Abstract
Background
Fatigue is a burdensome, under-recognized, multidimensional symptom experienced by patients with Crohn’s disease (CD). We evaluated the impact of mirikizumab on fatigue and the association between changes in select patient-reported outcomes and clinical measures with changes in fatigue from baseline to week 104 (W104).
Methods
Patients (N = 191) were randomized (2:1:1:2) to receive placebo (PBO), 200 mg, 600 mg, or 1000 mg of mirikizumab, administered intravenously (IV) every 4 weeks at W0, W4, and W8. Patients who achieved ≥1 point improvement in Simple Endoscopic Score for Crohn’s Disease (SES-CD) and received mirikizumab at W12 (rerandomized maintenance cohort) were rerandomized to continue induction IV treatment assignment (IV-C) or received 300 mg of mirikizumab subcutaneously (SC) until W52. Nonrandomized maintenance cohort had endoscopic nonimprovers (1000 mg) and PBO patients (PBO/1000 mg) who received 1000 mg of mirikizumab until W52. Subjects from the maintenance period with clinical benefit received 300 mg SC Q4W from W52 to W104. The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire was used to assess fatigue, and the FACIT-F associations were assessed using Pearson correlation coefficient.
Results
At W12, mirikizumab groups reported improved FACIT-F scores compared with PBO, and improvement was maintained through W52 and W104. Changes in FACIT-F at W52 and W104 had strong correlations with changes at the same time point in quality of life (QoL) scores but lacked correlations with changes in inflammatory biomarkers.
Conclusions
Mirikizumab treatment significantly improved fatigue in patients with moderately to severely active CD, which was sustained to W104. The improvement in fatigue was correlated with improvement in clinical measures and was strongly correlated with improvement in QoL.
Keywords: Crohn’s disease, fatigue, quality of life
KEY MESSAGES.
What is already known?
Mirikizumab, an anti-IL23p19 monoclonal antibody, has demonstrated safety and efficacy in the treatment of patients with moderately to severely active CD.
What is new here?
Treatment with mirikizumab was associated with significantly improved FACIT-F scores that were sustained through W104, where improvement in fatigue correlated better with emotional, social, and mental well-being (strong correlation) concepts than with disease-related symptoms such as abdominal pain and CDAI score (moderate correlation).
How can this study help patient care?
Mirikizumab treatment improves the symptom of fatigue in patients with moderately to severely active CD, which may lead to better overall quality of life.
Introduction
Crohn’s disease (CD) is a chronic, debilitating, and progressive inflammatory disease of the gastrointestinal tract with typical symptoms of diarrhea, abdominal pain, anemia, malnutrition, and fatigue.1 Uncontrolled inflammation leads to disease progression including the development of fistulas, stenosis, and surgical resections.2 These factors negatively impact quality of life (QoL) including psychological and emotional concepts, social and family interactions, and work-related productivity.2–4 Thus, achieving improvement of these symptoms in addition to control of intestinal inflammation reaches the treatment target of normalized QoL and the absence of disability.5 Despite achieving clinical remission, some symptoms such as fatigue can continue to impact patients and their QoL.6
Fatigue may be described as a persistent sense of tiredness, weakness, or exhaustion that impacts physical and mental work but is not relieved by rest.7 Due to these subjective and objective components, fatigue is known to be complex and multifactorial, making it difficult to treat in patients. Up to 86% of patients with active CD report some degree of fatigue, while it has been recorded that up to 54% of patients with CD report fatigue while in some state of remission.8,9 Fatigue is generally poorly understood, is often under-reported, remains untreated, and significantly impairs QoL while being associated with disease activity and severity.10–14 It has been reported that 61% of patients feel that fatigue greatly impacts their QoL,15 including depression, work/school, housework, socializing, family life, and hobbies.16 Fatigue is also associated with psychological factors like stress, anxiety, and depression.17
Current treatment of CD includes corticosteroids, immunomodulators, biologics, and small molecules that each target different mechanisms of action.18,19 However, the efficacy of CD treatments is limited by primary nonresponse, intolerance, or loss of response to the biologic treatment.18–21 This suboptimal treatment is associated with disease progression, complications, impaired QoL, greater rates of surgery and hospitalization, and longer corticosteroid use, producing a significant unmet medical need for patients.22,23
Interleukin (IL)-23 plays a vital role in the amplification and maintenance of T helper 17 cells which is important in the pathogenesis of CD, making the IL-23p19 inhibitor class a prime candidate for enhanced durability and efficacy.24–26 Mirikizumab is a humanized immunoglobulin G4 which is a variant monoclonal antibody that binds to the p19 subunit of IL-23 and has shown efficacy and durability in the treatment of ulcerative colitis and CD.27,28
Given the efficacy and durability of mirikizumab, as well as the gap in treatment for fatigue in those with moderate to severe CD, we examined the effects of mirikizumab on fatigue from baseline to W104.
Materials and Methods
Study Design and Participants
Study I6T-MC-AMAG, SERENITY (NCT02891226) was a dose-finding, multicenter, parallel-arm, randomized, double-blind, placebo (PBO)-controlled trial (see Figure 1) that was done across 14 countries with enrollment starting in 2017; and the last patient visit was in 2021 (see Supplementary Figure 1 for CONSORT diagram).
Figure 1.
Study Design for SERENITY. Dashed lines indicate endoscopic nonimprovers (NI) while solid lines indicate endoscopic improvers at the end of Period 1. R1 = Randomization 1: patients were stratified based on previous exposure to biologic therapy for the treatment of CD. R2 = randomization 2: patients who received mirikizumab during induction and had endoscopic improvement were rerandomized in a 1:1 ratio at week 12 to continue their induction regimen or receive mirikizumab 300 mg SC Q4W with stratification based on endoscopic response. All patients who received PBO in Period 1 received mirikizumab 1000 mg IV Q4W. Abbreviations: CD, Crohn’s disease; IV, intravenous; MIRI, mirikizumab; PBO, placebo; Q4W, every 4 weeks; SC, subcutaneous.
Eligible patients were 18 to 75 years old who had a duration of moderately to severely active CD defined as abdominal pain (AP) ≥2 and/or stool frequency (SF) ≥ 4 at baseline along with a centrally read Simple Endoscopic Score for Crohn’s Disease (SES-CD) ≥7 for subjects with ileal-colonic, or ≥4 for subjects with isolated ileal disease within 14 days before the first dose of study treatment for ≥3 months.28 It was required that patients had received prior treatment for CD, which included a history of intolerance or inadequate response to treatments specified in the supplementary appendix.
Patients were ineligible if any of the following was met: complications of CD that could confound the evaluation of efficacy; any type of bowel resection or intra-abdominal surgery (see supplementary appendix for full list of eligibility requirements).
Randomization and Blinding
Induction period
Patients were randomized and allocated in a 2:1:1:2 ratio to PBO, 200 mg mirikizumab, 600 mg mirikizumab, or 1000 mg mirikizumab that was given intravenously (IV) Q4W through W12. The strata planned for approximately 30% and at least 50% of patients being respectively naïve and experienced to biologic CD therapy.
Maintenance period
To maintain the study blind, all patients were given both IV and subcutaneous (SC) dosing in a double-dummy design during the maintenance period (W12-52).
Rerandomized maintenance cohort
Patients who received mirikizumab during the first 12 weeks of the study and improved (at least 1 point decrease) in their SES-CD score from baseline at W12 were randomized to either (1) continue induction treatment assignment (IV of 200, 600, or 1000 mg mirikizumab Q4W) or (2) IV PBO Q4W and SC 300 mg mirikizumab Q4W administered through W52. The stratification for randomization was based on endoscopic improvement (defined as achieving ≥1 point improvement in SES-CD).
Nonrandomized maintenance cohort
Patients treated with mirikizumab during the induction period who did not improve in baseline SES-CD score at W12 (endoscopic nonimprovers) as well as patients who received PBO in the induction phase received 1000 mg of mirikizumab and SC PBO Q4W through W52 as previously described.28
Extension period
All patients who had clinical benefit per investigator continued treatment from W52 to W104 receiving 300 mg SC of mirikizumab Q4W open-label. Patients who did not have clinical benefit at W52 discontinued treatment and entered a follow-up period where they assessed their safety for an additional 16 weeks (W104 to W120).
Outcome Measures
We analyzed changes in fatigue and its association with QoL end points at W12, W52, and W104.
Fatigue was measured with the Functional Assessment of Chronic Illness Therapy (FACIT-Fatigue or FACIT-F), which is a 13-item instrument developed to measure fatigue in patients with chronic illness. The total score ranges from 0 to 52 using a 4-point Likert scale where 4 means “very much” and 0 means “not at all.”29
Endoscopic response was defined as a 50% reduction from baseline in SES-CD, and endoscopic remission was defined as SES-CD score of <4 for ileal-colonic disease or <2 for isolated ileal disease, and no subscore >1.
Quality of life end points included the following: Patient-Reported Outcome (PRO) remission (average daily Abdominal Pain Numeric Rating Scale [AP NRS]) score ≤1 and daily stool frequency [SF]) ≤2.5); change from baseline in the Inflammatory Bowel Disease Questionnaire (IBDQ) score (scores range from 32 to 224; a higher score indicates a higher QoL)30; Patient’s Global Rating of Change (a single item, patient-rated instrument that assesses the rating of change in symptoms where 1 is “very much better” and 7 is “very much worse”); Patient’s Global Rating of Severity (PGRS; 1-item patient-rated questionnaire that assesses rating of disease symptom severity over the past 24 hours); and the Medical Outcomes Study 36-item short form health survey (SF-36; a 36-item patient-completed measure that is a short, multipurpose assessment of health in the areas of physical functioning, role-physical, role-emotional, bodily pain, vitality, social functioning, mental health and general health).31
Statistical Analysis
Change in FACIT-F from baseline to W12 was compared between treatment groups using a mixed model for repeated measures. The model included treatment, geographic region, prior CD biologic therapy, visit, and visit-by-treatment interactions. At W12, mirikizumab treatment arms were compared with PBO with the least squares mean changes on the FACIT-F scale. The change from baseline to W52 and W104 in the FACIT-F are presented descriptively. A 2-sided alpha level of 0.10 was considered statistically significant. FACIT-F associations with patient-reported outcomes and clinical measures were assessed using the Pearson correlation coefficient at weeks 52 and 104. Cohen’s conventions were used to assess the strength of the correlation, and 95% confidence intervals were calculated for patients with baseline and post-baseline measures.
Results
Patient Demographics and Characteristics
Between December 2016 and September 2018, 191 of 526 screened patients met inclusion criteria and were randomized.28 Of these randomized patients, 92.1% (N = 176 of 191) completed the induction period (first 12 weeks of the study) with the baseline characteristics being similar across the treatment groups.28 Demographics and characteristics such as mean disease duration, baseline Crohn’s Disease Activity Index (CDAI), AP NRS, and SES-CD were all similar across treatment groups(Table 1).28
Table 1.
Baseline demographics and clinical characteristics.
| Mean (SD) unless otherwise specified | Treatment Groups | |||
|---|---|---|---|---|
| Miri | ||||
| Placebo (N = 64) | 200 mg (N = 31) | 600 mg (N = 32) | 1000 mg (N = 64) | |
| Age, years | 39.0 (13.0) | 38.1 (11.8) | 40.4 (13.3) | 37.7 (13.1) |
| Male, n (%) | 28 (43.8) | 17 (54.8) | 14 (43.8) | 34 (53.1) |
| Race-White, n (%) | 55 (85.9) | 28 (90.3) | 24 (75.0) | 52 (81.3) |
| Disease duration, years | 10.2 (9.8) | 8.9 (7.4) | 10.8 (9.7) | 8.6 (6.7) |
| Disease location, n (%) | ||||
| Ileal | 11 (17.2) | 6 (19.4) | 5 (15.6) | 11 (17.2) |
| Colonic | 25 (39.1) | 14 (45.2) | 10 (31.3) | 26 (40.6) |
| Ileocolonic | 28 (43.8) | 11 (35.5) | 17 (53.1) | 27 (42.2) |
| Simple endoscopic score for Crohn’s disease (SES-CD) | 11.9 (5.6) | 14.4 (7.9) | 15.2 (7.4) | 13.1 (6.8) |
| PRO scores | ||||
| Stool frequency | 6.4 (3.1) | 7.4 (3.0) | 6.4 (3.8) | 6.6 (5.5) |
| Abdominal pain | 1.9 (0.6) | 2.0 (0.6) | 1.7 (0.7) | 1.9 (0.6) |
| Crohn’s Disease Activity Index (CDAI) | 304.7 (93.1) | 348.3 (92.1) | 298.2 (103.7) | 304.5 (94.4) |
| Previous biologic usea, n (%) | 43 (67.2) | 19 (61.3) | 19 (59.4) | 39 (60.9) |
| Previous biologic failure,b n (%) | 36 (56.3) | 15 (48.4) | 16 (50.0) | 31 (48.4) |
| Prior vedolizumab use, n (%) | 14 (21.9) | 5 (16.1) | 5 (15.6) | 6 (9.4) |
| Prior anti-TNF use, n (%) | ||||
| 0 | 25 (39.1) | 14 (45.2) | 14 (43.8) | 26 (40.6) |
| 1 | 16 (25.0) | 10 (32.3) | 9 (28.1) | 22 (34.4) |
| 2 | 22 (34.4) | 7 (22.6) | 5 (15.6) | 14 (21.9) |
| 3+ | 1 (1.6) | 0 | 4 (12.5) | 2 (3.1) |
| Concomitant oral corticosteroid use, n (%) | 21 (32.8) | 14 (45.2) | 7 (21.9) | 15 (23.4) |
| Concomitant immunosuppressant use, n (%) | 19 (29.7) | 12 (38.7) | 10 (31.3) | 21 (32.8) |
| IBDQ | 113.88 (37.07) | 104.77 (34.31) | 127.03 (35.47) | 120.31 (32.40) |
| hsCRP (median, Q1, Q3) | 6.8 (1.8, 19.0) | 7.4 (2.3, 31.4) | 6.8 (2.7, 20.7) | 4.5 (2.7, 15.5) |
| FCP (median, Q1, Q3) | 799.5 | 877 | 822.5 | 773 |
| (256.5, 1945.5) | (225.0, 4359.0) | (355.0, 2302.5) | (293.0, 1634.0) | |
Intent-to-treat population.
aAlthough prior induction dosing of ustekinumab (UST) use was allowed, no patients had prior UST treatment.
bInadequate response, loss of response, or intolerance to medication.
Patients with prior biologic exposure that were not biologic failures discontinued treatment for the following reasons: cannot afford, treatment availability, subject decision, completed treatment, and other.
Abbreviations: SD, standard deviation; N, patient number in treatment group; n, patient number in demographic; PRO, patient reported outcomes; TNF, tumor necrosis factor; IBDQ, Inflammatory Bowel Disease Questionnaire; hsCRP, high-sensitivity C-reactive protein; FCP, fecal calprotectin.
Induction Period (Week 0-12)
Change from baseline in FACIT-Fatigue
At W12, all mirikizumab treatment groups had significantly higher least squares mean change from baseline in FACIT-F scores compared with PBO (mirikizumab 200 mg 10.81 ± 1.73, P < .001; mirikizumab 600 mg: 9.09 ± 1.72, P = .004; mirikizumab 1000 mg 9.62 ± 1.22, P < .001; PBO: 2.90 ± 1.21; Figure 2a).
Figure 2.
FACIT-Fatigue assessment in the induction period (week 0-12) including change from baseline in FACIT-Fatigue (A), correlation of FACIT-F improvement with changes in patient-reported outcomes (B), and correlation of FACIT-F improvement with changes in clinical measures (C). aA negative correlation means an improvement in PGRS or abdominal pain NRS. bData are log-transformed.
Correlation of FACIT-Fatigue improvement with changes in patient-reported outcomes
In all patients (N = 191), the change in FACIT-F at W12 showed strong correlations with changes in IBDQ total score (ranging from 0.63 to 0.78, P < .0001), and IBDQ bowel symptoms (ranging from 0.48 to 0.68, P < .0001), systemic systems (ranging from 0.58 to 0.75, P < .0001), emotional function (ranging from 0.58 to 0.75, P < .0001), and social function domains (ranging from 0.50 to 0.70, P < .0001), as well as the SF-36 Mental (ranging from 0.52 to 0.71, P < .0001) and Physical Component Summary scores (ranging from 0.51 to 0.70, P < .0001). Moderate correlations were observed with changes in PGRS and AP NRS (PGRS: ranging from −0.51 to −0.25, P < .0001; AP NRS: ranging from −0.49 to −0.22, P < .0001). Positive correlations for IBDQ total score, IBDQ domain scores, and SF-36 Summary scores and negative correlations for PGRS and AP NRS indicate improvement (refer to Methods for scoring systems, Figure 2b and Table 2).
Table 2.
Correlation of FACIT-F improvement with changes in patient-reported outcomes.
| Week 12 (N = 191) |
Week 52 (N = 176) |
Week 104 (N = 137) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pearson corr | 95% CI | P | Pearson corr | 95% CI | P | Pearson corr | 95% CI | P | |
| IBDQ total scorea | 0.714 | (0.632-0.781) | <0.0001 | 0.791 | (0.721-0.845) | <0.0001 | 0.816 | (0.746-0.867) | <0.0001 |
| IBDQ bowel symptoms domaina | 0.588 | (0.480-0.678) | <0.0001 | 0.709 | (0.617-0.782) | <0.0001 | 0.692 | (0.587-0.774) | <0.0001 |
| IBDQ systemic symptoms domaina | 0.669 | (0.577-0.745) | <0.0001 | 0.777 | (0.703-0.835) | <0.0001 | 0.802 | (0.728-0.857) | <0.0001 |
| IBDQ emotional function domaina | 0.672 | (0.580-0.747) | <0.0001 | 0.733 | (0.647-0.800) | <0.0001 | 0.793 | (0.716-0.851) | <0.0001 |
| IBDQ social function domaina | 0.608 | (0.503-0.695) | <0.0001 | 0.674 | (0.574-0.754) | <0.0001 | 0.690 | (0.585-0.773) | <0.0001 |
| SF-36 Physical Component Summary Scorea | 0.614 | (0.511-0.700) | <0.0001 | 0.613 | (0.500-0.705) | <0.0001 | 0.603 | (0.477-0.705) | <0.0001 |
| SF-36 Mental Component Summary Scorea | 0.621 | (0.519-0.706) | <0.0001 | 0.687 | (0.590-0.764) | <0.0001 | 0.717 | (0.618-0.793) | <0.0001 |
| Patient’s Global Rating of Severityb | -0.389 | (-0.512, -0.250) | <0.0001 | -0.469 | (-0.591, -0.327) | <0.0001 | -0.459 | (-0.595, -0.297) | <0.0001 |
| Abdominal Pain NRSb | -0.365 | (-0.491, -0.224) | <0.0001 | -0.421 | (-0.550, -0.272) | <0.0001 | -0.400 | (-0.546, -0.229) | <0.0001 |
Abbreviations: CI = confidence interval; corr = correlation; FACIT-F = Functional Assessment of Chronic Illness Therapy-Fatigue; IBDQ = Inflammatory Bowel Disease Questionnaire; N = number of patients in the analysis (including patients with nonmissing change scores); NRS = Numeric Rating Scale; PGRS = patient’s global rating of severity; SF-36 = Medical Outcomes Study 36-item short form health survey.
aA positive correlation indicates an improvement in outcome is associated with an improvement in FACIT-F (IBDQ total score, IBDQ domain scores, SF-36 PCS and MCS).
bA negative correlation indicates an improvement in outcome is associated with an improvement in FACIT-F (PGRS, AP NRS).
Correlation strength is defined as the following: > 0.5 is strong, 0.3 to ≤ 0.5 is moderate, 0.1 to < .3 is weak, < .1 is insubstantial.
Correlation of FACIT-Fatigue with clinical measures, biomarkers, and endoscopic data
The mean change in FACIT-F scores for patients with and without endoscopic response at W12 were 8.1 and 7.2, respectively (P = .33). In patients (N = 191) during the induction period, change in FACIT-F demonstrated a moderate but statistically significant correlation with change at the same time point in CDAI total score, AP NRS, and SF (CDAI total score: ranging from −0.53 to −0.26, P < .0001; AP NRS: ranging from −0.50 to −0.24, P < .0001; SF: ranging from −0.48 to −0.21, P < .0001). Weak but statistically significant correlations were observed with fecal calprotectin and C-reactive protein (Fecal calprotectin (log): ranging from −0.42 to −0.11, P = .0015; C-reactive protein: ranging from −0.31 to −0.01, P = .0331; Figure 2c and Table 3).
Table 3.
Correlation of change in FACIT-F with change in clinical measures.
| Clinical measure | Week 12 (N = 191) |
Week 52 (N = 176) |
Week 104 (N = 137) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pearson corr | 95% CI | P | Pearson corr | 95% CI | P | Pearson corr | 95% CI | P | |
| CDAI total score | −0.404 | (−0.530, −0.259) | <0.0001 | −0.492 | (−0.614, −0.346) | <0.0001 | −0.465 | (−0.604, −0.300) | <0.0001 |
| Hematocrit (%) | −0.039 | (−0.194, 0.119) | 0.6319 | 0.152 | (−0.016, 0.312) | 0.0756 | 0.109 | (−0.074, 0.284) | 0.2426 |
| Hemoglobin | −0.011 | (−0.167, 0.145) | 0.8916 | 0.120 | (−0.049, 0.282) | 0.1638 | 0.129 | (−0.053, 0.303) | 0.1640 |
| Fecal calprotectin (log) | −0.269 | (−0.419, −0.105) | 0.0015 | −0.175 | (−0.342, 0.003) | 0.0544 | −0.155 | (−0.336, 0.038) | 0.1155 |
| C-reactive protein (log) | −0.165 | (−0.310, −0.013) | 0.0331 | −0.151 | (−0.310, 0.016) | 0.0756 | −0.275 | (−0.433, −0.100) | 0.0024 |
| Abdominal pain average score | −0.380 | (−0.504, −0.240) | <0.0001 | −0.438 | (−0.565, −0.292) | <0.0001 | −0.352 | (−0.506, −0.176) | 0.0001 |
| Stool frequency average score | −0.354 | (−0.481, −0.212) | <0.0001 | −0.290 | (−0.437, −0.128) | 0.0006 | −0.338 | (−0.494, −0.161) | 0.0003 |
| SES-CD total score | −0.146 | (−0.290, 0.004) | 0.0572 | −0.076 | (−0.238, 0.090) | 0.3702 | |||
Abbreviations: CDAI, Crohn’s Disease Activity Index; corr, correlation; N, number of patients in the analysis (including patients with nonmissing change scores); FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; SES-CD, Simplified Endoscopic Activity Score for Crohn’s Disease. A negative correlation indicates an improvement in outcome is associated with an improvement in FACIT-F.
Maintenance Period (Week 12-52)
Change from baseline in FACIT-Fatigue
Of the 191 patients who began the induction period, 92.1% (176 of 191) went on to the maintenance period (W12-52). Due to the small sample sizes in maintenance, all W12 endoscopic improvers who were rerandomized to IV (200, 600, or 1000 mg of mirikizuamb) in maintenance were pooled (referred to as IV-C); and all patients who were rerandomized to SC (300 mg of mirikizumab) arms were pooled (referred to as IV-SC). The improvements observed during W0-12 were sustained through W52 in patients who received mirikizumab during induction (Figure 3a). The PBO/1000 mg group had improvements in the FACIT-F score approaching that of mirikizumab groups at W52.
Figure 3.
FACIT-Fatigue assessment in the maintenance period (week 12-52) including change from baseline in FACIT-Fatigue (A), correlation of FACIT-F improvement with changes in patient-reported outcomes (B), and correlation of FACIT-F improvement with changes in clinical measures (C). aA negative correlation means an improvement in PGRS or abdominal pain NRS. bData are log-transformed.
Correlation of FACIT-Fatigue improvement with changes in patient-reported outcomes
In all patients in the maintenance period (N = 176), the change in FACIT-F at W52 sustained strong correlations with changes in IBDQ total score and IBDQ domain scores (bowel symptoms, systemic symptoms, emotional function, and social function), along with SF-36 Mental and Physical Component Summary scores (IBDQ total score: ranging from 0.72 to 0.85, P < .0001; IBDQ bowel symptoms: ranging from 0.62 to 0.78, P < .0001; IBDQ systemic symptoms: ranging from 0.70 to 0.84, P < .0001; IBDQ emotional function: ranging from 0.65 to 0.80, P < .0001; IBDQ social function: ranging from 0.57 to 0.75, P < .0001; SF-36 Physical Component Summary: ranging from 0.50 to 0.71, P < .0001; SF-36 Mental Component Summary: ranging from 0.59 to 0.76, P < .0001). Like W12, moderate correlations were observed with changes in PGRS and AP NRS at W52 (PGRS: ranging from −0.59 to −0.33, P < .0001; AP NRS: ranging from −0.55 to −0.27, P < .0001; Figure 3b and Table 2).
Correlation of FACIT-Fatigue with clinical measures, biomarkers, and endoscopic data
The mean change in FACIT-F scores for patients with and without endoscopic response at W52 were 13.9 and 13.7, respectively (P = .54). In patients at W52, change in FACIT-F was moderately but significantly correlated with change in CDAI total score, AP NRS, and SF (CDAI total score: ranging from −0.61 to −0.35, P < .0001; AP NRS: ranging from −0.57 to −0.29, P < .0001; SF: ranging from −0.44 to −0.13, P = .0006; Figure 3c and Table 3).
Maintenance Extension (Week 52-104)
Change from baseline in FACIT-Fatigue
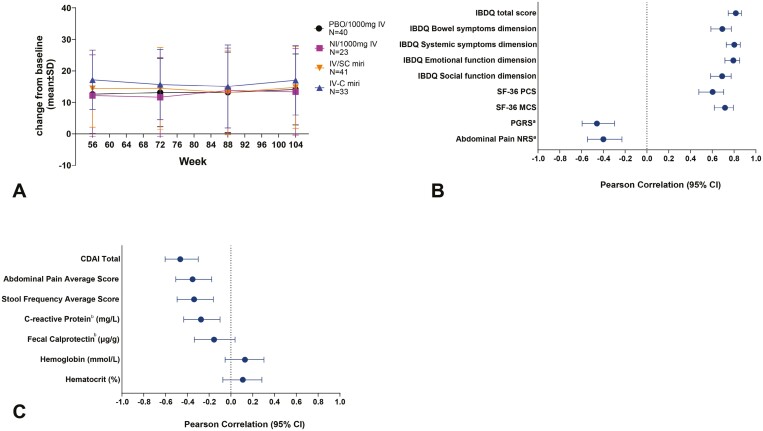
Of the 176 patients who entered the maintenance period at W52, 77.8% (137 of 176) entered the extension, and 70.5% (124 of 176) completed treatment through W104. FACIT-F improvements reported at W52 were maintained through W104 (Figure 4a).
Figure 4.
FACIT-Fatigue assessment in the extension period (week 52-104) including change from baseline in FACIT-Fatigue (A), correlation of FACIT-F improvement with changes in patient-reported outcomes (B), and correlation of FACIT-F improvement with changes in clinical measures (C). aA negative correlation means an improvement in PGRS or abdominal pain NRS. bData are log-transformed.
Correlation of FACIT-Fatigue improvement with changes in patient-reported outcomes
In the extension period, the correlations in patient-reported outcomes were similar to what was observed at W52. The change in FACIT-F had strong correlations with changes in IBDQ total score and IBDQ domain scores (bowel symptoms, systemic symptoms, emotional function, social function), and SF-36 Mental and Physical Component Summary scores (IBDQ total score: ranging from 0.75 to 0.87, P < .0001; IBDQ bowel symptoms: ranging from 0.59 to 0.77, P < .0001; IBDQ systemic symptoms: ranging from 0.73 to 0.86, P < .0001; IBDQ emotional function: ranging from 0.72 to 0.85, P < .0001; IBDQ social function: ranging from 0.59 to 0.77, P < .0001; SF-Physical Component Summary score: ranging from 0.48 to 0.71, P < .0001; SF-Mental Component Summary score: ranging from 0.62 to 0.79, P < .0001). Moderate correlations were maintained at W104 with changes in PGRS and AP NRS (PGRS: ranging from −0.60 to −0.30, P < .0001; AP NRS: ranging from −0.55 to −0.23, P < .0001; Figure 4b and Table 2).
Correlation of FACIT-Fatigue with clinical measures and biomarkers
In all patients (N = 137) at W104, change in FACIT-F was moderately correlated with change in CDAI total score, AP NRS, and SF (CDAI total score: ranging from −0.60 to −0.30, P < .0001; AP NRS: ranging from −0.51, −0.18, P = .0001; SF: ranging from −0.49, −0.16, P = .0003; Figure 4c and Table 3).
Discussion
In this phase 2, randomized, dose-ranging study, mirikizumab treatment in patients with moderately to severely active CD resulted in sustained FACIT-F score improvements from baseline at W12, W52, and W104. Compared with PBO, mirikizumab IV treatment at all doses (200 mg, 600 mg, and 1000 mg) was associated with significant improvement in FACIT-F during the induction period. In the maintenance and extension periods, patients who were endoscopic improvers at W12 and went on to receive mirikizumab treatment had sustained FACIT-F improvement up to W104 (Supplementary Figure 2). The nonrandomized groups (NI and PBO-treated patients from the induction period) also reached efficacy similar to the randomized group as early as week 24 of the trial (Supplementary Figure 2). At W52, all patients were treated with 300 mg SC mirikizumab up to W104, where all improvements were sustained in the rerandomized and nonrandomized groups with no clinically relevant differences in fatigue-related end points (Figure 4a). These data indicate that SC treatment with mirikizumab for a year after the initial induction and maintenance periods results in sustainable fatigue improvement in both patients who responded or did not respond initially (but achieved a delayed response) to mirikizumab treatment. Additionally, there were no statistically significant differences in FACIT-F in patients with or without endoscopic response or endoscopic remission (Table S1).
In our results, we observed that improvement in fatigue with mirikizumab treatment correlated with improvement in a few physical symptoms (such as abdominal pain, stool frequency, and total CDAI) as well as with multiple mental aspects including emotional, social, and mental well-being up to W104. Interestingly, improvement in fatigue was not associated with objective markers of disease such as endoscopic and biomarker improvements, highlighting that fatigue may perpetuate when inflammation is controlled. Despite fatigue being a typical symptom of iron deficiency anemia in IBD patients, we observed that fatigue in patients with CD was not related to anemia (Table 3). This indicates that the effect of mirikizumab on fatigue improvement in patients with CD is possibly through the improvement of psychological well-being and not directly due to alterations in the inflammatory pathway. One hypothesis is that the altered gut microbiome is impacting the gut-brain axis, where a change in the composition of bacteria in the gut increases systemic inflammation and in turn increases the permeability of the blood-brain axis and can lead to increased perception of fatigue symptoms.32 Another potential bias in our observations could have been due to the variation in qualitative interviews where patients may have had their symptoms vary between screening periods. Additionally, our observation of fatigue improvement had positive correlations with subjective tests such as IBDQ and SF-36; however, fatigue improvement was not associated with objective markers of disease. These observations could be related to the inherent difficulties in fatigue-related research stemming from the lack of conclusive studies on the relationship between fatigue and CD. Fatigue is multidimensional, containing physical, cognitive, and affective components where patients may exhibit only 1 or more of these categories.8 Further investigations into the bidirectional impact of brain-gut interactions could help explain the relationship between subjective perception of well-being and improvement in physical symptoms in CD.
A meta-analysis analyzed 7 therapies for fatigue in IBD and found that the mechanisms of the drugs that resulted in improved fatigue are not fully understood but are likely due to a reduction of pro-inflammatory cytokines.33 Mirikizumab was found to improve fatigue with a strong correlation to improvement in mental well-being up to W104, which is consistent with other biologic therapies.34,35 It has been observed with infliximab, which targets tumor necrosis factors, that the reduction of fatigue in patients with CD correlated with improved mental health but not with inflammation, similar to the findings with mirikizumab.36 Another IL-23p19 inhibitor, risankizumab, has also been shown to improve fatigue measured with FACIT-F, but no correlation with inflammatory cytokines was reported.37 Additional studies such as Villoria et al38 reported no correlation between fatigue scores (measured with FACIT-F), and CRP looking at 202 IBD patients and Jonefjall et al39 reported that patients with IBD in deep remission did not have significant differences in CRP in patients who had fatigue (measured with Multidimensional Fatigue Inventory) from those who did not have fatigue.
Limitations of the SERENITY study include that the data were from a dose-finding phase 2 trial enrolling a small and predominately white patient population, which restricts the generalizability of the results. Additionally, due to the re-randomization in period 2 of the study, there were small patient numbers per treatment group and no true PBO group in the maintenance and extension periods. Since the FACIT-F is a patient-reported outcome measuring fatigue over the last 7 days, recall bias may exist. Other limitations include variables between treatment groups that are difficult to control for such as sleep quality, anxiety, and depression, as these factors have been shown to impact fatigue in IBD patients.40 An additional limitation (or limiting variable) may be the use of nonpharmacological treatments of fatigue, such as concurrent psychiatric therapy and treatment aimed at decreasing sleep disturbances.32 Other limitations of the study are those inherent to post hoc analyses of clinical trial data. These findings will be validated in the VIVID-1 phase 3 study (NCT03926130).
Treatment with mirikizumab in patients with moderately to severely active CD was associated with significantly improved FACIT-F scores that were sustained through W104, where improvement in fatigue correlated better with emotional, social, and mental well-being than with disease-related indicators such as AP NRS, SF, and CDAI score. Overall, these outcomes demonstrate that mirikizumab treatment results in improvement in fatigue as early as 12 weeks and is sustained up to 104 weeks.
Supplementary Material
Acknowledgments
Writing support was provided by Tristan Williams, Ph.D., employee of Eli Lilly and Company; medical review was provided by Konstantinos Tsilkos, MD, employee of Eli Lilly and Company; and statistical review was provided by Jayme R. Gonzales, MS, employee of Eli Lilly and Company.
Contributor Information
Miguel Regueiro, Department of Gastroenterology, Hepatology and Nutrition, Cleveland Clinic, Cleveland, OH, USA.
Monika Fischer, Division of Gastroenterology and Hepatology, Indiana University, Indianapolis, IN, USA.
Peter Bossuyt, Imelda GI Clinical Research Centre, Imelda General Hospital, Bonheiden, Belgium.
Kim McGinnis, Eli Lilly and Company, Indianapolis, IN, USA.
Marijana Protic, Eli Lilly and Company, Indianapolis, IN, USA.
Theresa Hunter Gibble, Eli Lilly and Company, Indianapolis, IN, USA.
Tommaso Panni, Eli Lilly and Company, Indianapolis, IN, USA.
Lai Shan Chan, Eli Lilly and Company, Indianapolis, IN, USA.
Toshifumi Hibi, Kitasato Institute Hospital Center for Advanced Inflammatory Bowel Disease Research and Treatment, Minato-ku, Tokyo, Japan.
David T Rubin, Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, IL, USA.
The study was compliant with the International Conference on Harmonisation guideline on good clinical practice. All informed consent forms and protocols were approved by appropriate ethical review boards before initiation of the study. All patients provided written informed consent before receiving the study drug.
Funding
This work was supported by Eli Lilly and Company.
Conflicts of Interest
M.R.: Advisory Boards and Consultant (both) for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, Boehringer Ingelheim Pharmaceuticals Inc. (BIPI)
M.F.: Advisor, Review Panel Member, Speakers Bureau, or DSMB for AbbVie, BMS, Lilly, Ferring, Janssen, Pfizer, Rebiotix, Scioto, Seres
P.B.: Advisory Committee/Board Member, Grant/Research Support or Speakers Bureau for Abbvie, Amgen, Arena, BMS, CAG, Celltrion, CIRC, Dr. Falk Benelux, Lilly, EPGS, Galapagos, Globalport, Janssen, Materia Prima, Pentax, Pfizer, PSI-CRO, Roche, Scope, Takeda, Tetrameros, Viatris
K.M.: Employee and Stockholder of Lilly
M.P.: Employee and Stockholder of Lilly
T.H.G.: Employee and Stockholder of Lilly
T.P.: Employee and Stockholder of Lilly
L.S.C.: Former Employee and Stockholder of Lilly
T.H. has received lecture fees from, Abbvie GK, EA Pharma Co., Ltd., Janssen Pharmaceutical K.K., JIMRO Co., Ltd., Mitsubishi-Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sandoz K.K. Takeda Pharmaceutical Co., Ltd., Zeria Pharmaceutical Co.,Ltd., advisory/consultancy fees from Abbvie GK, Celltrion Healthcare Japan K.K., EA Pharma Co.,Ltd., Eli Lilly Japan K.K., Gilead Sciences K.K., Janssen Pharmaceutical K.K., Mitsubishi-Tanabe Pharma Corporation, Nichi-Iko Pharmaceutical, Nippon Kayaku Co.,Ltd., Takeda Pharmaceutical Co.,Ltd., Zeria Pharmaceutical Co., Ltd. and research grants from Abbvie GK, Activaid, Alfresa Pharma Corporation, Bristol-Myers Squibb K.K., Eli Lilly Japan K.K., Ferring Pharmaceuticals Co., Ltd., Gilead Sciences K.K., JIMRO Co., Ltd., JMDC Inc., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Miyarisan Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Pfizer Japan Inc., Zeria Pharmaceutical Co., Ltd.
D.T.R.: Reports speaker or consultant fees from AbbVie, Abgenomics, Allergan, Arena Pharmaceuticals, Bellatrix Pharmaceuticals, Boehringer Ingelheim, BMS, CDx Diagnostics, Syneos, Check-Cap, Dizal Pharmaceuticals, Genentech (Roche), Gilead Sciences, Ichnos Sciences (formerly Glenmark Pharmaceuticals), InDex Pharmaceuticals, Iterative Scopes, Janssen, Lilly, Materia Prima, Narrow River Management, Pfizer, Prometheus Laboratories, Reistone, Takeda, and Techlab; and research grants from Takeda.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, except pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and EU and after primary publication acceptance, whichever is later. No expiration date for data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- 1. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380(9853):1590-1605. doi: https://doi.org/ 10.1016/S0140-6736(12)60026-9 [DOI] [PubMed] [Google Scholar]
- 2. Roda G, Chien Ng S, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Primers. 2020;6(1):22. doi: https://doi.org/ 10.1038/s41572-020-0156-2 [DOI] [PubMed] [Google Scholar]
- 3. Wilburn J, McKenna SP, Twiss J, Kemp K, Campbell S. Assessing quality of life in Crohn’s disease: development and validation of the Crohn’s Life Impact Questionnaire (CLIQ). Qual Life Res. 2015;24(9):2279-2288. doi: https://doi.org/ 10.1007/s11136-015-0947-1 [DOI] [PubMed] [Google Scholar]
- 4. Gower-Rousseau C, Sarter H, Savoye G, et al. ; International Programme to Develop New Indexes for Crohn's Disease (IPNIC) group. Validation of the Inflammatory Bowel Disease Disability Index in a population-based cohort. Gut. 2017;66(4):588-596. doi: https://doi.org/ 10.1136/gutjnl-2015-310151 [DOI] [PubMed] [Google Scholar]
- 5. Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD. STRIDE-II: an update on the selecting therapeutic targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi: https://doi.org/ 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 6. Romkens TE, van Vugt-van Pinxteren MW, Nagengast FM, van Oijen MG, de Jong DJ. High prevalence of fatigue in inflammatory bowel disease: a case control study. J Crohns Colitis. 2011;5(4):332-337. doi: https://doi.org/ 10.1016/j.crohns.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 7. Barsevick AM, Cleeland CS, Manning DC, et al. ; ASCPRO (Assessing Symptoms of Cancer Using Patient-Reported Outcomes). ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage. 2010;39(6):1086-1099. doi: https://doi.org/ 10.1016/j.jpainsymman.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Langenberg DR, Gibson PR. Systematic review: fatigue in inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32(2):131-143. doi: https://doi.org/ 10.1111/j.1365-2036.2010.04347.x [DOI] [PubMed] [Google Scholar]
- 9. Regueiro M, Hunter T, Lukanova R, et al. Burden of fatigue among patients with ulcerative colitis and Crohn’s disease: results from a global survey of patients and gastroenterologists. Adv Ther. 2023;40(2):474-488. doi: https://doi.org/ 10.1007/s12325-022-02364-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. IsHak WW, Pan D, Steiner AJ, et al. Patient-reported outcomes of quality of life, functioning, and GI/psychiatric symptom severity in patients with inflammatory bowel disease (IBD). Inflamm Bowel Dis. 2017;23(5):798-803. doi: https://doi.org/ 10.1097/MIB.0000000000001060 [DOI] [PubMed] [Google Scholar]
- 11. Cohen BL, Zoega H, Shah SA, et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther. 2014;39(8):811-822. doi: https://doi.org/ 10.1111/apt.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graff LA, Walker JR, Lix L, et al. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin Gastroenterol Hepatol. 2006;4(12):1491-1501. doi: https://doi.org/ 10.1016/j.cgh.2006.09.027 [DOI] [PubMed] [Google Scholar]
- 13. Huppertz-Hauss G, Hoivik ML, Jelsness-Jorgensen LP, et al. Fatigue in a population-based cohort of patients with inflammatory bowel disease 20 years after diagnosis: the IBSEN study. Scand J Gastroenterol. 2017;52(3):351-358. doi: https://doi.org/ 10.1080/00365521.2016.1256425 [DOI] [PubMed] [Google Scholar]
- 14. Czuber-Dochan W, Norton C, Bassett P, et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J Crohns Colitis. 2014;8(11):1398-1406. doi: https://doi.org/ 10.1016/j.crohns.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 15. Rubin DT, Sninsky C, Siegmund B, et al. International perspectives on management of inflammatory bowel disease: opinion differences and similarities between patients and physicians from the IBD GAPPS Survey. Inflamm Bowel Dis. 2021;27(12):1942-1953. doi: https://doi.org/ 10.1093/ibd/izab006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regueiro M, Delbecque L, Hunter T, et al. Experience and measurement of fatigue in adults with Crohn’s disease: results from qualitative interviews and a longitudinal 2-week daily diary pilot study. J Patient Rep Outcomes. 2023;7(1):75. doi: https://doi.org/ 10.1186/s41687-023-00612-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Czuber-Dochan W, Ream E, Norton C. Review article: description and management of fatigue in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37(5):505-516. doi: https://doi.org/ 10.1111/apt.12205 [DOI] [PubMed] [Google Scholar]
- 18. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG Clinical Guideline: management of Crohn’s Disease in Adults. Am J Gastroenterol. 2018;113(4):481-517. doi: https://doi.org/ 10.1038/ajg.2018.27 [DOI] [PubMed] [Google Scholar]
- 19. Gomollon F, Dignass A, Annese V, et al. ; ECCO. 3rd European evidence-based consensus on the diagnosis and management of crohn’s disease 2016: part 1: diagnosis and Medical Management. J Crohns Colitis. 2017;11(1):3-25. doi: https://doi.org/ 10.1093/ecco-jcc/jjw168 [DOI] [PubMed] [Google Scholar]
- 20. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33(9):987-995. doi: https://doi.org/ 10.1111/j.1365-2036.2011.04612.x [DOI] [PubMed] [Google Scholar]
- 21. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011;106(4):674-684. doi: https://doi.org/ 10.1038/ajg.2011.60 [DOI] [PubMed] [Google Scholar]
- 22. Rubin DT, Mody R, Davis KL, Wang CC. Real-world assessment of therapy changes, suboptimal treatment and associated costs in patients with ulcerative colitis or Crohn’s disease. Aliment Pharmacol Ther. 2014;39(10):1143-1155. doi: https://doi.org/ 10.1111/apt.12727 [DOI] [PubMed] [Google Scholar]
- 23. Gordon JP, McEwan PC, Maguire A, Sugrue DM, Puelles J. Characterizing unmet medical need and the potential role of new biologic treatment options in patients with ulcerative colitis and Crohn’s disease: a systematic review and clinician surveys. Eur J Gastroenterol Hepatol. 2015;27(7):804-812. doi: https://doi.org/ 10.1097/MEG.0000000000000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Croxford AL, Kulig P, Becher B. IL-12-and IL-23 in health and disease. Cytokine Growth Factor Rev. 2014;25(4):415-421. doi: https://doi.org/ 10.1016/j.cytogfr.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 25. Gheita TA, El G, II, El-Fishawy HS, Aboul-Ezz MA, Kenawy SA. Involvement of IL-23 in enteropathic arthritis patients with inflammatory bowel disease: preliminary results. Clin Rheumatol. 2014;33(5):713-717. doi: https://doi.org/ 10.1007/s10067-013-2469-y [DOI] [PubMed] [Google Scholar]
- 26. Globig AM, Hennecke N, Martin B, et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-gamma+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis. 2014;20(12):2321-2329. doi: https://doi.org/ 10.1097/MIB.0000000000000210 [DOI] [PubMed] [Google Scholar]
- 27. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Ulcerative Colitis. Gastroenterology. 2020;158(3):537-549.e10. doi: https://doi.org/ 10.1053/j.gastro.2019.08.043 [DOI] [PubMed] [Google Scholar]
- 28. Sands BE, Peyrin-Biroulet L, Kierkus J, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022;162(2):495-508. doi: https://doi.org/ 10.1053/j.gastro.2021.10.050 [DOI] [PubMed] [Google Scholar]
- 29. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: https://doi.org/ 10.1186/1477-7525-1-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96(3):804-810. doi: https://doi.org/ 10.1016/0016-5085(89)90905-0 [DOI] [PubMed] [Google Scholar]
- 31. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: https://doi.org/ 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 32. Nocerino A, Nguyen A, Agrawal M, et al. Fatigue in inflammatory bowel diseases: etiologies and management. Adv Ther. 2020;37(1):97-112. doi: https://doi.org/ 10.1007/s12325-019-01151-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skjellerudsveen BM, Skoie IM, Dalen I, Grimstad T, Omdal R. The effect of biological treatment on fatigue in inflammatory bowel disease: a systematic review and meta-analysis. Drugs. 2023;83(10):909-921. doi: https://doi.org/ 10.1007/s40265-023-01888-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vermeire S, Loftus EV, Jr, Colombel JF, et al. Long-term efficacy of vedolizumab for Crohn’s Disease. J Crohns Colitis. 2017;11(4):412-424. doi: https://doi.org/ 10.1093/ecco-jcc/jjw176 [DOI] [PubMed] [Google Scholar]
- 35. Panes J, Vermeire S, D’Haens GR, et al. ; STARDUST study group. Ustekinumab improves health-related quality of life in patients with moderate-to-severe Crohn’s disease: results up to week 104 of the STARDUST trial. United European Gastroenterol J. 2023;11(5):410-422. doi: https://doi.org/ 10.1002/ueg2.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Minderhoud IM, Samsom M, Oldenburg B. Crohn’s disease, fatigue, and infliximab: is there a role for cytokines in the pathogenesis of fatigue? World J Gastroenterol. 2007;13(14):2089-2093. doi: https://doi.org/ 10.3748/wjg.v13.i14.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peyrin-Biroulet L, Ghosh S, Lee SD, et al. Effect of risankizumab on health-related quality of life in patients with Crohn’s disease: results from phase 3 MOTIVATE, ADVANCE and FORTIFY clinical trials. Aliment Pharmacol Ther. 2023;57(5):496-508. doi: https://doi.org/ 10.1111/apt.17242 [DOI] [PubMed] [Google Scholar]
- 38. Villoria A, Garcia V, Dosal A, et al. Fatigue in out-patients with inflammatory bowel disease: prevalence and predictive factors. PLoS One. 2017;12(7):e0181435. doi: https://doi.org/ 10.1371/journal.pone.0181435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jonefjall B, Simren M, Lasson A, Ohman L, Strid H. Psychological distress, iron deficiency, active disease and female gender are independent risk factors for fatigue in patients with ulcerative colitis. United European Gastroenterol J. 2018;6(1):148-158. doi: https://doi.org/ 10.1177/2050640617703868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bernstein CN, Fisk JD, Dolovich C, et al. Understanding predictors of fatigue over time in persons with Inflammatory Bowel Disease: the importance of depressive and anxiety symptoms. Am J Gastroenterol. 2024;119(5):922-929. doi: https://doi.org/ 10.14309/ajg.0000000000002630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, except pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and EU and after primary publication acceptance, whichever is later. No expiration date for data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.