Abstract
There is a rapid global rise in obesity, and the link between obesity and diabetes remains somewhat obscure. We identified an adipocytokine, designated as visceral adipose tissue-derived serpin (vaspin), which is a member of serine protease inhibitor family. Vaspin cDNA was isolated by from visceral white adipose tissues (WATs) of Otsuka Long-Evans Tokushima fatty (OLETF) rat, an animal model of abdominal obesity with type 2 diabetes. Rat, mouse, and human vaspins are made up of 392, 394, and 395 amino acids, respectively; exhibit ≈40% homology with α1-antitrypsin; and are related to serine protease inhibitor family. Vaspin was barely detectable in rats at 6 wk and was highly expressed in adipocytes of visceral WATs at 30 wk, the age when obesity, body weight, and insulin levels peak in OLETF rats. The tissue expression of vaspin and its serum levels decrease with worsening of diabetes and body weight loss at 50 wk. The expression and serum levels were normalized with the treatment of insulin or insulin-sensitizing agent, pioglitazone, in OLETF rats. Administration of vaspin to obese CRL:CD-1 (ICR) (ICR) mice fed with high-fat high-sucrose chow improved glucose tolerance and insulin sensitivity reflected by normalized serum glucose levels. It also led to the reversal of altered expression of genes relevant to insulin resistance, e.g., leptin, resistin, TNFα, glucose transporter-4, and adiponectin. In DNA chip analyses, vaspin treatment resulted in the reversal of expression in ≈50% of the high-fat high-sucrose-induced genes in WATs. These findings indicate that vaspin exerts an insulin-sensitizing effect targeted toward WATs in states of obesity.
Keywords: metabolic syndrome, diabetes, insulin resistance, mesenteric, white adipose tissue
Recently, there has been a worldwide increase in the incidence of obesity associated with a metabolic syndrome known as type 2 diabetes, the development of which seems to be as a result of high-caloric diet intake and physical inactivity (1, 2); and predicted estimates suggest that the population with this syndrome may double to ≈300 million by the year 2025 (3). One of the critical determinants for the development of this obesity may be an increase in the regional distribution of body fat, i.e., abdominal obesity. The latter often shows clustering of atherogenic risk factors (4, 5), i.e., hypertension, dyslipidemia, alterations in coagulation and inflammatory cytokine profiles, and hyperinsulinemic insulin resistance. As a consequence, there is an expected increase in morbidity and mortality of cardiovascular disease (6-9). Vigorous efforts have been made to delineate the relationship between increased adiposity and insulin resistance. In this regard, some molecules, including free fatty acids, leptin (10, 11), TNF-α (12, 13), acylation-stimulating protein (14, 15), adiponectin (16, 17), and resistin (18), which are secreted by the adipocytes, may modulate the sensitivity of insulin, the action of which activates multiple signaling events after phosphorylation of insulin receptor and several other molecules in type 2 diabetes (19, 20). To address the causal relationship between insulin resistance and obesity, various animal models, e.g., Otsuka Long-Evans Tokushima fatty (OLETF) rats, have been used by many investigators.
The OLETF rat is an animal model of type 2 diabetes, which is characterized by abdominal obesity, insulin resistance, hypertension, and dyslipidemia (21). Previously, we isolated a ≈135-bp cDNA fragment during differential screening of the genes up-regulated in visceral adipose tissues of obese OLETF rats and down-regulated in nonobese and diabetes-resistant Long-Evans Tokushima Otsuka (LETO) rats (22). This cDNA fragment exhibited partial homology with the serine protease inhibitor belonging to the serine protease inhibitor (serpin) family. In this study, we describe the isolation of the full-length cDNA of this potential inhibitor, designated as “vaspin” (visceral adipose tissue-derived serpin). In addition, preparation of its putative protein and characterization of its biological properties by using in vitro and in vivo rat and mouse model systems are included in this communication.
Methods
Isolation and Cloning of Vaspin. The 135-bp DNA fragment, designated as OL-64, isolated by representational difference analysis of cDNA (22, 23), was used to isolate the full-length cDNA of vaspin. Poly(A)+ mRNA was isolated from visceral adipose tissues of 8-wk-old LETO and 30-wk-old OLETF rats (Otsuka Pharmaceutical Tokushima Research Institute, Tokushima, Japan), and cDNA was prepared by using Moloney murine leukemia virus reverse transcriptase (MMLV-RT, RNase H-) and Oligo(dt)25d(G/C/A) as primer. The cDNA was used to generate rat, mouse, and human vaspin full-length cDNAs by using a Marathon cDNA amplification kit (Clontech).
Expression and Purification of Recombinant Human Vaspin (rhVaspin). The full-length vaspin cDNA along with (His-6)-tag was ligated into pET-15b vector and transformed into Escherichia coli host BL21 (DE3) by using the pET expression system (Novagen). (His-6)-tagged rhVaspin was purified by Ni-NTA affinity chromatography. After digestion with Factor-Xa (Sigma) to remove (His-6)-tag, rhVaspin was further purified by cation-exchange chromatography. Contaminating endotoxin was removed by using an acticlean etox column (Sterogene Bioseparations, Carlsbad, CA). The final endotoxin concentration of purified rhVaspin was <10 units/mg of protein, as measured by the limulus amoebocyte lysate assay (Seikagaku, Tokyo).
Preparation of Adenoviral Expression Vector. Full-length mouse vaspin cDNA was used to generate adenoviral vector, AxCAmOL64 with β-galactosidase, AxCAiLacZ, as control by using adenovirus expression kit (Takara Shuzo, Kyoto) as described (24). HEK293 cells were transfected with adenoviral vectors, and the culture medium was saved and analyzed by SDS/PAGE. The identity of the putative vaspin protein band was confirmed by N-terminal amino acid sequence analysis.
Vaspin Polyclonal Ab Production, Immunoblot, and Immunohistochemistry. rhVaspin, prepared from an E. coli host, was used to generate rabbit polyclonal Abs. Western blot analyses were performed by using by the enhanced chemiluminescence blotting detection system (Amersham Pharmacia), and immunohistochemistry procedures were carried out as described (25).
Northern Blot and Quantitative PCR Analysis. Northern blot analyses were performed by using 20 μg of total RNA, isolated from various adipose tissues, as described (22). Real-time PCR assays were performed by using an Applied Biosystems 7700 sequence detector. Total RNA was reverse-transcribed with random hexamers and by using the TaqMan reverse-transcription reagents kit (Applied Biosystems). The mRNA expression was determined by Syber green, and statistical analyses of mRNA data were performed by Student's t test.
DNA Chip Analysis. RNA (2-5 μg) was used for reverse transcription, and double-stranded cDNA was prepared and used as a template to synthesize the biotinylated cRNA probe. The probes were hybridized with the rat genome U34 set (Affymetrix, Santa Clara, CA). Expression values for each probe set and background binding levels were determined by Affymetrix microarray suite software. Principal component analyses were carried out by using all of the ≈24,000 genes arrayed on the DNA chip (26). For hierarchical clustering analysis, 1,319 genes were included with significant variation across the samples (27).
In Vivo Experiments with Rats. For expression studies, 4-wk-old OLETF and LETO rats were housed in a room with 12-h light/dark cycles, and animals were allowed free access to food and water. OLETF rats were divided into four groups, with 15 animals in each group: (i) control OLETF rats; (ii) OLETF rats receiving pioglitazone (TZD, 1 mg/kg body weight per day); (iii) OLETF rats subjected to voluntary exercise (EXE, access to running wheel); and (iv) insulin-treated OLETF rats (INS). In the INS group, neutral protamine Hagedorn insulin (10-40 units/animal) was administered to maintain fasting glucose levels of 80-150 mg/dl. At 6, 30, and 50 wk of age, sera, plasma, and urine samples were collected. Five animals in each group were killed after overnight fasting, and various tissues were harvested. White adipose tissues (WATs) were digested with collagenase in Krebs-Ringer Hepes buffer, and mature adipocytes and vascular stromal cells were separated and subjected to RNA isolation and immunoblotting (28).
In Vivo Experiments with Mice. Male CRL:CD-1 (ICR) (ICR) mice (8-wk-old) were purchased from Charles River Breeding Laboratories. At 10 wk of age, glucose and insulin tolerance tests were performed. rhVaspin (1 mg/kg weight) or vehicle was administered twice, at 7:00 a.m. and 7:00 p.m., i.p., to ICR mice, 10/group; and the mice were fasted after the first injection. Glucose (2 g/kg weight) was administered 2 h after the second injection, and blood glucose and plasma insulin levels were measured at 0-120 min. For the insulin-tolerance test, ICR mice, 10 per group, received two doses of rhVaspin or vehicle at 7:00 a.m. and 7:00 p.m. They were fasted 2 h before the second injection. After a total of 4 h of fasting, insulin (0.5 unit/kg body weight) was administered, and blood glucose was then determined. In the above experiments, the mice received corn starch chow containing 11% fat in total calories and corn starch (Research Diets, New Brunswick, NJ). Similarly, at 18 wk of age, glucose and insulin tolerance tests were performed, as described above, for ICR mice on standard (STD) chow. Additional ICR mice, 10 per group, that received sucrose chow containing 58% fat in total calories and sucrose (high fat high sucrose, HFHS) were used for glucose and insulin tolerance tests. Their WATs were harvested for isolation of RNA, quantitative RT-PCR, and DNA chip analyses.
Results
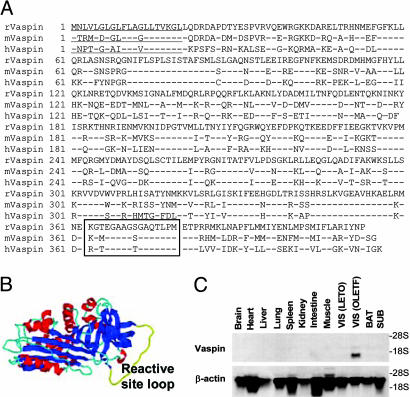
Vaspin Is a Visceral Adipose Tissue-Derived Serpin. The rat, mouse, and human vaspin cDNAs had ORFs of 1,236, 1,242, and 1,245 nucleotides, respectively (Fig. 1A). The putative proteins included 412, 414, and 415 amino. The analysis of the deduced amino acid sequence of rat, mouse, and human orthologs predicted a hydrophobic N-terminal signal peptide (underscored), and N-terminal Edman sequencing revealed that mouse vaspin protein begins at the Leu-21 residue. The reactive loop site (boxed) extends from 364 to 379 residues (Fig. 1 A). Homology analyses indicated that vaspin has 40.5% identity with α1-antitrypsin. Automated protein structure homology-modeling by swiss-model (http://swiss-model.expasy.org) predicted the presence of three β-sheets (blue), nine α-helices (red), and one reactive center loop (yellow), features characteristic of serpin gene family (Fig. 1B). A single transcript was observed in visceral WATs of 30-wk-old obese OLETF rats [VIS(OLTEF)], and it was absent in 6-wk-old lean LETO rats [VIS(LETO)] and in subdermal (SUB) and brown fatty tissues (Fig. 1C). No vaspin mRNA expression was observed in other nonadipose tissues from OLETF rats, suggesting its role in abdominal obesity as observed in this metabolic syndrome. Similarly, no mRNA transcript was observed in nonadipose tissues from human and mouse organs (data not shown).
Fig. 1.
Amino acid sequence, structural analyses, and gene expresion of vaspin in various tissues. (A) Amino acid sequence of rat, mouse, and human vaspins. Signal peptides are underlined, and reactive site loop is boxed. (B) Automated protein structure homology modeling by swiss-model predicted the presence of three β-sheets (blue), nine α-helices (red), and one reactive site loop (yellow). (C) Northern blot analyses of vaspin in various organs of obese 30-wk-old OLETF and visceral adipose tissue of lean 6-wk-old LETO rats. A single transcript is observed in visceral (VIS) fat of OLETF rats. BAT, brown adipose tissue.
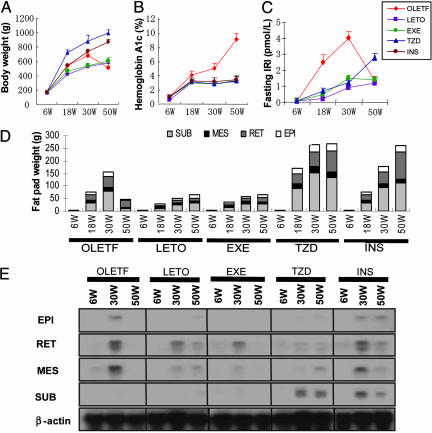
Regulation of Vaspin Gene Expression in Genetically Obese OLETF Rats. To investigate the regulation of vaspin gene expression in various WATs, OLETF rats were subjected to various regimens up to 50 wk of age, i.e., EXE, TZD, and insulin therapy. Body and fat-pad weights of OLETF rats peaked at 30 wk of age (Fig. 2 A and D), the time notable hyperinsulinemia was observed (Fig. 2C). Thereafter, these control OLETF rats progressively lost weight for the next 20 wk along with the decline in immunoreactive insulin levels (Fig. 2C) and rise in HbA1c (Fig. 2B). In TZD and INS groups, OLETF rats progressively gained body and fat-pad weights, and insulin levels increased for the entire duration of the experiment, more so in TZD group (Fig. 2 A, C, and D). However, plasma glucose levels were comparable to that in the diabetes-resistant LETO rats (data not shown). Vaspin mRNA was highly expressed in visceral mesenteric (MES) and retroperitoneal WATs and to a lesser extent in epididymal WAT in 30-wk-old OLETF rats (Fig. 2E), corresponding to the peak of their body and fat weights and hyperinsulinemia with insulin resistance (Fig. 2C), whereas expression was absent at the age of both 6 and 50 wk. Vaspin mRNA was barely detected in SUB WATs of OLETF and LETO rats during the entire period. EXE suppressed vaspin mRNA expression in visceral WATs comparable to the levels of diabetes-resistant LETO rats. Long-term administration of TZD notably up-regulated vaspin mRNA expression in SUB WATs at 30 wk of age and maintained its levels up to 50 wk, while reducing in visceral WATs, i.e., retroperitoneal and MES fat (Fig. 2E). Similarly, insulin therapy up-regulated expression of vaspin in SUB WATs along with a partial reduction in visceral WATs at 30 wk of age.
Fig. 2.
Effect of pioglitazone and insulin on various physiological parameters in OLETF and related strains of rats and expression of vaspins. (A) Body weight of OLETF, LETO, and OLETF rats with EXE, and OLETF rats administered with TZD and insulin (INS). A decline in the body weight of OLETF rats is observed at 50 wk of age after peaking at 30 wk. (B) Hemoglobin A1c levels progressively rise up to 50 wk in OLTEF rats. (C) Fasting immunoreactive insulin (IRI) levels peak at 30 wk and then decline at 50 wk in OLTEF rats. (D) Fat-pad weights peaked at 30 wk in OLETF rats, whereas with TZD and insulin treatment, they progressively rose up to 50 wk. Data included in A-D are mean ± SEM (n = 5). (E) Northern blot analyses of vaspin mRNA in WATs. At 30 wk, the expression is high in visceral retroperitoneal (RET) and MES WATS in OLTEF rats. The expression is high at 30 and 50 wk in SUB WATs from OLTEF rats treated with TZD, whereas the expression of visceral WATs of vaspin was decreased. Insulin had a partial effect on the mRNA expression of vaspin. EPI, epidydimal WATs.
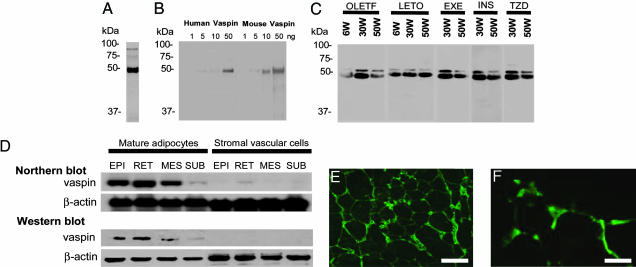
Adipocyte-Specific Expression of Vaspin and Its Serum Levels. Western blot analyses of the supernatants from cultured 293T cells transfected with adenoviral vector, AxCAmOL64, yielded a prominent ≈45-kDa band (Fig. 3A). A similar-size band was observed when analyses were performed on purified recombinant mouse and human vaspin with a detection limit of ≈5 ng (Fig. 3B). Vaspin was also detected in the sera of OLETF and LETO rats. By Western blot analyses, a major ≈45 kDa and a minor ≈50 kDa bands in rat sera were detected (Fig. 3C). The vaspin serum levels increased in OLETF rats compared with LETO rats at 30 wk of age, the period with peak insulin resistance (Fig. 3C). In OLETF rats, vaspin serum levels were markedly reduced when OLETF rats developed severe hyperglycemia at 50 wk of age. Insulin and TZD treatments seem to cause increase in vaspin serum levels in OLETF rats at 50 wk of age, and the bands were of similar intensity as seen in EXE group (Fig. 3C). The presence of two bands (≈45 and ≈50 kDa) in the sera may suggest processing or cleavage of vaspin by unknown serine proteases in the serum, which may be increased in states of insulin resistance or abdominal obesity. Alternatively, vaspin may have complexed to low Mr peptide in the blood to yield a high Mr ≈50-kDa protein band. To ensure that vaspin circulating in the blood is produced exclusively by adipocytes, WATs of OLETF rats were separated into mature adipocytes and vascular stromal cells by collagenase digestion, and Western and Northern blot analyses were performed. Both mRNA and protein expression of vaspin were found in mature adipocytes (Fig. 3D). The expression of vaspin in the adipocytes was further confirmed by immunohistochemistry of MES WAT, where it was found to be localized in their cytoplasm and was absent in the stromal endothelial/vascular cells (Fig. 3 E and F).
Fig. 3.
Expression of vaspin in 293T cells, adipocytes, and stromal vascular cells. (A) Western blot analysis of mouse vaspin by using supernatants of 293T cultured cells transfected with AxCAmOL64 and polyclonal antivaspin Ab. A prominent band of ≈45 kDa is observed. (B) Western blots of purified recombinant human and mouse vaspins, derived from E. coli by using pET expression system. (C) Western blot analyses of vaspin by using sera of OLETF and LETO rats, and OLETF rats with EXE, OLETF rats administered TZD and insulin (INS). A major ≈45-kDa band and a minor ≈50-kDa band are seen at 30 wk. Band intensity is notably less at 50 wk in OLTEF rats, but it seems to be normalized with insulin and TZD treatments. (D) Expression of vaspin in mature adipocytes and stromal vascular cells isolated from epidydimal (EPI), retroperitoneal (RET), MES, and SUB WATs as analyzed by Northern and Western blot analyses. Expression is confined to adipocytes of visceral WATs and not to stromal endothelial or vascular cells. (E and F) Expression in the adipocytes was confirmed by immunofluorescence microscopy in 30-wk-old OLETF rats. (Scale bars: E, 100 μm; F, 25 μm.)
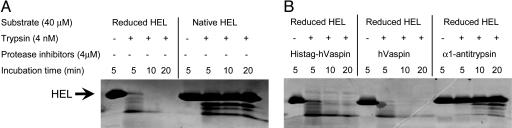
Vaspin Dose Not Inhibit Known Common Serine Proteases. Protein analysis using ExPASY Proteomics tools indicated that human vaspin consists of 395 (minus signal peptide) amino acids with a Mr of 45.2 kDa and theoretical pI 9.26. During the isolation of rhVaspin in the E. coli system, only ≈10% vaspin was found in the soluble fraction and ≈90% in the inclusion bodies. Both fractions yielded identical-size protein bands and thus were combined to investigate the biological activity of vaspin by using denatured and reduced hen-egg lysozyme as substrates. The reduced form is relatively sensitive to trypsin treatment (Fig. 4A), and it was used as a substrate for assay of rhVaspin inhibitory activity. Normally, proteolytic activity of trypsin toward reduced hen-egg lysozyme is inhibited by α1-antitrypsin (Fig. 4B). However, rhVaspin in the presence or absence of (His-6)-tag failed to inhibit protease activity of trypsin (Fig. 4B). Similarly, inhibitory activity of rhVaspin was not detected for other known common proteases, such as elastase, urokinase, factor Xa, collagenase, and dipeptidyl peptidase (data not shown).
Fig. 4.
Effect of vaspin on tryptic activity. (A) Reduced hen-egg lysozyme (HEL), and not native HEL, is sensitive to trypsin treatment. (B) The proteolytic activity of trypsin is normally blocked by its known inhibitor, α1-antitrypsin; however, rhVaspin with or without (His)6-tag failed to inhibit the tryptic activity.
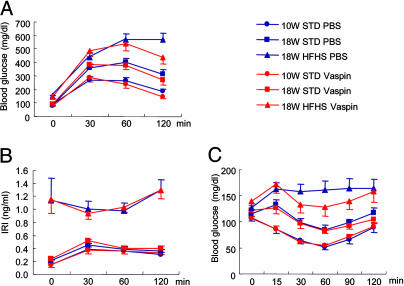
Vaspin Sensitizes Insulin Action in HFHS Chow-Induced Obese ICR Mice. Because vaspin expression is regulated in genetically obese OLETF rats (Figs. 1, 2, 3, 4), the role of vaspin, causative or protective, was assessed in the development of type 2 diabetes in diet-induced obese ICR mice fed with HFHS chow. The ICR mice were used because they do not develop obesity when fed with normal chow. The ICR mice fed with HFHS chow developed hyperglycemia, hyperinsulinemia, and obesity at 18 wk of age (Fig. 5 A and B), which is reminiscent of metabolic syndrome in humans with no genetic influence. Administration of rhVaspin to 18-wk-old ICR mice with HFHS chow significantly reduced their glucose levels 120 min after i.p. injection of glucose (Fig. 5A, asterisk). However, immunoreactive insulin levels were not altered by the injection of vaspin, and the animals remained relatively hyperinsulinemic (Fig. 5B). Except for HFHS, blood glucose levels were lowered for 90 min after an i.p. injection of insulin in all other groups fed STD diets (Fig. 5C). Interestingly, a concomitant injection of vaspin to HFHS-chow fed ICR mice reduced blood glucose levels (Fig. 5C, asterisks) (P < 0.01), suggesting sensitization of insulin action by rhVaspin and thereby negating or overcoming insulin resistance in diet-induced obese mice.
Fig. 5.
Profiles of glucose tolerance and insulin sensitization tests after administration of vaspin and insulin. (A) Glucose tolerance test. rhVaspin or PBS was i.p. injected into ICR mice fed STD chow or HFHS chow before glucose administration. Glucose levels were significantly reduced with the administration of vaspin (*, P < 0.01). (B) Insulin levels during the glucose tolerance test were unaltered, and HFHS mice remained hyperinsulinemic. (C) Insulin tolerance test. rhVaspin or PBS was i.p. injected into mice before insulin administration. Blood glucose levels were lowered in the HFHS group receiving insulin plus vaspin (*, P < 0.05; **, P < 0.01). All data are mean ± SEM (n = 10).
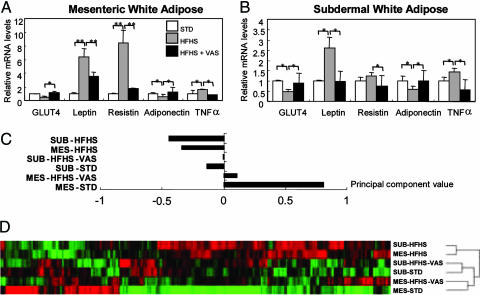
Vaspin Reverses Gene Expression Profile of WATs in Diet-Induced Obese Mice. Real-time RT-PCR was used to determine target organs responsive to the vaspin-sensitizing action of insulin in diet-induced obese mice. RNAs were isolated from MES and SUB WATs of three groups of mice: 18-wk-old STD chow, HFHS-chow-fed ICR mice, and HFHS-chow ICR mice treated with biweekly i.p. injections of rhVaspin for 2 wk. In MES and SUB WATs, treatment with vaspin in HFHS chow ICR mice suppressed the expression of leptin, resistin, and TNFα, whereas it increased that of the glucose transporter-4 and adiponectin (Fig. 6 A and B), suggesting rhVaspin administration reverses the expression of the genes related to insulin resistance. Affymetrix Genechip revealed that rhVaspin administration reversed the mRNA levels in 49.9% and 52.8% of the genes with altered expression induced by HFHS chow in MES and SUB WATs, respectively. HFHS chow induced similar trends both in MES and SUB WATs that were characterized by negative values for the principal component (Fig. 6C). In contrast, MES WAT in mice with STD chow had positive value. The treatment of HFHS-chow-fed mice with vaspin increased the principal component values both in MES and SUB WATs (Fig. 6C). Hierarchical clustering analysis was consistent with changes in the principal component values dividing in two distinct groups, i.e., mice fed with the HFHS chow group and STD chow on separate branches of the dendrogram (Fig. 6D). Both vaspin-treated MES and SUB WATs were on the same branches as that of the STD diet group (Fig. 6D). Thus, it seems that vaspin treatment improves the insulin sensitivity by normalizing the gene expression mainly in the WATs.
Fig. 6.
Effect of vaspin on leptin, resistin, TNFα, GLUT4, and adiponectin in obese ICR mice fed with HFHS chow. Vaspin administration reverses gene expression profile of WATs. (A and B) Injection of rhVaspin suppressed gene expression of leptin, resistin, and TNFα and increased the expression of glucose transporter-4 and adiponectin in obese ICR mice with HFHS chow. Data are mean ± SEM (n = 5). *, P < 0.05; **, P < 0.01. (C) Principal component analysis showing that the HFHS-chow-induced gene expression profile in MES and SUB fats is distinct from that of ICR mice with STD chow and after injection of rhVaspin (VAS). (D) Hierarchical clustering analysis seems to divide in two distinct groups, HFHS and STD chow groups. Both the MES and the SUB gene expression profile in rhVaspin-treated ICR mice fed with HFHS chow is on the same branch as mice fed STD chow.
Discussion
Serpins are a superfamily of proteins whose membership is based on the presence of a core domain consisting of three β-sheets and nine α-helices. Literature data include ≈500 serpins and a phylogenetic analysis divides serpins into 16 classes and 10 highly diverged “orphans” (29, 30). The structure of the cleaved α1-antitrypsin implies that serpins are “suicidal inhibitors” and the mechanism of proteinase inhibition may differ from other known classical inhibitors. The inhibitory activity of vaspin, though unknown but having a reactive site loop besides β-sheets and α-helices (Figs. 1 and 4), would suggest that it belongs to serpin family. Traditionally, during inhibition the reactive site loop of α1-antitrypsin is cleaved by the target proteinases with the formation of a covalently bound serpin-proteinase complex and as a result the serpins undergo a conformational change (31). This conformational transition is related to inherent molecular flexibility in the serpins, but it also renders them susceptible to point mutations with the formation of anomalous intermolecular linkages and polymers. Of interest, some of the conformational diseases are related to the mutation in serpins and are known as serpinopathies (32). These disorders include those affecting the clotting, fibrinolytic, and complement systems; however, the role of the majority of serpins in various pathobiologic processes awaits further investigations (33).
Vaspin expression, although specific to visceral adipose tissues, also has a circulating form, and the levels of both increase at the peak of obesity and insulin resistance, i.e., at 30 wk, and decrease with the worsening of diabetes in OLETF rats, i.e., at 50 wk (Figs. 1, 2, 3). Because administration of TZD, like insulin, significantly up-regulated vaspin mRNA and serum levels and maintained them until 50 wk of age, it would suggest the increase may be a compensatory response to antagonize the action of other unknown proteases up-regulated in obesity and in states of insulin resistance. In other words, the up-regulation of vaspin may have a defensive action against insulin resistance. To attest to such a contention, rhVaspin was administered to diet-induced obese mice, and this maneuver significantly improved insulin sensitivity and glucose tolerance (Fig. 5). But the addition of rhVaspin into in vitro 3T3L1 cell culture (data not shown) or administration into in vivo lean mice did not alter glucose uptake or glucose tolerance (Fig. 5). Thus, it is reasonable to speculate that the production of vaspin may antagonize the action of unknown proteases derived from fat or other tissues, which impair the insulin action. Such a protective or ameliorative relationship is well known in other systems, e.g., α1-antitrypsin and neutrophil elastase. α1-antitrypsin is an acute-phase protein derived from liver, the concentration of which rises during inflammation; it thereby inhibits neutrophil elastase to prevent tissue damage in target organs (30).
The effects of human vaspin administration on the gene expression profile of various tissues including WATs (Fig. 6), liver, and skeletal muscle (data not shown) indicated that WATs may be a major target organ for vaspin, although its target proteases are presently unknown (Fig. 4). Interestingly, the mRNA levels of various genes expressed in WATs, including, glucose transporter-4, leptin, resistin, and adiponectin, which are related to this metabolic syndrome, returned to normal levels after vaspin administration. Similarly, unsupervised principal component and hierarchical clustering analysis of Genechip data indicated that vaspin also reversed the global gene expression of WATs in ICR mice, with HFHS chow-induced obesity to insulin-sensitive WATs observed in ICR mice with STD chow (Fig. 6). These observations suggest that vaspin may have an insulin-sensitizing effect mainly on WATs. However, administration of vaspin in the in vitro culture of cells derived from these organs did not alter insulin-induced glucose uptake (data not shown), suggesting that vaspin modulates insulin action conceivably only in the presence of its target proteases in WATs. WATs include two cell types, adipocytes and stromal vascular cells; vaspin is mainly confined to the adipocytes and thus affects their biology (Fig. 3). It is conceivable that vaspin may have a paracrine effect on the stromal endothelial cell. The latter may be regarded as its target in an analogous manner to another proteinase inhibitor, PAI-1, which is derived from MES fat and modulates the endothelial biology of blood vessels (34). Certainly additional studies are needed to delineate whether vaspin accelerates or decelerates micro- and macrovascular complications of diabetes mellitus that are related to lipid signaling (35).
Conclusion
Vaspin is a visceral adipose tissue-derived factor with potential antiprotease properties, and it ameliorates certain aberrations seen in the diabetic/obesity metabolic syndrome by sensitizing insulin action, especially in WATs. It is hoped that the findings of the present study provide an impetus for the identification of the potential protease substrate leading to the development of antiprotease inhibitor therapy, which could facilitate the improvement of insulin sensitivity in this metabolic syndrome.
Acknowledgments
This work was supported by the Uehara Memorial Foundation; the Naito Foundation; the ONO Medical Foundation; Grants-in-Aid for Scientific Research 14571025 and 17590827 from the Ministry of Education, Science, and Culture, Japan (to J.W.); Grant-in-Aid for Scientific Research 14370319 from the Ministry of Education, Science, and Culture, Japan (to H.M.); and National Institutes of Health Grants DK28492 and DK60635 (to Y.S.K.).
Abbreviations: Serpin, serine protease inhibitor; vaspin, visceral adipose tissue-derived serpin; rhVaspin, recombinant human vaspin; WAT, white adipose tissue; OLETF, Otsuka Long-Evans Tokushima fatty; LETO, Long-Evans Tokushima Otsuka; HFHS, high fat high sucrose; EXE, voluntary exercise; TZD, pioglitazone; INS, insulin-treated OLETF rats; MES, mesenteric; SUB, subdermal; STD, standard; ICR, CRL:CD-1 (ICR).
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF245398, AY326419, and AY326420).
References
- 1.Butler, D. (2004) Nature 428, 244. [DOI] [PubMed] [Google Scholar]
- 2.James, P. T., Leach, R., Kalamara, E. & Shayeghi, M. (2001) Obes. Res. 9, Suppl. 4, 228S-233S. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet, P. (2003) Diabetes Metab. 29, S9-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunitomi, M., Wada, J., Takahashi, K., Tsuchiyama, Y., Mimura, Y., Hida, K., Miyatake, N., Fujii, M., Kira, S., Shikata, K., et al. (2002) Int. J. Obes. Relat. Metab. Disorders 26, 361-369. [DOI] [PubMed] [Google Scholar]
- 5.Seida, A., Wada, J., Kunitomi, M., Tsuchiyama, Y., Miyatake, N., Fujii, M., Kira, S., Takahashi, K., Shikata, K. & Makino, H. (2003) Int. J. Obes. Relat. Metab. Disorders 27, 1325-1331. [DOI] [PubMed] [Google Scholar]
- 6.Stevens, J., Cai, J., Pamuk, E. R., Williamson, D. F., Thun, M. J. & Wood, J. L. (1998) N. Engl. J. Med. 338, 1-7. [DOI] [PubMed] [Google Scholar]
- 7.Kannel, W. B., Cupples, L. A., Ramaswami, R., Stokes, J., III, Kreger, B. E. & Higgins, M. (1991) J. Clin. Epidemiol. 44, 183-190. [DOI] [PubMed] [Google Scholar]
- 8.Rexrode, K. M., Carey, V. J., Hennekens, C. H., Walters, E. E., Colditz, G. A., Stampfer, M. J., Willett, W. C. & Manson, J. E. (1998) J. Am. Med. Assoc. 280, 1843-1848. [DOI] [PubMed] [Google Scholar]
- 9.Pouliot, M. C., Despres, J. P., Nadeau, A., Moorjani, S., Prud'Homme, D., Lupien, P. J., Tremblay, A. & Bouchard, C. (1992) Diabetes 41, 826-834. [DOI] [PubMed] [Google Scholar]
- 10.Ebihara, K., Ogawa, Y., Masuzaki, H., Shintani, M., Miyanaga, F., Aizawa-Abe, M., Hayashi, T., Hosoda, K., Inoue, G., Yoshimasa, Y., et al. (2001) Diabetes 50, 1440-1448. [DOI] [PubMed] [Google Scholar]
- 11.Wolf G., Chen S., Han D. C. & Ziyadeh F. N. (2002) Am. J. Kidney Dis. 39, 1-11. [DOI] [PubMed] [Google Scholar]
- 12.Hotamisligil, G. S., Peraldi, P., Budavari, A., Ellis, R., White, M. F. & Spiegelman, B. M. (1996) Science 271, 665-668. [DOI] [PubMed] [Google Scholar]
- 13.Uysal, K. T., Wiesbrock, S. M., Marino, M. W. & Hotamisligil, G. S. (1997) Nature 389, 610-614. [DOI] [PubMed] [Google Scholar]
- 14.Xia, Z., Sniderman, A. D. & Cianflone, K. (2002) J. Biol. Chem. 277, 45874-45879. [DOI] [PubMed] [Google Scholar]
- 15.Cianflone, K., Xia, Z. & Chen, L. Y. (2003) Biochim. Biophys. Acta 1609, 127-143. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi, T., Kamon, J., Waki, H., Imai, Y., Shimozawa, N., Hioki, K., Uchida, S., Ito, Y., Takakuwa, K., Matsui, J., et al. (2003) J. Biol. Chem. 278, 2461-2468. [DOI] [PubMed] [Google Scholar]
- 17.Maeda, N., Shimomura, I., Kishida, K., Nishizawa, H., Matsuda, M., Nagaretani, H., Furuyama, N., Kondo, H., Takahashi, M., Arita, Y., et al. (2002) Nat. Med. 8, 731-737. [DOI] [PubMed] [Google Scholar]
- 18.Steppan, C. M., Bailey, S. T., Bhat, S., Brown, E. J., Banerjee, R. R., Wright, C. M., Patel, H. R., Ahima, R. S. & Lazar, M. A. (2001) Nature 409, 307-312. [DOI] [PubMed] [Google Scholar]
- 19.Senthil, D., Faulkner, J. L., Choudhury, G. G., Kasinath, B. S. & Abboud, H. E. (2001) Biochem. J. 360, 87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaue, H., Nishizawa, A., Ogawa, W., Teshigawara, K., Mori, T., Takashima, Y., Noda, T. & Kasuga, M. (2003) J. Biol. Chem. 278, 38870-38874. [DOI] [PubMed] [Google Scholar]
- 21.Kawano, K., Hirashima, T., Mori, S., Saitoh, Y., Kurosumi, M. & Natori, T. (1992) Diabetes 41, 1422-1428. [DOI] [PubMed] [Google Scholar]
- 22.Hida, K., Wada, J., Zhang, H., Hiragushi, K., Tsuchiyama, Y., Shikata, K. & Makino, H. (2000) J. Lipid Res. 41, 1615-1622. [PubMed] [Google Scholar]
- 23.Hubank, M. & Schatz, D. G. (1994) Nucleic Acids Res. 22, 5640-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyake, S., Makimura, M., Kanegae, Y., Harada, S., Sato, Y., Takamori, K., Tokuda, C. & Saito, I. (1996) Proc. Natl. Acad. Sci. USA 93, 1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, H., Wada, J., Hida, K., Tsuchiyama, Y., Hiragushi, K., Shikata, K., Wang, H., Lin, S., Kanwar, Y. S. & Makino, H. (2001) J. Biol. Chem. 276, 17132-17139. [DOI] [PubMed] [Google Scholar]
- 26.Alter, O., Brown, P. O. & Botstein, D. (2000) Proc. Natl. Acad. Sci. USA 97, 10101-10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. (1998) Proc. Natl. Acad. Sci. USA 95, 14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joost, H. G. & Schurmann, A. (2001) Methods Mol. Biol. 155, 77-82. [DOI] [PubMed] [Google Scholar]
- 29.Silverman, G. A., Bird, P. I., Carrell, R. W., Church, F. C., Coughlin, P. B., Gettins, P. G., Irving, J. A., Lomas, D. A., Luke, C. J., Moyer, R. W., et al. (2001) J. Biol. Chem. 276, 33293-33296. [DOI] [PubMed] [Google Scholar]
- 30.Gettins, P. G. (2002) Chem. Rev. 102, 4751-4804. [DOI] [PubMed] [Google Scholar]
- 31.Ye, S. & Goldsmith, E. J. (2001) Curr. Opin. Struct. Biol. 11, 740-745. [DOI] [PubMed] [Google Scholar]
- 32.Lomas, D. A. & Carrell, R. W. (2002) Nat. Rev. Genet. 3, 759-768. [DOI] [PubMed] [Google Scholar]
- 33.Worrall, D. M., Blacque, O. E. & Barnes, R. C. (1999) Biochem. Soc. Trans. 27, 746-750. [DOI] [PubMed] [Google Scholar]
- 34.Shimomura, I., Funahashi, T., Takahashi, M., Maeda, K., Kotani, K., Nakamura, T., Yamashita, S., Miura, M., Fukuda, Y., Takemura, K., et al. (1996) Nat. Med. 2, 800-803. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan, R. & Nadler, J. L. (2003) Front. Biosci. 8, S783-S795. [DOI] [PubMed] [Google Scholar]