Abstract
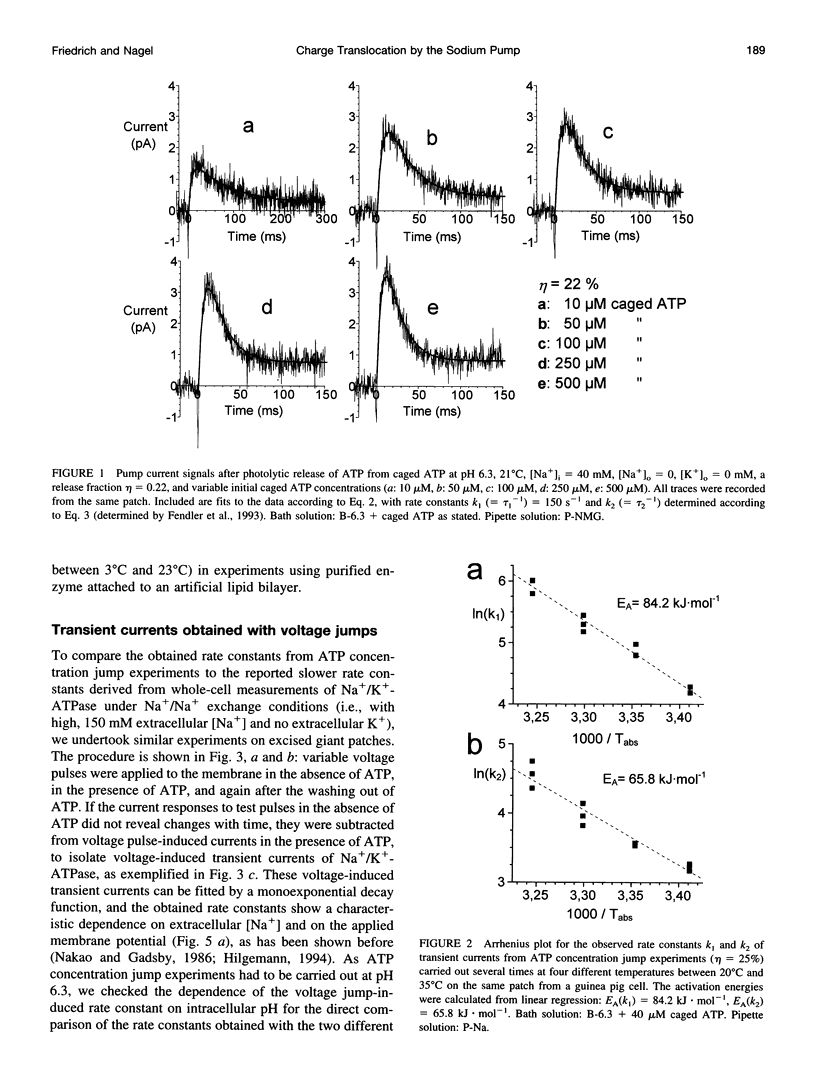
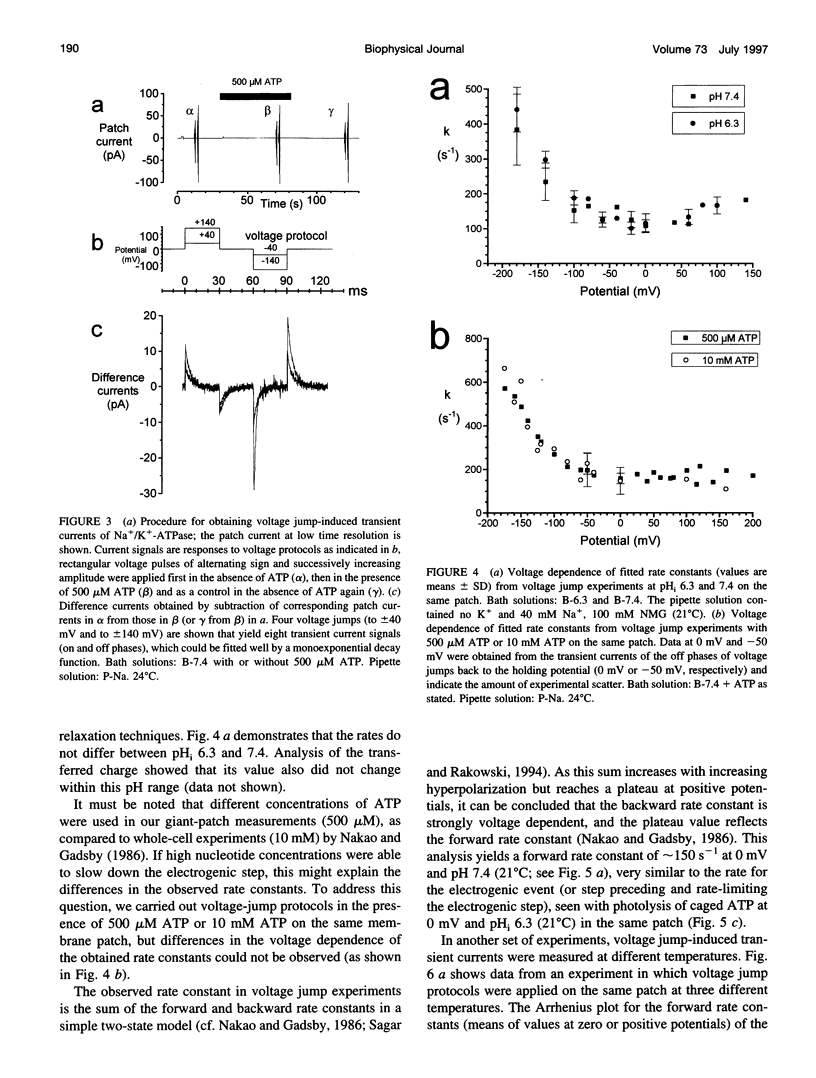
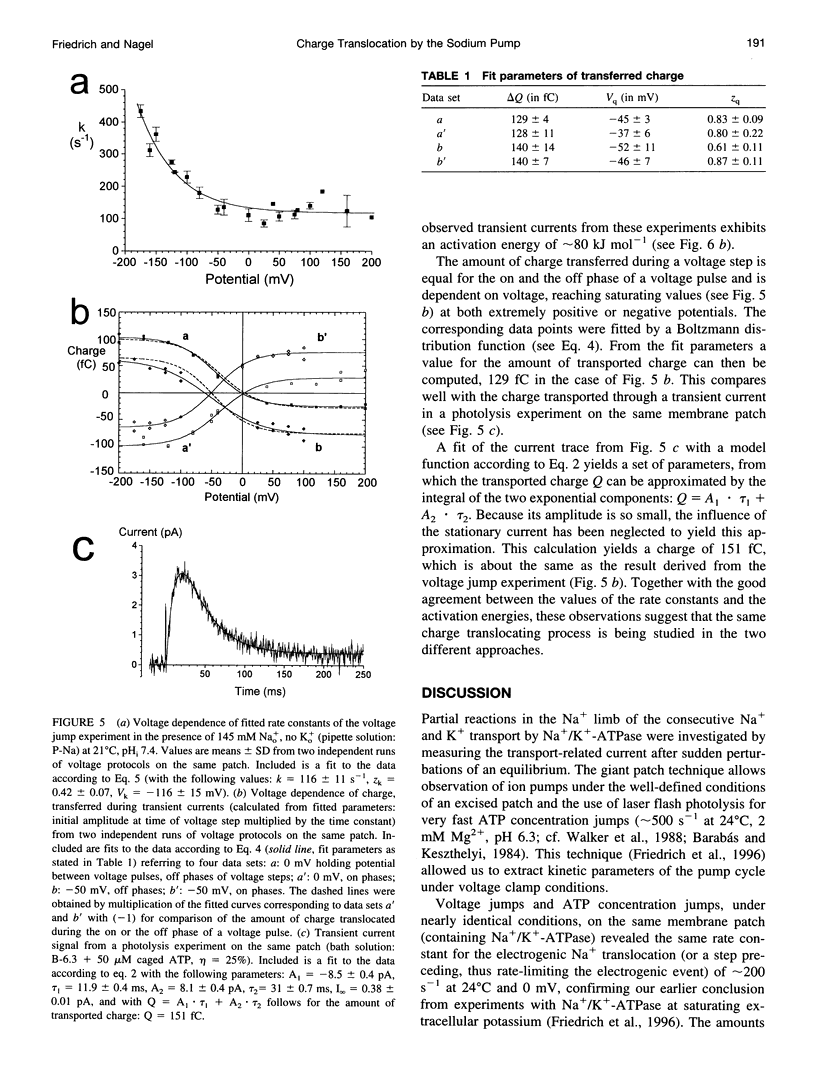
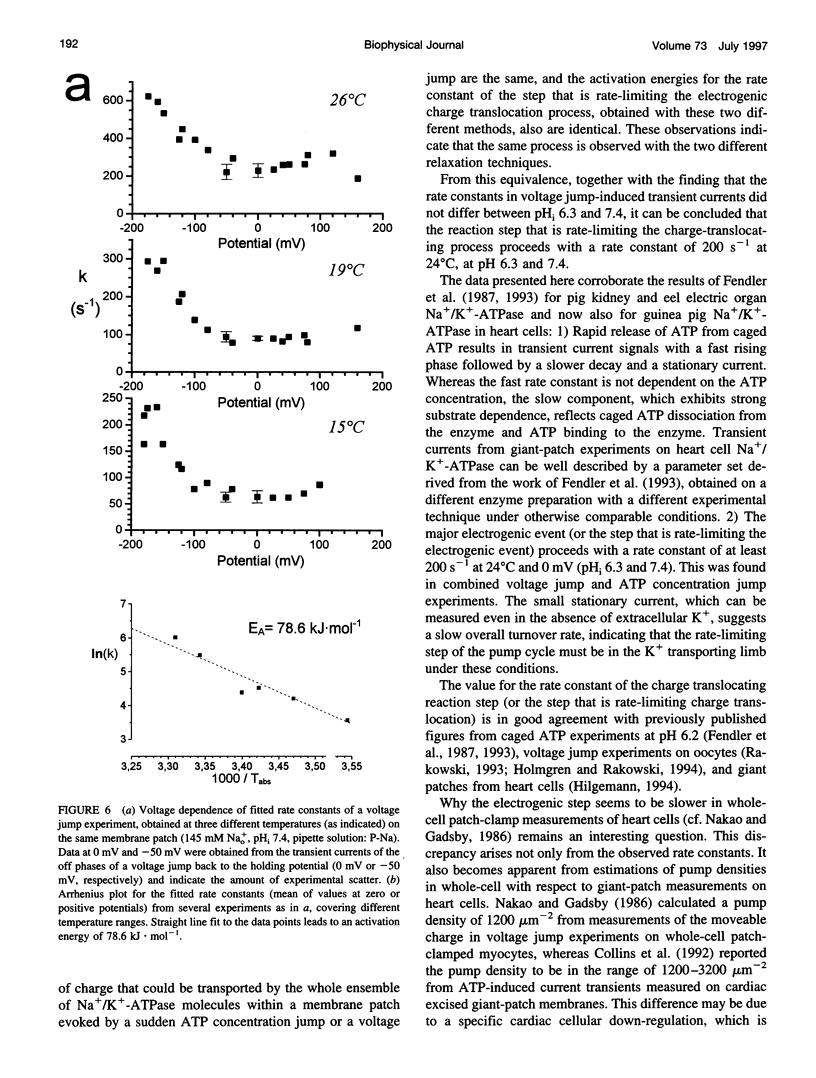
Using the giant patch technique, we combined two fast relaxation methods on excised patches from guinea pig cardiomyocytes to compare the rate constants of the involved reaction steps. Experiments were done in the absence of intra- or extracellular K+. Fast ATP concentration jumps were generated by photolysis of caged ATP at pH 6.3 with laser flash irradiation at a wavelength of 308 nm and 10 ns duration, as described previously. Transient outward currents with a fast rising phase, followed by a slower decay and a small stationary current, were obtained. Voltage pulses were applied to the same patch in the presence or absence of intracellular ATP. Subtraction of the voltage jump-induced currents in the absence of ATP from those taken in the presence of ATP yielded monoexponential transient current signals, which were dependent on external Na+ but did not differ between intracellular pH (pHi) values 6.3 or 7.4. Rate constants showed a characteristic voltage dependence, i.e., saturating at positive potentials (approximately 200 s-1, 24 degrees C) and exponentially rising with increasing negative potentials. Rate constants of the fast component from transient currents obtained after an ATP concentration jump agree well with rate constants from currents obtained after a voltage jump to zero or positive potentials (pHi 6.3), and the two exhibit the same activation energy of approximately 80 kJ.mol-1. For a given membrane patch, the amount of charge that is moved across the plasma membrane is roughly the same for each of the two relaxation techniques.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Borlinghaus R., Läuger P. Fast charge translocations associated with partial reactions of the Na,K-pump: II. Microscopic analysis of transient currents. J Membr Biol. 1987;97(3):179–191. doi: 10.1007/BF01869221. [DOI] [PubMed] [Google Scholar]
- Barabás K., Keszthelyi L. Temperature dependence of ATP release from "caged" ATP. Acta Biochim Biophys Acad Sci Hung. 1984;19(3-4):305–309. [PubMed] [Google Scholar]
- Borlinghaus R., Apell H. J., Läuger P. Fast charge translocations associated with partial reactions of the Na,K-pump: I. Current and voltage transients after photochemical release of ATP. J Membr Biol. 1987;97(3):161–178. doi: 10.1007/BF01869220. [DOI] [PubMed] [Google Scholar]
- Clarke R. J., Apell H. J., Läuger P. Pump current and Na+/K+ coupling ratio of Na+/K+-ATPase in reconstituted lipid vesicles. Biochim Biophys Acta. 1989 Jun 6;981(2):326–336. doi: 10.1016/0005-2736(89)90044-8. [DOI] [PubMed] [Google Scholar]
- Collins A., Somlyo A. V., Hilgemann D. W. The giant cardiac membrane patch method: stimulation of outward Na(+)-Ca2+ exchange current by MgATP. J Physiol. 1992 Aug;454:27–57. doi: 10.1113/jphysiol.1992.sp019253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendler K., Grell E., Bamberg E. Kinetics of pump currents generated by the Na+,K+-ATPase. FEBS Lett. 1987 Nov 16;224(1):83–88. doi: 10.1016/0014-5793(87)80427-1. [DOI] [PubMed] [Google Scholar]
- Fendler K., Grell E., Haubs M., Bamberg E. Pump currents generated by the purified Na+K+-ATPase from kidney on black lipid membranes. EMBO J. 1985 Dec 1;4(12):3079–3085. doi: 10.1002/j.1460-2075.1985.tb04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendler K., Jaruschewski S., Hobbs A., Albers W., Froehlich J. P. Pre-steady-state charge translocation in NaK-ATPase from eel electric organ. J Gen Physiol. 1993 Oct;102(4):631–666. doi: 10.1085/jgp.102.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbush B., 3rd Na+ movement in a single turnover of the Na pump. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5310–5314. doi: 10.1073/pnas.81.17.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T., Bamberg E., Nagel G. Na+,K(+)-ATPase pump currents in giant excised patches activated by an ATP concentration jump. Biophys J. 1996 Nov;71(5):2486–2500. doi: 10.1016/S0006-3495(96)79442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Nakao M., Bahinski A., Nagel G., Suenson M. Charge movements via the cardiac Na,K-ATPase. Acta Physiol Scand Suppl. 1992;607:111–123. [PubMed] [Google Scholar]
- Gadsby D. C., Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W. Channel-like function of the Na,K pump probed at microsecond resolution in giant membrane patches. Science. 1994 Mar 11;263(5152):1429–1432. doi: 10.1126/science.8128223. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W. Giant excised cardiac sarcolemmal membrane patches: sodium and sodium-calcium exchange currents. Pflugers Arch. 1989 Nov;415(2):247–249. doi: 10.1007/BF00370601. [DOI] [PubMed] [Google Scholar]
- Holmgren M., Rakowski R. F. Pre-steady-state transient currents mediated by the Na/K pump in internally perfused Xenopus oocytes. Biophys J. 1994 Mar;66(3 Pt 1):912–922. doi: 10.1016/s0006-3495(94)80867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Forbush B., 3rd, Hoffman J. F. Rapid photolytic release of adenosine 5'-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978 May 16;17(10):1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Lafaire A. V., Schwarz W. Voltage dependence of the rheogenic Na+/K+ ATPase in the membrane of oocytes of Xenopus laevis. J Membr Biol. 1986;91(1):43–51. doi: 10.1007/BF01870213. [DOI] [PubMed] [Google Scholar]
- Nagel G., Fendler K., Grell E., Bamberg E. Na+ currents generated by the purified (Na+ + K+)-ATPase on planar lipid membranes. Biochim Biophys Acta. 1987 Jul 23;901(2):239–249. doi: 10.1016/0005-2736(87)90120-9. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986 Oct 16;323(6089):628–630. doi: 10.1038/323628a0. [DOI] [PubMed] [Google Scholar]
- Rakowski R. F. Charge movement by the Na/K pump in Xenopus oocytes. J Gen Physiol. 1993 Jan;101(1):117–144. doi: 10.1085/jgp.101.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski R. F., Gadsby D. C., De Weer P. Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon. J Gen Physiol. 1989 May;93(5):903–941. doi: 10.1085/jgp.93.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar A., Rakowski R. F. Access channel model for the voltage dependence of the forward-running Na+/K+ pump. J Gen Physiol. 1994 May;103(5):869–893. doi: 10.1085/jgp.103.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer W., Apell H. J., Wuddel I., Läuger P. Conformational transitions and change translocation by the Na,K pump: comparison of optical and electrical transients elicited by ATP-concentration jumps. J Membr Biol. 1989 Aug;110(1):67–86. doi: 10.1007/BF01870994. [DOI] [PubMed] [Google Scholar]
- Wuddel I., Apell H. J. Electrogenicity of the sodium transport pathway in the Na,K-ATPase probed by charge-pulse experiments. Biophys J. 1995 Sep;69(3):909–921. doi: 10.1016/S0006-3495(95)79965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


