ABSTRACT
Background and Aim
The burden of cirrhosis and other chronic liver diseases has changed in recent years due to shifts in the contributing aetiologies. We estimated the burden of cirrhosis and other chronic liver diseases, including etiological and regional differences, across 204 countries and territories from 2010 to 2021.
Approach and Results
We analysed temporal trends in the burden of cirrhosis and other chronic liver diseases utilising data from the Global Burden of Disease Study 2021. We estimated annual frequencies and age‐standardised rates (ASRs) of incident cases, deaths and disability‐adjusted life‐years (DALYs) by sex, country, World Health Organisation region and its contributing aetiologies. In 2021, there were an estimated 58 417 006 incident cases, 1 425 142 deaths and 46 417 777 DALYs related to cirrhosis and other chronic liver diseases. From 2010 to 2021, there was a rise in age‐standardised incidence rates (ASIRs) (APC: +0.35%) but age‐standardised death rates (ASDRs) (APC: −1.74%) and age‐standardised disability‐adjusted life‐years (ASDALYs) (APC: −1.85%) declined. Cirrhosis related to metabolic dysfunction‐associated steatohepatitis (MASH) contributed to 48 310 981 incident cases in 2021 and was largely responsible for the overall increase in ASIRs from 2010 to 2021. Cirrhosis and other chronic liver diseases related to MASH were the only aetiology with a rise in ASIR (APC: +0.86%). Age‐standardised deaths related to all aetiologies of cirrhosis and other chronic liver diseases declined during the study period. Age‐standardised deaths and DALYs related to MASH increased in the Americas, unlike all other world regions where they declined or remained stable.
Conclusions
Age‐adjusted deaths related to cirrhosis and other chronic liver diseases are declining. However, the age‐adjusted incidence of cirrhosis and other chronic liver diseases is increasing, driven by increases in the incidence of MASH.
Keywords: chronic liver disease, cirrhosis, global burden
Summary.
The global burden of cirrhosis and chronic liver diseases is changing.
Despite a general decline in age‐adjusted deaths, there has been an increase in age‐adjusted incidence, which is largely attributed to the increasing incidence of metabolic dysfunction‐associated steatohepatitis.
1. Introduction
Cirrhosis and chronic liver disease are major global health concerns, ranking among the top 10 leading causes of death in Africa, Southeast Asia, Europe and the eastern Mediterranean in 2023 [1]. The primary causes of cirrhosis and other chronic liver diseases include hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol‐related liver disease (ALD) and metabolic dysfunction‐associated steatohepatitis (MASH) [2]. However, the epidemiology of the causes of liver diseases has changed dramatically in the recent decade [3].
The burden of HBV is declining largely due to successful vaccination programs and the increasing availability of effective antiviral therapy [4]. However, antiviral coverage remains poor in many regions, with the World Health Organisation (WHO) estimating that only 2.6% of all people with HBV receive antiviral therapy [5]. For HCV, direct‐acting antivirals (DAAs), which were introduced in the past decade, have made HCV cure possible for the vast majority of patients [6, 7, 8]. However, the effectiveness of DAAs has been hampered by limited accessibility, with only 20% of infected patients receiving therapy [5]. In contrast, ALD has seen a significant rise in mortality [9] and remains the leading cause of alcohol‐related deaths worldwide [10, 11]. The COVID‐19 pandemic has exacerbated this issue, with studies reporting a 24% increase in alcohol consumption among patients with alcohol use disorder (AUD). MASH, previously known as non‐alcoholic steatohepatitis (NASH) [12], is the inflammatory form of metabolic‐dysfunction‐associated steatotic liver disease (MASLD) that can progress to cirrhosis and liver cancer [13]. The prevalence of MASLD has grown rapidly, increasing from 17.6% in 1990 to 23.4% in 2019, making it one of the most prominent liver diseases in recent years [14].
These changes in the underlying causes of liver disease have led to a corresponding shift in the landscape of cirrhosis and other chronic liver diseases. There are limited data from a global perspective utilising the Global Burden of Disease Study 2021 (GBD 2021). Therefore, we provide updated estimates on the incidence, deaths and disability‐adjusted life years (DALYs) related to cirrhosis and other chronic liver diseases, as well as the contributing liver aetiologies across 204 countries and territories from 2010 to 2021.
2. Methods
2.1. Data Source
This study used data from the GBD 2021 [15]. The GBD 2021 study provides estimates for 371 diseases and injuries in 204 countries and territories. Information on incidence, deaths and DALYs, including stratification into SDI and WHO region [15], were obtained using the Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd‐results‐tool), which is a data catalogue created and supported by the Institute for Health Metrics and Evaluation (IHME).
2.2. Estimation Methods
In the GBD study, cirrhosis and other chronic liver diseases were divided into five main aetiologies. This included cirrhosis and other chronic liver diseases due to alcohol use, chronic hepatitis B, chronic hepatitis C, non‐alcoholic fatty liver disease and cirrhosis related to other causes [16]. Notable diseases from ‘cirrhosis related to other causes’ included autoimmune hepatitis, toxic liver disease, other forms of inflammatory liver disease and non‐specific chronic hepatitis [16]. Recent studies have demonstrated concordance between NAFLD and MASLD [17] and therefore term ‘non‐alcoholic fatty liver disease’ and data collected in this category were classified and referred to using the new nomenclature of MASLD [18]. General estimation methods for the GBD 2021 have been previously described. Data sources were identified from vital registration, verbal autopsy, registry, survey, police or surveillance data across all countries and territories [15]. For GBD 2021, improvements were made upon previous studies; this included updated Bayesian algorithms to reduce noise and implementation of a non‐zero floor to address distorted shapes and trends [19]. In addition, COVID‐19 estimates and other pandemic‐related mortalities (OPRMs) were included as new additions in GBD 2021 [15].
Incidences were modelled using DisMod‐MR 2.1 (Disease Modelling Meta‐Regression; version 2.1), which is a Bayesian disease modelling meta‐regression tool that generates estimates of values such as incidence, prevalence, remission and others [15]. However, for some causes, spatiotemporal Gaussian process regression (ST‐GPR) disease models were used instead of DisMod‐MR 2 [15]. For mortality estimates, GBD 2021 uses the ICD, 11th edition, whereby each death is assigned to the underlying cause that initiated events leading to death. Cause‐of‐death estimates were modelled via the Cause of Death Ensemble model (CODEm), which uses an ensemble of statistical models while also systematically testing combinations of covariates based on their out‐of‐sample predictive validity [15, 19]. It then combines results to estimate deaths by location, age, sex and year for a given cause. The cause‐of‐death database could only estimate overall cirrhosis and other chronic liver diseases and not its five causes. Instead, DisMod‐MR 2.1 splits the parent cirrhosis and other chronic liver disease mortality estimates and used liver cancer aetiological proportion models as covariates [16]. DALY estimates for GBD 2021 were informed by 100 983 data sources (including 19 189 sources newly used in 2021) [15]. Years lived with disability (YLDs) and years of life lost (YLLs) were summed to calculate DALYs by location, age, sex, year and cause [15].
Sociodemographic index (SDI) is a composite indicator representing the geometric mean of three parameters: the lag‐distributed income per capita, average years of schooling and the fertility rate in females younger than 25 years for a given location [15]. SDI scores were rescaled from 0 (lowest income and years of schooling, and highest fertility) to 100 (highest income and years of schooling, and lowest fertility) [15].
GBD 2021 metrics were estimated as counts, all‐age and age‐specific rates per 100 000 population, and age‐standardised rates per 100 000 population, calculated using the GBD standard population structure. All calculations were conducted 500 times to generate draw‐level estimates [15].
2.3. Statistical Analysis
Final estimates represent the mean estimate across 500 draws, and 95% uncertainty intervals (UIs) are represented by the 2·fifth and 97·fifth percentile values across the draws [15]. Uncertainty was propagated at each step in the estimation process.
The Joinpoint Regression Programme 5.2.0 was used to obtain annual percent change (APC) and its respective 95% confidence interval. When the annualised rate of change and the lower boundary of its 95% CI were both positive, this was considered an increasing trend. By contrast, when the annualised rate of change and the upper boundary were negative, this was considered as a decreasing trend. Results with a p‐value ≤ 0.05 were considered statistically significant. Percentage change between 2010 and 2021 within any group was calculated as (value in 2021—value in 2010)/value in 2010.
3. Results
3.1. Global Burden of Cirrhosis and Other Chronic Liver Diseases in 2021
Globally, in 2021, there were an estimated 58 417 006 (95% UI: 54231381–62 796 051) incident cases, 1 425 142 (95% UI: 1308121–1 563 089) deaths and 46 417 777 (95% UI: 43056397–50 687 931) DALYs related to cirrhosis and other chronic liver diseases (Supporting Information S1–, S3). In 2021, the age‐standardised incident rates (ASIRs), age‐standardised death rates (ASDRs) and age‐standardised disability‐adjusted life years (ASDALYs) were 724.31 per 100 000 population (95% UI: 672.98–779.55), 16.64 per 100 000 population (95% UI: 15.28–18.26) and 545.07 per 100 000 population (95% UI: 506.31–594.99), respectively. Between 2010 and 2021, there was a +18% increase in incident cases, a +7% increase in deaths and a +1% increase in DALYs. During the study period, ASIRs increased (APC: +0.35%, 95% CI: 0.30 to 0.40; p < 0.001), but the ASDRs (APC: −1.74%, 95% CI: −1.81 to −1.67; p < 0.001) and ASDALYs (APC: −1.85%, 95% CI: −1.92 to −1.77; p < 0.001) declined. In addition, for all three APC graphs (Supporting Information S4), there was a slight rise in recent years, especially from 2018 to 2021.
3.2. Burden of Cirrhosis and Other Chronic Liver Diseases by WHO Region
The incident cases, deaths and DALYs of cirrhosis and other chronic liver diseases by WHO regions are summarised in Supporting Information S1–, S3 and Figure 1. Southeast Asia had the highest number of incident cases (14 809 109; 95% UI: 13658110–16 064 676), deaths (460 403; 95% UI: 370585–562 696) and DALYs (15 924 598; 95% UI: 12975188—19 492 194) related to cirrhosis and other chronic liver diseases. However, for age‐standardised rates, eastern Mediterranean had the highest ASIR of 1057.21 (95% UI: 986.21–1125.43) per 100 000, while Africa had the highest ASDRs (31.44 per 100 000 population, 95% UI: 27.61–35.62) and the highest ASDALYs (933.8 per 100 000 population, 95% UI: 796.55–1074.47). From 2010 to 2021, the Americas had the greatest increase in the number of deaths (+22%) and DALYs (+16%), whereas Africa had the highest increase in incident cases (+27%).
FIGURE 1.

Global incident cases/age‐standardised incident rates, deaths/age‐standardised death rates and DALYs/age‐standardised DALYs rates of cirrhosis and other chronic liver diseases from 2010 to 2021, stratified by WHO region. (A) Age‐standardised incident rates due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by the WHO region. (B) Contribution by WHO region of incident cases due to cirrhosis and other chronic liver diseases in 2010 and 2021. (C) Trends in age‐standardised incident rates due to cirrhosis and other chronic liver diseases from 2010 to 2021, split by the WHO region. (D) Age‐standardised death rates due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by the WHO region. (E) Contribution by the WHO region of deaths due to cirrhosis and other chronic liver diseases in 2010 and 2021. (F) Trends in age‐standardised death rates due to cirrhosis and other chronic liver diseases from 2010 to 2021, split by the WHO region. (G) Age‐standardised DALYs due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by the WHO region. (H) Contribution by the WHO region of DALYs due to cirrhosis and other chronic liver diseases in 2010 and 2021. (I) Trends in age‐standardised DALYs due to cirrhosis and other chronic liver diseases from 2010 to 2021, split by the WHO region.
From 2010 to 2021, there was an increase in ASIRs in Europe, the Americas, Southeast Asia and the western Pacific, with the greatest increase in the western Pacific of APC: +0.49%, 95% CI: 0.44 to 0.55; p < 0.001. ASIRs declined in Africa (APC: −0.26%, 95% CI: −0.34 to −0.17; p < 0.001) and the eastern Mediterranean region (APC: −0.05%, 95% CI: −0.07 to −0.02; p = 0.002). During the study period, ASDRs and ASDALYs declined in all world regions. The greatest decline in ASDR (APC: −2.5%, 95% CI: −2.72 to −2.27; p < 0.001) and ASDALYs (APC: −2.46%, 95% CI: −2.65 to −2.27; p < 0.001) occurred in the eastern Mediterranean region (Figure 1F,I).
3.3. Burden of Cirrhosis and Other Chronic Liver Diseases by SDI
Middle SDI countries had the highest incident cases (19 806 401; 95% UI: 18363991–21 307 955) and deaths (441 526; 95% UI: 403365–485 997) related to cirrhosis and other chronic liver diseases, but low SDI countries had the highest ASIRs (778.48 per 100 000 population, 95% UI: 725.4–841.1) and ASDRs (29.74, 95% UI: 26.54–33.48) per 100 000 and ASDALYs of 907.41 (95% UI: 807.08–1024) per 100 000 (Supporting Information S1–, S3). The low DI countries had the greatest percentage increase in incident cases (+30%) and DALYs (+9%) (Figure 2B,H), whereas the middle SDI countries had the largest percentage increase in the number of deaths (+14%) (Figure 2E). For the rate of both deaths and DALYs, all SDI groups showed a declining trend with negative APC values. The greatest decline in ASDRs (APC: −2.70%, 95% CI: −3.17 to −2.23; p < 0.001) and ASDALYs (APC: −2.94%, 95% CI: −3.47 to −2.4; p < 0.001) occurred in high–middle SDI countries.
FIGURE 2.

Global/age‐standardised incident rates, deaths/age‐standardised death rates and DALYs/age‐standardised DALYs rates of cirrhosis and other chronic liver diseases from 2010 to 2021, stratified by SDI. (A) Age‐standardised incident rates due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by SDI. (B) Contribution by SDI of incident cases due to cirrhosis and other chronic liver diseases in 2010 and 2021. (C) Trends in age‐standardised incident rates due to cirrhosis and other chronic liver diseases from 2010 to 2021, split by SDI. (D) Age‐standardised death rates due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by SDI. (E) Contribution by SDI of deaths due to cirrhosis and other chronic liver diseases in 2010 and 2021. (F) Trends in age‐standardised death rates due to cirrhosis and other chronic liver diseases from 2010 to 2021, split by SDI. (G) Age‐standardised DALYs due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by SDI. (H) Contribution by SDI of DALYs due to cirrhosis and other chronic liver diseases in 2010 and 2021. (I) Trends in age‐standardised DALYs due to cirrhosis and other chronic liver diseases from 2010 to 2021, split by SDI.
3.4. Burden of Cirrhosis and Other Chronic Liver Diseases by Gender
Males experienced higher ASIR (767.36; 95% UI: 714.63–823.52), ASDR (23.13; 95% UI: 21.12–25.52) and ASDALY (762.35; 95% UI: 702.4–846.3) compared to females (Supporting Information S1–, S3). When broken down to specific aetiologies, ASIR, ASDR and ASDALY cirrhosis and other chronic liver diseases due to alcoholic, HBV and HCV were much higher in males (Figure 3, Supporting Information S5–, S7).
FIGURE 3.

Global age‐standardised incident rates, age‐standardised death rates and age‐standardised DALYs rates of cirrhosis and other chronic liver diseases from 2010 to 2021, stratified by gender. (A) Age‐standardised incident rates due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by SDI. (B) Age‐standardised death rates due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by SDI. (C) Age‐standardised DALY due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by SDI.
3.5. Burden of Cirrhosis and Other Chronic Liver Diseases by Country/Territory
The ASDRs related to cirrhosis and other chronic liver diseases in each country/territory in 2021 are summarised in Figure 4. Moldova (ASDR: 75.4, 95% UI: 67.89–83.64), Romania (ASDR: 55.61, 95% UI: 48.96–61.65) and Turkmenistan (51.55, 95% UI: 40.78–64.51) were the countries with the highest ASDR values (Supporting Information S8). ASIRs and ASDALYs in each country/territory in 2021 are also summarised in Figure 4.
FIGURE 4.
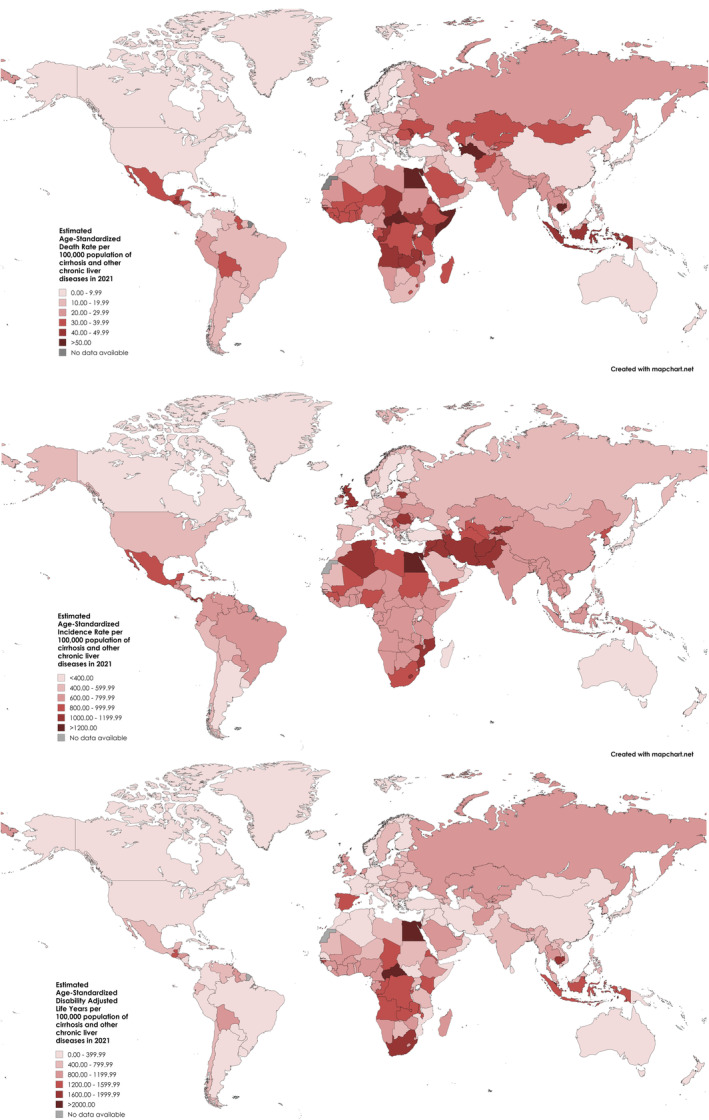
Map depicting the ASIR, ASDR and ASDALY across the countries.
3.6. Trends in the Aetiologies of Cirrhosis and Other Chronic Liver Diseases
MASH had the greatest number of incident cases (48 310 981; 95% UI: 4419137–52 313 165) and ASIRs (592.78, 95% UI: 542.23–643.24) per 100 000. For deaths and DALYs, the most significant contributor was cirrhosis due to hepatitis B with 431 964 (95% UI: 365199–502 419) deaths and 13 882 280 (95% UI: 11749493—15 998 380) DALYs (Supporting Information S1–, S3, Figure 5). Between 2010 and 2021, cirrhosis due to MASH had the highest percentage increase in incidence (+24%), deaths (+27%) and DALYs (+23%).
FIGURE 5.
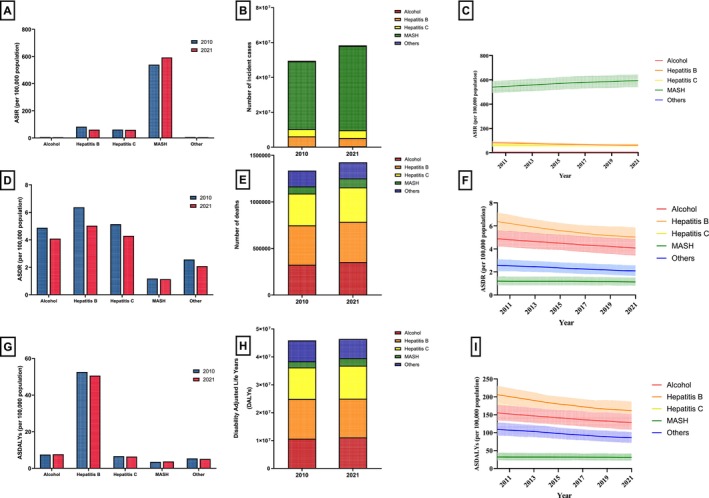
Global incident/age‐standardised incident rates, deaths/age‐standardised death rates and DALYs/age‐standardised DALYs rates of cirrhosis and other chronic liver diseases from 2010 to 2021, stratified by aetiology. (A) Age‐standardised incident rates due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by aetiology. (B) Contribution by aetiology of incident cases due to cirrhosis and other chronic liver diseases in 2010 and 2021. (C) Trends in age‐standardised incident rates due to cirrhosis and other chronic liver diseases from 2010 to 2021, split by aetiology. (D) Age‐standardised death rates due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by aetiology. (E) Contribution by aetiology of deaths due to cirrhosis and other chronic liver diseases in 2010 and 2021. (F) Trends in age‐standardised death rates due to cirrhosis and other chronic liver diseases from 2010 to 2021, split by aetiology. (G) Age‐standardized DALYs due to cirrhosis and other chronic liver diseases in 2010 and 2021, split by aetiology. (H) Contribution by aetiology of DALYs due to cirrhosis and other chronic liver diseases in 2010 and 2021. (I) Trends in age‐standardised DALYs due to cirrhosis and other chronic liver diseases from 2010 to 2021, split by aetiology.
ASIR declined in all aetiologies aside from cirrhosis due to MASH (APC: +0.86%, 95% CI: 0.82 to 0.90; p < 0.001) (Figure 5C), with cirrhosis due to HBV experiencing the greatest decrease (APC: −2.72%, 95% CI: −3 to −2.44; p < 0.001). All aetiologies also experienced a decrease in ASDR and ASDALY (Figure 5F,I). Cirrhosis due to HBV had the largest decrease in ASDRs (APC: −2.14%, 95% CI: −2.23 to −2.04; p < 0.001), whereas ASDALYs was most significantly decreased in cirrhosis due to other causes (APC: −2.19%, 95% CI: −2.36 to −2.01; p < 0.001).
When further stratified by WHO regions (Figure 6, Supporting Information S9–, S11), Africa had a significantly higher ASIR for cirrhosis related to HBV (ASIR: 130.77, 95% UI: 109.58–150.31) and cirrhosis related to HCV (ASIR: 104.82, 95% UI: 87.05–123.93; p < 0.001). In addition, ASIRs (APC: +0.52%, 95% CI: 0.35 to 0.68; p < 0.001) of alcohol‐associated cirrhosis in the Americas increased, unlike all other regions where ASIRs remained stable or declined. In the Americas, the ASDR (APC: +1.05, 95% CI: 0.86 to 1.25; p < 0.001) and ASDALY (APC: +1.19, 95% CI: 0.97 to 1.41; p < 0.001) of MASH‐related cirrhosis also increased in contrast to other regions where ASDR and ASDALY remained stable or declined.
FIGURE 6.

Contribution of aetiologies stratified by the WHO region. (A) Contributions of aetiologies to the incident cases for cirrhosis and chronic liver diseases, stratified by the WHO region. (B) Contributions of aetiologies to the deaths for cirrhosis and chronic liver diseases, stratified by the WHO region. (C) Contributions of aetiologies to the DALYs for cirrhosis and chronic liver diseases, stratified by the WHO region. (D) Contributions of aetiologies to the ASIR for cirrhosis and chronic liver diseases, stratified by the WHO region. (E) Contributions of aetiologies to the ASDR for cirrhosis and chronic liver diseases, stratified by the WHO region. (F) Contributions of aetiologies to the ASDALY for cirrhosis and chronic liver diseases, stratified by the WHO region.
In stratified analysis by SDI and aetiology (Figure 7, Supporting Information S12–, S14), low SDI regions had the highest ASIR (116.33, 95% UI: 97.56–134.49), ASDR (10.75 95% UI: 8.8–12.78) and ASDALY (322.71, 95% UI: 264.21–386.61) for cirrhosis related to HBV. Cirrhosis related to HCV followed a similar trend with low SDI regions having the highest ASIR (93.28, 95% UI: 77.38–110.68), ASDR (8.29, 95% UI: 6.82–10.13) and ASDALY (249.30, 95% UI: 203.40–302.86). ASIRs related to MASH increased across all SDI classes, most evidently in the high–middle SDI group (APC: +1.17, 95% CI: 1.11 to 1.224; p < 0.001).
FIGURE 7.

Contribution of aetiologies stratified by SDI. (A) Contributions of aetiologies to the incident cases for cirrhosis and chronic liver diseases, stratified by SDI. (B) Contributions of aetiologies to the deaths for cirrhosis and chronic liver diseases, stratified by SDI. (C) Contributions of aetiologies to the DALYs for cirrhosis and chronic liver diseases, stratified by SDI. (D) Contributions of aetiologies to the ASIR for cirrhosis and chronic liver diseases, stratified by SDI. (E) Contributions of aetiologies to the ASDR for cirrhosis and chronic liver diseases, stratified by SDI. (F) Contributions of aetiologies to the ASDALY for cirrhosis and chronic liver diseases, stratified by SDI.
4. Discussion
4.1. Main Findings
Between 2010 and 2021, there was a substantial increase in incident cases and deaths related to cirrhosis and chronic liver diseases. Age‐standardised incidence increased between 2010 and 2021, but age‐standardised deaths and DALYs declined during the study period. Cirrhosis related to MASH was the only aetiology with an increasing trend in age‐standardised incidence. By contrast, age‐standardised incidence rates from all other aetiologies declined, including HBV, HCV and alcohol. However, age‐standardised deaths related to all aetiologies of cirrhosis and other chronic liver diseases declined during the study period. Age‐standardised deaths and DALYs related to MASH increased in the Americas, unlike all other world regions where they declined or remained stable, which could be due to the rapidly rising obesity rates in the Americas [20, 21]. Collectively, these data suggest that age‐standardised death rates from cirrhosis and other chronic liver diseases are declining, but incidence rates are increasing, largely driven by MASH.
The decrease in death rates yet increase in incidence could reflect that despite an increase in cirrhosis and other chronic liver diseases, there have been more efficacious management strategies. This is most evident in the management of its contributing aetiologies such as in HBV and HCV [22]; however, advances in treating the complications of cirrhosis and other chronic liver diseases through pharmacological or procedural techniques may have allowed for improvement in recent years [23].
The decline in the burden of viral hepatitis highlights the effectiveness of vaccinations for HBV, and the impact of DAAs and elimination strategies for HCV [5, 24, 25]. Notably, the region of the Americas, which achieved a HBV third dose vaccination rate of 70% the earliest (2000) [26], presented with the lowest ASIR in both 2010 and 2021, showing the efficacy of vaccination in decreasing the incidence of HBV. However, the effectiveness of DAA in HCV is less clear, with the American and European region not having lower ASDR and ASDALY due to HCV despite having the earliest implementation of DAA in 2013 and 2014, respectively [27]. Despite this progress, the burden remains high in low SDI countries, related to gaps in linkage to care and limited vaccination coverage [5, 28, 29]. Underdiagnosis and undertreatment remain a major issue in viral hepatitis [5, 30], further exacerbated by the COVID‐19 pandemic [31]. The burden of alcohol‐associated cirrhosis appears to be declining; however, these data require cautious interpretation as underreporting is likely. However, the rapidly rising age‐adjusted incidence of cirrhosis related to MASH is sobering and calls for renewed focus to combat the metabolic risk factors contributing to this widespread disease [32].
In recent years, namely from 2018 to 2021, there has also been a slight increase in the APC in ASIR, ASDR and ASDALY. This could be attributed to the COVID‐19 pandemic, which saw a rise in obesity [33, 34] and possibly alcohol consumption [35]. In addition, concurrent COVID‐19 and cirrhosis or chronic liver disease were also associated with higher mortality, especially in unvaccinated individuals [36, 37], possibly resulting in an increase in ASDR.
4.2. In Context With Current Literature
This study builds on previous analyses of the burden of cirrhosis and other chronic liver diseases using GBD 2017 and GBD 2019 data, providing a comprehensive analysis of the global and regional burden of cirrhosis and other chronic liver diseases and its aetiologies [3, 16, 38]. Compared to previous GBD studies, GBD 2021 includes estimates for COVID‐19 and OPRMs, offering relevant and up‐to‐date insights into potential changes in trends due to the pandemic. The overall trends in cirrhosis and other chronic liver diseases and the contribution of various aetiologies in this study are supported by other country or region‐specific studies [39, 40, 41]. A previous study focused on liver‐related complications from MASLD from 1990 to 2021 [42]. The current study builds upon this by providing data for incidence, mortality and DALYs from the major aetiologies of liver disease from 2010 to 2021.
4.3. Limitations
The limitations of this study largely depend on the quality and validity of the data provided by GBD 2021. Data standardisation and estimate methods were used to account for missing data [12], which introduces uncertainty and variability. This was particularly evident in low SDI countries [19], which have increasingly contributed to the global burden of alcohol and MASH in the recent decades [43, 44]. Furthermore, low and middle SDI countries might have large health gaps as a result of a lack of well‐functioning health information systems [45], resulting in inaccurate estimates. Underreporting of alcohol consumption could also contribute to lower estimates. People with multiple aetiologies of liver disease were not accounted for in the GBD 2021 study. In addition, the GBD study did not distinguish between liver‐related deaths versus non‐liver related deaths; hence, these data require cautious interpretation.
4.4. Implications for Clinical Care and Research
Despite a decline in age‐adjusted death rates, the burden of cirrhosis remains high. These data have important implications for healthcare policymakers and public health specialists. National policies and health promotion initiatives aimed at preventing cirrhosis, such as by targeting the metabolic risk factors of MASH, are lacking [46, 47]. Increasing access to therapies for MASH, such as resmetirom, may alleviate the burden of MASH while awaiting more effective therapies [48, 49]. Despite the decline in the burden of cirrhosis related to HBV and HCV, its burden remains enormous, calling for renewed efforts towards eliminating viral hepatitis. Improving vaccination coverage, screening and linkage to care, especially in lower SDI countries, is likely to further reduce the burden of viral hepatitis [50, 51, 52, 53]. Public health policies directed against heavy alcohol use [54] may further reduce the burden of alcohol‐associated liver disease [55, 56].
In conclusion, death rates from cirrhosis are declining. However, the overall incidence is increasing, driven by MASH. Age‐standardised deaths and DALYs related to MASH increased in the Americas, unlike all other world regions where they declined or remained stable. Concerted efforts targeted at the metabolic risk factors to curb the rising incidence of MASH, increasing linkage to care for viral hepatitis and reducing heavy alcohol consumption are required to sustain the decline in the global burden of cirrhosis.
Author Contributions
All authors approve the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki. The study was exempt from IRB review as no confidential patient information was involved.
Conflicts of Interest
C.H.N. has served as a consultant for Boxer Capital. R.L. serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol‐Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. D.H., co‐founder of LipoNexus Inc., has served as an advisory board member for Gilead. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting information
Supporting Information S1.
Supporting Information S2.
Supporting Information S3.
Supporting Information S4.
Supporting Information S5.
Supporting Information S6.
Supporting Information S7.
Supporting Information S8.
Supporting Information S9.
Supporting Information S10.
Supporting Information S11.
Supporting Information S12.
Supporting Information S13.
Supporting Information S14.
Acknowledgements
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, acquisition of data or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content and (3) final approval of the version to be submitted. No writing assistance was obtained in the preparation of the manuscript. The manuscript, including related data, figures and tables, has not been previously published and the manuscript is not under consideration elsewhere.
Handling Editor: Alejandro Forner
Funding: R.L. received funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835) and John C. Martin Foundation (RP124). D.H. received funding support from the Singapore Ministry of Health's National Medical Research Council (MOH‐001370).
Contributor Information
Mark Muthiah, Email: mdcmdm@nus.edu.sg.
Daniel Q. Huang, Email: daniel_huang@nus.edu.sg.
Data Availability Statement
No dataset was used in this study. All articles in this manuscript are publicly available from Medline and Embase.
References
- 1. Devarbhavi H., Asrani S. K., Arab J. P., Nartey Y. A., Pose E., and Kamath P. S., “Global Burden of Liver Disease: 2023 Update,” Journal of Hepatology 79, no. 2 (2023): 516–537, 10.1016/j.jhep.2023.03.017. [DOI] [PubMed] [Google Scholar]
- 2. Smith A., Baumgartner K., and Bositis C., “Cirrhosis: Diagnosis and Management,” American Family Physician 100, no. 12 (2019): 759–770. [PubMed] [Google Scholar]
- 3. Huang D. Q., Terrault N. A., Tacke F., et al., “Global Epidemiology of Cirrhosis – Aetiology, Trends and Predictions,” Nature Reviews. Gastroenterology & Hepatology 20, no. 6 (2023): 388–398, 10.1038/s41575-023-00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2019 Hepatitis B Collaborators , “Global, Regional, and National Burden of Hepatitis B, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019,” Lancet Gastroenterology & Hepatology 7, no. 9 (2022): 796–829, 10.1016/s2468-1253(22)00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization , Global Hepatitis Report 2024: Action for Access in Low‐ and Middle‐Income Countries (World Health Organization, 2024). [Google Scholar]
- 6. Brunner N. and Bruggmann P., “Trends of the Global Hepatitis C Disease Burden: Strategies to Achieve Elimination,” Journal of Preventive Medicine and Public Health 54, no. 4 (2021): 251–258, 10.3961/jpmph.21.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carrat F., Fontaine H., Dorival C., et al., “Clinical Outcomes in Patients With Chronic Hepatitis C After Direct‐Acting Antiviral Treatment: A Prospective Cohort Study,” Lancet 393, no. 10179 (2019): 1453–1464, 10.1016/s0140-6736(18)32111-1. [DOI] [PubMed] [Google Scholar]
- 8. Jeong D., Wong S., Karim M. E., et al., “Treatment of HCV With Direct‐Acting Antivirals on Reducing Mortality Related to Extrahepatic Manifestations: A Large Population‐Based Study in British Columbia, Canada,” Lancet Regional Health 29 (2024): 100658, 10.1016/j.lana.2023.100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Åberg F., Jiang Z. G., Cortez‐Pinto H., and Männistö V., “Alcohol‐Associated Liver Disease‐Global Epidemiology,” Hepatology 80, no. 6 (2024): 1307–1322, 10.1097/hep.0000000000000899. [DOI] [PubMed] [Google Scholar]
- 10. Chaudhry H., Sohal A., Iqbal H., and Roytman M., “Alcohol‐Related Hepatitis: A Review Article Review,” World Journal of Gastroenterology 29, no. 17 (2023): 2551–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang D. Q., Mathurin P., Cortez‐Pinto H., and Loomba R., “Global Epidemiology of Alcohol‐Associated Cirrhosis and HCC: Trends, Projections and Risk Factors,” Nature Reviews. Gastroenterology & Hepatology 20, no. 1 (2023): 37–49, 10.1038/s41575-022-00688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rinella M. E., Lazarus J. V., Ratziu V., et al., “A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature,” Hepatology 78, no. 6 (2023): 1966–1986, 10.1097/HEP.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang D. Q., Noureddin N., Ajmera V., et al., “Type 2 Diabetes, Hepatic Decompensation, and Hepatocellular Carcinoma in Patients With Non‐Alcoholic Fatty Liver Disease: An Individual Participant‐Level Data Meta‐Analysis,” Lancet Gastroenterology & Hepatology 8, no. 9 (2023): 829–836, 10.1016/s2468-1253(23)00157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan W. K., Chuah K. H., Rajaram R. B., Lim L. L., Ratnasingam J., and Vethakkan S. R., “Metabolic Dysfunction‐Associated Steatotic Liver Disease (MASLD): A State‐of‐the‐Art Review,” Journal of Obesity & Metabolic Syndrome 32, no. 3 (2023): 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. GBD 2021 Diseases and Injuries Collaborators , “Global Incidence, Prevalence, Years Lived With Disability (YLDs), Disability‐Adjusted Life‐Years (DALYs), and Healthy Life Expectancy (HALE) for 371 Diseases and Injuries in 204 Countries and Territories and 811 Subnational Locations, 1990‐2021: A Systematic Analysis for the Global Burden of Disease Study 2021,” Lancet 403, no. 10440 (2024): 2133–2161, 10.1016/s0140-6736(24)00757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. GBD 2017 Cirrhosis Collaborators , “The Global, reGional, and National Burden of Cirrhosis by Cause in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017,” Lancet Gastroenterology & Hepatology 5, no. 3 (2020): 245–266, 10.1016/s2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong V. W. and Lazarus J. V., “Prognosis of MAFLD vs. NAFLD and Implications for a Nomenclature Change,” Journal of Hepatology 75, no. 6 (2021): 1267–1270, 10.1016/j.jhep.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 18. Rinella M. E., Lazarus J. V., Ratziu V., et al., “A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature,” Annals of Hepatology 29, no. 1 (2024): 101133. [DOI] [PubMed] [Google Scholar]
- 19. GBD 2021 Causes of Death Collaborators , “Global Burden of 288 Causes of Death and Life Expectancy Decomposition in 204 Countries and Territories and 811 Subnational Locations, 1990‐2021: A Systematic Analysis for the Global Burden of Disease Study 2021,” Lancet 403, no. 10440 (2024): 2100–2132, 10.1016/s0140-6736(24)00367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y., Beydoun M. A., Min J., Xue H., Kaminsky L. A., and Cheskin L. J., “Has the Prevalence of Overweight, Obesity and Central Obesity Levelled Off in the United States? Trends, Patterns, Disparities, and Future Projections for the Obesity Epidemic,” International Journal of Epidemiology 49, no. 3 (2020): 810–823, 10.1093/ije/dyz273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen J., Narayan K. M. V., and Cunningham S. A., “Incidence of Obesity Across Adulthood in the United States, 2001–2017—A National Prospective Analysis,” American Journal of Clinical Nutrition 117, no. 1 (2023): 141–148, 10.1016/j.ajcnut.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshiji H., Nagoshi S., Akahane T., et al., “Evidence‐Based Clinical Practice Guidelines for Liver Cirrhosis 2020,” Journal of Gastroenterology 56, no. 7 (2021): 593–619, 10.1007/s00535-021-01788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh J., Ebaid M., and Saab S., “Advances in the Management of Complications From Cirrhosis,” Gastroenterology Report 12 (2024): goae072, 10.1093/gastro/goae072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J., Liang W., Jing W., and Liu M., “Countdown to 2030: Eliminating Hepatitis B Disease, China,” Bulletin of the World Health Organization 97, no. 3 (2019): 230–238, 10.2471/blt.18.219469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nelson N. P., Jamieson D. J., and Murphy T. V., “Prevention of Perinatal Hepatitis B Virus Transmission,” Journal of the Pediatric Infectious Diseases Society 3, no. 1 (2014): S7–s12, 10.1093/jpids/piu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization , “WHO Immunization Data Portal.”
- 27. Seifert L. L., Perumpail R. B., and Ahmed A., “Update on Hepatitis C: Direct‐Acting Antivirals,” World Journal of Hepatology 7, no. 28 (2015): 2829, 10.4254/wjh.v7.i28.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hsu Y. C., Huang D. Q., and Nguyen M. H., “Global Burden of Hepatitis B Virus: Current Status, Missed Opportunities and a Call for Action,” Nature Reviews. Gastroenterology & Hepatology 20, no. 8 (2023): 524–537, 10.1038/s41575-023-00760-9. [DOI] [PubMed] [Google Scholar]
- 29. Zeng D. Y., Li J. M., Lin S., et al., “Global Burden of Acute Viral Hepatitis and its Association With Socioeconomic Development Status, 1990‐2019,” Journal of Hepatology 75, no. 3 (2021): 547–556, 10.1016/j.jhep.2021.04.035. [DOI] [PubMed] [Google Scholar]
- 30. Bixler D., Zhong Y., Ly K. N., et al., “Mortality Among Patients With Chronic Hepatitis B Infection: The Chronic Hepatitis Cohort Study (CHeCS),” Clinical Infectious Diseases 68, no. 6 (2018): 956–963, 10.1093/cid/ciy598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He C. Q., Sun B. H., Yu W. T., An S. Y., Qiao B. J., and Wu W., “Evaluating the Impact of COVID‐19 Outbreak on Hepatitis B and Forecasting the Epidemiological Trend in Mainland China: A Causal Analysis,” BMC Public Health 24, no. 1 (2024): 47, 10.1186/s12889-023-17587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang A. H., Tincopa M. A., Tavaglione F., et al., “Prevalence of Steatotic Liver Disease, Advanced Fibrosis and Cirrhosis Among Community‐Dwelling Overweight and Obese Individuals in the USA,” Gut 73 (2024): 2045–2053, 10.1136/gutjnl-2024-332917. [DOI] [PubMed] [Google Scholar]
- 33. Nour T. Y. and Altintaş K. H., “Effect of the COVID‐19 Pandemic on Obesity and Its Risk Factors: A Systematic Review,” BMC Public Health 23, no. 1 (2023): 1018, 10.1186/s12889-023-15833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Restrepo B. J., “Obesity Prevalence Among U.S. Adults During the COVID‐19 Pandemic,” American Journal of Preventive Medicine 63, no. 1 (2022): 102–106, 10.1016/j.amepre.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Capasso A., Jones A. M., Ali S. H., Foreman J., Tozan Y., and DiClemente R. J., “Increased Alcohol Use During the COVID‐19 Pandemic: The Effect of Mental Health and Age in a Cross‐Sectional Sample of Social Media Users in the U.S,” Preventive Medicine 145 (2021): 106422, 10.1016/j.ypmed.2021.106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keskin O., Oral H., Sahin T., Kav T., and Parlak E., “The Impact of COVID‐19 Disease on the Natural Course of Cirrhosis: Before and After Starting Vaccination,” Frontiers in Medicine 9 (2022): 1039202, 10.3389/fmed.2022.1039202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagarajan R., Krishnamoorthy Y., Rajaa S., and Hariharan V. S., “COVID‐19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta‐Analysis,” Preventing Chronic Disease 19 (2022): E53, 10.5888/pcd19.210228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu X. N., Xue F., Zhang N., et al., “Global Burden of Liver Cirrhosis and Other Chronic Liver Diseases Caused by Specific Etiologies From 1990 to 2019,” BMC Public Health 24, no. 1 (2024): 363, 10.1186/s12889-024-17948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Genowska A., Zarębska‐Michaluk D., Tyszko P., et al., “Trends of Infections and Mortality due to Hepatitis B Virus (2005‐2022) and the Potential Impact of the COVID‐19 Pandemic: A Population‐Based Study in Poland,” Clinical and Experimental Hepatology 9, no. 3 (2023): 286–296, 10.5114/ceh.2023.131225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hofmeister M. G., Zhong Y., Moorman A. C., Samuel C. R., Teshale E. H., and Spradling P. R., “Temporal Trends in Hepatitis C–Related Hospitalizations, United States, 2000–2019,” Clinical Infectious Diseases 77, no. 12 (2023): 1668–1675, 10.1093/cid/ciad425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonzalez‐Chagolla A., Olivas‐Martinez A., Ruiz‐Manriquez J., et al., “Cirrhosis Etiology Trends in Developing Countries: Transition From Infectious to Metabolic Conditions. Report From a Multicentric Cohort in Central Mexico,” Lancet Regional Health – Americas 7 (2022): 100151, 10.1016/j.lana.2021.100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu F., Liu J., She B., Yang H., Ji F., and Zhang L., “Global Trends and Inequalities of Liver Complications Related to Metabolic Dysfunction‐Associated Steatotic Liver Disease: An Analysis From 1990 to 2021,” Liver International (2024), 10.1111/liv.16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Danpanichkul P., Suparan K., Dutta P., et al., “Disparities in Metabolic Dysfunction‐Associated Steatotic Liver Disease and Cardiometabolic Conditions in Low and Lower Middle‐Income Countries: A Systematic Analysis From the Global Burden of Disease Study 2019,” Metabolism: Clinical and Experimental 158 (2024): 155958, 10.1016/j.metabol.2024.155958. [DOI] [PubMed] [Google Scholar]
- 44. Danpanichkul P., Chen V. L., Chaiyakunapruk N., et al., “Socio‐Economic Association of Alcohol Use Disorder and Cardiovascular and Alcohol‐Associated Liver Disease From 2010 to 2019,” Alimentary Pharmacology & Therapeutics 60, no. 3 (2024): 340–349, 10.1111/apt.18095. [DOI] [PubMed] [Google Scholar]
- 45. Zhao L., Cao B., Borghi E., et al., “Data Gaps Towards Health Development Goals, 47 Low‐ and Middle‐Income Countries,” Bulletin of the World Health Organization 100, no. 1 (2022): 40–49, 10.2471/blt.21.286254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karlsen T. H., Sheron N., Zelber‐Sagi S., et al., “The EASL‐Lancet Liver Commission: Protecting the Next Generation of Europeans Against Liver Disease Complications and Premature Mortality,” Lancet 399, no. 10319 (2022): 61–116, 10.1016/s0140-6736(21)01701-3. [DOI] [PubMed] [Google Scholar]
- 47. Lazarus J. V., Mark H. E., Villota‐Rivas M., et al., “The Global NAFLD Policy Review and Preparedness Index: Are Countries Ready to Address This Silent Public Health Challenge?,” Journal of Hepatology 76, no. 4 (2022): 771–780, 10.1016/j.jhep.2021.10.025. [DOI] [PubMed] [Google Scholar]
- 48. Petta S., Targher G., Romeo S., et al., “The First MASH Drug Therapy on the Horizon: Current Perspectives of Resmetirom,” Liver International 44, no. 7 (2024): 1526–1536. [DOI] [PubMed] [Google Scholar]
- 49. Keam S. J., “Resmetirom: First Approval,” Drugs 84, no. 6 (2024): 729–735, 10.1007/s40265-024-02045-0. [DOI] [PubMed] [Google Scholar]
- 50. World Health Organization , Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030 (World Health Organization, 2022). [Google Scholar]
- 51. Cox A. L., El‐Sayed M. H., Kao J. H., et al., “Progress Towards Elimination Goals for Viral Hepatitis,” Nature Reviews. Gastroenterology & Hepatology 17, no. 9 (2020): 533–542, 10.1038/s41575-020-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nguyen M. H., Wong G., Gane E., Kao J. H., and Dusheiko G., “Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy,” Clinical Microbiology Reviews 33, no. 2 (2020): e00046‐19, 10.1128/cmr.00046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen S., Mao W., Guo L., Zhang J., and Tang S., “Combating Hepatitis B and C by 2030: Achievements, Gaps, and Options for Actions in China,” BMJ Global Health 5, no. 6 (2020): e002306, 10.1136/bmjgh-2020-002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Komro K. A. and Toomey T. L., “Strategies to Prevent Underage Drinking,” Alcohol Research & Health 26, no. 1 (2002): 5–14. [PMC free article] [PubMed] [Google Scholar]
- 55. Kilian C., Lemp J. M., Llamosas‐Falcón L., et al., “Reducing Alcohol Use Through Alcohol Control Policies in the General Population and Population Subgroups: A Systematic Review and Meta‐Analysis,” EClinicalMedicine 59 (2023): 101996, 10.1016/j.eclinm.2023.101996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Díaz L. A., Fuentes‐López E., Idalsoaga F., et al., “Association Between Public Health Policies on Alcohol and Worldwide Cancer, Liver Disease and Cardiovascular Disease Outcomes,” Journal of Hepatology 80, no. 3 (2024): 409–418, 10.1016/j.jhep.2023.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1.
Supporting Information S2.
Supporting Information S3.
Supporting Information S4.
Supporting Information S5.
Supporting Information S6.
Supporting Information S7.
Supporting Information S8.
Supporting Information S9.
Supporting Information S10.
Supporting Information S11.
Supporting Information S12.
Supporting Information S13.
Supporting Information S14.
Data Availability Statement
No dataset was used in this study. All articles in this manuscript are publicly available from Medline and Embase.


