences on barnacle reproduction by controlling for possible influences of pre-recruitment conditions or varying conspecific densities. Pitted plastic settlement plates were used to generate a common cohort of similar-aged recruits living at a standardized density. The 10 × 10-cm plates were made of opaque gray 6.35-mm-thick polyvinylchloride and attached to the substrata with 6.35-cm stainless steel lag screws. The plates had a regular array of shallow pits (1 mm in diameter, 0.3 mm deep) spaced at 1-cm intervals (n = 81 pits per plate). Barnacles prefer to settle in these pits (29), enabling us to control recruit densities. Barnacles settled onto the plates at a common source [SH, +1 m above mean lower low water (MLLW)]. In October 2002, 2 mo after settlement, the plates were sorted randomly into groups (n = 26-27 replicates) and outplanted to the mid-intertidal zone (+2 m above MLLW) over a 50-m stretch of exposed shore at each site. Plates were photographed monthly to track survival, and new recruits were removed as needed to prevent crowding of the original recruits. In April 2003, when the original recruits were 8 mo old, the plates were collected, photographed, and stored at -20°C for several months before processing. In the laboratory, the original recruits were identified based on the photographs. We recorded the dimensions of each original recruit and scored it as brooding or not.
Observations of Natural Populations. We surveyed natural populations of B. glandula at two sites nested within each cape (FC, DBN, SH, and BC) during six sampling periods in 2002-2003. We collected information on barnacle abundance, size structure, brooding frequency, and calculated larval production per 100 cm2. At each site, transects were stratified by intertidal zone (mid vs. high). Through the biological center of the zone, we ran a 50-m transect. All barnacles within 12 randomly selected 100- cm2 quadrats were collected. Animals were frozen in the field and stored at -20°C until laboratory processing. In the laboratory, barnacles with 2+ mm basal diameters were counted to estimate density per 100 cm2. We measured the dimensions of up to 50 animals per quadrat and scored each as brooding or not. The number of larvae per brood was enumerated for the barnacles sampled in April 2003 (33). These data were used to estimate the number of larvae produced per brooding barnacle for the experimental and surveyed animals.
Recruitment. To investigate the relationship between the timing of larval production and recruitment of new individuals to the benthos, recruitment was monitored during six sampling periods that corresponded to the natural population surveys. Settlement plates [10 × 10-cm, 5 mm-thick polyvinylchloride plates coated with Saf-T-Walk, a rubbery plastic with a textured surface (29)] were deployed at two sites within each region and replaced monthly at each site (n = 5). The Cape Foulweather sites were FC and Boiler Bay (BB, <1 km south of FC), and the Cape Perpetua sites were Yachats Beach (YB) and SH. B. glandula metamorphs were identified and counted under a dissecting microscope.
Data Analysis. All analyses were conducted with jmp in 4.0 (SAS Institute, Cary, NC). When possible, nested ANOVA with cape and site nested within cape was used. Cape was a fixed factor, and site was a random factor. When missing data led to biased estimates of the cape effect in nested models (34), linear contrasts or t tests were used instead to test for cape effects. For all analyses, data were transformed as needed to meet ANOVA assumptions.
For both the experimental and survey data, larval production per barnacle was estimated as a function of barnacle size and shape. Although intertidal zone, site, and barnacle morphology had context-dependent effects on larval production per barnacle, the mean predicted number of larvae generated by the full model (Table 1, top) and the reduced model (Table 1, bottom) that include only barnacle size and shape were not significantly different (paired t test: t <0.0001, P = 0.50, df = 137). This indicated that relative to cape, site and intertidal zone had minor effects on larval production per barnacle. Consequently, site and zone were omitted from the predictive model of larval production per barnacle. We used this model [ln(#larvae per barnacle) = 4.59 + 0.304 (basal diameter) + 0.505 (height: basal diameter)] to estimate larval production per barnacle.
Table 1. Multiple regression results for larval production per barnacle based on the natural population surveys.
| Source of variation | R2 | df | SS | F | P | VC* | Percent of total |
|---|---|---|---|---|---|---|---|
| In(number of larvae produced per barnacle) | |||||||
| Basal diameter | 0.50 | 1 | 24.359 | 56.376 | <0.0001 | – | – |
| Height: basal diameter | 1 | 6.718 | 15.547 | 0.0001 | – | – | |
| Site | 2 | 0.698 | 0.141 | 0.876 | –0.055 | –11.424 | |
| Zone | 1 | 0.043 | 0.025 | 0.887 | – | – | |
| Site × zone | 2 | 5.454 | 6.311 | 0.002 | 0.106 | 21.952 | |
| Error | 130 | 56.171 | – | – | 0.432 | 89.472 | |
| In(number of larvae per barnacle) | |||||||
| Basal diameter | 0.44 | 1 | 43.155 | 92.318 | <0.0001 | – | – |
| Height: basal diameter | 1 | 7.197 | 15.396 | 0.0001 | – | – | |
| Error | 135 | 63.107 | – | – | – | – |
SS, Sum of Squares.
VC, variance components, which were calculated by using the traditional estimated mean squares approach because of the presence of negative variance components
For the among-site comparison of larval production per individual barnacle in the experiment, only estimates from brooding animals were used. The two sites without brooding animals (DBN and DBS) were not included in the statistical analysis of larval production per barnacle, because this would have unduly under-estimated mean individual production per cape. For the population-level analysis, larval production per 100 cm2 was calculated as the sum of the estimated larval production per barnacle for all brooding original recruits per plate. In contrast to the individual analysis, the two sites without brooding barnacles were included in the population-level analysis. This difference accounts for the divergence between the arithmetic mean larval production per 100 cm2 and adjusted [least-square (LS)] means reported in Table 2.
Table 2. Larval production in experimental barnacle populations.
| Site | n | Production per barnacle* | Adjusted production per barnacle† | Production per 100 cm2 | Adjusted production per 100 cm2‡ |
|---|---|---|---|---|---|
| FC | 26 | 859.922 (20.976) | 952.543 (1.169) | 363.006 (252.103) | 0.768 (0.763) |
| DBN | 20 | 0.000 (0.000) | –§ | 0.000 (0.00) | 0.053 (0.886) |
| DBS | 25 | 0.000 (0.000) | –§ | 0.000 (0.00) | 0.044 (0.781) |
| YB | 26 | 2,120.321 (195.786) | 2,052.518 (1.108) | 1,149.143 (511.974) | 6.323 (0.763) |
| SH | 26 | 2,111.875 (123.984) | 2,049.081 (1.060) | 6,637.057 (1,756.030) | 163.876 (0.763) |
| BC | 26 | 2,018.534 (167.504) | 1,948.094 (1.076) | 2,493.098 (1,167.812) | 24.576 (0.763) |
Arithmetic mean (standard errors)
Adjusted production per barnacle calculated as: exp(LS mean). LS means (standard errors) were calculated from an ANOVA based on In-transformed values
Adjusted production per 100 cm2 was calculated as: exp(LS mean)–1. LS means (standard errors) were calculated from a nested ANOVA based on In-transformed values
Because no barnacles were brooding at DBN or DBS, these sites were not included in the statistical analysis
For the survey data, to calculate the mean cumulative larval production for each site, we summed the mean larval production values per 100 cm2 over the six sampling periods. Larval production per 100 cm2 was calculated for each zone×site×sampling period combination as the product of the mean estimated number of larvae produced per barnacle in a given quadrat and the estimated number of brooding barnacles per 100 cm2. For sampling periods where density was not estimated (June and August 2002), the annual mean density for a given zone and site combination was substituted.
Results
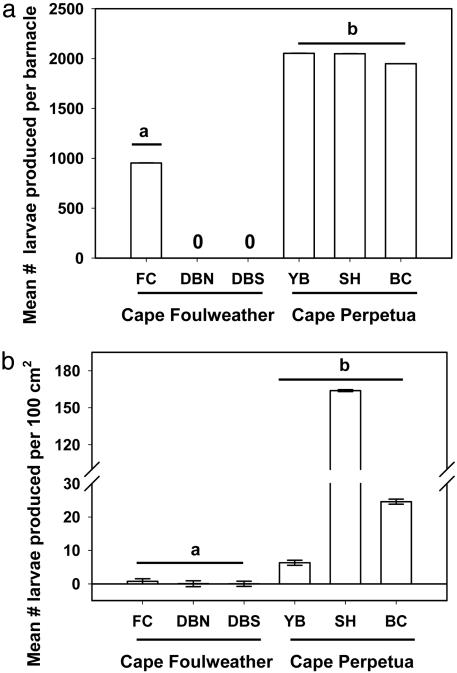
At the end of the experiment, the mean estimated larval production per barnacle was greater in the region of higher primary productivity than in the region of lower productivity (Fig. 2a). Mean larval production per barnacle in the Cape Perpetua populations was 2.4× greater (95% confidence interval: 0.7-7.8×) than for those at Cape Foulweather (Table 2, fourth column).
Fig. 2.
Larval production in the experiment per barnacle (a) and per 100 cm2 (b). LS means and standard errors based on ln-transformed data are shown (standard errors are too small to be visible in a at this scale). Means with dissimilar letters were different (linear contrasts by cape for a, F = 21.561, P < 0.0001, df = 1, 29; b, F = 7.907, P = 0.048, df = 1, 143). See Methods for full site names. No brooding animals were found at DBN or DBS, so the LS means in b were very small.
At the population level, larval production per 100 cm2 was greater for the Cape Perpetua experimental populations than for those at Cape Foulweather (Fig. 2b). Larval production per 100 cm2 was 122.6× greater (95% confidence interval: -3.8× to 7.68 × 104×) for the Cape Perpetua populations than for those at Cape Foulweather (Table 2, last column). Among-site differences also existed. For example, no larvae were produced at DBN and DBS in the Cape Foulweather region. Also, SH in the Cape Perpetua region produced 6.7-213.4× more larvae per 100 cm2 than experimental populations at the other three sites with brooding individuals (Table 2, last column).
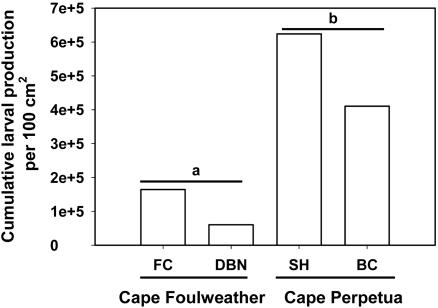
To evaluate the relevance of the experiment to natural population dynamics, we simultaneously surveyed natural barnacle populations in the mid- and high-intertidal zones at two sites nested within each cape and calculated the mean cumulative larval production over the six sampling periods. We found that mean cumulative larval production per 100 cm2 in the Cape Perpetua natural populations was 4.6× greater (95% confidence interval: -3.5× to 12.8×) than that in the Cape Foulweather populations (Fig. 3; Table 3, last row).
Fig. 3.
Cumulative larval production per 100 cm2 in the natural populations. Means with dissimilar letters were different (t test by cape: F = -3.409, P = 0.076, df = 2). See Methods for full site names.
Table 3. Larval production per 100 cm2 in the natural populations.
| Production per 100 cm2
|
|||||
|---|---|---|---|---|---|
| Zone and date | n | FC | DBN | SH | BC |
| Mid zone | |||||
| June 2002 | * | 9,183 (4,018) | 3,108 (790) | 49,464 (17,868) | 6,107 (4,197) |
| August 2002 | 12 | 2,055 (826) | 450 (228) | 16,698 (8,026) | 7,046 (3,061) |
| November 2002 | 12 | 0 (0) | 0 (0) | 983 (983) | 0 (0) |
| February 2003 | 12 | 7,307 (3,033) | ? ? | 21,600 (9,468) | 50,699 (21,025) |
| April 2003 | 12 | 12,395 (6,327) | ? ? | 23,503 (15,239) | 55,080 (29,722) |
| June 2003 | 12 | 8,238 (5,375) | 1,052 (809) | 23,870 (12,583) | 38,186 (11,511) |
| Mid-zone total | 39,177 | 4,610 | 136,118 | 157,118 | |
| High zone | |||||
| June 2002 | † | 482 (338) | ? ? | 30,194 (16,747) | 6,723 (4,935) |
| August 2002 | ‡ | 1,122 (1,122) | 0 (0) | 147,741 (42,148) | 1,746 (1,746) |
| November 2002 | 12 | 0 (0) | 380 (380) | 0 (0) | 634 (634) |
| February 2003 | 12 | 79,328 (2,206) | 45,020 (10,651) | 112,669 (23,907) | 193,711 (34,247) |
| April 2003 | 12 | 42,616 (1,338) | 10,390 (3,362) | 116,512 (30,690) | 49,619 (10,566) |
| June 2003 | 12 | 1,520 (1,520) | 0 (0) | 80,449 (23,077) | 684 (684) |
| High-zone total | 125,068 | 55,790 | 487,565 | 253,117 | |
| Cumulative production§ | 164,245 | 60,400 | 623,683 | 410,235 | |
Results are presented as arithmetic means (standard errors). Missing data are denoted by ?.
Sample sizes varied. FC, DBN, and SH (n = 12) and BC (n = 11)
Sample sizes varied. FC and BC (n = 12), SH (n = 5), and DBN (n = 0)
Sampled sizes varied. DBN, SH, and BC (n = 12) and FC (n = 9)
Cumulative production per 100 cm2 was calculated as the sum of all available means
Considerable differences also existed among sites and between zones in the natural populations. Cumulative larval production varied markedly among the sites: SH cumulative larval production per 100 cm2 was 1.5-10× greater than at the other sites (Table 3, last row). High-zone production accounted for an average of 77% of the cumulative larval production (Table 3). Again, within the high zone, site differences were evident. SH's high-intertidal cumulative larval production was 2-10× that of the other populations, because SH high-zone barnacles continued to produce a substantial number of larvae during the summer, whereas high-intertidal populations at the other sites did not.
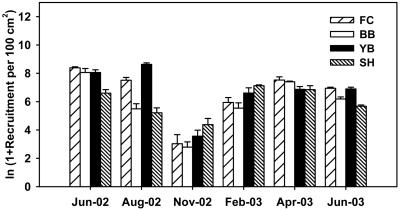
Recruitment of B. glandula on the Oregon coast occurred throughout the year in 2002-2003 and did not differ by cape (Fig. 4; ANOVA on ln-transformed means: F = 0.074, P = 0.812, df = 1, 92). Recruitment varied through time (ANOVA: F = 12.110, P = 0.001, df = 5, 92), with a low point in November 2002, and the rank order of sites changed through time (ANOVA: F = 7.985, P < 0.0001, df = 10, 92).
Fig. 4.
Monthly mid-intertidal recruitment of B. glandula. Ln-transformed means + standard errors are shown. See Methods for full site names.
Discussion
These results demonstrate that not all intertidal sites are equivalent in terms of their reproductive capacity, at least for this species. Reproductive hotspots within the Cape Perpetua region were documented and were associated with more productive nearshore ocean conditions in both natural and experimental populations. Natural barnacle populations in the region of higher primary productivity (Cape Perpetua) produced almost 5× more offspring than those in the region of lower productivity (Cape Foulweather). When variation in age and size classes and pre-recruitment history were controlled for in the experiment, the association was much more striking: mean larval production per individual in the Cape Perpetua experimental populations was >2× that in the Cape Foulweather populations, and mean larval production per 100 cm2 was >120× greater in the Cape Perpetua populations. Given that the differences between the capes in primary productivity and intertidal community dynamics have persisted for >10 years (compare ref. 13 with ref. 14), it is likely that the variation in reproduction we documented is equally persistent.
We have observed similar qualitative differences in reproduction with another ecological important intertidal species, the competitively dominant mussel Mytilus californianus (B.A.M., unpublished work). Mussels exhibit very high fecundity at SH (within the region of higher primary productivity) and low reproductive capacity at FC and BB (within the region of lower productivity).
Although these comparative studies were designed to test the role of the variation in primary productivity between the two capes, other factors (e.g., differences in wave exposure, tidal height, air or water temperature, water flow, or zooplankton abundance) also are known to influence organismal functioning and thus population and community dynamics (35). Wave exposure and tidal height were controlled in these studies, eliminating the first two factors. Barnacles living under higher water temperatures would be expected to grow more rapidly (29) and thus achieve larger carapace sizes (that would enable them to brood more larvae). However, Sanford and Menge (29) found that water temperatures measured at sites within each cape (BB, Cape Foulweather; SH, Cape Perpetua) varied concurrently through time. In terms of air temperature, higher temperatures would be predicted to cause greater physiological stress and thus to impede growth and reproductive output. Yet Menge et al. (28) observed that mean air temperature actually was higher at SH than at BB. Greater water flow rates, which in turn could result in more rapid delivery of phytoplankton, would be predicted to increase barnacle growth and reproduction. But Menge et al. (28) observed the opposite trend: mean flow rates were greater at a site of lower primary productivity (BB) than at a site of higher productivity (SH). Finally, although cape-specific data on abundance of zooplankton (another potential food resource for barnacles) are not available, the lack of significant differences in recruitment between the capes (Fig. 4) suggests that at least this component of the zooplankton assemblage does not vary between the capes. Given the lack of support for the alternatives raised above, our findings are most consistent with the initial hypothesis that bottom-up forcing is responsible for the observed differences in barnacle larval production.
We also found striking among-site differences in larval production in B. glandula within each region. In particular, SH natural and experimental populations produced many more larvae than those at other sites. Also, natural barnacle populations at DBN and DBS produced very few larvae in comparison with the other Cape Foulweather site (FC), and experimental populations produced none at all. We have no data on the variation in abiotic conditions that suggest that DBN and DBS are particularly difficult places for barnacles to live relative to the other Cape Foulweather site, FC, or that SH is a particularly benign environment relative to the other sites within the Cape Perpetua region. In terms of potential variation in competitive interactions, the degree of conspecific crowding, a proxy for intraspecific competition in B. glandula, did not vary between DBN and FC or between SH and BC (33). Also whelk densities (genus Nucella) were similar across sites nested within the capes (H.M.L., unpublished work), suggesting that predation intensity did not vary. Alternatively, SH experimental populations hypothetically might have outperformed those at the other sites, if the animals were locally adapted to that site. Recall that the experimental plates were seeded at SH. However, previous work by Sanford and Menge (29) showed that B. glandula transplanted from a common Cape Foulweather source (BB) showed a similar response to that observed here, with transplanted animals at SH growing faster than those at BB. Also, Bertness et al. (3) found similar trends: barnacles living in phytoplankton-rich Narragan-sett Bay grew to a larger size and had 10× the reproductive output of animals living on the open coast (3). Again, our results support our hypothesis that reproduction is a phenotypically plastic trait in barnacles that can be strongly influenced by variation in primary productivity. Although SH's strikingly high larval production is consistent with the bottom-up hypothesis (as are the lower values from the sites with lower primary productivity), clearly much remains to be explained regarding the mechanisms underlying variation in benthic-pelagic coupling of marine communities within capes or headlands (1-10s of kilometers) (see also refs. 14 and 36).
Considerable differences in larval production also existed between zones. High-zone production accounted for an average of 77% of the cumulative larval production in natural populations. However, the timing of high-intertidal production relative to recruitment of barnacles in this region suggests that many of the high-zone offspring may be “wasted.” Seventy percent of the high-zone larvae were produced in the early spring (February and April 2003) (Table 3). Recruitment of B. glandula on the Oregon coast occurred throughout the year in 2002-2003, although for the past 15 years at least, the major recruitment pulses of B. glandula have tended to occur during the summer and fall (B.A.M., unpublished work). Thus it is likely that mid-intertidal animals contributed substantially to the observed recruitment pulses in summer 2002, when high-zone production was so low. Also, it is unlikely that these summer recruits resulted from the high-zone adults, because barnacle larvae are thought to spend 2-4 weeks in the plankton (37) rather than the 3-4 months that would be required for high-intertidal offspring to account for the usual heavy settlement in late summer. Estimates of larval production based on barnacle cover may overestimate the contribution of high-zone animals to future recruitment.
Our results demonstrate that variation in bottom-up factors, specifically primary productivity, can have considerable influence on population dynamics. Because barnacles interact as competitors, prey, and habitat modifiers with other members of the rocky intertidal community, it is likely that the bottom-up effects we documented extend to the community level as well. Barnacles have played a central role in quantitative models of benthic community dynamics (38, 39). The data reported here could be used to improve these models, particularly by incorporating more realistic estimates of how larval production varies through space and time.
Differential production of offspring across 10s of kilometers is important to consider when managing coastal and nearshore marine ecosystems. Some sites likely will produce many more offspring than others. Reproductive hotspots, like those documented here, are preferable locations in which to site marine reserves and other marine protected areas, because they are more likely to act as sources (than sites producing fewer larvae) and provide offspring for unprotected sites in the region. Managers would do well to “hedge their bets” by spreading reserves and other protected areas over a network of sites (40), based on the best available information.
Our results highlight the need to move beyond merely mapping species and habitat distributions toward a more mechanistic understanding of coastal marine ecosystem functioning. To date, information on ecological processes has rarely been incorporated into reserve design and other marine conservation planning efforts (41). Along with evolutionary mechanisms, ecological processes (e.g., reproduction, recruitment, dispersal, species interactions, disturbance, and allochthonous inputs of nutrients and other resources) underlie the biodiversity patterns upon which conservation and management strategies currently are focused. Consequently, ecological processes, particularly those associated with spatially dynamic oceanographic features, need to be integrated into marine reserve design and other area-based management efforts to ensure persistence of target populations, species, and biological communities. In addition to considering variable reproduction and other ecological processes during the design phase, monitoring of reserves should be structured so that information on key ecological processes can be incorporated into future management efforts in an adaptive manner.
Acknowledgments
We thank J. Rich, A. Guerry, M. Noble, S. Oda, M. O'Donnell, W. Wood, B. Helmuth, M. Denny, and G. Somero for assistance and J. Witman, R. Emlet, V. Weis, and S. Heppell for comments on the manuscript. G. Boehlert and the Hatfield Marine Science Center, B. Abbott, and the Oregon Parks and Recreation Department, Oregon State University, provided access to facilities and research sites. This research was supported by a Mamie Markham Research Award, Environmental Defense, the National Science Foundation, Oregon State University, the University Club of Portland, the Andrew W. Mellon Foundation, and the Partnership for Interdisciplinary Studies of Coastal Oceans (PISCO) through funding from the David and Lucile Packard Foundation. This is PISCO publication number 187.
Abbreviations: FC, Fogarty Creek; DBN, Depoe Bay North; DBS, Depoe Bay South; SH, Strawberry Hill; BB, Boiler Bay; LS, least square; BC, Bob Creek
References
- 1.Pulliam, H. R. (1988) Am. Nat. 132, 652-661. [Google Scholar]
- 2.National Research Council (2001) Marine Protected Areas: Tools for Sustaining Ocean Ecosystems (Natl. Academy Press, Washington, DC).
- 3.Bertness, M. D., Gaines, S. D., Bermudez, D. & Sanford, E. (1991) Mar. Ecol. Prog. Ser. 75, 91-100. [Google Scholar]
- 4.Barnes, H. & Barnes, M. (1968) J. Exp. Mar. Biol. Ecol. 2, 135-153. [Google Scholar]
- 5.Best, P. B. (2001) Mar. Ecol. Prog. Ser. 220, 277-289. [Google Scholar]
- 6.Dugan, J. E., Wenner, A. M. & Hubbard, D. M. (1991) J. Exp. Mar. Biol. Ecol. 150, 63-81. [Google Scholar]
- 7.Hughes, T. P., Baird, A. H., Dinsdale, E. A., Molchaniwskyj, N. A., Pratchett, M. S., Tanner, J. E. & Willis, B. L. (2000) Ecology 81, 2241-2249. [Google Scholar]
- 8.Rogers-Bennett, L., Haaker, P. L., Karpov, K. A. & Kushner, D. J. (2002) Conserv. Biol. 16, 1308-1317. [Google Scholar]
- 9.Gerber, L. R., Botsford, L. W., Hastings, A., Possingham, H. P., Gaines, S. D., Palumbi, S. R. & Andelman, S. (2003) Ecol. App. 13, S47-S64. [Google Scholar]
- 10.Hughes, T. P., Baird, A. H., Dinsdale, E. A., Molchaniwskyj, N. A., Pratchett, M. S., Tanner, J. E. & Willis, B. L. (1999) Nature 397, 59-63. [Google Scholar]
- 11.Connolly, S. R., Menge, B. A. & Roughgarden, J. (2001) Ecology 82, 1799-1813. [Google Scholar]
- 12.Palumbi, S. R. (2004) Annu. Rev. Environ. Res. 29, 31-68. [Google Scholar]
- 13.Menge, B. A., Berlow, E. L., Blanchette, C. A., Navarrete, S. A. & Yamada, S. B. (1994) Ecol. Monog. 64, 249-286. [Google Scholar]
- 14.Menge, B. A., Blanchette, C., Raimondi, P., Freidenburg, T., Gaines, S., Lubchenco, J., Lohse, D., Hudson, G., Foley, M. & Pamplin, J. (2004) Ecol. Monog. 74, 663-684. [Google Scholar]
- 15.Barth, J. A., Pierce, S. D. & Smith, R. L. (2000) Deep-Sea Res. 47, 783-810. [Google Scholar]
- 16.Witman, J. D., Genovese, S. J., Bruno, J. F., McLaughlin, J. W. & Pavlin, B. I. (2003) Ecol. Monog. 73, 441-462. [Google Scholar]
- 17.Witman, J. D., Etter, R. J. & Smith, F. (2004) Proc. Natl. Acad. Sci. USA 101, 15664-15669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter, M. D. & Price, P. W. (1992) Ecology 73, 724-732. [Google Scholar]
- 19.Power, M. E. (1992) Ecology 73, 733-746. [Google Scholar]
- 20.Menge, B. A. (1992) Ecology 73, 755-765. [Google Scholar]
- 21.Menge, B. A., Lubchenco, J., Bracken, M. E. S., Chan, F., Foley, M. M., Freidenburg, T. L., Gaines, S. D., Hudson, G., Krenz, C., Leslie, H., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 12229-12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connell, J. H. (1961) Ecol. Monog. 31, 61-104. [Google Scholar]
- 23.Menge, B. A. (1976) Ecol. Monog. 46, 355-393. [Google Scholar]
- 24.Paine, R. T. (1966) Am. Nat. 100, 65-75. [Google Scholar]
- 25.Leslie, H. M. (2005) Ecology, in press.
- 26.Strathmann, M. F. (1985) Reproduction and Development of Marine Invertebrates of the Northern Pacific Coast (Univ. of Washington Press, Seattle).
- 27.Morgan, S. G. (2001) in Marine Community Ecology, eds. Bertness, M. D., Gaines, S. D. & Hay, M. E. (Sinauer, Sunderland, MA), pp. 159-182.
- 28.Menge, B. A., Daley, B. A., Wheeler, P. A., Dahlhoff, Sanford, E. & Strub, P. T. (1997) Proc. Natl. Acad. Sci. USA 94, 14530-14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanford, E. & Menge, B. A. (2001) Mar. Ecol. Prog. Ser. 209, 143-157. [Google Scholar]
- 30.Grantham, B. A., Chan, F., Nielsen, K. J., Fox, D. S., Barth, J. A., Huyer, A., Lubchenco, J. & Menge, B. A. (2004) Nature 429, 749-754. [DOI] [PubMed] [Google Scholar]
- 31.Kosro, P. M., Barth, J. A. & Strub, P. T. (1997) Oceanography 10, 53-56. [Google Scholar]
- 32.Barnes, H. (1959) Can. J. Zool. 37, 231-236. [Google Scholar]
- 33.Leslie, H. M. (2004) Ph.D. thesis (Oregon State University, Corvallis).
- 34.Quinn, G. P. & Keough, M. J. (2002) Experimental Design and Data Analysis for Biologists (Cambridge Univ. Press, Cambridge, U.K.).
- 35.Menge, B. A. & Branch, G. M. (2001) in Marine Community Ecology, eds. Bertness, M. D., Gaines, S. D. & Hay, M. E. (Sinauer, Sunderland, MA), pp. 221-252.
- 36.Freidenburg, T. L. (2002) Ph.D. thesis (Oregon State University, Corvallis).
- 37.Strathmann, R., Branscomb, E. S. & Vedder, K. (1981) Oecologia 48, 13-18. [DOI] [PubMed] [Google Scholar]
- 38.Connolly, S. R. & Roughgarden, J. (1998) Am. Nat. 151, 311-326. [DOI] [PubMed] [Google Scholar]
- 39.Connolly, S. R. & Roughgarden, J. (1999) Ecol. Monog. 69, 277-296. [Google Scholar]
- 40.Lubchenco, J., Palumbi, S. R., Gaines, S. D. & Andelman, S. (2003) Ecol. App. 13, S3-S7. [Google Scholar]
- 41.Leslie, H. M. (2005) Conserv. Biol., in press.