Abstract
Multidrug resistance in bacteria poses a significant threat to global health, creating an urgent need for new sources of antibiotics. Nut grass or Cyperus rotundus L., a common Asian medicinal herbal remedy, is gaining increasing attention in the scientific community as a potential source of antimicrobial agents. In this study, endophytic fungi living in this plant were isolated, macro- and micrologically identified, and assessed for their antibacterial properties on both Gram-positive and Gram-negative bacteria. As a result, seven types of endophytic fungi with potential antibacterial activities were obtained from Vietnamese Cyperus rotundus L. These endophytic strains could inhibit Gram-positive bacteria, including Bacillus subtilis, Methicilin-susceptible Staphylococcus aureus (MSSA), and Methicilin-resistant Staphylococcus aureus (MRSA). In particular, the most potent fungus could effectively inhibit not only MRSA but also Escherichia coli, and Pseudomonas aeruginosa. Furthermore, a significant impact of the culture medium on the biomass’ antibacterial activity was observed and the Potato Dextrose Agar (PDA) and Czapek-Dox (Cz) media were shown to be the most appropriate culture medium. Altogether, endophytic fungi isolated from Cyperus rotundus L. were shown to be a promising source for antibiotics to tackle the problem of antibiotic resistance.
Keywords: Cyperus rotundus L., Endophytic fungi, Antibiotics, Multidrug resistance
Highlights
-
•
Multidrug resistance in bacteria is a critical global health threat.
-
•
Cyperus rotundus L. emerges as a potential source for new antibiotics.
-
•
Seven endophytic fungi from Cyperus rotundus L. showed promising antibacterial activity.
-
•
The fungi inhibited Gram-positive bacteria, including MRSA.
-
•
Culture medium significantly impacts antibacterial activity.
1. Introduction
The efficacy of treating infectious diseases, particularly nosocomial bacterial infections, is on a decline as these pathogens continuously develop new resistance mechanisms to antibiotics [1,2]. The prevalence of multidrug-resistant (MDR) and pan-drug-resistant (PDR) bacteria has undeniably brought up extensive concerns about the degree of antibiotic treatment efficacy. For instance, recently, an estimated 1.27 million deaths in 2019 was proven to be associated with antimicrobial resistance (AMR) [1,2]. Consequently, conventional antibiotic treatment regimens require higher doses and more frequent dosing intervals to prevent antimicrobial resistance due to selection and mutagenesis [3].
When antibiotics are administered, they eliminate the bacteria that are susceptible, while those with resistance mutations survive and multiply. As a result, these resistant bacteria become more common in the population over time through natural selection. This practice is ultimately unsustainable in the long run due to higher risks of systemic exposures to antibiotics and can lead to a series of adverse side effects, including cardiovascular issues, neurotoxicity, and the development of new resistance [4].
Furthermore, it has been observed that the number of casualties from failed antibiotic therapies or MDR and PDR infections would be on the rise in the absence of appropriate treatments for preventing and treating drug-resistant infectious diseases [5]. To improve the quality-adjusted life years for patients together with the need to upgrade first-line regimens, numerous MDR and PDR infections are included in the list of priority pathogens for research and development of novel antibiotics by the World Health Organization (WHO) [6]. Furthermore, scientists are urgently searching for more effective alternatives to antibiotics to combat these microorganisms.
Due to these aforementioned reasons, research on potential antibacterial agents extracted from medicinal plants is a widely recognized trend among scientists. In addition, these plants are also the hosts to a wide range of microbial domains. Among different natural resources for antibiotics, bioactive compounds derived from endophytic fungus, are increasingly considered as a reliable and promising alternative to chemosynthetic drugs [[7], [8], [9]]. In modern medicine, endophytes or microorganisms living in medicinal herbs have been utilized as sources of secondary metabolites for drugs [7]. To be specific, endophytes are naturally found within the endospheric region of a plant cell therefore, posing no pathological consequences to host plants [10]. In nature, many studies have demonstrated a symbiotic relationship between the host plants and endophytes, in which, the host offers protection and food while the endophytes act as chemical guardians fostering plant growth, increasing the host's tolerance for abiotic and biotic challenges and thus improving disease-resisting capacity [[11], [12], [13]].
Natural compounds with intriguing bioactivities, such as antibacterial, larvicidal, antioxidant, anti-inflammatory, and phytotoxic activities can be obtained from plants' endophytic fungi [14]. By adopting a combinatory approach of different analytical methods including morphological, phylogenetic, and metabolomics, a growing number of bioactive compounds with distinctive chemical structures have been extracted and identified from endophytic fungi in recent years. These substances have been isolated from a variety of previously investigated plants, including angiosperms, bryophytes, pteridophytes, and herbage [14]. The majority of endophytic fungi produce beneficial secondary metabolites including lignans, phenylpropanoids, lactones, phenols, steroids, terpenoids, alkaloids, and isocoumarin derivatives [15].
Nut grass, scientifically known as Cyperus rotundus of Cyperaceae (C. rotundus), is often included in folk remedies, primarily distributed in Vietnam due to its pharmacological properties [16]. In addition, its rhizome and leaves can be used to produce essential oils, phenylpropanoids, triterpenoids, and alkaloids with antimicrobial activities [17]. Nevertheless, few studies have so far investigated the bioactivities of endophytic fungi found in nut grass. Therefore, this study was performed to isolate and identify endophytic fungi from nut grass and to search for endophytic fungi capable of producing bioactive substances with potent antimicrobial activities for “hard-to-treat” infections.
2. Materials and methods
2.1. Material
Trypton Soybean Agar (TSA), Sabouraud Dextrose Agar (SDA), Potato Dextrose Agar (PDA), Czapek-Dox Agar (Cz) were purchased from Himedia (USA).
2.2. Microbial strains
Five bacterial strains employed in this study are.
Methicillin-resistant Staphylococcus aureus (MRSA) ATCC 44300
Methicillin-susceptible Staphylococcus aureus (MSSA) ATCC 25923
Pseudomonas aeruginosa ATCC 27853
Escherichia coli ATCC 25922
Bacillus subtilis ATCC 6633
2.3. Sample collection and verification
Leaves and rhizomes of healthy, mature C. rotundus were collected in Ho Chi Minh City, Vietnam, and were phytologically verified according to Vietnamese pharmacopeia V. The herbarium voucher number of nut grass is B20240711 which was deposited at the Faculty of Pharmacy, University of Medicine and Pharmacy at Ho Chi Minh City.
Afterward, the samples were washed with tap water before being cut into small parts. The surfaces of the obtained samples were then sterilized according to a defined protocol (described below).
2.4. Surface sterilization
Due to the presence of complex epiphytic microbiota on the plants’ surface, a serial antiseptic solution was used to remove epiphytic microbiota without interfering with its endophytic microbiota [18,19]. The samples were cut into small pieces (2–3 cm) by a sterile knife. Every piece was immersed alternatively in 5 % (v/v) bleach (sodium hypochlorite) for 2 min, 70 % (v/v) ethanol for 2 min and distilled water for 5 min. At the end, the pieces were dried out in biosafety cabinet.
2.5. Isolation of endophytic fungi
To culture endophytic fungi, PDA culture medium was used. The surface-sterilized samples were aseptically cut into 1–2 cm pieces, placed directly on the culture medium plates, and incubated at 28 °C for seven days. The plates were checked periodically, and the growth of endophytic fungi colonies from the tissues was monitored daily. Different colonies with specific fungal characteristics were introduced and subcultured onto three different media including PDA, SDA, and Cz. Pure cultures were obtained after two to three consecutive subcultures and transferred into fresh isolation media. Finally, each fungal colony for a distinct strain was collected and stored in distilled water at 28 °C for further analysis.
2.6. Evaluation of antimicrobial activities
The antimicrobial activities of obtained endophytic fungi were screened against five pathogenic microorganisms (S. aureus MRSA, S. aureus MSSA, P. aeruginosa, E. coli, and B. subtilis) using the disk diffusion assay method [20]. Initially, four or five colonies of test bacteria were suspended in 2 mL of sterile saline by using a sterile inoculating loop. The suspension was adjusted to the 0.5 McFarland standard or optical density (OD) equivalent to 0.1 at 600 nm. The bacterial suspension was streaked onto TSA dishes. For endophytic fungi samples, fungal colonies on three culture media were cut using a sterile cork borer with a diameter of 6 mm, and then were directly set on the TSA medium. The plates were incubated at 37 °C for 24 h. The zone of growth inhibition was determined. All tests were performed in triplicate.
2.7. Macroscopicand microscopic studies of endophytic fungus
To classify endophytic fungi, different macroscopic and microscopic techniques were applied. Macroscopic vegetative characteristics, including, color, texture and topography were assessed. Diffuse pigmentation of structures together with color and topography of the colony's dorsum were microscopically analyzed for their reproductive structures, using microculture and/or sporulation methods [21].
2.8. Molecular identification
The fungal strain was identified by DNA amplification and sequencing of the internal transcribed spacer (ITS) region using molecular biology protocol. The endophytic strains were cultured in PDA medium for five days, the fungal threads were transferred into Eppendorf tubes (Biologix, USA) containing 100 μL of cetyltrimethylammonium bromide (CTAB). The procedures were performed according to previously published protocols including cell lysis, RNA digestion by RNase A, removal of precipitates and cell waste, DNA shearing, precipitation, and purification [21].
Then, the isolated DNA was amplified by polymerase chain reaction (PCR). The PCR was performed using the MyTaq™ DNA PCR kit (Meridian, USA). As primers, ITS 1-F (with 5′TCCGTAGGTGAACCTGCGG3′ base sequence) and ITS2-R (with 5′GCTGCGTTCTTCATCGATGC3′ base sequence) were mixed with the MyTaq™ DNA Polymerase PCR kit and DNA template with a total volume of 50 μL. The mixture was then added to the thermal cycler using the programmed PCR (BioRad, USA). The ITS gene sequences were compared with the sequences retrieved from EzBioCloud and GenBank, NCBI databases using BLAST tool (Basic Local Alignment Search Tool) at http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi.
3. Results
3.1. Isolation of endophytic fungi
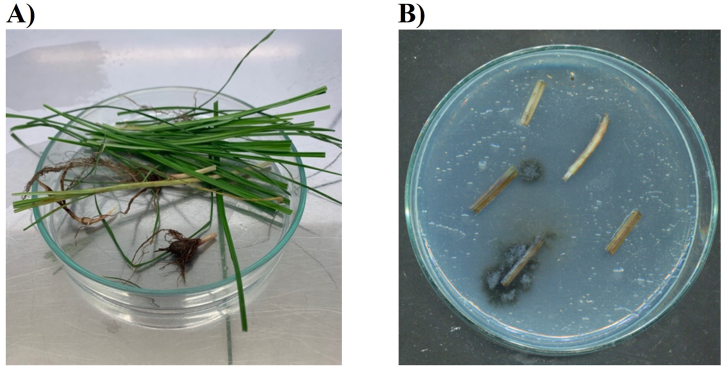
A total of seven endophytic fungi were isolated from the leaves and the rhizome of healthy and mature C. rotundus, coded as strain HP-L1, HP-L2, HP-L3, HP-L4, HP-R1, HP-R2, HP-R3, and cultured on three different media sources SDA, PDA and Cz as shown in Fig. 1.
Fig. 1.
Isolation of endophytic fungi from Cyperus rotundus L. Cyperaceae. A) Fresh Cyperus rotundus L. Cyperaceae, B) Endophytic fungi.
3.2. Antimicrobial screening of endophytic fungi from C. rotundus
To investigate the antibacterial activity of the obtained endophytic fungi, the agar disc diffusion assay was applied for five pathogenic bacteria, including both Gram-positive (MRSA, MSSA and B. subtilis) and Gram-negative (E. coli and P. aeruginosa). As shown in Fig. 2A–G, all of the isolated endophytic fungi presented significant antimicrobial activity. Nevertheless, this antibacterial activity was more pronounced on Gram-positive bacteria than on Gram-negative strains. Indeed, the diameters of the antibacterial zones of MRSA, MSSA and B. subtilis were more than 15 mm for all of the isolated endophytic fungi, especially, that of HP-L1 was shown to be the highest one (more than 30 mm). In contrast, E. coli was less susceptible whereby an inhibitory zone was observed for five of the seven endophtic strains (HP-L1, HP-L3, HP-L4, HP-R1, HP-R2). Especially, only one out of seven strains (HP-L1) exhibited an antibacterial activity on P. aeruginosa. The diameter for this endophytic fungal strain HP-L1 was greater than 20 mm. In comparison, the HP-L2 isolate showed a lower diameter (15–20 mm) of antibacterial zones whereas the other five isolates (HP-L3, HP-L4, HP-R1, HP-R2, HP-R3) revealed no antibacterial zones against P. aeruginosa bacteria.
Fig. 2.
Antimicrobial activity against tested microorganism strains of obtained endophytic fungi from Cyperus rotundus: A) strain HP-L1, B) strain HP-L2, C) HP-L3, D) HP-L4, E) HP-R1, F) HP-R2, G) HP-R3. The diameter (in mm) represents the ‘antibacterial zone’.
As expected, the culture medium was shown to have a significant impact on the antibacterial properties of endophytic fungal strains. The results showed that for all tested endophytic strains, the strains cultured on PDA and Cz media possessed more effective antibacterial capacity than that of SDA.
3.3. Macroscopic and microscopic of fungal isolates
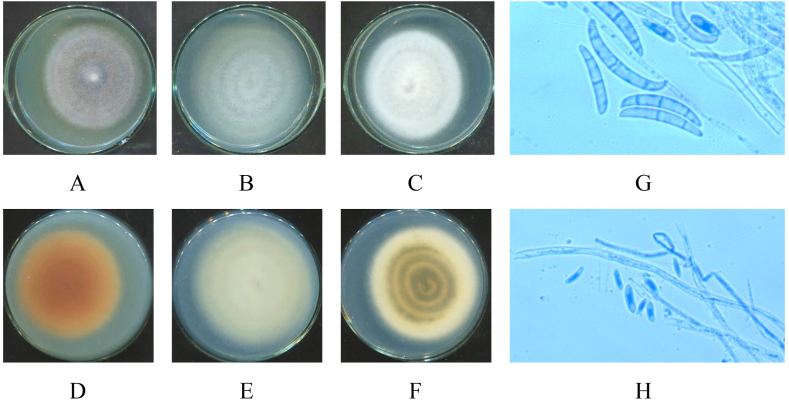
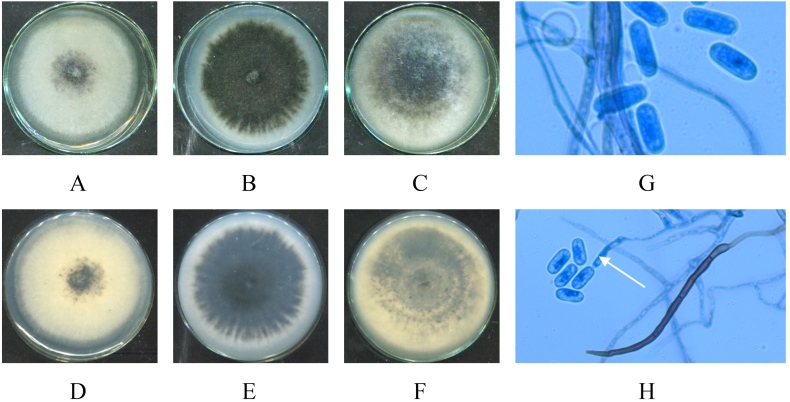
Colonies on SDA, PDA and Cz media of seven isolated strains: HP-L1, HP-L2, HP-L3, HP-L4, HP-R1, HP-R2, HP-R3 were macroscopically and microscopically analyzed. The results are shown in Fig. 3(A–K), Fig. 4(A–H), Fig. 5(A–H), Fig. 6(A–H), Fig. 7(A–H), Fig. 8(A–H), and Fig. 9(A–H), respectively.
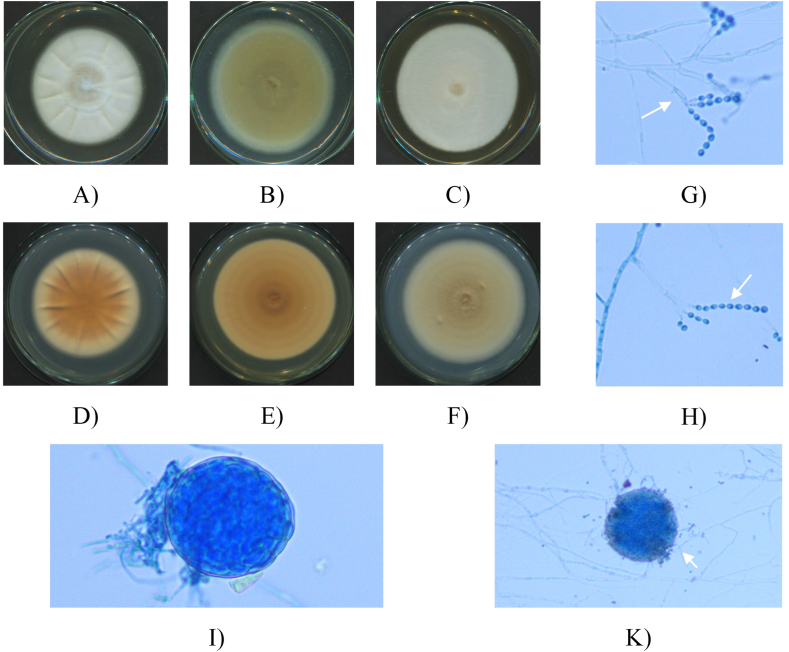
Fig. 3.
Macro and micro characteristics of Penicillium setosum isolate (HP-L1): 7-day old upper surface colonies (A–C) and reverse sides (D–F) on different culture media SDA (A, D), PDA (C, F) and Cz (B, E) at 28 °C; Mature cleistothecium (I); Cleistothecium squashed open to show several asci with ascospores (K); Conidiophores with phialides (G); Chains of sphere or ovoid conidia (H) (White arrow) under microscope (x40).
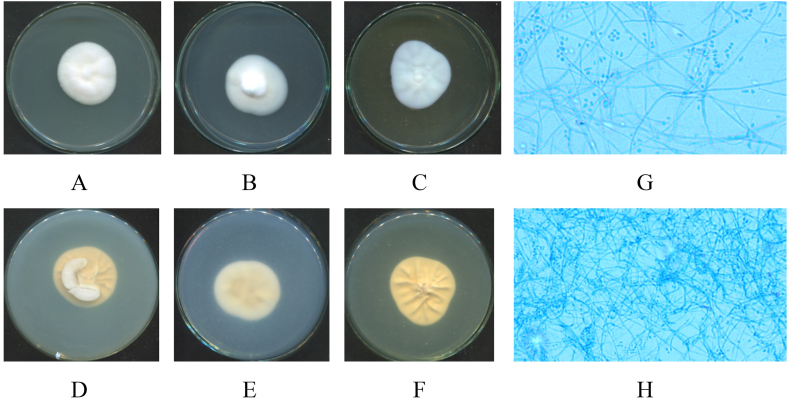
Fig. 4.
Macro and micro morphology of Simplicillium obclavatum isolate (HP-L2): 7-day old upper surface colonies (A–C) and reverse sides (D–F) on different culture media SDA (A, D), PDA (B, E) and Cz (C, F) at 28 °C; mycelium and conidia under microscope (x40) (G, H).
Fig. 5.
Macro and micro morphology of Neottiosporina sp. isolate (HP-L3): 7-day old upper surface colonies (A–C) and reverse sides (D–F) on different culture media SDA (A, D), PDA (B, E) and Cz (C, F) at 28 °C; mycelium and conidia under microscope (x40) (G, H).
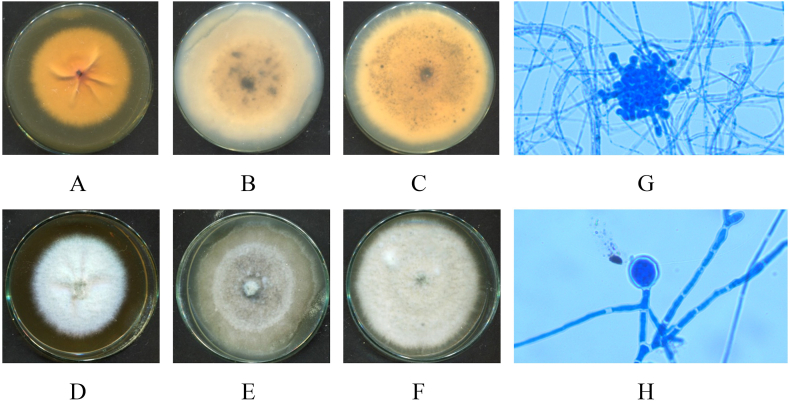
Fig. 6.
Macro and micro morphology of Curvularia sp. isolate (HP-L4): 7-day old upper surface colonies (A–C) and reverse sides (D–F) on different culture media SDA (A, D), PDA (B, E) and Cz (C, F) at 28 °C; Conidiophores and conidia (G); mycelia (H) under microscope (x40).
Fig. 7.
Macro and micro morphology of Ceratobasidium sp. isolate (HP-R1): 7-day old upper surface colonies (A–C) and reverse sides (D–F) on different culture media SDA (A, D), PDA (B, E) and Cz (C, F) at 28 °C; mycelia (G, H) under microscope (x40).
Fig. 8.
Macro and micro morphology of Fusarium keratoplasticum isolate (HP-R2): 7-day old upper surface colonies (A–C) and reverse sides (D–F) on different culture media SDA (A, D), PDA (B, E) and Cz (C, F) at 28 °C; macroconidia (G); microconidia (H) under microscope (x40).
Fig. 9.
Macro and micro morphology of Collectotrichum crassipes isolate (HP-R3): 7-day old upper surface colonies (A–C) and reverse sides (D–F) on different culture media SDA (A, D), PDA (B, E) and Cz (C, F) at 28 °C; conidia (G, H); conidiogenous cells (H) (white arrow) under microscope (x40).
3.4. Molecular identification of fungal isolates
All seven isolated strains (HP-L1, HP-L2, HP-L3, HP-L4, HP-R1, HP-R2 and HP-R3) were identified by DNA sequence analysis based on mega BLAST software provided by the U.S. National Centre for Biotechnology Information (NCBI).
As shown in the results (Table 1Fig. 10), seven different species for seven endophytic fungal isolates were found in the leaves and rhizomes of C. rotundus L., including, Penicillium sp. (HP-L1), Simplicillium sp. (HP-L2), Colletotrichum sp. (HP-R3), Fusarium sp. (HP-R2), Curvularia sp. (HP-L4), Ceratobasidium sp. (HP-R1), and Neottiosporina sp. (HP-L3).
Table 1.
NCBI blast results of the fungal isolates recovered from Cyperus rotundus L.
| Isolates | Description | Matched Sequence ID | Query Coverage | E-value | Percent Identity |
|---|---|---|---|---|---|
| HP-L2 | Simplicillium obclavatum strain UAS 046 | FJ156235 | 99 % | 0.0 | 99.11 % |
| HP-L1 | Penicillium setosum CBS 144865 | NR_185521.1 | 99 % | 0.0 | 99.44 % |
| HP-L3 | Neottiosporina sp. GZ-2024a strain RUFCC2454 | PP989220.1 | 96 % | 0.0 | 100 % |
| HP-L4 | Curvularia sp. | LC422832.1 | 100 % | 0.0 | 99.82 % |
| HP-R1 | Ceratobasidium sp. | OP604252.1 | 92 % | 1E-148 | 84.24 % |
| HP-R2 | Fusarium keratoplasticum isolate L-2320/2012 | MN540855.1 | 99 % | 0.0 | 100 % |
| HP-R3 | Colletotrichum crassipes 11F015 | LC387814.1 | 100 % | 0.0 | 99.39 % |
Fig. 10.
A phylogenetic tree constructed for fungal species isolated from Cyperus rotundus L.
4. Discussion
Endophytic fungi exhibit a significant level of taxonomic diversity and have the ability to inhabit various tissues, influencing the morphological and physiological functions of host plants through diverse mechanisms. Throughout their life cycle, these fungi produce bioactive substances with poorly understood biological control properties. Indeed, they possess various biological control properties that benefit their host plants. They can produce bioactive compounds that inhibit the growth of plant pathogens and reduce oxidative stress, thereby protecting the plant from diseases. Additionally, endophytes can enhance plant resistance to pests and herbivores by producing defensive chemicals. They also help induce systemic resistance in plants, making them more resilient to various stresses. Consequently, there is a growing interest in unraveling the complex ecological and biological functions of endophytic fungi [13,22,23]. Cyperus rotundus, a common medicinal plant which is utilized in many countries due to its pharmacological activities, has been found to possess potent antimicrobial activity against both Gram-positive and Gram-negative bacteria [24].
As per the findings of this study, this property of endophytic fungi from Vietnamese Cyperus rotundus was validated. Firstly, the results showed that the number of endophytic fungi in Vietnamese nut grass was lower than that of other countries with similar climate characteristic such as, Srilanka or Indonesia (7 versus over 10 strains) [25,26]. To explain this difference, not only geographical conditions (temperature, humidity) play an important role but also soil conditions, especially the nutrient content, as well as the presence of different pests in Vietnam may be important attributes. The fact that our isolate HP-R1 is only about 84 % related to Ceratobasidium and Rhizoctonia highlights its significant genetic distinction. Among Ceratobasidium species, some isolates showed strong antimicrobial activity. This uniqueness offers promising potential for novel antibiotic sources, as the genetic variation may translate into new bioactive compounds with potent antimicrobial properties. Harnessing this genetic divergence could lead to breakthrough treatments in combating antibiotic-resistant pathogens. Moreover, these findings have inspired future research work on endophytes from plants in other countries that originate from the same plant species but may display diverse antimicrobial activity.
Among the collected endophytes, four were isolated from leaves, and three from roots, indicating that no apparent preference for a specific plant tissue was significant. In addition, the culture medium of isolated fungi was shown to have a significant impact on its antibacterial activity. Indeed, PDA and Cz media are recommended for a good antibacterial capacity. This difference may be attributed to the presence of potato extract (PDA) and nitrate components (Cz) that falicitate the development of fungal spores. Moreover, the fact that 100 % of the isolated endophytic fungi displayed potent antibacterial activity suggests that most endophytic fungi living in Vietnamese nut grass, have the potential to synthesize antibacterial secondary metabolites. Especially, among the obtained endophytic strains, the HP-L1 isolate displayed a potential antimicrobial activity for all five of the tested bacteria, even on the most threatening nosocomial bacterial infections: MRSA and P. aeruginosa [27,28].
This finding also suggests that the endophylic fungi isolated from Vietnamese nut grass possesses a better anti-bacterial activity against both gram positive and gram negative strains, with an emphasis on “hard-to-treat” species [26]. The results highlight the plausibility for natural sources in the discovery of novel antibacterial agents. Furthermore, this is also the first study in Vietnam and in mainland Southeast Asia that focuses on this potent source of antibiotics.
5. Conclusions
The increasing problem of antibiotic resistance and the limitations in naturally synthesizing bioactive compounds in plants due to metabolic and environmental constraints make it crucial to explore biotechnological approaches for producing these compounds. This study highlights the potential of Vietnamese C. rotundus as a reservoir for antibacterial endophytic fungi. The results reveal that endophytic fungi isolated from this plant could serve as a potent resource of novel antibiotics against both Gram-positive and Gram-negative bacteria, especially for MRSA and P. aeruginosa. Future studies will focus on examining the structure of secondary metabolites produced by these fungi and the optimal culture conditions required for obtaining potential antibacterial agents. To scale up fermentation processes, the effects of nutritional factors and harvest timing on the production of antimicrobial compounds from endophytes will be investigated. Following the optimization of culture conditions, endophytic fungi will be fermented to maximize the yield of antibacterial compounds of which will be isolated using chromatographic techniques, and their chemical structures will be determined.
CRediT authorship contribution statement
Bac V.G. Nguyen: Writing – original draft, Methodology, Conceptualization. Linh X.T. Tran: Investigation. Anh-Tu Ha-Nguyen: Writing – review & editing. Minh-Tri Le: Validation. Thanh-Hoa Vo: Data curation. Gia Phong Vu: Writing – review & editing, Formal analysis, Data curation. Phuoc-Vinh Nguyen: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization.
Data availability
Data included in article/supplementary material is referenced in the article.
Funding
This research is funded by Vietnam National University Ho Chi MinhCity (VNU-HCM) under grant number No. C2024-44-11.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2025.e41920.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salam MdA., Al-Amin MdY., Salam M.T., Pawar J.S., Akhter N., Rabaan A.A., Alqumber M.A.A. Antimicrobial resistance: a growing serious threat for global public health. Healthcare. 2023;11:1946. doi: 10.3390/healthcare11131946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basak S., Singh P., Rajurkar M. Multidrug resistant and extensively drug resistant bacteria: a study. Journal of Pathogens. 2016;2016:1–5. doi: 10.1155/2016/4065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raymond B. Five rules for resistance management in the antibiotic apocalypse, a road map for integrated microbial management. Evol Appl. 2019;12:1079–1091. doi: 10.1111/eva.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heianza Y., Ma W., Li X., Cao Y., Chan A.T., Rimm E.B., Hu F.B., Rexrode K.M., Manson J.E., Qi L. Duration and life-stage of antibiotic use and risks of all-cause and cause-specific mortality: prospective cohort study. Circ. Res. 2020;126:364–373. doi: 10.1161/CIRCRESAHA.119.315279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray C.J.L., Ikuta K.S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., Johnson S.C., Browne A.J., Chipeta M.G., Fell F., Hackett S., Haines-Woodhouse G., Kashef Hamadani B.H., Kumaran E.A.P., McManigal B., Achalapong S., Agarwal R., Akech S., Albertson S., Amuasi J., Andrews J., Aravkin A., Ashley E., Babin F.-X., Bailey F., Baker S., Basnyat B., Bekker A., Bender R., Berkley J.A., Bethou A., Bielicki J., Boonkasidecha S., Bukosia J., Carvalheiro C., Castañeda-Orjuela C., Chansamouth V., Chaurasia S., Chiurchiù S., Chowdhury F., Clotaire Donatien R., Cook A.J., Cooper B., Cressey T.R., Criollo-Mora E., Cunningham M., Darboe S., Day N.P.J., De Luca M., Dokova K., Dramowski A., Dunachie S.J., Duong Bich T., Eckmanns T., Eibach D., Emami A., Feasey N., Fisher-Pearson N., Forrest K., Garcia C., Garrett D., Gastmeier P., Giref A.Z., Greer R.C., Gupta V., Haller S., Haselbeck A., Hay S.I., Holm M., Hopkins S., Hsia Y., Iregbu K.C., Jacobs J., Jarovsky D., Javanmardi F., Jenney A.W.J., Khorana M., Khusuwan S., Kissoon N., Kobeissi E., Kostyanev T., Krapp F., Krumkamp R., Kumar A., Kyu H.H., Lim C., Lim K., Limmathurotsakul D., Loftus M.J., Lunn M., Ma J., Manoharan A., Marks F., May J., Mayxay M., Mturi N., Munera-Huertas T., Musicha P., Musila L.A., Mussi-Pinhata M.M., Naidu R.N., Nakamura T., Nanavati R., Nangia S., Newton P., Ngoun C., Novotney A., Nwakanma D., Obiero C.W., Ochoa T.J., Olivas-Martinez A., Olliaro P., Ooko E., Ortiz-Brizuela E., Ounchanum P., Pak G.D., Paredes J.L., Peleg A.Y., Perrone C., Phe T., Phommasone K., Plakkal N., Ponce-de-Leon A., Raad M., Ramdin T., Rattanavong S., Riddell A., Roberts T., Robotham J.V., Roca A., Rosenthal V.D., Rudd K.E., Russell N., Sader H.S., Saengchan W., Schnall J., Scott J.A.G., Seekaew S., Sharland M., Shivamallappa M., Sifuentes-Osornio J., Simpson A.J., Steenkeste N., Stewardson A.J., Stoeva T., Tasak N., Thaiprakong A., Thwaites G., Tigoi C., Turner C., Turner P., Van Doorn H.R., Velaphi S., Vongpradith A., Vongsouvath M., Vu H., Walsh T., Walson J.L., Waner S., Wangrangsimakul T., Wannapinij P., Wozniak T., Young Sharma T.E.M.W., Yu K.C., Zheng P., Sartorius B., Lopez A.D., Stergachis A., Moore C., Dolecek C., Naghavi M. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeleke B.S., Babalola O.O. Pharmacological potential of fungal endophytes associated with medicinal plants: a review. J Fungi (Basel) 2021;7:147. doi: 10.3390/jof7020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva D.P.D., Cardoso M.S., Macedo A.J. Endophytic fungi as a source of antibacterial compounds-A focus on gram-negative bacteria. Antibiotics (Basel) 2022;11:1509. doi: 10.3390/antibiotics11111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasrija P., Girdhar M., Kumar M., Arora S., Katyal A. Endophytes: an unexplored treasure to combat Multidrug resistance. Phytomedicine. 2022;2 doi: 10.1016/j.phyplu.2022.100249. [DOI] [Google Scholar]
- 10.Singh V.K., Kumar A. Secondary metabolites from endophytic fungi: production, methods of analysis, and diverse pharmaceutical potential. Symbiosis. 2023:1–15. doi: 10.1007/s13199-023-00925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson D. Endophyte: the evolution of a term, and clarification of its use and definition. Oikos. 1995;73:274. doi: 10.2307/3545919. [DOI] [Google Scholar]
- 12.Chaudhary P., Agri U., Chaudhary A., Kumar A., Kumar G. Endophytes and their potential in biotic stress management and crop production. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.933017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana D.C., de Paula S., Torres A.G., de Souza V.H.M., Pascholati S.F., Schmidt D., Dourado Neto D. Endophytic fungi: biological control and induced resistance to phytopathogens and abiotic stresses. Pathogens. 2021;10:570. doi: 10.3390/pathogens10050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao Z., Zhang W., Wu C., Feng H., Peng Y., Shahid H., Cui Z., Ding P., Shan T. Diversity and antibacterial activity of fungal endophytes from Eucalyptus exserta. BMC Microbiol. 2021;21:155. doi: 10.1186/s12866-021-02229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manganyi M.C., Ateba C.N. Untapped potentials of endophytic fungi: a review of novel bioactive compounds with biological applications. Microorganisms. 2020;8:1934. doi: 10.3390/microorganisms8121934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhar P., Dhar D.G., Rawat A.K.S., Srivastava S. Medicinal chemistry and biological potential of Cyperus rotundus Linn.: an overview to discover elite chemotype(s) for industrial use. Ind. Crop. Prod. 2017;108:232–247. doi: 10.1016/j.indcrop.2017.05.053. [DOI] [Google Scholar]
- 17.Peerzada A.M., Ali H.H., Naeem M., Latif M., Bukhari A.H., Tanveer A. Cyperus rotundus L.: traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2015;174:540–560. doi: 10.1016/j.jep.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Thomas G., Kay W.T., Fones H.N. Life on a leaf: the epiphyte to pathogen continuum and interplay in the phyllosphere. BMC Biol. 2024;22:168. doi: 10.1186/s12915-024-01967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodyear N. Effectiveness of five-day-old 10% bleach in a student microbiology laboratory setting. Clin. Lab. Sci. 2012;25:219–223. doi: 10.29074/ascls.25.4.219. [DOI] [PubMed] [Google Scholar]
- 20.Tenover F.C. Reference Module in Biomedical Sciences. Elsevier; 2015. Antimicrobial susceptibility testing. [DOI] [Google Scholar]
- 21.Dos Reis J.B.A., Lorenzi A.S., Do Vale H.M.M. Methods used for the study of endophytic fungi: a review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022;204:675. doi: 10.1007/s00203-022-03283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segaran G., Sathiavelu M. Fungal endophytes: a potent biocontrol agent and a bioactive metabolites reservoir. Biocatal. Agric. Biotechnol. 2019;21 doi: 10.1016/j.bcab.2019.101284. [DOI] [Google Scholar]
- 23.Bhardwaj M., Kailoo S., Khan R.T., Khan S.S., Rasool S. Harnessing fungal endophytes for natural management: a biocontrol perspective. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1280258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilani-Jaziri S., Bhouri W., Skandrani I., Limem I., Chekir-Ghedira L., Ghedira K. Phytochemical, antimicrobial, antioxidant and antigenotoxic potentials of Cyperus rotundus extracts. South Afr. J. Bot. 2011;77:767–776. doi: 10.1016/j.sajb.2011.03.015. [DOI] [Google Scholar]
- 25.Ambarwati S. Antibiotic produced by streptomycetes associated with rhizosphere of purple nut sedge (Cyperus rotundus L.) in Surakarta, Indonesia. Afr. J. Microbiol. Res. 2012;6 doi: 10.5897/AJMR11.832. [DOI] [Google Scholar]
- 26.Ratnaweera P.B., Walgama R.C., Jayasundera K.U., Herath S.D., Abira S., Williams D.E., Andersen R.J., De Silva E.D. Antibacterial activities of endophytic fungi isolated from six Sri Lankan plants of the family Cyperaceae. Bangladesh J. Pharmacol. 2018;13:264. doi: 10.3329/bjp.v13i3.36716. [DOI] [Google Scholar]
- 27.Maddiboyina B., Roy H., Ramaiah M., Sarvesh C.N., Kosuru S.H., Nakkala R.K., Nayak B.S. Methicillin-resistant Staphylococcus aureus: novel treatment approach breakthroughs. Bull. Natl. Res. Cent. 2023;47:95. doi: 10.1186/s42269-023-01072-3. [DOI] [Google Scholar]
- 28.Losito A.R., Raffaelli F., Del Giacomo P., Tumbarello M. New drugs for the treatment of Pseudomonas aeruginosa infections with limited treatment options: a narrative review. Antibiotics (Basel) 2022;11:579. doi: 10.3390/antibiotics11050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material is referenced in the article.