Abstract
The duration of songs and the intervals between these songs are more variable when wild, adult, free-ranging chipping sparrows sing at dawn than when they sing during the day. The more variable delivery is used to interact with males, and the stereotyped delivery is used to attract females. In captive birds, however, the variability observed at dawn persists during the day. We quantified the expression of an immediate early gene, ZENK, in wild and captive birds and found that the level of song-associated ZENK expression in two song nuclei, Area X and lMAN, was positively related to variability in song duration and intersong interval and could be dissociated from the social context in which the song occurred. Thus, a combination of field and laboratory approaches helped us identify nuclei, context, and behavioral features associated with a change in gene expression thought to be a marker of behavioral variability.
Keywords: dawn chorus, vocal communication, ZENK, sexual selection
The level of expression of an immediate early gene (IEG) in some avian song nuclei is determined not just by the ongoing behavior, song (1-4), but also by the context in which it occurs (5). Because natural contexts are often hard to reproduce in captivity, there is a premium, when studying behavior-associated gene expression, to relate observations in captive animals to observations in the wild. The goal of this study was to compare IEG expression in free and captive singing chipping sparrows, Spizella passerina.
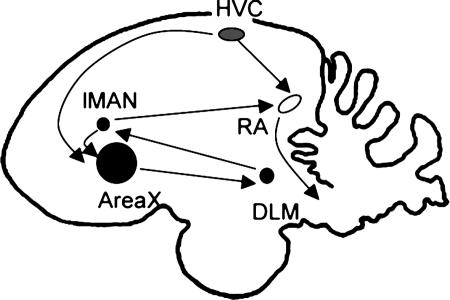
The characterization of IEG expression in free ranging birds was pioneered by Jarvis et al. (4, 6). Just like these earlier studies, we focused on a particular IEG, ZENK, the acronym for the gene known in other species as Zif-268, Egr-1, NGFIA, and Krox-24 (2). ZENK encodes a DNA-binding protein capable of regulating the expression of other genes and is thought to be part of a genomic program that coordinates long-term cellular changes in response to neuronal activation (7, 8). We looked at ZENK expression in four song system nuclei: HVC, RA, Area X, and lMAN (Fig. 1) of male chipping sparrows singing in captivity and in the wild. Such a comparison between free-ranging and captive conspecifics had not been previously attempted.
Fig. 1.
Schematic sagittal view of the chipping sparrow brain, showing some of the song system nuclei, their relative sizes, and interconnections. The anterior pathway (in black) originates in HVC and from there goes to Area X, then DLM, LMAN, and RA. The posterior pathway (in gray) also originates from HVC and from there goes to RA, then brainstem nuclei and muscles of the respiratory tract and the syrinx. Because HVC is a member of both these pathways, its shading of gray is intermediate. Area X, a part of the avian basal ganglia, is disproportionately large when compared with other song nuclei.
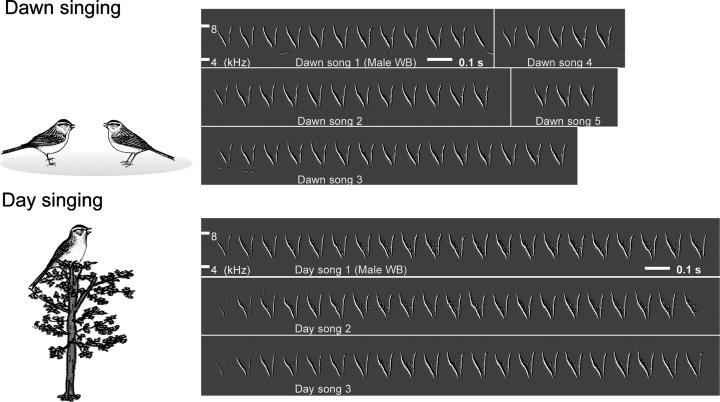
Male chipping sparrows have a simple song that differs in manner of delivery between dawn and the rest of the day. At dawn, territorial males stand on the ground at their territorial boundary and sing a song of variable length at short, variable intervals, while interacting with neighboring males (Fig. 2). Dawn singing occurs during the entire breeding season. During the day, males deliver the same song from the top of a tree near the center of the territory, but at this time, song length and intersong intervals are constant. Daytime singing stops when territorial males pair with a female (9). We compared ZENK expression associated with dawn and daytime singing.
Fig. 2.
Examples of dawn and daytime song of the same, free-ranging chipping sparrow and the context in which they occur. The same single, stereotyped syllable is used in both cases, but songs produced at dawn are more variable in duration than those produced during the remainder of the day.
Materials and Methods
Field Experiment. We collected nine wild, adult, free-ranging, male chipping sparrows near Millbrook, NY, from May to July. Five of these birds were captured and killed after dawn singing and four after daytime singing. Dawn singing in chipping sparrows consists of 15-30 min of intense singing before sunrise. The daytime singing occurs after sunrise and throughout the day (9).
Dawn song in the five males lasted from 15 to 28 min; three of the five birds were unpaired, and two were paired. After song recording, the five males were captured by playing back of conspecific song to draw birds to the mist net started ≈10 min after the last song for the birds that sang less and immediately after the last song for the birds that sang more. All birds were captured 0.5-8.5 min after song playback began; none was killed sooner than 30 min after the onset of singing. Earlier observations suggested such timing would suffice to induce a song-associated rise in IEG expression (3, 10).
The four birds captured after day singing were caught at several different times between 0930 and 1200 hours; by the time they were caught, they had sung for 3.5-6 h. The singing perch, rate of singing, and song duration are very constant throughout the day (from 0600 to 1500 hours) (9). Before each bird was captured, its song was recorded for 1 h and notes made on other ongoing behavior. Immediately after capture, birds were killed by decapitation, and their brain was removed and stored in dry ice. Songs were recorded by using a Marantz 222 tape recorder with SE62 microphone and a Saul Mineroff parabola.
Laboratory Experiment. Thirteen 5- to 9-day-old nestlings were collected from the field in June and July and hand raised; after independence, each juvenile was housed singly in a separate cage. The cages were arranged in a circle around the cage of a wild-caught adult that sang profusely. In this caging configuration, all juveniles could see and hear this adult and also interact with each other. Many of the juveniles imitated the song of the tutor and, at maturity, they all sang a single song type whose pattern was similar to that of wild-type song (11). During the first fall and winter, several cages with two to three female chipping sparrows were in the same room as the males. The room in which our birds were housed had two windows that let in outdoor light. Electrical lights turned on and off to coincide with sunrise and sunset. Because of this lighting arrangement, the captive birds were exposed to the gradual brightening and dimming of light that occurs outdoors at dawn and dusk.
During the following spring, at ≈1 year of age, each of the 13 laboratory-reared birds had a single crystallized song. Females were then removed, and the yearling sparrows sang both at dawn (before the electrical lights went on) and during the day. These 13 hand-reared birds were assigned to one of four groups: (i) Three birds caged singly were kept in the same room; this group was called “socially interactive.” These birds sang at dawn for 15-20 min, and were killed 7-15 min after they stopped singing and at least 30 min after the onset of singing. (ii) Four males held in this socially interactive manner were killed between 0930 and 1200 hours, 3.5-6 h after the onset of daytime singing. (iii) Three “social isolates” were housed singly, each in a separate cage, from where they could not see or hear other birds. (iv) Three birds in a “silent control group” were killed at dawn at about the same time as those in the wild; they were discouraged from singing by one of the researchers being in the room with them. We included this fourth group to test whether the ZENK expression that occurs in song nuclei at dawn is associated with the time of day (waking up) or with singing. Birds in all groups were killed by decapitation. The brain was removed and stored in a -80°C freezer. Procedures were approved by the The Rockefeller University's Animal Care committee.
Song Analysis. In the laboratory, the song of each male was recorded and analyzed by using automatic computer recording software produced by T. J. Gardner in our laboratory. Song recordings made in the wild with a Marantz tape recorder were digitized and analyzed with the same software.
Song quantification used each bird's last 15 min of recorded song because one of the free-ranging wild birds recorded and collected at dawn produced only 15 min of song. We believe that there would be no difference in song features between the last 15 min of daytime singing and singing before that period because a previous study showed that after sunrise, the song rate and song length of chipping sparrows remain constant (9) and they were constant during the last hour of recording (W.-c.L., unpublished data).
Quantified song features: (i) duration, in seconds, of each song; (ii) variability in song duration, defined as: coefficient of variance = (SD/mean) × 100; (iii) mean song rate per minute, obtained by counting all songs produced during the last 15 min during which the bird was recorded and dividing by 15; (iv) variability (coefficient of variance, as defined for feature ii) in song rate during this 15-min period, this value is the SD for the 15 entries, one for each minute; (v) Amount of singing (number of songs × mean song duration per unit time); and (vi) stability of song syllables. Syllable data included duration, pitch, entropy, and frequency modulation. Data for all of the above features were obtained from 800 to 1,000 syllables drawn from dawn and daytime songs. We used sound analysis pro (12) for analyzing the acoustic features of syllables.
Choice of Immediate Early Gene. We chose to focus on ZENK because prior normative studies conducted on songbirds focused on this gene. These studies showed ZENK expression to be quickly up-regulated in song nuclei when a bird sings and just as quickly down-regulated when singing stops. For example, ZENK expression in Area X is already maximal 30 min after the onset of singing and remains at that level if the bird continues to sing for another 30 min; however, if a 30-min period of silence follows the initial 30 min of singing and the bird is then killed, ZENK expression levels return to the baseline (3). Birds killed after dawn singing had engaged in this behavior for 15-28 min, and for them at least 30 min had elapsed between the onset of singing and when they were killed. Birds killed after daytime singing had, by then, engaged in this behavior for 3.5-6.5 h.
In Situ Hybridization. After a bird was killed, its brain was removed and processed for in situ hybridization (13, 14) by using 33P-labeled riboprobes. Serial sagittal 10-um sections were cut throughout the entire brain. Zebra finch ZENK and glutamate receptor NR2A riboprobes were made from T7 and SP6 promoter sites of pGEMTeasy with Promega RNA polymerases. Frozen sections were fixed in 3% paraformaldehyde in PBS (pH 7.0), acetylated, dehydrated in an ascending ethanol series, and air dried. The hybridization solution was then placed on each slide. This hybridization solution was made as follows: 50% formamide/2× standard saline phosphate (SSPE)/EDTA/2 μg/ul yeast tRNA/1 μg/ul polyA/0.4 μg/ul BSA/100 mM DTT. We then added to the hybridization solution with 33P-labeled probes (80 cpm per slide) and then the solution was incubated at 62°C for 13-15 h under mineral oil. Excess probe was removed by washing in 2× SSPE at room temperature for 1 h, then washed with 2× SSPE for 30 min. The next wash was for 1 h at 65°C in a solution of 50% formamide with 0.1× 2-mercaptoethanol; the last wash was in 0.1× SSPE twice at 65°C for 30 min each. Slides were then dehydrated in an ascending ethanol series and exposed to x-ray film (Bio-Max, Eastman Kodak, Rochester, NY) for 2 days.
To quantify gene expression, we first identified the region of interest by using adjacent sections stained with cresyl violet or reacted with the glutamate receptor gene, NR2A (15). We used unsaturated x-ray film (exposed only for 2 days) and quantified outcomes by using published procedures in refs. 15 and 16. Song nuclei and adjacent nonvocal areas, nidopallium adjacent to ventral HVC; nonauditory arcopallium abutting RA; nidopallium rostral to lMAN; and striatum immediately caudal to Area X (17), were outlined with a selection tool, and the average pixel density was calculated in the vocal and adjacent nonvocal tissue by using the photoshop (Adobe Systems, San Jose, CA) histogram function. Results from each nucleus were the average of five to six sections from the right half of the brain in each bird.
Pixel density (shade of gray) was quantified in three ways: as an absolute value, as the ratio of pixel density in each nucleus/adjacent area, and as the ratio of pixel density in that nucleus divided by that found in HVC (e.g., Area X/HVC). We used comparably sized areas adjacent to each nucleus for the ZENK expression ratio.
Statistical Analysis. One-way ANOVAs tested for differences in ZENK expression among the groups of free and captive birds singing at dawn or during the day. We used the Wilcoxon two-tailed matched-pairs signed-ranks test when comparing dawn and daytime singing. We used two-way ANOVA to test the effect of time of day and housing (free or captive) and their interaction on ZENK expression. Analysis of syllable variability was used for each bird in two-way ANOVA paired comparisons of dawn and day singing.
Results
Anatomy. The relative sizes of HVC, RA, Area X, and lMAN are shown schematically in Fig. 1. Compared with the relative sizes of these nuclei in canaries or zebra finches (18-20), with more complex learned songs, the song nuclei of chipping sparrows are relatively smaller, except for Area X that is disproportionately large when compared with the other song nuclei of the chipping sparrow.
The Song of Wild and Captive Birds. Male captive chipping sparrows, like free-ranging wild birds, sang at dawn while standing on the ground; in this case, the floor of their cage. During the daytime, the wild birds sang from a higher elevation, perched in a bush or tree, and captive chipping sparrows perched while singing in their cages (Table 1). Captive birds kept in a same room and interacting socially showed a similar behavior, with one male consistently being the first one to start singing at dawn; captive isolates did not engage in dawn singing, perhaps because at that time of day (dawn), song is an interactive behavior or because they didn't perceive a gradual change in illumination, i.e., no twilight. Finally, the song syllables that any one bird, captive or wild, used in dawn and daytime singing were the same, so that this part of their behavior did not differ between these two contexts. In these ways, there are marked similarities between singing behavior in the wild and under captivity (Fig. 2).
Table 1. ZENK gene expression in four-song nuclei among six experimental groups.
| Wild
|
Captive
|
Two-way ANOVA, P
|
|||||
|---|---|---|---|---|---|---|---|
| Dawn | Day | Dawn* (silent) | Dawn (sing) | Day (social) | Day (isolate) | ||
| HVC | 149.1 ± 14 | 131.5 ± 10 | 78.3 ± 6 | 141 ± 17 | 135 ± 14 | 137 ± 16 | NS |
| RA | 131.4 ± 20 | 110.8 ± 15 | 76.5 ± 7 | 125 ± 22 | 119 ± 21 | 117 ± 17 | NS |
| IMAN | 171.1 ± 16 | 101.9 ± 7 | 81.2 ± 4 | 154 ± 11 | 140 ± 19 | 135 ± 22 | <0.01 |
| Area X | 195.3 ± 11 | 91.2 ± 6 | 77.7 ± 5 | 171 ± 16 | 165 ± 24 | 172 ± 20 | <0.01 |
| HVC/cN | 2.0 ± 0.4 | 1.6 ± 0.5 | 1 ± 0.1 | 2.2 ± 0.2 | 1.9 ± 0.3 | 1.8 ± 0.5 | NS |
| RA/A | 1.9 ± 0.4 | 1.3 ± 0.2 | 1 ± 0.2 | 2.1 ± 0.2 | 1.7 ± 0.4 | 1.6 ± 0.4 | <0.05 |
| IMAN/rN | 2.2 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.1 | 2.1 ± 0.3 | 1.8 ± 0.5 | 1.8 ± 0.4 | <0.01 |
| X/St | 2.4 ± 0.7 | 0.8 ± 0.2 | 0.9 ± 0.3 | 2.6 ± 0.3 | 1.9 ± 0.3 | 2.0 ± 0.4 | <0.01 |
| X/HVC | 1.5 ± 0.1 | 0.6 ± 0.2 | 1 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.1 | 1.3 ± 0.2 | <0.01 |
| IMAN/HVC | 1.2 ± 0.2 | 0.7 ± 0.2 | 1 ± 0.2 | 1.1 ± 0.2 | 1 ± 0.1 | 1 ± 0.1 | <0.025 |
The first four measurements are based on the absolute expression value (i.e., mean pixel density) in each song nucleus. The next four measurements are the ratio of ZENK expression in each song nucleus relative to that in its surrounding area. The last two measurements correspond to ZENK expression in Area X and IMAN relative to that in HVC. All three types of measurements revealed that in the free-ranging wild birds, singing at dawn ZENK expression in Area X and IMAN was, in absolute or relative terms, higher than that in wild birds singing during the day, but these dawn/day differences did not occur among singing captive birds in the laboratory. We used two-way ANOVA to test the difference of gene expression level in four song nuclei among five experimental groups, but we excluded the silent control birds because ZENK expression in them was always very low. NS, not significant.
Dawn silent group (captive birds) was not included in two-way ANOVA analysis.
ZENK Expression Levels in the Song Nuclei. Table 2 shows the mean and SDs of ZENK expression in the four song nuclei studied in each of the experimental groups. These values are presented as average pixel density, as density relative to those in the surrounding area and as density relative to expression in HVC. Fig. 3 shows examples of ZENK expression in one song nucleus, Area X.
Table 2. Song features and behavioral correlates of dawn and daytime singing between wild (free-ranging) and captive birds.
| Wild birds
|
Captive birds
|
||||
|---|---|---|---|---|---|
| Dawn (n = 5 males) | Day (n = 4) | Dawn (n = 3) | Day-social (n = 4) | Day-isolate (n = 3) | |
| Perch height, m | 0.5 ± 0.3 | 7.4 ± 1.6 | On the floor | On the perch | On the perch |
| Amount of singing, sec | 353.5 ± 24.3 | 232.8 ± 27.6 | 321.7 ± 19.7 | 249.3 ± 26.5 | 244 ± 33.9 |
| Song rate, songs/min | 16.8 ± 3.8 | 7.5 ± 0.8 | 15.2 ± 3.1 | 7.8 ± 1.9 | 8.3 ± 2.3 |
| CV of song rate, % | 31.5 | 10.3 | 28.4 | 22.7 | 20.3 |
| Song duration, s | 1.4 ± 0.5 | 2.5 ± 0.3 | 1.6 ± 0.4 | 2.2 ± 0.4 | 2.3 ± 0.4 |
| CV of song duration, % | 30.3 | 12 | 24.2 | 19.3 | 18.5 |
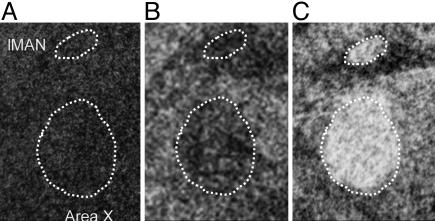
Fig. 3.
In situ hybridization of ZENK expression in three individuals. (A) Silent, captive individual at dawn. (B) Free-ranging individual singing during the day; LMAN is shown above Area X. The outlines of these two nuclei where their boundaries correspond to the drop in ZENK expression. Differences in background between A and B are probably due to the fact that bird A was very quiet in the laboratory, whereas bird B was in a richer outdoor setting and more active. Yet ZENK expression in A and B were similarly low. (C) Free-ranging individual singing at dawn.
ZENK expression in the song nuclei of the three individuals that were killed at dawn and did not sing was very low and not above that in adjacent tissue. By contrast, ZENK expression levels in HVC, RA, Area X, and lMAN of birds engaged in dawn singing in the wild and in captivity were well above those in their surrounding areas and sufficient to make these nuclei stand out as discrete entities.
The absolute levels of ZENK expression in HVC and RA of singing birds (i.e., excluding the three silent ones) were not differentially affected by dawn vs. daytime conditions or by whether the birds sang in captivity or in the wild (one-way ANOVA, for HVC, P = 0.46; RA, P = 0.13). ZENK levels in lMAN and Area X, differed, however among the singing groups (one-way ANOVA for Area X, P = 0.002; lMAN, P = 0.004). ZENK expression was particularly high in the Area X and lMAN of birds singing at dawn, but in the captive birds, although this level of expression was maintained during daytime singing, in the free-ranging wild birds it dropped markedly (Fig. 4). The group differences seen when using the ratio of ZENK expression in a song nucleus/adjacent tissue were similar to those seen when using absolute levels of ZENK expression with one exception. When using the ratio song nucleus/adjacent tissue, ZENK expression in RA also became significantly lower in wild birds engaged in daytime singing (Table 2). The next question, then, is what accounts for the group differences in ZENK expression seen in Area X and lMAN.
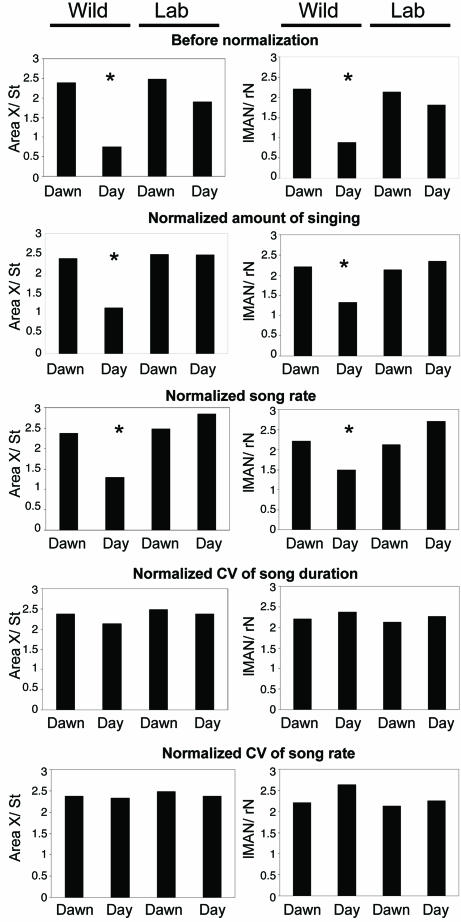
Fig. 4.
The ratio of ZENK expression between song nucleus and adjacent tissue among four different experimental groups before and after normalization for variability in song duration and variability in song rate. After we normalized for the amount of singing and the rate of singing, there is still a significant difference in ZENK expression between dawn and daytime singing wild birds in Area X and lMAN. However, when we normalized for differences in variability of song duration and the rate of singing, the depressed ZENK expression in the Area X and lMAN of free-ranging wild birds singing during the day is brought up to that seen at dawn.
Song Features Related to ZENK Expression. Amount of singing per unit time and the mean rate of singing were significantly higher at dawn than during the remainder of the day in both the captive and wild sparrows (Wilcoxon matched-pairs, two-tailed signed-ranks test for amount of singing, P < 0.005; for song rate, P < 0.01; see also Table 1). However, even when we normalized for the amount of singing and the rate of singing, there still were no group differences in ZENK expression for HVC and RA, and the differences between groups seen in Area X and lMAN persisted (Fig. 4). Therefore, these two behavioral traits do not explain the Area X and lMAN differences in ZENK expression between free birds singing at dawn or during the day.
The one consistent difference that we observed between the song of wild and captive birds occurred during daytime singing. Variability in song rate and song duration was high at dawn in both captive and free-ranging birds and remained relatively high during the day in the captive birds, whether they were communally housed or isolated; however, this variability was low during the day in the free-ranging birds (Table 1). Normalizing for variability in song duration and for variability in song rate eliminated the group differences seen for ZENK expression in Area X and lMAN (Fig. 4).
None of the five syllable features of free-ranging and captive birds differed when the dawn and daytime songs of a same bird were compared (P > 0.05). Thus song variability differences between dawn and day singing were restricted, apparently, to song duration and singing rate.
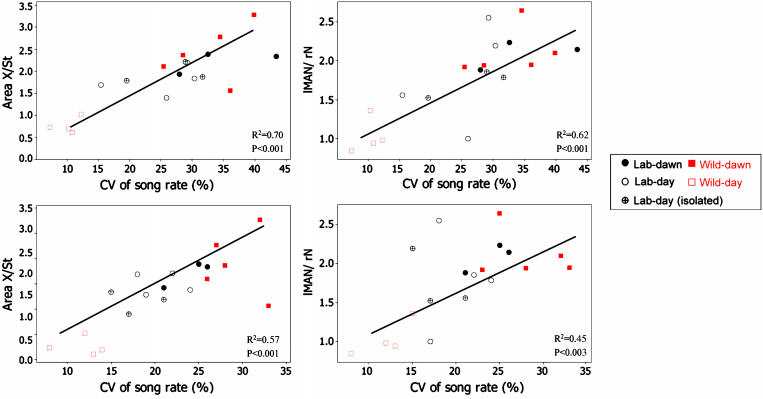
Interestingly, ZENK expression in the various song nuclei of the captive birds was similar regardless of whether these birds were singing, during the day in a socially interactive or in an isolated setting (Table 1). As for the captive birds held communally, the levels of ZENK expression in Area X and lMAN of the isolates singing during the day was significantly higher than that in free-ranging wild birds (Table 1). Our observations offer no evidence that social stimulation, per se, played a role in determining the level of ZENK expression in these nuclei, but we found strong evidence of a correlation between the level of ZENK expression and variability in song delivery (Fig. 5).
Fig. 5.
ZENK expression in Area X and lMAN divided by that in the adjacent tissue. These values are shown in relation to the coefficient of variance of song duration (Lower) and the rate of singing (Upper).
Discussion
Our results indicate that the song-associated level of ZENK expression in the Area X and lMAN of wild, free-ranging chipping sparrows is high at dawn and lower during the day, whereas in hand-reared, captive birds, song-associated ZENK expression is high at dawn and high during the day. These differences between dawn and day and free and captive cannot be related to differences in amount of singing, mean rate of singing, or syllable stereotypy.
Male chipping sparrows singing at dawn use their song differently from those singing during the day. During dawn song, males flexibly change their song duration and intersong interval to match that of neighbors singing a few feet away; such adjustments in song delivery can result in both birds overlapping or alternating their song. In this way, dawn countersinging requires that both birds focus on the ongoing interaction and adjust their output in a manner that does not occur during day song (9). During the day, free, territorial males usually sing from the top of a tree at the center of their territories, rarely interacting with neighboring males. Might the greater social complexity that accompanies dawn singing provide, by itself, the explanation for the higher levels of ZENK expression in Area X and lMAN? Probably not because our adult, captive isolates singing by themselves during the day, unable to hear or see other individuals, also showed high levels of ZENK expression in these two nuclei (Table 1).
We wondered whether higher auditory stimulation at dawn (because other males sing more at dawn) than during the day might contribute to the level of ZENK expression in Area X and lMAN of our chipping sparrows, but we do not think this hypothesis was the case. Those levels of expression were similar in the free and captive birds singing at dawn and in the captive isolates singing during the day, although birds in the latter group heard only their voice. Clearly, there was something about wild birds singing during the day that depressed the relative level of ZENK expression in Area X and lMAN; this “something” seemed to be diluted or absent in our captive birds singing at about the same time and housed communally or as isolates.
We wondered whether levels of ZENK expression might decrease after hours of daytime singing, as described for the habituation of the ZENK response that occurs after a same song is played back many times (1). We do not think so. The amount of daytime singing was comparable in our wild and captive birds, yet ZENK expression dropped in the Area X and lMAN of the free birds but remained significantly higher in the captive ones.
We identified only two behavioral variables that predict the level of song-induced ZENK expression in lMAN and Area X: (i) variability in song duration and (ii) variability in the interval between successive songs. Both were high at dawn in captive and wild birds and in captive birds singing during the day but low in wild birds singing during the day. High variability in these two parameters was associated with high levels of ZENK expression in lMAN and Area X and low song variability was associated with low levels of ZENK expression. Our results are correlative and are not proof of a causal link. When we normalized for these two variables, then the differences in ZENK expression between free birds singing at dawn and during the day disappeared (Fig. 4).
We found a likely causal link between ZENK expression and the manner of singing in prior studies. Bottjer et al. (21) reported that bilateral lMAN lesions in juvenile male zebra finches, Taeniopygia guttata, prevented them from imitating a model and developing normal song, although the same lesions in adults had apparently no effect on song. Subsequently, it was shown that the primary effect of such lesions was to induce premature song stereotypy in juveniles, an effect that presumably stood in the way of song learning (22). Recently, neurons in the lMAN of juvenile songbirds have been shown to have highly variable patterns of firing when these birds sing. The firing of individual lMAN neurons bears no relation to a particular song syllable or part of song, This quasi-random firing, which is much more active in singing than in silent birds, is thought to drive variability in the song of juvenile and adult zebra finches, where the phenomenon was discovered, so that when this firing is suppressed by lesion of lMAN or by infusion into lMAN of action potential blockers, song becomes immediately much more stereo-typed (23, 24). Apparently, whereas the HVC to RA descending pathway (Fig. 1) provides the pattern of learned song (18, 25, 26), the lMAN → RA connection regulates the variability in execution that, during development, is necessary for vocal learning to occur. It follows that higher levels of ZENK expression in lMAN, driven by the increased firing of lMAN neurons, will be associated with greater variability in song delivery, which is what we observed. This rationale fully accounts for the fact that ZENK expression in lMAN is equally low in adult chipping sparrows prevented from singing and in free birds producing highly stereotyped day song.
The neurophysiological observations on zebra finches that related the firing of lMAN neurons to song variability focused on variability in syllable structure and serial order of syllables, both important for reinforcement learning and song imitation (23, 24). However, when our free chipping sparrows delivered their song in a variable temporal manner at dawn, they maintained throughout high syllable stereotypy and the invariant serial order of a monotonous repetition. In these birds, lMAN involvement, as inferred from ZENK expression, was related to the flexible temporal execution of the behavior. RA neurons that project to medullary relays involved with song production receive, in their dendrites, interspersed inputs from HVC and lMAN; although the lMAN inputs are numerically smaller than those from HVC, they may nonetheless regulate the firing of their target RA neurons (27). Thus, increased activity in lMAN may occur in either of two contexts: (i) stochastic jitter associated with learning (23, 24) and (ii) more flexible temporal delivery, which occurs when birds countersing. It would be of interest, to know whether the lMAN firing pattern is the same in both cases, vested on the same lMAN neurons, and distributed to the same RA recipients. In our minds, both kinds of lMAN involvement in behavioral plasticity could point to very different anterior forebrain pathway roles.
There is a precedent for linking ZENK expression in lMAN and Area X with manner of song delivery. In zebra finch males, ZENK expression in Area X and lMAN is high when the birds sing undirected song but very low when they direct the same song at a female (3). Behavioral observations indicate that the directed song is more narrowly stereotyped than the undirected one (23). Although there are no electrophysiological recordings from chipping sparrows, we suspect that the diurnal changes in ZENK expression seen in the lMAN and Area X of free chipping sparrows can be accounted for by underlying differences in neural activity.
The cortical-basal ganglia-thalamus-cortical loop of mammals is strikingly similar to the anterior forebrain pathway of songbirds, of which Area X (homologous to the striatum) and lMAN are part (28-31). In the mammalian circuit, the basal ganglia are involved with the sequencing of behavior, fine motor control, and associated cognitive functions (32-36). It seems possible that in the free chipping sparrows, the anterior forebrain pathway is similarly engaged during dawn singing but less so during the remainder of the day. If this interpretation is correct then, we must suppose that, for reasons unknown, this function remains more engaged during the day in the captive birds. It could be that this result is a way in which the birds were housed and reared in the laboratory, which may be accompanied by hormonal changes, to which the song system, including nucleus lMAN, is sensitive (37, 38). Because the neurons of lMAN receive input, through the thalamus, from Area X and send input to Area X and RA, these circuit relations may provide the mechanism for the similar changes in ZENK expression observed in Area X and lMAN and, to a lesser extent, in RA.
Taken together, our observations suggest that the more variable delivery of song is the one that involves Area X and lMAN to a greater extent, as suggested by the high levels of ZENK expression there, whereas the more invariant (and possibly “automatic”) rendering of the song involves this anterior pathway less. Although the involvement of Area X and lMAN in song learning has been known for some years, we now suspect that this involvement is needed, too, for the subtle differences in song variability and timing that occur during vocal interactions after the song has been mastered. As an aside, it is tempting to speculate that female zebra finches and chipping sparrows, the apparent target of very stereotyped singing, prefer stereotyped over variable song, whereas variable song is reserved for the more contentious interactions among males.
Important brain functions might be blurred in studies that draw all their data from captive animals reared and housed under unnatural conditions. In an earlier study that looked at new neuron recruitment in the brain of captive and free songbirds, this concern proved justified (39). The insights offered in this article could not have been gleaned from captive or free individuals alone, but we found a comparison of the two most helpful.
Acknowledgments
We thank Erich Jarvis and Kazuhiro Wada (Duke University, Durham, NC) and Robert Agate and Moritz Hertel (The Rockefeller University) for teaching us the in situ hybridization technique; Kazuhiro Wada for providing us with the ZENK and glutamate receptor NR2A probes, for advising us on quantification of x-ray film, and for his valuable comments; Tim Gardner for the automatic sound recording software; the Institute of Ecosystem Studies (Millbrook, NY), for kindly allowing us to use their land for field experiments; Meng-ping Tu, Dania Vallinova and, particularly, Debbie Gardner for help with hand-raising sparrows and Daun Jackson, Sharon Sepe, and Helen Ecklund for the daily care of all our birds; and Michale Fee and Erich Jarvis for making helpful editorial suggestions. This study was supported by Public Health Service Grant MH18343, the Mary Flagler Cary Charitable Trust, and the Phipps Family Foundation. At the time this work was done, W.-c.L. was supported by a fellowship from the Li Memorial Fund.
Abbreviation: IEG, immediate early gene.
References
- 1.Mello, C. V., Nottebohm, F. & Clayton, D. F. (1995) J. Neurosci. 15, 6919-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mello, C. V., Vicario, D. S. & Clayton, D. F. (1992) Proc. Natl. Acad. Sci. USA 89, 6818-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvis, E. D. & Nottebohm, F. (1997) Proc. Natl. Acad. Sci. USA 94, 4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvis, E. D., Ribeiro, S., da Silva, M. L., Ventura, D., Vieillard, J. & Mello, C. V. (2000) Nature 406, 628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis, E. D., Scharff, C., Grossman, M. R., Ramos, J. A. & Nottebohm, F. (1998) Neuron 21, 645-647. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis E. D., Schwabl, H., Ribeiro, S., Mello, C. V. (1997) NeuroReport 8, 2073-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan, J. & Curran, T. (1989) Trends Neurosci. 12, 423-433. [DOI] [PubMed] [Google Scholar]
- 8.Clayton, D. F. (2000) Learn. Mem. 74, 185-216. [DOI] [PubMed] [Google Scholar]
- 9.Liu, W. C. (2004) Anim. Behav. 68, 39-44. [Google Scholar]
- 10.Mello, C. V. & Clayton, D. F. (1994) J. Neurosci. 14, 6652-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, W. C. & Kroodsma, D. E. (1999) Anim. Behav. 57, 1275-1286. [DOI] [PubMed] [Google Scholar]
- 12.Tchernichovski, O., Nottebohm, F., Ho, C. E., Pesaran, B. & Mitra, P. P. (2000) Anim. Behav. 59, 1167-1176. [DOI] [PubMed] [Google Scholar]
- 13.Mello, C. V., Jarvis, E. D., Denisenko, N. & Rivas, M. (1997) in Methods in Molecular Biology: Differential Display Methods and Protocols, eds. Liang, P. & Pardee, A. B. (Humana, Totowa, NJ), pp. 205-217. [DOI] [PubMed]
- 14.Jacobs, E. C., Arnold, A. P. & Campagnoni, A. T. (1999) Dev. Neurosci. 21, 453-472. [DOI] [PubMed] [Google Scholar]
- 15.Wada, K., Sakaguchi, H., Jarvis, E. D. & Hagiwara, M. (2004) J. Comp. Neurol. 476, 44-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haesler, S., Wada, K., Nshdejan, A., Morrisey, E. E., Lints, T., Jarvis, E. D. & Scharff, C. (2004) J. Neurosci. 24, 3164-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiner, A., Bruce, L., Butler, A., Csillag, A., Kuenzel, W., Medina, L., Paxinos, G., Perkel, D., Shimizu, T., Striedter, G., et al. (2004) J. Comp. Neurol. 473, 377-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nottebohm, F., Stokes, T. M. & Leonard, C. M. (1976) J. Comp. Neurol. 165, 457-486. [DOI] [PubMed] [Google Scholar]
- 19.Nottebohm, F., Kelley, D. B. & Paton, J. A. (1982) J. Comp. Neurol. 207, 344-357. [DOI] [PubMed] [Google Scholar]
- 20.Bottjer, S. W., Halsema, K. A., Brown, S. A. & Miesner, E. A. (1989) J. Comp. Neurol. 279, 312-326. [DOI] [PubMed] [Google Scholar]
- 21.Bottjer, S. W., Miesner, E. A. & Arnold, A. P. (1984) Science 224, 901-903. [DOI] [PubMed] [Google Scholar]
- 22.Scharff, C. & Nottebohm, F. (1991) J. Neurosci. 11, 2896-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao, M. H., Doupe, A. J. & Brainard, M. S. (2005) Nature 433, 638-643. [DOI] [PubMed] [Google Scholar]
- 24.Olveczky, B. P., Andalman, A. S. & Fee, M. S. (2005) PLoS Bio. 3, e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahnloser, R. H.R., Kozhevnikov, A. A. & Fee, M. S. (2002) Nature 419, 65-70. [DOI] [PubMed] [Google Scholar]
- 26.Yu, A. C. & Margoliash, D. (1996) Science 273, 1871-1875. [DOI] [PubMed] [Google Scholar]
- 27.Canady, R. A., Burd, G. D., DeVoogd, T. J. & Nottebohm, F. (1988) J. Neurosci. 8, 3770-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bottjer, S. W. & Johnson, F. (1997) J. Neurobiol. 33, 602-618. [DOI] [PubMed] [Google Scholar]
- 29.Garrillo, G. D. & Doupe, A. J. (2004) J. Comp. Neruol. 473, 415-437. [DOI] [PubMed] [Google Scholar]
- 30.Perkel, D. J. (2004) Ann. N.Y. Acad. Sci. 1016, 736-748. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis, E. D. (2004) Ann. N. Y. Acad. Sci. 1016, 749-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albin, R. L., Young, A. B. & Penney, J. B. (1989) Trends Neurosci. 12, 366-375. [DOI] [PubMed] [Google Scholar]
- 33.DeLong, M. R. (1990) Trends Neurosci. 13, 281-285. [DOI] [PubMed] [Google Scholar]
- 34.Graybiel, A. M. (1995) Curr. Opin. Neurobiol. 5, 733-741. [DOI] [PubMed] [Google Scholar]
- 35.Graybiel, A., Aosaki, T., Flaherty, A. W. & Kimura, M. (1994) Science 265, 1826-1831. [DOI] [PubMed] [Google Scholar]
- 36.Marsden, C. D. & Obeso, J. A. (1994) Brain 117, 877-897. [DOI] [PubMed] [Google Scholar]
- 37.Arnold, A. P., Nottebohm, F. & Pfaff, D. W. (1976) J. Comp. Neurol. 165, 487-512. [DOI] [PubMed] [Google Scholar]
- 38.Arnold, A. P. & Saltiel, A. (1979) Science 205, 702-705. [DOI] [PubMed] [Google Scholar]
- 39.Barnea, A. & Nottebohm, F. (1994) Proc. Natl. Acad. Sci. USA 91, 11217-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]