Prions are unconventional infectious agents that cause fatal neurological illnesses such as Creutzfeldt-Jakob disease, bovine spongiform encephalopathy, and scrapie. Many hypotheses have been advanced to explain the chemical composition of infectious prions and the mechanism of their formation in the neurons of infected hosts, but none has yet been proven. Perhaps the most provocative proposal has been the “protein-only” hypothesis, which posits that the infectious agent is composed exclusively of a misfolded, host-encoded protein called the prion protein (PrP). However, three decades of investigation have yielded no direct experimental proof for this stringent hypothesis. Moreover, various biochemical studies have suggested that nonproteinaceous cofactors may be required to produce infectious prions, possibly by forming physical complexes with PrP (1–4). On page 1132 of this issue, Wang et al. demonstrate the importance of cofactors for producing recombinant infectious prions in vitro (5). Another study by Li et al. suggests that endogenous cofactors may also infl uence the strain properties of prions in cells (6).
Misfolded PrP that is associated with disease can convert normal PrP into an aberrant form. In a subset of cases, aggregates of misfolded PrP form amyloid fibers, which can accumulate and form plaques in the brain. A central prediction of the protein-only hypothesis is that it should be possible to generate prions with high specific infectivity by chemically refolding PrP in vitro in the absence of other cellular components. The specific infectivity of an infectious agent is measured by an end-point titration bioassay of a known quantity of the agent in susceptible, wild-type hosts. Previous studies have shown that using chemical denaturants to fold purified recombinant PrP (produced by genetically engineered bacteria) into amyloid fibrils yields products with minimal infectivity (7, 8). Although end-point titration was not performed in these studies, extremely low specific infectivity may be inferred because wildtype rodents failed to develop clinical disease when inoculated with samples of highly concentrated protein.
By contrast, a mixture of native PrP and lipid molecules purified from noninfected hamster brain, plus synthetic polyadenylic acid RNA molecules, resulted in the de novo formation of prions whose specific infectivity was comparable to that of naturally occurring prions (3). Although these results indicated that nonproteinaceous cofactors were necessary for generating native prions, it remained unknown whether the addition of such cofactors could facilitate the production of infectious prions from recombinant PrP (9).
In a major advance, Wang et al. report that mixing recombinant PrP with total liver RNA and synthetic 1-palmitoyl-2-oleoyl-phosphatidylglycerol (POPG) lipid molecules produces bona fide infectious prions ( 5). Building upon their earlier work showing that POPG promotes the conversion of PrP into an aberrant, protease-resistant conformation (2), Wang et al. used the protein misfolding cyclic amplification technique ( 10) to generate recombinant prions de novo. Remarkably, the resulting recombinant prions were infectious to wildtype mice and displayed unique strain characteristics (prion strains are self-propagating variants with distinct PrP conformations and infectious phenotypes). Although an endpoint titration bioassay was not performed, a 100% fatality rate among inoculated animals and a short incubation period between inoculation and disease onset suggest high specific infectivity. The contrast between the bioassay results obtained with recombinant prions formed with lipid and polyanionic cofactors and those obtained using recombinant PrP amyloid fibrils (7, 8) argues that cofactors likely facilitated the formation of prions with high specific infectivity from recombinant PrP (see the figure).
Extra ingredients.
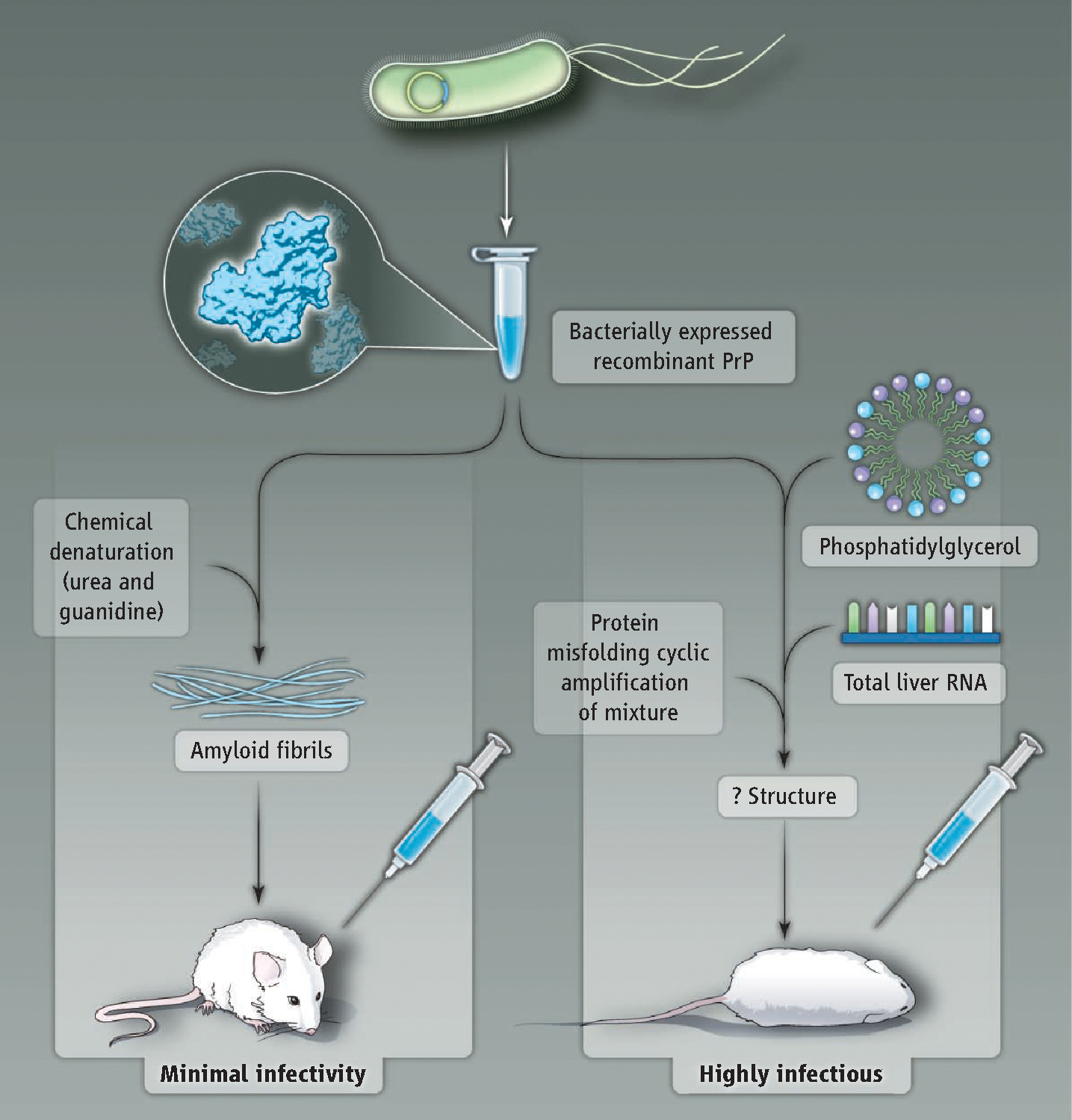
Two different biochemical protocols yield recombinant PrP with different infectivity. (Left) Minimally infectious amyloid fibrils are formed by incubating recombinant PrP with chemical denaturants. (Right) Mixing recombinant PrP with phospholipid and RNA produces highly infectious prions.
If endogenous cofactors participate in prion conversion, it is reasonable to anticipate that they might also constrain PrP structure and infl uence the properties of different prion strains in cells. Consistent with this possibility, Li et al. observed that infecting different cell types with prions can cause phenotypic “mutation” and selection of prion strains, as detected by a new rapid strain-typing assay. It may be that cell type–dependent differences in non-PrP factors could be responsible for the observed evolution of prion strains because all of the cell types examined expressed identical endogenous PrP molecules.
Wang et al. and Li et al. each have developed powerful methods that can be used to answer critical questions in future studies. It will be important to determine whether cofactors are essential components of an infectious complex, or simply catalyze the formation of prions exclusively composed of PrP. Identifying the endogenous cofactors that facilitate the formation of prion strains in different cell types is also of interest. And a biophysical comparison of the protein structures of the infectious recombinant prions produced by Wang et al. and the minimally infectious PrP amyloid (produced by chemically induced refolding of recombinant PrP) could reveal the structural features that encode prion infectivity. After decades of speculation, it may finally be possible to determine the molecular basis of prion infectivity experimentally.
References
- 1.Wong C et al. , EMBO J. 20, 377 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang F et al. , Biochemistry 46, 7045 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Deleault NR, HarrEis BT, Rees JR, Supattapone S, Proc. Natl. Acad. Sci. U.S.A. 104, 9741 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geoghegan JC et al. , J. Biol. Chem. 282, 36341 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Wang X, Yuan C-G, Ma J, Science 327, 1132 (2010); published online 28 January 2010. ( 10.1126/science.1183748). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C, Science 327, 869 (2010); published online 31 December 2009 ( 10.1126/science.1183218). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legname G et al. , Science 305, 673 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Makarava N et al. , Acta Neuropathol. 119, 177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JI, Surewicz K, Gambetti P, Surewicz WK, FEBS Lett. 583, 3671 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castilla J, Saa P, Hetz C, Soto C, Cell 121, 195 (2005). [DOI] [PubMed] [Google Scholar]


