Abstract
Free-living amebas are widely distributed in soil and water, particularly members of the genera Acanthamoeba and Naegleria. Since the early 1960s, they have been recognized as opportunistic human pathogens, capable of causing infections of the central nervous system (CNS) in both immunocompetent and immunocompromised hosts. Naegleria is the causal agent of a fulminant CNS condition, primary amebic meningoencephalitis; Acanthamoeba is responsible for a more chronic and insidious infection of the CNS termed granulomatous amebic encephalitis, as well as amebic keratitis. Balamuthia sp. has been recognized in the past decade as another ameba implicated in CNS infections. Cultivation of these organisms in vitro provides the basis for a better understanding of the biology of these amebas, as well as an important means of isolating and identifying them from clinical samples. Naegleria and Acanthamoeba can be cultured axenically in cell-free media or on tissue culture cells as feeder layers and in cultures with bacteria as a food source. Balamuthia, which has yet to be isolated from the environment, will not grow on bacteria. Instead, it requires tissue culture cells as feeder layers or an enriched cell-free medium. The recent identification of another ameba, Sappinia diploidea, suggests that other free-living forms may also be involved as causal agents of human infections.
INTRODUCTION
In contrast to gastroenteric infections caused by parasitic amebas as represented by Entamoeba histolytica, a relatively small number of infections are caused by free-living amebas, organisms normally found in soil or water. The genera included in this category are Naegleria, Acanthamoeba, and, more recently, Balamuthia. Representatives of these genera are pathogenic but not parasitic. They cause disease by being in the right place at the right time or by taking advantage of a host with impaired immune defenses. In other words, they are opportunistic pathogens. A number of reviews are available concerning the biology and pathogenic potential of these amebas (13, 18, 39, 40, 48, 50-52, 64, 70, 78).
Naegleria spp. as Infectious Agents
Naegleria is associated with primary amebic meningoencephalitis (PAM), a fulminating, rapidly fatal infection of the central nervous system (CNS). Naegleria fowleri is the causal agent of most PAM infections, but other species of Naegleria having pathogenic potential have been described (Naegleria australiensis and Naegleria italica). Currently, there are more than a dozen species of Naegleria that have been recognized based upon small subunit ribosomal DNA. The type habitat for N. fowleri is a natural or man-made lake, a thermally polluted body of water, or an inadequately chlorinated swimming pool where the amebas can feed upon bacteria and proliferate. With respect to humans, mostly children, teenagers, and young adults in good health are infected by swimming or washing in such waters, where amebas enter the nostrils, migrate along the olfactory nerves to the cribriform plate, and gain access to the CNS. Amebas proliferate rapidly and cause extensive damage to neural tissue (Fig. 1A). Diagnosis is difficult and is dependent upon recognition of the amebas in the cerebrospinal fluid; not infrequently, trophic amebas are dismissed as leukocytes. Because of the rapid onset, delayed diagnosis, destructive nature of the disease, and dearth of effective antimicrobial agents, death is an almost invariable consequence of infection.
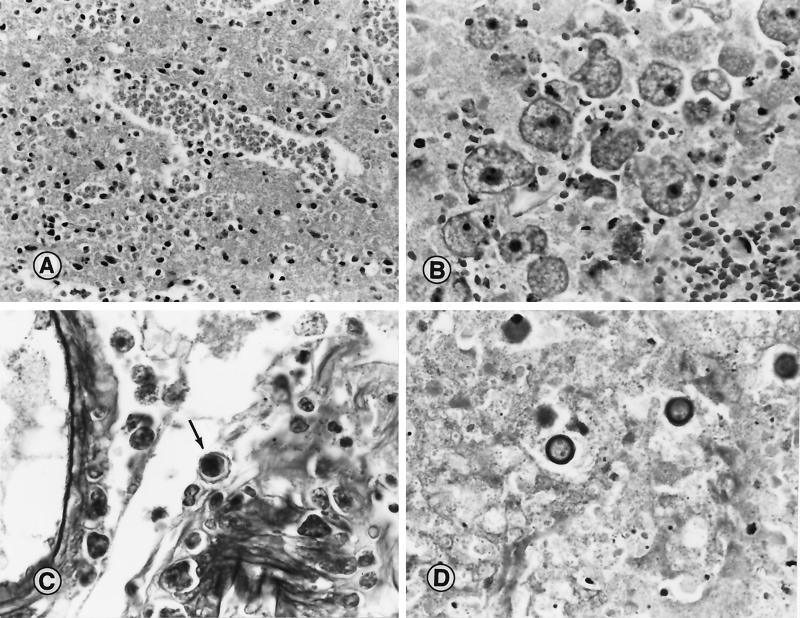
FIG. 1.
(A) Section of cerebral cortex from a patient with PAM due to N. fowleri. A large cluster of trophic amebas is seen in the brain tissue. Magnification, ×210; original magnification, ×230. (B) Section of brain tissue from a patient with Balamuthia GAE. Typical trophic amebas are seen within the necrotic CNS tissue. Magnification, ×210; original magnification, ×230. (C) Tissue section showing cysts of A. castellanii within the wall of a blood vessel and the perivascular space. Note the double wrinkled wall seen around one of the cysts (arrow). Magnification, ×370; original magnification, ×400. (D) Cysts of B. mandrillaris in necrotic brain tissue from a patient with amebic encephalitis. Magnification, ×520; original magnification, ×560. (Micrographs copyright A. J. Martinez.)
Acanthamoeba spp. as Infectious Agents
Acanthamoeba spp. also infect the CNS, causing granulomatous amebic encephalitis (GAE). Much more so than Naegleria, Acanthamoeba is ubiquitous in the environment, with amebas being widely disseminated in soil and water. Unlike the healthy individuals acquiring Naegleria infections, persons contracting Acanthamoeba infections of the CNS are compromised hosts, suffering from concurrent diseases such as AIDS (30, 71) or other conditions such as alcoholism that predispose them to opportunistic infections. The portal of entry of ameba can vary. It may be intranasal, allowing amebas to migrate directly to the CNS, or entry can be via a break in the skin or through the respiratory tract, with subsequent spread of amebas to the CNS by a hematogenous route. The disease assumes a chronic status, leading to slow deterioration. Diagnosis is most often made by postmortem examination of brain tissue (Fig. 1C).
Another major class of infection caused by Acanthamoeba spp. is amebic keratitis. This condition was first noted in individuals suffering corneal trauma due to injury to the corneal surface that became infected with amebas (43, 49). More commonly, amebic keratitis occurs in contact lens users when, due to improper maintenance and poor sanitary precautions, amebas proliferate in the ophthalmic solutions or in the lens cases and are transferred to the corneal surface when the lens is inserted. These infections are localized, and amebic spread to the CNS from corneal sites has not been reported.
Balamuthia spp. as Infectious Agents
Balamuthia is another free-living ameba that causes GAE in humans and other animals (Fig. 1B and D). First described as an isolate from the brain of a pregnant mandrill baboon that died in a zoological park (76), Balamuthia infections have been reported in individuals with compromised health status, with AIDS patients among those diagnosed (3, 30), as well as in immunocompetent individuals. While this ameba undoubtedly occurs in nature, it has been isolated only from CNS tissue of individuals or animals that have died from the infection (see Addendum in Proof). Diagnosis in most cases has been made postmortem. The portal of entry of the ameba is not known but, as with Acanthamoeba, is likely to be through the nostrils or through breaks in the skin with hematogenous spread.
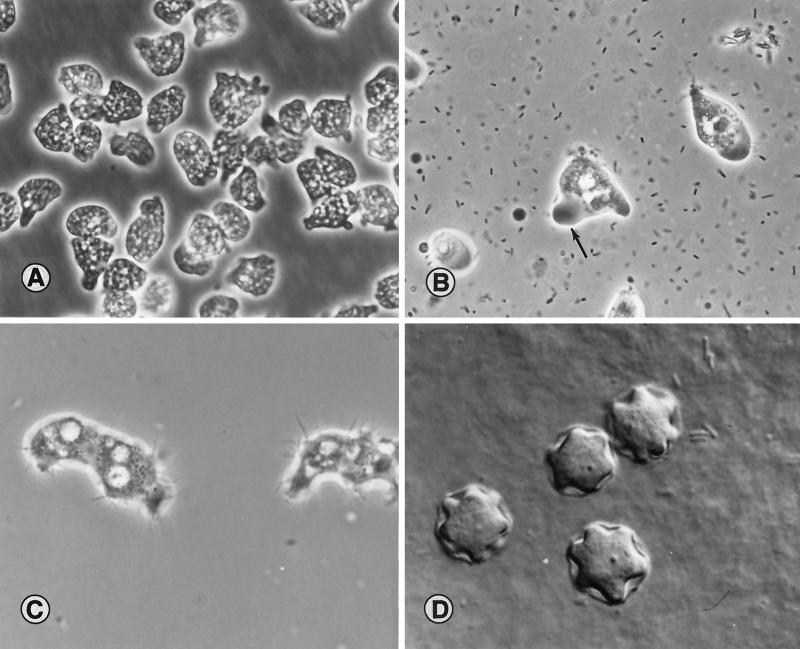
Aspects of Naegleria morphology are seen in Fig. 2A and B. Amebas growing in axenic culture fill with vacuoles containing culture medium (Fig. 2A). When feeding on bacteria, Naegleria amebas are more likely to exhibit the typical slug-like morphology of the genus, with a definite antero-posterior polarity (Fig. 2B). Trophic Acanthamoeba are recognized by the projecting pseudopods around the periphery of the ameba (Fig. 2C). Cysts of Acanthamoeba are surrounded by a thick wall and a stellate ameba within the cyst (Fig. 2D). The junction of the arm of the star and the cyst wall is the location of a pore, which is used when the cyst germinates, releasing the ameba. Balamuthia amebas, seen in Fig. 3, are less well-defined in their morphology. The shape of the amebas is dependent to a great extent on the manner of cultivation (cell-free medium or tissue culture feeder cells) and age of the culture; trophic forms show a high degree of pleomorphism.
FIG. 2.
(A) N. fowleri, from a patient with PAM, growing in axenic medium in tissue culture flasks. Amebas contain numerous fluid-filled vacuoles and appear larger than their counterparts growing on bacteria. The cultures were photographed in situ on an inverted microscope. Magnification, ×960; original magnification, ×1,000. (Micrograph copyright T. H. Dunnebacke.) (B) N. fowleri amebas in a wet-mount microscope slide. The prominent clear, ectoplasmic pseudopod is seen (arrow). Adjacent particles are bacteria. Magnification, ×960; original magnification, ×1,000. (C) Trophic A. castellanii ameba from culture. Amebas show the characteristic projecting pseudopods (acanthopodia) over the surface. Magnification, ×960; original magnification, ×1,000. (D) Interference-contrast image of cysts of Acanthamoeba sp. isolated from a human brain biopsy specimen. The cysts are on the surface of an agar plate. Magnification, ×1440; original magnification, ×1,500. (Micrographs B to D copyright G. S. Visvesvara.)
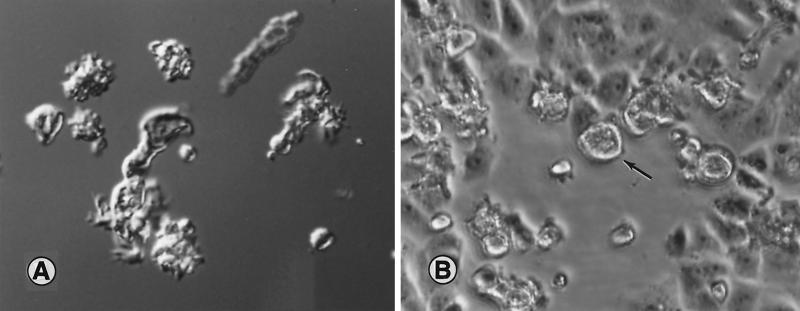
FIG. 3.
(A) Interference-contrast micrograph of trophic Balamuthia amebas in a wet-mount preparation. The amebas are pleomorphic and are likely to show varied morphology depending on whether they are in a cell-free medium or feeding on tissue culture cells. Magnification, ×380; original magnification, ×400. (B) Light micrograph of monkey kidney cell monolayer in a tissue culture flask. The open area seen in the micrograph is where the cells have been fed upon by Balamuthia amebas (arrow), about 24 h after the amebas were inoculated into the flask. The photograph was taken in situ on the stage of an inverted microscope. Magnification, ×190; original magnification, ×200.
Other Amebas as Infectious Agents
A recent report (B. B. Gelman, S. J. Rauf, R. Nader, V. Popov, J. Borkowski, G. Chaljub, H. W. Nauta, and G. S. Visvesvara, Letter, JAMA 285:2450-2451, 2001) has identified Sappinia diploidea, a soil ameba, as the cause of amebic encephalitis in a human. The report suggested that the portal of entry was the respiratory tract and that, because the patient recovered, this ameba was probably less virulent than the other amebas described above. Given the increasing numbers of immunocompromised and debilitated hosts, it is likely that more of the free-living amebas will be recognized as opportunistic infectious agents.
GROWTH IN BACTERIZED OR XENIC CULTURES
General Guidelines
Naegleria spp. and Acanthamoeba spp. can be readily cultivated on either nonnutrient agar or agar media containing low concentrations of nutrients (e.g., peptone 0.05%, yeast extract 0.05%, glucose 0.1%) in the presence of living or killed bacteria. In general, the bacteria of choice include nonmucoid strains of Klebsiella pneumoniae, Enterobacter spp. (Enterobacter aerogenes and Enterobacter cloacae), and Escherichia coli. The presence of a mucoid capsule around bacteria appears to impede phagocytosis by amebas and leads to bacterial overgrowth of the ameba population. Nutrients, particularly glucose, enhance bacterial overgrowth and inhibit ameba proliferation. A number of studies have examined the suitability of different bacteria as food sources for soil amebas (see, for example, reference 80). Balamuthia, however, will not grow with bacteria as a food source but has been grown from brain tissue by providing tissue culture cells as a feeder layer (76). Some of the early attempts at axenic growth of the free-living amebas have made use of heat-killed bacteria as an intermediate stage between xenic and axenic cultivation (56).
Acanthamoeba and Endosymbionts
For reasons that are not known, Acanthamoeba, more so than other soil amebas, is often likely to harbor endosymbiotic bacteria. While their presence is not strictly related to cultivation of amebas, bacterial endosymbionts that have been detected in Acanthamoeba isolates are of considerable interest (24). These bacteria may have a role in promoting virulence of amebas (16, 26). Acanthamoeba has been implicated as a potential host for Legionella spp., the causal agent of legionellosis and Pontiac fever; Legionella sp. has been recently isolated from Acanthamoeba taken from soil samples (59). Other pathogenic or potentially pathogenic bacteria that have been described in associations with Acanthamoeba spp. are Mycobacterium avium (72), Afipia felis (B. La Scola and D. Raoult, Letter, Lancet 353:1330, 1999), Listeria monocytogenes (47), Burkholderia pseudomallei (37), E. coli serotype O157 (8), Chlamydia spp. and Chlamydia-like bacteria (23, 25), and Vibrio cholerae (74).
GROWTH IN AXENIC CULTURES
General Guidelines
To various degrees, free-living amebas can be readily established in axenic culture from initially bacterized cultures by providing an enriched nutrient medium with antibiotics (penicillin-streptomycin and gentamicin) added to kill off contaminating bacteria. The basic nutrient medium that is used for Acanthamoeba typically contains peptone, yeast extract, and glucose, in concentrations generally higher than those used for growth in bacterized cultures (e.g., peptone 2.0%, yeast extract 0.5%, glucose 0.5%). For nonpathogenic Naegleria spp., the nutrient medium contains peptone and yeast extract, with or without glucose, with liver extract and calf serum added as additional supplements. Balamuthia is a more fastidious organism and requires a heavily supplemented basic medium (66). The three genera of amebas can be grown axenically in the presence of tissue culture monolayers. In each of these instances, the tissue culture cells provide a feeder layer for the actively phagocytic amebas—in reality a predator-prey relationship. It is worth noting that potentially pathogenic Acanthamoeba organisms have been isolated from tissue cultures, where they are present as contaminants. In the course of growing poliomyelitis virus in monkey kidney cell cultures for vaccine preparation, Culbertson et al. (18, 19) observed areas of tissue culture monolayer destruction and subsequently isolated an ameba (later named Acanthamoeba culbertsoni) from dead and dying mice inoculated with culture supernatant.
Naegleria spp.
Long known as a nonpathogenic species with a life cycle comprised of a trophic ameboid stage, a nonfeeding and nondividing flagellate stage, and a dormant cyst stage, Naegleria gruberi amebas were established axenically in an enriched medium consisting of peptone or Proteose Peptone, yeast extract, liver concentrate (4), and additional supplements. These supplements included calf serum as Balamuth medium (4), killed bacteria (55, 60, 61, 63), chicken embryo extract (65), folic acid (46, 54), or hemin (7). It was somewhat surprising, therefore, to find that the pathogenic isolates, once they were recognized as such, required a simpler nutrient medium based on peptone and calf serum (10, 13, 57). Ultimately, pathogenic isolates were determined to be members of a new species, N. fowleri, with less demanding nutritional requirements for growth (Fig. 2A). For example, Cerva (10, 11) grew his isolates in a medium made up of Casitone and serum, a medium that would not support growth of N. gruberi. Willaert (85) used Cerva's original formulation and one supplemented with folic acid and biotin for growth of Naegleria for immunoelectrophoretic studies. A medium (Table 1) developed by Chang (13-15) was originally formulated for growth of nematodes, and while it supported growth of pathogenic isolates, it was not successful in growing the nonpathogenic N. gruberi. The medium consisted of casein, glucose, fetal calf serum, and fresh yeast extract (14). This medium was used successfully by De Jonckheere (21) for cultivation of Naegleria spp., pathogenic as well as nonpathogenic strains. Chang (13) also experimented with sheep blood and liver extract as additives to this basal medium. Another medium that was successful in supporting growth of pathogenic Naegleria was developed by Nelson (cited in references 83 and 86) and consisted of glucose (1.0 g), liver infusion or liver digest (2.0 g), fetal calf serum (50.0 ml), and distilled water (450.0 ml) (pH ≈ 6.5). (The medium was modified from its original formulation because some of the components are unavailable.) Haight and John (32) later compared growth of N. fowleri in Balamuth, Chang, Cerva, and Nelson media in agitated and unagitated cultures, concluding that optimal growth of amebas occurred with agitated cultures in Nelson medium. They also found variation in cell yields among 10 different isolates of N. fowleri growing in Nelson medium (32). John (40) reported cell yields of 3 × 109 amebas/ml were achieved with a generation time of 5.5 h. Cline et al. (17) developed a modification of Balamuth and Nelson media (40, 84, 86) that would support growth of both N. gruberi and N. fowleri, giving cell yields of about 106 amebas/ml. This medium contained Proteose Peptone, yeast extract, liver digest, and glucose and was supplemented with both calf serum and hemin (17, 50).
TABLE 1.
Undefined medium SCGYEM for cultivation of N. fowleria
| Component | Amt or concn |
|---|---|
| Organic | |
| Isoelectric casein | 10.0 g |
| Glucose | 2.5 g |
| Yeast extract | 5.0 g |
| Fetal calf serum | 100.0 ml |
| Penicillin-streptomycin | 200.0 μg/ml (each) |
| Distilled water | 900.0 ml |
| Buffering | |
| Na2HPO4 | 1.325 g |
| KH2PO4 | 0.8 g |
A partially defined medium based on Willaert's medium (84) was formulated by Cursons et al. (20). This medium contained Casitone, glucose, rutin, l-methionine, l-histidine, folic acid, thiamine·HCl, vitamin B12, and hemin; generation times of both pathogenic and nonpathogenic isolates of Naegleria were about 15 h in this medium (20). Nerad et al. (58) developed a chemically defined medium (Table 2) that would support the growth of N. fowleri, Naegleria lovaniensis, and, with a 10-fold increase in the metals and several other changes, the more nutritionally exacting N. australiensis. They obtained cell yields of better than 106 amebas/ml with generation times of about 20 h.
TABLE 2.
Defined medium for pathogenic Naegleria spp.a
| Component | Concn (mg/liter) |
|---|---|
| Amino acids | |
| l-Alanine | 460.0 |
| l-Asparagine | 600.0 |
| l-Aspartic acid | 1,170.0 |
| l-Arginine | 940.0 |
| l-Cysteine · HCl | 40.0 |
| l-Glutamic acid | 1,865.0 |
| l-Glutamine | 600.0 |
| Glycine | 2,705.0 |
| l-Histidine | 200.0 |
| l-Isoleucine | 525.0 |
| l-Leucine | 760.0 |
| l-Lysine · HCl | 880.0 |
| l-Methionine | 970.0 |
| l-Phenylalanine | 480.0 |
| l-Proline | 1,200.0 |
| dl-Serine | 600.0 |
| l-Threonine | 530.0 |
| l-Tryptophan | 140.0 |
| l-Tyrosine | 180.0 |
| l-Valine | 700.0 |
| Nucleic acid precursors | |
| Adenine | 10.0 |
| Adenosine 3′,2′-monophosphoric acid | 15.0 |
| Guanosine | 10.0 |
| Guanosine 3′,2′-monophosphoric acid | 10.0 |
| Hypoxanthine | 10.0 |
| Cytosine · H2O | 10.0 |
| Cytidine 3′,2′-monophosphoric acid | 25.0 |
| Thymine | 10.0 |
| Thymidine | 16.0 |
| Uracil | 10.0 |
| Uridine 3′,2′-monophosphoric acid | 15.0 |
| Vitamins | |
| p-Aminobenzoic acid | 1.0 |
| Biopterin | 0.01 |
| d-Biotin | 0.01 |
| Choline chloride | 1.0 |
| Folic acid | 1.0 |
| Hemin | 10.0 |
| i-Inositol | 1.0 |
| Nicotinamide | 1.0 |
| Ca pantothenate | 1.0 |
| Pyridoxal · HCl | 1.0 |
| Pyridoxamine · diHCI | 1.0 |
| Riboflavin | 1.0 |
| Thiamine | 1.0 |
| dl-Thioctic acid | 0.5 |
| B12 | 0.001 |
| Salts and metals | |
| CaCl2 | 40.0 |
| MgSO4 · 7H2O | 800.0 |
| KH2PO4 | 362.0 |
| Na2HPO4 | 500.0 |
| FeCl2 · 4H2O | 0.0105 |
| FeSO4 · 7H2O | 0.0105 |
| MnSO4 · H2O | 0.0095 |
| ZnSO4 · 7H2O | 0.0220 |
| (NH4)Mo7O24 · 4H2O | 0.0036 |
| CuSO4 · 5H2O | 0.0016 |
| NH4VO3 | 0.00046 |
| CoSO4 · 7H2O | 0.00048 |
| H3BO3 | 0.00057 |
| NiSO4 · 6H2O | 0.00045 |
| CrK(SO4) · 12H2O | 0.00096 |
| BaCl2 · 2H2O | 0.00050 |
| Other | |
| d-Glucose | 8,000.0 |
| Na citrate | 100.0 |
| l-Ornithine.HCl | 40.0 |
| Putrescine.diHCl | 1.0 |
| Orotic acid | 20.0 |
| dl-Citruline | 8.0 |
| 5-Sulfosalicylic acid | 1.0 |
This defined medium was described previously by Nerad et al. (58).
Haight and John (33) found quantitative differences in growth of N. fowleri with 17 different types of sera added to Nelson medium. Calf, pig, monkey, newborn calf, and dialyzed calf sera gave growth of ca. 106 amebas/ml, while human and fetal calf sera, cerebrospinal fluid, and hemin gave less growth of 5 × 104 to 1 × 105 amebas/ml. John (40) suggested that the presence of iron in lakes and streams may be a factor in enhancing growth of N. fowleri. Enrichment of the medium with various lipid components at 100 μg/ml improved growth of amebas over the calf serum control (33).
Several studies have examined the relationship between in vitro cultivation and loss of virulence of N. fowleri. Hu et al. (34) found that loss of virulence correlated with a loss of pathogenic protein synthesis patterns in axenically grown amebas. Induction of gene activity correlating with virulence occurred when amebas fed upon tissue culture cells but not when they fed upon bacteria or when they were grown in axenic culture (35). Cultivation of amebas in the presence of cholesterol (100 μg/ml) over a 6-month period resulted in a loss of virulence (42). John (40) noted that virulence of N. fowleri is affected by growth temperature (30 to 37°C produced more virulent amebas), growth phase (late logarithmic to early stationary phase amebas were more virulent for mice), and strain.
Although N. gruberi is not a pathogenic ameba, it is of interest to contrast its nutritional requirements with those of pathogenic isolates. Growth parameters for N. gruberi growing in rotary cultures in an enriched medium were defined by Weik and John (84). They reported a biphasic pattern of logarithmic growth, with generation times of 7 and 19 h during the two phases. Cell yields were 5 × 106 amebas/ml.
Fulton and coworkers (27) prepared a semidefined medium and, later (28), a chemically defined medium that would support the growth of a variant strain of N. gruberi with a doubling time of 8 to 19 h. The medium, however, would not support growth of the parent stock of the variant. Initially, peptone was replaced by l-methionine and a serum fraction was required. The defined medium contained 14 amino acids of which 11 were essential, six vitamins, glucose, hematin, guanosine, uracil, glycerol, and sodium pyruvate. This medium was pared down to eliminate components that were not essential for growth, with a consequent drop in yield from 5 × 106 to about 106 amebas/ml and a doubling time of 12 to 15 h (28).
Acanthamoeba spp.
Unlike the situation with Naegleria amebas, where there appears to be a difference in nutritional requirements between pathogens and nonpathogens, sharp distinctions are not evident with Acanthamoeba spp. For the most part, pathogenic and nonpathogenic species of Acanthamoeba grow well in the same media.
Acanthamoeba are better able to tolerate a range of growth conditions than Naegleria spp. They readily survive over a wide range of osmolarities, both in vivo and in vitro, having been isolated from marine and fresh waters, from tissue culture media where they occur as contaminants, and from soil. They appear to be nutritionally less exacting than Naegleria amebas, in that they readily go from bacterized to axenic cultures without the prolonged adaptation or selection that often occurs with Naegleria.
A basic medium that supports growth of Acanthamoeba spp. consists of Proteose Peptone or peptone, yeast extract, and glucose (PPYG or PYG, respectively). Neff (56) isolated a widely used strain of Acanthamoeba castellanii and grew it axenically in Proteose Peptone (0.75%), yeast extract (0.75%), and glucose (1.5%). What varies in most formulations for growth of Acanthamoeba are the concentrations of these components. Jensen et al. (38) used the basic medium components with rotary agitation to obtain cell yields of A. castellanii of 3 × 107 amebas/ml, with a 6-h generation time.
Defined media have been devised for several species. Adam (1) prepared a medium for the Neff strain containing 18 amino acids, acetate as a carbon source, and the vitamins B12 and thiamine. Working with several species, including the Neff strain, Band (5) later demonstrated a need for biotin. Subsequently, Band (6) formulated a medium with seven amino acids; glucose or sodium acetate as a carbon source; and the vitamins B12, thiamine, and biotin for Hartmannella (Acanthamoeba) rhysodes. Adam and Blewett (2) compared carbohydrate utilization of different strains of A. castellanii in a basal medium containing 5 (arginine, leucine, isoleucine, methionine, and valine) of the 10 essential amino acids and found variations in use of sucrose, melibiose, mannitol, and raffinose as carbon energy sources. These defined media supported growth, but often with extended generation times from 40 to >60 h. In order to produce a higher growth rate, Byers et al. (9) formulated a defined medium (Table 3) based on these earlier studies. Their media, DGM-21A and DGM-21B, gave generation times of about 13 and 16 h, respectively, and cell yields of 2 × 106 to 3 × 106 amebas/ml. The two media differed in vitamin content and salts, with DGM-21B lacking four vitamins (ascorbic, folic, and thioctic acids and riboflavin) and four salts present in DGM-21A. They reported that glucose was necessary for enhancing growth rate, while acetate, which was present in the two media, had a minor affect on growth rate. Omission of the carbon sources induced encystment of the amebas (9). (Griffiths and Hughes [31], among others, have explored methods to induce encystment, for example, by suspending trophic amebas in a MgCl2 solution.) The medium of Byers et al. (9) has been used for growing a variety of Acanthamoeba species and strains for development of a genus- and subgenus-specific fluorescent oligonucleotide probe (73). Ingalls and Brent (36) formulated a defined medium for Acanthamoeba polyphaga containing 11 amino acids; the vitamins B12, biotin, and thiamine; and glucose. Acetate could not substitute for glucose as a carbon source.
TABLE 3.
Defined medium DGM-21A for Acanthamoeba spp.a
| Component | Concn |
|---|---|
| Amino acids (g/liter) | |
| l-Alanine | 0.2 |
| l-Asparagine | 0.5 |
| l-Aspartic acid | 0.3 |
| l-Arginine.HCl | 1.0 |
| l-Cysteine | 0.2 |
| l-Cystine | 0.1 |
| l-Glutamic acid | 0.5 |
| l-Glutamine | 0.5 |
| Glycine | 1.5 |
| l-Histidine.HCl | 0.2 |
| l-Isoleucine | 0.6 |
| l-Leucine | 0.9 |
| l-Lysine · HCl | 1.0 |
| l-Methionine | 0.3 |
| dl-Phenylalanine | 0.9 |
| l-Proline | 0.8 |
| l-Serine | 0.2 |
| l-Threonine | 0.5 |
| l-Tryptophan | 0.2 |
| l-Tyrosine | 0.2 |
| l-Valine | 0.7 |
| Vitamins (mg/liter) | |
| Biotin | 0.3 |
| Folic acid | 0.2 |
| Ascorbic acid | 2.0 |
| Riboflavin | 1.0 |
| Thiamine · HCl | 0.01 |
| Thioctic (lipoic) acid | 0.4 |
| B12 | 0.01 |
| Salts (g/liter) | |
| CaCl2 · 2H2O | 0.0074 |
| MgSO4 · 7H2O | 0.25 |
| KH2PO4 | 0.27 |
| NaHCO3b | 0.25 |
| NH4Cl | 0.0005 |
| NH4 formate | 0.0006 |
| FeSO4 · 7H2O | 0.009 |
| Trace elements (mg/liter) | |
| ZnSO4 · 7H2O | 1.00 |
| MnCl2 · 4H2O | 2.30 |
| (NH4)Mo7O24.4H2O | 0.40 |
| CoCl2 | 0.017 |
| CuSO4 | 0.0033 |
| H3BO3 | 0.10 |
| Na2EDTA | 0.01 |
| Other components (g/liter) | |
| Glucose | 15.0 |
| Na acetate | 2.5 |
| Ethanolb | 0.5 |
Medium DGM-21A was described previously by Byers et al. (9). Individual groupings of components were prepared as stock solutions. pH before autoclaving, 6.5.
Introduced from vitamin stock solution.
Weekers et al. (82) used laboratory fermentors with aeration to scale up growth of A. castellanii, in a Proteose Peptone and glucose medium (10.5 liters). Growth was monitored over a time period of 20 to 30 days, with generation times during the exponential phase varying from 67 to 90 h, depending on the degree of buffering in the medium. Cell yields were about 4 × 105 amebas/ml, or 3 g of cells (wet weight). They found a buildup of ammonia and a concomitant pH increase (from 6.5 to >7), although neither the increasing ammonia concentration (to ca. 5 mM) nor glucose depletion (12 to 19%) was responsible for terminating exponential growth. They invoked Pigon's hypothesis (62) of a growth-inhibitory exudate produced in Acanthamoeba cultures to explain termination of exponential growth and onset of the death phase in cultures. Weekers and Vogels (81) used a chemostat for axenic cultivation of A. castellanii with cell yields of about 3 × 106 amebas/ml with a generation time of about 25 h.
In recent years, a large number of strains and species of Acanthamoeba have been isolated from clinical samples. Many of these can be grown on the basic PPYG medium used for the free-living isolates. De Jonckheere (22) used the ability of Acanthamoeba spp. to grow in a medium of casein, glucose, and yeast extract at 37°C as an indicator of virulence. Some strains and possibly new species, however, required a richer medium consisting of the PPYG base supplemented with calf serum and a vitamin mixture (67). Even at that, growth of these strains did not match that of the soil and water isolates of nonpathogenic Acanthamoeba. Although isolated from human hosts, a number of these amebas grew better at 30°C than at 37°C. Generation times ranged from about 10 to >40 h (67).
Shukla et al. (68), working with A. culbertsoni, a pathogenic species isolated from tissue culture, tested a variety of peptones, Proteose Peptone, protein hydrolysates, and vitamin supplements, reporting yields of 1 × 107 to 2 × 107 amebas/ml with Proteose Peptone, yeast extract, and glucose. In a later paper, Shukla et al. (69) presented a defined medium for A. culbertsoni that gave approximately the same cell yields as their earlier media. The optimal medium (Table 4) contained 11 amino acids; the vitamins biotin, B12, and thiamine; and glucose and sodium citrate. Generation time in this medium was about 10 h, with cell yields of 2 × 107 to 3 × 107 amebas/ml. Another variation contained seven amino acids (omitting histidine, lysine, threonine, and tryptophan); cell yield was somewhat lower, and the generation time extended to about 23 h.
TABLE 4.
Defined medium M-11 for A. culbertsonia
| Component | Concn |
|---|---|
| Amino acids (mg/liter) | |
| l-Arginine · HCl | 825.0 |
| Glycine | 1,500.0 |
| l-Histidine · HCl | 160.0 |
| l-Isoleucine | 600.0 |
| l-Leucine | 900.0 |
| l-Lysine · HCl | 1,250.0 |
| l-Methionine | 300.0 |
| l-Phenylalanine | 900.0 |
| l-Threonine | 500.0 |
| l-Tryptophan | 200.0 |
| l-Valine | 700.0 |
| Other (g/liter) | |
| Glucose | 1.8 |
| Na citrate | 1.0 |
| Vitamins (mg/liter) | |
| Biotin | 0.25 |
| B12 | 0.00125 |
| Thiamine · HCl | 1.25 |
| Salts (g/liter) | |
| CaCl2 · 2H2O | 0.0588 |
| MgSO4 · 7H2O | 0.985 |
| KH2PO4 | 0.340 |
| Na2HPO4 · 2H2O | 0.445 |
| (NH4)2SO4 · FeSO4.6H2O | 0.0196 |
| Trace elements (mg/liter) | |
| MnCl2 · 4H2O | 2.3 |
| ZnSO4 · 7H2O | 1.0 |
| (NH4) Mo7O24.4H2O | 0.4 |
| CuSO4 · 5H2O | 0.0033 |
| CoCl2 | 0.017 |
| H3BO3 | 0.1 |
| Na2EDTA | 0.01 |
Medium M-11 was described previously by Shukla et al. (69). Initial pH, 6.5.
Balamuthia mandrillaris
The ameba B. mandrillaris was only recently described (76), the type species having been isolated from the brain of a mandrill baboon (79). Additional isolates have been obtained postmortem from humans in compromised health (a chronic alcoholic suffering seizures and an amputee with skin abscesses, both elderly), as well as from apparently immunocompetent humans (mostly young children). Another isolate in culture was from a horse (44). Attempts to culture these amebas on bacteria, either living or dead, were unsuccessful. The amebas were isolated from samples of brain tissue using monolayers of African green monkey kidney cells, upon which the amebas fed and proliferated (Fig. 3B). Schuster and Visvesvara (66) established these strains, including the baboon isolate, in an enriched axenic medium (BM-3) containing Biosate peptone, Torula RNA, and yeast and liver extracts supplemented with calf serum, hemin, lipid mixture, vitamin mixture, nonessential amino acids, taurine, and glucose (Table 5; Fig. 3A). Generation times were 20 to 28 h and yields were about 106 amebas/ml, with differences between strains. Michel and Janitschke (53) developed cell-free cultures of the baboon isolate using a modified Chang medium (13, 21) for Naegleria. The medium was prepared at a relatively high salt concentration (8‰) using sea salt. Their generation times were 32 to 36 h, with cell yields of about 8 × 105 amebas/ml.
TABLE 5.
Complex growth medium BM-3 for B. mandrillarisa
| Component | Amt |
|---|---|
| Basal medium | |
| Biosate peptone | 2.0 g |
| Yeast extract | 2.0 g |
| Torula yeast RNA | 0.5 g |
| Double-distilled water | 345.0 ml |
| Supplements | |
| Hanks' balanced salts, 10× | 34.0 ml |
| 5% Liver digest in Hanks' salts | 100.0 ml |
| MEM vitamin mixture, 100× | 5.0 ml |
| Lipid mixture, 1,000× (Gibco) | 0.5 ml |
| MEM nonessential amino acids, 100× | 5.0 ml |
| 10% Glucose | 5.0 ml |
| Hemin, 2 mg/ml | 0.5 ml |
| 0.5% Taurine | 5.0 ml |
| Calf serum | 50.0 ml |
Medium BM-3 was described previously in reference 66. pH adjusted to 7.2.
Unsuccessful attempts have been made to isolate Balamuthia from environmental water and soil samples. The ameba grows slowly in culture (generation time of ≥25 h), and probably does not compete effectively against other soil amebas, soil fungi, or accompanying bacteria. John and Howard (41) have isolated pathogenic (for mice) leptomyxid amebas from pond water using nonnutrient agar and E. coli as a food source. Although they have a superficial similarity to Balamuthia amebas, the leptomyxids were different from Balamuthia as indicated by immunofluorescence staining patterns. Thus, isolation and identification of Balamuthia from environmental samples remains a challenge to an understanding of the ecological niche and mode of infection of this newest protozoal agent of amebic meningoencephalitis.
CULTURING AMEBAS AS A DIAGNOSTIC TOOL
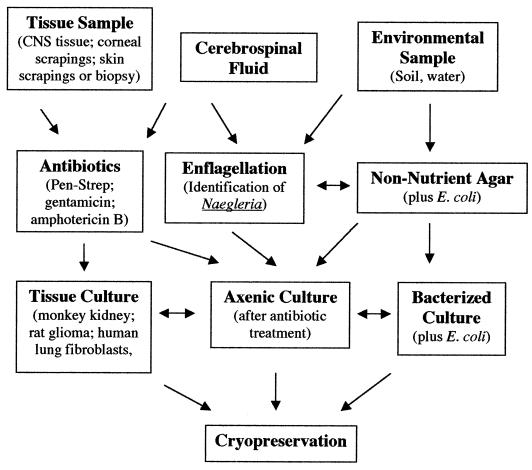
This section deals with techniques for isolating amebas from clinical or environmental samples. This is often the most readily available procedure for confirming an amebic infection and identifying the ameba that caused it. Cerva (12) has summarized methods and materials needed for isolation and cultivation of Naegleria amebas from clinical specimens, and Visvesvara (75) has dealt with techniques applicable to all three genera of opportunistic free-living amebas. A general flowchart for isolating amebas from clinical and environmental samples is presented in Fig. 4.
FIG. 4.
The chart presents a general scheme for isolation of free-living pathogenic amebas from clinical samples or from environmental sources. The goal is to establish the amebas either in an axenic culture (cell-free growth medium), with a tissue culture feeder layer, or in a xenic culture with a suitable bacterial strain as a food source. Although two different tissue culture types are noted (monkey kidney or rat glioma cells), other cell types (e.g., human lung fibroblasts) are suitable as feeder layers. When possible, cryopreservation of ameba strains is recommended as an alternative to regular subculturing and as a backup in the event of loss of a culture.
Clinical Samples
N. fowleri, the cause of PAM, is most readily isolated from CSF of the patient. It can also be isolated from brain tissue, particularly the olfactory lobes in the case of biopsy or autopsy. Acanthamoeba is more likely to be isolated from brain tissue or skin lesions (tissue or swab specimens). Strains that cause amebic keratitis are isolated from corneal scrapings. Balamuthia has been isolated from brain tissue, upon either biopsy or, more typically, autopsy. Acanthamoeba is generally not found in CSF, and Balamuthia has not been recovered from CSF.
The procedure for growing Naegleria and Acanthamoeba from clinical specimens is the use of a nonnutrient agar spread with E. coli or some other nonmucoid bacteria (40, 75). Amebas begin feeding on bacteria and soon grow to cover the agar surface in 1 to 2 days at 37°C. The presence of the amebas can be ascertained by examining the agar surface using an inverted microscope or with a conventional microscope by inverting the plate on the stage and focusing through the agar using a 10× objective.
Naegleria amebas, as seen in a wet-mount preparation on a microscope slide, have a characteristic limacine (slug-like) pattern of locomotion (Fig. 2B), with a clear, ectoplasmic pseudopod at the anterior end of the ameba. This characteristic appearance is of help in identifying Naegleria in CSF but is difficult to observe in amebas on agar plates. As an additional aid in identification, Naegleria amebas transform into flagellates when suspended in distilled water (75). To induce enflagellation, CSF containing presumptive Naegleria amebas can be diluted directly and suspended in 1 ml of distilled water. Flagellates, usually with two anterior flagella, should appear in <1 h. Not all amebas transform and, once transformed, revert to the ameboid stage. Visvesvara (75) estimates that 30 to 50% transformation occurs with isolates of N. fowleri. John (40), in reviewing published reports, gave a range of 0 to 55% enflagellation for different isolates of N. fowleri. If dealing with amebas on an agar surface, the amebas can be scraped off the agar with a bacteriological loop and transferred to 1 ml of distilled water to induce the same type of enflagellation response.
Balamuthia does not feed on bacteria, requiring the use of a tissue culture monolayer (such as monkey kidney [ATCC CRL 1586] or rat glioma [ATCC CCL 107] cells) as a feeder layer (Fig. 3). Small macerated pieces of brain tissue are introduced directly into the culture flasks, and the flasks are checked for evidence of ameba growth on the cell layer using an inverted microscope. Unlike the other amebas, Balamuthia is slow to adapt to culture conditions, and several weeks may be required before the amebas begin proliferating (76). Once adapted, however, Balamuthia grows well on the tissue culture monolayers. It is usually necessary to add antibiotics to the flask to prevent bacterial and fungal contamination, either from the brain specimen itself or from the laboratory environment. Penicillin-streptomycin (at 100 U/ml and 100 μg/ml, respectively) or gentamicin (100 μg/ml) is commonly used to prevent bacterial growth, and amphotericin B (Fungizone) is used to prevent fungal growth. Except for amphotericin B, these antimicrobials do not adversely affect ameba growth, and higher concentrations may be used as needed to eliminate persistent contaminants. Naegleria and, to a lesser extent, Balamuthia are sensitive to amphotericin B, which should be used sparingly (<1 μg/ml) or not at all in these cultures. The technique outlined for Balamuthia can also be used for Naegleria and Acanthamoeba, which, like Balamuthia, actively feed upon tissue culture cells.
Cultures of Naegleria and Acanthamoeba can be maintained with bacteria as a food source (xenic cultures) or, by addition of antibiotics to destroy bacteria, can be established in cell-free (axenic) cultures (Fig. 2A and B). Appropriate media for axenic cultivation are described above in the section on axenic cultivation. Balamuthia can be maintained on tissue culture cells or can be established in a cell-free medium (Fig. 3).
In attempting isolation of amebas from clinical materials, it is helpful to have positive control cultures in parallel as a check on the methodology and ability to recognize amebas in the samples. Table 6 lists several strains of free-living amebas available from the American Type Culture Collection (ATCC) that can be used as controls. Some of these are nonpathogenic isolates (A. castellanii, the Castellani and Neff strains; N. gruberi EG strain), while others are isolates from patients with amebic encephalitis or amebic keratitis.
TABLE 6.
Selected species of free-living pathogenic and nonpathogenic amebas available from the ATCCa
| Species | ATCC accession no. | Comments | Pathogenicity |
|---|---|---|---|
| A. castellanii | 30010 | Classic strain (Neff isolate) | Nonpathogenic |
| A. castellanii | 30011 | Classic strain (Castellani isolate) | Nonpathogenic |
| A. culbertsoni | 30171 | Tissue culture contaminant | Pathogenic |
| A. polyphaga | 30461 | Corneal isolate (Jones et al.) | Pathogenic |
| N. fowleri | 30174 | Isolated from human CSF (HB, strain) | Class III pathogen |
| N. gruberi | 30133 | Soil isolate (EG) | Nonpathogenic |
| B. mandrillaris | 50209 | Original isolate from mandrill baboon | Class III pathogen |
The ATCC can be accessed via the Internet at www.atcc.org.
Subculturing Schedules
Table 7 presents suggested time intervals for subculturing amebas. Culture schedules can vary with temperature, bacterial growth, and the presence of cysts in the culture. The time intervals described in the table are for stock cultures. Cultures being used in experiments would require more-frequent transfers (days instead of weeks) as, for example, the use of logarithmic phase cultures in growth experiments, etc. Amebas, once they encyst, tend to survive over longer culture intervals. Naegleria cultures, however, appear more sensitive than Acanthamoeba and Balamuthia to culture conditions (pH changes and waste accumulation), and if not transferred regularly, cultures may abruptly “crash.” Some cultured strains lose their ability to form cysts after prolonged subculturing. Pathogenic amebas, which grow optimally at 37°C, will also grow at 30°C and will even grow at room temperature, but at a lower rate. Keeping cultures at ∼20°C will prolong the intervals at which cultures have to be transferred.
TABLE 7.
Recommended subculturing schedules for stock cultures of free-living amebas
| Ameba culture | Type of culture | Culture schedule (wk) at:
|
|||
|---|---|---|---|---|---|
| ∼20°C | Room temperature (∼25°C) | 30°C | 37°C | ||
| Acanthamoeba spp. | Bacterized | 4 | 3-4 | 1-2 | 1 |
| Axenic | 4 | 3-4 | 2-3 | 2 | |
| Naegleria spp. | Bacterized | 4 | 3-4 | 2 | 1-2 |
| Axenic | 2 | 1-2 | 1-2 | 1 | |
| B. mandrillaris | Axenic | 3 | 2-3 | 2-3 | 2 |
| With tissue culture cells | 1 | 1 | |||
Balamuthia amebas will remain viable in cell-free cultures for weeks, but it is best to transfer them at ∼2-week intervals. For cultures maintained on tissue culture cell monolayers, cultures have to be fed or transferred at approximately weekly intervals since the amebas will rapidly consume the available food supply.
Cryopreservation of Strains
In lieu of maintaining cultures by routine subculturing, storage of cultures by freezing is an alternative. Harvested amebas (approximately 2 × 106/ml) in growth medium are mixed 1:1 with cell-culture-grade 20% dimethyl sulfoxide (DMSO) to give a final concentration of DMSO of 10% (G. S. Visvesvara, personal communication). This mixture is frozen and subsequently stored in liquid nitrogen. John (40) used a mixture of 12% DMSO, 20% heat-inactivated serum, 10% glucose, growth medium, and 106 amebas/ml in the exponential growth phase for cryopreservation of N. fowleri. He reported that viability of frozen cultures was 38% after 6 months, down from 64% at 1 month.
Testing for Pathogenic Potential
If an ameba isolate is from a clinical specimen, it is clearly pathogenic (brain tissue) or potentially pathogenic (corneal isolates). Environmental samples of amebas can be either pathogenic or nonpathogenic. Ability of these isolates to survive and grow at 37°C or higher temperatures is an indication that the strain could also grow at mammalian body temperature. Mouse inoculation, usually intranasal with a suspension of amebas to be tested, is a relatively reliable way of determining pathogenicity of an isolate (Fig. 5). Young mice are more likely to develop infections than older mice (40). Intracerebral inoculation is another route (12). Death of mice can occur within <1 to 4 weeks, depending upon the ameba, the dose, and the virulence of a particular strain (77). Subculturing of a strain can lead to loss of virulence for mice; conversely, virulence can be restored by mouse passage or even subculturing on tissue culture cells.
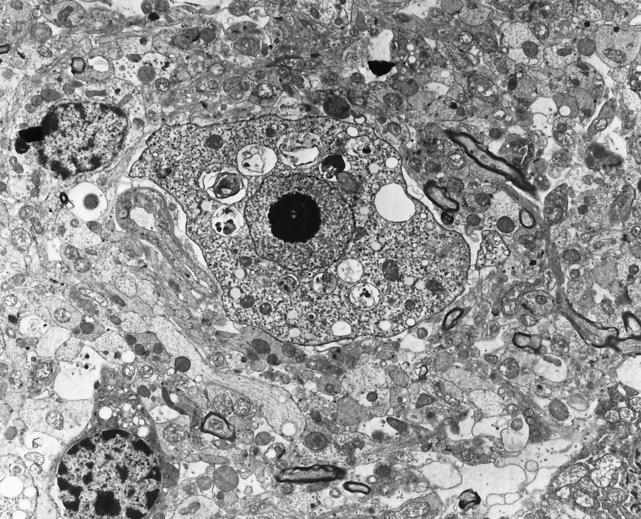
FIG. 5.
Transmission electron micrograph of N. fowleri in brain tissue of a mouse that had been experimentally inoculated with a suspension of the amebas. The characteristic nuclear morphology of the ameba can be seen as a large nucleus with a centrally located nucleolus (vesicular nucleus). Original magnification, ×7,500. (Micrograph copyright A. J. Martinez.)
Specimen Handling for Cultivation
Freezing of clinical specimens should be avoided, particularly with samples (CSF and brain tissue) that might contain Naegleria, and specimens should be processed as quickly as possible. All three amebas encyst, but Naegleria cysts are more fragile than those of Acanthamoeba and Balamuthia. Acanthamoeba and Balamuthia, however, have been isolated from frozen brain tissue (G. S. Visvesvara, personal communication). The latter two amebas encyst within brain tissue, but Naegleria does not (Fig. 1C and D).
Laboratory Safety
Another consideration in handling samples and cultures of pathogenic free-living amebas is the safety of laboratory personnel working with the cultures. There have been no reported infections with free-living amebas of laboratory workers, either from specimen samples or from cultures of organisms. Except for extreme carelessness, it is unlikely that aerosols would be produced within the laboratory during routine handling of materials and cultures. Care should be taken by personnel to avoid getting culture materials on the skin or in open cuts or abrasions. Gloves should be worn when handling materials, and surgical masks may be used, particularly when performing animal inoculation studies. If available, it is advisable to handle cultures in a biological safety cabinet. This not only protects personnel from chance contact with amebas but also minimizes the chance of contamination of cultures by airborne molds, bacteria, and yeasts. Laboratory personnel wearing contact lenses should be instructed about precautions in working with Acanthamoeba spp., since corneal infections can be caused by any number of different species, even those without a history of pathogenesis.
Environmental Samples
In attempting to establish the source of an amebic infection, it is often necessary to screen environmental samples for the presence of amebas. The most commonly examined samples are soil (garden soils or soils in flower pots) or water (from lakes, ponds, home aquaria, hot tubs, humidifiers, heating and ventilating air conditioning units, and eye wash irrigation stations, etc.). For instances in which Acanthamoeba keratitis may be related to contact lens wear, the lens case may be examined and cultured for amebas. Unlike clinical specimens, these samples will almost certainly contain bacteria and fungi, other protozoa, and, perhaps, metazoa (nematode worms or aquatic arthropods). The difficulty in working with such samples is to encourage growth of the amebas but inhibit the growth of the other soil organisms.
The basic procedure is the use of nonnutrient agar with E. coli as a food source, as described for clinical specimens. For environmental samples, use of enriched media suitable for axenic cultures should be avoided as they will stimulate heavy growth of contaminating bacteria and fungi and prevent ameba growth. Once the amebas are established in a bacterized culture, antibiotics can be added to kill off bacteria and the amebas can be transferred to the appropriate axenic medium.
Both Naegleria and Acanthamoeba have been isolated from a variety of environmental samples by employing the techniques described. Balamuthia, however, has yet to be isolated from the environment, and its niche in nature remains to be defined.
CONCLUSIONS
Free-living amebas are recognized as opportunistic agents of disease. Cultures of these amebas are useful in defining their basic nutritional requirements, testing efficacy of antimicrobial agents for therapeutic value, understanding their phylogenetic relationships, and perfecting diagnostic techniques for rapid identification of isolates. Naegleria and Acanthamoeba are readily isolated from environmental samples (soil and water), but the isolation of Balamuthia remains an important goal. Its slow growth, its apparent inability to feed on bacteria, and the presence of highly competitive soil fauna (fungi, bacteria, other protozoa, and metazoa) have complicated the task. Though it is generally regarded as free living, this cannot be know for certain until the organism is isolated from soil or water samples.
It is likely that, on a global scale, many cases of amebic encephalitis go undiagnosed and unreported. Development of techniques to improve on isolation and cultivation of these amebas will help in obtaining a more accurate assessment of the extent of these infections.
ADDENDUM IN PROOF
A Balamuthia ameba associated with a fatal case of amebic encephalitis in northern California has been isolated from soil of a potted plant in the home of the patient (F. L. Schuster, T. H. Dunnebacke, C. Glaser, D. Vugia, A. Bakardjiev, P. Azimi, M. Maddux-Gonzalez, G. S. Visvesvara, 54th Annu. Meet. Soc. Protozool., 2002). The ameba is morphologically similar to the ameba isolated postmortem from the patient, gave positive immunofluorescence reactions with serum from the patient and with rabbit anti-Balamuthia serum, and had a similar antimicrobial sensitivity profile. The environmental isolate is in culture on tissue culture cells and in a cell-free medium. This represents the first isolation of Balamuthia from the environment.
Acknowledgments
I thank G. S. Visvesvara (Division of Parasitic Diseases, Centers for Disease Control and Prevention) for comments and suggestions in the preparation of the manuscript. Thanks, too, go to T. H. Dunnebacke (California Department of Health Services), A. J. Martinez (University of Pittsburgh School of Medicine), and G. S. Visvesvara for providing photomicrographic material included in the paper.
REFERENCES
- 1.Adam, K. M. G. 1959. The growth of Acanthamoeba sp. in a chemically defined medium. J. Gen. Microbiol. 21:519-529. [DOI] [PubMed] [Google Scholar]
- 2.Adam, K. M. G., and D. A. Blewett. 1967. Carbohydrate utilization by the soil amoeba Hartmannella castellanii. J. Protozool. 14:227-282. [DOI] [PubMed] [Google Scholar]
- 3.Anzil, A. P., C. Rao, M. A. Wrzolek, G. S. Visvesvara, J. H. Sher, and P. B. Kozlowsky. 1991. Amebic meningoencephalitis in a patient with AIDS caused by a newly recognized opportunistic pathogen. Arch. Pathol. Lab. Med. 115:21-25. [PubMed] [Google Scholar]
- 4.Balamuth, W. 1964. Nutritional studies on axenic cultures of Naegleria gruberi. J. Protozool. 11(Suppl.):19-20. [Google Scholar]
- 5.Band, R. N. 1961. Biotin, a growth requirement for four soil amoebae. Nature 192:674. [DOI] [PubMed] [Google Scholar]
- 6.Band, R. N. 1962. The amino acid requirements of the soil ameba Hartmannella rhysodes Singh. J. Protozool. 9:377-379. [DOI] [PubMed] [Google Scholar]
- 7.Band, R. N., and W. Balamuth. 1974. Hemin replaces serum as a growth requirement for Naegleria. Appl. Microbiol. 28:64-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker, J., T. J. Humphrey, and M. W. Brown. 1999. Survival of Escherichia coli 0157 in a soil protozoan. Implications for disease. FEMS Microbiol. Lett. 173:291-295. [DOI] [PubMed] [Google Scholar]
- 9.Byers, T. J., R. A. Akins, B. J. Maynard, R. A. Lefken, and S. M. Martin. 1980. Rapid growth of Acanthamoeba in defined media; induction of encystment by glucose-acetate starvation. J. Protozool. 27:216-219. [DOI] [PubMed] [Google Scholar]
- 10.Cerva, L. 1969. Amoebic meningoencephalitis: axenic culture of Naegleria. Science 163:576. [PubMed] [Google Scholar]
- 11.Cerva, L. 1978. Some further characteristics of the growth of Naegleria fowleri and Naegleria gruberi in axenic culture. Folia Parasitol. (Prague) 25:1-8. [PubMed] [Google Scholar]
- 12.Cerva, L. 1980. Laboratory diagnosis of primary amoebic meningo-encephalitis and methods for the detection of limax amoebae in the environment. Folia Parasitol. (Prague) 27:1-9. [PubMed] [Google Scholar]
- 13.Chang, S. L. 1971. Small, free-living amebas: cultivation, quantitation, identification, classification, pathogenesis, and resistance. Curr. Top. Comp. Pathobiol. 1:201-254. [DOI] [PubMed] [Google Scholar]
- 14.Chang, S. L. 1974. Etiological, pathological, epidemiological, and diagnostical considerations of primary meningoencephalitis. Crit. Rev. Microbiol. 3:135-159. [Google Scholar]
- 15.Chang, S. L. 1978. Resistance of pathogenic Naegleria to some common physical and chemical agents. Appl. Enivron. Microbiol. 35:368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirillo, J. D., S. L. Cirillo, L. Yan, L. E. Bermudez, S. Falkow, and L. S. Tompkins. 1999. Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 67:4427-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cline, M., F. Marciano-Cabral, and S. G. Bradley. 1983. Comparison of Naegleria fowleri and Naegleria gruberi cultivated in the same nutrient medium. J. Protozool. 30:387-391. [DOI] [PubMed] [Google Scholar]
- 18.Culbertson, C. G. 1971. The pathogenicity of soil amebas. Annu. Rev. Microbiol. 25:231-254. [DOI] [PubMed] [Google Scholar]
- 19.Culbertson, C. G., J. W. Smith, and J. R. Minner. 1958. Acanthamoeba: observations on animal pathogenicity. Science 127:1506. [DOI] [PubMed] [Google Scholar]
- 20.Cursons, R. T. M., J. J. Donald, T. J. Brown, and E. A. Keys. 1979. Cultivation of pathogenic and nonpathogenic free-living amebae. J. Parasitol. 65:189-191. [PubMed] [Google Scholar]
- 21.De Jonckheere, J. 1977. Use of an axenic medium for differentiation between pathogenic and nonpathogenic Naegleria fowleri isolates. Appl. Enivron. Microbiol. 33:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Jonckheere, J. 1980. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl. Environ. Microbiol. 39:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essig, A., M. Heinemann, U. Simnacher, and R. Marre. 1997. Infection of Acanthamoeba castellanii by Chlamydia pneumoniae. Appl. Environ. Microbiol. 63:1396-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritsche, T. R., R. K. Gautom, S. Seyedirashti, D. L. Bergeron, and T. D. Lindquist. 1993. Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lenses. J. Clin. Microbiol. 31:1122-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritsche, T. R., M. Horn, M. Wagner, R. P. Herwig, K. H. Schleifer, and R. K. Gautom. 2000. Phylogenetic diversity among geographically dispersed Chlamydiales endosymbionts recovered from clinical and environmental isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 66:2613-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritsche, T. R., D. Sobele, and R. K. Gautom. 1998. Enhancement of in vitro cytopathogenicity by Acanthamoeba spp. following acquisition of bacterial endosymbionts. FEMS Microbiol. Lett. 166:231-236. [DOI] [PubMed] [Google Scholar]
- 27.Fulton, C. 1974. Axenic cultivation of Naegleria gruberi. Requirement for methionine. Exp. Cell Res. 88:365-370. [DOI] [PubMed] [Google Scholar]
- 28.Fulton, C., C. Webster, and J. S. Wu. 1984. Chemically defined media for cultivation of Naegleria gruberi. Proc. Natl. Acad. Sci. USA 81:2406-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Gordon, S. M., J. P. Steinberg, M. DuPuis, P. E. Kozarsky, J. F.Nickerson, and G. S. Visvesvara. 1992. Culture isolation of Acanthamoeba species and leptomyxid amebas from patients with amebic meningoencephalitis, including two patients with AIDS. Clin. Infect. Dis. 15:1024-1030. [DOI] [PubMed] [Google Scholar]
- 31.Griffiths, A. J., and D. E. Hughes. 1968. Starvation and encystment of a soil ameba Hartmannella castellanii. J. Protozool. 15:673-677. [DOI] [PubMed] [Google Scholar]
- 32.Haight, J. B., and D. T. John. 1980. Growth of Naegleria fowleri in several axenic media. Folia Parasitol. (Prague) 27:207-212. [PubMed] [Google Scholar]
- 33.Haight, J. B., and D. T. John. 1982. Varying the serum component in axenic cultures of Naegleria fowleri. Proc. Helminthol. Soc. Wash. 49:127-134. [Google Scholar]
- 34.Hu, W.-N., R. N. Band, and W. J. Kopachik. 1991. Virulence-related protein synthesis in Naegleria fowleri. Infect. Immun. 59:4278-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu, W-N., W. Kopachik, and R. N. Band. 1992. Cloning and characterization of transcripts showing virulence-related gene expression in Naegleria fowleri. Infect. Immun. 60:2418-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingalls, C. S., and M. M. Brent. 1983. Defined minimal growth medium for Acanthamoeba polyphaga. J. Protozool. 30:606-608. [DOI] [PubMed] [Google Scholar]
- 37.Inglis, T. J. J., P. Rigby, T. A. Robertson, N. S. Dutton, M. Henderson, and B. J. Chang. 2000. Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect. Immun. 68:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen, T., W. G. Barnes, and D. Meyers. 1970. Axenic cultivation of large populations of Acanthamoeba castellanii (JBM). J. Parasitol. 56:904-906. [PubMed] [Google Scholar]
- 39.John, D. T. 1982. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu. Rev. Microbiol. 36:101-123. [DOI] [PubMed] [Google Scholar]
- 40.John, D. T. 1993. Opportunistically pathogenic free-living amebae, p. 143-246. In J. P. Kreier and J. R. Baker (ed.), Parasitic protozoa, 2nd ed., vol. 3. Academic Press, New York, N.Y. [Google Scholar]
- 41.John, D. T., and M. J. Howard. 1995. Seasonal distribution of pathogenic free-living amebae in Oklahoma waters. Parasitol. Res. 81:193-201. [DOI] [PubMed] [Google Scholar]
- 42.John, D. T., and C. V. McCutchen. 1995. Reduction in virulence of Naegleria fowleri following growth with cholesterol. Folia Parasitol. (Prague) 42:236-238. [PubMed] [Google Scholar]
- 43.Jones, D. B., G. S. Visvesvara, and N. M. Robinson. 1975. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans. Ophthalmol. Soc. U. K. 95:221-232. [PubMed] [Google Scholar]
- 44.Kinde, H., G. S. Visvesvara. B. C. Barr, R. W. Nordhausen, and P. H. W. Chiu. 1998. Amebic meningoencephalitis caused by Balamuthia mandrillaris (leptomyxid ameba) in a horse. J. Vet. Diagn. Investig. 10:378-381. [DOI] [PubMed] [Google Scholar]
- 45.Reference deleted.
- 46.LaVerde, A. V., and M. M. Brent. 1980. Simplified soluble media for the axenic cultivation of Naegleria. Protistologica 16:11-15. [Google Scholar]
- 47.Ly, T. M., and H. E. Muller. 1990. Ingested Listeria monocytogenes survive and multiply in protozoa. J. Med. Microbiol. 33:51-54. [DOI] [PubMed] [Google Scholar]
- 48.Ma, P., G. S. Visvesvara, A. J. Martinez, F. H. Theodore, P.-M. Daggett, and T. K. Sawyer. 1990. Naegleria and Acanthamoeba infections: review. Rev. Infect. Dis. 12:490-513. [DOI] [PubMed] [Google Scholar]
- 49.Ma, P., E. Willaert, K. B. Juechter, and A. R. Stevens. 1981. A case of keratitis due to Acanthamoeba in New York, New York, and features of 10 cases. J. Infect. Dis. 143:662-667. [DOI] [PubMed] [Google Scholar]
- 50.Marciano-Cabral, F. 1988. Biology of Naegleria spp. Microbiol. Rev. 52:114-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez, A. J. 1985. Free-living amebas: natural history, prevention, diagnosis, pathology, and treatment of disease. CRC Press, Inc., Boca Raton, Fla.
- 52.Mehlotra, R. K., and O. P. Shukla. 1997. Pathogenic free-living amoebae: cultivation, nutritional requirements and chemotherapy of infections. Ind. J. Microbiol. 37:113-123. [Google Scholar]
- 53.Michel, R., and K. Janitschke. 1996. Axenic and monoxenic cultivation of Balamuthia mandrillaris (Visvesvara et al. 1993) Leptomyxidae, p. 100-102. In Christian Gottfried Ehrenberg Festschrift. Leipziger Universitätsverlag, Leipzig, Germany.
- 54.Napolitano, J. J., and H. R. Gamble. 1978. Folic acid stimulation of axenically grown Naegleria gruberi. Protistologica 14:183-187. [Google Scholar]
- 55.Napolitano, J. J., A. V. LaVerde, and H. R. Gamble. 1977. Cultivation of Naegleria using alcohol killed bacteria. Acta Protistol. 16:207-217. [Google Scholar]
- 56.Neff, R. J. 1957. Purification, axenic cultivation, and description of a soil amoeba. Acanthamoeba sp. J. Protozool. 4:176-182. [Google Scholar]
- 57.Nelson, E. C., and M. M. Jones. 1970. Culture isolation of agents of primary amebic meningoencephalitis. J. Parasitol. 56:248. (Abstract.)5445820 [Google Scholar]
- 58.Nerad, T. A., G. Visvesvara, and P.-M. Daggett. 1983. Chemically defined media for the cultivation of Naegleria: pathogenic and high temperature tolerant species. J. Protozool. 30:383-387. [DOI] [PubMed] [Google Scholar]
- 59.Newsome, A. L., T. M. Scott, R. F. Benson, and B. S. Fields. 1998. Isolation of an amoeba naturally harboring a distinctive Legionella species. Appl. Environ. Microbiol. 64:1688-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Dell, W. D., and M. M. Brent. 1974. Nutritional study of three strains of Naegleria gruberi. J. Protozool. 21:129-133. [DOI] [PubMed] [Google Scholar]
- 61.O'Dell, W. D., and A. R. Stevens. 1973. Quantitative growth of Naegleria in axenic culture. Appl. Microbiol. 25:621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pigon, A. 1970. Hartmannella: growth controlling substances in culture medium. Protoplasma 70:405-414. [Google Scholar]
- 63.Schuster, F. L. 1961. Axenic cultivation of Naegleria gruberi. J. Protozool. 8:19. (Abstract.) [Google Scholar]
- 64.Schuster, F. L. 1979. Small amebas and ameboflagellates, p. 215-285. In M. Levandowsky and S. H. Hutner (ed.), Biochemistry and physiology of protozoa, vol. 1. Academic Press, New York, N.Y. [Google Scholar]
- 65.Schuster, F. L., and G. Svilha. 1968. Ribonucleoprotein-containing vesicles in cysts of Naegleria gruberi. J. Protozool. 15:752-758. [DOI] [PubMed] [Google Scholar]
- 66.Schuster, F. L., and G. S. Visvesvara. 1996. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J. Clin. Microbiol. 34:385-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schuster, F. L., and G. S. Visvesvara. 1998. Efficacy of novel antimicrobials against clinical isolates of opportunistic amebas. J. Eukaryot. Microbiol. 45:612-618. [DOI] [PubMed] [Google Scholar]
- 68.Shukla, O. P., S. M. Kaul, and R. K. Mehlotra. 1989. Development of improved media for axenic cultivation of Acanthamoeba culbertsoni, Singh and Das 1970. Ind. J. Exp. Biol. 27:785-791. [PubMed] [Google Scholar]
- 69.Shukla, O. P., S. M. Kaul, and R. K. Mehlotra. 1990. Nutritional studies on Acanthamoeba culbertsoni and development of chemically defined medium. J. Protozool. 37:237-242. [DOI] [PubMed] [Google Scholar]
- 70.Singh, B. N., and G. D. P. Dutta. 1984. Small free-living aerobic amoebae: soil as a suitable habitat, isolation, culture, classification, pathogenicity, epidemiology and chemotherapy. Ind. J. Parasitol. 8:1-23. [Google Scholar]
- 71.Sison, J. P., C. A. Kemper, M. Loveless, D. McShane, G. S. Visvesvara, and S. C. Deresinski. 1995. Disseminated Acanthamoeba infection in patients with AIDS: case reports and review. Clin. Infect. Dis. 20:1207-1216. [DOI] [PubMed] [Google Scholar]
- 72.Steinert, M., K. Birkness, E. White, B. Fields, and F. Quinn. 1998. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl. Environ. Microbiol. 64:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stothard, D. R., J. Hay, J. M. Schroeder-Diedrich, D. V. Seal, and T. J. Byers. 1999. Fluorescent oligonucleotide probes for clinical and environmental detection of Acanthamoeba and the T4 18S rRNA gene sequence type. J. Clin. Microbiol. 37:2687-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thom, S. D. Warhurst, and B. S. Drasar. 1992. Association of Vibrio cholerae with fresh water amoebae. J. Med. Microbiol. 35:303-306. [DOI] [PubMed] [Google Scholar]
- 75.Visvesvara, G. S. 1999. Pathogenic and opportunistic free-living amebae, p. 1383-1390. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D. C.
- 76.Visvesvara, G. S., A. J. Martinez, F. L. Schuster, G. J. Leitch, S. V. Wallace, T. K. Sawyer, and M. Anderson. 1990. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J. Clin. Microbiol. 28:2750-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Visvesvara, G. S., S. S. Mirra, F. H. Brandt, D. M. Moss, H. M. Mathews, and A. J. Martinez. 1983. Isolation of two strains of Acanthamoeba castellanii from human tissue and their pathogenicity and isoenzyme profiles. J. Clin. Microbiol. 18:1405-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Visvesvara, G. S., R. C. Neafie, and A. J. Martinez. 1997. Pathogenic and opportunistic free-living amebae, p. 257-267. In C. R. Horsbrugh, Jr., and A. M. Nelson (ed.), Pathology of emerging infections. American Society for Microbiology, Washington, D.C.
- 79.Visvesvara, G. S., F. L. Schuster, and A. J. Martinez. 1993. Balamuthia mandrillaris, n. g., n. sp., an agent of amebic meningoencephalitis in humans and other animals. J. Eukaryot. Microbiol. 40:504-514. [DOI] [PubMed] [Google Scholar]
- 80.Weekers, P. H. H., P. L. E. Bodelier, J. P. H. Wijen, and G. D. Vogels. 1993. Effects of grazing by the free-living soil amoebae Acanthamoeba castellanii, Acanthamoeba polyphaga, and Hartmannella vermiformis on various bacteria. Appl. Environ. Microbiol. 59:2317-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weekers, P. H. H., and G. D. Vogels. 1994. Axenic cultivation of the free-living soil amoebae, Acanthamoeba castellanii and Hartmannella vermiformis in a chemostat. J. Microbiol. Methods 19:13-18. [Google Scholar]
- 82.Weekers, P. H. H., J. P. H. Wijen, B. P. Lomans, and G. D. Vogels. 1996. Axenic mass cultivation of the free-living soil amoeba. Acanthamoeba castellanii in a laboratory fermentor. Antonie Leeuwenhoek 69:317-322. [DOI] [PubMed] [Google Scholar]
- 83.Weik, R. R., and D. T. John. 1977. Agitated mass cultivation of Naegleria fowleri. J. Parasitol. 63:868-871. [PubMed] [Google Scholar]
- 84.Weik, R. R., and D. T. John. 1977. Cell size, macromolecular composition, and O2 consumption during agitated cultivation of Naegleria gruberi. J. Protozool. 24:196-200. [DOI] [PubMed] [Google Scholar]
- 85.Willaert, E. 1976. Etude immuno-taxonomique des genres Naegleria et Acanthamoeba (Protozoa:Amoebida). Acta Zool. Pathol. Antverp 65:1-239. [PubMed] [Google Scholar]
- 86.Wong, M. M., S. L. Karr, Jr., and W. Balamuth. 1975. Experimental infections with pathogenic amebae in laboratory primate hosts. I. (A) A study on susceptibility to Naegleria fowleri. J. Parasitol. 61:199-208. [PubMed] [Google Scholar]