Abstract
Pathological response (PR) is an oncological outcome measure that indicates the therapeutic response to neoadjuvant therapy. In clinical trials involving neoadjuvant or perioperative interventions, overall survival and disease/event-free survival are typically the primary outcome measures. Although some evidence suggests that pathological complete response (pCR) can serve as a surrogate marker for the primary endpoint in prospective trials, it remains uncertain whether pCR is a true surrogate marker for patients with cancer undergoing curative resection across all solid tumours. Here, we review the role of PR as a surrogate marker and its associated methodological issues in the era of perioperative immune checkpoint inhibitors.
Key words: immune checkpoint inhibitor, immunotherapy, pathological response, neoadjuvant therapy, perioperative therapy
Highlights
-
•
PR is associated with event-free survival (EFS) and disease-free survival in triple-negative breast cancer and non-small-cell lung cancer.
-
•
Increased pCR rates did not translate into EFS benefits in gastric and gastroesophageal junction cancers.
-
•
Distinguishing patient- and trial-level surrogacy is crucial for PR as a surrogate endpoint for survival.
-
•
EFS and overall survival remain the standard endpoints until PR is confirmed as a robust surrogate marker.
Introduction
Neoadjuvant (preoperative) and adjuvant (post-operative) systemic therapies are crucial components of locoregional treatment for various types of cancer. Currently, post-operative systemic therapy is a standard of care across different solid tumour origins. Neoadjuvant therapy and perioperative systemic therapy (pre- and post-operative therapy) have been implemented in clinical practice aiming to achieve tumour shrinkage, eliminate micrometastatic disease, enhance the physical condition before major operation, and assess the in vivo sensitivity of cancer to treatment.
Currently, pre- and/or postoperative immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) antibodies are considered standard regimens in triple-negative breast cancer (TNBC),1 non-small-cell lung cancer (NSCLC),2, 3, 4, 5, 6 renal cell carcinoma,7 urothelial cancer,8,9 and melanoma.10, 11, 12, 13, 14 From a theoretical perspective, neoadjuvant ICIs present an advantage in tumour immunology. In particular, inhibition of PD-1/PD-L1 before resection can lead to the expansion of tumour-specific T-cell clones already residing within the tumour microenvironment. This, in turn, enables stimulation of the priming phase of the immune system and increases immune surveillance activity.15,16 Following curative resection, tumour-specific T cells may persist in an active state, and any remaining microscopic residual disease may be monitored and eliminated, potentially leading to an enhanced cure rate. Figure 1 shows the rationale of neoadjuvant immunotherapy.
Figure 1.
The rationale of neoadjuvant immunotherapy. Neoadjuvant immunotherapy induces a broader and stronger T-cell response by targeting the entire tumour. Intact lymph nodes may further enhance antitumour immunity. By contrast, upfront resection reduces tumour volume, limiting the neoantigen burden and potentially impairing T-cell activation and expansion by immune checkpoint inhibitors (ICIs). Following tumour resection, activated and more diverse T cells continue surveillance and eliminate micrometastases.
Histopathological evaluation of pathological response (PR), especially pathological complete response (pCR), has been utilised as an outcome measure for patients treated with neoadjuvant therapy because it reflects the in vivo therapeutic effect of systemic therapy. Outcomes can be obtained rapidly compared with event-free survival (EFS) or overall survival (OS), potentially accelerating the progress of clinical trials and the introduction of novel agents into clinical practice. Before the introduction of ICIs, extensive data supported the correlation between PR and OS and/or EFS in breast,17,18 lung,19, 20, 21 urothelial,22 and rectal cancers23 in the prognosis of individual patients. However, the use of ICIs as opposed to conventional chemotherapy raises uncertainty regarding the relationship between PR and long-term outcomes. In this review, we aimed to examine PR as a surrogate marker for survival within the context of the neoadjuvant ICI era.
Evaluation of pathological response in neoadjuvant immune checkpoint inhibitors
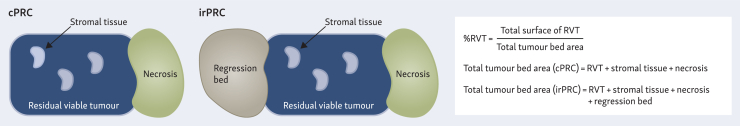
The definition of PR criteria has not been harmonised across different tumour types, unlike the radiological evaluation with RECIST.24 There are different PR scoring systems for breast,25 lung,26 upper and lower gastrointestinal,27, 28, 29 bladder,30 and ovarian cancers,31 each developed for evaluating the effects of neoadjuvant therapy using cytotoxic agents with or without radiotherapy. Table 1 presents a description of representative PR evaluation systems. However, in melanoma, where preoperative therapy is a relatively recent approach, a consensus guideline for neoadjuvant immunotherapy was reported in 2018.32 Novel immune-related pathologic response criteria have been developed specifically for neoadjuvant immunotherapy,33 serving as a pan-tumour pathologic scoring system aimed at evaluating the therapeutic effect of neoadjuvant PD-1/PD-L1 inhibitors. They evaluate the features of PR in immune-mediated tumour regression, regardless of tumour location and histological characteristics. Tumour response is evaluated based on the percentage of immune-related residual tumour volume (%irRTV), calculated by dividing the area of the residual tumour by the sum of the tumour area, necrosis area, and regression. Figure 2 shows a schema of residual viable tumour volume percentage defined by PR criteria. In a prespecified exploratory analysis of the phase III CHECKMATE 816 trial, comparing neoadjuvant nivolumab plus chemotherapy with chemotherapy alone in NSCLC cases, it was reported that RTV at the primary site predicted EFS.34 Notably, each 1% increase in RTV was associated with a 0.017 increase in the hazard ratio (HR) for EFS. The immune-related pathologic response criteria have been utilised in various phase I and II trials (NCT05977907, NCT05733715, and NCT03878979) to assess neoadjuvant immunotherapy across different tumour types.
Table 1.
Representative pathological response evaluation systems
| Tumour type | Criteria name | Description | Category |
|---|---|---|---|
| Gastric cancer | Mandard Tumour Regression Grade (TRG) system28 |
|
TRG1 TRG2 TRG3 TRG4 TRG5 |
| Becker classification27 |
|
TRG1a TRG1b TRG2 TRG3 |
|
| Breast cancer | Residual cancer burden (RCB)25 | RCB was calculated as a continuous index combining pathologic measurements of primary tumour (size and cellularity) and nodal metastases (number and size)
|
RCB 0 RCB 1 RCB 2 RCB 3 |
| Definition of pCR by the US FDA (https://www.fda.gov/media/83507/download) |
|
||
| NSCLC | Junker et al.35 | %RVT = viable tumour/(viable tumour + stromal tissue + necrosis)
|
Grade I Grade IIa Grade IIb Grade III |
| Pataer et al.36 | %RVT = viable tumour/(viable tumour + stromal tissue + necrosis) | ||
| Melanoma (for immunotherapy) | Tetzlaff et al.32 | %RVT = area of residual viable tumour/area of total assessed cross-sectional tumour bed. The definition of total tumour bed is the area of viable tumour or tumoural regression (necrosis with or without clusters/sheets of pigmented macrophages and fibrosis/fibroinflammatory stroma) | |
| Pan-tumour (for immunotherapy) | Immune-related pathologic response (irPR)33 | %RVT = total tumour area/total tumour bed area. The definition of total tumour bed area is RVT + tumour-associated stroma + necrosis + regression bed |
AJCC, American Joint Committee on Cancer; NSCLC, non-small-cell lung cancer; pCR, pathological complete response; RD, residual disease; US FDA, United States Food and Drug Administration; %RVT, % residual viable tumour.
Figure 2.
Schema of % residual viable tumour (%RVT) defined by pathologic response criteria. The %RVT in cases assessed using neoadjuvant chemotherapy pathologic response criteria (cPRC) and immune-related pathologic response criteria (irPRC) is illustrated. %RVT is calculated as the ratio of the residual viable tumour area to the total tumour bed area. In irPRC assessments, the tumour regression bed is included in the total tumour bed area. Distinct histological features observed within the regression bed include lymphoid infiltrates, tertiary lymphoid structures, plasma cells, foamy macrophages, cholesterol clefts, proliferative fibrosis, and neovascularization.
The concept of major PR (MPR), known as ‘near pCR’, has recently emerged. In an analysis of a clinical trial involving neoadjuvant chemotherapy in NSCLC, MPR was defined as ≤10% residual viable tumour in both the primary tumour and sampled lymph nodes following neoadjuvant chemotherapy.19 In current prospective clinical trials, both pCR and MPR are being applied as endpoints. The International Association for the Study of Lung Cancer is currently working on releasing a consensus guideline, conducting reproducibility studies, and developing an artificial intelligence-driven digital pathology system for histopathological evaluation following neoadjuvant immunotherapy.
Patient- and trial-level surrogacy
When discussing surrogate markers for survival, it is essential to distinguish between two different concepts: ‘patient-level’ and ‘trial-level’ surrogacy.37 Patient-level surrogacy refers to a potential surrogate marker that is associated with the true endpoint, such as survival, in individual patients. In this context, the surrogate marker has prognostic value, meaning that it can predict outcomes for individual patients. Conversely, trial-level surrogacy indicates that the treatment effect on the surrogate endpoint is reliably predictive of the true endpoint, reflecting its predictive value across studies. Patient-level surrogacy can be evaluated using any type of dataset, whether from randomised or nonrandomised studies. By contrast, demonstrating the robustness of trial-level surrogacy requires meta-analysis, preferably with individual patient data, that includes multiple studies assessing the treatment effects on both the potential surrogate marker and the true endpoint.38,39 In colon cancer, the ACCENT group demonstrated that disease-free survival (DFS) with a 2-3-year follow-up is an excellent predictor of 5-year OS.40,41 In their study, they analysed individual patient data from 20 898 cases enrolled in 18 randomised trials. They applied correlation analysis, linear regression models, and Cox proportional hazards regression models. Subsequently, they carried out surrogacy testing, validation studies, and concordance evaluations for trial-level surrogacy. By contrast, an integrative analysis of 12 randomised studies involving 11 995 breast cancer patients found that pCR had strong patient-level surrogacy for EFS [HR 0.44, 95% confidence interval (CI) 0.39-0.51],18 but very weak trial-level surrogacy for EFS (R2 = 0.03) Other similar results have been reported in breast cancer.42,43 Given that neoadjuvant immunotherapy is a relatively new treatment strategy, evaluating whether pCR is a trial-level surrogate endpoint is challenging, particularly where multiple randomised trials investigating neoadjuvant immunotherapy are lacking.
Neoadjuvant immunotherapy from clinical studies
Table 2 summarises the notable clinical evidence focusing on neoadjuvant immunotherapy in NSCLC, breast cancer, melanoma, and gastric/gastroesophageal junction (G/GOJ) cancer.
Table 2.
Summary of selected clinical trials of neoadjuvant immune checkpoint inhibitors
| Cancer type | Form of therapy | Trial (phase) | Target population | Regimen | OS | DFS/EFS/RFS | pCR | MPR |
|---|---|---|---|---|---|---|---|---|
| Non-small-cell lung cancer | Neo ICI followed by adj CTx | Pilot study (n = 21)44,45 | Stage IIA-IIIA (N2) (AJCC 8th) | Nivo | (60 months) 80% | (60 months) 60% | 10% | 45% (95% CI 23% to 68%) |
| Neo chemo-immunotherapy followed by adjuvant ICI. | CHECKMATE 8163 (III) | Stage IB-IIIA (AJCC 7th) | CTx + Nivo (NAC), Nivo (Adj) | NR versus NR (HR 0.57; P = 0.008) | 31.6 months versus 20.8 months (HR 0.63; P = 0.005) | 24% versus 2.2% (OR 13.94; P < 0.001) | 36.9% versus 8.9% (OR 5.70) | |
| KEYNOTE-6716 (II) | Stage IIA-IIIA (N2), (AJCC 8th) | CTx + Pemb (NAC), Pemb (Adj) | (24 months) 80.9% versus 77.6% (P = 0.02, which did not meet the significance criterion) | (24 months) 62.4% versus 40.6% (HR 0.58, 95% CI 0.46-0.72; P < 0.001) | 18.1% and 4.0%; difference, 14.2% (95% CI 10.1% to 18.7%; P < 0.0001) | 30.2% and 11%; difference, 19.2% (95% CI 13.9% to 24.7%; P < 0.0001) | ||
| AEGEAN4 (III) | Stage IIA-IIIA (N2), (AJCC 8th) | CTx + durvalumab (NAC), durvalumab (Adj) | Not reported | NR versus 25.9 months (HR 0.68, 95% CI 0.53-0.88; P = 0.004) | 17.2% versus 4.3%; difference, 13% (95% CI 8.7% to 17.6%; P < 0.001) | 33.3% versus 12.3%; difference, 21% (95% CI 15.1% to 26.9%; P < 0.001) | ||
| Neotorch46 (III) | Stage IIA-IIIB (N2), (AJCC 8th) | CTx + toripalimab (NAC), CTx + toripalimab followed by toripalimab alone (Adj) | NR versus 30.4 months (HR 0.62, 95% CI 0.38-1.00); P = 0.05) | NR versus 19.3 months (HR 0.50, 95% CI 0.33-0.76); P < 0.001 | 24.8% versus 1.0%; difference, 23.7% (95% CI 17.6% to 29.8%), P < 0.001 | Not reported | ||
| Triple-negative breast cancer | Neo chemo-immunotherapy followed by adjuvant ICI | KEYNOTE-52247, 48, 49 (III) | Stage II-III (AJCC 7th) | CTx + Pemb (NAC), Pemb (Adj) | (60 months) 86.6% versus 81.7% (P = 0.002) | (60 months) 81.2% (95% CI 78.3% to 83.8%) versus 72.2% (95% CI 67.4% to 76.4%) | 64.0% (95% CI 60.2% to 67.6%) versus 54.7% (95% CI 49.1% to 60.1%), P = 0.0021 | Not reported |
| ER-positive breast cancer | Neo chemo-immunotherapy followed by adjuvant ICI | KEYNOTE-75650 (III) | T1c-T2cN1-2 or T3-4cN0-2 | CTx + Pemb (NAC), Pemb (Adj) | Not reported | Not reported | 24.3% versus 15.6% (95% CI 4.2% to 12.8%; P = 0.0005) | Not reported |
| CHECKMATE7FL51 (III) | T1c-T2N1-2 or T3-4cN0-2, Grade 3 with ER ≥ 1% or grade 2 with ER 1%-10% | CTx + Nivo (NAC), Nivo (Adj) | Not reported | Not reported | 24.5% versus 13.8% (OR 2.05, 95% CI 1.29-3.27, P = 0.0021) | Not reported | ||
| Melanoma | Neoadjuvant ICI | PRADO52 (II) | stage III (AJCC 7th) | Ipi + Nivo, two cycles | (24 months) 95% (entire cohort) | (24 months) 93% (MPR), 71% (pPR), 64% (pNR) | 48% | 61% (95% CI 50% to 70%) |
| OpACIN-neo53,54 (II) | stage III | Ipi + Nivo | Not reported | (36 months) 95% versus 37% (pPR-pCR or pNR, P < 0.001 | 47% (arm A), 57% (arm B), 23% (arm C) | 33% (arm A), 70% (arm B), 46% (arm C) | ||
| Southwest Oncology Group S180155 (II) | Stage IIIB-D, oligometastatic stage IV (AJCC 8th) | Adj Pemb or neo + adj Pemb | Numerical results were not reported | (24 months), 72% versus 49% (P = 0.04) | 21% in the neoadjuvant therapy arm | Not reported | ||
| Neoadjuvant ICI followed by ICI or observation | NADINA (III)56 | Stage III or recurrent resectable | Adj Nivo or neoadjuvant IPI + adj Nivo (no MPR) or observation (MPR) | Not reported | (12 months), 83.7% versus 57.2% (P < 0.001) | 47.2% | 59.0% | |
| Gastric/oesophagogastric junctional adenocarcinoma | Perioperative (neo and adj) chemo-immunotherapy | KEYNOTE-58557,58 (III) | T3-T4 or N+ | CTx + Pemb (perioperative) | (Main cohort) 71.8 months versus 55.7 months (HR 0.86, 95% CI 0.71-1.06) | (Main cohort) 44.4 months versus 25.5 months (HR 0.81, 95% CI 0.67-0.99; P = 0·0198)a | (Main cohort) 12.9% versus 2.0% (95% CI 7.5% to 14.8%; P < 0·00001), (FLOT cohort) 17.0% versus 6.8% estimated difference [10.2% (95% CI 1.3% to 19.7%)] | 31.5% versus 22.2%b |
| MATTERHORN59 (III) | T2N0-3 or T0-4N+ | CTx + durvalumab | Not reported | Not reported | 19% versus 7% (OR 3.08; P < 0.00001) | 27% versus 14% (OR 2.19; P < 0.00001) | ||
| DANTE60 (II/III) | T2N0-3 or T0-4N+ | CTx + atezolizumab | Not reported | Not reported | 24% versus 15%b | 48% versus 39%c |
adj, adjuvant; AJCC, American Joint Committee on Cancer; CI, confidence interval; CTx, chemotherapy; DFS, disease-free survival; EFS, event-free survival; ER, oestrogen receptor; FLOT, 5-fluorouracil, leucovorin, oxaliplatin, and docetaxel; HR, hazard ratio; ICI, immune-check point inhibitor; IPI, ipilimumab; MPR, major pathological response; NAC, neoadjuvant chemotherapy; Nivo, nivolumab; NR, not reached; OR, odds ratio; OS, overall survival; pCR, pathological complete response; Pemb, pembrolizumab; pNR, pathological non-response; pPR, pathological partial response; RFS, relapse-free survival.
Not statistically significant [significance criterion (P = 0.0178)].
Pathological evaluation was carried out using Mandard tumour regression (MPR was defined as grade 1 + 2).
Pathological evaluation was carried out using Becker’s criteria.
Non-small-cell lung cancer
For many years, cisplatin-based adjuvant chemotherapy has been considered the standard of care for patients with stage II-III NSCLC.61,62 A pilot study conducted in 2018 assessed neoadjuvant nivolumab monotherapy for clinical stage IIA-IIIA NSCLC, revealing a 10% pCR rate and a 45% MPR rate.44 Long-term follow-up data (median follow-up 63 months) showed that 89% of patients with MPR remained disease-free, while 54% of patients without MPR experienced tumour relapse or death.45
CHECKMATE 816 was the first randomised phase III trial comparing the effects of neoadjuvant nivolumab plus chemotherapy alone (without adjuvant immunotherapy) with neoadjuvant chemotherapy alone. This trial reported a significant improvement in EFS, the primary endpoint, in the nivolumab plus chemotherapy arm (Table 2). Moreover, both the pCR and MPR rates were notably higher in the nivolumab–chemotherapy group.3 At the prespecified first analysis, the presence of a statistically significant OS benefit remained uncertain (P = 0.008 and 0.02, respectively, boundary cut-off was 0.003). In the nivolumab–chemotherapy group, the median EFS was not reached among patients who achieved pCR, whereas it was 26.6 months among those who did not achieve pCR (HR 0.13, 95% CI 0.05-0.37). Notably, PR evaluation using %RTV revealed a superior predictive value for survival compared with radiological evaluation or circulating tumour DNA testing. However, an exploratory analysis revealed a longer EFS even among patients who did not achieve pCR with nivolumab. EFS was 26.6 months with nivolumab plus chemotherapy and 18.4 months with chemotherapy alone, regardless of the pCR status (HR for EFS 0.84, 95% CI 0.61-1.17), suggesting that perioperative nivolumab may be effective beyond achieving pCR. This trial also confirmed a differential pathological and clinical efficacy associated with PD-L1 expression status, consistent with observations in the metastatic setting.
Another phase III study, the KEYNOTE-671 trial, evaluated neoadjuvant chemotherapy plus pembrolizumab and chemotherapy in resectable stage II-IIIB NSCLC. The study successfully met its primary endpoint (HR 0.58, 95% CI 0.46-0.72; P < 0.001).6 The pCR rates for pembrolizumab plus chemotherapy and chemotherapy alone were 18.1% and 4.0%, respectively (95% CI 10.1% to 18.7%; P < 0.0001). Furthermore, the MPR rates were 30.2% and 11.0% (95% CI 13.9% to 24.7%; P < 0.0001), respectively. An exploratory analysis similar to CHECKMATE 816 revealed enhanced EFS benefit among patients who achieved MPR (HR 0.54, 95% CI 0.24-1.22) and pCR (HR 0.33, 95% CI 0.09-1.22).34 Notably, a prespecified second analysis with a median follow-up of 36.8 months demonstrated significantly longer OS in the chemotherapy plus pembrolizumab group (HR 0.72, 95% CI 0.56-0.93; P = 0.00517).63 Interestingly, similar to the observations in CHECKMATE 816, an exploratory analysis revealed superior EFS in the pembrolizumab group, irrespective of attaining MPR or pCR. In addition, four other registrational trials of perioperative ICIs have all suggested enhanced EFS attributable to PR.4,46,64,65
Based on the findings of the aforementioned pivotal studies, neoadjuvant immuno-chemotherapy with or without adjuvant ICIs has now become the standard of care for NSCLC without an oncogenic driver mutation. These trials have consistently demonstrated enhanced EFS, strongly correlated with an increase in the MPR or pCR. However, it is plausible that improved patient outcomes with ICI treatment may not necessarily require MPR or pCR, given the observed association between the depth of PR and EFS with PD-L1 expression.
A recent meta-analysis encompassing 2940 patients from seven randomised phase III trials investigating neoadjuvant ICI demonstrated that pCR and MPR were correlated with 2-year EFS rates.66 The correlation coefficients (R2) for pCR and MPR with 2-year EFS were 0.82 (95% CI 0.66-0.94) and 0.81 (95% CI 0.63-0.93), respectively. However, trial-level surrogacy was found to be limited and imprecise with R2 for the association of PR with EFS and OS being 0.58 (95% CI 0.00-0.97) and 0.61 (95% CI 0.00-0.97), respectively. Overall, the best available data suggest that PR is associated with prognosis in patients treated with neoadjuvant ICIs (patient-level surrogacy), but there is no clear evidence that PR is a trial-level surrogate marker.
Breast cancer
Neoadjuvant chemotherapy has been a standard of care for patients with breast cancer for over two decades. Following neoadjuvant chemotherapy, pCR is a well-established patient-level prognostic marker for long-term survival in early breast cancer,17 with the strongest correlations observed in the triple-negative and human epidermal growth factor receptor-2 (HER2) positive subtypes.18 Although the prognostic value of pCR was confirmed as patient-level surrogacy by pooled analysis, the validity of trail-level surrogacy remains unclear. Residual cancer burden (RCB) has been used as another tool to evaluate PR. RCB categorises PR into four grades, where RCB 0 corresponds to pCR and RCB 3 signifies negligible therapeutic effect from neoadjuvant therapy, consistently aligning with prognosis.25
ICIs have been investigated in early breast cancer, with regulatory approvals in TNBC based on the results of the KEYNOTE-522 trial. This pivotal randomised phase III trial evaluated the addition of pre- and post-operative pembrolizumab to standard multiagent chemotherapy for stage 2-3 TNBC,1 demonstrating the superiority of immuno-chemotherapy over chemotherapy in terms of both co-primary endpoints: pCR and EFS.47 More recently, an OS benefit has also been reported with pembrolizumab plus chemotherapy with an absolute difference in 5-year OS rates of 4.9%.48 A prespecified OS subgroup analysis for pCR showed that in patients with pCR, there was little difference in survival (absolute difference in 5-year OS rates: 0.7%). However, in patients without pCR, there was more difference in OS (absolute difference in 5-year OS rates: 6.1%).48 An exploratory analysis of KEYNOTE-522 evaluated EFS by RCB49 and reported that EFS declined with higher RCB grades. Moreover, the addition of pembrolizumab appears to shift RCB to lower RCB grades and increase the chance of achieving pCR. However, in this exploratory analysis, the HRs for EFS of the pembrolizumab arms in RCB 0, 1, 2, and 3 were 0.70 (95% CI 0.38-1.31), 0.92 (95% CI 0.39-2.20), 0.52 (95% CI 0.32-0.82), and 1.24 (95% CI 0.69-2.23), respectively. This suggests that the benefit of pembrolizumab was not proportional to the degree of RCB, with the most significant difference in EFS between the placebo and pembrolizumab arms observed in the RCB 2 group. PR, as defined by RCB, could be a patient-level surrogate marker for EFS with a 36-month EFS rate in the pembrolizumab arm, with the RCB 0 category being 94.7%; however, further evaluation in an independent dataset is required.
More recently, perioperative ICIs have also been evaluated in the most common oestrogen receptor-positive (ER+), HER2-negative subtype. Preliminary results from two phase III studies, KEYNOTE-75650 and CHECKMATE 7FL,51 both reported an improvement in the pCR rate when pembrolizumab or nivolumab, respectively, was added to neoadjuvant chemotherapy. However, as the EFS results are not yet mature, these trials have not yet influenced the changes in clinical practice. Notably, the benefits of pembrolizumab appeared to be most marked in patients with low levels of ER expression, for whom standard adjuvant endocrine therapy has more limited benefit. While nivolumab showed a correlation with certain biomarkers, such as PD-L1 expression, this correlation was not consistently observed across both studies.
The addition of ICIs to chemotherapy for operable breast cancer led to an increased PR rate in both ER-positive breast cancer and TNBC with significantly longer EFS and OS in TNBC. Although the role of pCR as a trial-level surrogacy remains uncertain, based on patient-level surrogacy of PR, the benefit of response-adapted adjuvant therapy after neoadjuvant chemo-immunotherapy is currently being explored in several large clinical trials (NCT02954874, NCT05812807, NCT02926196, NCT04595565, NCT05633654, NCT04434040, and NCT05629585). Response-guided therapy is already a standard of care for HER2-positive breast cancer, with adjuvant therapy switched to the antibody–drug conjugate trastuzumab emtansine for patients with residual disease after neoadjuvant therapy.67 This strategy is an excellent example of using PR as a patient-level surrogacy for tailoring therapy.
Melanoma
Consecutive clinical trials in melanoma have demonstrated significant efficacy of ipilimumab,68,69 nivolumab,14 and pembrolizumab70 as adjuvant therapies. Consequently, adjuvant ICI (pembrolizumab or nivolumab) has now become the standard of care for high-risk stage II and stage III melanoma.10,14,70 Following pioneering trials that demonstrated the efficacy and broader T-cell response in the tumour microenvironment through neoadjuvant pembrolizumab71 or ipilimumab and nivolumab,72 neoadjuvant ICI is currently under active investigation. Table 2 summarises clinical evidence focusing on neoadjuvant immunotherapy in melanoma. The OpACIN-Neo trial53 investigated three different doses and timings of ipilimumab and nivolumab for resectable stage III melanomas. Of the 86 patients, 52% and 74% achieved radiological response and PR based on the International Neoadjuvant Melanoma Consortium scoring system.32 Interestingly, none of the responders experienced a recurrence, a finding that was sustained during the 69-month follow-up.54 In another phase II study, the PRADO trial, favourable relapse-free survival (RFS) and OS were reported in patients achieving pCR after neoadjuvant ipilimumab and nivolumab therapy compared with those who did not.52 In this trial, response-guided adjuvant therapy was administered based on PR. Patients who achieved MPR in their index lymph nodes did not undergo therapeutic lymph node dissection or adjuvant therapy. By contrast, patients with a pathological partial response underwent therapeutic lymph node dissection alone, whereas those with no histological response underwent therapeutic lymph node dissection and adjuvant therapy with ICIs or molecular targeted therapy (BRAF/MEK inhibition). Among the 99 patients, those achieving a major PR (pCR or near pCR) demonstrated a 24-month RFS rate of 93%, with only one patient developing distant metastasis.
A randomised phase II study (SWOG S1801) investigated two immunotherapy strategies for resectable stage III or IV melanoma.55 This study compared adjuvant pembrolizumab with neoadjuvant plus adjuvant pembrolizumab and showed that the neoadjuvant plus pembrolizumab arm had a significantly longer EFS. This finding confirmed the potent activity of neoadjuvant ICIs and provided a clear rationale for further investigations in melanoma. Exploratory analyses of the 2-year RFS by PR showed 97% for pCR, 80% for near pCR, 73% for pathological partial response, and 48% for no PR. Taken together, these findings suggest a correlation between survival and PR in resected melanoma treated with neoadjuvant pembrolizumab.73 A phase III NADINA study investigated the standard of care (adjuvant nivolumab) versus neoadjuvant ipilimumab plus nivolumab in patients with stage III melanoma.56 Only patients in the neoadjuvant therapy group who did not achieve a pCR or MPR received adjuvant therapy. Specifically, these patients were treated with nivolumab if they had BRAF wild type, or with a combination of dabrafenib and trametinib if they had the BRAF V600E or V600K mutation. Notably, the 12-month RFS rate in the neoadjuvant arm was correlated with the extent of PR (95.1%, 76.1%, and 57.0% among patients with MPR, partial response, and nonresponse, respectively), suggesting that PR is a promising patient-level surrogate marker for survival in these patients.
Pooled data analyses from six prospective trials including 141 patients treated with neoadjuvant ICIs [ipilimumab plus nivolumab (n = 104); anti-PD-1 monotherapy (n = 37)] showed excellent RFS among patients with pCR.74 The 2-year RFS rate was 96%, compared with 64% (P < 0.001) in patients without pCR. Additionally, patients with pathological near pCR and partial response had a more favourable prognosis than nonresponders (P < 0.001). To the best of our knowledge, PR seems to be a patient-level prognostic marker in melanoma; however, robust evidence that PR is a trial-level surrogate marker for survival is lacking.
Gastric and gastroesophageal junction cancer
Perioperative chemotherapy is considered the standard of care in Western countries for G/GOJ adenocarcinoma with a diminishing role for preoperative chemoradiation. Initially, an epirubicin, cisplatin plus 5-fluorouracil-based regimen was introduced based on the results of The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) study75 and FFCD 9703 trial.76 Later, the combination regimen of 5-fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) became the current standard of care for locally advanced (clinical stage II-III), operable G/GOJ adenocarcinoma.77
Before the ICI era, there was some evidence suggesting that PR, graded as TRG (summarised in Table 1), was correlated with OS or EFS.78,79 In the FLOT4 trial, the FLOT arm demonstrated significantly better pCR rate and DFS, suggesting the potential of PR as a surrogate marker for survival. However, neither subgroup analyses nor post hoc analyses were presented to evaluate the prognostic impact of pCR or TRG. Moreover, post hoc analysis from the MAGIC trial revealed that TRG is not a prognostic marker, with histological nodal status more closely associated with long-term survival.80,81 Overall, there is no robust evidence to support PR as a surrogate for survival at the patient level, and even less so at the trial level. Meta-analysis using individual patient data enrolled in randomised studies is the ideal solution to clarify whether patient- and trial-level surrogacy exists in resectable G/GOJ adenocarcinoma.
Currently, perioperative platinum plus fluoropyrimidine therapy or FLOT in conjunction with ICIs is being actively investigated. The KEYNOTE-585 study assessed perioperative chemotherapy and pembrolizumab or chemotherapy alone in locally advanced resectable G/GOJ adenocarcinoma.57 This study utilised two backbone chemotherapy regimens: cisplatin plus fluoropyrimidine doublet regimen (main cohort, n = 805) and FLOT (FLOT cohort, n = 203). In the main cohort, the pembrolizumab arm showed a significantly better pCR rate. No significant improvement in EFS or OS was seen with the addition of pembrolizumab, thus increased pCR rate did not translate into a survival advantage. The results of the FLOT cohort, reported separately, also showed a better pCR rate in the FLOT plus pembrolizumab arm than in the FLOT plus placebo arm (17.0% versus 6.8%). However, EFS or OS advantages were not achieved in the pembrolizumab arm (EFS: HR 0.79, 95% CI 0.52-1.22; OS: HR 1.04, 95% CI 0.66-1.66),58,82 showing a similar outcome to the main cohort. Post hoc analysis for patients achieving PR also did not show significantly longer EFS (HR 0.6, 95% CI 0.4-1.0) or OS (HR 0.7, 95% CI 0.4-1.2).83
Other randomised studies investigating the role of durvalumab (MATTERHORN study,59,84 NCT04592913) or atezolizumab (DANTE study,60 NCT03421288) in combination with the FLOT regimen are currently underway. Although interim analyses revealed that the arms receiving ICI plus chemotherapy achieved significantly higher pCR rates, the survival data are still awaited. Overall, there are no data to conclusively establish an association between PR and survival in perioperative ICIs in G/GOJ adenocarcinoma, unlike other cancers.
Further perspectives
The application and investigation of neoadjuvant chemo-immunotherapy are rapidly advancing, recently becoming a standard of care in NSCLC and TNBC. Promising phase II and III data reported in gastric cancer and melanoma, along with early phase III data in ER positive breast cancer, may represent a potential expansion of the role of neoadjuvant and perioperative ICIs.
PR is an informative measure in patients who have received neoadjuvant therapy. While clinical evidence from TNBC, NSCLC, and melanoma suggests that PR appears to be a significant prognostic marker for EFS with neoadjuvant ICI, the extent to which this measure can be universally adopted for EFS and OS across different solid tumours, such as G/GOJ adenocarcinoma, remains uncertain. Although a significant improvement in pCR rate was observed, this did not translate into prolonged EFS or OS in G/GOJ cancer thus far. Nevertheless, further randomised phase III data are anticipated. In this review, we have discussed that PR appears to function as a prognostic marker (patient-level surrogacy) in certain tumour types treated with neoadjuvant therapy, such as breast cancer, NSCLC, and melanoma. However, the establishment of trial-level surrogacy for PR remains unproven. The discrepancy between patient- and trial-level surrogacy continues to be a topic of ongoing debate in oncology.
Two possibilities may explain this discrepancy: direct treatment effects and the existence of confounding factors.37 The former refers to the situation where a treatment effect influences both the surrogate marker and survival outcomes. For example, a treatment might induce long-term toxicity that affects OS. The latter pertains to confounding factors that may independently affect both the surrogate marker and survival. For instance, an unknown prognostic factor might be associated with both PR and survival independently of the treatment effect. Although randomization in clinical trials aims to eliminate confounding factors, it is impossible to entirely exclude these possibilities.
From a biological perspective, the efficacy of systemic therapy might vary in primary lesions and micrometastasis. Currently, evaluation of post-operative minimal residual disease by circulating tumour DNA is reported to predict colon cancer relapse with high accuracy.85 In terms of patient- and trial-level surrogacy, both PR and minimal residual disease analysis should be investigated in the tumour type treated with perioperative systemic therapy in future trials.42
Conclusions
Although PR has been used as a short-term outcome in early-phase clinical trials to select novel agents to move forward to phase III testing, its inconsistent correlation with survival outcomes presents methodological challenges in clinical trial design, particularly when applied across all solid tumours in the era of neoadjuvant immune checkpoint inhibition and other agents with novel mechanisms of action. For now, it remains imperative that prognostic outcome measures, such as EFS and OS, form the basis for changes in clinical practice until the utility of PR as a surrogate marker is robustly confirmed for each cancer subtype.
Acknowledgements
All authors acknowledge that this work was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
SP has served in a consulting role or on the advisory boards for AnHeart, Amgen, AstraZeneca, Bayer, Blueprint, BMS, Boehringer Ingelheim, Daiichi Sankyo, EQRx, GlaxoSmithKline, Guardant Health, Janssen, Lilly, Merck KGaA, MSD, Novartis, Pfizer, PharmaMar, Roche, Sanofi, Takeda, Seattle Genetics, Trizel, and Turning Point Therapeutics; and has received research funding from Elsevier, Medscape, and VJ Oncology. AO has received research funding from Pfizer and Roche; served on advisory boards for AstraZeneca, Daichi-Sankyo, Pfizer, Roche, and Seagen; received travel support for congresses from AstraZeneca, Novartis, Lilly, and Roche; and received honoraria from AstraZeneca, Daichi-Sankyo, Esai, Gilead, Lily, Pfizer, Roche, and Seagen. JL has served in a consulting role or on the advisory boards for iOnctura, Apple Tree, Merck, BMS, Eisai, Debiopharm, Incyte, Pfizer, Novartis, and MSD; received research funding from Achilles, BMS, MSD, Novartis, Pfizer, Achilles Therapeutics, Roche, Nektar Therapeutics, Covance, Immunocore, Pharmacyclics, and Aveo; and received honoraria from Eisai, Novartis, Incyte, Merck, touchIME, touchEXPERTS, Pfizer, Royal College of Physicians, Cambridge Healthcare Research, Royal College of General Practitioners, VJOncology, Agence Unik, BMS, Immatics, Insighter, and GCO. IC has served in a consulting role or on the advisory boards for Eli-Lilly, Bristol Meyers Squibb, MSD, Roche, Merck-Serono, Astra-Zeneca, OncXerna, Boehringer Ingelheim, Incyte, Astella, GSK, SOTIO, Eisai, Daiichi-Sankyo, Taiho, Servier, Seagen, Turning Point Therapeutics, Novartis, Takeda, Elevation Oncology, and BeiGene; received research funding from Eli-Lilly and Janssen-Cilag; and received honoraria from Eli-Lilly, Eisai, and Servier. KS and AG have declared no conflicts of interest.
References
- 1.Schmid P., Cortes J., Pusztai L., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien M., Paz-Ares L., Marreaud S., et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274–1286. doi: 10.1016/S1470-2045(22)00518-6. [DOI] [PubMed] [Google Scholar]
- 3.Forde P.M., Spicer J., Lu S., et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymach J.V., Harpole D., Mitsudomi T., et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672–1684. doi: 10.1056/NEJMoa2304875. [DOI] [PubMed] [Google Scholar]
- 5.Felip E., Altorki N., Zhou C., et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–1357. doi: 10.1016/S0140-6736(21)02098-5. [DOI] [PubMed] [Google Scholar]
- 6.Wakelee H., Liberman M., Kato T., et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491–503. doi: 10.1056/NEJMoa2302983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choueiri T.K., Tomczak P., Park S.H., et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–694. doi: 10.1056/NEJMoa2106391. [DOI] [PubMed] [Google Scholar]
- 8.Bajorin D.F., Witjes J.A., Gschwend J.E., et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102–2114. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powles T., Catto J.W.F., Galsky M.D., et al. Perioperative durvalumab with neoadjuvant chemotherapy in operable bladder cancer. N Engl J Med. 2024;14:1773–1786. doi: 10.1056/NEJMoa2408154. [DOI] [PubMed] [Google Scholar]
- 10.Eggermont A.M.M., Blank C.U., Mandala M., et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 11.Grossmann K.F., Othus M., Patel S.P., et al. Adjuvant pembrolizumab versus IFNα2b or ipilimumab in resected high-risk melanoma. Cancer Discov. 2022;12(3):644–653. doi: 10.1158/2159-8290.CD-21-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luke J.J., Rutkowski P., Queirolo P., et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399(10336):1718–1729. doi: 10.1016/S0140-6736(22)00562-1. [DOI] [PubMed] [Google Scholar]
- 13.Kirkwood J.M., Del Vecchio M., Weber J., et al. Adjuvant nivolumab in resected stage IIB/C melanoma: primary results from the randomized, phase 3 CheckMate 76K trial. Nat Med. 2023;29(11):2835–2843. doi: 10.1038/s41591-023-02583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber J., Mandala M., Del Vecchio M., et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 15.Topalian S.L., Taube J.M., Pardoll D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367(6477) doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell J.S., Hoefsmit E.P., Smyth M.J., Blank C.U., Teng M.W.L. The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clin Cancer Res. 2019;25(19):5743–5751. doi: 10.1158/1078-0432.CCR-18-2641. [DOI] [PubMed] [Google Scholar]
- 17.Rastogi P., Anderson S.J., Bear H.D., et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 18.Cortazar P., Zhang L., Untch M., et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 19.Hellmann M.D., Chaft J.E., William W.N., Jr., et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42–e50. doi: 10.1016/S1470-2045(13)70334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dooms C., Verbeken E., Stroobants S., Nackaerts K., De Leyn P., Vansteenkiste J. Prognostic stratification of stage IIIA-N2 non-small-cell lung cancer after induction chemotherapy: a model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol. 2008;26(7):1128–1134. doi: 10.1200/JCO.2007.13.9550. [DOI] [PubMed] [Google Scholar]
- 21.Rosner S., Liu C., Forde P.M., Hu C. Association of pathologic complete response and long-term survival outcomes among patients treated with neoadjuvant chemotherapy or chemoradiotherapy for NSCLC: a meta-analysis. JTO Clin Res Rep. 2022;3(9) doi: 10.1016/j.jtocrr.2022.100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfister C., Gravis G., Fléchon A., et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol. 2021;79(2):214–221. doi: 10.1016/j.eururo.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Maas M., Nelemans P.J., Valentini V., et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Symmans W.F., Peintinger F., Hatzis C., et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 26.Travis W.D., Dacic S., Wistuba I., et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709–740. doi: 10.1016/j.jtho.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker K., Mueller J.D., Schulmacher C., et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98(7):1521–1530. doi: 10.1002/cncr.11660. [DOI] [PubMed] [Google Scholar]
- 28.Mandard A.M., Dalibard F., Mandard J.C., et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Lo D.S., Pollett A., Siu L.L., Gallinger S., Burkes R.L. Prognostic significance of mesenteric tumor nodules in patients with stage III colorectal cancer. Cancer. 2008;112(1):50–54. doi: 10.1002/cncr.23136. [DOI] [PubMed] [Google Scholar]
- 30.Voskuilen C.S., Oo H.Z., Genitsch V., et al. Multicenter validation of histopathologic tumor regression grade after neoadjuvant chemotherapy in muscle-invasive bladder carcinoma. Am J Surg Pathol. 2019;43(12):1600–1610. doi: 10.1097/PAS.0000000000001371. [DOI] [PubMed] [Google Scholar]
- 31.Böhm S., Faruqi A., Said I., et al. Chemotherapy response score: development and validation of a system to quantify histopathologic response to neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J Clin Oncol. 2015;33(22):2457–2463. doi: 10.1200/JCO.2014.60.5212. [DOI] [PubMed] [Google Scholar]
- 32.Tetzlaff M.T., Messina J.L., Stein J.E., et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol. 2018;29(8):1861–1868. doi: 10.1093/annonc/mdy226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein J.E., Lipson E.J., Cottrell T.R., et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade. Clin Cancer Res. 2020;26(3):545–551. doi: 10.1158/1078-0432.CCR-19-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deutsch J.S., Cimino-Mathews A., Thompson E., et al. Association between pathologic response and survival after neoadjuvant therapy in lung cancer. Nat Med. 2024;30(1):218–228. doi: 10.1038/s41591-023-02660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junker K., Langner K., Klinke F., Bosse U., Thomas M. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest. 2001;120(5):1584–1591. doi: 10.1378/chest.120.5.1584. [DOI] [PubMed] [Google Scholar]
- 36.Pataer A., Kalhor N., Correa A.M., et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2012;7(5):825–832. doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buyse M., Saad E.D., Burzykowski T., Regan M.M., Sweeney C.S. Surrogacy beyond prognosis: the importance of ‘trial-level’ surrogacy. Oncologist. 2022;27(4):266–271. doi: 10.1093/oncolo/oyac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie W., Halabi S., Tierney J.F., et al. A systematic review and recommendation for reporting of surrogate endpoint evaluation using meta-analyses. JNCI Cancer Spectr. 2019;3(1) doi: 10.1093/jncics/pkz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buyse M., Molenberghs G., Burzykowski T., Renard D., Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1(1):49–67. doi: 10.1093/biostatistics/1.1.49. [DOI] [PubMed] [Google Scholar]
- 40.Sargent D.J., Wieand H.S., Haller D.G., et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23(34):8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 41.Sargent D.J., Patiyil S., Yothers G., et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25(29):4569–4574. doi: 10.1200/JCO.2006.10.4323. [DOI] [PubMed] [Google Scholar]
- 42.Conforti F., Pala L., Bagnardi V., et al. Surrogacy of pathologic complete response in trials of neoadjuvant therapy for early breast cancer: critical analysis of strengths, weaknesses, and misinterpretations. JAMA Oncol. 2022;8(11):1668–1675. doi: 10.1001/jamaoncol.2022.3755. [DOI] [PubMed] [Google Scholar]
- 43.Conforti F., Pala L., Sala I., et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ. 2021;375 doi: 10.1136/bmj-2021-066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forde P.M., Chaft J.E., Smith K.N., et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosner S., Reuss J.E., Zahurak M., et al. Five-year clinical outcomes after neoadjuvant nivolumab in resectable non-small cell lung cancer. Clin Cancer Res. 2023;29(4):705–710. doi: 10.1158/1078-0432.CCR-22-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu S., Zhang W., Wu L., et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: The neotorch randomized clinical trial. JAMA. 2024;331(3):201–211. doi: 10.1001/jama.2023.24735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmid P., Cortes J., Dent R., et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–567. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 48.Schmid P., Cortes J., Dent R., et al. Overall survival with pembrolizumab in early-stage triple-negative breast cancer. N Engl J Med. 2024;391(21):1981–1991. doi: 10.1056/NEJMoa2409932. [DOI] [PubMed] [Google Scholar]
- 49.Pusztai L., Denkert C., O’Shaughnessy J., et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann Oncol. 2024;35(5):429–436. doi: 10.1016/j.annonc.2024.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Cardoso F., McArthur H.L., Schmid P., et al. LBA21 KEYNOTE-756: phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2− breast cancer. Ann Oncol. 2023;34:S1260–S1261. [Google Scholar]
- 51.Loi S., Curigliano G., Salgado R.F., et al. LBA20 A randomized, double-blind trial of nivolumab (NIVO) vs placebo (PBO) with neoadjuvant chemotherapy (NACT) followed by adjuvant endocrine therapy (ET) ± NIVO in patients (pts) with high-risk, ER+ HER2− primary breast cancer (BC) Ann Oncol. 2023;34:S1259–S1260. [Google Scholar]
- 52.Reijers I.L.M., Menzies A.M., van Akkooi A.C.J., et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: the PRADO trial. Nat Med. 2022;28(6):1178–1188. doi: 10.1038/s41591-022-01851-x. [DOI] [PubMed] [Google Scholar]
- 53.Rozeman E.A., Menzies A.M., van Akkooi A.C.J., et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–960. doi: 10.1016/S1470-2045(19)30151-2. [DOI] [PubMed] [Google Scholar]
- 54.Versluis J.M., Menzies A.M., Sikorska K., et al. Survival update of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma in the OpACIN and OpACIN-neo trials. Ann Oncol. 2023;34(4):420–430. doi: 10.1016/j.annonc.2023.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Patel S.P., Othus M., Chen Y., et al. Neoadjuvant-adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med. 2023;388(9):813–823. doi: 10.1056/NEJMoa2211437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blank C.U., Lucas M.W., Scolyer R.A., et al. Neoadjuvant nivolumab and ipilimumab in resectable stage III melanoma. N Engl J Med. 2024;391(18):1696–1708. doi: 10.1056/NEJMoa2402604. [DOI] [PubMed] [Google Scholar]
- 57.Shitara K., Rha S.Y., Wyrwicz L.S., et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024;25(2):212–224. doi: 10.1016/S1470-2045(23)00541-7. [DOI] [PubMed] [Google Scholar]
- 58.Shitara K., Rha S.Y., Wyrwicz L.S., et al. LBA3 Final analysis of the phase III KEYNOTE-585 study of pembrolizumab plus chemotherapy vs chemotherapy as perioperative therapy in locally-advanced gastric and gastroesophageal junction cancer. Ann Oncol. 2024;35 [Google Scholar]
- 59.Janjigian Y.Y., Al-Batran S.E., Wainberg Z.A., et al. LBA73 Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): interim results of the global, phase III MATTERHORN study. Ann Oncol. 2023;34:S1315–S1316. [Google Scholar]
- 60.Lorenzen S., Götze T.O., Thuss-Patience P., et al. Perioperative atezolizumab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel for resectable esophagogastric cancer: interim results from the randomized, multicenter, phase II/III DANTE/IKF-s633 trial. J Clin Oncol. 2024;42(4):410–420. doi: 10.1200/JCO.23.00975. [DOI] [PubMed] [Google Scholar]
- 61.Winton T., Livingston R., Johnson D., et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 62.Kenmotsu H., Yamamoto N., Yamanaka T., et al. Randomized phase III study of pemetrexed plus cisplatin versus vinorelbine plus cisplatin for completely resected stage II to IIIA nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(19):2187–2196. doi: 10.1200/JCO.19.02674. [DOI] [PubMed] [Google Scholar]
- 63.Spicer J.D., Gao S., Liberman M., et al. LBA56 Overall survival in the KEYNOTE-671 study of perioperative pembrolizumab for early-stage non-small-cell lung cancer (NSCLC) Ann Oncol. 2023;34:S1297–S1298. [Google Scholar]
- 64.Yue D., Wang W., Liu H., et al. LBA58 Pathological response to neoadjuvant tislelizumab (TIS) plus platinum-doublet (PtDb) chemotherapy (CT) in resectable stage II-IIIA NSCLC patients (pts) in the phase III (Ph3) RATIONALE-315 trial. Ann Oncol. 2023;34:S1299. [Google Scholar]
- 65.Cascone T., Awad M.M., Spicer J.D., et al. Perioperative nivolumab in resectable lung cancer. N Engl J Med. 2024;390(19):1756–1769. doi: 10.1056/NEJMoa2311926. [DOI] [PubMed] [Google Scholar]
- 66.Hines J.B, Cameron R.B., Esposito A., et al. Evaluation of major pathologic response and pathologic complete response as surrogate end points for survival in randomized controlled trials of neoadjuvant immune checkpoint blockade in resectable in NSCLCJ. Thorac Oncol. 2024;19:1108–1116. doi: 10.1016/j.jtho.2024.03.010. [DOI] [PubMed] [Google Scholar]
- 67.von Minckwitz G., Huang C.S., Mano M.S., et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 68.Tarhini A.A., Lee S.J., Hodi F.S., et al. Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American intergroup E1609. J Clin Oncol. 2020;38(6):567–575. doi: 10.1200/JCO.19.01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eggermont A.M.M., Chiarion-Sileni V., Grob J.J., et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 70.Eggermont A.M.M., Blank C.U., Mandalà M., et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):643–654. doi: 10.1016/S1470-2045(21)00065-6. [DOI] [PubMed] [Google Scholar]
- 71.Huang A.C., Orlowski R.J., Xu X., et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461. doi: 10.1038/s41591-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blank C.U., Rozeman E.A., Fanchi L.F., et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661. doi: 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]
- 73.Patel S., Othus M., Wright P., et al. LBA48 Pathologic response and exploratory analyses of neoadjuvant-adjuvant versus adjuvant pembrolizumab (PEM) for resectable stage IIIb-IV melanoma from SWOG S1801. Ann Oncol. 2023;34:S1288. [Google Scholar]
- 74.Menzies A.M., Amaria R.N., Rozeman E.A., et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC) Nat Med. 2021;27(2):301–309. doi: 10.1038/s41591-020-01188-3. [DOI] [PubMed] [Google Scholar]
- 75.Cunningham D., Allum W.H., Stenning S.P., et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 76.Ychou M., Boige V., Pignon J.P., et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 77.Lordick F., Carneiro F., Cascinu S., et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–1020. doi: 10.1016/j.annonc.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Wan T., Zhang X.F., Liang C., Liao C.W., Li J.Y., Zhou Y.M. The prognostic value of a pathologic complete response after neoadjuvant therapy for digestive cancer: systematic review and meta-analysis of 21 studies. Ann Surg Oncol. 2019;26(5):1412–1420. doi: 10.1245/s10434-018-07147-0. [DOI] [PubMed] [Google Scholar]
- 79.Lorenzen S., Thuss-Patience P., Al-Batran S.E., et al. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol. 2013;24(8):2068–2073. doi: 10.1093/annonc/mdt141. [DOI] [PubMed] [Google Scholar]
- 80.Smyth E.C., Fassan M., Cunningham D., et al. Effect of pathologic tumor response and nodal status on survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy trial. J Clin Oncol. 2016;34(23):2721–2727. doi: 10.1200/JCO.2015.65.7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Athauda A., Nankivell M., Langer R., et al. Pathological regression of primary tumour and metastatic lymph nodes following chemotherapy in resectable OG cancer: pooled analysis of two trials. Br J Cancer. 2023;128(11):2036–2043. doi: 10.1038/s41416-023-02217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Batran S.E., Shitara K., Folprecht G., et al. Pembrolizumab plus FLOT vs FLOT as neoadjuvant and adjuvant therapy in locally advanced gastric and gastroesophageal junction cancer: interim analysis of the phase 3 KEYNOTE-585 study. J Clin Oncol. 2024;42(suppl 3):247. [Google Scholar]
- 83.Shitara K., Bang Y.J., Wyrwicz L.S., et al. Neoadjuvant/adjuvant pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (pbo) + chemo for gastric/gastroesophageal junction (G/GEJ) adenocarcinoma: major pathologic response (mPR) in KEYNOTE-585. J Clin Oncol. 2024;42(suppl 16):4073. [Google Scholar]
- 84.Janjigian Y.Y., Van Cutsem E., Muro K., et al. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol. 2022;18(20):2465–2473. doi: 10.2217/fon-2022-0093. [DOI] [PubMed] [Google Scholar]
- 85.Tie J., Cohen J.D., Lahouel K., et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med. 2022;386(24):2261–2272. doi: 10.1056/NEJMoa2200075. [DOI] [PMC free article] [PubMed] [Google Scholar]