Abstract
Hepatocellular carcinoma is considered to be the fifth most rampant type of cancer in the whole world and has a high death rate. The hypoxic microenvironment is one of the typical features of tumor tissues and has an important impact on the activity of multiple signaling pathways. It is of great significance to study the effects of hypoxia on the pathophysiological processes and molecular mechanisms of HCC. Signalling pathways associated with SHP2 were analysed using bioinformatics. Detection of relevant protein expression using Western blotting. Tube formation assay was used in evalution of the angiogenic potential. The concentrations of MDA and SOD were measured by ELISA. The cell migration and invasion ability were measured with a scratch wound assay and transwell assay in SMMC-7721, HepG2 and Huh-7 cells. The effect of hypoxia on the growth of hepatocellular carcinoma was examined using subcutaneous graft tumors and HE staining experiments in nude mice. Bioinformatics analysis of SHP2 negatively correlates with the PI3K signalling pathway. Hypoxia promotes the concentration of MDA and inhibited the concentration of SOD. Hypoxia may up-regulate NOX2, NOX4 and p-PI3K and down-regulate the treatment of p-SHP2. Compared with NC group, the expression of SHP2 and p-SHP2 was inhibited in SHP2 KD group and the expression of p-PI3K, HIF1α, COX2, FOXM1, β-catenin and MMP9 was promoted. However, the differences of the expression of p-PI3K, HIF1α, COX2, FOXM1, β-catenin and MMP9 between the two groups were abolished after the addition of PI3K inhibitor. The angiogenesis, migration and invasion abilities were significantly increased in SHP2 KD group compared with NC group. Similarly, after the addition of PI3K inhibitor, the difference of these abilities between the two groups was eliminated. Hypoxia can promote the growth of hepatocellular carcinoma. Hypoxia can activate the oxidative stress-mediated SHP2/PI3K signaling pathway to promote angiogenesis, migration, and invasion in hepatocellular carcinoma, thus advancing the development of hepatocellular carcinoma.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-89137-3.
Keywords: Hepatocellular carcinoma, Oxidative stress, SHP2, PI3K, Angiogenesis
Subject terms: Cancer, Cell biology
Introduction
The invasion and metastasis rates of hepatocellular carcinoma (HCC) are notably elevated. It ranks as the fifth most prevalent malignancy and the second leading cause of cancer mortality globally. In our nation, approximately half of all global HCC cases are recorded1. Presently, surgical resection remains the primary treatment for HCC; however, the 5-year postoperative recurrence and metastasis rate exceeds 70%2. Additionally, around 80% of HCC patients have advanced to invasion and metastasis at diagnosis, thereby missing the opportunity for radical surgical intervention3. Studies indicate that the pronounced invasiveness of HCC is the main driver of postoperative recurrence and metastasis4. Consequently, investigating the mechanisms underlying HCC invasion and metastasis holds substantial theoretical importance and potential clinical value for enhancing prevention, treatment, and patient prognosis.
Hypoxia is prevalent in solid tumors, contributing to tumor cell death and influencing metabolism, gene expression, and post-translational modifications5. Under hypoxic conditions, tumor cells can undergo adaptive changes that enhance their proliferation, migration, and invasion6–10. Although the liver has a rich blood supply and receives dual blood supply from the portal vein and hepatic artery, hypoxia is particularly prominent in the growth of hepatocellular carcinoma. Research demonstrates that hypoxia can enhance the invasive and migratory capabilities of HCC cells by activating HIF-1α-related signaling pathways and upregulating transcription factors11,12. The precise mechanisms of hypoxia-induced invasion and metastasis in liver cancer cells remain incompletely understood. Previous studies have primarily focused on the regulation of pathways such as EGFR, HIF-α-VEGF, Rab11-FIP4, mTOR-PRAS40, and the transcription factor NF-κB. However, interventions targeting these pathways have not effectively curbed liver cancer cell invasion and metastasis, suggesting the existence of other critical regulatory pathways and mechanisms13,14.
Research has revealed that reversible tyrosine phosphorylation regulates intercellular communication, cell differentiation, growth, proliferation, cytoplasmic RNA synthesis, DNA synthesis during cell cycle regulation, nerve transmission, embryogenesis, angiogenesis, and plays a vital role in the development and metastasis of numerous genetic disorders and malignancies15,16. The human PTPN11 gene encodes the non-receptor tyrosine phosphatase SHP2, which contains Src homology domains. Intriguingly, PTPN11 was previously identified as an oncogene17. In fact, somatic gain-of-function mutations in PTPN11 have been found in 50% of Noonan syndrome cases and certain leukemias, leading to overactivation of the Ras-Erk signaling pathway. However, PTPN11 mutations are relatively rare in solid tumors, with SHP2’s oncogenic properties showing tissue specificity. SHP2’s role in cancer has been documented in breast, gastric, and lung cancers. Recently, SHP2 has been shown to act as a tumor suppressor in several cancers, including HCC cell lines, where it downregulates inflammatory signaling pathways beyond colorectal cancer18,19. Therefore, elucidating hypoxia’s role and molecular mechanisms in HCC, and its potential association with SHP2, may offer novel targets for cancer therapy.
Methods
Bioinformatics
Used R (4.2.1) version with R packages: geoquery [2.64.2], limma [3.52.2], ggplot2 [3.3.6], ComplexHeatmap [2.13.1]. The HCC dataset GSE76427 was downloaded from the GEO database using the GEOquery package. Missing values in the dataset were imputed using the impute package. The data were then normalized again using the normalizeBetweenArrays function from the limma package. Differential gene expression analysis was conducted with thresholds set at |LogFC| > 1 and p.adj < 0.05. Gene co-expression scatter plots were generated for the selected differentially expressed genes, and the results of the differential analysis were visualized using the ggplot2 package.
Cell culture
The human hepatocellular carcinoma cell lines SMMC-7721, HepG2 and Huh-7 were purchased from Procell Life Technology Co., Ltd. (WuHan, China). SMMC-7721, HepG2 and Huh-7 cells were cultured in RPMI1640 (PM150110) medium supplemented with 10% FBS (164210-50) and 1% P/S (PB180120), and SMMC-7721 cells were cultured in a normal incubator and a tri-gas incubator with 1% O2 separately20,21. Dovitinib, Antioxidant NAC, oxidant H2O2 and SHP2 agonist trichomide A (Purchased from MCE) were used respectively. The culture medium of cells in the hypoxia group was supplemented with 3% FBS and 1% P/S, and subsequent experiments were performed in Anaerobic Work Station (AW200SG).
Cell transfection
According to the designed shRNA sequence, six template DNA strands were designed, and the mode was as follows: BamH I-Sense-loop (TTC AAG ACG) Antisense-stop signal-EcoR I-Hind III. The sequence information was as follows: 5′-GAT CCG TGA TTA CTA TGA CCT GTA TTT CAA GAG AAT ACA GGT CAT AGT AAT CAC TTT TTT GAA TTC-A-3′, Antisense: 5′-AGC TTG AAT TCA AAA AAG TGA TTA CTA TGA CCT GTA TTC TCT TGA AAT ACA GGT CAT AGT AAT CAC G-3′; The template was annealed at 95 °C to form shRNA with small hairpin structure. The large fragment of pSilencer4.1/NC plasmid was recovered after BamH I and Hind III digestion. The annealed shRNA template DNA was ligated in the BamH I and Xho I sites of the pSilencer 4 vector. 1-CMV neo to build the recombinant plasmid. The above recombinant plasmid was then electroporate into E. coli DH5 α, and screened in LB medium containing 50 mg/L ampicillin. The single colony was selected and cultured. The plasmids were extracted and named as pSilencer4.1 SHP2 and pSilencer4.1/NC, respectively as negative controls. The recombinant plasmid was screened by agarose gel electrophoresis after EcoR I digestion22. The sequencing of recombinant plasmid was completed by Invitrogen Corporation Shanghai Representative Office.
SMMC-7721, HepG2 and Huh-7 cells were maintained in RPMI 1640 medium contain 10% fetal animal serum, 37 °C with 5% CO2 and these cells were passaged 2 to 3 times a week. Exponentially growing cells were inoculated in a 6-well plate at a concentration of 1 × 106/well. When the cell density reached 80–90%, the recombinant plasmids pSilencer4.1SHP2 and pSilencer4.1/NC were transfected into SMMC-7721 and HepG2 cells by X-fect transfection kit. The operation was in accordance with the instructions of XfectTM Transfection Reagents of Clontech. The cells where subsequently split into NC group and SHP2 KD group and treated with PI3K inhibitor LY294002. And we used 200 µmol/L CoCl2 added to the culture medium to treat the cells to create hypoxic conditions. The cells were divided into CoCl2 + NC group, CoCl2 + SHP2 KD group, CoCl2 + NC + LY294002 group and CoCl2 + SHP2 KD + LY294002 group.
ELISA
After the kit has been placed at room temperature for 60 min, open it and remove the desired slats from it. It is necessary to set up standard wells (50 µl of different concentrations of standards/well), blank wells (50 µl of sample dilution/well) and sample wells (50 µl of sample to be tested/well). 100 µl of horseradish peroxidase (HRP)-labeled antibody was added to each well (except for the blank wells), and then the reaction wells were sealed with a sealing film, followed by placing the slats in a thermostat at 37 °C for 60 min. The liquid was discarded, and the slats were patted dry by placing them on absorbent paper. Then, 350 µl of washing solution was added to each well, and the plate was allowed to stand for 1 min, then the washing solution was shaken off, and the plate was patted dry on absorbent paper, and this operation was repeated three times. After adding 50 µl of substrate A and 50 µl of substrate B to each well, the plate was incubated at 37 °C for 15 min, and finally 50 µl of termination solution was added to each well, and the OD value at 450 nm of each well was measured on the machine as soon as possible. The first step is to plot the standard curve; the horizontal coordinate is the concentration of the standard, and the vertical coordinate is the corresponding OD value. Then the concentrations of MDA and SOD were calculated according to the curve equation.
Western blotting
SMMC-7721 cells were treated with lysate (RIPA lysate and protease inhibitor in a 100:1 ratio). In accordance with the instructions for gel preparation, deionized water, 30% acrylamide, 1 Tris-HCl buffer (PH6.8), SDS, AP (Ammonium persulphate), and TEMED were added in order to prepare the upper layer of the gel (concentrated gel), which was mixed well and added slowly into a glass plate (taking care to avoid air bubbles), and then inserted into a comb, and the gel was allowed to gel for about 20 min at room temperature before it could be withdrawn from the comb and the samples were started. Filled up the electrophoresis liquid in the electrophoresis rack, blew off the bubbles on the liquid surface, added electrophoresis liquid in the electrophoresis tank to the scale line, covered the electrophoresis lid, paid attention to the red to red, black to black, otherwise the current was opposite. Set the electrophoresis apparatus into a stabilized state, and adjusted the voltage to 80 V so that the sample passed through the concentrated gel for about 35 min, electrophoresis to the appropriate position of the separated gel and ended the electrophoresis. The separated proteins were transferred from the gel to a PVDF membrane using the wet transfer method. Transferring the membrane took 90 min and the current was set at 200 mA. Next, PVDF membrane was blocked in TBST solution supplemented with 5% skim milk at the room temperature for 1 h, while NOX2, NOX4, SHP-2, p-SHP2, p-PI3K, HIF1α, COX2, FOXM1, β-catenin and MMP9, GAPDH antibodies were diluted at 1:1000 ratio with TBST solution. After dilution, they were added to the corresponding PVDF membrane and incubated at 4 ℃. The next day, the membranes were washed with TBST solution for 3 times continuously, from where it was transferred to its related secondary antibody chamber for 1 h incubation, then washed with TBST solution for 3 times. Then the color was developed with ECL and some photographs were captured. Experimental results were analyzed using imageJ software22.
Tube formation assay
SMMC-7721 and HUVEC were co-cultured, and when HUVEC reached more than 80% confluence, basal medium without FBS was given and starved for 8 h. Matrigel (BD: The reagent of Orginal Seed No. 190145 and Orginal Seed No. 356234 was put in the 4℃ refrigerator to thaw and was spread into the pre-cooled 24-well plate by using the pre-cooled gun tip in the ultra-clean bench and the pre-cooled reagent, so Matrigel was evenly distributed, and solidified at 37 °C for 30 min. Then, HUVEC cells were treated with trypsin and the number of the cells was counted and resuspended in 100 µl of free serum containing media and adjust to 5 × 105 cells/100µl. Thus 100 µl cell suspension was added onto the 24-well plate/cm2 and under an inverted microscope within 4 h pictures were taken23.
Scratch wound assay
SMMC-7721, HepG2 and Huh-7 cells were so next into a 6-well plate. Incubation was performed at 37 °C for 24 h in a 5% CO2 incubator. When the confluence rate has reached 70%, a 200 µl sterile pipette was used for the healing experiment, and the angle between the scratch tip and cells was as small as possible to ensure that the width of each group’s scratch was approximately similar. The cells were washed with PBS and allowed to incubate for 24 h. Different pictures were then taken within 0, and 48 h to assess the number of healthy distance24.
Transwell
A trans-pore chamber with a 24-hole, 8.0 μm pore membrane was used. SMMC-7721 cells and HepG2 cells, 1 × 105 cells/well were plated in 100 µl of serum-free medium; 600 µl completed medium was placed in the lower chamber as a chemo attractant. After the 24 h incubation at 37 °C, the cells on the upper surface of the membrane were wiped off with a cotton tipped swab while those adhering to the lower sides of the membrane were migratory cells. Following that, the cells were washed two times and then fixed with 4% paraformaldehyde and stained with 0.1% crystal violet solution. Finally, the cells were then allowed to be visualized through the filter using an inverted fluorescence microscope. Besides, medium in each well was replaced with 100 µl of 1:8 DMEM diluted matrigel for 6 h, invaded cells were fixed for 48 h and seeded onto the membrane for invasion experiment25.
Animal
Sixteen nude mice were obtained from Henan SKBS Biotechnology Co. Ltd with the genetic background of BALB/c, at the age of 8 weeks, and male. The animals were kept under correct temperature and humidity conditions, and sufficient water and food were provided (proper temperature: 25 °C, proper humidity: usually 50%, day-night cycle: 12/12). Nude mice were randomly placed in cages. The last, I divided the naked mice into control group and hypoxia group, each group includes eight mice. Nude mice in the hypoxia group were placed in the hypoxia experimental system Ox-100 for incubation. Nude mice were sacrificed with CO2, and the anxiety of nude mice was reduced as much as possible during the euthanasia process, and the death of nude mice was defined 30 min following the nude mice being unable to breathe. All animal protocols were conduct with the permission of the Animal Care and Use Committee of the Fourth Hospital of Hebei Medical University (Ethical Number: No.2023KS241; Approval date: 2023.11.30).
Subcutaneous tumor bearing experiment in nude mice
To establish a syngeneic mouse tumor model, a suspension of SMMC-7721 (4 × 105 cells in 100 µl) was implanted subcutaneously onto the dorsal side of an 8-week-old male mouse. The experimental procedure was carried out in Anaerobic Work Station (AW200SG). Tumor volume was measured at 18th day using digital calipers and calculated according to the following formula: Thus, at intermediate level of saturation V = 0.52 × L × W2 (V, tumor volume; L, the longest diameter; W, vertical diameter). This group of tumors was taken at 18 day post cell injection and fixed in 4% PFA for subsequent histological studies26.
HE staining
Sliced the paraffin-embedded blocks and baked them at 60 °C until the wax melted. Placed the slides in xylene solution and shook them slowly for 10 min to remove the wax. Replaced the xylene with new solution and repeated this operation three times. Immersed the slides in 100% ethanol twice, then in 90%, 80%, and 70% ethanol solutions. Rinsed the slides with tap water and dried them with blotting paper. Added drops of hematoxylin staining solution and stained for 15 min. Rinsed with tap water to remove the excess hematoxylin and blotted them dry. Differentiated the slides with 1% hydrochloric acid in ethanol for 10 s and immediately rinsed them with tap water. Baked the slides at 60 °C for 1 min, then rinsed again with tap water. Added drops of eosin stain and stained for 30 s. Rinsed with tap water, dried at 37 °C, and sealed the slides with neutral gum.
Statistical analysis
GraphPad prism 9.0 was employed for the data analysis and processing purposes. All the collected data were presented in the form of means standard deviation (x ± s). Comparisons between multiple groups were analyzed by ANOVA, and two-by-two comparisons were made using the t-test. I am going to remove data samples that do not fit the experiment’s set criteria. P < 0.05 was taken as the level of significance.
Results
Bioinformatics analysis results
The samples of the dataset GSE76427 were distributed into two categories, normal group and group with cancer for the 119 sets of samples. The Significant expressed genes: out of 42 screened genes 42 are up-regulated and 129 are down-regulated. Among them, the differential gene ERK was found to be a marker protein of the RAS-AMPK pathway, JAK2 and STAT3 were marker proteins of the JAK-STAT pathway, and PI3K was marker proteins of the PI3K-AKT pathway. The gene co-expression scatter plot revealed that SHP2 promotes the expression of ERK, SHP2 promotes the expression of JAK2 and STAT3, and SHP2 inhibits the expression of PI3K. It indicated that SHP2 activated the RAS-AMPK and JAK-STAT pathways and inhibited the PI3K-AKT pathway (Fig. 1).
Fig. 1.
Bioinformatics analysis results. (A) Differential gene volcano plot; (B) SHP2 and ERK correlation scatter plot; (C) SHP2 and JAK2 correlation scatter plot; (D) SHP2 and STAT3 correlation scatter plot; (E) SHP2 and PI3K correlation scatter plot; (F) SHP2 and AKT correlation scatter plot.
Hypoxia can promote oxidative stress and affect the activation of SHP2 and PI3K
The first Western blotting experiment revealed the differences of relative protein expression of NOX2, NOX4 and p-PI3K were statistically increased in the hypoxia group than that of normal group, while the relative protein expression of p-SHP2 was statistically reduced in the hypoxia group than that of normal group. Whereas, the relative protein expression of NOX2, NOX4, p-SHP2, and p-PI3K of two groups have no significant differences after addition of antioxidant NAC; The differences in relative protein expression of NOX2, NOX4, p-SHP2 and p-PI3K were also eliminated after the addition of prooxidant H2O2. After treatment with the SHP2 agonist trichomide A, that was still significant difference in the relative protein expression of NOX2 and NOX4 remained and the removal of relative protein expression of p-SHP2 and p-PI3K was not different. In contrast, there was no significant difference in the relative protein expression of t-SHP2 and t-PI3K between the groups. The results of ELISA experiments showed that the concentration of MDA was significantly higher and the concentration of SOD was significantly lower in the hypoxia group relative to the normal group. In these two groups, the concentration of MDA was significantly reduced and the concentration of SOD was significantly increased after the use of NAC. There was no significant difference in the concentrations of MDA and SOD between these two groups. normal and hypoxic groups showed a significant increase in the concentration of MDA and a significant decrease in the concentration of SOD after treatment with H2O2. There was no significant difference in the concentrations of MDA and SOD between these two groups. normal and hypoxic groups showed a significant decrease in the concentration of MDA and an increase in the concentration of SOD after treatment with Trichomide A. The concentration of MDA was significantly higher in the normal and hypoxic groups than in the hypoxic group after treatment with H2O2. The concentration of MDA was significantly higher in the hypoxic Trichomide A group compared to the normal + Trichomide A group, while there was no significant difference in the concentration of SOD (Fig. 2). The outcomes indicated that hypoxia could enhance the level of oxidative stress, which would suppress the extent of phosphorylation of SHP2 and encouraged the phosphorylation of PI3K.
Fig. 2.
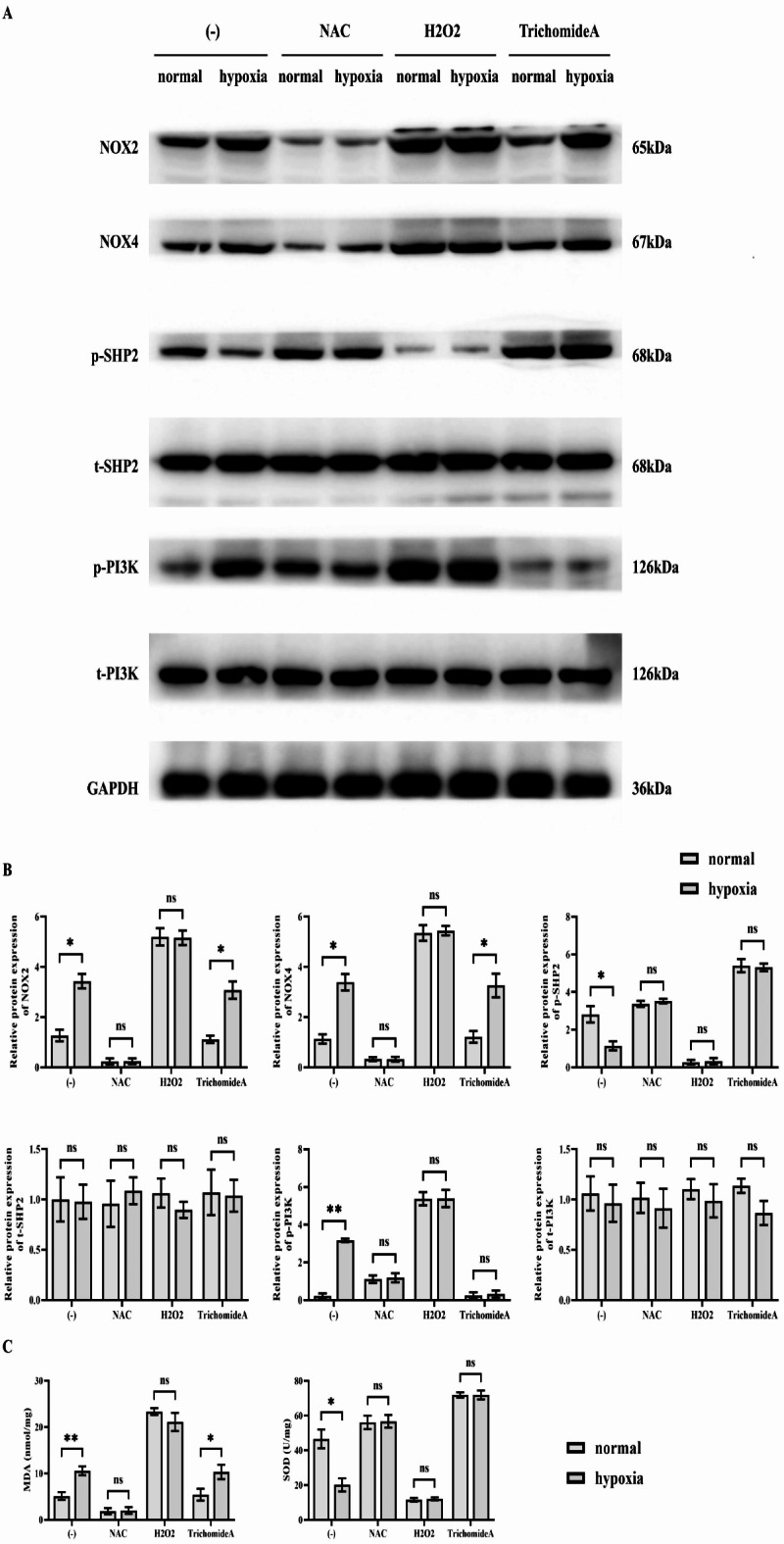
Results of Western blotting to detect NOX2, NOX4, p-SHP2 and p-PI3K. (A) SMMC-7721 cells were subjected to hypoxia and then treated with the antioxidant NAC, the oxidant H2O2, and the SHP2 agonist Trichomide A. Corrective experiments were performed to observe the effects of hypoxia on the relevant signalling pathways in SMMC-7721 cells. Protein banding plots of NOX2, NOX4, p-SHP2, t-SHP2, p-PI3K and t-PI3K detected by Western blot assay; (B) Statistical plot of relative protein expression of NOX2, NOX4, p-SHP2, t-SHP2, p-PI3K and t-PI3K detected by Western blot assay. GAPDH was used as a control protein; (C) Plot of results of ELISA assay for MDA and SOD concentrations. Data are expressed as mean ± SEM; N = 3; **P < 0.01; nsP > 0.05.
Specific knockout of SHP2 in hypoxic environment can promote PI3K phosphorylation and expression of HIF1α and COX2
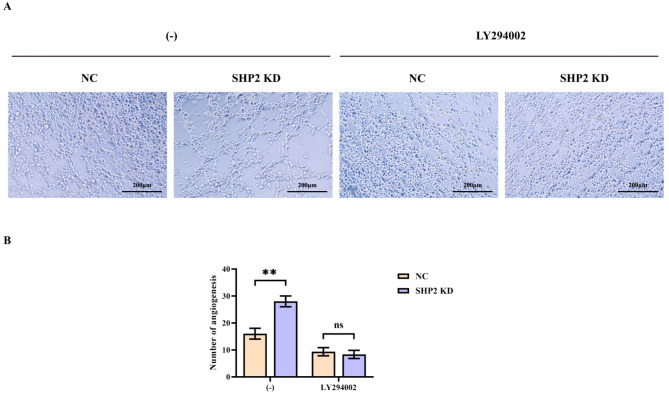
HIF-1 induced the expression of numerous genes contributing to the process of energy metabolism, angiogenesis, apoptosis, and other proteins that enhanced oxygen supply and metabolic compensation to hypoxic conditions. This was considered a global regulator of the steady-state response of cells and the entire organism to hypoxia. Accordingly, HIF-1 was vital in the pathophysiology of embryonic vascularization, tumor angiogenesis and ischemic diseases. COX, also known as prostaglandin synthase, has two isozymes, COX1 and COX2. This study demonstrated that the high expression of COX2 in tumor tissues would impact the occurrence and development of tumors in different manners through modulating the downstream metabolites to promote the proliferation and migration ability of tumor cells as well as angiogenesis, and by supporting apoptosis inhibition, which exerted a significant role in disease growth and metastasis. Protein samples used in western blotting analysis of SHP2 KD group had significantly lower protein density of t-SHP2 and p-SHP2 comparing to NC group; However, the density of p-PI3K, HIF1α and COX2 for SHP2 KD group was significantly higher than that of NC group. However, after the addition of the PI3K inhibitor LY294002, the difference of the relative protein expression of p-PI3K, HIF1α, and COX2 was diminished. In contrast, there was no significant difference in the relative protein expression of t-PI3K between the groups (Fig. 3). As it exhibited in the tube formation assay, the number of vascularization turned to be higher in SHP2 KD group than that in NC group, however, after the administration of LY294002, it is no longer significantly different. Consequently, specific knockout of SHP2 in the hypoxic environment could promote PI3K phosphorylation and the expression of HIF1α and COX2, increasing angiogenesis capacity (Fig. 4).
Fig. 3.
Results of Western blotting for SHP2, p-SHP2, p-PI3K, HIF1α and COX2. (A) SMMC-7721 cells were subjected to hypoxia treatment before specific knockdown of SHP2 and corrective experiments using the PI3K inhibitor LY294002 were performed to explore the effects of the associated proteins in SMMC-7721 cells. Protein banding plots of t-SHP2, p-SHP2, p-PI3K, t-PI3K, HIF1α and COX2 detected by Western blot assay; (B) Statistical plot of relative protein expression of SHP2, p-SHP2, p-PI3K, t-PI3K, HIF1α and COX2 detected by Western blot assay. GAPDH was used as a control protein; Data are expressed as mean ± SEM; N = 3; **P < 0.01;nsP > 0.05.
Fig. 4.
The number of angiogenesis was measured by tube formation assay. (A) SMMC-7721 cells were subjected to hypoxia treatment before specific knockdown of SHP2, and corrective experiments using the PI3K inhibitor LY294002 were performed to explore the effects on the angiogenic capacity of SMMC-7721 cells. Diagram of angiogenesis experimental results; (B) Statistical graph of the number of blood vessels formed; Data are expressed as mean ± SEM; N = 3; **P < 0.01;nsP > 0.05.
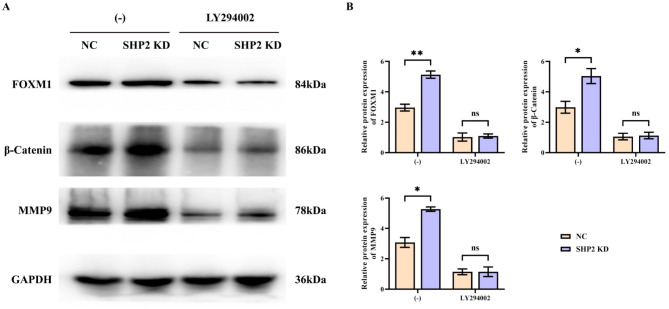
Specific knockdown of SHP2 in hypoxic environment can promote the expression of FOXM1, β-catenin and MMP9
The FOXM1 was assumed to regulate the cell division and differentiation together with stabilizing the genome. According to experimental and clinical studies, FOXM1 was overexpressed in various human cancers and served as one of the master regulators of various cancer-related processes, including angiogenesis, invasion, and metastasis. β-Catenin, as an adhesion junction protein, formed an adhesion junction complex with E-cadherin and alpha Catenin, which maintained normal tissue structure and morphogenesis by regulating cell growth and intercellular adhesion. In addition, β-Catenin was critical in embryonic development, tissue homeostasis, and tumorigenesis. The level of FOXM1, β-catenin and MMP9 protein was up-regulated in SHP2 KD group than that in NC group. Nevertheless, this difference is abolished when combined LY294002, the relative protein expression of FOXM1, β-catenin and MMP9 of the NC group shifted to the same level with the SHP2 KD group (Fig. 5).
Fig. 5.
Results of Western blotting for FOXM1, β-catenin and MMP9. (A) SMMC-7721 cells were subjected to hypoxia treatment before specific knockdown of SHP2 and corrective experiments using the PI3K inhibitor LY294002 were performed to explore the effects of proteins related to the migratory capacity of SMMC-7721 cells. Protein banding plots of FOXM1, β-catenin and MMP9 detected by Western blot assay; (B) Statistical plot of relative protein expression of FOXM1, β-catenin and MMP9 detected by Western blot assay. GAPDH was used as a control protein; Data are expressed as mean ± SEM; N = 3; **P < 0.01; nsP > 0.05.
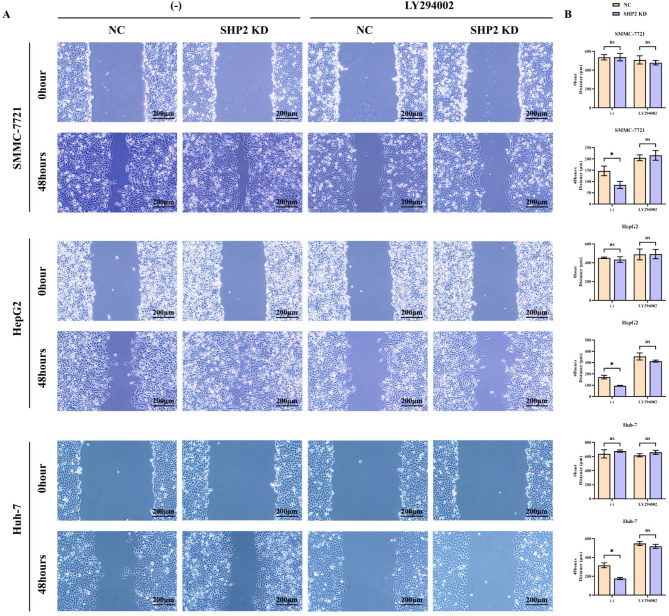
Hypoxia enhances migration and invasion of hepatocellular carcinoma cells, but specific silencing of SHP2 not
Scratch wound assay results showed that after 48 h, the distance between cells in the SHP2 KD group was significantly reduced compared to the NC group in SMMC-7721, HepG2 and Huh-7 cells. After the addition of LY294002, the distance between the cells in the two groups increased significantly and there was no significant difference (Fig. 6). The results of the Transwell experiment showed that in SMMC-7721, HepG2 and Huh-7 cells, compared with the NC group, the number of cells that migrated and invaded was significantly higher in the SHP2 KD group. After the addition of LY294002, the number of migrating and invading cells in the two groups was significantly reduced and there was no significant difference (Fig. 7). This indicates that specific interference with SHP2 under hypoxic conditions can mediate PI3K to enhance the migration and invasion of hepatocellular carcinoma cells.
Fig. 6.
Scratch wound assay was used to detect the ability of cell migration. (A) SMMC-7721, HepG2 and Huh-7 cells were subjected to hypoxia treatment before specifically knocking down SHP2, and corrective experiments using the PI3K inhibitor LY294002 were performed to explore the effects on the migratory capacity of SMMC-7721, HepG2 and Huh-7 cells. Plot of scratch wound assay results of SMMC-7721, HepG2 and Huh-7 cells; (B) Statistics of the scratch spacing for SMMC-7721, HepG2 and Huh-7 cells. Data are expressed as mean ± SEM; N = 3; **P < 0.01; nsP > 0.05.
Fig. 7.
Transwell assay detected the ability of cell migration and invasion. (A) SMMC-7721, HepG2 and Huh-7 cells were subjected to hypoxia treatment before specifically knocking down SHP2, and corrective experiments using the PI3K inhibitor LY294002 were performed to explore the effects of migratory and invasive capacities of SMMC-7721, HepG2 and Huh-7 cells. Transwell results of SMMC-7721, HepG2 and Huh-7 cells; (B) Statistics of migration and invasion of SMMC-7721, HepG2 and Huh-7 cells. Data are expressed as mean ± SEM; N = 3; **P<0.01; nsP>0.05.
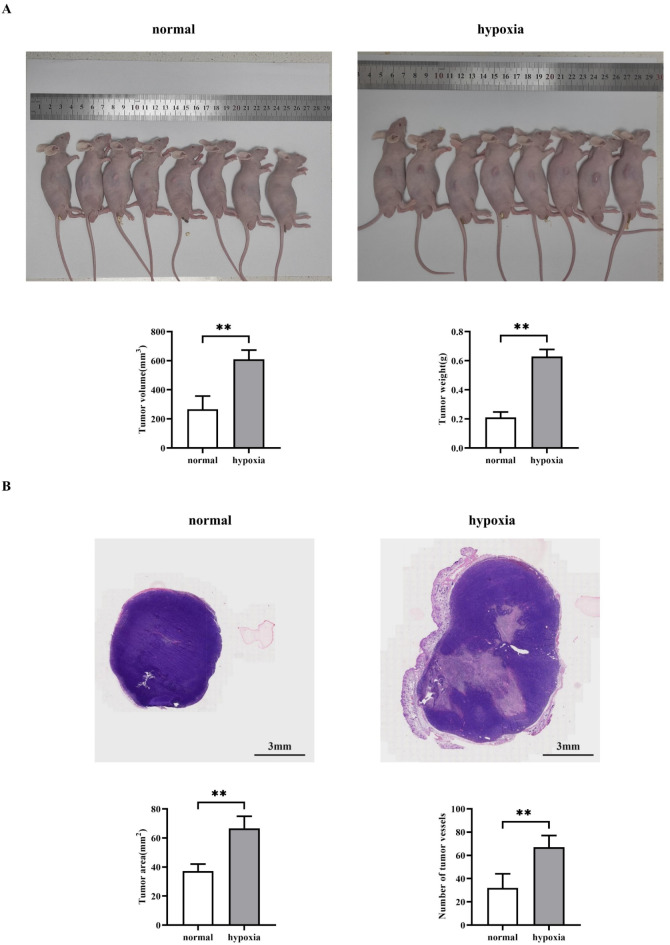
Hypoxia promotes the development of hepatocellular carcinoma
In order to verify the conclusions of the in vitro experiment, we verified by establishing a nude mouse tumor-bearing experiment, this proved that the density of hepatocellular carcinoma in the hypoxia group was higher than that of the normal group. The results of HE staining experiments showed that the tumor area as well as the number of tumor blood vessels were significantly higher in the hypoxia group relative to the normal group. It is explained that hypoxia can promote the growth and metastasis of hepatocellular carcinoma (Fig. 8).
Fig. 8.
Nude mouse tumor-bearing experiments to detect the effect of hypoxia on hepatocellular carcinoma. (A) SMMC-7721 cells were placed in a hypoxic environment, and then the effects of hypoxia on the growth and metastasis of hepatocellular carcinoma as well as the tumor volume and statistical graphs of the tumor experiments were detected using the nude mice subcutaneous xenograft assay; (B) Graphs of the results of HE staining experiments as well as graphs of the statistical results of the area of tumor sections as well as the number of tumor blood vessels. Data are expressed as mean ± SEM; N = 8; **P < 0.01; nsP > 0.05.
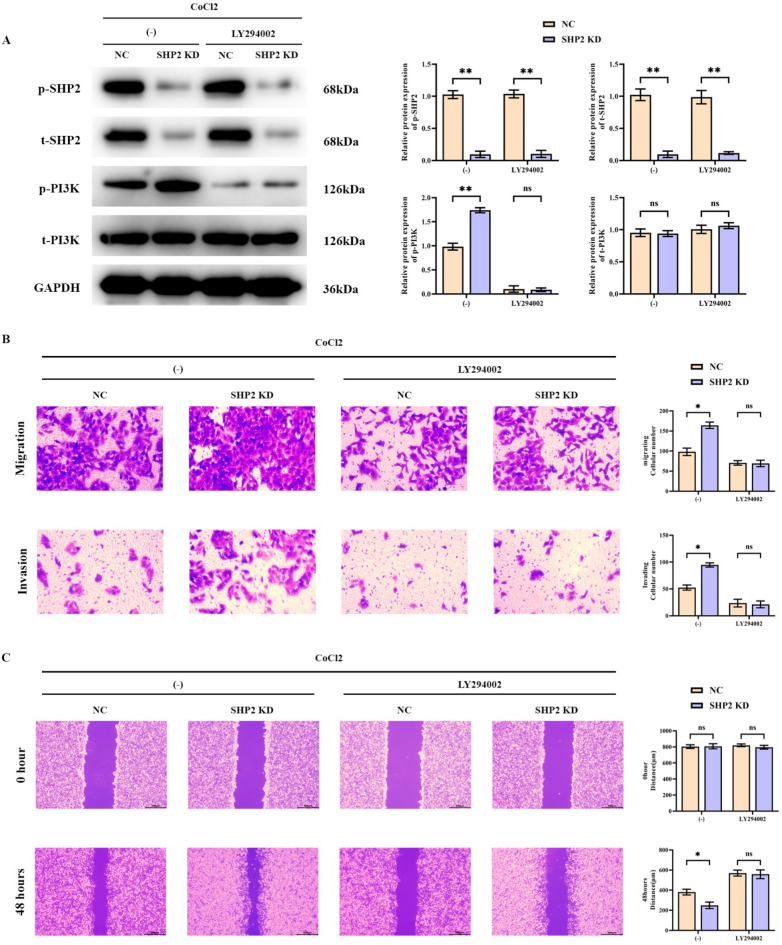
Finally we used CoCl2 to create hypoxic conditions for experiments to support the current findings. western blot experiments showed that relative to the CoCl2 + NC group, the relative protein expression of p-SHP2 and t-SHP2 was significantly lower in the CoCl2 + SHP2 KD group, the relative protein expression of p-PI3K was significantly higher, and t-PI3K’s relative protein expression did not change significantly. Relative protein expression of p-SHP2 and t-SHP2 was significantly lower in the CoCl2 + NC + LY294002 group relative to the CoCl2 + SHP2 KD + LY294002 group, and there was no significant change in the relative protein expression of p-PI3K and t-PI3K. transwell experiments showed that relative to the CoCl2 + NC group, the The number of migrating and invading cells was significantly higher in the CoCl2 + SHP2 KD group. There was no significant change in the number of migrating and invading cells in the CoCl2 + SHP2 KD + LY294002 group relative to the CoCl2 + NC + LY294002 group. Scratch wound assay results showed a significant decrease in cell spacing in the CoCl2 + SHP2 KD group at 48 h relative to the CoCl2 + NC group. There was no significant change in cell spacing at 48 h in the CoCl2 + SHP2 KD + LY294002 group relative to the CoCl2 + NC + LY294002 group (Fig. 9).
Fig. 9.
EGb761 mediated SHP2/PI3K signaling pathway inhibits migration and invasion in hepatocellular carcinoma. (A) Western blot protein bands of p-SHP2, t-SHP2,p-PI3K and t-PI3K and relative protein expression statistics; (B) Transwell results of migration and invasion ability of SMMC-7721 cells; (C) Scratch wound assay to detect the migration ability of SMMC-7721 cells and cell spacing statistics. **P < 0.01; *P < 0.05; nsP > 0.05.
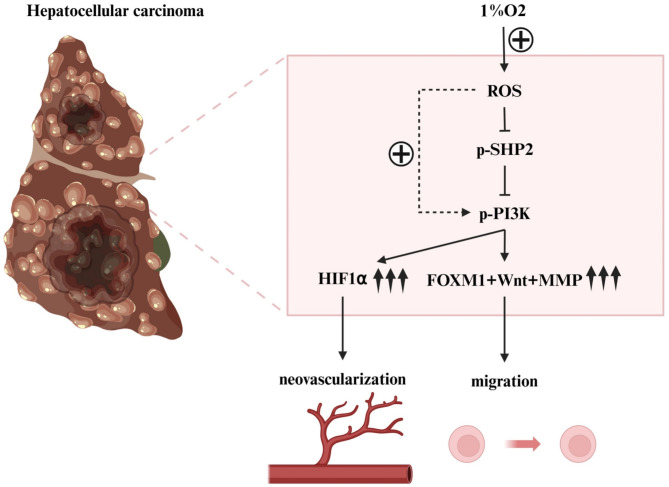
Therefore, it can be deduced that hypoxia could incite the oxidative stress-mediated SHP2/PI3K signal p.a. and enhance the angiogenesis, migration and invasion of hepatocellular carcinoma, thereby promoting the growth and metastasis of hepatocellular carcinoma (Fig. 10). The datasets used in the current study may be requested from the authors of the papers under study provided that s reasonable request is made. The raw data which was used in this study has also been provided in Supplementary Table 1.
Fig. 10.
Hypoxia can activate oxidative stress-mediated SHP2/PI3K signaling pathway to promote angiogenesis, migration and invasion of hepatocellular carcinoma, thus promoting the growth and metastasis of hepatocellular carcinoma.
Discussion
Primary liver cancer ranks as the fifth most prevalent cancer worldwide, with hepatocellular carcinoma (HCC) accounting for 70–85% of liver cancer cases. Globally, the incidence of HCC ranks fifth among malignant tumors in men and seventh in women. Moreover, it is the third leading cause of cancer-related deaths worldwide1,2. Surgical resection remains the most effective treatment for liver cancer, yet the resection rate is notably low (10–30%) because the majority of patients are diagnosed at an advanced stage. Despite advancements in diagnostic and surgical techniques, high mortality persists due to the elevated rates of metastasis and postoperative recurrence, with the overall 5-year survival rate not surpassing 30%4. Thus, investigating the mechanisms of liver cancer metastasis and recurrence, identifying predictive markers, and developing targeted intervention therapies have significant theoretical and clinical value in improving the prognosis of liver cancer patients.
Hypoxia is a common physiological phenomenon and one of the significant features of microenvironment of many solid tumor cells5. In the dataset GSE76427, 119 sets of samples were divided into normal and cancer groups. The data analysis of the gene chips revealed that forty two genes were up regulated while one hundred and twenty nine genes were down regulated. Among them, ERK is a marker protein for RAS-AMPK pathway, JAK2 and STAT3 are marker proteins for JAK-STAT pathway, and PI3K is a marker protein for PI3K pathway. Gene co-expression scatter plot analysis showed that SHP2 promoted the expression of ERK, JAK2 and STAT3 but inhibited the expression of PI3K. These results suggest that SHP2 plays a key regulatory role in cancer development by activating the RAS-AMPK and JAK-STAT pathways and inhibiting the PI3K pathway. In this experimental study, The quantitative change of following protein blots, hypoxia increased the relative protein expression of NOX2, NOX4 and p-PI3K, Thus, consequently, levels of the relative protein expression remained to be the same with p-SHP2 as with p-PI3K. However, similar to the H2O2 treatment, the difference between the relative protein expression amounts of NOX2, NOX4, p-SHP2 and p-PI3K was abolished as well. Nevertheless, the difference of relative protein expression amounts of NOX2 and NOX4 came to an end not after added the SHP2 agonist trichomide A treatment and the relative protein expression amounts of p-SHP2 and p-PI3K remained almost the same. This suggested that hypoxia could increase the levels of oxidative stress and that oxidative stress could reduce the ability of SHP2 to become activated thus leading to the inhibition of PI3K.
Hypoxia inducible factors are special group of transcription factor with multiple family members that is involved in the regulation of a large number of genes under hypoxic stress. HIFs respond to the decrease of intracellular oxygen concentration by regulating the action of target genes. This stress response is beneficial to the body in some physiological processes such as embryonic development and some physiological processes such as ischemic injury, but it is harmful to the occurrence and development of tumors27. HIF-1 is vital during embryonic vascularization and new vessel formation in tumors as well as in the processes related to ischemic diseases. COX, also known as prostaglandin synthase, has two isoenzymes, COX1 and COX2. Some of the findings have been that high expression of COX-2 is present in tumor tissues, and this influences or can influence occurrence and development of tumors in several ways, encourages growth of tumor cells besides causing its migration, promotes formation of new blood vessels and inhibits apoptosis. Therefore, it plays a prominent role in disease progression28,29. In particular, the relative protein expression of SHP2 and p-SHP2 was decreased in NC group as contrasted with SHP2 KD group, while the relative protein expression of p − PI3K, HIF 1α and COX 2 was elicited in SHP2 KD group. However, the relative protein expression of p-PI3K, HIF1α and COX2 difference was not existed anymore after the LY294002 was added. The tube formation assay also revealed that the number of blood vessels formed in the SHP2 KD group was increased more than that in the NC group, however, the increment was canceled out after adding LY294002.
FoxM1 has been demonstrated to be up-regulated in many human malignancies and is the master regulator of cancer associated events like angiogenesis, invasion and metastasis. β-Catenin is also an adhesive Connexin which along with E-cadherin and alpha Catenin constituting an adhesive junction complex which help in the normal growth of tissue and morphogenisis and controls cell growth and intercellular connections30–32. This protein is relevant in embryonic development, tissue remodelling and also tumourigenesis. Compared with NC group. Cytosolic FOXM1, β-catenin and MMP9 protein level were all higher in the SHP2 KD group compared to the control group. However, when the cells were treated with the PI3K inhibitor LY294002, the relative protein expression of FOXM1, β- The difference of FOXM1, β- catenin and MMP9 was eliminated. The result of scratch wound assay indicated that at the 48 h, the value of scratch spacing of SMMC-7721 cells and HepG2 cells of SHP2 KD group was lower than that of NC group while the numbers of migrating and invading cells were much higher. Following the incorporation of a PI3K inhibitor LY294002, there was no differences in cell scratch spacing, migration, and number of invading cells. It suggested that when the hypoxic environment is selected, there is selective knockout in SHP2, which will enhance HCC cells to migrate and invade. The experiment of nude mouse bearing hepatocellular carcinoma indicated that the tumor volume in hypoxia group was significantly increased than that in normal group. It is explained that hypoxia can promote the growth and metastasis of hepatocellular carcinoma. This study provides new insights into how hypoxia-activated oxidative stress promotes hepatocellular carcinoma (HCC) growth and metastasis through the SHP2/PI3K signaling pathway, however, there are still limitations that need to be overcome in future studies. Although this study revealed the mechanism of action of the SHP2/PI3K signaling pathway under hypoxic conditions, it has not been validated in clinical samples. In the future, it is necessary to analyze the expression of SHP2 and PI3K in HCC patients and their correlation with prognosis and treatment response in combination with clinical data, so as to improve the clinical application value of the study. SHP2/PI3K signaling pathways may be regulated by a variety of factors in the intracellular and external environment, and this study may not cover all related regulatory mechanisms. Further research should explore more upstream and downstream regulators and their interactions to fully understand the role of this signaling pathway in HCC. By overcoming these limitations, future studies will be able to more comprehensively understand the mechanism of hypoxia-activated oxidative stress in the growth and metastasis of HCC, providing a more solid scientific basis for the diagnosis and treatment of HCC.
Overall, hypoxia can activate the oxidative stress-mediated SHP2/PI3K signaling pathway, promoting angiogenesis, migration, and invasion of HCC. These findings offer a new target for HCC treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Z.N. and S.W. contributed to the conception of the study. Q.Z. performed the experiments. H.C. contributed significantly to analysis and manuscript preparation. B.Y. performed the data analyses and wrote the manuscript. S.W. helped perform the analysis with constructive discussions.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal protocols were approved by the Animal Care and Use Committee of the Fourth Hospital of Hebei Medical University (Ethical Number: No.2023KS241).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zuo, Z. et al. Construction of a ceRNA network in hepatocellular carcinoma and comprehensive analysis of immune infiltration patterns. Am. J. Transl Res.13 (12), 13356–13379 (2021). [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin.70, 7–30 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Khemlina, G., Ikeda, S. & Kurzrock, R. The biology of hepatocellular carcinoma: implications for genomic and immune therapies. Mol. Cancer. 16, 149 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto Marques, H., Gomes da Silva, S., De Martin, E., Agopian, V. G. & Martins, P. N. Emerging biomarkers in HCC patients: current status. Int. J. Surg.82S, 70–76 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Zonneveld, M. I., Keulers, T. G. H. & Rouschop, K. M. A. Extracellular vesicles as transmitters of Hypoxia Tolerance in Solid Cancers. Cancers (Basel). 11 (2), 154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu, X. H. et al. Recent research on methods to improve Tumor Hypoxia Environment. Oxid. Med. Cell. Longev.2020, 5721258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu, N. et al. Hypoxia-induced LINC00674 facilitates hepatocellular carcinoma progression by activating the NOX1/mTOR signaling pathway. J. Cancer. 13 (11), 3177–3188 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang, Y. et al. Hypoxia signaling in cancer: implications for therapeutic interventions. MedComm (2020). 4 (1), e203 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou, Z. et al. Synthesis and in vitro and in vivo evaluation of hypoxia-enhanced 111In-bombesin conjugates for prostate cancer imaging. J. Nucl. Med.54 (9), 1605–1612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou, Y. et al. Autophagy inhibits chemotherapy-induced apoptosis through downregulating bad and Bim in hepatocellular carcinoma cells. Sci. Rep.4, 5382 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo, Q. et al. Plexin-B3 expression stimulates MET signaling, breast cancer stem cell specification, and lung metastasis. Cell. Rep.42 (3), 112164 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Zuo, D., Liu, G., Deng, M. & Gao, Y. Efficacy of nedaplatin combined with docetaxel in patients with nasopharyngeal carcinoma and its influence on ECRG4 and VEGF expressions. J. BUON. 25 (4), 1976–1981 (2020). [PubMed]
- 13.Zuo, G. L. et al. Activation of HIFa pathway in mature osteoblasts disrupts the integrity of the osteocyte/canalicular network. PLoS One. 10 (3), e0121266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou, B. et al. The expression of FAP in hepatocellular carcinoma cells is induced by hypoxia and correlates with poor clinical outcomes. J. Cancer. 9 (18), 3278–3286 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang, Y., Li, S., Wang, Y., Zhao, Y. & Li, Q. Protein tyrosine kinase inhibitor resistance in malignant tumors: molecular mechanisms and future perspective. Signal. Transduct. Target. Ther.7 (1), 329 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu, J. et al. PTPN9 promotes cell proliferation and invasion in Eca109 cells and is negatively regulated by microRNA-126. Oncol. Lett.14 (2), 1419–1426 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu, D. et al. Osteosarcoma cell proliferation suppression via SHP-2-mediated inactivation of the JAK/STAT3 pathway by tubocapsenolide A. J. Adv. Res.34, 79–91 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou, J., Guo, H., Zhang, Y., Liu, H. & Dou, Q. Prognostic significance of SHP2 (PTPN11) expression in solid tumors: a meta-analysis. PLoS One. 17 (1), e0262931 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuo, C. et al. Chemotherapy effectiveness and prognosis of gastric Cancer influenced by PTPN11 polymorphisms. Cell. Physiol. Biochem.39 (4), 1537–1552 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Zhao, J. et al. Effects of sinapic acid combined with cisplatin on the apoptosis and autophagy of the hepatoma cells HepG2 and SMMC-7721. Evid. Based Complement. Altern. Med.2021, 6095963 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wo, L. et al. LncRNA HABON promoted liver cancer cells survival under hypoxia by inhibiting mPTP opening. Cell. Death Discov.. 8 (1), 171 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai, G., Nan, Y., Zhao, H. & Hu, Q. SHP2 mediates STAT3/STAT6 signaling pathway in TAM to inhibit proliferation and metastasis of lung adenocarcinoma. Aging (Albany NY). 16 (2024). [DOI] [PMC free article] [PubMed]
- 23.Dong, S. et al. Arsenic trioxide inhibits angiogenesis of hepatocellular carcinoma after insufficient radiofrequency ablation via blocking paracrine angiopoietin-1 and angiopoietin-2. Int. J. Hyperth.39 (1), 888–896 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Gau, D. M. & Roy, P. Single cell migration assay using human breast cancer MDA-MB-231 cell line. Bio Protoc.10 (8), e3586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, R. et al. TRPM2 promotes pancreatic cancer by PKC/MAPK pathway. Cell. Death Dis.12 (6), 585 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, F. et al. Hedyotis diffusa injection induces ferroptosis via the Bax/Bcl2/VDAC2/3 axis in lung adenocarcinoma. Phytomedicine104, 154319 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Zhou, C. H., Zhang, X. P., Liu, F. & Wang, W. Modeling the interplay between the HIF-1 and p53 pathways in hypoxia. Sci. Rep.5, 13834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piasecka, D. et al. Upregulation of HIF1-α via an NF-κB/COX2 pathway confers proliferative dominance of HER2-negative ductal carcinoma in situ cells in response to inflammatory stimuli. Neoplasia22 (11), 576–589 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maturu, P. et al. Role of Cyclooxygenase-2 pathway in creating an immunosuppressive microenvironment and in initiation and progression of Wilms’ Tumor. Neoplasia19 (3), 237–249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu, X., Yu, M., Wang, K., Zou, W. & Zhu, L. FoxM1 affects adhesive, migratory, and invasive abilities of human retinoblastoma Y-79 cells by targeting matrix metalloproteinase 2. Acta Biochim. Biophys. Sin (Shanghai). 52 (3), 294–301 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Zhu, X. et al. The FoxM1-ABCC4 axis mediates carboplatin resistance in human retinoblastoma Y-79 cells. Acta Biochim. Biophys. Sin (Shanghai). 50 (9), 914–920 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Zhu, X. et al. FoxM1 is upregulated in osteosarcoma and inhibition of FoxM1 decreases osteosarcoma cell proliferation, migration, and invasion. Cancer Manag. Res.12, 9857–9867 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.