Abstract
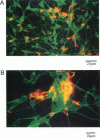
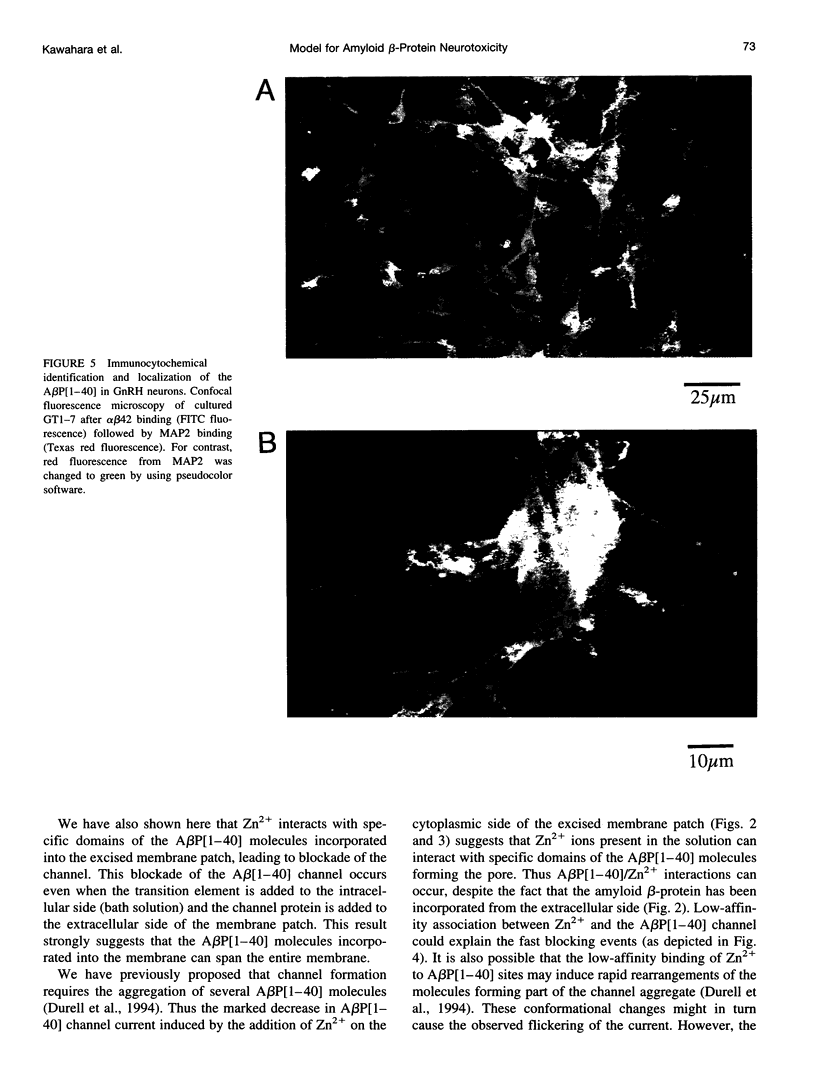
We have previously shown that the 40-residue peptide termed amyloid beta-protein (A beta P[1-40]) in solution forms cation-selective channels across artificial phospholipid bilayer membranes. To determine whether A beta P[1-40] also forms channels across natural membranes, we used electrically silent excised membrane patches from a cell line derived from hypothalamic gonadotrophin-releasing hormone GnRH neurons. We found that exposing either the internal or the external side of excised membrane patches to A beta P[1-40] leads to the spontaneous formation of cation-selective channels. With Cs+ as the main cation in both the external as well as the internal saline, the amplitude of the A beta P[1-40] channel currents was found to follow the Cs+ gradient and to exhibit spontaneous conductance changes over a wide range (50-500 pS). We also found that free zinc (Zn2+), reported to bind to amyloid beta-protein in solution, can block the flow of Cs+ through the A beta P[1-40] channel. Because the Zn2+ chelator o-phenanthroline can reverse this blockade, we conclude that the underlying mechanism involves a direct interaction between the transition element Zn2+ and sites in the A beta P[1-40] channel pore. These properties of the A beta P[1-40] channel are rather similar to those observed in the artificial bilayer system. We also show here, by immunocytochemical confocal microscopy, that amyloid beta-protein molecules form deposits closely associated with the plasma membrane of a substantial fraction of the GnRH neurons. Taken together, these results suggest that the interactions between amyloid beta-protein and neuronal membranes also occur in vivo, lending further support to the idea that A beta P[1-40] channel formation might be a mechanism of amyloid beta-protein neurotoxicity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arispe N., Pollard H. B., Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1-40)] in bilayer membranes. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N., Pollard H. B., Rojas E. Zn2+ interaction with Alzheimer amyloid beta protein calcium channels. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1710–1715. doi: 10.1073/pnas.93.4.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N., Pollard H. B., Rojas E. beta-Amyloid Ca(2+)-channel hypothesis for neuronal death in Alzheimer disease. Mol Cell Biochem. 1994 Nov 23;140(2):119–125. doi: 10.1007/BF00926750. [DOI] [PubMed] [Google Scholar]
- Arispe N., Rojas E., Pollard H. B. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush A. I., Multhaup G., Moir R. D., Williamson T. G., Small D. H., Rumble B., Pollwein P., Beyreuther K., Masters C. L. A novel zinc(II) binding site modulates the function of the beta A4 amyloid protein precursor of Alzheimer's disease. J Biol Chem. 1993 Aug 5;268(22):16109–16112. [PubMed] [Google Scholar]
- Bush A. I., Pettingell W. H., Multhaup G., d Paradis M., Vonsattel J. P., Gusella J. F., Beyreuther K., Masters C. L., Tanzi R. E. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994 Sep 2;265(5177):1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R., Arispe N., Rojas E., Pollard H. B. Theoretical models of the ion channel structure of amyloid beta-protein. Biophys J. 1994 Dec;67(6):2137–2145. doi: 10.1016/S0006-3495(94)80717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Deerinck T. J., Ouyang Y., Beck C. F., Tanksley S. J., Walton P. D., Airey J. A., Sutko J. L. Identification and localization of ryanodine binding proteins in the avian central nervous system. Neuron. 1990 Aug;5(2):135–146. doi: 10.1016/0896-6273(90)90304-x. [DOI] [PubMed] [Google Scholar]
- Fraser S. P., Suh Y. H., Djamgoz M. B. Ionic effects of the Alzheimer's disease beta-amyloid precursor protein and its metabolic fragments. Trends Neurosci. 1997 Feb;20(2):67–72. doi: 10.1016/s0166-2236(96)10079-5. [DOI] [PubMed] [Google Scholar]
- Frautschy S. A., Baird A., Cole G. M. Effects of injected Alzheimer beta-amyloid cores in rat brain. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8362–8366. doi: 10.1073/pnas.88.19.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993 Dec 17;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Kosik K. S. Alzheimer's disease: a cell biological perspective. Science. 1992 May 8;256(5058):780–783. doi: 10.1126/science.1589757. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., McKee A. C., Yankner B. A., Beal M. F. In vivo neurotoxicity of beta-amyloid [beta(1-40)] and the beta(25-35) fragment. Neurobiol Aging. 1992 Sep-Oct;13(5):537–542. doi: 10.1016/0197-4580(92)90053-z. [DOI] [PubMed] [Google Scholar]
- Malouf A. T. Effect of beta amyloid peptides on neurons in hippocampal slice cultures. Neurobiol Aging. 1992 Sep-Oct;13(5):543–551. doi: 10.1016/0197-4580(92)90054-2. [DOI] [PubMed] [Google Scholar]
- Martone M. E., Zhang Y., Simpliciano V. M., Carragher B. O., Ellisman M. H. Three-dimensional visualization of the smooth endoplasmic reticulum in Purkinje cell dendrites. J Neurosci. 1993 Nov;13(11):4636–4646. doi: 10.1523/JNEUROSCI.13-11-04636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P. L., Windle J. J., Goldsmith P. C., Padula C. A., Roberts J. L., Weiner R. I. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990 Jul;5(1):1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Dawes L. R., Yankner B. A., Benowitz L. I., Rodriguez W., Higgins G. A. Genetics and biology of the Alzheimer amyloid precursor. Prog Brain Res. 1990;86:257–267. doi: 10.1016/s0079-6123(08)63182-9. [DOI] [PubMed] [Google Scholar]
- Pollard J. R., Arispe N., Rojas E., Pollard H. B. A geometric sequence that accurately describes allowed multiple conductance levels of ion channels: the "three-halves (3/2) rule". Biophys J. 1994 Aug;67(2):647–655. doi: 10.1016/S0006-3495(94)80525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Hidalgo J., Carroll P. B., Li M. X., Atwater I. A new class of calcium channels activated by glucose in human pancreatic beta-cells. FEBS Lett. 1990 Feb 26;261(2):265–270. doi: 10.1016/0014-5793(90)80568-4. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Sharp A. H., Dawson T. M., Ross C. A., Fotuhi M., Mourey R. J., Snyder S. H. Inositol 1,4,5-trisphosphate receptors: immunohistochemical localization to discrete areas of rat central nervous system. Neuroscience. 1993 Apr;53(4):927–942. doi: 10.1016/0306-4522(93)90478-x. [DOI] [PubMed] [Google Scholar]
- Spergel D. J., Catt K. J., Rojas E. Immortalized GnRH neurons express large-conductance calcium-activated potassium channels. Neuroendocrinology. 1996 Feb;63(2):101–111. doi: 10.1159/000126946. [DOI] [PubMed] [Google Scholar]
- Spergel D. J., Krsmanovic L. Z., Stojilkovic S. S., Catt K. J. L-type Ca2+ channels mediate joint modulation by gamma-amino-butyric acid and glutamate of [Ca2+]i and neuropeptide secretion in immortalized gonadodropin-releasing hormone neurons. Neuroendocrinology. 1995 May;61(5):499–508. doi: 10.1159/000126873. [DOI] [PubMed] [Google Scholar]
- Tabaton M., Nunzi M. G., Xue R., Usiak M., Autilio-Gambetti L., Gambetti P. Soluble amyloid beta-protein is a marker of Alzheimer amyloid in brain but not in cerebrospinal fluid. Biochem Biophys Res Commun. 1994 May 16;200(3):1598–1603. doi: 10.1006/bbrc.1994.1634. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Yankner B. A. Commentary and perspective on studies of beta amyloid neurotoxicity. Neurobiol Aging. 1992 Sep-Oct;13(5):615–616. doi: 10.1016/0197-4580(92)90067-8. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]