Abstract
The hemoflagellates, Trypanosoma spp. and Leishmania spp., are causal agents of a number of parasitic diseases having a major impact on humans and domestic animals over vast areas of the globe. Among the diseases are some of the most pernicious and deadly of human afflictions: African sleeping sickness, Chagas' disease, kala-azar, and Oriental sore. The organisms have complex, pleomorphic life cycles typically involving a vertebrate and an invertebrate host, the latter serving as a vector. In the vertebrate host, they are primarily blood and tissue parasites. In their transition from one host to another, the hemoflagellates undergo morphological, physiological, and biochemical changes that facilitate their growth and subsequent transmission. A major goal in the study of the hemoflagellates has been the cultivation in vitro of both vertebrate and invertebrate stages of the organisms. The first types of media used in their cultivation, and still useful for establishment of cultures, were undefined and contained a complex of ingredients. These gave way to semidefined formulations which included tissue culture media as a base and, as a next step, addition of tissue culture cells as a feeder layer to promote parasite growth. More recently developed media are completely defined, having replaced the feeder cells with various supplements. Serum, a sometimes-variable component of the media, can be replaced by various serum substitutes. This review focuses on the hemoflagellates that infect humans, describing stages in the development of media leading to the fully defined formulations that are now available for the cultivation of many of these organisms.
INTRODUCTION
The hemoflagellates include medically significant protozoan parasites of humans as well as other vertebrates. Two genera of the group that have a global impact on human health are Leishmania and Trypanosoma. The former genus contains species responsible for cutaneous, mucocutaneous, and visceral leishmaniases; the latter genus includes the agents of Chagas' disease in the Western hemisphere and African sleeping sickness.
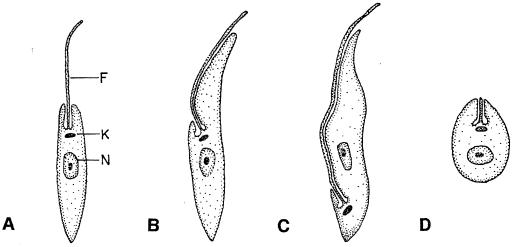
A defining feature of the hemoflagellates—and of the order Kinetoplastida—is a unique DNA-containing organelle, the kinetoplast, associated with the mitochondrion of the organism. Representatives of the hemoflagellates are pleomorphic, adopting a variety of stages in the course of their life cycles. The stages differ morphologically from one another in shape, location of the kinetoplast, and the extent of the flagellum (Fig. 1). The flagellum may appear as a much-shortened structure (typical of the amastigote intracellular stages of Leishmania spp. and Trypanosoma cruzi), an extensive organelle traversing the length of the cell as a so-called undulating membrane (the trypomastigote bloodstream forms of Trypanosoma spp.), or something more typical of flagellated protozoa (the promastigote stage of Leishmania spp.). In addition to morphological differences, there are physiological, biochemical, and metabolic distinctions reflecting the vertebrate (definitive host) or insect (vector) habitats of the stages. The vertebrate-to-insect alternation requires a switch from poikilothermic to homeothermic hosts, with the shift up in temperature imposing stress upon the parasite. An insect acquires infective parasites during a blood meal from an infected mammal. The parasite undergoes a developmental cycle within the insect to produce a stage infective for mammals, which is then transmitted through an insect bite (via the proboscis) or through deposit on the skin surface with subsequent contamination of the break in the skin by the parasite. At least one species, Trypanosoma equiperdum, exhibits dyskinetoplasty in which the kinetoplast has become a rudimentary structure, a condition that may also arise spontaneously in cultured organisms. This species is sexually transmitted between horses and is not dependent upon an insect vector. Other species, such as Trypanosoma evansi, are transmitted mechanically by tabanid flies. In any case, uptake of the infective stage initiates the developmental cycle within the mammalian host. There are reports of genetic exchange among the trypanosomatids, but specifics have yet to be defined (34, 92). Lee and Hutner (60) have generalized the nutritional requirements of the kinetoplastid assemblage to include heme; 6-hydroxymethylpterine and related pteridines; high levels of folic acid; the vitamins thiamine, nicotinic acid, pantothenate, riboflavin, and biotin; and the amino acids required by animals.
FIG. 1.
Schematic illustrations of the hemoflagellate stages discussed in this review. Morphological distinctions include the length of the flagellum and its location, and the position of the kinetoplast with respect to the nucleus of the cell. (A) Promastigote stage of Leishmania spp. found within the insect vector; (B) epimastigote stage typical of Trypanosoma spp. in the insect vector; (C) trypomastigote stage typical of the bloodstream form of Trypanosoma spp.; (D) intracellular amastigote stage of T. cruzi and Leishmania spp. Abbreviations: N, nucleus; K, kinetoplast; F, flagellum. Adapted from reference 60 with permission of the publisher.
Comprehensive reviews of the hemoflagellates can be found in a number of books and monographs, including those of Baker (5), Hoare (49), and Trager (98), and the parasitology text by Roberts and Janovy (82). This review focuses on recent attempts at axenic cultivation of hemoflagellates but will refer to earlier studies which aid in continuity or clarity. Emphasis is placed on those hemoflagellates responsible for human disease, with some attention given to those infecting livestock. Some fundamental features of in vitro cultivation are omitted from descriptions of media and procedures that follow: (i) heat inactivation of sera used in medium preparation to prevent complement-induced cell lysis, (ii) use of 5% CO2-95% air atmosphere for cultivation of hemoflagellates in the presence of tissue culture cells, and (iii) addition of penicillin-streptomycin or gentamicin to media to prevent or minimize contamination of cultures. Another point noted only in the context of different media is the importance of clonal populations for cultivation, as well as for molecular and biochemical studies (65). Many of the following studies, particularly those of more recent vintage, have made use of such cloned cultures.
TRYPANOSOMA SPP.
Overview
The parasitic agents of African sleeping sickness and Chagas' disease take a heavy toll on human lives. Their location in the vector determines the nature of transmission of the infective stage. Salivarian transmission is via the insect proboscis. In stercorarian transmission, the infective stage is deposited in feces on the skin while the vector is feeding; the trypanosomes enter via a break in the skin or through mucous membranes, including the conjunctiva of the eyes. In general, trypanosomes having an anterior station (salivarian forms) in the insect vector have been more difficult to culture than the stercorarian trypanosomes having a posterior station (49, 95).
Interest in cultivation of the trypanosomes developed early in the 20th century beginning with the Novy-McNeal medium for Trypanosoma brucei brucei (72) as modified by Nicolle (71), which consisted of blood agar slants inoculated with infected materials, and is referred to as NNN medium. Parasite growth occurred in the water that had condensed on the agar surface. More recently, a fluid overlay of Locke's solution containing glucose over a nutrient-blood agar base has been employed as a diphasic medium (95). Embryonated eggs have also been used successfully for the cultivation of trypanosomes (79). Reviews by Pipkin (79) and Tobie (94) describe the development of attempts at in vitro cultivation. While capable of maintaining cultures and even supporting heavy growth, diphasic cultures presented problems for harvesting cells for studies of trypanosomal antigenic components, metabolic and biochemical activities, and antimicrobial sensitivities.
In culture, mammalian bloodstream forms of the trypanosomes (trypomastigote stage) revert to the stage found in the vector (49). Thus, another goal of cultivation was to devise in vitro conditions that would retain the bloodstream morphology and physiology of the trypanosomes. This is of particular importance in testing drugs for trypanocidal activity, where antimicrobial sensitivities can be affected by metabolic and synthetic pathways. Another consideration is that the in vitro parasite retains its infectivity for the mammalian host. Tobie (94) noted that trypanosomes of the T. brucei brucei group rapidly lose infectivity in culture, but not those of the T. lewisi group. As with many other pathogenic protozoa as well as bacteria, prolonged in vitro cultivation may result in the loss of infectivity and/or virulence for the host. In the case of the trypanosomes, virulence can be restored in many instances by passage through a vertebrate host. For example, prolonged in vitro growth (14 months) of T. evansi, one of the T. brucei brucei complex of trypanosomes, led to reduction or loss of the kinetoplast (dyskinetoplasty) and a loss of infectivity for mice (108). To address these problems, a number of partially defined media were formulated for in vitro growth of the hemoflagellates, as was the use of a feeder layer of tissue culture cells to provide nutrients for trypanosomal growth. As a more recent development in media formulations, completely defined media have become available for some species of trypanosomes, while others remain refractory to in vitro cultivation (88).
The major focus in cultivating trypanosomes has been on the T. brucei complex, which includes T. brucei brucei, T. brucei rhodesiense, and T. brucei gambiense. T. brucei brucei infects cattle, causing a disease called nagana; T. brucei rhodesiense and T. brucei gambiense infect humans, the former causing a virulent form of sleeping sickness and the latter causing a more chronic form (27). Other species of African trypanosomes (Trypanosoma congolense, T. evansi, T. equiperdum, Trypanosoma simiae, and Trypanosoma vivax) infect cattle, sheep, goats, horses, pigs, camels, and antelope (42). Brun and Jenni (16), de Raadt and Seed (27), Gray et al. (36), Hill and Hirumi (42), Soltys and Woo (88), and Woo (100) have reviewed earlier research on culture techniques for the mammalian and insect forms of African trypanosomes.
To be sure, differences exist between species and strains of trypanosomes regarding their ability to grow in vitro (47). Hill and Hirumi (42) list a number of strains of African trypanosomes and their histories; a somewhat less informative description of strains and species is found in the protist catalog of the American Type Culture Collection (69). For the most part, this review does not include strain distinctions in discussions of medium composition. The interested reader is advised to consult the original literature.
Trypanosoma brucei Complex
The trypanosome is present in the mammalian host as an elongate, slender trypomastigote (posterior kinetoplast, undulating membrane, rudimentary mitochondrion, and no oxidative metabolism [Fig. 1C]), which transforms into a stumpy trypomastigote that is acquired by the insect vector. In the insect midgut, the trypomastigote changes to an epimastigote (anterior kinetoplast, anterior flagellum with partial undulating membrane, functional mitochondrion with oxidative metabolism [Fig. 1B]) and, ultimately, an infective metacyclic trypomastigote in the salivary glands, which is transmitted back to the mammalian host. The presence of a variable glycoprotein coat on the bloodstream trypomastigote stage of the parasite is the basis for their evading the host immune response, enabling them to survive by antigenic shift (78).
Trypanosomes destined for in vitro cultivation are best isolated from naturally infected animals, rather than from laboratory animals infected with syringe-passaged material (42). Lumsden (65) noted the importance of obtaining hemoflagellates as close to their wild origins as possible since change can occur in populations with prolonged cultivation in vitro. Cultivation has progressed from the use of crude media to supplemented tissue culture media such as medium 199 (24), to tissue culture media plus mammalian feeder cells (45), to replacement of the feeder layer with supplements such as thiol compounds (9, 29, 30), and ultimately, to replacement of the serum additive necessary for trypanosome growth (48).
Vector stage cultivation: undefined media.
Pittam (80) cultured vector stages of T. brucei brucei and T. brucei rhodesiense in a complex undefined medium consisting of a base containing tryptose, casein hydrolysate, liver digest, and glucose, to which was added a human blood filtrate. Cell yield in this medium was 3 × 107 cells/ml from an inoculum of 3 × 106 cells in 4 days at about 27°C. Pittam used medium volumes as high as 10 liters for cultivation as a source of trypanosomes for lipid analysis.
A monophasic culture medium for T. brucei brucei was developed by Cross and Manning (24), based on horse blood lysate and broth concentrate (tryptose, casein hydrolysate, liver digest, and glucose), and was the starting point for a semidefined medium utilizing tissue culture medium 199 with casein hydrolysate plus supplements. This medium evolved into a blood-free medium, HX25, with hemin replacing an erythrocyte fraction, and in turn, into a complex defined medium built upon medium 199 with vitamin and amino acid mixtures replacing casein (Table 1). Cell growth was 106 to 107 cells/ml over a 20- to 30-day period.
TABLE 1.
Defined medium HX25 for cultivation of T. bruceia
| Component> | Concn |
|---|---|
| Inorganic salts (mg/liter) | |
| HEPES | 19,000.0 |
| NaCl | 1,500.0 |
| KH2PO4 | 1,000.0 |
| NaHCO3 | 800.0 |
| Trisodium citrate · 2H2O | 600.0 |
| Sodium acetate · 3H2O | 540.0 |
| Sodium succinate · 6H2O | 270.0 |
| Organic (mg/liter) | |
| d(+)-Glucosamine · HCl | 220.0 |
| EDTA, disodium salt | 80.0 |
| Adenosine | 20.0 |
| Guanosine | 20.0 |
| Cytidine | 20.0 |
| Uridine | 20.0 |
| Hemin | 10.0 |
| Tween 40 | 5.0 |
| Folic acid | 10.0 |
| dl-α-Tocopherol | 4.0 |
| Vitamin B12 | 1.0 |
| dl-α-Lipoic acid (oxidized form) | 0.4 |
| Menadione | 0.4 |
| Coenzyme Q 6 | 0.4 |
| Coenzyme Q 10 | 0.4 |
| trans-Retinoic acid | 0.4 |
| Amino acids (mg/liter) | |
| l-Alanine | 160.0 |
| l-Arginine · HCl | 200.0 |
| l-Asparagine | 100.0 |
| l-Aspartic acid | 400.0 |
| l-Cysteine · HCl | 100.0 |
| l-Cystine | 40.0 |
| l-Glutamic acid | 670.0 |
| l-Glutamine | 100.0 |
| Glycine | 100.0 |
| l-Histidine · HCl | 160.0 |
| l-Isoleucine | 300.0 |
| l-Leucine | 460.0 |
| l-Lysine · HCl | 400.0 |
| l-Methionine | 100.0 |
| l-Phenylalanine | 250.0 |
| l-Proline | 580.0 |
| l-Serine | 160.0 |
| l-Threonine | 120.0 |
| l-Tryptophan | 90.0 |
| l-Tyrosine | 160.0 |
| l-Valine | 360.0 |
| Supplementary vitamin mixture (mg/liter) | |
| d-Biotin | 1.0 |
| d-Calcium pantothenate | 1.0 |
| Choline chloride | 1.0 |
| Folic acid | 1.0 |
| l-Inositol | 2.0 |
| Nicotinamide | 1.0 |
| Pyridoxal · HCl | 1.0 |
| Riboflavin | 0.1 |
| Thiamine · HCl | 1.0 |
| Other (ml/liter) | |
| Medium 199, 10× | 88.0 |
| Linoleic acid-albumin complex | 6.0 |
| Supplementary vitamin mixture, 100× | 10.0 |
Medium HX25 was described previously by Cross and Manning (24). pH adjusted to 7.40 with NaOH.
Bloodstream stage cultivation. (i) Cultivation with tissue culture feeder layers.
Le Page (63) made use of tissue culture cells to support growth of the bloodstream stage of T. brucei brucei. Mouse L-line cells were used in NCTC 109 culture medium with 10% presucking calf serum. Up to fivefold increases in cell number with a generation time of 6 to 9 h were reported, but cultures could not be sustained beyond 20 to 30 h.
Bovine fibroblast-like cells growing in RPMI 1640 tissue culture medium plus 20% fetal calf serum served as a feeder layer for growth of T. brucei brucei (45). Trypanosomes retained features of the bloodstream form (including surface antigen and mitochondrial morphology) as well as infectivity for mammalian hosts. This led to a succession of other studies employing tissue culture media with feeder cell layers. Hill et al. (43, 44) employed buffalo lung and Chinese hamster lung cells as feeder layers for cultivation of T. brucei brucei and T. brucei rhodesiense in RPMI 1640 plus 20% fetal calf serum. Growth was from 2 × 106 to 5 × 106 cells/ml. The trypanosomes were morphologically similar to bloodstream forms with regard to surface coat and presence of l-α-glycerophosphate oxidase, a mitochondrial enzyme found in the bloodstream stage; under these conditions, the trypanosomes were infective for mice and rats. In attempting to define the synergistic relationship between bloodstream trypanosomes and bovine lung feeder cells, Tanner (93), by use of electron microscopy, looked for close contacts between the trypanosomes and the tissue culture monolayer but saw none. Trypanosomal growth did not occur, however, when trypanosomes were separated from the feeder cell layer by a 0.45-μm-pore-size Millipore filter, nor did freeze-thawed bovine lung cells support growth (93). The latter observation established the importance of living cells for the cultivation of the trypanosomes.
Brun et al. (17, 18) used modified Eagle tissue culture medium (MEM) in Earle's salts and 15% rabbit serum to culture T. brucei brucei, T. brucei rhodesiense, and T. brucei gambiense on feeder layers of rabbit and vole fibroblast-like cells (Table 2). Commercial sources of rabbit serum were found to be unsatisfactory. They reported that MEM gave better results than RPMI 1640 medium and that freshly obtained rabbit and human sera were better than fetal calf serum for supporting growth (17). As with the previous studies (43, 44), the culture forms of the trypanosomes were infective for vertebrates.
TABLE 2.
Medium for cultivation of feeder layer fibroblast-like cells and bloodstream-stage trypanosomesa
| Component | Amt |
|---|---|
| MEM with Earle's salts without l-glutamine | 450.0 ml |
| Double-distilled water | 45.0 ml |
| MEM nonessential amino acids, 100× | 5.0 ml |
| HEPES | 3.6 g |
| Glucose | 1.0 g |
| l-Glutamine | 150.0 mg |
| Heat-inactivated rabbit serum | 15.0% |
Medium described previously by Brun et al. (17). pH 7.5 after adjustment with 4 N NaOH
T. brucei gambiense has been grown in RPMI 1640-based media with murine bone marrow or rat astroglial feeder cell layers by Balber (8) and by Yabu and Takayanagi (101), respectively. Balber obtained yields of about 2 × 106 cells/ml, with a doubling time of about 6 h. In his system, Balber (8) found that adherent clusters of epithelioid-adipocyte cells produced trypanosome growth, while fibroblasts alone or nonadherent cells would not support growth. Astroglial cell feeder layers gave trypanosome yields of 7 × 106 to 8 × 106 cells/ml, with 7- to 8-h doubling times (101).
About 20 different cell lines have been used as feeder layers to support growth of trypanosomes of the T. brucei group (Table 1 in Hill and Hirumi; [42]), and include bovine-derived cells (from heart, lung, peritoneum, pericardium, and aorta), blood cells (from white rabbit and bovine), and more-conventional cell types such as African green monkey kidney, Chinese hamster lung, buffalo lung, and miscellaneous others such as mouse L-line and dog kidney cells. The cell types used represent mostly fibroblasts but also included epithelial and endothelial cells. In a study that focused on differentiation of bloodstream trypomastigotes into procyclic stages, typical of trypanosomes found in the tsetse fly gut, Simpson et al. (87) used human fetal lung fibroblasts and human epithelial cells as feeder layers. They noted that trypanosomes growing in the fibroblast culture system were sequestered in intercellular spaces of the cell sheet, while those growing in cultures with epithelial layers were found in the supernatant fluid and were more readily observed and harvested.
(ii) Cultivation without feeder layers.
With a better understanding of the relationship between the feeder layers and trypanosomes, it was now possible to contrast growth in the presence and absence of tissue culture cells. Baltz et al. (9) cultured bloodstream forms of the T. brucei brucei complex with and without macrophage feeder layers, as well as T. equiperdum and T. evansi, in a semidefined medium of MEM in Earle's salts with rabbit or human serum at 10%. Additional supplements included pyruvate and a reducing agent (either 2-mercaptoethanol or thioglycerol), hypoxanthine, thymidine, and a feeder layer of mouse peritoneal macrophages. The presence of a reducing agent at 0.2 mM was found to be essential for trypanosome growth. They also noted differences in the sera used: commercial sera were less satisfactory than fresh sera, horse and pig sera were less satisfactory than rabbit serum, and human serum was toxic for the non-human trypanosome strains and even for a strain of T. brucei rhodesiense. Growth of 10 strains of trypanosomes in the semidefined medium with freshly prepared rabbit serum ranged from <1 × 106 to 4 × 106 cells/ml, with generation times of 6 to 32 h. Baltz et al. (9) characterized the steps in transition of trypanosomes from animal host to in vitro growth, recognizing an initiation phase with use of a feeder layer of macrophages, a period of adaptation with or without a feeder layer depending upon the trypanosomal strain, and finally, maintenance of the strain in the absence of the feeder layer.
Duszenko et al. (29) stressed the importance of a reducing agent for cultivation of bloodstream T. brucei brucei. They concluded that reducing agents in the medium were converting cystine to cysteine and that cysteine was the growth factor provided to the trypanosomes by the tissue culture feeder cells; providing cysteine eliminated the need for the feeder cell layer. Optimal concentrations of cysteine and 2-mercaptoethanol were determined to be 10−3 and 10−6 M, respectively. The cysteine level was also critical; a cysteine concentration that was too high (2 to 3 × 10−4 M) was toxic to trypanosomes, presumably by undergoing auto-oxidation to generate hydrogen peroxide. The peroxide effect could be countered by addition of catalase, which is lacking in bloodstream trypanosomes. However, the medium required replenishment of 2-mercaptoethanol twice daily in order to maintain a sufficient supply of cysteine. Brun and Jenni (16) postulated that the function of the feeder layer was to provide a reducing condition in the culture, a source of growth factors for trypanosome proliferation, detoxification of the added serum, and modulation of the gas phase in the culture.
Bloodstream stages of T. brucei brucei and T. brucei rhodesiense from mice were cultured by Kaminsky et al. (56) at 37°C with mouse fibroblasts as a feeder layer. Transformation into procyclic forms typical of the insect vector was effected by transfer of feeder culture trypomastigotes into medium with insect tissue explants or cells (Glossina morsitans, Phormia regina, or Anopheles gambiae) at 27°C and that they would develop into epimastigote and procyclic stages infective for mice. Reversion to bloodstream forms occurred when the trypomastigotes were returned to medium with mouse fibroblast feeder layers. Thus, it was possible to complete the trypanosome life cycle in vitro. Addressing direct isolation of bloodstream trypanosomes (T. brucei brucei and T. evansi) from animals with light infections, Zweygarth and coworkers (106, 107) employed combinations of commercial tissue culture media (MEM, Leibovitz's L-15, Ham's F-12, Iscove's modified Dulbecco Medium [IMDM], and SFRE 199-2) with supplements (including 2-mercaptoethanol) to enhance growth from sparse inocula. With or without feeder layers of bovine, pig, and mouse cells, they were able to establish cultures of most trypanosome stocks, although one T. brucei brucei stock (CP 271 from a goat) gave inconsistent results. In general, MEM was the most-useful medium, while RPMI 1640 and Leibovitz's medium were not. T. brucei brucei cultures gave doubling times of 13 to 15 h in MEM; doubling times of T. evansi cultures were about 25 h. Zweygarth and coworkers were able to establish cultures from inocula in which trypanosomes were not detectable in wet blood films (<103 trypanosomes/ml). Ross and Taylor (83) made use of this system to culture isolates of T. evansi to test for trypanosome sensitivity to 11-oxatetradecanoic acid, a myristic acid analog. Myristic acid serves to anchor the variable surface glycoproteins to the trypanosomes, and replacement with the fatty acid analog inhibited growth.
In a study building upon earlier work with reducing agents (29), Duszenko et al. (30) reiterated that cysteine was essential for in vitro growth of bloodstream stages of T. brucei brucei and that copper ions in the growth medium catalyzed its auto-oxidation to cystine. In order to prevent this from happening, a copper chelator, bathocuproine sulfonate (BCS), was added to the medium. Thus, the reducing agent could be dispensed with, since it was not essential for growth as long as cysteine was present. They suggested that cysteine was needed for synthesis of protein as well as glutathione and trypanothione, which are protective against oxidative stress. Using T. brucei gambiense, Yabu et al. (102) found that, in the absence of added BCS, 65 to 70% of the cysteine underwent oxidation within 1 h and confirmed cysteine toxicity for trypanosomes at high concentrations in the absence of the copper chelator.
Reduced serum and serum-free cultivation.
Preparation of media without a serum component that would promote growth has been an attractive goal in hemoflagellate cultivation. Serum is expensive, highly variable, and may contain factors that are trypanocidal. Thus, eliminating serum from media would be a major advance toward standardization of in vitro growth. Drawing upon studies of Baltz et al. (9) and Duszenko et al. (29), Hirumi and Hirumi (46) established strains of T. brucei brucei and T. brucei gambiense (now reclassified as T. brucei rhodesiense IL-2343 [48]) in a low serum protein medium. Starting with Iscove's modified tissue culture medium as a base, cysteine, hypoxanthine, thymidine, sodium pyruvate, and the copper chelator BCS were added to formulate medium HMI-18 (Table 3). Bloodstream forms of the parasites were established in HMI-9 supplemented with 10% fetal calf serum and 10% of the low serum protein product, Serum Plus. Trypanosomes were weaned from calf serum, replacing it with Serum Plus, until cultures grew in 20% Serum Plus. Serum Plus contained 13 μg of serum protein/ml, as well as growth-promoting factors, hormones, glucose, hemoglobin, and endotoxin. Cell yields of 2 × 106 to 3 × 106 cells/ml were obtained with the trypanosomes retaining their surface coat and infectivity for mice. A variation of this medium containing fetal calf serum was later modified by Hirumi et al. (48), with gradual reduction of serum from 10 to 1% over <90 days for growth of bloodstream T. brucei brucei and T. evansi. Serum was ultimately replaced by several serum-derived components, including fatty acid-free bovine serum albumin, bovine α2-macroglobulin, and bovine β-lipoprotein (Table 4). Growth to >2 × 106 cells/ml occurred with doubling times of 10.6 and 10.9 h in serum-free medium compared to doubling times of 8.4 and 9.5 h in serum-containing medium for T. brucei brucei and T. evansi, respectively.
TABLE 3.
Low serum protein medium HMI-18 for cultivation of T. bruceia
| Componentb | Concn |
|---|---|
| BCS | 0.05 mM |
| l-Cysteine | 1.5 mM |
| Hypoxanthine | 1.0 mM |
| 2-Mercaptoethanol | 0.2 mM |
| Sodium pyruvate | 1.0 mM |
| Thymidine | 0.16 mM |
| Serum Plus | 20.0% |
Medium HMI-18 was described previously by Hirumi and Hirumi (48).
Added to Iscove’s modified Dulbecco medium.
TABLE 4.
Serum replacement supplement for cultivation of T. brucei and T. evansia
| Component | Concn (mg/liter) |
|---|---|
| d-Biotin | 1.0 |
| Retinol | 0.1 |
| β-Alanine | 80.0 |
| l-Anserine nitrate salt | 17.0 |
| o-Phosphorylethanolamine | 30.0 |
| Sarcosine | 57.0 |
| l-Ornithine hydrochloride | 3.0 |
| Taurine | 6.0 |
| ATP · Na2 | 5.5 |
| 2′-dCMP | 3.5 |
| 2′-dGMP | 3.5 |
| 5-Methyltetrahydrofolic acid | 0.5 |
| Bovine α2-macroglobulin | 0.2 |
| Bovine β-lipoprotein | 0.1 |
| Fatty acid-free bovine serum albumin | 5,000.0 |
Serum replacement supplement was described previously by Hirumi et al. (48).
Other growth-promoting factors.
Hide et al. (41) have shown the presence of an epidermal growth factor (EGF) receptor in T. brucei brucei, raising the possibility that this and other cytokine or cytokine-like substances might regulate parasite growth in the host. Addition of EGF to MEM supplemented with cysteine and BCS was found to boost significantly proliferation of the bloodstream stage of T. brucei brucei (91). The optimal range for EGF was 20 to 200 nM, many times higher than the reported concentration of EGF in human blood (0.02 to 0.3 nM). According to Sternberg and McGuigan (91), EGF was probably acting as a mitogen. The disparity between the mitogenic stimulatory effect and the bloodstream concentration of EGF suggested that there may be other low-molecular-weight compounds in blood that stimulate proliferation of bloodstream trypanosomes in early stages of infection of the mammalian host. Coppens et al. (23) demonstrated specific receptors for low-density lipoprotein and transferrin in T. brucei brucei, with these compounds being taken up by receptor-mediated endocytosis. This would satisfy the need of bloodstream trypanosomes for cholesterol via the low-density lipoprotein receptor and the need for iron via the transferrin receptor. These and additional cytokine factors and their possible role in growth stimulation of trypanosomes have been reviewed by Barcinski and Costa-Moreira (10).
Hamm et al. (38) used a cell-free axenic medium to examine differentiation of the bloodstream trypomastigote stage of T. brucei to the procyclic stage found in the insect vector. The transformation medium was composed of MEM supplemented with glucose, pyruvate, glutamine, proline, hemin, cis-aconitate, citric acid, and fetal calf serum at 27°C. Indicative of a shift from glycolysis to oxidative metabolism typical of the vector form, oxidative metabolism in stationary phase trypanosomes was inhibited 44% by cyanide and was accompanied by an increase in the number of cristae in the mitochondria as detected by electron microscopy. Hesse et al. (40) observed an oscillating pattern of growth and differentiation in T. brucei brucei grown axenically in cysteine-BCS-supplemented MEM. They found that growth limitation was not due to depletion of essential growth factors in the medium but rather to a parasite-derived substance, likened to a cytokine, that triggered differentiation in the trypanosome population. In the presence of this factor, slender trypomastigotes characteristic of the bloodstream stage transformed into short, stumpy, nondividing cells; this transformation was typical of high-density bloodstream populations of trypanosomes.
Cell numbers produced for the T. brucei brucei complex in vitro are in the range of 106 to 107 trypanosomes/ml. Brun and Jenni (16) have noted, however, that bloodstream trypanosome populations achieve yields of about 109 cells/ml. This suggests that, despite the successes of in vitro systems, there is still room for improvement to boost cell growth.
T. (SCHIZOTRYPANUM) CRUZI
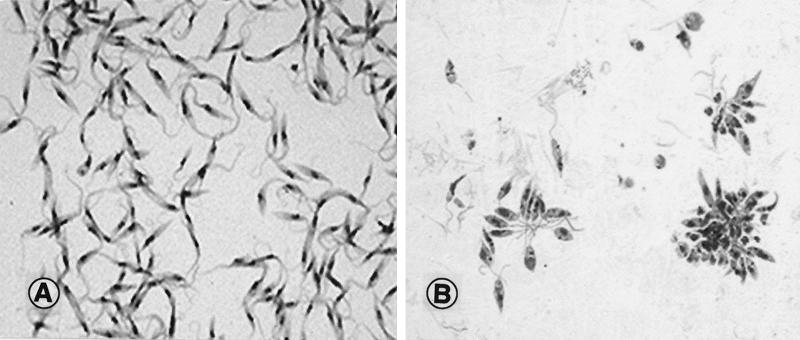
T. cruzi is the etiologic agent of Chagas' disease. Reviews of research on T. cruzi and its relatives are found in the work of Fife (33) and Baker (6). The trypomastigote stage infects the mammalian host by contaminative transmission, invades tissue cells, and transforms into the amastigote form (rounded shape, short flagellum [Fig. 1D]), which is the reproductive stage in the mammalian host. Unlike the salivarian trypanosomes, these forms exhibit oxidative metabolism as bloodstream trypomastigotes. Upon release from ruptured cells, trypomastigotes are acquired by blood-sucking insects, in which they transform into epimastigotes (anterior kinetoplast, elongate anterior flagellum [Fig. 1B and 2A ]) and multiply in the insect midgut, becoming infective metacyclic trypomastigotes.
FIG. 2.
(A) Epimastigote stage of T. cruzi from culture, the stage normally seen in the insect vector. Flagella can be seen arising from the slender organisms. The dark-staining body within the cell is the nucleus. Magnification, ×1,000. (B) Promastigote stage of L. donovani from culture, the stage normally seen in the insect vector. The organisms are clustered in the forms of rosettes, held together by their intertwined flagella. Magnification, ×800.
Vector stage cultivation. (i) Undefined media.
Citri and Grossowicz (22) initially employed a diphasic medium containing tomato juice, human hemoglobin extract, and human plasma for growth of T. cruzi. This medium was the basis for formulating a partially defined medium containing casein hydrolysate, hematin, and serum albumin, in which tomato juice was replaced by 16 growth factors. Serum albumin, while not essential for growth, presumably served as a detoxifying agent. Cell yields were 5 × 107 cells/ml. Steiger and Steiger (90) also found serum albumin helpful in promoting growth of Leishmania spp. (see below) and suggested that it might promote pinocytotic activity of the cells.
Zeledon (103) used a medium based upon brain heart infusion and 10% sheep blood, boiled and filtered, to culture T. cruzi. The medium was selective in that Trypanosoma rangeli, would not grow. Cell yields were about 4 × 107 cells/ml.
(ii) Semidefined and defined media.
Axenic growth of the insect stage in the T. cruzi life cycle was accomplished by a number of investigators. de Azevedo and Roitman (26) modified the medium HX25 (Table 1) developed by Cross and Manning (24) for T. brucei brucei to culture a strain of T. cruzi and obtained yields of about 2 × 107 cells/ml. Hendricks et al. (39) used medium 199 and Grace's and Schneider's insect tissue culture media with 30% fetal calf serum for growth of T. cruzi and T. rangeli as well as Leishmania sp. Avila et al. (3) developed a completely defined medium which supported the growth of five different strains of T. cruzi; bovine liver catalase at a relatively high concentration (3 mg/ml) served as an amino acid source. Other macromolecules that also served as amino acid sources were lactoperoxidase, horseradish peroxidase, and bovine hemoglobin. With these amino acid sources yields of 2 × 107 cells/ml were obtained. Subsequently, Avila et al. (4) formulated a minimal medium containing bovine liver catalase as an amino acid source, with the vitamins choline and folic acid and glucose. This medium, while supporting growth of seven different T. cruzi isolates, did not support growth of T. rangeli and Leishmania spp. It has been noted, however, that catalase may contain protein, DNA, and RNA (74). Sadigursky and Brodskyn (84) produced serum- and blood-free medium (LIT) which supported growth of T. cruzi, as well as Leishmania spp. (see below), giving yields of about 107 cells/ml. The medium contained a base of liver infusion broth, tryptose, and glucose, to which was added a concentrated (20×) mixture of RPMI 1640 and medium 199.
A cell-free fluid medium (F-69) was developed by S. Pan (77) which successfully grew not only the flagellate stage of T. cruzi at 27°C but also the intracellular amastigote stage at 37°C (see below). Composition of the medium included medium 199; Trypticase; hemin; the nucleotides ATP, ADP, and AMP; a vitamin mixture; and 10% fetal calf serum (Table 5). Yields of epimastigotes at 27°C were about 9 × 107 cells/ml and were about 3 × 107 cells/ml for amastigotes at 37°C.
TABLE 5.
Enriched medium F-69 for growth of T. cruzi amastigotes and epimastigotesa
| Component | Amt |
|---|---|
| Basal medium | |
| Distilled water | 22.0 ml |
| Medium 199, 10× | 10.0 ml |
| 0.8% glucose | 25.0 ml |
| 0.1 M sodium pyruvate | 2.0 ml |
| 5% Trypticase | 10.0 ml |
| Vitamin mixtureb | 1.0 ml |
| Biotin (2 mg/ml) | 1.0 ml |
| Folic acid (10 mg/ml) | 5.0 ml |
| Vitamin B12 (1 mg/ml) | 1.0 ml |
| 4% methyl cellulose | 5.0 ml |
| Hemin (50 mg/ml; in 0.01 N NaOH) | 5.0 ml |
| Penicillin G-streptomycin (100,000 U/ml) | 0.1 ml |
| ATP, ADP, AMP solutionb | 10.0 ml |
| 4.2% NaHCO3 | 3.0 ml |
| Vitamin mixture | |
| p-Aminobenzoic acid | 30 mg |
| d-Calcium pantothenate | 40 mg |
| Choline chloride | 30 mg |
| Isoinositol | 30 mg |
| Nicotinamide | 50 mg |
| Nicotinic acid | 20 mg |
| Pyridoxal · HCl | 20 mg |
| Pyridoxine · HCl | 20 mg |
| Pyridoxamine · 2HCl | 20 mg |
| Riboflavin-5-phosphate-Na · 2H2O | 4 mg |
| Thiamine | 20 mg |
| Distilled water | 100 ml |
| ATP, ADP, AMP solution | |
| ATP | 50 mg |
| ADP | 10 mg |
| AMP | 10 mg |
| Glutathione (reduced) | 20 mg |
| l-Cysteine · HCl | 5 mg |
| Ascorbic acid | 5 mg |
| Distilled water | 100 ml |
Enriched medium F-69 was described previously by S. Pan (77).
Described in this table.
(iii) Serum-free cultivation.
O'Daly et al. (74) approached the formulation of a synthetic T. cruzi medium by examining amino acid concentrations in trypanosome extracts. Suitabilities of media were also compared at a range of temperatures from 26 to 37°C. Their enriched synthetic medium (ESM) was tested with either 5% fetal calf serum or a peptide fraction of serum. Growth in serum-containing ESM produced somewhat higher cell yields (3 to 6 × 107 cells/ml, with a generation time of 40 to 45 h) than ESM with peptide (about 1 × 107 cells/ml, with a generation time of 45 to 48 h). The peptide fraction was able to replace serum and contained one glutamic acid, one lysine, and two alanines. By single component deletions from ESM, they also demonstrated a need for 10 amino acids (2 fewer at 26°C) and nine vitamins. The enhanced nutritional need with increased temperatures was ascribed to reduced permeability or heat lability of the enzymes.
Merlen et al. (68) developed a defined medium that was free of serum and macromolecules of serum origin for Leishmania spp. (see below) but that also supported growth of T. cruzi at about 8 × 107 cells/ml. This medium, that of Sadigursky and Brodskyn (82), and medium F-69 (77) were described as hyperosmotic, but that did not appear to adversely affect cell growth. Pan (77) suggested that the high osmolarity of the medium may have aided in the epimastigote-to-trypomastigote-to-amastigote transformations.
Baker (6) summarized several procedures that have been developed to support transformation of T. cruzi epimastigotes into metacyclic trypomastigotes. However, the factors which allow the metacyclogenesis of T. cruzi in vitro are not particularly well understood; Baker (6) does indicate that metacyclogenesis will not occur under conditions that support exponential growth.
Tissue (amastigote) stage cultivation. (i) Whole-tissue-based cultivation.
Since T. cruzi in the mammalian host is found intracellularly as an amastigote, tissue culture was employed early on as a means of growing the organisms in vitro. For example, Kofoid et al. (58) used embryonic heart muscle from rodents to study infection of mammalian tissue in 1935. These early attempts are described by Neva et al. (70), Pipkin (79), and Tobie (94).
(ii) Cultivation with tissue cells.
Neva et al. (70) used a variety of cell cultures to examine effects of temperature on growth of flagellate and amastigote stages of T. cruzi from NNN cultures and from mouse blood. The basic maintenance medium for tissue cells contained bovine embryonic fluids, beef embryo extract, and horse serum. Cell cultures included a number of human, chick, rat, and bovine cell types (amnion, kidney, heart, lung, and foreskin), but they found that embryonic human skin-muscle cultures gave the best results in terms of percentage of cells in culture that became infected. The optimal temperature for infection was 33°C; 38°C also produced intracellular infections but at lower percentages. They noted, too, that the preference for somewhat-lower-than-normal body temperature may have reflected infectivity of T. cruzi for mammals such as the armadillo and the opossum, whose body temperatures are below 37°C. Bayles et al. (13) used chicken embryo fibroblasts in medium 199 plus 20% calf serum to produce intracellular parasites. They found 8 to 22% of cells were infected over the 5-day incubation period of their experiments, 5 days being the length of one intracellular cycle. Hudson et al. (53) utilized two cell lines, M4 and S2, to culture a mouse-derived clone of epimastigotes, M4 being a fibrosarcoma line and S2 being a muscle-derived line. Growth in M4 was limited to one round of parasite replication, while growth in S2 produced continuous cultures of trypomastigotes and amastigotes. Based upon ultrastructure, ability to grow at 37°C, electrophoretic similarity of polypeptides, and oxidation of palmitate to CO2, the authors concluded that their in vitro-produced cells were indeed amastigotes and not just rounded epimastigotes.
(iii) Cell-free media.
Villalta and Kierszenbaum (99) used discontinuous density gradient centrifugation to separate T. cruzi amastigotes from trypomastigotes growing in Vero cell cultures. Amastigotes were then established in cell-free cultures in modified Leibovitz's L-15 medium (59), giving yields of >107 cells/ml, with generation times of 18 to 26 h for three different strains of T. cruzi. Although the trypanosomes were grown in 5% CO2, they also grew without CO2, but with somewhat longer generation times (17 to 36 h). Cultured amastigotes appeared morphologically identical by electron microscopy to amastigotes living within Vero cells, and they actively incorporated tritiated uridine as an indication of metabolic activity (99).
LEISHMANIA SPP.
Overview
The genus Leishmania contains a number of species pathogenic for humans, including Leishmania donovani, Leishmania tropica, Leishmania chagasi, Leishmania major, Leishmania mexicana, Leishmania braziliensis, and Leishmania infantum. They also occur in reservoir hosts such as dogs, rodents, and other mammals (85). Leishmanias can be divided into Old and New World species, the former being responsible for cutaneous and visceral leishmanial infections (Oriental sore, kala-azar) and the latter being responsible for mucocutaneous infections (82). Compared to the Trypanosoma life cycle, that of Leishmania is relatively simple. These organisms are transmitted to the mammalian host by the bite of a sandfly (Phlebotomus sp.), where they develop in the midgut as promastigotes (anterior kinetoplast, elongate anterior flagellum [Fig. 1A and 2B]). In the mammalian host, the leishmanias occupy an intracellular niche as amastigotes (Fig. 1D), mainly within macrophages of the host, where they exist within a parasitophorous vacuole protected from and unaffected by cellular digestive enzymes. Amastigotes are also known as LD bodies (for William Leishman and Charles Donovan, the investigators who identified the disease and its etiological agent). The parasite has long been cultured in the promastigote or insect vector stage. In vitro axenic growth of amastigotes has been relatively recently achieved.
As in the case of the trypanosomes, there are a large number and variety of strains and species, many with complex ancestries (55, 69). Conventional classifications of leishmanias have been based upon clinical manifestations caused by different strains or species (64), but this is an unreliable basis for species identification. More recent taxonomic treatments have made use of molecular, biochemical, and antigenic properties in addition to classical morphological features of the parasites (85). Variation is also found within species as to the ability to grow in particular media, with some strains being more readily amenable to in vitro cultivation. Even the vertebrate source of the inoculum can be a factor in successful cultivation (97).
Leishmanias have been readily cultured as promastigotes (the insect stage) in a variety of media at temperatures below 28°C (7, 75). NNN medium, first used for isolation of the agent of Oriental sore by Nicolle (71), and other diphasic-type media are routinely used for maintenance, producing yields of 107 to 108 cells/ml (19). Indeed, the original formulation of NNN medium remains useful for establishment of strains in culture. Initial isolation of a strain is apt to be most difficult, and the use of traditional diphasic blood media is recommended (32). A number of media developed for trypanosomes as well as commercially available media, either directly or with modifications, have also been used successfully to culture Leishmania promastigotes. Blood is an essential component of these media, with rabbit blood preferable to other types (19, 32). Use of an anticoagulant is recommended, as is heat inactivation of the blood (32). Culture techniques and methods for leishmanias are found in a number of monographs and book chapters, including works by Chang and Fish (19), Chang et al. (20), Evans (32), and Schnur and Jacobson (86). Broader coverage of life cycles, epidemiology, metabolic activity, and pathology, etc., can be found in the works of Janovy (55), Lumsden (65), Roberts and Janovy (82), Schnur and Greenblatt (85), and Zuckerman and Lainson (105). The reader is referred to these works for background information, with this review stressing more recent studies on in vitro cultivation and, where relevant to cultivation and growth conditions, aspects of transformation into the amastigote stage of the Leishmania life cycle.
Vector Stage (Promastigote) Cultivation
Undefined media.
In their review of Leishmania cultivation, Chang and Fish (19) list approximately two dozen formulations of diphasic media that have appeared in the literature since the initial use of NNN medium by Nicolle (71). The solid phases of these media are enriched with a variety of organics, including peptone, beef infusion, glucose, tryptose, liver extract, brain heart infusion, individual amino acids, nutrient or Trypticase soy agars, and, of course, blood—chiefly rabbit blood at concentrations ranging from 2.5 to 50% (Table 7 in the work of Chang and Fish [19]). The liquid phase in this system was generally either the water condensate forming on the agar surface or Locke's solution (95). Chang and Fish estimate yields in diphasic cultures of 106 to 108 cells/ml. Harvesting large numbers of cells from diphasic media presents difficulties, as does contamination of the yields with components from the solid phase of the culture system. Thus, there has been a preference for liquid media for promastigote cultivation. These liquid (monophasic) media have evolved through stages as undefined media (many of the same components found in the solid phase of diphasic media), semidefined media (making use of commercially available insect and mammalian tissue culture media with undefined supplements), and defined media (in which all the components are known). Three such defined media that were described and compared by Chang and Fish (Table 10 in reference [19]) were medium C of Trager (96) for Leishmania tarentolae from lizards, RE I and RE III media of Steiger and Steiger (89, 90) for L. donovani and L. braziliensis, and medium HOSMEM-II (Table 6) of Berens and Marr (14) for L. donovani. Evans (32) and Trager (98) have noted, however, that the hemoflagellate that grew in medium C and was described as L. tarentolae was probably a trypanosome (Trypanosoma platydactyli) and not Leishmania.
TABLE 6.
Defined medium HOSMEM-II for cultivation of L. donovani promastigotesa
| Componentb | Amt |
|---|---|
| MEM from powder | 10.58 g |
| NaHCO3 | 1.0 g |
| MEM amino acids, 50× | 10.0 ml |
| MEM nonessential amino acids, 100× | 10.0 ml |
| 1% Na pyruvate | 11.0 ml |
| 30 mM MOPSd | 6.28 g |
| Glucose | 2.0 g |
| Biotin | 0.1 mg |
| p-Aminobenzoic acid | 1.0 mg |
| Solution Ac | 10.0 ml |
| Solution Bc | 2.5 ml |
| Double-distilled water | 800.0 ml |
| Solution A | |
| Hypoxanthine | 150 mg |
| Ascorbic acid | 2 mg |
| Vitamin B12 | 2 mg |
| Fatty acid-free bovine albumin fraction IV | 150 mg |
| Thioctic acid | 4 mg |
| Menadione | 4 mg |
| Retinol acetate | 4 mg |
| Double-distilled water | 91 ml |
| Solution B | |
| Hemin | 250 mg |
| Folic acid | 500 mg |
| 0.05 N NaOH | 50 ml |
| Double-distilled water | 50 ml |
Medium HOSMEM-II was described previously by Berens and Marr (14).
For components apart from those in solutions A and B, pH was adjusted to 7.2 with 5 N NaOH, volume was brought to 1 liter, and the mixture was filter sterilized. Solution B was added prior to use of the medium. The pH of the medium was adjusted to 8 with 1 N HCl.
Defined in this table.
MOPS, morpholinepropanesulfonic acid.
A medium containing peptone and yeast extract with 10% fetal calf serum was developed by Limoncu et al. (64) as an inexpensive alternative to those containing various types of tissue culture media. This medium was used successfully for cultivation of L. infantum and L. tropica, with cell yields (about 106 cells/ml) comparable to those obtained in their laboratory with NNN medium. Brain heart infusion as a base supplemented with a high concentration of folic acid (100 mg/ml) was used by Kar (57) for cultivation of primary isolates of L. donovani from kala-azar patients. In the absence of folic acid, the medium was inhibitory for growth. The high concentration of folic acid needed to reverse inhibition suggested that it was not functioning as a vitamin, but in some other undefined capacity. The medium with agar added served as a means for cloning promastigotes.
Defined media.
A defined medium which, with agar added, could be used for cloning L. donovani and L. tropica promastigotes was developed by Iovannisci and Ullman (54). The medium consisted of Dulbecco's MEM, to which was added Tween 80, hemin, biotin, bovine serum albumin fraction V, and a purine. The purine requirement was satisfied by any of about a dozen different compounds (e.g., guanine, adenine, hypoxanthine, etc.).
Steiger and Steiger (89, 90) formulated defined media RE I (89) and RE III (90) to support growth of L. donovani and L. braziliensis. The initial RE I medium contained 17 amino acids, and this was decreased to 14 amino acids in RE III. In addition, RE III contained glucose, adenosine, vitamin mix, lipoic acid, and bovine albumin. Bovine albumin, while not essential for growth, improved doubling times in the cultures (see also Cross and Manning [24]). Growth yields in RE III were about 5 × 107 promastigotes/ml for the two leishmanias at 27°C, with doubling times of about 17 h.
Melo et al. (67) modified a medium for T. cruzi (AR-103 of de Azevedo and Roitman, cited by Trager [98]) to produce a defined medium (MD-29) capable of supporting growth of 19 stocks of Leishmania promastigotes, most of which were New World species. ATP and AMP were omitted from and ferric nitrate was added to medium MD-29. Inocula were amastigotes from infected hamsters or promastigotes already in culture. Cell yields were on the order of 107 promastigotes/ml over a 9-day period at 25°C.
McCarthy-Burke et al. (66) formulated defined media based upon commercial tissue culture media for L. donovani promastigotes. They used medium 199 supplemented with folic acid, hemin, and a vitamin mix, or RPMI 1640 with the same additives plus glutamine and adenosine. Both media gave similar cell yields of 3 to 4 × 107 cells/ml which, in turn, were comparable to yields in 199 plus 10% fetal calf serum. Generation time in the RPMI-based medium was about 16 h, while the comparable figure for the medium 199-based medium was about 9 h.
Beginning with an ESM formulated for T. cruzi (see above), O'Daly and Rodriguez (73) prepared a chemically defined medium lacking protein yet capable of supporting growth of 17 leishmanial strains, including the following species: L. donovani, L. brasiliensis, L. mexicana, and Leishmania garnhami. Cell yields were 2 to 4 × 107 cells/ml at 26°C, but the medium would not support growth at temperatures above 26°C. ESM contained 34 amino acids and related intermediates, 23 vitamins, nucleotides, and tetrahydrofolic acid. All strains tested required the amino acids arginine, isoleucine, leucine, lysine, phenylalanine, tryptophan, tyrosine, and valine and the vitamin riboflavin. Needs for other amino acids, vitamins, and nucleotides showed up in subsequent passages of the promastigotes, probably as the result of depletion of intracellular pools. Differences were noted between strains regarding ability to grow in ESM, but they were able to tailor the medium for different strains. Leishmanias retained their virulence for hamsters after growth in ESM, transforming into amastigotes in lesions.
More recently, Merlen et al. (68) devised a defined medium (CDM/LP) for promastigotes of 26 strains of Leishmania and two strains of T. cruzi epimastigotes, which eliminated serum and serum-related macromolecules (Table 7). Growth yields were on the order of 107 cells/ml over about 1 week of growth at 26°C. Proteinase profiles of promastigotes grown in RPMI 1640 with serum and those of promastigotes grown in the serum-free medium were compared using polyacrylamide gel electrophoresis and found to be similar. Likewise, polypeptide and antigenic profiles were similar. Serum-free promastigotes retained infectivity for macrophages (up to 100%) in vitro, as well as for hamsters and mice.
TABLE 7.
Defined medium CDM/LP for cultivation of Leishmania sp. promastigotesa
| Component | Concn |
|---|---|
| Vitamins | |
| l-Ascorbic acid | 0.06 μM |
| d-Biotin | 1.50 μM |
| Folic acid | 3.00 μM |
| p-Aminobenzoic acid | 9.00 μM |
| Nicotinic acid | 0.04 μM |
| Nicotinamide | 9.40 μM |
| d-Pantothenic acid | 0.90 μM |
| Pyridoxine · HCl | 5.40 μM |
| Pyridoxal · HCl | 1.00 μM |
| Riboflavin | 0.56 μM |
| Thiamine · HCl | 0.34 μM |
| Cyanocobalamine | 4.00 μM |
| Calciferol | 0.05 μM |
| Menadione · 3H2O | 0.01 μM |
| l-α-Tocopherol | 0.01 μM |
| Retinol | 0.07 μM |
| Inositol | 0.25 μM |
| Choline | 24.0 μM |
| Cholesterol | 0.10 μM |
| Essential amino acids | |
| l-Arginine | 1.90 mM |
| l-Cysteine · HCl | 0.12 μM |
| l-Cystine · H2O | 0.60 mM |
| l-Glutamine | 2.30 mM |
| l-Histidine · HCl · H2O | 0.13 mM |
| l-Isoleucine | 0.48 mM |
| l-Leucine | 0.60 mM |
| l-Lysine · HCl | 0.32 mM |
| l-Methionine | 0.14 mM |
| l-Phenylalanine | 0.16 mM |
| l-Threonine | 0.29 mM |
| l-Tryptophan | 46.00 μM |
| l-Tyrosine | 0.17 mM |
| l-Valine | 0.27 mM |
| Nonessential amino acids | |
| d-Alanine | 0.11 mM |
| Asparagine | 0.36 mM |
| l-Aspartic acid | 0.26 mM |
| l-Glutamic acid | 0.34 mM |
| Glycine | 0.28 μM |
| l-Proline | 0.20 mM |
| l-Serine | 0.42 mM |
| Glutathione | 3.60 μM |
| Hydroxyproline | 0.17 mM |
| Salts, sugars, and nucleotides | |
| NaCl | 139.00 mM |
| KCl | 7.00 mM |
| CaCl2 | 0.72 mM |
| MgSO4 · 7H2O | 0.61 mM |
| Fe(NO3)3 · 9H2O | 0.36 μM |
| Na2HPO4 | 6.22 mM |
| NaHCO3 | 24.00 mM |
| KH2PO4 | 0.09 mM |
| Glucose | 13.30 mM |
| Hemin | 7.70 mM |
| HEPES | 20.00 mM |
| Na acetate | 0.12 mM |
| d-Ribose | 0.66 mM |
| 2-Deoxyribose | 0.74 mM |
| Tween 80 | 4.00 mg/liter |
| ATP (Na2) | 0.36 μM |
| Adenosine sulfate | 8.60 μM |
| Guanine · HCl | 0.32 μM |
| Hypoxanthine | 0.52 μM |
| Xanthine (Na) | 0.50 μM |
| Uracil | 0.62 μM |
| Thymine | 0.48 μM |
Medium CDM/LP was described previously by Marlen et al. (68). pH adjusted to 7.2 before filter sterilization.
Serum-free media.
Sadigursky and Brodskyn (84) produced an undefined medium for Leishmania amazonensis and L. chagasi promastigotes. As already noted in the section on T. cruzi, the liquid medium (LIT) contained neither blood nor serum and supported leishmanial growth, as well as growth of T. cruzi. The medium contained liver infusion broth, tryptose, glucose and 1% of a 20× mixture of tissue culture media 199 and RPMI 1640. Growth yields in this medium were comparable to those obtained in other standard media containing fetal calf serum, with numbers approaching 107 cells/ml.
L. major promastigotes were cultured in a semisynthetic medium without fetal calf serum by Ali et al. (1). The medium contained beef extract, peptone, and casein hydrolysate, with glucose and proline added. Growth yields (about 107 cells/ml) were comparable, though growth was somewhat slower, to those in RPMI 1640 medium with 10% serum and 2% urine (see below).
Growth-stimulatory agents.
Addition of filter-sterilized human urine at 2% to Schneider's Drosophila medium with 10% fetal calf serum had a stimulatory effect upon growth of 11 different stocks of Leishmania spp. Howard et al. (52) found that cell yields were about 108 cells/ml and, further, that cultures could be established from lesions with as few as 10 amastigotes/ml. The growth-promoting factor in the urine was unknown, though the authors speculated that it might be substances such as EGF (see above). Filter-sterilized urine at a concentration of 5% was used by Armstrong and Patterson (2) for cultivation of L. braziliensis in a medium 199-based medium. They found that the 5% urine produced growth equivalent to the addition of 5% fetal calf serum, with cell yields of >107 promastigotes/ml
Several studies have examined the stimulatory effect of cytokines or cytokine-like substances upon in vitro growth of promastigotes. Growth of L. amazonensis was enhanced by the presence of granulocyte-macrophage colony-stimulating factor (21), and an insulin-like growth factor promoted growth of L. panamensis, L. chagasi, and L. mexicana (35). Inoculum size was also demonstrated as a factor influencing growth patterns of leishmanias (61). Using several different media, neither L. chagasi or L. braziliensis would grow from dilute inocula (about 104 organisms/ml in 50 ml of medium). Lemesre et al. (63) also noted basic differences in growth curves with an L. infantum-L. chagasi pattern of growth characterized by stationary phase after 5 days of growth, while the L. braziliensis-L. amazonensis-L. mexicana species were still in logarithmic phase growth.
What is a promastigote?
Bates (11) sounded a cautionary note regarding the use of axenically cultivated promastigotes for biological and biochemical studies. He raised the question of how closely cultured promastigotes relate to those within the sandfly host. Based on properties such as infectivity and ability to undergo stage-related transformations, he suggested that there is little evidence that they are the same.
Tissue Stage (Amastigote) Cultivation
Induction of amastigote stage.
Attempts at in vitro axenic cultivation of Leishmania amastigotes made use of heat shock and pH to transform promastigotes into amastigotes, essentially replicating the shift in temperature that the promastigote experienced in passing from the insect to the mammalian host (104). Heat-shock, however, resulted in cultures which had finite life spans (<5 days) and could not be serially passaged (11, 76). Variation exists between Leishmania spp. on susceptibility to heat shock-induced transformation (37, 81, 104). Heat shocked leishmanias were more likely to be abnormal promastigotes rather than bona fide amastigotes, in contrast to the real-life situation, in which insect-borne promastigotes are injected into the mammalian host at about 37°C, penetrating a macrophage, and proliferating (80). Although many of the authors whose works are cited below equivocate by calling these extracellular stages amastigote-like or axenic amastigotes, for simplicity we refer to them here as amastigotes.
Metacyclogenesis, the process by which an insect stage promastigote transforms into a metacyclic promastigote preadapted for survival in the vertebrate host was the subject of a study by Cysne-Finkelstein et al. (25). Working with L. amazonensis metacyclic promastigotes growing in Schneider's Drosophila medium with 20% fetal calf serum, pH 5.5 at 32°C, they produced amastigote cultures with no spontaneous reversion. They found that the percentage of transformation into amastigotes, as high as 100%, reflected the percentage of metacyclic forms typical of a particular culture, as indicated by complement-induced lysis. These amastigote cultures were maintained for 2 years.
Balanco et al. (7) grew L. braziliensis promastigotes to stationary phase in a 1:1 mixture of Dulbecco's MEM and Leibovitz's L-15 medium with 20% fetal calf serum (22°C) for transformation. Cells were suspended in UM-54 medium based upon medium 199 with additions of glucose, Trypticase, glutamine, hemin, and fetal calf serum. Promastigotes were then subjected to a regimen of increasing temperatures at approximately weekly intervals: 28, 30, 31, and 32°C. Ultimately, a population of amastigotes was produced that was capable of growing at 33 and 34°C, and giving cell yields of about 108 cells/ml. These amastigotes lacked the paraflagellar rod characteristic of the promastigote stage and were infective for macrophages.
Puentes et al. (81) isolated amastigotes of L. guyanensis and L. panamensis from infected U937 human macrophage-like cells in RPMI 1640 medium with 20% fetal calf serum at a pH of 5.0, establishing them in culture using the same medium at pH 5.5 and 34°C in 5% CO2. Amastigote growth over a period of 9 to 10 days produced populations of 4 × 107 to 5 × 107 cells/ml, with doubling times of 20 to 22 h. Among the criteria used for amastigote characterization were morphological features, surface antigens, thymidine incorporation, cysteine proteinase production, amastigote-specific gene markers, and, finally, infectivity for U937 cells (81).
Whole-tissue-based cultivation.
Since amastigotes develop within cells, it is not surprising that the earliest attempts at their cultivation in vitro should make use of cells that mimicked their normal habitat in the mammalian host. Tissue explants of hamsters, guinea pigs, and chicken embryos have been employed in a variety of culture media, as have peritoneal macrophages and macrophage cell lines (19, 20). The mouse macrophage line J744G8 has been used in many of the following studies to test infectivity of cultured amastigotes for macrophages. Although intracellular cultivation of amastigotes was successful, problems arose for obtaining good cell yields that were free of contaminating host material. This type of culture system has been supplanted by the use of cell-free fluid media for raising amastigotes.
Semidefined media.
Pan's success (75) in producing a stable amastigote stage of L. pifanoi that could be serially passaged was an important accomplishment. The intracellular stages were grown axenically in a heavily enriched medium (Table 8) consisting of tissue culture medium 199, supplemented with Trypticase, hemin, glucose, water-soluble vitamins, nucleotides, and 25% fetal calf serum at a temperature of 33°C. Yields approaching 108 cells/ml were obtained, with a doubling time of about 24 h. Amastigotes developed from promastigotes—up to 99% of the cells transformed—as the pH of the medium became more acidic, from an initial pH of 7.2 to pH 6. Pan also succeeded (75) in transformation and serial subculture of strains of L. braziliensis and L. mexicana. Transformed cells were identified as amastigotes on the basis of their morphological appearance by light- and electron-microscopy, ability to be passaged at elevated temperatures, and their infectivity for cultured macrophages and animals.
TABLE 8.
Cell-free medium JH-31 for cultivation of the intracellular amastigote stage of L. mexicanaa
| Component | Amt |
|---|---|
| Basal solution | |
| 8.0% glucose | 2.5 ml |
| Medium 199, 10× | 10.0 ml |
| 5.0% Trypticase | 10.0 ml |
| Vitamin mixtureb | 2.0 ml |
| Biotin (4 mg/100 ml; in 0.083% HCl) | 2.0 ml |
| Folic acid (10 mg/100 ml; in 0.008% NaOH) | 5.0 ml |
| Vitamin B12 (1 mg/100 ml) | 2.0 ml |
| 0.1 M Na pyruvate | 3.0 ml |
| 4.0% methyl cellulose | 5.0 ml |
| Penicillin (100,000 U/ml) | 0.1 ml |
| Hemin (50 mg/100 ml; in 0.04% NaOH) | 5.0 ml |
| Nucleotide mixtureb | 10.0 ml |
| Fetal bovine serum | 33.5 ml |
| 3% l-glutamine | 2.0 ml |
| Vitamin mixture | |
| p-Aminobenzoic acid | 30 mg |
| d-Ca pantothenate | 40 mg |
| Choline chloride | 30 mg |
| Isoinositol | 30 mg |
| Nicotinamide | 50 mg |
| Nicotinic acid | 20 mg |
| Pyridoxal · HCl | 20 mg |
| Pyridoxine · HCl | 20 mg |
| Pyridoxamine · 2HCl | 20 mg |
| Riboflavin-5-phosphate Na · 2H2O | 4 mg |
| Thiamine · HCl | 20 mg |
| Double-distilled water | 100 ml |
| Nucleotide mixture | |
| ATP | 200 mg |
| ADP | 100 mg |
| AMP | 100 mg |
| Glutathione (reduced) | 20 mg |
| l-Cysteine · HCl | 20 mg |
| Ascorbic acid | 20 mg |
| Double-distilled water | 100 ml |
| Final mediumc | |
| Basal solution | 92.1 ml |
| 1.0 M HEPES | 2.5 ml |
| 4.2% NaHCO3 | 10.0 ml |
| Double-distilled water | 29.5 ml |
Eperon and McMahon-Pratt (31) were able to successfully culture two additional Leishmania spp., L. panamensis and L. braziliensis, as amastigotes at temperatures of 32 and 28°C, respectively. Using Schneider's Drosophila medium with 20% fetal calf serum (considerably less rich than the medium used by Pan [75]) and beginning with a mix of morphological forms at 24°C, they adapted organisms to higher temperatures in 2°C increments. L. panamensis ultimately gave yields of >108 cells/ml; L. braziliensis gave yields of about 108 cells/ml. The amastigote nature of the cells was demonstrated by microscopy, by infectivity for a macrophage line, and by the use of amastigote-specific monoclonal antibodies. Eperon and McMahon-Pratt (31) noted that high concentrations of gentamicin (≥50 μg/ml), the antimicrobial used in their medium, eventually killed the amastigotes. In the same vein, Pan (75) and Pan et al. (76) suggested eliminating or reducing antibiotic (penicillin or gentamicin) added to media, because of sensitivity, particularly at the early stages of amastigote adaptation to culture conditions.
Doyle et al. (28) cultured a strain of L. donovani as amastigotes from transformed promastigotes, using medium 199 with 10% fetal calf serum at 37°C with passages at 2- to 3-day intervals. They reported that their culture forms corresponded to amastigotes in ultrastructural morphology, freeze fracture appearance of membrane particle patterns, and metabolic activity. They were unable, however, to establish several other L. donovani strains in culture as amastigotes, nor would a strain of L. chagasi grow in this axenic system.
Bates et al. (12) established in vitro cultures of amastigotes using L. mexicana taken directly from lesions of infected laboratory mice. They employed Schneider's Drosophila medium with 20% fetal calf serum, with an optimal initial pH of 5.4 and a temperature of 32 to 33°C. Cell yields were 108 cells/ml or slightly less, with doubling times of 18 to 22 h. Amastigotes were characterized on morphological bases (including specialized lysosomes called megasomes), the presence of cysteine proteinases, and infectivity for mice and a macrophage cell line. They compared the preference for a growth medium with an acid pH to the habitat of the amastigote within the parasitophorous vacuole of the macrophage, actually a phagolysosome with a decidedly acid pH (12). Bates (11) noted that in addition to being cultured at acidic pHs, some strains grew better when provided with a higher concentration of CO2 (6.5% CO2-93.5% air) than that generally used for tissue culturing (5% CO2-95% air).
Working with L. amazonensis, Hodgkinson et al. (51) effected transformation of promastigotes growing in Schneider's Drosophila medium with 20% fetal calf serum at pH 4.6 at 24°C. Amastigotes were produced upon transfer to 32°C, with yields of about 106 cells/ml and a generation time of 17 h. The species was tolerant of a relatively acidic pH, but pH of the medium rose to about 5 after 4 days of growth. They noted that, in order for the transformation to occur, a growth period at 24°C was necessary. The resultant amastigotes were reported to be infectious for mice, peritoneal macrophages, and a macrophage cell line. In a later study of in vitro maintenance of L. amazonensis amastigotes isolated from mouse cutaneous lesions, Hodgkinson and Soong (50) reported that amastigotes continued to reproduce after 96 h in Schneider's medium with serum, pH 4.6 at 31°C. Cell yields and metabolic activity, however, began to decline in the cultures after 48 to 72 h, limiting the usefulness of the culture system to short-term studies of amastigotes. Absence of paraxial rod protein—a flagellar component found in the promastigote—in these amastigotes was demonstrated by use of a monoclonal antibody specific for it. In the latter study (50), immunofluorescence for promastigote antigen GP 46 was found in <4% of the stained cells grown at pH 4.6 and 31°C after 96 h. In contrast, 97 to 100% of cells kept at a pH of 7.3 exhibited promastigote GP 46. Thus, both pH and temperature were critical in controlling the amastigote-to-promastigote transformation. Indicating the sensitivity to temperature for different species, Dwyer (cited in the work of Pan et al. [76]) and Leon et al. (62) suggest that Old World leishmanias tolerate higher temperatures (37 to 39°C) than New World species (28 to 33°C).
What is an amastigote?
The question of what is a “genuine” amastigote, in contrast to a rounded promastigote, has been considered by many of the above authors (see particularly references 11, 37, and 76). Morphological, biochemical, molecular, and antigenic criteria have been cited, as have the ability to grow at mammalian body temperature and infect the host's cells. Most or all of these criteria appear to have been satisfied in the studies that are described above. In a useful summation of information about extracellular in vitro amastigotes, Pan et al. (76) enumerated cultural and other considerations. Use of a growth medium that produces luxuriant cultures is critical, and obviously there are strain differences in terms of suitable media. The calf serum employed is another important consideration. Heat-inactivated (56°C for 60 min) fetal calf serum is the additive of choice, though the concentration used may vary from strain to strain but is usually set at 20%. They note that the medium and its various additions, such as vitamin mix, must be freshly prepared. These components have a finite shelf life in spite of freezing. Finally, in transformation protocols, the transition phases should be stepwise and gradual, rather than abrupt as in the heat-shock procedure. This refers not only to temperature, but also to the pH of the medium in which the transformation is to take place.
HEMOFLAGELLATE CULTIVATION AS DIAGNOSTIC TOOL
Trypanosomiases
For the hemoflagellates found in the bloodstream, probably the simplest and most direct means of detecting their presence is by examination of a Giemsa-stained blood smear under the microscope. In some cases, particularly if isolation of the parasite is required for confirmation, it is desirable to cultivate of the organism. The most convenient approach to cultivation is through the use of NNN medium (71, 72). The medium, because of its relatively short shelf life (2 to 4 weeks with refrigeration) and the infrequent need for its use, would not be stocked in clinical laboratories. However, the Centers for Disease Control and Prevention (CDC) (Division of Parasitic Diseases, Atlanta, Ga.) can provide NNN medium for inoculation with blood or skin biopsy specimens, which can be returned to CDC for workup (Frank Steurer, CDC, personal communication). In other parts of the world, major reference laboratories may provide a similar service.
Leishmaniases
The procedure for isolating leishmanias from suspected cases of cutaneous, mucocutaneous, and visceral leishmaniases is basically similar to that for trypanosomes. In this case it is the amastigote found within host cells, or the promastigote that develops from it, that would be sought. Punch biopsies or needle aspirates serve as the inocula for NNN medium or Schneider's Drosophila medium with 30% fetal bovine serum (15). Other procedures such as splenic puncture and liver biopsy can also yield material but are invasive and less likely to be performed.
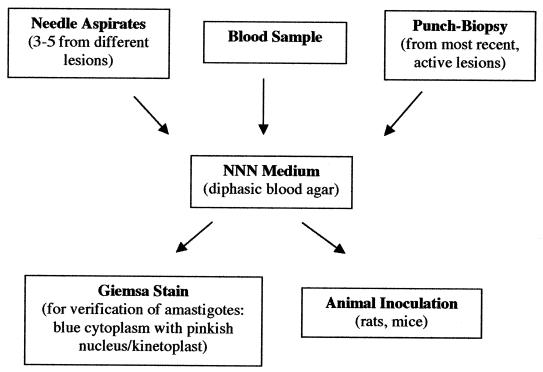
A generic scheme for cultivation of blood and tissue stages of hemoflagellates is presented in Fig. 3. Specimens are obtained as blood samples, needle aspirates, and punch or deep organ biopsies. The ease of detecting parasites in the case of African trypanosomiasis will vary with the subspecies of parasite and the stage of the disease (febrile or afebrile) (15). For leishmanial diseases, some organs such as the spleen may serve as a better source of material for cultivation than blood and skin tissues.
FIG. 3.
Generalized scheme for isolation of representatives of T. cruzi and the African trypanosome complex from blood and other tissues of a vertebrate host. Samples are taken from blood as well as lymphoid tissue and transferred to NNN diphasic blood agar medium. Once established in an enriched medium, trypanosomes can be inoculated into animals or transferred into semidefined or defined culture medium. Isolates behave differently from one another, and it may not be possible to establish all isolates in defined media. The crude media are a good backup in case the organism will not grow under more defined circumstances.
A listing of strains available from the American Type Culture Collection which can serve as positive controls for clinical specimen processing is included (Table 9). Few laboratories would be able or would want to maintain control cultures in laboratory animals. Thus, appropriate media can be employed for in vitro maintenance. Laboratory personnel are cautioned in handling in vitro, in vivo, or clinical specimens as these hemoflagellates are highly pathogenic and should be treated with all precautions observed for dealing with blood-borne pathogens.
TABLE 9.
Selected strains of clinically significant hemoflagellates from the American Type Culture Collection cataloga
| Hemoflagellate | ATCC accession no. | Comments |
|---|---|---|
| T. brucei brucei | 30118 | Shipped frozen; maintained in rat with transfer at 4-day intervals; isolated from tsetse fly |
| T. brucei gambiense | 30025 | As for ATCC 30118; human isolate |
| T. brucei rhodesiense | 30027 | As for ATCC 30118; human isolate |
| T. cruzi (amastigote) | 30208 | Shipped frozen; maintained in rat with transfer at 4-day intervals |
| L. donovani (amastigote) | 30498 | Shipped frozen; maintained in hamster with transfer at 30- to 45-day intervals; human isolate |
| L. donovani (promastigote) | 30142 | Shipped frozen; maintained in vitro on diphasic blood agar medium; human isolate |
All strains listed are pathogenic and require a permit for ordering from the United States Public Health Service. ATCC, American Type Culture Collection (www.atcc.org).
CONCLUSIONS
In vitro cultivation of hemoflagellates of the genera Trypanosoma and Leishmania has progressed from crude media with undefined ingredients to fully defined media with serum substitutes capable of supporting good to excellent growth of the organisms. Most of the media used for in vitro cultivation of hemoflagellates as cited in this review are summarized in Table 10. These cultures have been useful in determining the nutritional requirements of these flagellates and provide a basis for selecting or designing antimicrobial agents that can be tailored to specific pathways, examining the trypanosomal mechanism of antigenic variation, defining immunogenic antigens, and developing attenuated cultures that can be used for protecting humans and cattle from hemoflagellate-caused diseases.
TABLE 10.
Summary of media, hemoflagellates, and life cycle hosts cited in this work
| Species | Type of medium | Reference(s) concerning:
|
|
|---|---|---|---|
| Vertebrate host | Insect host | ||
| T. brucei complex | Undefined | 71, 72 | 24, 80 |
| Semi-defined | 9, 17, 18, 40, 83, 106, 107 | 24 | |
| Defined | 24 | 24 | |
| Tissue culture | 8, 9, 42-45, 56, 63, 87, 101 | ||
| Low or no serum | 46, 48 | ||
| T. cruzi | Undefined | 22, 103 | |
| Semidefined | 99 | 39, 77 | |
| Defined | 3, 4, 26 | ||
| Tissue culture | 13, 53, 70, 99 | ||
| Low or no serum | 68, 74, 84 | ||
| Leishmania spp. | Undefined | 64, 71 | |
| Semidefined | 7, 12, 28, 31, 50, 51, 75, 77 | 2 | |
| Defined | 14, 39, 54, 66-68, 73, 89, 90 | ||
| Tissue culture | 19, 20 | 70 | |
| Low or no serum | 1, 84 | ||
REFERENCES
- 1.Ali, S. A., J. Iqbal, B. Ahmad, and M. Masoom. 1997. A semisynthetic fetal calf serum-free liquid medium for in vitro cultivation of Leishmania promastigotes. Am. J. Trop. Med. Hyg. 59:163-165. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, T. C., and J. L. Patterson. 1994. Cultivation of Leishmania braziliensis in an economical serum-free medium containing human urine. J. Parasitol. 80:1030-1032. [PubMed] [Google Scholar]
- 3.Avila, J. L., A. Bretana, M. A. Casanova, A. Avila, and F. Rodriquez. 1979. Trypanosoma cruzi: defined medium for continuous cultivation of virulent parasites. Exp. Parasitol. 48:27-35. [DOI] [PubMed] [Google Scholar]
- 4.Avila, J. L., R. Perez-Kepp, and A. Bretana. 1983. A minimal medium for the cultivation of infective Trypanosoma cruzi epimastigotes. J. Gen. Microbiol. 129:285-291. [DOI] [PubMed] [Google Scholar]
- 5.Baker, J. R. 1969. Parasitic protozoa. Hutchinson University Library, London, United Kingdom.
- 6.Baker, J. R. 1987. Trypanosoma cruzi and other stercorarian trypanosomes, p. 76-93. In A. E. R. Taylor and J. R. Baker (ed.), In vitro methods for parasite cultivation. Academic Press, New York, N.Y.
- 7.Balanco, J. M. F., E. M. F. Pral, S. da Silva, A. T. Bijovsky, R. A. Mortara, and S. C. Alfieri. 1998. Axenic cultivation and partial characterization of Leishmania braziliensis amastigote-like stages. Parasitology 116:103-113. [DOI] [PubMed] [Google Scholar]
- 8.Balber, A. E. 1983. Primary murine bone marrow cultures support continuous growth of infectious human trypanosomes. Science 220:421-423. [DOI] [PubMed] [Google Scholar]
- 9.Baltz, T., D. Baltz, C. Giroud, and J. Crockett. 1985. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense, and T. gambiense. EMBO J. 4:1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barcinski, M. A., and M. E. Costa-Moreira. 1994. Cellular response of protozoan parasites to host-derived cytokines. Parasitol. Today 10:352-355. [DOI] [PubMed] [Google Scholar]
- 11.Bates, P. A. 1993. Axenic cultivation of Leishmania amastigotes. Parasitol. Today 9:143-146. [DOI] [PubMed] [Google Scholar]
- 12.Bates, P. A., C. D. Robertson, L. Tetley, and G. H. Coombs. 1992. Axenic cultivation and characterization of Leishmania mexicana amastigote-like forms. Parasitology 105:193-202. [DOI] [PubMed] [Google Scholar]
- 13.Bayles, A., J. A. Waitz, and P. E. Thompson. 1966. Growth of Trypanosoma cruzi in cultures of chick embryo cells, and effects of furazolidone and tris (p-aminophenyl) carbonium chloride. J. Protozool. 13:110-114. [DOI] [PubMed] [Google Scholar]
- 14.Berens, R. L., and J. J. Marr. 1978. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J. Parasitol. 64:160. [PubMed] [Google Scholar]
- 15.Bruckner, D. A., and J. A. Labarca. 1999. Leishmania and Trypanosoma. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 16.Brun, R., and L. Jenni. 1987. Salivarian trypanosomes: bloodstream forms (Trypomastigotes), p. 94-117. In A. E. R. Taylor and J. R. Baker (ed.), In vitro methods for parasite cultivation. Academic Press, New York, N.Y.
- 17.Brun, R., L. Jenni, M. Schonenberger, and K.-F. Schell. 1981. In vitro cultivation of bloodstream forms of Trypanosoma brucei, T. rhodesiense, and T. gambiense. J. Protozool. 28:470-479. [DOI] [PubMed] [Google Scholar]
- 18.Brun, R., L. Jenni, M. Tanner, M. Schonenberger, and K.-F. Schell. 1979. Cultivation of vertebrate infective forms derived from metacyclic forms of pleomorphic Trypanosoma brucei stocks. Acta Trop. 36:387-390. [PubMed] [Google Scholar]
- 19.Chang, K.-P., and W. R. Fish. 1983. Leishmania, p. 111-153. In J. B. Jensen (ed.), In vitro cultivation of protozoan parasites. CRC Press, Inc., Boca Raton, Fla.
- 20.Chang, K.-P., C. A. Nacy, and R. D. Pearson. 1986. Intracellular parasitism of macrophages in leishmaniasis: in vitro systems and their applications. Methods Enzymol. 132:603-626. [DOI] [PubMed] [Google Scholar]
- 21.Charlab, R., C. Blaineau, D. Schechtman, and M. A. Barcinski. 1990. Granulocyte-macrophage colony-stimulating factor is a growth-factor for promastigotes of Leishmania mexicana amazonensis. J. Protozool. 37:352-357. [DOI] [PubMed] [Google Scholar]
- 22.Citri, N., and N. Grossowicz. 1955. A partially defined culture medium for Trypanosoma cruzi and some other haemoflagellates. J. Gen. Microbiol. 13:273-278. [DOI] [PubMed] [Google Scholar]
- 23.Coppens, I., F. R. Opperdoes, P. J. Courtoy, and P. Baudhuin. 1987. Receptor-mediated endocytosis in the bloodstream form of Trypanosoma brucei. J. Protozool. 34:465-473. [DOI] [PubMed] [Google Scholar]
- 24.Cross, G. A. M., and J. C. Manning. 1973. Cultivation of Trypanosoma brucei sspp. in semi-defined and defined media. Parasitology 67:315-331. [DOI] [PubMed] [Google Scholar]
- 25.Cysne-Finkelstein, L., R. M. Temporal, F. A. Alves, and L. L. Leon. 1998. Leishmania amazonensis: long-term cultivation of axenic amastigotes is associated to metacyclogenesis of promastigotes. Exp. Parasitol. 89:58-62. [DOI] [PubMed] [Google Scholar]
- 26.de Azevedo, H. P., and I. Roitman. 1977. Growth of the Y strain of Trypanosoma cruzi in an HX-25-modified defined medium. J. Parasitol. 63:485. [PubMed] [Google Scholar]
- 27.de Raadt, P., and J. R. Seed. 1977. Trypanosomes causing disease in man in Africa, p. 175-237. In J. P. Kreier (ed.), Parasitic protozoa, vol. 1. Academic Press, New York, N.Y. [Google Scholar]
- 28.Doyle, P. S., J. C. Engel, P. F. P. Pimenta, P. P. da Silva, and D. M. Dwyer. 1991. Leishmania donovani: long-term culture of axenic amastigotes at 37°C. Exp. Parasitol. 73:326-334. [DOI] [PubMed] [Google Scholar]
- 29.Duszenko, M., M. A. J. Ferguson, G. S. Lamont, M. R. Rifkin, and G. A. M. Cross. 1985. Cysteine eliminates the feeder cell requirements for cultivation of Trypanosoma brucei bloodstream forms in vitro. J. Exp. Med. 162:1256-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duszenko, M., K. Mühlstädt, and A. Broder. 1992. Cystein is an essential growth factor for Trypanosoma brucei bloodstream forms. Mol. Biochem. Parasitol. 50:269-274. [DOI] [PubMed] [Google Scholar]
- 31.Eperon, S., and D. McMahon-Pratt. 1989. I. Extracellular cultivation and morphological characterization of amastigote-like forms of Leishmania panamensis and L. braziliensis. J. Protozool. 36:502-510. [DOI] [PubMed] [Google Scholar]
- 32.Evans, D. A. 1987. Leishmania, p. 52-75. In A. E. R. Taylor and J. R. Baker (ed.), In vitro methods for parasite cultivation. Academic Press, New York, N.Y.
- 33.Fife, E. H., Jr. 1977. Trypanosoma (Schizotrypanum) cruzi, p. 135-173. In J. P. Kreier (ed.), Parasitic protozoa, vol. 1. Academic Press, New York, N.Y. [Google Scholar]
- 34.Gibson, W., and J. Stevens. 1999. Genetic exchange in the Trypanosomatidae. Adv. Parasitol. 43:1-46. [DOI] [PubMed] [Google Scholar]
- 35.Gomes, C. M. C., H. Goto, C. E. P. Corbett, and M. Gidlund. 1997. Insulin-like growth factor-1 is a growth promoting factor for Leishmania promastigotes. Acta Trop. 64:225-228. [DOI] [PubMed] [Google Scholar]
- 36.Gray, M. A., H. Hirumi, and P. R. Gardiner. 1987. Salivarian trypanosomes: insect forms, p. 118-152. In A. E. R. Taylor and J. R. Baker (ed.), In vitro methods for parasite cultivation. Academic Press, New York, N.Y.
- 37.Gupta, N., N. Goyal, and A. K. Rastogi. 2001. In vitro cultivation and characterization of axenic amastigotes of Leishmania. Trends Parasitol. 17:150-153. [DOI] [PubMed] [Google Scholar]
- 38.Hamm, B., A. Schindler, D. Mecke, and M. Duszenko. 1990. Differentiation of Trypanosoma brucei bloodstream trypomastigotes from long slender to short stumpy-like forms in axenic culture. Mol. Biochem. Parasitol. 40:13-22. [DOI] [PubMed] [Google Scholar]
- 39.Hendricks, L. D., D. E. Wood, and M. E. Hajduk. 1978. Haemoflagellates: commercially available liquid media for rapid cultivation. Parasitology 76:309-316. [DOI] [PubMed] [Google Scholar]
- 40.Hesse, F., P. M. Selzer, K. Mühlstadt, and M. Duszenko. 1995. A novel cultivation technique for long-term maintenance of bloodstream form trypanosomes in vitro. Mol. Biochem. Parasitol. 70:157-166. [DOI] [PubMed] [Google Scholar]
- 41.Hide, G., A. Gray, C. M. Harrison, and A. Tait. 1989. Identification of an epidermal growth factor receptor homologue in trypanosomes. Mol. Biochem. Parasitol. 36:51-60. [DOI] [PubMed] [Google Scholar]
- 42.Hill, G. C., and H. Hirumi. 1983. African trypansomes, p. 193-219. In J. B. Jensen (ed.), In vitro cultivation of protozoan parasites. CRC Press, Inc., Boca Raton, Fla.
- 43.Hill, G. C., S. Shimer, B. Caughey, and S. Sauer. 1978. Growth of infective forms of Trypanosoma (T.) brucei on buffalo lung and Chinese hamster lung tissue culture cells. Acta Trop. 35:201-207. [PubMed] [Google Scholar]
- 44.Hill, G. C., S. P. Shimer, B. Caughey, and L. S. Sauer. 1978. Growth of infective forms of Trypanosoma rhodesiense in vitro, the causative agent of African trypanosomiasis. Science 202:763-765. [DOI] [PubMed] [Google Scholar]
- 45.Hirumi, H., J. J. Doyle, and K. Hirumi. 1977. African trypanosomes: cultivation of animal-infective Trypanosoma brucei in vitro. Science 196:992-994. [DOI] [PubMed] [Google Scholar]
- 46.Hirumi, H., and K. Hirumi. 1989. Continuous culture of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:85-989. [PubMed] [Google Scholar]
- 47.Hirumi, H., and K. Hirumi. 1991. In vitro cultivation of Trypanosoma congolense bloodstream forms in the absence of feeder cell layers. Parasitology 102:225-236. [DOI] [PubMed] [Google Scholar]
- 48.Hirumi, H. S. Martin, K. Hirumi, N. Inoue, H. Kanbara, A. Saito, and N. Suzuki. 1997. Cultivation of bloodstream forms of Trypanosoma brucei and T. evansi in a serum-free medium. Trop. Med. Int. Health 2:240-244. [DOI] [PubMed] [Google Scholar]
- 49.Hoare, C. A. 1972. The trypanosomes of mammals. Blackwell Scientific Publications, Oxford, United Kingdom.
- 50.Hodgkinson, V. H., and L. Soong. 1997. In vitro maintenance of Leishmania amastigotes directly from lesions: advantages and limitations. J. Parasitol. 83:953-956. [PubMed] [Google Scholar]
- 51.Hodgkinson, V. H., L. Soong, S. M. Duboise, and D. McMahon-Pratt. 1996. Leishmania amazonensis: cultivation and characterization of axenic amastigote-like organisms. Exp. Parasitol. 83:94-105. [DOI] [PubMed] [Google Scholar]
- 52.Howard, M. K., M. M. Pharoah, F. Ashall, and M. A. Miles. 1991. Human urine stimulates growth of Leishmania in vitro. Trans. R. Soc. Trop. Med. Hyg. 85:477-479. [DOI] [PubMed] [Google Scholar]
- 53.Hudson, L., D. Snary, and S.-J. Morgan. 1984. Trypanosoma cruzi: continuous cultivation with murine cell lines. Parasitology 88:283-294. [PubMed] [Google Scholar]
- 54.Iovannisci, D. M., and B. Ullman. 1983. High efficiency plating method for plating Leishmania promastigotes in semidefined or completely-defined medium. J. Parasitol. 69:633-636. [PubMed] [Google Scholar]
- 55.Janovy, J., Jr. 1987. Physiology and biochemistry, p. 177-210. In W. Peters and R. Killick-Kendrick (ed.), The leishmaniases in biology and medicine, vol. 1. Academic Press, New York, N.Y. [Google Scholar]
- 56.Kaminsky, R., E. Beaudoin, and I. Cunningham. 1988. Cultivation of the life cycle stages of Trypanosoma brucei sspp. Acta Trop. 45:33-43. [PubMed] [Google Scholar]
- 57.Kar, K. 1997. Folic acid the essential supplement to brain heart infusion broth for cultivation and cloning of Leishmania donovani promastigotes. Parasitology 115:231-235. [DOI] [PubMed] [Google Scholar]
- 58.Kofoid, C. A., F. D. Wood, and E. McNeil. 1935. The cycle of Trypanosoma cruzi in tissue culture of embryonic heart muscle. Univ. Calif. Publ. Zool. 41:23-24. [Google Scholar]
- 59.Lanar, D. E. 1979. Growth and differentiation of Trypanosoma cruzi cultivated with a Triatoma infestans embryo cell line. J. Protozool. 26:457-462. [DOI] [PubMed] [Google Scholar]
- 60.Lee, J. J., and S. H. Hutner. 1985. Order 11. Kinetoplastida Honigberg, 1963 Emend Vickerman, 1976, p. 141-155. In J. J. Lee, S. H. Hutner, and E. C. Bovee (ed.), An illustrated guide to the protozoa. Society of Protozoologists, Lawrence, Kans.
- 61.Lemesre, J. L., F. Darcy, M. Kweider, A. Capron, and F. Santoro. 1988. Requirements of defined cultivation conditions for standard growth of Leishmania promastigotes in vitro. Acta Trop. 45:99-108. [PubMed] [Google Scholar]
- 62.Leon, L. L., M. J. Soares, and R. M. Temporal. 1995. Effects of temperature on promastigotes of several species of Leishmania. J. Eukaryot. Microbiol. 42:219-223. [DOI] [PubMed] [Google Scholar]
- 63.Le Page, R. W. F. 1967. Short term cultivation of Trypanosoma brucei in vitro. Nature 216:1141-1142. [DOI] [PubMed] [Google Scholar]
- 64.Limoncu, M. E., I. C. Balcioglu, K. Yereli, Y. Ozbel, and A. Ozbilgin. 1997. A new experimental in vitro culture medium for cultivation of Leishmania species. J. Clin. Microbiol. 35:2430-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lumsden, W. H. R. 1974. Leishmaniasis and trypanosomiasis: the causative organisms compared and contrasted, p. 3-21. In Ciba Foundation symposium 20: trypansomiasis and leishmaniasis with special reference to Chagas' disease. Elsevier, Amsterdam, The Netherlands.
- 66.McCarthy-Burke, C., P. A. Bates, and D. M. Dwyer. 1991. Leishmania donovani: use of two different, commercially available, chemically defined media for the continuous in vitro cultivation of promastigotes. Exp. Parasitol. 73:385-387. [DOI] [PubMed] [Google Scholar]
- 67.Melo, N. M., H. P. de Azevedo, I. Roitman, and W. Mayrink. 1985. A new defined medium for cultivating Leishmania promastigotes. Acta Trop. 42:137-141. [PubMed] [Google Scholar]
- 68.Merlen, T., D. Sereno, N. Brajon, F. Rostand, and J.-L. Lemesre. 1999. Leishmania spp.: completely defined medium without serum and macromolecules (CDM/LP) for the continuous in vitro cultivation of infective promastigote forms. Am. J. Trop. Med. Hyg. 60:41-50. [DOI] [PubMed] [Google Scholar]
- 69.Nerad, T. 1993. Catalog of protists, 18th ed. American Type Culture Collection, Manassas, Va.
- 70.Neva, F. A., M. F. Malone, and B. R. Meyers. 1961. Factors influencing the intracellular growth of Trypanosoma cruzi in vitro. Am. J. Trop. Med. Hyg. 10:140-149. [DOI] [PubMed] [Google Scholar]
- 71.Nicolle, C. 1908. Culture du parasite du bouton d'Orient. C. R. Acad. Sci. 146:842-843. [Google Scholar]
- 72.Novy, F. G., and W. J. McNeal. 1904. On the cultivation of Trypanosoma brucei. J. Infect. Dis. 1:1-30. [Google Scholar]
- 73.O'Daly, J. A., and M. B. Rodriquez. 1988. Differential growth requirements of several Leishmania spp. in chemically defined culture media. Acta Trop. 45:109-126. [PubMed] [Google Scholar]
- 74.O'Daly, J. A., M. B. Rodriquez, and G. Garlin. 1987. Trypanosoma cruzi: growth requirements at different temperatures in fetal bovine serum or peptide supplemented media. Exp. Parasitol. 64:78-87. [DOI] [PubMed] [Google Scholar]
- 75.Pan, A. 1984. Leishmania mexicana: serial cultivation of intracellular stages in a cell-free medium. Exp. Parasitol. 58:72-80. [DOI] [PubMed] [Google Scholar]
- 76.Pan, A. A., S. M. Duboise, S. Eperon, L. Rivas, V. Hodgkinson, Y. Traub-Cseko, and D. McMahon-Pratt. 1993. Developmental life cycle of Leishmania—cultivation and characterization of cultured extracellular amastigotes. J. Eukaryot. Microbiol. 40:213-223. [DOI] [PubMed] [Google Scholar]
- 77.Pan, S. C.-T. 1978. Trypanosoma cruzi: intracellular stages grown in a cell-free medium at 37°C. Exp. Parasitol. 45:215-224. [DOI] [PubMed] [Google Scholar]
- 78.Pays, E., and L. Vanhamme. 1996. Developmental regulation of gene expression in African trypanosomes, p. 88-114. In D. F. Smith and M. Parsons (ed.), Molecular biology of parasitic protozoa. IRL Press, Oxford, United Kingdom.
- 79.Pipkin, A. C. 1960. Avian embryos and tissue culture in the study of parasitic protozoa. II. Protozoa other than Plasmodium. Exp. Parasitol. 9:167-203. [DOI] [PubMed] [Google Scholar]
- 80.Pittam, M. D. 1970. Medium for in vitro culture of Trypanosoma rhodesiense and Trypanosoma brucei. Comp. Biochem. Physiol. 33:127-128. [Google Scholar]
- 81.Puentes, F., D. Diaz, R. D. Hoya, J. A. Gutierrez, J. M. Lozano, M. E. Patarroyo, and A. Moreno. 2000. Cultivation and characterization of stable Leishmania guyanensis complex axenic amastigotes derived from infected U937 cells. Am. J. Trop. Med. Hyg. 63:102-110. [DOI] [PubMed] [Google Scholar]
- 82.Roberts, L. S., and J. Janovy, Jr. 1996. Foundations of parasitology, 5th ed. Wm. C. Brown Publishers, Dubuque, Iowa.
- 83.Ross, C. A., and A. M. Taylor. 1994. Trypanocidal activity of a myristic acid analog in axenic cultures of Trypanosoma evansi. Parasitol. Res. 80:147-153. [DOI] [PubMed] [Google Scholar]
- 84.Sadigursky, M., and C. I. Brodskyn. 1986. A new liquid medium without blood and serum for culture of hemoflagellates. Am. J. Trop. Med. Hyg. 35:942-944. [DOI] [PubMed] [Google Scholar]
- 85.Schnur, L. F., and C. L. Greenblatt. 1995. Leishmania, p. 1-160. In J. P. Kreier (ed.), Parasitic protozoa, vol. 10. Academic Press, New York, N.Y. [Google Scholar]
- 86.Schnur, L. F., and R. L. Jacobson. 1987. Appendix III. Parasitological techniques, p. 499-541. In W. Peters and R. Killick-Kendrick (ed.), The leishmaniases in biology and medicine, vol. 1. Academic Press, New York, N.Y. [Google Scholar]
- 87.Simpson, A. M., D. Hughes, and L. Simpson. 1985. Trypanosoma brucei: differentiation of in vitro-grown bloodstream trypanomastigotes into procyclic forms. J. Protozool. 32:672-677, 685. [DOI] [PubMed] [Google Scholar]
- 88.Soltys, M. A., and P. T. K. Woo. 1977. Trypanosomes producing disease in livestock in Africa, p. 239-268. In J. P. Kreier (ed.), Parasitic protozoa, vol. 1. Academic Press, New York, N.Y. [Google Scholar]
- 89.Steiger, R. F., and E. Steiger. 1976. A defined medium for cultivating Leishmania donovani and L. braziliensis. J. Parasitol. 62:1010-1011. [PubMed] [Google Scholar]
- 90.Steiger, R. F., and E. Steiger. 1977. Cultivation of Leishmania donovani and Leishmania braziliensis in defined media: nutritional requirements. J. Protozool. 24:437-441. [DOI] [PubMed] [Google Scholar]
- 91.Sternberg, J. M., and F. McGuigan. 1994. Trypanosoma brucei: mammalian epidermal growth factor promotes the growth of the African trypanosome bloodstream form. Exp. Parasitol. 78:422-424. [DOI] [PubMed] [Google Scholar]
- 92.Swindle, J., and A. Tait. 1996. Trypanosomatid genetics, p. 6-34. In D. F. Smith and M. Parsons (ed.), Molecular biology of parasitic protozoa. IRL Press, Oxford, United Kingdom.
- 93.Tanner, M. 1980. Studies on the mechanisms supporting the continuous growth of Trypanosoma (Trypanozoon) brucei as bloodstream-like form in vitro. Acta Trop. 37:203-220. [PubMed] [Google Scholar]
- 94.Tobie, E. J. 1964. Cultivation of mammalian trypanosomes. J. Protozool. 11:418-423. [DOI] [PubMed] [Google Scholar]
- 95.Tobie, E. J., T. von Brand, and B. Mehlman. 1950. Cultural and physiological observations on Trypanosoma rhodesiense and Trypanosoma gambiense. J. Parasitol. 36:48-54. [PubMed] [Google Scholar]
- 96.Trager, W. 1957. Nutrition of a hemoflagellates (Leishmania tarentolae) having an interchangeable requirement for choline or pyridoxal. J. Protozool. 4:269-276. [Google Scholar]
- 97.Trager, W. 1974. Nutrition and biosynthetic capabilities of flagellates: problems of in vitro cultivation and differentiation, p. 225-254. In Ciba Foundation symposium 20: trypanosomiasis and leishmaniasis with special reference to Chagas' disease. Elsevier, Amsterdam, The Netherlands.
- 98.Trager, W. 1986. Living together. The biology of animal parasitism. Plenum Press, New York, N.Y.
- 99.Villalta, F., and F. Kierszenbaum. 1982. Growth of isolated amastigotes of Trypanosoma cruzi in cell-free medium. J. Protozool. 29:570-576. [DOI] [PubMed] [Google Scholar]
- 100.Woo, P. T. K. 1977. Salivarian trypanosomes producing disease in livestock outside of sub-Saharan Africa, p. 270-296. In J. P. Kreier (ed.), Parasitic protozoa, vol. 1. Academic Press, New York, N.Y. [Google Scholar]
- 101.Yabu, Y., and T. Takayanagi. 1987. The effective long-term culture and cloning system for Trypanosoma gambiense bloodstream forms in vitro. Parasitol. Res. 73:381-383. [DOI] [PubMed] [Google Scholar]
- 102.Yabu, Y., T. Takayanagi, and S. Sato. 1989. Long-term culture and cloning system for Trypanosoma brucei gambiense bloodstream forms in semi-defined medium in vitro. Parasitol. Res. 76:93-97. [DOI] [PubMed] [Google Scholar]
- 103.Zeledon, R. 1959. Differentiation of Trypanosoma rangeli and Schizotrypanum cruzi in a liquid medium, with notes on the nutrition of flagellates. J. Parasitol. 45:652. [Google Scholar]
- 104.Zilberstein, D., and M. Shapira. 1994. The role of pH and temperature in the development of Leishmania parasites. Annu. Rev. Microbiol. 48:449-470. [DOI] [PubMed] [Google Scholar]
- 105.Zuckerman, A., and R. Lainson. 1977. Leishmania, p. 57-133. In J. P. Kreier (ed.), Parasitic protozoa, vol. 1. Academic Press, New York, N.Y. [Google Scholar]
- 106.Zweygarth, E., and R. Kaminsky. 1990. Direct in vitro isolation of Trypanosoma brucei brucei and T. b. evansi from disease hosts with low parasitaemia. Trop. Med. Parasitol. 41:56-58. [PubMed] [Google Scholar]
- 107.Zweygarth, E., R. Kaminsky, and J. K. Cheruiyot. 1989. A simple and rapid method to initiate Trypanosoma brucei brucei and T. brucei evansi bloodstream form cultures. Acta Trop. 46:205-206. [DOI] [PubMed] [Google Scholar]
- 108.Zweygarth, E., R. Kaminsky, and P. Webster. 1991. Trypanosoma brucei evansi: dyskinetoplasia and loss of infectivity after long-term in vitro cultivation. Acta Trop. 48:95-99. [DOI] [PubMed] [Google Scholar]