Abstract
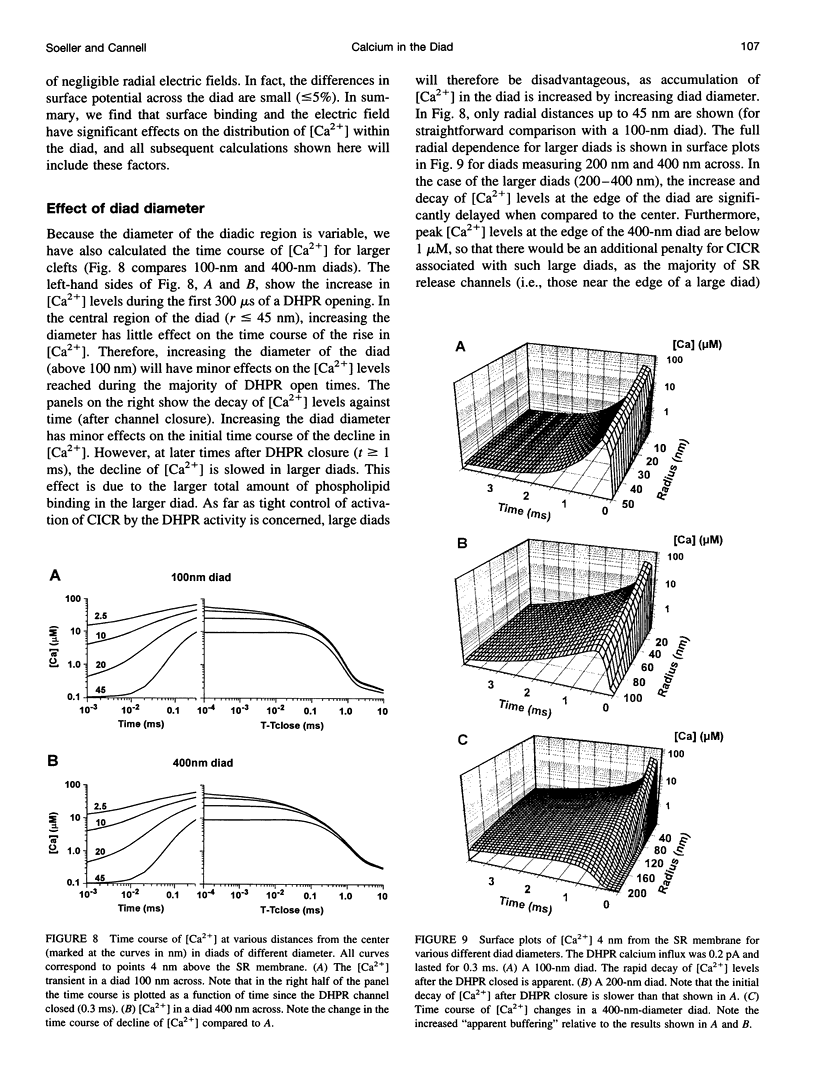
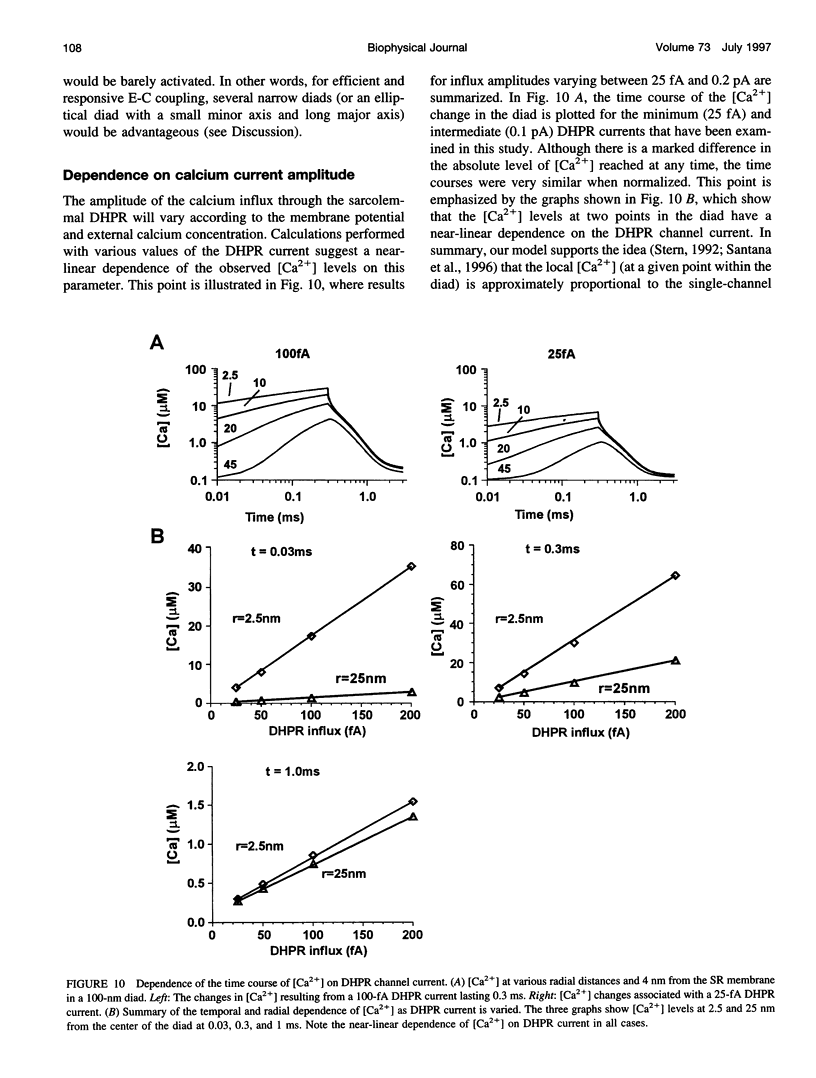
Computer simulation was used to investigate the calcium levels after sarcolemmal calcium influx through L-type calcium channels (DHPRs) into the narrow diadic space of cardiac muscle. The effect of various cytosolic and membranebound buffers, diad geometry, DHPR properties (open time and current), and surface charge were examined. The simulations showed that phospholipid binding sites on the sarcolemmal membrane are the major buffer affecting free calcium ([Ca2+]) levels in the diad. The inclusion of surface charge effects calculated from Gouy-Chapman theory resulted in a marked decrease in [Ca2+] levels at all times and a faster decay of [Ca2+] after termination of DHPR influx. For a DHPR current of 200 fA, [Ca2+] at the center of the diad reached peak levels of approximately 73 microM. In larger diads (> or = 400 nm diameter), [Ca2+] decayed more slowly than in smaller diads (100-200 nm diameter), although peak [Ca2+] levels reached during typical DHPR open times were similar. For a wide range of DHPR single-channel current magnitudes (Ica = 25-200 fA), [Ca2+] levels in the diad were approximately proportional to ICa. The decrease in calculated [Ca2+] levels due to the effects of surface charge can be interpreted as resulting from an effective "volume expansion" of the diad space. Furthermore, the layer of increased [Ca2+] close to the sarcolemmal membrane can act as a fast buffer.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bers D. M., Philipson K. D., Peskoff A. Calcium at the surface of cardiac plasma membrane vesicles: cation binding, surface charge screening, and Na-Ca exchange. J Membr Biol. 1985;85(3):251–261. doi: 10.1007/BF01871520. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. The control of calcium release in heart muscle. Science. 1995 May 19;268(5213):1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Soeller C. Numerical analysis of ryanodine receptor activation by L-type channel activity in the cardiac muscle diad. Biophys J. 1997 Jul;73(1):112–122. doi: 10.1016/S0006-3495(97)78052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E., Lehninger A. L. A survey of the interaction of calcium ions with mitochondria from different tissues and species. Biochem J. 1971 May;122(5):681–690. doi: 10.1042/bj1220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Affolter H. Insulation of the conduction pathway of muscle transverse tubule calcium channels from the surface charge of bilayer phospholipid. J Gen Physiol. 1986 Jun;87(6):933–953. doi: 10.1085/jgp.87.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., McNutt N. S. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969 Jul;42(1):1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssmann W. G., Girardier L. A study of the T system in rat heart. J Cell Biol. 1970 Jan;44(1):1–19. doi: 10.1083/jcb.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyde M. J., Robertson S. P., Johnson J. D., Solaro R. J., Potter J. D. The calcium and magnesium binding sites on cardiac troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1980 Dec 25;255(24):11688–11693. [PubMed] [Google Scholar]
- Isenberg G., Han S. Gradation of Ca(2+)-induced Ca2+ release by voltage-clamp pulse duration in potentiated guinea-pig ventricular myocytes. J Physiol. 1994 Nov 1;480(Pt 3):423–438. doi: 10.1113/jphysiol.1994.sp020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Peskoff A. Calcium concentration and movement in the diadic cleft space of the cardiac ventricular cell. Biophys J. 1996 Mar;70(3):1169–1182. doi: 10.1016/S0006-3495(96)79677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner M., Cafiso D., Marcelja S., McLaughlin S. Electrostatics of phosphoinositide bilayer membranes. Theoretical and experimental results. Biophys J. 1990 Feb;57(2):335–349. doi: 10.1016/S0006-3495(90)82535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A., McLaughlin A., McLaughlin S. The adsorption of divalent cations to phosphatidylglycerol bilayer membranes. Biochim Biophys Acta. 1981 Jul 20;645(2):279–292. doi: 10.1016/0005-2736(81)90199-1. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995 May 19;268(5213):1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
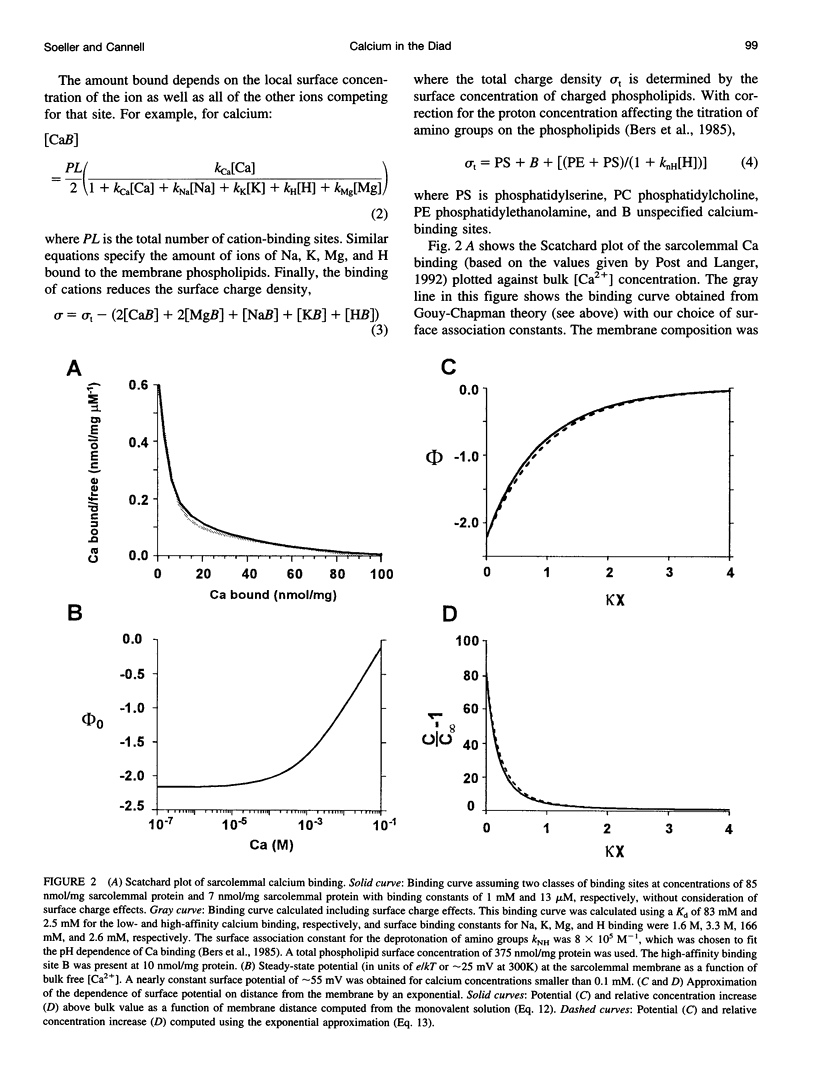
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local, stochastic release of Ca2+ in voltage-clamped rat heart cells: visualization with confocal microscopy. J Physiol. 1994 Oct 1;480(Pt 1):21–29. doi: 10.1113/jphysiol.1994.sp020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Peskoff A., Bers D. M. Electrodiffusion of ions approaching the mouth of a conducting membrane channel. Biophys J. 1988 Jun;53(6):863–875. doi: 10.1016/S0006-3495(88)83167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskoff A., Post J. A., Langer G. A. Sarcolemmal calcium binding sites in heart: II. Mathematical model for diffusion of calcium released from the sarcoplasmic reticulum into the diadic region. J Membr Biol. 1992 Jul;129(1):59–69. doi: 10.1007/BF00232055. [DOI] [PubMed] [Google Scholar]
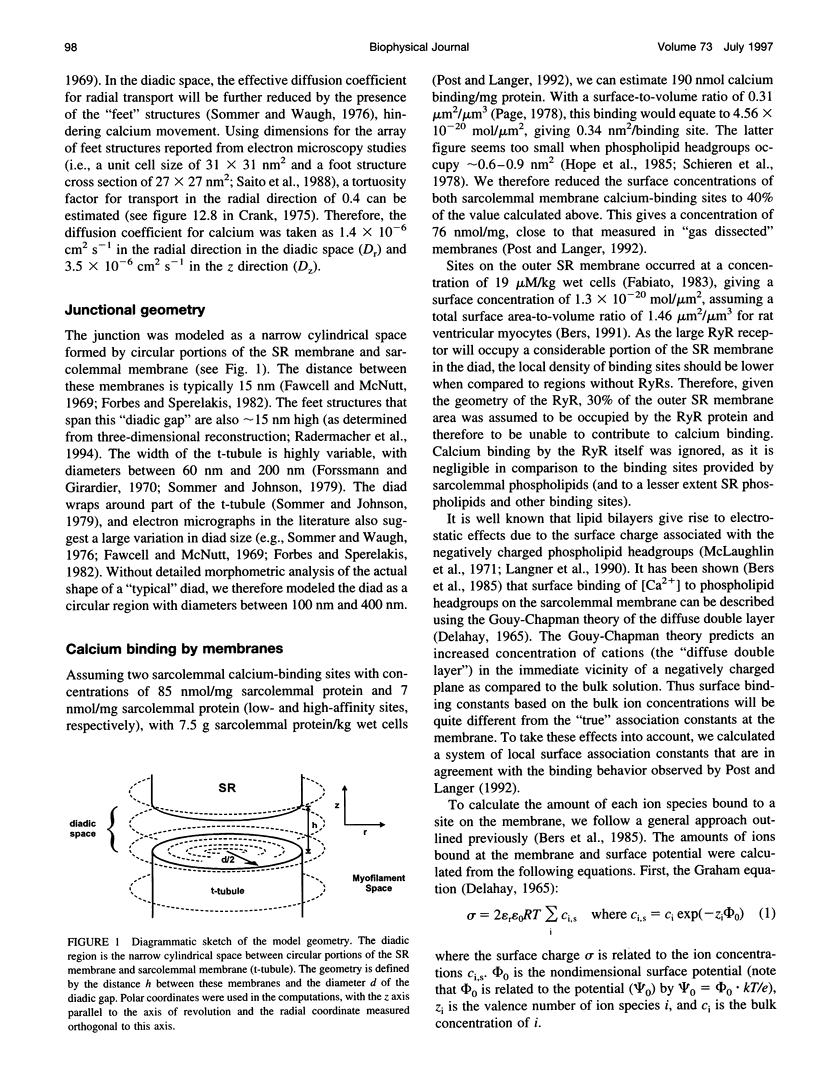
- Post J. A., Langer G. A. Sarcolemmal calcium binding sites in heart: I. Molecular origin in "gas-dissected" sarcolemma. J Membr Biol. 1992 Jul;129(1):49–57. doi: 10.1007/BF00232054. [DOI] [PubMed] [Google Scholar]
- Radermacher M., Rao V., Grassucci R., Frank J., Timerman A. P., Fleischer S., Wagenknecht T. Cryo-electron microscopy and three-dimensional reconstruction of the calcium release channel/ryanodine receptor from skeletal muscle. J Cell Biol. 1994 Oct;127(2):411–423. doi: 10.1083/jcb.127.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose W. C., Balke C. W., Wier W. G., Marban E. Macroscopic and unitary properties of physiological ion flux through L-type Ca2+ channels in guinea-pig heart cells. J Physiol. 1992 Oct;456:267–284. doi: 10.1113/jphysiol.1992.sp019336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Inui M., Radermacher M., Frank J., Fleischer S. Ultrastructure of the calcium release channel of sarcoplasmic reticulum. J Cell Biol. 1988 Jul;107(1):211–219. doi: 10.1083/jcb.107.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana L. F., Cheng H., Gómez A. M., Cannell M. B., Lederer W. J. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996 Jan;78(1):166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- Schieren H., Rudolph S., Finkelstein M., Coleman P., Weissmann G. Comparison of large unilamellar vesicles prepared by a petroleum ether vaporization method with multilamellar vesicles: ESR, diffusion and entrapment analyses. Biochim Biophys Acta. 1978 Aug 3;542(1):137–153. doi: 10.1016/0304-4165(78)90240-4. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. D., Wagner J., Keizer J. Validity of the rapid buffering approximation near a point source of calcium ions. Biophys J. 1996 Jun;70(6):2527–2539. doi: 10.1016/S0006-3495(96)79824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer J. R., Waugh R. A. The ultrastructure of the mammalian cardiac muscle cell--with special emphasis on the tubular membrane systems. A review. Am J Pathol. 1976 Jan;82(1):192–232. [PMC free article] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]