Abstract
Cultivation of luminal protistan parasites has a long history. In this review we discuss the methods and media that are most widely used for the establishment and maintenance of the following organisms in culture: Entamoeba histolytica, Giardia intestinalis, Trichomonas vaginalis, Dientamoeba fragilis, Blastocystis hominis, and Balantidium coli. While cultivation is of limited importance in the diagnostic laboratory, it is essential to most research laboratories, and it is toward the latter that this review is primarily aimed.
INTRODUCTION
Although cultivation of intestinal protists has a long history, not all parasites are amenable to growth in vitro. Without the ability to cultivate organisms few basic studies can be performed beyond morphological or pathological descriptions, and cultivation is a prerequisite for studies that require large numbers of cells. This review discusses methods for cultivation of luminal parasitic protists. This is a diverse group of organisms, but many of the principles and even the media used are often common among species.
Although the roles in disease of Entamoeba histolytica, Giardia intestinalis, Balantidium coli, and Trichomonas vaginalis are undisputed, some may be surprised by the inclusion of Dientamoeba fragilis and Blastocystis hominis in this review of parasitic protists of clinical importance. Opinion is divided on the roles (if any) of these species in human disease, and certainly the data are still unclear. We thought it appropriate to include these organisms for two reasons: (i) they are often grown in the same or similar media as the other organisms listed above and (ii) we hope to stimulate further interest in these relatively obscure organisms. It is now clear that significant genetic variability exists in both species (10, 37), which may presage a situation similar to that which led to the recognition of Entamoeba dispar as a species distinct from E. histolytica, with a consequent increase in interest in the organism.
In the clinical diagnostic laboratory setting, cultivation of most of these organisms plays a minor role. Microscopy is still the first choice for identification of these protists in stool samples. However, cultivation is one of the diagnostic methods of choice for T. vaginalis (8, 51). For E. histolytica (32) and G. intestinalis (46), stool antigen capture enzyme-linked immunosorbent assay kits are available commercially, and for all these species, PCR-based stool diagnosis is a current research tool that may become the method of choice in the future.
Establishment of all these organisms in culture is far from a routine procedure and is usually less sensitive than microscopy as a detection mechanism. In contrast to bacteria these organisms are difficult, expensive, and labor-intensive to maintain in the diagnostic laboratory, and thus there is little incentive to do so. Cultures are therefore primarily research tools rather than diagnostic tools.
GENERAL CONSIDERATIONS
There are three basic types of culture systems: xenic, in which the parasite is grown in the presence of an undefined flora; monoxenic, in which the parasite is grown in the presence of a single additional species; and axenic, in which the parasite is grown in the absence of any other metabolizing cells. The term “polyxenic” is sometimes erroneously used as a synonym for xenic; however, polyxenic should refer only to cultures in which the identities of all the species present are known (26). The species of parasitic protist discussed below are all isolated from sources often rich in bacteria and/or fungi. Controlling or eliminating bacterial and fungal growth is crucial to success in cultivating the parasites of interest. This is true even in xenic culture, in which reaching a balance between the needs of the bacterial flora and those of the eukaryote is important.
While T. vaginalis and G. intestinalis can be established directly into axenic cultures, E. histolytica and B. hominis have never been grown axenically without first being established in culture with other organisms, usually with a complex undefined bacterial flora. In contrast, D. fragilis and B. coli have never been grown successfully in axenic culture to our knowledge (Table 1). Axenization of E. histolytica is a laborious and lengthy process (see below). Organisms in xenic culture are first adapted to monoxenic growth, usually with a trypanosomatid flagellate as an associate, before they are weaned from a phagocytic lifestyle to one in which the nutrients are obtained largely by pinocytosis (16); the whole process often takes months. Perhaps surprisingly, then, the media used to cultivate T. vaginalis, G. intestinalis, and E. histolytica in the axenic state are almost identical. It is for this reason that these three unrelated parasites are often considered together.
TABLE 1.
Selection of cultivation conditions and media for luminal parasitesa
| Organism | Xenic culture (media) | Monoxenic culture (media) | Axenic culture (media) |
|---|---|---|---|
| Entamoeba histolytica | √ (LE, Robinson's, TYSGM-9) | √ (TYI-S-33, YI-S, LYI-S-2) | √ (TYI-S-33, YI-S, LYI-S-2) |
| Giardia intestinalis | × | × | √ (TYI-S-33, YI-S) |
| Trichomonas vaginalis | × | × | √ (TYI-S-33, YI-S) |
| Dientamoeba fragilis | √ (LE, Robinson's) | × | × |
| Blastocystis hominis | √ (LE, Robinson's, TYSGM-9) | × | √ (LE, IMDM) |
| Balantidium coli | √ (LE, Robinson's) | √ (LE, Robinson's) | × |
Symbols: √, cultivation method is recommended; ×, cultivation method is either not possible or not recommended.
HISTORICAL BACKGROUND
E. histolytica
This parasite was first established in culture by Boeck and Drbohlav in 1925 in a diphasic egg slant medium they had developed for isolation of intestinal flagellates (7). A modification of this medium (Locke-egg [LE]) is still in use today (see below). Their success refuted the Promethean view of E. histolytica as an obligate tissue parasite. Dobell and Laidlaw (25) introduced the use of rice starch as a carbohydrate source, which remains a component of all media for xenic cultivation to this day. If soluble sugars were used they would be metabolized rapidly by the bacteria, and this would prevent the necessary bacterium-ameba balance from being reached in culture. Several other diphasic media were subsequently developed with serum, agar, or egg extracts in the slants (18). Monophasic media that have been developed include the egg yolk infusion medium of Balamuth (3), Jones's medium (38), and TYSGM-9 of Diamond (17). Currently the most widely used media for xenic cultivation of E. histolytica are the diphasic LE and Robinson's (54) media and the monophasic TYSGM-9 (17). These three media are detailed below. Additional recipes are given in Diamond's review (18). These media can also be used for the cultivation of other species of Entamoeba and Endolimax nana with various degrees of success.
Monoxenic cultivation of E. histolytica with a single bacterium was first accomplished by Cleveland and Sanders (11) in a diphasic medium. The most widely used medium for this type of culture is the monobacterial modified Shaffer-Frye medium (MS-F) (53). Monoxenic cultivation with a trypanosomatid was first achieved using Trypanosoma cruzi in a diphasic medium (52).
Monoxenic cultivation is of limited use today except as a transitional stage between xenic and axenic cultures. Crithidia fasciculata and T. cruzi are the associated organisms of choice for monoxenic cultivation (15, 16). For media designed for use in monoxenic cultivation, see Diamond's review (18). However, in at least some cases axenic culture media have worked well for monoxenic cultivation.
Axenic cultivation of E. histolytica was first accomplished by Diamond in 1961 (14). The diphasic medium used was complex—a serum-enriched nutrient agar slant overlaid with a broth supplemented with chicken embryo extract and vitamins. It was not until Diamond introduced the monophasic medium TP-S-1 in 1968 (16) that axenic cultures of E. histolytica started to be widely used. TP-S-1 was superseded by TYI-S-33 in 1978 (22), and this is currently the most widely used medium for axenic cultivation of E. histolytica. Diamond et al. later described YI-S as an alternative to TYI-S-33 (20); these two media are described below. A homemade axenic culture medium (PEHPS [55]) made from liver and pancreas extracts has been described as an alternative to the above media which rely on commercially produced products. A partially defined medium is also available (21) but is not further considered here. Methods for obtaining cloned colonies in agar have also been published (30, 49), as have micromanipulation methods for cloning xenic (27) and axenic (23) cultured amebae.
The main components of the E. histolytica axenic culture media are a source of peptides and amino acids (Trypticase or casein digest peptone), nucleic acids (yeast extract), carbohydrate (glucose), lipids (serum), and vitamins. Most of these components are also part of the axenic media for the other parasites considered here.
Because of its emerging importance, especially with respect to diagnosis, it is appropriate to mention E. dispar here. Earlier called “nonpathogenic E. histolytica” but now recognized as a distinct species (19), E. dispar can be grown in xenic culture just as easily as E. histolytica. However, most isolates grow poorly in monoxenic culture, and few have been reported in axenic culture (9, 41). It appears that E. dispar may be less able than E. histolytica to obtain its nutrients in a particle-free medium.
G. intestinalis
The nomenclature of Giardia is controversial. In using the species name “intestinalis” we are following the suggestion of Kulda and Nohynková (42), but this organism is also called Giardia lamblia and Giardia duodenalis. This parasite was first cultivated by Karapetyan in 1960 in a mixed culture with Candida guilliermondi and chick fibroblasts. He later reported monoxenic cultivation of a rabbit isolate with Saccharomyces cerevisiae (39). Meyer was the first to report axenic cultivation of Giardia from small mammals in 1970 (47) and later also grew the parasite from human material (48). Bingham and Meyer (6) reported that treatment of mature cysts with hydrochloric acid (pH 2) improved excystation of the parasite, allowing the axenic cultivation of the parasite without resorting to prior xenic or monoxenic cultivation of trophozoites obtained by duodenal biopsy. Visvesvara (66) found that axenic G. intestinalis grew luxuriantly in the Entamoeba medium TP-S-1 that had been filter sterilized rather than autoclaved. Finally, TP-S-1 was superseded by filter-sterilized and modified TYI-S-33, in which the amount of cysteine was doubled and bovine bile was added (40). This is currently the medium used for cultivation of G. intestinalis almost exclusively, although the organism also will grow well in similarly modified YI-S (20). Serum-free media have been described previously (70), and a method for obtaining cloned colonies in agar has been published (31). Media that induce encystation of axenic cultures have also been described (29, 45).
T. vaginalis
T. vaginalis has a much longer record of axenic cultivation than the other parasites previously discussed, having first been obtained in axenic culture by Trussel in 1940 (65). Because xenic and monoxenic cultivations are rarely used today, they will not be discussed here; further details can be found in the work of Taylor and Baker (64). In most cases, T. vaginalis is now isolated directly as an axenic culture through the incorporation of antifungal and antibacterial antibiotics into the growth medium. Two media are widely used for the isolation and axenic cultivation of T. vaginalis—TYM (13, 18) and its modifications (e.g., see reference 34) and TYI-S-33, the latter of which is modified from its original formulation as devised for E. histolytica by lowering of the pH. YI-S will also support luxuriant growth of T. vaginalis when similarly modified (20) but has not been tested as an isolation medium. Defined media for cultivation of T. vaginalis have been described elsewhere (44) but will not be further considered here. Methods for obtaining cloned colonies in agar plates have also been published (34, 35, 56).
A caveat worth mentioning here is the use of the term “Diamond's medium” for axenic cultivation of T. vaginalis. We have seen cases in which it has been used instead of TYM, TYI-S-33, or Hollander's modification of TYM (34). In some cases, the actual medium used is not clear (51).
D. fragilis
According to Dobell (24), D. fragilis was probably first grown in a short-term, mixed culture in 1925 by Thomson and Robertson, but the first “mono-protist” cultures were produced by Dobell and Svensson in 1929. In contrast to the experience of most recent investigators, Dobell also found that D. fragilis was “one of the easiest of the human intestinal amoebae to isolate and grow in vitro” (24). Perhaps the medium used is responsible for this marked difference: Dobell used a diphasic medium of his own devising, an inspissated horse serum slant overlaid with diluted egg whites in Ringer's solution and supplemented with rice starch (HSre+S). He also reported that it grew best at 41°C, a much higher temperature than routinely would be used for the isolation of intestinal protists.
As mentioned above, this organism has not been reported in axenic culture. Whether this is due to lack of effort or inherent difficulties with the organism is not clear. Although generally regarded as difficult to establish in long-term culture, D. fragilis can often be grown for a few subcultures before dying out. In our experience the isolate that gives the most luxuriant growth is isolate Bi/Pa (available from the American Type Culture Collection; ATCC 30948) growing in LE medium with two bacterial species, although it also grows in other xenic media.
B. hominis
Long considered a fungus, B. hominis is now known to be the only human parasite belonging to the kingdom Chromista (59), which includes the oomycetes and diatoms among others, and is thus strictly speaking not a protist at all. However, it is a very common infection in humans and grows luxuriantly in all xenic media used for the isolation of Entamoeba species and D. fragilis, and it can be a major problem if it is the other organisms that are wanted (see below). The first report of xenic cultivation was by Barret (4) in a serum-saline medium. It was first grown axenically by Zierdt and Williams (72) in prereduced LE medium with a serum overlay and in a strictly anaerobic environment. Subsequently, a modification of this medium has been described (43). A completely different medium based on Iscove's modified Dulbecco's medium (IMDM) has been used by one group (33) which has also described cloning in soft agar using the medium (61, 62, 63).
B. coli
The first report of cultivation of the ciliate B. coli is that of Barret and Yarbrough (5), using the simple saline-serum medium used for B. hominis (above). The media that have been used for its xenic cultivation include many of the same ones used for E. histolytica, including LE, Robinson's, and TYSGM-9 (all below); Balamuth's (3); Jones's (38); and Dobell's HSre+S. The only reported axenic cultivation of a Balantidium was of a reptilian isolate (cited in reference 71), but B. coli apparently can be “maintained monoxenically with Escherichia coli or other intestinal bacteria without much difficulty” (71). In contrast to the other species discussed here, B. coli will grow over a broad temperature range (25 to 40°C [12]).
MEDIA
Xenic Culture Media
Rice starch.
Purified rice starch is important for growth of E. histolytica in all the following media. To prepare (18), place 500 mg of powdered rice starch into each of several culture tubes (16 by 125 mm) and heat at 150°C, with loose caps, in a dry oven for 2.5 h with the starch distributed along the length of the horizontal tubes. Sterilization of the rice starch prevents alteration of the bacterial flora when it is added to the culture and is thus recommended. Autoclaving is not an acceptable alternative to dry heat, as the starch will swell with the moisture.
After cooling, tighten the caps and store at room temperature. To prepare for use, add 9.5 ml of sterile distilled water or phosphate-buffered saline (PBS) to one tube and vortex to resuspend. Distribute 1 ml of the resuspended starch to each of 10 tubes containing 9 ml of sterile water or PBS, and refrigerate. The final concentration of diluted rice starch is 5 mg/ml. Before use, resuspend the rice by vortexing or vigorous shaking and pipette the desired volume into culture tubes with medium, making sure that the stock rice stays in suspension. Different isolates require various amounts of rice starch, but 0.2 ml (1 mg) is often a suitable amount to add per culture tube.
Entamoeba will not ingest all forms of rice. Most important is the size of the rice particle, as it must be within the ameba's ability to phagocytize it. A source for a very reliable rice starch is listed below under “Sources of Medium Materials.”
Diphasic media. (i) LE medium.
To prepare LE medium (NIH modification of Boeck and Drbohlav's medium [67]), first prepare Locke's solution by dissolving the following in 1 liter: 8.0 g of sodium chloride; 0.2 g of calcium chloride; 0.2 g of potassium chloride; 0.01 g of magnesium chloride; 2.0 g of sodium phosphate, dibasic; 0.4 g of sodium bicarbonate; and 0.3 g of potassium phosphate, monobasic. Autoclave 15 min at 121°C under a pressure of 15 lb/in2. Cool to room temperature and remove any precipitate by filtration (Whatman no. 1 paper). Reautoclave to sterilize.
To prepare the egg slant, surface sterilize fresh hens' eggs by flaming in 70% ethanol and break into a graduated cylinder. Add 12.5 ml of Locke's solution per 45 ml of egg. Emulsify in a Waring-type blender and filter through gauze into a flask. Place under vacuum to draw out all air bubbles. Add 5-ml amounts of the emulsified egg to standard culture tubes (16 by 125 mm) and autoclave at 100°C for 10 min with the tubes at an angle that produces a 12- to 15-mm (ca. 0.5-in.) butt. The resulting egg slants should be free of bubbles. Cool to room temperature and overlay slants with 6 ml of Locke's solution and autoclave 15 min at 121°C under a pressure of 15 lb/in2. After cooling the slants to room temperature, tighten the caps and refrigerate for up to 6 months,
(ii) Robinson's medium.
Robinson's medium (54) is a complex medium that has nevertheless found widespread use for the isolation of enteric amebae. To prepare Robinson's medium, prepare the six following stock solutions.
(a) 0.5% erythromycin Prepare 0.5% erythromycin in distilled water and filter sterilize. Refrigerate.
(b) 20% Bacto Peptone. Prepare 20% Bacto Peptone in distilled water. Autoclave and refrigerate.
(c) 10× phthalate solution stock. To prepare 10× phthalate solution stock, mix 102 g of potassium hydrogen phthalate and 50 ml of 40% sodium hydroxide. Bring to 1 liter at pH 6.3. Autoclave for 15 min at 121°C under a pressure of 15 lb/in2. Store at room temperature. Dilute 1:10 with sterile water before use.
A stock of phthalate-Bacto Peptone can be made by adding 1.25 ml of 20% Bacto Peptone per 100 ml of 1× phthalate solution. Store refrigerated.
(d) 10× R medium stock. To prepare 10× R medium stock, dissolve the following in distilled water: 25.0 g of sodium chloride; 10.0 g of citric acid; 25.0 g of potassium phosphate, monobasic; 5.0 g of ammonium sulfate; 0.25 g of magnesium sulfate · 7H2O; and 20 ml of 85% lactic acid solution. Bring to 500 ml. Dilute stock 1:10, adjusting pH to 7.0. Autoclave for 15 min at 121°C under a pressure of 15 lb/in2 in 20-ml amounts.
(e) BR medium. To prepare BR medium, inoculate 1× R medium with a standard E. coli strain such as O111. Incubate at 37°C for 48 h and store at room temperature (good for several months).
(f) BRS medium. To prepare BRS medium, add an equal volume of heat-inactivated bovine serum to BR medium and incubate at 37°C for 24 h. Store at room temperature (good for several months).
To prepare agar slants, many people use screw-cap glass bijou bottles (total volume, 7 ml), but we have also used standard culture tubes with good success. Autoclave a solution of 1.5% Noble agar in 0.7% sodium chloride-distilled water for 15 min at 121°C under a pressure of 15 lb/in2. Dispense in 5-ml (tube) or 3-ml (bottle) amounts, reautoclave, and slant until cool and set. For slants in tubes, use an angle that produces a 12- to 15-mm (ca. 0.5-in.) butt. When cool, tighten lids and store at room temperature or refrigerated.
To one tube or bottle add the following: 3 ml of 1× phthalate-Bacto Peptone, 1 ml of BRS medium, and 50 μl of erythromycin. This must be done on the same day as inoculation. Note that although erythromycin is added to Robinson's medium at every subculture, this does not lead to a monoxenic culture as occasionally stated. Additional antibiotic treatment would be needed for this to occur.
Monophasic media. (i) TYSGM-9.
To prepare TYSGM-9 (17), dissolve the following: 2.8 g of potassium phosphate, dibasic; 0.4 g of potassium phosphate, monobasic; 7.5 g of sodium chloride; 2.0 g of casein digest peptone; and 1.0 g of yeast extract. Bring to 950 ml with distilled water. Dispense in 95-ml amounts and add 0.2 g of porcine gastric mucin to each bottle. Autoclave for 15 min at 121°C with 15 lb pressure and store refrigerated. Before use, add 0.1 ml of a filter-sterilized 5% stock of Tween 80 in distilled water and 5 ml of heat- inactivated adult bovine serum. Dispense in 8-ml amounts into culture tubes (16 by 125 mm).
(ii) Robinson's medium.
The liquid overlay from Robinson's medium (54) as described above can be used as a monophasic growth medium. This is especially useful once isolates are established in culture. The phthalate-Bacto Peptone, BRS medium, and erythromycin solutions are mixed in the same proportions and dispensed into tubes or bottles to approximately two-thirds full.
Axenic Culture Media
One constant problem facing those who rely on axenic cultures is the fastidiousness of these organisms. Although the others are also affected to a significant degree, this is especially true of E. histolytica. Lot-to-lot variations in several components of the axenic culture media in particular can have profound effects on the ability of a medium to support growth of the organisms; some lots may even be toxic. Trypticase (casein digest peptone), yeast extract, and serum are the medium components most commonly affected, but the quality of the distilled water and even the type of glass used in making the culture tubes can cause problems (18) (screw-cap borosilicate glass tubes should be used when possible). For this reason, we highly recommend that those wishing to undertake axenic cultivation of these organisms test the ability of each new lot of reagent to support growth before starting to use it.
E. histolytica. (i) TYI-S-33.
To prepare TYI-S-33 (22), dissolve the following in this order in 600 ml of deionized or glass-distilled water: 1.0 g of potassium phosphate, dibasic; 0.6 g of potassium phosphate, monobasic; 2.0 g of sodium chloride; 20.0 g of casein digest peptone; 10.0 g of yeast extract; 10.0 g of glucose; 1.0 g of l-cysteine hydrochloride; 0.2 g of ascorbic acid; and 1.0 ml of ferric ammonium citrate, brown form (22.8 mg/ml)
Bring the final volume to 880 ml and pH to 6.8 using 1 N sodium hydroxide solution (ca. 7.5 ml). Dispense in 88-ml amounts into 125-ml glass bottles and autoclave for 15 min at 121°C under a pressure of 15 lb/in2. Sterile TYI base can be stored frozen at −20°C for several months.
Vitamin mix 18 (21), unlike earlier mixtures used in the axenic culture of E. histolytica, contains only those vitamins known to be required by the parasite. It is also available commercially (Biofluids, Inc.).
(a) Step 1. Prepare the following four solutions and then combine. (i) Dissolve 45 mg of niacinamide, 4 mg of pyridoxal hydrochloride, 23 mg of calcium pantothenate, 5 mg of thiamine hydrochloride, and 1.2 mg of vitamin B12 in a final volume of 25 ml of water. (ii) Dissolve 7 mg of riboflavin in water using the minimum amount of 0.1 N sodium hydroxide, bringing the final volume to 45 ml. (iii) Dissolve 5.5 mg of folic acid in water using the minimum amount of 0.1 N sodium hydroxide, bringing the final volume to 45 ml. (iv) Dissolve 2 mg of d-biotin in water bringing the final volume to 45 ml.
If the combined solution is cloudy, it indicates that the pH is too high due to too much sodium hydroxide having been used in solutions ii and iii and the mixture must be discarded.
(b) Step 2. Dissolve 1 mg of dl-6-8-thioctic acid (oxidized form) in 5 ml of 95% ethanol. Add 500 mg of Tween 80, bringing the volume to 30 ml with water.
Combine the solutions from steps 1 and 2, bring the final volume to 200 ml with distilled water, and sterilize through a 0.22-μm-pore-size filter. Store in 100-ml amounts at 4°C for up to 6 months. The solution is light sensitive. The above recipe provides enough vitamin mix 18 to make 10 liters of complete TYI-S-33.
To complete TYI-S-33 medium for use in axenic cultivation add 2.0 ml of vitamin mix 18 and 10 to 15 ml of heat-inactivated adult bovine serum to each 88 ml of TYI broth. Note that fetal bovine serum is not acceptable, as fetuin is toxic to the parasite. Use complete medium within 7 days. Dispense into screw-cap borosilicate glass culture tubes (16 by 125 mm). The tubes should be filled to ca. 80% capacity (including inoculum). In most cases 13 ml of TYI-S-33 per tube is the correct amount, as inocula for axenic E. histolytica are generally small in established cultures. The percentage of serum used varies among isolates but is usually either 10 or 15%.
(ii) YI-S.
YI-S (20) was developed as an alternative to TYI-S-33 due to difficulties in obtaining lots of casein digest peptone that would support adequate growth of E. histolytica. The recipe for YI-S is identical to that of TYI-S-33 except that casein digest peptone is replaced weight for weight by additional yeast extract, making the final concentration of yeast extract 3%. YI-S is not without its own problems, as the lot of yeast extract used is crucial to successful cultivation using this medium.
The development of YI-S had one unexpected benefit. The first isolate of E. dispar to be axenized would grow only in YI-S, not in TYI-S-33 (9). The other reported axenic isolates of E. dispar use a very different medium (41), indicating that YI-S may not be suitable for axenic cultivation of all E. dispar.
(iii) LYI-S-2.
In the course of developing YI-S, several combinations of liver digest and yeast extract were studied. One of these, designated LYI-S-2 (containing liver digest, yeast extract, iron, and serum), was found to result in growth equal to that in TYI-S-33. Intent on producing a medium with as few biological ingredients as possible, the medium containing only yeast extract, YI-S, was extensively tested and published. No difference in the ability of YI-S and LYI-S-2 to support growth of E. histolytica was observed (unpublished results). After publication of YI-S, further testing within our laboratory and by others disclosed the fact that some lots of yeast extract would not support any growth of the ameba while with others growth was very poor. In the case of the latter it was found that substitution of a small amount of liver digest for an equal amount of yeast extract enhanced growth considerably. LYI-S-2 is recommended when a given lot of yeast extract will support some growth, though poorly, of E. histolytica. LYI-S-2 is identical to YI-S except that weight for weight it contains 0.5% neutralized liver digest and only 2.5% yeast extract. It has been used in the long-term cultivation of several isolates of E. histolytica and a number of other Entamoeba species, with yields similar to those observed with the more widely used TYI-S-33 and YI-S (unpublished data).
G. intestinalis. (i) TYI-S-33 (Keister's modification).
For TYI-S-33 (Keister's modification [40]), prepare TYI broth exactly as for E. histolytica with the following changes. (i) Increase the amount of l-cysteine hydrochloride to 2.0 g/liter. (ii) Add 500 mg of dehydrated bovine bile per liter. (iii) Adjust the pH to 7.0 to 7.1. (iv) Sterilize by filtration through a 0.22-μm-pore-size filter. Do not autoclave!
Complete medium is made by addition of bovine serum to 10%.
(ii) YI-S.
YI-S will also support good growth of G. intestinalis with exactly the same modifications as for TYI-S-33.
T. vaginalis. (i) TYM.
TYM (13, 18) was the starting point for development of axenic media for E. histolytica—hence the similarity in components. However, in general T. vaginalis is less sensitive to lot variation than the other two parasites. One point that should be made is that this medium was devised as a general medium for growing trichomonads. More species of trichomonads have been grown axenically in TYM than in any other medium. The original recipe also added 0.5 g of Bacto Agar to the medium (0.05%). This improves isolation and growth but is not strictly necessary.
Dissolve the following in 600 ml of distilled water: 20.0 g of casein digest peptone (Trypticase); 10.0 g of yeast extract; 5.0 g of maltose; 1.0 g of l-cysteine hydrochloride; 0.2 g of ascorbic acid; 0.8 g of potassium phosphate, dibasic; and 0.8 g of potassium phosphate, monobasic.
Adjust pH to 6.0. Bring to 900 ml with distilled water and dispense in 90-ml amounts. Autoclave for 15 min at 121°C under a pressure of 15 lb/in2 to sterilize. TYM base can be stored frozen at −20°C for several months. To complete the medium, add 10 ml of heat-inactivated bovine serum.
(ii) Hollander's modification of TYM.
Hollander's modification of TYM (34) differs from TYM by the replacement of cysteine with additional ascorbic acid and the addition of potassium chloride, potassium carbonate, and iron sulfate. The recipe is as follows. Hollander also includes agar to 0.05%. Dissolve the following in 600 ml of distilled water: 20.0 g of casein digest peptone (Trypticase); 10.0 g of yeast extract; 5.0 g of maltose; 1.0 g of ascorbic acid; 1.0 g of potassium chloride; 1.0 g of potassium bicarbonate; 1.0 g of potassium phosphate, monobasic; 0.5 g of potassium phosphate, dibasic; and 0.1 g of ferrous sulfate. Continue as described above.
(iii) TYI-S-33 and YI-S.
To prepare TYI-S-33 and YI-S (18, 20), prepare the TYI or YI basal medium exactly as for E. histolytica, except adjust the pH to 6.0 before autoclaving.
B. hominis. (i) LE medium.
There are two versions of LE medium in the literature that have been used for axenic cultivation of B. hominis. Zierdt and Williams (72) used the same LE medium as described above except with the addition of 20% human or horse serum to the Locke's solution overlay. In the version of Lanuza et al. (43) the medium is modified by addition of 0.5% glucose to the Locke's solution, which is prepared at double the concentration of the recipe given before. The glucose-supplemented 2× Locke's solution for the overlay is mixed in equal volumes with fetal horse or calf serum. In both cases the medium is prereduced in an anaerobic environment for 48 h before use.
(ii) IMDM.
IMDM for axenic cultivation of B. hominis was first used by Ho et al. (33) and is available commercially from a number of sources. It is prepared with 10% horse serum and is also prereduced before use.
ESTABLISHMENT OF CULTURES
It is very important to remember that a negative culture result does not mean that the patient is uninfected. None of the organisms being considered here produce cultures 100% of the time from microscopy-positive samples, and in the case of E. histolytica the success rate appears to be between 50 and 70% in most laboratories, based on personal communications. It is also important to remember that what grows in culture is not necessarily the organism seen by microscopy.
E. histolytica
General considerations.
E. histolytica needs to be established in xenic culture. The most-common source of material will be stool samples, and this is what is assumed below. In rare instances rectal biopsy specimens or liver abscess aspirates have been the starting point for cultures. In the latter case, since the abscess is sterile, addition of a bacterial flora is necessary before inoculation of amebae into xenic culture. Such material has also been used for the direct establishment of E. histolytica into monoxenic cultures with either a bacterium (28) or a trypanosomatid (69) as the associate.
Unless a stool sample is from a patient with dysentery, it is likely that the amebae will be in the encysted form. This allows for several approaches to the establishment of cultures.
Elimination of unwanted organisms.
One of the banes of xenic cultivation is the likelihood of unwanted organisms overgrowing the desired ameba. The most-frequent source of this problem is B. hominis, which may be the most-common parasitic infection of humans. This organism is often missed on stool examination but grows luxuriantly in all the media used to cultivate xenic Entamoeba. Some authors control the growth of B. hominis with acriflavin as first described by Dobell and Laidlaw (25), but this also has an adverse effect on the bacterial flora and, directly or indirectly, on the ameba of interest. We have successfully used two methods to eliminate B. hominis from Entamoeba cultures.
The first method also was described by Dobell and Laidlaw in 1926 (25). In this method, cysts are treated with 0.1 N hydrochloric acid at room temperature for 10 min, washed thoroughly with distilled water, and reinoculated into culture medium to which a suitable bacterial flora has been added. The acid kills the bacteria, any fungi, B. hominis, intestinal trichomonads, and any nonencysted amebae while leaving the cysts intact and viable. We have found that it is not necessary in most cases for the cysts to be mature. We do not know whether the cysts complete their maturation upon inoculation or whether immature cysts respond to the stimulus and excyst directly. The cysts used can be either from stool or cultures; Entamoeba cultures in LE medium in particular frequently produce small numbers of cysts spontaneously.
The bacterial flora used in the above method is separated from another xenic culture by inoculating into culture medium, without rice starch, a small amount of supernatant from an established culture, subculturing twice, and refrigerating the flora for 48 h. The successful separation of the flora can be checked by inoculating a substantial volume into fresh medium with rice starch and checking for amebal growth. The flora can be stored at 4°C indefinitely.
The second method is that of Smedley (60) and is used when B. hominis appears in cultures after inoculation. It does not rely on cysts being present and so has advantages over the method of Dobell and Laidlaw in that respect. However, the method may need to be repeated a couple of times before the B. hominis is completely eliminated. Cultures are pelleted, and the pellet, which contains a mixture of all the organisms present, is resuspended in distilled water at room temperature for 15 min. The material is then repelleted and inoculated into fresh culture medium. Perhaps surprisingly, many Entamoeba trophozoites survive this treatment while B. hominis generally does not. A few cells or cysts of B. hominis may survive and start to grow, and the procedure will then need to be repeated. The advantages of Smedley's method are its simplicity and the fact that no separate bacterial flora is needed.
Other unwanted organisms such as fungi and trichomonads will usually disappear from xenic cultures after several passages. However, occasional instances of balanced mixed cultures are known.
Isolation.
In our experience LE medium has proven to be the best medium for primary isolation of Entamoeba species from stool, although we have limited experience with Robinson's medium, which is widely used by others for this purpose. TYSGM-9 can also be used for isolation, but its primary utility is in generating large numbers of amebae from established cultures. The numbers of amebae obtained from the two diphasic media are generally low in comparison with TYSGM-9, but their success in primary isolation of amebae from microscopically positive stool is higher (58; unpublished observations). In all cases, rice starch is added to the medium before inoculation, as are the antibiotics when needed.
Material for inoculation of xenic cultures can be prepared in several ways (18). Most commonly, stool samples are emulsified in saline and passed through a mesh to remove most of the larger particulates from the material before addition to the culture medium. However, small-pea-size pieces of fecal material can be added directly to the medium. It is always a good idea to include portions of the stool that appear mucoid or bloody if these are present. Stool fractionation by flotation in zinc sulfate (1) or sucrose (68) is also used, as this reduces the amount of debris while concentrating the cysts present in the sample. However, zinc sulfate shrinks the cysts and may damage them, resulting in lower isolation rates. Cyst purification on Percoll gradients (2) is very successful when using culture material as a starting point and could well be adapted to isolation from stool.
We routinely use more than one medium, if available, and set up duplicate cultures in which one has antibiotics added and the other does not. Penicillin-streptomycin and erythromycin are the antibiotics of choice, as they appear to have little direct effect on the amebae. However, the widespread occurrence of antibiotic resistance in bacteria makes it impossible to generalize about the amount and type of antibiotics necessary to control the growth and rice-splitting activity of human bacterial flora.
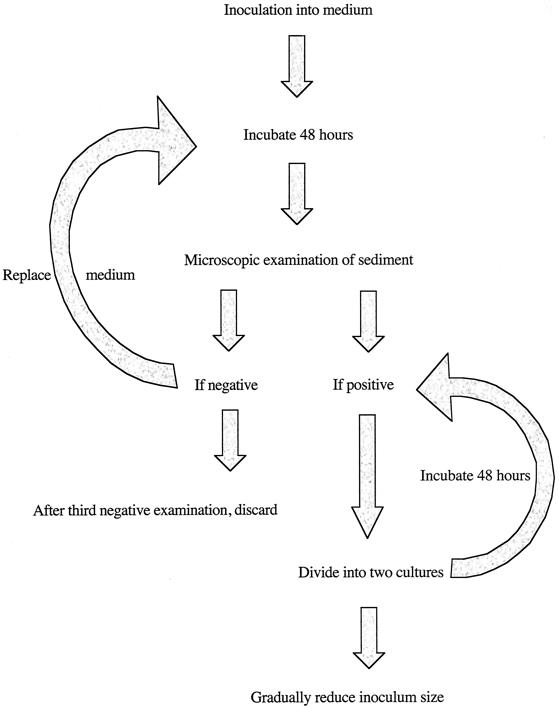
Culture tubes, containing medium and rice starch, to which stool-derived material has been added, are incubated vertically at 35.5°C for 48 h before examination (Fig. 1). A drop of sediment can be extracted from the tube for examination on a microscope slide. Alternatively, cultures can be examined in situ by slanting the tubes and using an inverted microscope. Amebae can be observed adhering to the walls of the glass culture tubes above the fecal material and above the slant in diphasic media. In situ examination is much easier in monophasic medium due to its relative clarity.
FIG. 1.
Flow diagram illustrating the stages in establishing luminal protists in culture. This procedure is relevant to all the species discussed in the present work, although the medium used and source of material will vary.
If no growth is observed at 48 h, a blind passage should be made. Most of the liquid overlying the sediment is discarded to leave less than 1 ml in the tube. The sediment is resuspended in the remaining fluid and transferred to a fresh culture tube with medium and rice starch (and antibiotic if appropriate). After incubation for an additional 48 h the culture is reexamined as described above. If no amebae are seen a further 48-h incubation is warranted, and this is followed by reexamination. If there are still no amebae seen, the culture is discarded as negative.
If cultures are positive for amebae, it is usually helpful to centrifuge the cultures in a swinging-bucket rotor (275 × g, 3 min) and divide the pellet among the recipient tubes. This can be done by chilling the culture tubes for 5 min in an ice-water bath, inverting several times to detach adherent amebae, and transferring the liquid phase to an empty culture tube before centrifugation. Cultures in LE medium can also be pelleted in situ, but in our experience the agar slant of Robinson's medium is not as conducive to this approach. As growth improves, centrifugation is no longer necessary as measured inocula (<2 ml) can be transferred to the fresh medium.
Axenization.
As previously mentioned, axenization of E. histolytica is a long and laborious procedure involving gradual adaptation of the parasite to a new way of life. A brief overview of the method is given here. A more complete description can be found in the works of Diamond (16, 18). The first step is to grow the organisms in monoxenic culture and is achieved by washing the xenic amebae in PBS to remove as many bacteria as possible and then placing the trophozoites in a rich medium with their new food organism and antibiotics. The medium can be a specialized monoxenic culture medium as described previously (16), but we have also had success initiating such cultures using one of the axenic media such as TYI-S-33. The monoxenic associate we have used most frequently is C. fasciculata ReF-1:PRR (15) (ATCC 50083). This insect flagellate is grown as a stock culture at room temperature and added to the monoxenic culture of amebae at each subculture, as Crithidia does not grow at the incubation temperature of the amebae; the amount added varies. T. cruzi Culbertson (ATCC 30013) has also been used successfully as the associated organism but is not recommended due to the potential for infection, even though this strain is of very low virulence.
The antibiotics added vary both in type and amount depending on the sensitivities of the flora in which the amebae were growing. We have used a cocktail of rifampin, amikacin, oxytetracycline, and cefotaxime with good success. Except for the first agent, these have little effect on the amebae. The initial concentration is often as high as 0.1 mg/ml of culture medium. After 24 h, the cells are pelleted by centrifugation and the medium is replaced. As the ameba cell numbers increase, the cell pellet can be divided between two tubes. By reducing the antibiotic concentration gradually in one of a pair of tubes to test for bacterial growth, sterility can be achieved gradually while at the same time the numbers of amebae are increasing. At least two subcultures in the absence of antibiotics should be performed before the cultures can be considered free of bacteria. This can be verified using standard aerobic and anaerobic testing procedures for bacteria, including mycoplasmas, and fungi (18).
Established monoxenic cultures, those in which growth is reproducible and bacteria are absent, are then used to initiate axenic cultures. This uses the same medium but with no Crithidia added. After a few subcultures the flagellates disappear as a result of dilution and ingestion. It is often helpful, although not always necessary, to add a small amount of Noble agar to the tubes at each subculture (0.01%, wt/vol; for example, adding 0.25 ml of a melted 0.5% stock agar solution to 13 ml of medium in a tube). It appears to form a substrate for the amebae. In addition, the tubes should be incubated vertically rather than at 5° to the horizontal, as this appears less “stressful” to the cells. Often the culture will flourish initially and then numbers will crash. It is at this crisis point that the cultures are most vulnerable. As long as a few live cells persist, it is worth continuing to replace the medium every few days. With luck, the numbers will gradually start to increase again, and eventually addition of the agar will no longer be needed. When established, the axenic cultures can be incubated at 5° to the horizontal and eventually should reach cell concentrations of 150,000 to 300,000 per ml on a twice-per-week subculture schedule.
Principles of inducing encystment of E. histolytica.
The methods of inducing encystment of E. histolytica are based on Dobell and Laidlaw's discovery that “… .cyst-production may sometimes be temporarily increased by cultivating the amoebae in starch-free media for one or two generations, and then transferring them to media containing this substance: but the results are uncertain, and the number of cysts produced in any culture cannot be predicted” (25). Each point these authors make holds true to this day, and anyone attempting to induce encystment must bear these points in mind at all times. To date, cysts of this species have been induced only in xenic culture.
No one as far as we can determine has published a detailed account of the process of inducing encystation. Here we present a protocol used for many years in the NIH Laboratory of Parasitic Diseases but never previously published in detail.
Three things are of special concern in obtaining cysts: the media, bacterial flora, and rice starch. Some media are better than others for this purpose. LE medium is the one with which we and others have had excellent results (see above).
The accompanying bacterial flora present in a xenic culture plays an important role in the process of encystment. Here again some are better than others. It is good practice for those requiring a steady source of large numbers of cysts to isolate and maintain the bacterial flora of a xenic culture in which cysts regularly form spontaneously. A technique for doing this is presented above (under “Elimination of unwanted organisms”).
(i) Protocol: encystment.
(a) Day 1. Begin the process with three amebae-rich 48-h cultures in LE medium. Harvest them by chilling the culture tubes for 5 min in an ice-water bath, invert the tubes 10 times to mix contents and free amebae adhering to the glass and egg slant, and centrifuge for 3 min at 275 × g. Remove and discard all but 1 ml of the spent overlay. Resuspend pelleted amebae, pool, and transfer equal amounts to six tubes of LE medium without rice. Incubate the cultures in an upright position for 72 h.
(b) Day 4. Harvest each of the six cultures: Chill, remove and discard all but 1 ml of overlay. Mix remaining overlay of each culture and transfer equal amounts to two tubes of LE medium without rice. There will now be 12 cultures. Incubate 48 h.
(c) Day 6. Harvest the 12 cultures and subculture as on day 4. Incubate the 24 cultures for 48 h.
(d) Day 8. Carefully remove the overlay from each culture, leaving only enough to cover the sediment at the interface of the egg slant and overlay. Collect the sediments from three cultures and transfer to one tube of medium to which rice has been added. Repeat with the remaining cultures. Incubate the resulting eight cultures for 48 h.
(e) Day 10. Remove a small drop of sediment from each culture, stain with Lugol's iodine solution, and search for presence of quadrinucleate cysts. If found, harvest cultures as on day 1. Remove overlay, leaving only the sediment. Pool sediments and wash two times with distilled water. Cysts will remain viable from 10 to 14 days when stored at 4°C. If cysts are not found, incubate an additional 24 h.
(ii) Protocol: excystment of cysts induced in vitro.
Inducing E. histolytica to excyst is relatively easy compared to getting the ameba to encyst. How this is accomplished depends on the goal. If the goal is to propagate the amebae in a xenic environment, then the medium in which the cysts were induced is used, in this case LE medium. If the goal is to excyst them in a bacteria-free environment, any of the monophasic liquid media devised for axenic culture can be used. In the latter case freshly prepared medium must be used. While the amebae will excyst in the axenic media, no one, as yet, has been able get them to encyst in this environment.
Best results are obtained when at least 50% of the cysts produced are in the quadrinucleate stage. Usually no more than 25% of the cysts can be expected to excyst.
To induce excystment, the cysts are first treated to remove unwanted organisms as recommended above. They are then placed in a tube of LE medium inoculated with a suitable bacterial flora for xenic growth or in a medium capable of sustaining axenic growth. Upon incubation most of the cysts capable of undergoing excystation will have done so by the end of 6 h.
Rexenization of axenically cultivated E. histolytica.
Occasions will arise when it is desirable to return axenized amebae to the xenic state. The following protocol has worked well in our hands.
(i) Inoculate three tubes of LE medium with a bacterial flora known to support xenic growth.
(ii) Chill a 72-h culture of axenically cultivated amebae in an ice-water bath for 5 min. Invert culture tube 10 times to dislodge amebae from glass surfaces. Centrifuge 3 min at 275 × g. Remove supernatant and discard.
(iii) Resuspend amebae in 1 ml of fresh medium for axenic culture, count cells, and inoculate the tubes of LE medium with 1 × 105, 2 × 105, and 4 × 105 amebae, respectively.
(iv) Incubate 48 h. Remove all but approximately 1 ml of overlay. Resuspend the sediment located at the interface of the slant and overlay. Examine a drop with a microscope. The majority of inoculated amebae will have died. Select the best of the three cultures and subculture.
(v) Subculture. The number of amebae transferred can be determined only by trial and error. In the early stages of establishing the culture, transfer one-half of the material from the old culture to each of two tubes of fresh medium (do not add additional bacteria after the initial inoculation of the medium). Later, as amebic growth improves, transfer smaller portions, e.g., one-third to one-fourth. Do not reduce the inoculum further. If only a few amebae are present, transfer all the sediment to a fresh tube of medium. Subculture three times per week as noted under “Maintenance of Cultures.”
G. intestinalis
Isolation.
Xenic and monoxenic cultures are not required for isolation of G. intestinalis. While cultures can be initiated from trophozoites obtained by duodenal biopsy, it is now much more common for cultures to be initiated with cysts collected from feces. Many different methods have been described for purification of Giardia cysts from stool. The one below is courtesy of J. T. Conrad and has elements in common with several others in the literature (e.g., see reference 6).
Stool samples are diluted 1:12 (vol/vol) in distilled water, and 20 ml of diluted stool is vortexed for 5 min with 3-mm-diameter glass beads to give a slurry. The material is then filtered through sterile nylon mesh (183-mesh count). On ice, layer 5 ml of the filtrate onto 10 ml of 1 M sucrose in a 50-ml centrifuge tube. Spin at 450 × g for 5 min. Pipette off the supernatant and retain. Dilute the supernatant in water to 50 ml and spin at 450 × g for 5 min. Decant and discard the liquid. On ice, resuspend the pellet in 2.5 ml of distilled water and layer on top of 10 ml of 0.5 M sucrose in a 15-ml centrifuge tube. Spin at 450 × g for 5 min. With a Pasteur pipette, remove the bottom 1 ml of liquid and place in a clean 15-ml centrifuge tube. Examine this material for the presence of Giardia cysts. Dilute to 10 ml with distilled water and spin at 450 × g for 5 min. Decant and discard the liquid. Resuspend pellet and use to initiate cultures.
Cultures are initiated in 13 ml of TYI-S-33 (Keister's modification) in culture tubes (16 by 125 mm) or other tubes filled to ca. 80% capacity. The TYI-S-33 should contain antibiotics and antifungals. One successful combination includes the following (per milliliter): 1 μg of amphotericin B (1-mg/ml stock), 1 mg of moxolactam (300-mg/ml stock), 1 mg of ticarcillin (400-mg/ml stock), 40 μg of gentamicin (40-mg/ml stock), and 200 U of penicillin (100,000-U/ml stock).
Some authors recommend “activating” the cysts with hydrochloric acid at pH 2 and washing in distilled water before inoculation. This should obviate the need for the antibiotics, but acid treatment is not necessary for successful establishment of axenic cultures. Culture tubes are incubated vertically at 35.5°C and inspected visually with an inverted microscope daily. The medium should be decanted and replaced with fresh medium and antibiotics daily for the first week and then every other day for up to 2 weeks. If no trophozoites are seen by then, the isolation is negative. The decanted medium may contain swimming trophozoites and can be retained if wanted. Most trophozoites should adhere to the glass, however. As growth improves, the antibiotic levels can be gradually reduced in concentration. To ascertain that the cultures are truly in the axenic state, parallel cultures should be set up after growth has stabilized, one set with and one without antibiotics. After two subcultures the antibiotic-free line should be examined for the presence of bacteria, mycoplasmas, and fungi using standard aerobic and anaerobic testing procedures (see reference 18).
Encystment.
In contrast to E. histolytica, in vitro encystment of G. intestinalis in axenic culture is a well-established procedure (reviewed in reference 36). Two main approaches have been used: exposure to bile salts and cholesterol starvation. It is likely that the former induces the latter. As we have no direct experience with these methods we simply refer the reader to the appropriate publications (29, 45).
T. vaginalis
Isolation.
Xenic and monoxenic cultures are not required for isolation of T. vaginalis. Axenic cultures are initiated in 13 ml of TYM or TYI-S-33 in standard culture tubes (16 by 125 mm) in one of two ways: (i) with sterile cotton swabs used to obtain material from the vagina or urethra or (ii) with sediments obtained after centrifugation following vaginal washing (18). Antibiotics and antifungals must be included. A combination of penicillin, streptomycin, and kanamycin plus mycostatin or nystatin at initial concentrations of 100 μg/ml is often successful. Addition of a small amount of agar to the medium (0.05%) often helps in the establishment of cultures. This can be included either at the medium preparation stage or by adding a 0.5% stock (0.1 ml/10 ml of medium) that has been melted and cooled to 45°C prior to use. Culture tubes are incubated vertically at 35.5°C and inspected visually with an inverted microscope daily for 4 days. If no trichomonads are seen after 4 days the isolation is negative. As growth of positive cultures improves, the antibiotic levels can be gradually reduced in concentration. To ascertain that the cultures are truly in the axenic state, parallel cultures should be set up after growth has stabilized, one set with and one without antibiotics. After two subcultures the antibiotic-free line should be examined for the presence of bacteria, mycoplasmas, and fungi using standard aerobic and anaerobic testing procedures (see reference 18).
A commercial and proprietary product called InPouch TV has been reported to give results comparable to those of the more widely used TYM medium for isolation and has the advantage of being self-contained and having a reasonable shelf life (8). Although we do not know its composition it presumably contains antibiotics and antifungals in addition to a growth medium.
D. fragilis, B. coli, and B. hominis
Isolation.
As mentioned above, B. hominis will grow in all of the media used for xenic growth of E. histolytica and is frequently isolated along with (or instead of!) the latter. Growth of B. hominis in xenic culture does not require the addition of rice starch to the medium. Otherwise, preparation of the medium and inoculation of material follow the same protocol as for E. histolytica.
Isolation of D. fragilis is more complicated. As its name suggests it is a more labile cell than the other species, in part because it has no known cyst form. Therefore, if isolation is to be successful the stool sample must be fresh. Studies have shown successful cultivation from feces stored at room temperature for up to 24 h but only up to 10 h for refrigerated feces (57). Cultures have been established in LE and Robinson's media, but as mentioned before it is difficult to keep many isolates for long. Whether this is a deficiency of the media or is dependent on the bacterial flora composition or on some other factor is not known.
Although more-complex methods have been described, simple inoculation of a small amount of fecal material into medium with rice starch as for E. histolytica appears to be sufficient to start cultures of B. coli. However, our own experience with this organism is limited.
Axenization of B. hominis.
The axenization of B. hominis is also quite laborious, and all authors appear to have started from established xenic cultures. Monoxenic cultures have not been found to be necessary. Xenic cells or Ficoll-metrizoic acid gradient-purified cells (43) are inoculated into LE-based axenic culture medium with a cocktail of antibiotics. Weekly subcultures with large inocula and fresh antibiotics are performed for up to 6 weeks before sterility is achieved. It is not clear whether direct axenization of B. hominis into liquid IMDM is possible. Established axenic cultures in LE medium were transferred to IMDM in the original publication (33). However, since then the axenization of B. hominis using IMDM-soft agar with addition of antibiotics has been described (50). The B. hominis organisms form colonies in the agar which can then be isolated in liquid IMDM with little or no bacterial contamination.
MAINTENANCE OF CULTURES
Established cultures of all parasites are handled in essentially the same way. Xenic cultures of E. histolytica and D. fragilis are routinely passaged at 48- to 72-h intervals; usually a Monday-Wednesday-Friday schedule is convenient. Occasionally cultures of these organisms will be found that do better with twice-weekly subculture. Xenic isolates of B. hominis and B. coli can usually be kept on this schedule also. The inoculum size for the longer incubation period should be smaller than that for shorter incubations. However, variation among isolates and flora means that no generalities can be made regarding the size of inocula or the amount of rice and antibiotics to be added to the medium for optimal growth. It is very much a case of trial and error combined with experience in evaluating growth of cultures that leads to successful establishment of these parasites in xenic culture. It is recommended that xenic cultures be passaged using two or more inoculum sizes to ensure a successful subculture. A significant threshold effect can sometimes be encountered, in which a certain inoculum size gives rise to a healthy culture but an inoculum smaller by as little as 50 μl may result in no growth.
Established axenic cultures of E. histolytica, G. intestinalis, and B. hominis are passaged at 72- and 96-h intervals, with a Monday-Friday schedule being convenient. Axenic T. vaginalis cultures usually need to be passaged three times a week. Again the inocula for the longer incubation period should be smaller than for the shorter incubation period, and again trial and error is needed to find out exactly what those are, due to significant variation among isolates and species (from <50 per ml for some T. vaginalis isolates to >106 per ml for axenic B. hominis). While counted inocula are desirable, established cultures become predictable and measured volume inocula can be used.
Visual inspection of every culture before subculture is recommended, since what appears to be a heavy culture may in fact contain many lysed cells, indicating that the inoculum previously used was too large. An increased inoculum volume may be warranted for the subsequent subculture to compensate for the dead amebae. Likewise, parallel duplicate cultures are recommended in case of inadvertent contamination or tube breakage. The unused culture can be kept at 33°C as a backup in case of problems.
The method for subculturing many types of cultures is essentially the same. Cultures are chilled in an ice-water bath for 5 min (xenic cultures and axenic E. histolytica) or 10 min (G. intestinalis) to release trophozoites attached to the glass culture tube. In T. vaginalis, B. coli, and B. hominis cultures, most organisms will be nonadherent and the tubes need not be chilled unless an accurate count is desired. Tubes are inverted several times to disperse the cells and a measured inoculum is passed aseptically to a culture tube containing fresh medium. The tubes are capped tightly and incubated at 36 to 37°C, either vertically (xenic cultures, T. vaginalis, and axenic B. hominis) or at 5° to the horizontal (established axenic cultures of E. histolytica and G. intestinalis). For axenic B. hominis the medium must be prereduced for 48 h before inoculation in an anaerobic jar.
SOURCES OF MEDIUM MATERIALS
While other sources are no doubt available, below are listed the suppliers of medium components used in our laboratories that have been tested for their ability to support growth of these parasites. However, it should be noted again that not all lots under the same catalog number will necessarily support growth of these organisms. The suppliers and their products are as follows: American Laboratories, Inc., Omaha, Nebr., gastric mucin (catalog no. 014); Becton Dickinson Co., Cockeysville, Md., BBL casein digest peptone (catalog no. 211921) and yeast extract (catalog no. 288620); Biofluids, Inc., Rockville, Md., vitamin mixture 18 (catalog no. 318) and adult bovine serum (catalog no. 203); VWR International, Poole, United Kingdom, starch rice powder (catalog no. 21150294); Sigma Aldrich, St. Louis, Mo., adult bovine serum (catalog no. B-2771) and yeast extract (catalog no. Y-1625); Oxoid Ltd., Basingstoke, United Kingdom, liver digest neutralized (LP027).
CONCLUSIONS
Cultivation of luminal protists has a limited role in the diagnostic laboratory, and no diagnosis should rely on cultivation alone. However, for many types of research project, large numbers of cells are needed and cultures are thus required. We have therefore provided detailed protocols based on our experience that will hopefully be helpful to those who intend to culture these organisms in vitro.
The organisms discussed here have a long history of cultivation but, in spite of this, establishing and growing them in the laboratory remain an art rather than a science. The procedures we discuss should therefore be viewed as recipes to be modified to meet individual tastes. Indeed, changes are likely to be forced upon us due to the increasing prevalence of antibiotic resistance in gut bacteria. This makes controlling or eliminating bacterial flora much more difficult.
One of the frustrations we face when cultivating axenic organisms in particular is the lot-to-lot variability in the medium components. An item from the same supplier with the same catalog number may support abundant growth one time and not the next. The reasons for this are unclear, but recognition of this variability is crucial to long-term success in cultivating these organisms. Part of the problem is that the components are not produced with these organisms in mind and are usually tested only for their ability to support bacterial or fungal growth. The more fastidious protists clearly detect differences that are unimportant to bacteria and fungi!
Finally, we hope that the difficulties associated with cultivation of these organisms will not prevent people from choosing to work with them. If the medium supports the growth of the organisms, routine maintenance of cultures is not difficult or time-consuming.
REFERENCES
- 1.Ash, R. L., and T. C. Orihel. 1987. Collection and preservation of feces, p. 5-14. In American Society of Clinical Pathologists (ed.), Parasites: a guide to laboratory procedures and identification. ASCP Press, Chicago, Ill.
- 2.Avron, B., R. Bracha, M. Deutch, and D. Mirelman. 1983. Entamoeba invadens and Entamoeba histolytica: separation and purification of precysts and cysts by centrifugation on discontinuous gradients of Percoll. Exp. Parasitol. 55:265-269. [DOI] [PubMed] [Google Scholar]
- 3.Balamuth, W. 1946. Improved egg yolk medium for cultivation of Entamoeba histolytica and other intestinal protozoa. Am. J. Clin. Pathol. 16:380-384. [DOI] [PubMed] [Google Scholar]
- 4.Barret, H. P. 1921. A method for the cultivation of Blastocystis. Ann. Trop. Med. Parasitol. 15:113-116. [Google Scholar]
- 5.Barret, H. P., and N. Yarbrough. 1921. A method for the cultivation of Balantidium coli. Am. J. Trop. Med. 1:161-165. [Google Scholar]
- 6.Bingham, A. K., and E. A. Meyer. 1979. Giardia excystation can be induced in vitro in acidic solutions. Nature (London) 277:301-302. [DOI] [PubMed] [Google Scholar]
- 7.Boeck, W. C., and J. Drbohlav. 1925. The cultivation of Endamoeba histolytica. Am. J. Hyg. 5:371-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borchardt, K. A., and R. F. Smith. 1991. An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin. Med. 67:149-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, C. G. 1995. Axenic cultivation of Entamoeba dispar Brumpt 1925. Entamoeba insolita Geiman and Wichterman 1937 and Entamoeba ranarum Grassi 1879. J. Eukaryot. Microbiol. 42:590-593. [DOI] [PubMed] [Google Scholar]
- 10.Clark, C. G. 1997. Extensive genetic diversity in Blastocystis hominis. Mol. Biochem. Parasitol. 87:79-83. [DOI] [PubMed] [Google Scholar]
- 11.Cleveland, L. R., and E. P. Sanders. 1930. The production of bacteria-free amoebic abscesses in the liver of cats and observations on the amoebae in various media with and without bacteria. Science 77:149-151. [DOI] [PubMed] [Google Scholar]
- 12.Cox, F. E. G. 1961. The cultivation of Balantidium coli throughout its viable temperature range. Ann. Trop. Med. Parasitol. 55:305-308. [DOI] [PubMed] [Google Scholar]
- 13.Diamond, L. S. 1957. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 43:488-490. [PubMed] [Google Scholar]
- 14.Diamond, L. S. 1961. Axenic cultivation of Entamoeba histolytica. Science 134:336-337. [DOI] [PubMed] [Google Scholar]
- 15.Diamond, L. S. 1968. Improved method for the monoxenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae with trypanosomatids. J. Parasitol. 54:715-719. [PubMed] [Google Scholar]
- 16.Diamond, L. S. 1968. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J. Parasitol. 54:1047-1056. [PubMed] [Google Scholar]
- 17.Diamond, L. S. 1982. A new liquid medium for xenic cultivation of Entamoeba histolytica and other lumen dwelling protozoa. J. Parasitol. 68:958-959. [PubMed] [Google Scholar]
- 18.Diamond, L. S. 1983. Lumen dwelling protozoa: Entamoeba, trichomonads, and Giardia, p. 67-109. In J. B. Jensen (ed.), In vitro cultivation of protozoan parasites. CRC Press, Boca Raton, Fla.
- 19.Diamond, L. S., and C. G. Clark. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 40:340-344. [DOI] [PubMed] [Google Scholar]
- 20.Diamond, L. S., C. G. Clark, and C. C. Cunnick. 1995. YI-S, a casein-free medium for axenic cultivation of Entamoeba histolytica, related Entamoeba, Giardia intestinalis and Trichomonas vaginalis. J. Eukaryot. Microbiol. 42:277-278. [DOI] [PubMed] [Google Scholar]
- 21.Diamond, L. S., and C. C. Cunnick. 1991. A serum-free, partly defined medium, PDM-805, for the axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and other Entamoeba. J. Protozool. 38:211-216. [DOI] [PubMed] [Google Scholar]
- 22.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 23.Diamond, L. S., C. F. T. Mattern, I. L. Bartgis, W. A. Daniel, and D. B. Keister. 1976. Viruses of Entamoeba histolytica. VI. A study of host range, p. 334-345. In B. Sepúlveda and L. S. Diamond (ed.), Proceedings of the International Conference on Amebiasis. Instituto Mexicano de Seguro Social, Mexico City, Mexico.
- 24.Dobell, C. 1940. Researches on the intestinal protozoa of monkeys and man. X. The life history of Dientamoeba fragilis: observations, experiments, and speculations. Parasitology 32:417-461. [Google Scholar]
- 25.Dobell, C., and P. P. Laidlaw. 1926. On the cultivation of Entamoeba histolytica and some other entozoic amoebae. Parasitology 18:283-318. [Google Scholar]
- 26.Dougherty, E. C. 1959. Introduction to axenic cultivation of invertebrate metazoa: a goal. Ann. N. Y. Acad. Sci. 77:27-54. [Google Scholar]
- 27.Farri, T. A. 1978. A simple technique for preparing clone cultures of Entamoeba histolytica. Trans. R. Soc. Trop. Med. Hyg. 72:205-206. [DOI] [PubMed] [Google Scholar]
- 28.Freedman, L., S. E. Maddison, and R. Elsdon-Dew. 1958. Monoxenic culture of Entamoeba histolytica derived from human liver abscesses. S. Afr. J. Med. Sci. 23:9-12. [PubMed] [Google Scholar]
- 29.Gillin, F. D., S. E. Boucher, S. S. Rossi, and D. S. Reiner. 1989. Giardia lamblia: the roles of bile, lactic acid, and pH in the completion of the life cycle in vitro. Exp. Parasitol. 69:164-174. [DOI] [PubMed] [Google Scholar]
- 30.Gillin, F. D., and L. S. Diamond. 1978. Clonal growth of Entamoeba histolytica and other species of Entamoeba in agar. J. Protozool. 25:539-543. [DOI] [PubMed] [Google Scholar]
- 31.Gillin, F. D., and L. S. Diamond. 1980. Clonal growth of Giardia lamblia trophozoites in a semi-solid agarose medium. J. Parasitol. 66:350-352. [PubMed] [Google Scholar]
- 32.Haque, H., N. U. Mollah, I. K. M. Ali, K. Alam, A. Eubanks, D. Lyerly, and W. A. Petri, Jr. 2000. Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J. Clin. Microbiol. 38:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho, L. C., M. Singh, G. Suresh, G. C. Ng, and E. H. Yap. 1993. Axenic culture of Blastocystis hominis in Iscove's modified Dulbecco's medium. Parasitol. Res. 79:614-616. [DOI] [PubMed] [Google Scholar]
- 34.Hollander, D. H. 1976. Colonial morphology of Trichomonas vaginalis in agar. J. Parasitol. 62:826-828. [PubMed] [Google Scholar]
- 35.Ivey, M. H. 1961. Growth characteristics of clones of Trichomonas vaginalis in solid medium. J. Parasitol. 47:539-544. [PubMed] [Google Scholar]
- 36.Jarroll, E. L., P. T. Macechko, P. A. Steimle, D. Bulik, C. D. Karr, H. van Keulen, T. A. Paget, G. Gerwig, J. Kamerling, J. Vliegenthart, and S. Erlandsen. 2001. Regulation of carbohydrate metabolism during Giardia encystment. J. Eukaryot. Microbiol. 48:22-26. [DOI] [PubMed] [Google Scholar]
- 37.Johnson, J. A., and C. G. Clark. 2000. Cryptic genetic diversity in Dientamoeba fragilis. J. Clin. Microbiol. 38:4653-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones, W. R. 1946. The experimental infection of rats with Entamoeba histolytica; with a method for evaluating the anti-amoebic properties of new compounds. Ann. Trop. Med. Parasitol. 40:130-140. [DOI] [PubMed] [Google Scholar]
- 39.Karapetyan, A. 1962. In vitro cultivation of Giardia duodenalis. J. Parasitol. 48:337-340. [PubMed] [Google Scholar]
- 40.Keister, D. B. 1983. Axenic cultivation of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77:487-488. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi, S., E. Imai, H. Tachibana, T. Fujiwara, and T. Takeuchi. 1998. Entamoeba dispar: cultivation with sterilized Crithidia fasciculata. J. Eukaryot. Microbiol. 45:3S-8S. [DOI] [PubMed] [Google Scholar]
- 42.Kulda, J., and E. Nohynková. 1995. Giardia in humans and animals. p. 225-422 In J. P. Kreier (ed.), Parasitic protozoa, vol. 10. Academic Press, San Diego, Calif. [Google Scholar]
- 43.Lanuza, M. D., J. A. Carbajal, J. Villar, and R. Borras. 1997. Description of an improved method for Blastocystis hominis culture and axenization. Parasitol. Res. 83:60-63. [DOI] [PubMed] [Google Scholar]
- 44.Linstead, D. 1981. New defined and semi-defined media for cultivation of the flagellate Trichomonas vaginalis. Parasitology 83:125-137. [DOI] [PubMed] [Google Scholar]
- 45.Lujan, H. D., M. R. Mowatt, L. G. Byrd, and T. E. Nash. 1996. Cholesterol starvation induces differentiation of the intestinal parasite Giardia lamblia. Proc. Natl. Acad. Sci. USA 93:7628-7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maraha, B., and A. G. Buiting. 2000. Evaluation of four enzyme immunoassays for the detection of Giardia lamblia antigen in stool specimens. Eur. J. Clin. Microbiol. Infect. Dis. 19:485-487. [DOI] [PubMed] [Google Scholar]
- 47.Meyer, E. A. 1970. Isolation and axenic cultivation of Giardia trophozoites from the rabbit and chinchilla. Nature (London) 207:1417-1418. [DOI] [PubMed] [Google Scholar]
- 48.Meyer, E. A. 1976. Giardia lamblia: isolation and axenic cultivation. Exp. Parasitol. 39:101-105. [DOI] [PubMed] [Google Scholar]
- 49.Mueller, D. E., and W. A. Petri, Jr. 1995. Clonal growth in Petri dishes of Entamoeba histolytica. Trans. R. Soc. Trop. Med. Hyg. 89:123. [DOI] [PubMed] [Google Scholar]
- 50.Ng, G. C., and K. S. Tan. 1999. Colony growth as a step towards axenization of Blastocystis isolates. Parasitol. Res. 85:678-679. [DOI] [PubMed] [Google Scholar]
- 51.Patel, S. R., W. Wiese, S. C. Patel, C. Ohl, J. C. Byrd, and C. A. Estrada. 2000. Systematic review of diagnostic tests for vaginal trichomoniasis. Infect. Dis. Obstet. Gynecol. 8:248-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips, B. P. 1950. Cultivation of Endamoeba histolytica with Trypanosoma cruzi. Science 111:8-9. [DOI] [PubMed] [Google Scholar]
- 53.Reeves, R. E., H. E. Meleney, and W. W. Frye. 1957. A modified Shaffer-Frye technique for the cultivation of Entamoeba histolytica and some observations on its carbohydrate requirements. Am. J. Hyg. 66:56-62. [DOI] [PubMed] [Google Scholar]
- 54.Robinson, G. L. 1968. The laboratory diagnosis of human parasitic amoebae. Trans. R. Soc. Trop. Med. Hyg. 62:285-294. [DOI] [PubMed] [Google Scholar]
- 55.Said-Fernandez, S., J. Vargas-Villarreal, J. Castro-Garza, B. D. Mata-Cardenas, L. Navarro-Marmolejo, G. Lozano-Garza, and H. Martinez-Rodriguez. 1988. PEHPS medium: an alternative for axenic cultivation of Entamoeba histolytica and E. invadens. Trans. R. Soc. Trop. Med. Hyg. 82:249-253. [DOI] [PubMed] [Google Scholar]
- 56.Samuels, R. 1962. Agar techniques for colonizing and cloning trichomonads. J. Protozool. 9:103-107. [Google Scholar]
- 57.Sawangjaroen, N., R. Luke, and P. Prociv. 1993. Diagnosis by faecal culture of Dientamoeba fragilis infections in Australian patients with diarrhoea. Trans. R. Soc. Trop. Med. Hyg. 87:163-165. [DOI] [PubMed] [Google Scholar]
- 58.Sehgal, R., M. Abd-Alla, A. H. Moody, P. L. Chiodini, and J. P. Ackers. 1995. Comparison of two media for the isolation and short-term culture of Entamoeba histolytica and E. dispar. Trans. R. Soc. Trop. Med. Hyg. 89:394. [DOI] [PubMed] [Google Scholar]
- 59.Silberman, J. D., M. L. Sogin, D. D. Leipe, and C. G. Clark. 1996. Human parasite finds taxonomic home. Nature (London) 380:398. [DOI] [PubMed] [Google Scholar]
- 60.Smedley, S. R. 1956. A method for freeing cultures of Entamoeba histolytica from contamination with Blastocystis. Trans. R. Soc. Trop. Med. Hyg. 50:232-233. [DOI] [PubMed] [Google Scholar]
- 61.Tan, S. W., M. Singh, K. T. Thong, L. C. Ho, K. T. Moe, X. Q. Chen, G. C. Ng, and E. H. Yap. 1996. Clonal growth of Blastocystis hominis in soft agar with sodium thioglycollate. Parasitol. Res. 82:737-739. [DOI] [PubMed] [Google Scholar]
- 62.Tan, K. S., G. C. Ng, E. Quek, J. Howe, N. P. Ramachandran, E. H. Yap, and M. Singh. 2000. Blastocystis hominis: a simplified, high-efficiency method for clonal growth on solid agar. Exp. Parasitol. 96:9-15. [DOI] [PubMed] [Google Scholar]
- 63.Tan, S. W., M. Singh, E. H. Yap, L. C. Ho, K. T. Moe, J. Howe, and G. C. Ng. 1996. Colony formation of Blastocystis hominis in soft agar. Parasitol. Res. 82:375-377. [DOI] [PubMed] [Google Scholar]
- 64.Taylor, A. E. R., and J. R. Baker. 1978. Methods of cultivating parasites in vitro. Academic Press, London, United Kingdom.
- 65.Trussel, R. E. 1940. Experimental and clinical Trichomonas vaginitis. J. Iowa State Med. Soc. 30:66-70. [Google Scholar]
- 66.Visvesvara, G. S. 1980. Axenic growth of Giardia lamblia in Diamond's TPS-1 medium. Trans. R. Soc. Trop. Med. Hyg. 74:213-215. [DOI] [PubMed] [Google Scholar]
- 67.Von Brand, T., C. R. Rees, L. Jacobs, and L. V. Reardon. 1943. Studies on reducing substances and gas formation in cultures of Endamoeba histolytica and a single species of symbiotic bacterium. Am. J. Hyg. 37:310-319. [Google Scholar]
- 68.Walderich, B., L. Mueller, R. Bracha, J. Knobloch, and G. D. Burchard. 1997. A new method for isolation and differentiation of native Entamoeba histolytica and E. dispar cysts from fecal samples. Parasitol. Res. 83:719-721. [DOI] [PubMed] [Google Scholar]
- 69.Wang, L. T., G. Jen, and J. H. Cross. 1973. Establishment of Entamoeba histolytica from liver abscess in monoxenic cultures with hemoflagellates. Am. J. Trop. Med. Hyg. 22:30-32. [DOI] [PubMed] [Google Scholar]
- 70.Wieder, S. C., D. B. Keister, and D. S. Reiner. 1983. Mass cultivation of Giardia lamblia in a serum-free medium. J. Parasitol. 69:1181-1182. [PubMed] [Google Scholar]
- 71.Zaman, V. 1993. Balantidium coli, p. 43-63. In J. P. Kreier and J. H. Baker (ed.), Parasitic protozoa, vol. 3. Academic Press, San Diego, Calif. [Google Scholar]
- 72.Zierdt, C. H., and R. L. Williams. 1974. Blastocystis hominis: axenic cultivation. Exp. Parasitol. 36:233-243. [DOI] [PubMed] [Google Scholar]