Abstract
The Ganoderma lucidum polysaccharides (GLPs) content, monosaccharide composition, molecular weight, sugar metabolism-related enzyme activities and in vitro antioxidant activities of Ganoderma lucidum fruiting body (GLFB) from cassava stalk cultivation at different growth stages (namely, differentiation stage, umbrella stage, maturity stage and spore stage) were determined. The results showed that the monosaccharides of GLPs in GLFB were mainly composed of galactose, glucose, and mannose. During the maturation of GLFB, the polysaccharide content, hexose kinase and phosphoglucose isomerase activities showed an increasing trend, the structure of functional groups did not change significantly, and the molecular weight of GLPs showed a tendency to increase (112 kDa up to 11358 kDa) and then decrease (11358 kDa down to 7678 kDa). Of the four growth stages, the spore stage had the highest GLPs content of 4.11%, the highest hexokinase (HK) and phosphoglucose isomerase (PGM) activities of 4051.4 and 322.1 nmol/(min/g), respectively, the highest total antioxidant activity (18.79 mmol/mL) and DPPH radical scavenging capacity (36.15%). This study provided a theoretical basis for the application of cassava stalks in GLFB cultivation and harvesting.
Keywords: Ganoderma lucidum, Cassava stalks, Polysaccharides, Enzymatic activity, Bioactivity
Subject terms: Carbohydrates, Polysaccharides
Introduction
Ganoderma lucidum is an important medicinal and food fungus in China1. GLFB is the general name of the whole plant, which consists of a cap and a stalk. GLPs are not only an important functional component of GLFB, but also one of the most important indicators of the quality of Ganoderma lucidum products2,3. GLPs have been demonstrated to play a role in immunomodulation, antitumor, hypolipidemic, and antichemical and immune liver injury4. Glucose, galactose, arabinose, mannose, xylose, fucose and rhamnose are the main monosaccharide compositions of GLPs, among which glucose, galactose and mannose account for the largest proportion. In addition, a variety of factors such as the cultivation substrate also change the composition and content of monosaccharides in GLPs5. Various monosaccharides in GLPs are linked by α or β conformational glycosidic bonds through 1→2, 1→3, 1→4 and 1→6, among which β-polysaccharide plays a major role in biological activity6.
The biological activities of GLPs, such as reducing blood lipid, regulating immunity and resisting tumor, are related to its structure and molecular weight7–10. It has been reported that the molecular weight distribution of GLPs ranges from thousands to millions of Da, and the proportion of each molecular weight is related to its variety and extraction technology, and the biological activity of GLPs in vitro are also related to its molecular weight11,12. GLPs were found to significantly reduce weight gain and blood lipid levels by Wang et al.13 GLPs effectively alleviated oxidative stress and inflammatory responses by activating the Nrf2-Keap1 and inhibiting the NF-κB signaling pathway, promoted reverse cholesterol transport through LXRα-ABCA1/ABCG1 signaling, and increased the expression of CYP7A1 and CYP27A1, which are responsible for the production of bile acids, as well as inhibited the synthesis of bile acids. Zhang et al.14 clarified that GLPs not only increase the reserves of HG and MG in mice and reduce the levels of BLA and BUN in the body, but also improve the ability of mice to scavenge free radicals and reactive oxygen species, and improve the activity of antioxidant enzymes and thus reduce the lipid peroxidation of the cells, which has better anti-fatigue ability, thus alleviating the damage caused by exercise fatigue to the body. In addition, GLPs were found to alleviate rheumatoid arthritis in rats by inhibiting the NF - κB and MAPK signaling pathways15, and mitigate colorectal cancer by altering the intestinal flora and modulating the gene expression of colon epithelial cells16. Zizyphus β-glucan GSP-2 can promote the release of NO by stimulating the proliferation of B lymphocytes in the mouse spleen, activating the phagocytosis function of RAW264.7 macrophages17, increasing the secretion of cytokines such as IL-2, IL-6, and IFN-γ18, modulating the secretion of enzymes such as mitogen activated protein kinase, and promoting pancreatic cancer19. Sugar absorption, nucleotide sugar donor synthesis, sugar chain connection and extracellular output are four steps of polysaccharide synthesis. Hexokinase (HK), phosphoglucomutase (PGM), glucose phosphate isomerase (PGI), mannose phosphate isomerase (PMI), GDP-mannose pyrophosphorylase (GMP) and UDP-glucose pyrophosphorylase (UGP) are key enzymes in sugar synthesis20. These enzymes control the flow of carbon sources to various nucleotide donors, forming various nucleotide sugars (NDP-sugar)21. The activities of PGI, PGM and UGP are related to the yield and monosaccharide composition of GLPs22. Zhou et al.23 showed that the changes of GLPs content during the growth of GLFB were positively correlated with the changes of PGI, PGM and UGP activities. Overexpression of PGM and UGP genes has achieved high yield of intracellular polysaccharide and extracellular polysaccharide24. The silencing of PGM and UGP genes led to the decrease of extracellular GLPs production25.
During the growth of GLFB, the activity of enzymes controlling sugar metabolism affects the synthesis of sugar, and the characteristics of sugar content and molecular structure affect its biological activity, and the types of carbon sources affect the growth of GLFB, and the cultivation of cassava stalk instead of sawdust is helpful to alleviate the excessive dependence of Ganoderma lucidum industry on forest resources26–28. Therefore, the structural characteristics, functional group characteristics, molecular weight distribution, sugar metabolizing enzyme activity and antioxidant activity in vitro of GLFB at different growth stages were investigated by gas chromatography-mass spectrometry (GC-MS), infrared spectroscopy (IR) and high-performance gel permeation chromatography (HPGPC). It is of great significance to provide basic data support for the application of cassava stalks in the cultivation of GLFB and guide the harvest of Ganoderma lucidum.
Materials and methods
Materials
Mannose, rhamnose, galacturonic acid, galactose, glucose, glucuronic acid, arabinose, xylose, fucose, glucosamine hydrochloride, N-acetyl-D-glucosamine, fructose, ribose, galactosamine hydrochloride, guluronic acid and mannuronic acid were purchased from Borui Sugar Biotechnology Co., Ltd. The molecular weight standards of sucrose, P5, P10, P20, P50, P100, P200, P400, P800 and P2000 were purchased from SHOWA DENKO company.
Ganoderma lucidum strains (Red Ganoderma lucidum) were provided by Jiangsu Gaoyou Edible Fungi Research Institute and stored at 4 ℃. The culture medium consists of 60% (w/w) cassava stalk chips, 30% (w/w) sawdust, 8% (w/w) wheat bran and 2% (w/w) lime. The differentiation stage, umbrella stage, maturity stage and spore stage of GLFB growth were defined as GS1, GS2, GS3 and GS4 respectively.
Determination of GLPs content
The content of GLPs were determined according to the method of Liu et al.12 Firstly, 0.1 mg/mL glucose standard solution was prepared. Secondly, a standard curve was established according to the reaction solution absorption value (A) of the different concentrations of glucose standard solution (C, mg/mL) with 1.0 mL 6% of phenol solution and 5.0 mL of concentrated sulfuric acid in a water bath at 100 ℃ at 490 nm. A = 8.8032×C + 0.0626 (R2 = 0.9996). Thirdly, 100 mg of GLFB, 5 mL of water and 20 mL of anhydrous ethanol were put into a 50 mL test tube. The sample solution was extracted in an ultrasonic extractor for 30 min, then centrifuged at 4000 r/min for 10 min after the extraction, and the supernatant was discarded. The insoluble matter was washed with 10 mL 80% ethanol solution and centrifuged. The insoluble matter and 50 mL of water were transferred into a round-bottomed flask with water, and extracted at 100 ℃ for 30 min, repeat twice. After cooled to room temperature, the supernatant was filtered and transferred to a 200 mL volumetric flask, and the residue was washed for 2–3 times. finally, the volumetric flask was filled to the constant volume by water. The absorbance of solution was measured at 490 nm, and the content of GLPs (w, %) was calculated by the equation. w=(c1×V)/m1 × 0.9, where c1 represented the concentration of GLPs in the extract, mg/g. m1 represented the GLFB mass, mg. 0.9 represented the glucose correction coefficient, and V represented the constant volume of the extract, 50 mL.
Determination of monosaccharide composition in GLPs
The composition of monosaccharide in GLPs was determined by ion chromatography (ICS5000, ThermoFisher) with reference to Liu et al.12.
Preparation of standard solution: 16 of monosaccharide standards (including fucose, rhamnose, arabinose, galactose, glucose, xylose, mannose, fructose, ribose, galacturonic acid, glucuronic acid, galactosamine hydrochloride, glucosamine hydrochloride, N- acetyl -D glucosamine, guluronic acid and mannuronic acid) were prepared into standard mother solution. The concentration standard of each monosaccharide standard solution was taken as the mixed standard, and the spectrum was shown in Table 1. According to the absolute quantitative method, different monosaccharide mass concentrations were determined, and the molar ratio was calculated according to the molar mass of monosaccharide. C (standard) /A (standard) = C (sample) /A (sample). Wherein, C (standard) and C (sample) respectively represented the concentration of the standard and sample, and A (standard) and A (sample) respectively represented the peak area of the standard and sample.
Table 1.
Peak area and concentration parameters of monosaccharide mixed standard solution.
| No. | Name | Name | Concentration (mg/L) | RT | Area |
|---|---|---|---|---|---|
| 1 | Fucose | Fuc | 2.5 | 5.809 | 7.605 |
| 2 | Galactosamine hydrochloride | GalN | 1.5 | 10.459 | 13.191 |
| 3 | Rhamnose | Rha | 2.5 | 10.875 | 5.483 |
| 4 | Arabinose | Ara | 1.875 | 11.434 | 7.914 |
| 5 | Glucosamine hydrochloride | GlcN | 2.5 | 12.900 | 19.511 |
| 6 | Galactose | Gal | 2.5 | 14.200 | 8.468 |
| 7 | Glucose | Glc | 2.5 | 16.042 | 13.453 |
| 8 | N-acetyl-D glucosamine | GlcNAc | 2.5 | 17.684 | 7.120 |
| 9 | Xylose | Xyl | 2.5 | 18.509 | 12.468 |
| 10 | Mannose | Man | 2.5 | 19.084 | 7.278 |
| 11 | Fructose | Fru | 7.5 | 21.592 | 6.545 |
| 12 | Ribose | Rib | 5 | 23.642 | 12.135 |
| 13 | Galactouronic acid | GalA | 2.5 | 44.159 | 4.575 |
| 14 | Guluronic acid | GulA | 5 | 44.709 | 9.965 |
| 15 | Glucuronic acid | GlcA | 2.5 | 46.709 | 5.345 |
| 16 | Mannuronic acid | ManA | 5 | 49.259 | 15.545 |
Preparation of GLPs solution: A 5 mg of GLFB powder and 3 M TFA 2 mL were placed in 15 mL ampoules and hydrolyzed at 120 ℃ for 3 h. A 1 mL of acid hydrolysis solution was put into 2 mL test tube and dried in nitrogen blower (UGC-24 M, Lichen Technology). After blow-drying, 5 mL of distilled water was added into the test tube, mixed by vortex to obtain sample solution 1. Then, 50 µL of sample solution 1 and 950 µL of deionized water were put into a 2 mL centrifuge tube, and the solution was centrifuged at 12,000 rpm for 5 min and the supernatant was collected for analysis.
Detection conditions of ion chromatograph: A 25 µL GLPs sample passed through Dionex CarbopacTM PA20 (3 × 150 mm) chromatographic column at the flow rate of 0.3 mL/min, and the sample was separated by the column and then detected by electrochemical detector. Mobile phases A, B and C were H2O, 15 mM NaOH and 15 mM NaOH and 100 mM NaAc respectively. Elution gradient: mobile phase A /B /C was eluted for 18 min at the ratio of 98.8:1.2:0, mobile phase A /B /C for 2 min at the ratio of 50:50: 0, mobile phase A /B /C for 30 min at the ratio of 50:50: 0, mobile phase C for 16 min and mobile phase B for 4 min.
Determination of molecular weight of GLPs
The determination of molecular weight and purity of GLPs were determined by high performance gel permeation chromatography (HPGPC) according to Liu et al.12.
Preparation of standard solution: A 5 mg of standard (dextran) was dissolved in 1mL 0.05 mol/L NaCl solution to prepare a 5 mg/mL solution, and then the standard solution was placed in a 1.8 mL injection vial.
Preparation of sample solution: A 5 mg of GLPs were dissolved in 1 mL of 0.05 mol/L NaCl solution to prepare a 5 mg/mL sample solution, which was ultrasonicated for 10 min and centrifuged at 12,000 rpm for 10 min. The collected supernatant was filtered with a 0.22 μm water-based microporous membrane, and then the sample was transferred to a 1.8 mL injection vial.
Detection parameters of HPGPC: 25 µl GLPs solution passed through a series gel column (8 × 300 mm) of BRT105-103-101 at a speed of 0.8 mL/min, and eluted with 0.05 mol/L NaCl solution for 60 min. The signal value of the peak was detected in RID-20 A differential detector. The linear regression equation of retention time (RT, min) with peak molecular weight (Mp), weight average molecular weight (Mw) and number average molecular weight (Mn) was established. Lg Mp = −0.238 RT + 11.86, R2 = 0.99. lg Mw=−0.243 RT + 12.03, R2 = 0.99. lg Mn=−0.238 RT + 11.88, R2 = 0.99.
Infrared spectrum characteristics of GLPs
The functional groups of GLPs were determined by infrared spectrometer (Thermo Nicolet iN10, Thermo Fisher Scientific, USA) with reference to Liu et al.12 The concentration of GLPs solution was diluted to 1 mg/mL, the scanning range was 4000 –400 cm− 1, the resolution was 4 cm− 1, and the total reflection mode (ATR) was adopted.
Determination of enzyme activity
GLFB at different growth stages were picked, cooled by liquid nitrogen, and ground into powder in a grinder. Then, the activities of hexokinase (HK) and phosphoglucose isomerase (PGM) were determined.
Determination of HK activity: The activity of HK was measured by HK kit (ADS-F-T001, Shenzhen anpei biology science and technology Co., Ltd) method with reference to Chen et al.2 A 0.1 g of GLFB powder and 1mL extract were homogenized in ice bath, and then the extract was centrifuged at 4 ℃ for 10 min at 12,000 rpm. The supernatant was taken and diluted 10 times with distilled water, and stored it in an ice bath.
Reactants 2 and 3 were preheated at 25 ℃ for 5 min before use. An 80 µL of the sample diluted 10 times, 40 µL of reagent 2 and 680 µL of reagent 3 were added into a 1 mL quartz cell, and mixed them evenly. When the absorption values of the samples measured at the absorption wavelength of 340 nm at the 1 min and 16 min were A1 and A2, respectively. Then, △A = A2-A1, and the distilled water was zeroed. The activity of HK (nmol/min/g fresh weight) = [Δ A÷(ε × d) ×V2 × 109] ÷ (w× V1 ÷ V) ÷ t = 1071.8 × Δ A ÷ w.
Where ε was the molar extinction coefficient of NADPH, 6.22 × 103 L/ (mol /cm). d was the length of 1 mL quartz cell, 1 cm. V, V1 and V2 were the extraction volume (1 mL), sample volume (0.08 mL) and total volume of reaction system (8 × 10− 4 L) respectively. W was the sample mass, g. T was the reaction time, 15 min.
Determination of PGM activity: The activity of PGM was measured by PGM kit (ADS-F-FM032, Shenzhen anpei biology science and technology Co., Ltd) method with reference to Xia et al.29 A 0.1 g of GLFB powder and 1mL extract were homogenized in ice bath, and then the extract was centrifuged at 4 ℃ for 10 min at 12,000 rpm. The supernatant was taken and diluted 10 times with distilled water, and stored it in an ice bath.
Reactants 1, 2, 3, 4 and 5 were preheated at 37℃ for 5 min before use and the sample solution was diluted 10 times. A 40 µL of sample solution, 20 µL of reagent 1, 20 µL of reagent 2, 40 µL of reagent 3 and 640 µL of reagent 4 were added into a 1mL quartz cell, and mixed them evenly and incubated for 10 min at 37 ℃. Then, the 40 µL of reagent 5 were mixed at 37 ℃. When the absorption values of the samples measured at the absorption wavelength of 450 nm at the 1 min and 11 min were A1 and A2, respectively. △A = A2-A1, and the distilled water was zeroed. The activity of PGM (nmol/min/g fresh weight) = [(ΔA + 0.0095) ÷ 0.0317] ÷(W×V1 ÷ V) ÷ T = 78.86 × (ΔA + 0.0095) ÷ W. Where V and V1 were respectively the volume of the extract (1 mL) and the sample (0.01 mL), W was the sample mass, and T was the reaction time (10 min).
Determination of in vitro antioxidant activity
The determination of total antioxidant capacity (T-AOC) and DPPH free radical scavenging capacity refer to the method described by Liu et al.12.
Results and analysis
Polysaccharide content and monosaccharide composition of GLFB at different growth stages
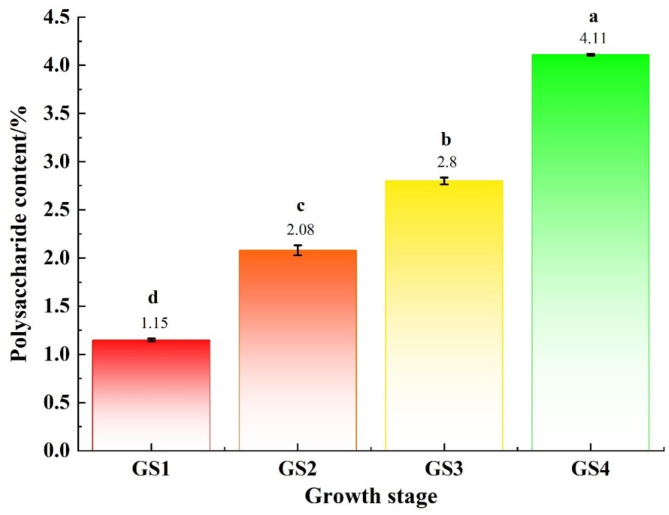
The results of polysaccharide content and monosaccharide composition of GLFB at different growth stages were shown in Fig. 1, and Table 2 respectively. As could be seen from Fig. 1, the GLPs content of GLFB was gradually increasing during the ripening process, and the GLPs content was the highest (4.11%) in spore stage (GS4), which was about 4 times higher than that in GS1. The content of GLPs in GLFB obtained in four growth stages was significantly different. It could be seen from Table 2 that monosaccharides in GLPs were mainly composed of galactose (111.86–247.08 µg/mg), glucose (168.01–248.45 µg/mg), mannose (14.58–50.72 µg/mg), fucose (12.08–30.57 µg/mg), glucuronic acid (7.18–16.07 µg/mg) and glucosamine hydrochloride (2.75–3.61 µg/mg). Among them, the contents of galactose and glucose were the highest, and the content of galactose in GLPs was the largest in the growth stages of differentiation stage (GS1), umbrella stage (GS2) and maturity stage (GS3). The glucose content was the highest in the spore stage (GS4). The contents of galactose, glucose, mannose, glucuronic acid and fucose increased at first and then decreased, and reached the maximum at the umbrella stage (GS2).
Fig. 1.
Variation of GLPs content in GLFB at different growth stages.
Table 2.
Monosaccharide composition of GLPs in GLFB at different growth stages.
| GS1 | GS2 | GS3 | GS4 | |
|---|---|---|---|---|
| Name | µg/mg | µg/mg | µg/mg | µg/mg |
| Fucose | 27.12 | 30.57 | 20.93 | 12.08 |
| Glucosamine hydrochloride | 2.8 | 2.75 | 3.44 | 3.61 |
| Galactose | 247.08 | 276.62 | 211.83 | 111.86 |
| Glucose | 226.17 | 248.45 | 175.25 | 168.01 |
| Mannose | 43.88 | 50.72 | 33.30 | 14.58 |
| Glucuronic acid | 12.23 | 16.07 | 12.99 | 7.18 |
Infrared spectral characteristics of GLPs from GLFB at different growth stages
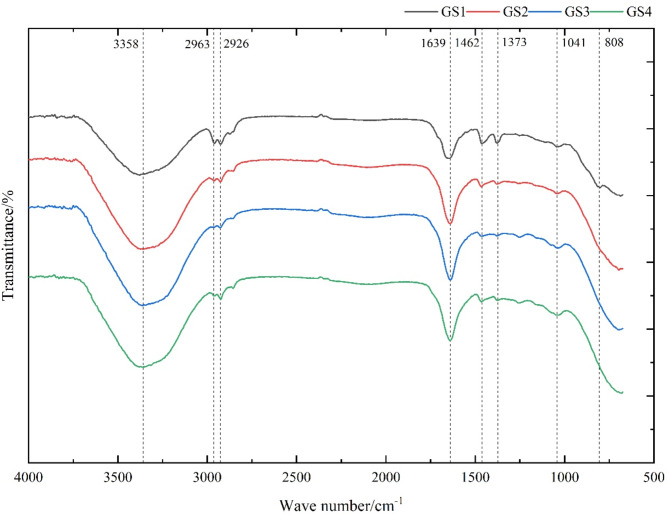
The infrared spectra of GLPs at different growth stages were shown in Fig. 2. As could be seen from Fig. 2, 3358 cm− 1, 2963 cm− 1, 2926 cm− 1, 1639 cm− 1, 1462 cm− 1, 1373 cm− 1, 1041 cm− 1 and 808 cm− 1 were the characteristic absorption peaks of GLPs, of which 3358 cm− 1 was the -OH stretching30, 2926 cm− 1 and 2963 cm− 1 was the C-H stretching vibrations with alkyl groups31, 1639 cm− 1 was the C = O stretching31, 1462 cm− 1 was caused by the variable angle vibration of -CH, 1373 cm− 1 was the absorption peak of -COOH, and 1041 cm− 1 was a common resonance absorption peak of pyranose cyclolactone and hydroxyl group. 808 cm− 1 was the characteristic absorption peak of mannose32. In addition, the peak at 1041 cm− 1 was due to the asymmetric stretching vibration of the C-O-C ether bond on the sugar ring, which constituted the characteristic absorption peak of sugar, and would also dextran the typical infrared spectrum signal33,34. The peak types of GLPs obtained at different growth stages were very similar, and the peak strength was different, which might be caused by the difference of monosaccharide content in GLPs.
Fig. 2.
IR spectra of GLPs from GLFB with different growth stages.
Molecular weight distribution of GLPs from GLFB at different growth stages
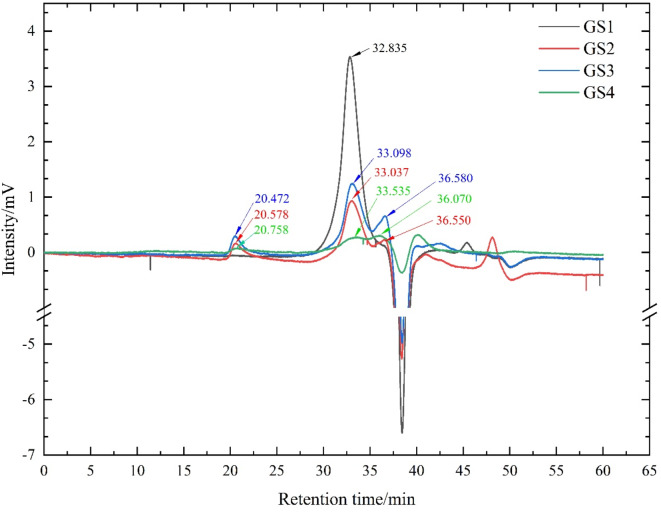
The difference of molecular weight distribution of GLPs in different growth stages were shown in Fig. 3. As could be seen from Fig. 3, there were differences in the number of peaks, retention time and strength of GLPs in the gel chromatography column in different growth stages of GLFB. The peak molecular weight (Mp), peak weight average molecular weight (Mw) and number average molecular weight (Mn) were three parameters to characterize the molecular weight of GLPs, as shown in Table 3. From Table 3, the molecular weight distribution of GLPs obtained at different growth stages was quite different, and Mw, Mn and Mp were all positively correlated. The greater the Mw, the greater the Mn and Mp. The Mw of GLPs obtained in GS1, GS2, GS3 and GS4 stages were 11,249 Da (accounting for 100%), 10,046 Da (accounting for 76.16%), 9709 Da (accounting for 70.38%) and 7603 Da (accounting for 51.44%) respectively. Different from the GS1, the GLPs from the GS2, GS3 and GS4 had a molecular weight of millions or even tens of millions of Daltons. The Mw value of GS3 was the largest, which was 11,358,056 Da, accounting for 7.365%, and the Mw of GS3 was 1,000 times that of GS1.
Fig. 3.
High performance gel permeation chromatography of GLPs at different growth stages. 38.3min is the peak of mobile phase.
Table 3.
Molecular weight parameters of GLPs in different growth stages.
| RT (min) | Lg Mp | Lg Mw | Lg Mn | Mp /Da | Mw /Da | Mn /Da | Peak area ratio% | |
|---|---|---|---|---|---|---|---|---|
| GS1 | 32.835 | 4.05 | 4.05 | 4.07 | 11,099 | 11,249 | 11,622 | 100 |
| GS2 | 20.578 | 6.96 | 7.03 | 6.98 | 9,171,408 | 10,703,998 | 9,603,643 | 11.643 |
| 33.037 | 4 | 4 | 4.02 | 9936 | 10,046 | 10,404 | 76.163 | |
| 36.55 | 3.16 | 3.15 | 3.18 | 1449 | 1407 | 1517 | 12.193 | |
| GS3 | 20.472 | 6.99 | 7.06 | 7.01 | 9,719,949 | 11,358,056 | 10,178,036 | 7.365 |
| 33.098 | 3.98 | 3.99 | 4 | 9609 | 9709 | 10,062 | 70.383 | |
| 36.58 | 3.15 | 3.14 | 3.17 | 1425 | 1384 | 1493 | 22.252 | |
| GS4 | 20.758 | 6.92 | 6.99 | 6.94 | 8,309,904 | 9,678,454 | 8,701,538 | 10.823 |
| 33.535 | 3.88 | 3.88 | 3.9 | 7563 | 7603 | 7919 | 51.444 | |
| 36.07 | 3.28 | 3.26 | 3.3 | 1885 | 1841 | 1974 | 37.733 |
Activities of HK and PGM in GLFB at different growth stages
The changes of HK and PGM activities in GLFB at different growth stages and the correlation of enzyme activities were shown in Fig. 4. Figure 4A showed that the activities of HK and PGM were gradually increasing during the growth of GLFB. The activities of HK and PGM in GS4 were highest, which were 4051.4 nmol/(min/g) and 322.1 nmol/(min/g), respectively. The activities of HK and PGM in GS4 were 85.6 and 221.2% higher than those in GS1, respectively. The results of significant analysis showed that there were significant differences in the activities of HK and PGM between the GS4 and the other three growth stages, but there were no significant differences in the activities of HK between GS1 and GS2, and PGM between GS2 and GS3.
Fig. 4.
Changes of HK and PGM activities in GLFB at different growth stages (A), HK and PGM correlation plot (B).
It could be seen from Fig. 4B that there was a significant correlation between the activities of HK and PGM in GLFB at different growth stages. HK was the first key enzyme in the metabolic pathway of GLPs, which was responsible for catalyzing glucose to glucose-6-phosphate. PGM was the key enzyme responsible for the branch of glucose − 6- phosphate from GLPs synthesis path to fructose-6-phosphate. There was a close positive correlation between glucose - 6- phosphate and during the growth of GLFB, which was consistent with the research report of Zhou et al.23 and Xu et al.35.
In vitro antioxidant activity of GLPs from GLFB at different growth stages
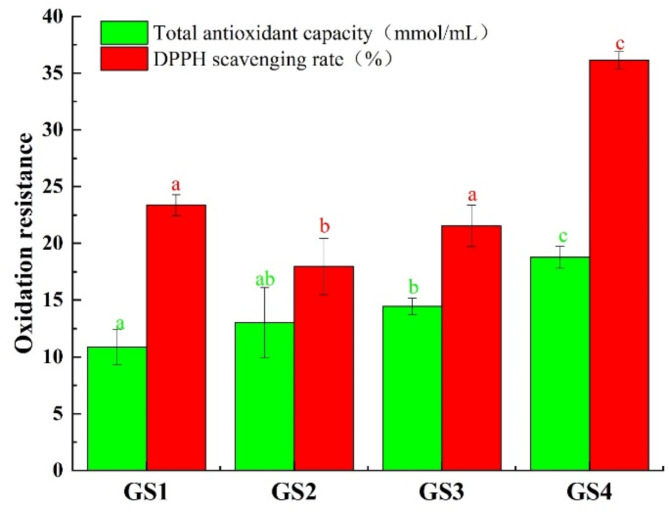
The total antioxidant capacity and DPPH radical scavenging capacity of GLPs in GLFB at different growth stages were shown in Fig. 5. As could be seen from Fig. 5, the total antioxidant capacity of GLPs showed an increasing trend during the growth of GLFB, and the total antioxidant capacity of GLPs was the highest at the GS4, which was 18.8 mmol/mL. The scavenging capacity of DPPH showed a trend of decreasing at first and then increasing, and the DPPH scavenging capacity was the highest in GS4, accounting for 36.2%. The results of significant analysis showed that in terms of total oxidation capacity, there was no significant difference between GS1 and GS2, but significant difference between GS1 and GS3 and GS4. There was no significant difference between GS1 and GS3. In terms of DPPH scavenging ability, there was no significant difference between GS1 and GS3, but significant difference between GS1 and GS2 and GS4. There was a significant difference between the GS2 and GS3 and GS4. There were also significant differences between GS3 and GS4.
Fig. 5.
Antioxidant activity of GLPs from GLFB at different growth stages. Small letters in green and red respectively represented the differences in total antioxidant capacity and DPPH scavenging rate of GLPs obtained at different growth stages.
Discussion
Carbon source was one of the important factors for the growth of Ganoderma lucidum. Shu36 reported that the addition of 25–75% mulberry branches had a significant effect on the growth rate of Ganoderma lucidum mycelium, the infection rate, the thickness and polysaccharide content of GLFB, and the highest GLPs was 1.75% when the addition of mulberry branches was 75%. At the same time, it was also revealed that the GLPs was not necessarily related to the agronomic shape of GLFB (including the thickness and diameter, etc.). Zhang et al.37 found that the addition of 10-80% of lotus seed shells affected the growth of GLFB, and the highest GLPs content was 1.057% with 80% addition. In this study, cassava stalk was used as carbon source to cultivate Ganoderma lucidum, and the GLPs content was 4.11%, which was better than that of mulberry branches and lotus seed shells reported. It might be that the differences in fiber and nutrient components in different cultivation substrates affected the polysaccharide metabolism of Ganoderma lucidum, thus affecting the growth of GLFB and its polysaccharide content.
The content, composition, molecular structure characteristics and related enzyme activities of polysaccharides in the GLFB from the different growth stages of Ganoderma lucidum were affected by the differential expression of enzymes related to sugar metabolism and genes related to controlling enzyme synthesis5,22,23. Li et al.38 showed that the contents of metabolites (polysaccharides, 12 triterpenoids, ergosterol, uridine and uracil, etc.) showed a downward trend with the continuous growth of GLFB. The GLPs content in bud stage was the highest. The changes of GLPs content during the growth of GLFB were positively correlated with the changes of PGI, PGM and UGP activities23. This study also clarified that with the continuous maturity of GLFB, the activities of HK and PGM enzymes in GLFB also showed an increasing trend, thus increasing the content of GLPs by regulating the activity of sugar synthase. The difference in enzyme activity between GLFB with cassava stalk as carbon source and GLFB with basswood as carbon source might be due to the influence of carbon source types on sugar synthesis.
The biological activity of GLPs was not only related to the species of GLFB, the culture medium and the extraction process, but also related to the maturity of GLFB39,40. Li et al.41 found that the different proportions of galactose and mannose had little effect on the yield and biomass of GLPs, while the proportion of carbon source had great influence on the activity of GLPs. Lu et al.11 reported that different concentrations of ethanol precipitated GLPs, which affected the antioxidant activity and other biological activities in vitro by changing the composition, functional group characteristics and molecular weight distribution of GLPs. Microwave extraction, alkali extraction and alcohol precipitation affect the biological activity of GLPs by changing its relative molecular weight42. The molecular weight of water-soluble GLPs was more than 100 thousand Da, while the alkali-soluble polysaccharide was less than 10 thousand Da, which lead to better antioxidant activity of water-soluble GLPs. Zhang et al.43 reported that polysaccharides mainly produce antioxidant activity through targeting NF-κ B and NRF2/Kea P1 were signaling pathways, and the length of molecular weight and other characteristics of polysaccharides would affect their water solubility and other properties, resulting in differences in biological activities. The results of this study showed that the molecular weight of GLPS showed an increasing trend in different growth stages. When the GLFB was in GS4 stage, the high molecular weight GLPs accounted for a higher proportion, and the antioxidant activity of GLPs was the highest.
Conclusion
In this study, the regulatory effects of the growth of GLFB on the monosaccharide composition, molecular weight distribution, hexokinase and glucose phosphate isomerase activities of GLPs and their antioxidant activities in vitro were revealed by gas chromatography-mass spectrometry, infrared spectroscopy and gel chromatography. The content of GLPs, the activities of HK and PGM were increasing, and the monosaccharide composition and molecular structure characteristics of GLPs were also changing dynamically in GLFB. The molecular structure characteristics also affected the in vitro antioxidant activity of GLPs. GLFB in GS4 had high polysaccharide content, strong enzyme activity and strong antioxidant activity. Therefore, picking the mature GLFB had better biological activity, which provided a scientific basis for the application of cassava stalks in Ganoderma lucidum cultivation. In the future, it was of great significance to further reveal the expression differences of genes related to polysaccharide synthesis in the process of Ganoderma lucidum maturation from gene expression and transcription.
Author contributions
S.J. Ma, and H.G. Huang; methodology: S.J. Ma, H.B. Liang and Y. Kuang; formal analysis: Y.L. Chen and Y.J. Liu; investigation: X.R. Pu and S.J. Ma; data curation: H.B. Liang and Y.J. Liu; writing—original draft preparation: S.J. Ma and Y.J. Liu; writing—review and editing: Y. Kuang and Y.J. Liu; project administration: Y.J. Liu. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hainan Provincial Natural Science Foundation of China (No. 320QN326), the 2021 Guangdong Science and Technology Innovation Strategy Special Fund (No. 2021A05160), the National Natural of Science Foundation of China (31101137), the Natural Science Foundation Zhanjiang, Guangdong (2020A03027, 2020A03006, 2021A05239, and 2021A05216), and the 2022 Guangdong Science and Technology Innovation Strategy Special Fund (No. 2022A05025).
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Kuang, Email: kyu06@sina.com.
Yijun Liu, Email: liuyijun-1@163.com.
References
- 1.Liu, Y. J. et al. Study on the nutrition quality change in different growth stages of Ganoderma lucidum. J. Yunnan Agric. Univ.35 (06), 1061–1066. 10.12101/j.issn.1004-390X(n).202002004 (2020). [Google Scholar]
- 2.Chen, Y. et al. Repressed central carbon metabolism and its effect on related metabolic pathways in cefoperazone/sulbactam-resistant Pseudomonas aeruginosa. Front. Microbiol.3 (13), 847634. 10.3389/fmicb.2022.847634 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang, Z. Y., Cui, F. J., Sun, L., Zan, X. Y. & Sun, W. J. Recent advances in Ganoderma lucidum polysaccharides: Structures/bioactivities, biosynthesis and regulation. Food Biosci.56, 103281. 10.1016/j.fbio.2023.103281 (2023). [Google Scholar]
- 4.Chen, S. et al. WU, Q. Structural characterization and hepatoprotective activity of an acidic polysaccharide from Ganoderma lucidum. Food Chem. X13, 100204. 10.1016/j.fochx.2022.100204 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng, L. et al. Effects of culture conditions on monosaccharide composition of Ganoderma lucidum exopolysaccharide and on activities of related enzymes. Carbohydr. Polym.133, 104–109. 10.1016/j.carbpol.2015.07.014 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Lu, X. et al. Function of ceramide synthases on growth, ganoderic acid biosynthesis and sphingolipid homeostasis in Ganoderma lucidum. Phytochemistry172, 112283. 10.1016/j.phytochem.2020.112283 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Fang, D. et al. Auricularia polytricha noodles prevent hyperlipemia and modulate gut microbiota in high-fat diet fed mice. Food Sci. Hum. Well.10 (4), 431–441. 10.1016/j.fshw.2021.04.005 (2021). [Google Scholar]
- 8.Ma, G. & Xia, X. Study on the improvement effect and mechanism of Ganoderma lucidum polysaccharides on diabetic nephropathy in mice. Nat. Prod. Res. Dev.36 (06), 938–945. 10.16333/j.1001-6880.2024.6.003 (2024). [Google Scholar]
- 9.Li, W. et al. Ganoderma lucidum polysaccharide supplementation significantly activates T-cell-mediated antitumor immunity and enhances anti-PD-1 immunotherapy efficacy in colorectal cancer. J. Agric. Food Chem.72 (21), 12072–12082. 10.1021/acs.jafc.3c08385 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Chen, Z. & Xiao, G. Total synthesis of nona-decasaccharide motif from Ganoderma sinense polysaccharide enabled by modular and one-pot stereoselective glycosylation strategy. J. Am. Chem. Soc.146 (25), 17446–17455. 10.1021/jacs.4c05188 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Lu, J. et al. Effect of gradient alcohol precipitation process on the structural characteristics and biological activity of Ganoderma lucidum poly-saccharides. Chin. J. Trop. Crops44 (04), 816–824. 10.3969/j.issn.1000-2561.2023.04.019 (2023). [Google Scholar]
- 12.Liu, Y. et al. Study on the structure and bioactivity of Ganoderma lucidum polysaccharides under cassava stalk stress. J. Fungi9 (5), 514. 10.3390/jof9050514 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, W., Zhang, Y., Wang, Z., Zhang, J. & Jia, L. Ganoderma lucidum polysaccharides improve lipid metabolism against high-fat diet-induced dyslipidemia. J. Ethnopharmacol.309, 116321. 10.1016/j.jep.2023.116321 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Zhang, R., Bai, H. J. & Liu, J. Structural analysis of Ganoderma tsugae polysaccharide and its alleviative effect on exercise-induced fatigue in mice. China Food Addit.34 (04), 69–78. 10.19804/j.issn1006-2513.2023.04.009 (2023). [Google Scholar]
- 15.Meng, M. et al. Ganoderma lucidum polysaccharide peptide (GLPP) attenuates rheumatic arthritis in rats through inactivating NF-κB and MAPK signaling pathways. Phytomed. Int. J. Phytother. Phytopharmacol.119, 155010. 10.1016/j.phymed.2023.155010 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Luo, J. et al. Ganoderma Lucidum polysaccharide alleviating colorectal cancer by alteration of special gut bacteria and regulation of gene expression of colonic epithelial cells. J. Funct. Foods47, 127–135. 10.1016/j.jff.2018.05.041 (2018). [Google Scholar]
- 17.Han, X. Q. et al. Structure elucidation and immunomodulatory activity of a beta glucan from the fruiting bodies of Ganoderma sinense. PloS ONE9 (7), e100380. 10.1371/journal.pone.0100380 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, Y., Zhou, S., Jiang, W., Huang, M. & Dai, X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol. Investig.2003 (32(3)), 201–215. 10.1081/imm-120022979 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Pan, Y. et al. Antioxidation of a proteoglycan from Ganoderma lucidum protects pancreatic β-cells against oxidative stress-induced apoptosis in vitro and in vivo. Int. J. Biol. Macromol.200, 470–486. 10.1016/j.ijbiomac.2022.01.044 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Ma, Z. et al. Reconstruction and analysis of a genome-scale metabolic model of Ganoderma lucidum for improved extracellular polysaccharide production. Front. Microbiol.9, 3076. 10.3389/fmicb.2018.03076 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, Q. Analysis of triterpenoid and polysaccharide high yield mechanism in Ganoderma lucidum based on omics technology. Jiangsu Wuxi Jiangnan Univ.10.27169/d.cnki.gwqgu.2020.001075 (2020). [Google Scholar]
- 22.Wei, Z. H. et al. Sucrose fed-batch strategy enhanced biomass, polysaccharide, and ganoderic acids production in fermentation of Ganoderma lucidum 5.26. Bioprocess Biosyst. Eng.39 (1), 37–44. 10.1007/s00449-015-1480-x (2016). [DOI] [PubMed] [Google Scholar]
- 23.Zhou, Z., Chen, X., Wang, L., Wang, Q. & Lan, J. Studies on the correlation between polysaccharides accumulation and sugar metabolizing enzymes in Ganoderma lucidum. Chin. Agric. Sci. Bull.28 (22), 253–257. 10.3969/j.issn.1000-6850.2012.22.045 (2012). [Google Scholar]
- 24.Xu, J. W. et al. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous α-phosphoglucomutase gene. Bioprocess Biosyst. Eng.38 (2), 399–405. 10.1007/s00449-014-1279-1 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Hu, Y. et al. Ganoderma Lucidum phosphoglucomutase is required for hyphal growth, polysaccharide production, and cell wall integrity. Appl. Microbiol. Biotechnol.102 (4), 1911–1922. 10.1007/s00253-017-8730-6 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Liu, L. et al. Influence of carbon and nitrogen sources on structural features and immunomodulatory activity of exopolysaccharides from Ganoderma lucidum. Process Biochem. 119. 10.1016/j.procbio.2022.05.016 (2022).
- 27.Liu, Y. F. et al. Characterization of polysaccharides from the fruiting bodies of two species of genus Ganoderma (Agaricomycetes) and determination of water-soluble β-D-Glucan using high-performance liquid chromatography. Int. J. Med. Mushrooms. 19 (1), 75–85. 10.1615/IntJMedMushrooms.v19.i1.80 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Artiningsih, T. Ligninolytic activity of Ganoderma strains on different carbon sources. Biodiversitas7 (4), 307–311. 10.13057/biodiv/d070402 (2006). [Google Scholar]
- 29.Xia, L., Li, Z., Zhang, X. & Zhang, L. Identification of phosphoglucomutase and UDP-Glucose pyrophosphorylase involved in biosynthesis of polysaccharide from Wolfiporia cocos. Chem. Bioeng.41 (06), 44–52. 10.3969/j.issn.1672-5425.2024.06.007 (2024). [Google Scholar]
- 30.Liu, Y. F. et al. Triple helix conformation of β-D-glucan from Ganoderma lucidum and effect of molecular weight on its immunostimulatory activity. Int. J. Biol. Macromol.114, 1064–1070. 10.1016/j.ijbiomac.2018.03.054 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Zhang, H. et al. Sulfated modification, characterization and property of a water-insoluble polysaccharide from Ganoderma atrum. Int. J. Biol. Macromol.79, 248–255. 10.1016/j.ijbiomac.2015.04.070 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Xia, C. H., Dai, Q., Fang, W. & Chen, H. Research on the IR spectrscopy of kinds of polysaccharide. J. Wuhan Univ. Technol.2 (01), 45–47. 10.3321/j.issn:1671-4431.2007.01.012 (2007). [Google Scholar]
- 33.He, J. Z. et al. Analysis of structural characteristics of polysaccharide from Ganoderma lucidum. Chin. J. Anal. Chem.38 (03), 372–376. (2018).
- 34.Ma,, Q. et al. Assessment of polysaccharides from mycelia of genus Ganoderma by mid-infrared and near-infrared spectroscopy. Sci. Rep.8 (1), 10. (2010). [DOI] [PMC free article] [PubMed]
- 35.Qian, L. et al. Polysaccharides content and activity of enzymes related to sugar metabolism from the fruiting bodies of Ganoderma lucidum at different growth stages. Storage Process19 (03), 128–131. 10.3969/j.issn.1009-6221.2019.03.020 (2019). [Google Scholar]
- 36.Shu, T. H. Effects of different cultivation materials on the growth and nutritional quality of Ganoderma lucidum fruiting body. Spec. Econ. Anim. Plant2023 (26(12)), 39–41. 10.3969/j.issn.1001-4713.2023.12.013 (2023). [Google Scholar]
- 37.Zhang, B. et al. Effects of lotus-seed-husk substrate on Ganoderma lingzhi growth and production benefits. Mycosystema43 (02), 90–101. 10.13346/j.mycosystema.230207 (2024). [Google Scholar]
- 38.Li, S. F. et al. Dynamic changes of bioactive substances at the whole basidioma growth stage of Ganoderma lingzhi cultivated on wood log. Mycosystema43 (02), 102–114. 10.13346/j.mycosystema.230186 (2024). [Google Scholar]
- 39.Zhang, H. et al. Review on structure and characterization methodology of polysaccharides from ganoderma. J. Chin. Inst. Food Sci. Technol.20 (01), 290–301. 10.16429/j.1009-7848.2020.01.038 (2020). [Google Scholar]
- 40.Xu, H. et al. Effect of Ganoderma Applanatum polysaccharides on MAPK/ERK pathway affecting autophagy in breast cancer MCF-7 cells. Int. J. Biol. Macromol.146, 353–362. 10.1016/j.ijbiomac.2020.01.010 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Li, J., Ding, C. Y., Gu, Z. H., Zhang, L. & Shi, G. Y. Effects of mixed carbon sources on production and antitumor activity of Ganoderma Lucidum exopolysaccharides by submerged culture. J. Food Sci. Biotechnol.36 (02), 129–135. 10.3969/j.issn.1673-1689.2017.02.003 (2017). [Google Scholar]
- 42.Han, W., Chen, L. R., Zheng, D. T., Bu, Y. L. & Yao, Z. Y. Comparative study on antioxidant activity of water soluble and alkali soluble polysaccharides from Ganoderma lucidum. J. Xuzhou Inst. Technology(Natural Sci. Edition). 37 (03), 33–40. 10.15873/j.cnki.jxit.000475 (2022). [Google Scholar]
- 43.Zhang, J., Wen, C., Zhang, H. & Duan, Y. Review of isolation, structural properties, chain conformation, and bioactivities of psyllium polysaccharides. Int. J. Biol. Macromol.139, 409–420. 10.1016/j.ijbiomac.2019.08.014 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.