Abstract
Actin is an essential component of the cytoskeleton in every eukaryotic cell. β-and γ-nonmuscle actin are over 99% identical to each other at the protein level but are encoded by different genes and play distinct roles in vivo. Blood cells, especially red blood cells (RBC), contain almost exclusively β-actin, and it has been generally assumed that this bias is dictated by the unique suitability of β-actin for RBC cytoskeleton function due to its specific amino acid sequence. Here we tested this assumption by analyzing the “β-coded γ-actin” (Actbcg) mouse model, in which the β-actin gene is edited by five-point mutations to produce γ-actin protein. Strikingly, despite lacking β-actin protein, Actbcg mice had no detectable phenotypes in RBCs, and no changes in the RBC shape, integrity, deformability, and molecular composition of their spectrin-based membrane skeleton. No actin-dependent changes were observed in platelets, another anucleate cell type enriched for β-actin. Our data show that, contrary to expectations, β-actin function in mature RBCs and platelets is independent of its protein sequence and therefore its enrichment in hematopoiesis and mature blood cells is likely driven entirely by its nucleotide-dependent functions.
The actin cytoskeleton in platelets and RBCs is composed almost exclusively of the β-actin isoforms, and the reasons for this bias are unknown.
The authors find that β-actin protein in these cells can be fully replaced with γ-actin without any structural or functional implications at the biochemical, cellular, and organizmal levels.
These results show that the unique enrichment of β-actin in the blood is functionally independent of the specific features of its protein sequence.
INTRODUCTION
Actin is an essential component of the cytoskeleton in every eukaryotic cell. The mammalian actin family contains six major genes, encoding two nonmuscle isoforms, cytoplasmic β-and γ-actin, as well as four distinct muscle isoforms. All actins are highly similar at the protein level and share >95% identity with each other. This similarity is especially striking when comparing the two nonmuscle actin isoforms, β-and γ-actin, which are over 99% identical in their amino acid sequence, with only four conservative substitutions within their N-termini (Kashina, 2020). Despite this high sequence identity, they play vastly different biological roles: knockout of β-actin in mice leads to early embryonic lethality, while the γ-actin knockout phenotype is much milder and does not strongly affect embryogenesis (Perrin and Ervasti, 2010).
For decades, different groups have been searching for the amino acid–level differences that could serve as a source of such strong functional distinction between two nearly identical proteins, until recently it was discovered that the uniquely essential role of β-actin in mouse embryogenesis actually lies at the nucleotide level. CRISPR/Cas9 gene editing of the mouse β-actin gene to encode γ-actin protein (β-coded γ-actin or Actbcg mice), leads to complete replacement of β-actin protein with γ-actin but does not interfere with mouse viability, in contrast to a straight β-actin gene knockout (Vedula et al., 2017; Patrinostro et al., 2018).
This result has introduced a paradigm-shifting concept into the field, by demonstrating that nucleotide sequences of actin isoforms can determine their major in vivo functions. Follow-up work proposed that one of the important determinants resides in the repertoire of silent substitutions in the actin coding sequence, which confers on β-actin the ability to undergo faster translation (Vedula et al., 2021). However, this mechanism does not negate the fact that the amino acid differences between actin isoforms may also play important biological roles. In fact, β-and γ-actin amino acid sequences are highly conserved in evolution and these two isoforms are 100% identical at the amino acid level in all bird and mammalian species, suggesting high evolutionary pressure on these specific N-terminal residues within each protein. This, in turn, implies that these sequences are highly physiologically important.
Actbcg mice represent a perfect model for dissecting these differences at the functional level. In these mice, any phenotypic changes arise due to the protein-level replacement of β- to γ-actin due to the functionality of their amino acid–level differences, while the lack of phenotypes in specific processes points to nucleotide-level functions. Thus, identifying the phenotypes of these mice would enable a complete understanding of the contribution of these determinants to actin's in vivo function.
Blood is the only tissue in the body whose cells contain almost exclusively β-actin. The only other actin isoform expressed in these cells to a detectable level is γ-actin, which by different estimates, constitutes less than 10% of the total actin pool (Fowler, 2013). This enrichment is believed to be functionally important, and thus, we predicted that full replacement of β- to γ-actin protein in these cells would have prominent amino acid–dependent phenotypic effects. We analyzed the effect of this replacement in RBC and platelets, the two cell types that constitute the majority of the blood cell mass and contain almost exclusively β-actin (80–95% of total actin [Fowler, 2013]). Because these cells contain no nuclei and are believed not to rely strongly on protein synthesis (Mills et al., 2017; Kumar et al., 2022), we reasoned that actin function in these cells must not strongly depend on the sequence and properties of its mRNA or gene and must be strongly amino acid dependent. However, contrary to these expectations we found no impact of this protein replacement on the RBC morphology, structural integrity, deformability, and the composition of the spectrin-based membrane skeleton. No corresponding changes were observed in platelets. Furthermore, Actbcg mice had overall normal blood counts, suggesting no major impairments in hematopoiesis. Thus, β-actin protein is fully interchangeable with γ-actin in RBCs and platelets, and the specific features of its amino acid sequence have no functional consequences in these cell types. These results lead us to the conclusion that contrary to expectations and prior assumptions in the field, β-actin enrichment in these cells is not functionally driven at the amino acid level.
RESULTS
Replacement of β-actin protein with γ-actin does not affect hematopoiesis, blood cell morphology, or blood counts
Actbcg mice completely lack β-actin protein and exhibit a corresponding increase in γ-actin protein levels in a variety of cells and tissues, due to its expression from the β-actin gene in addition to its native locus (Vedula et al., 2017). To confirm that this is the case in the blood, we analyzed mouse blood cell preparations (collected by centrifugation from whole blood [WB] samples) using actin isoform-specific antibodies (Dugina et al., 2009) (Figure 1). Both Western blot (Figure 1, left panels) and immunofluorescence (Figure 1, right panels) confirmed complete lack of β-actin protein in Actbcg samples and a corresponding increase in γ-actin signal. In addition, immunofluorescence showed no gross changes in RBC morphology or actin cytoskeleton in Actbcg (Figure 1, right panels).
FIGURE 1:
Blood cells from Actbcg mice contain no β-actin protein. (A) Schematic of gene editing to produce Atbcg mice. (B) Representative Western blots of WB cell preparations from wild type (Actb WT) and Actbcg mice. (C) Immunofluorescence staining of RBCs using actin isoform-specific antibodies.
Previous studies showed that, in addition to its cytoskeletal role, nuclear β-actin is required for transcriptional reprogramming during erythropoiesis through modulating GATA2 transcription levels (Tondeleir et al., 2013). In addition, analysis of public datasets aggregated at the Haemosphere web portal (https://www.haemosphere.org/) (Choi et al., 2019) shows that β-actin mRNA is highly enriched over any other actin isoform in every blood cell type, starting with multipotential progenitors and ending with terminally differentiated cell types (Supplemental Figures S1 and S2). This implies that β-actin expression is strongly biased toward the blood as a tissue, suggesting that it may be functionally important not only in the mature blood cells but also during early and late hematopoiesis. However, analysis of total blood counts in Actbcg mice revealed no gross changes in blood cell composition or morphology (Figure 2A and Figure 3), suggesting that hematopoiesis overall occurred normally in these mice. Because β-actin has been previously implicated specifically in erythropoiesis, we stained samples of spleen and bone marrow with antibodies to transferrin receptor to identify RBC progenitors but saw no obvious changes (Figure 2B).
FIGURE 2:
Replacement of β-actin protein with γ-actin does not affect overall blood cell morphology and RBC precursor distribution in the spleen and bone marrow. (A) Wright–Giemsa staining of blood smears from WT and Actbcg mice. (B) Sections of the spleen (top four panels) and bone marrow (imaged in the sternum, bottom two panels) stained for transferrin receptors to detect erythroid precursors. For the spleen images, boxed areas in the 10x magnification view are shown expanded at 20x in the lower images. Bar, 100 μm for 10x images (top two) and 50 μm for 20x images (bottom 4).
FIGURE 3:
Replacement of β-actin protein with γ-actin does not affect overall blood CBC. CBC for samples from WT and Actbcg (MUT) mice. Red and blue dots represent males and females, respectively. n = 10 biological replicates for each genotype. Significance was calculated by unpaired two-tailed Welch's t test without correction for multiple comparisons. Lines represent the median value.
We next performed complete blood counts (CBC) of blood samples from wild type (WT) and Actbcg mice to test for the overall parameters that are commonly analyzed in patients. Mice of both genders were analyzed, to ensure no bias. No changes were detected in any of these tests (Figure 3). Limited analysis with a subset of samples shown in Figure 3 was also compared with those from matched heterozygous mice derived from the same litters, expressing Actbcg from one allele and intact β-actin from the other allele. No changes were seen in any of the tested parameters (Supplemental Figure S3).
Thus, partial or total replacement of β-actin protein with γ-actin does not alter overall blood cell composition in mice. Indirectly, these results imply that β-to γ-actin protein replacement does not interfere with hematopoiesis.
Replacement of β-actin protein with γ-actin does not affect the RBC properties
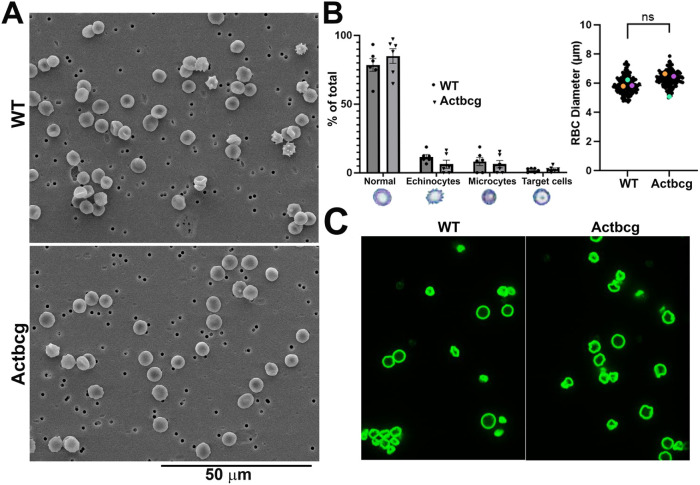
We next tested whether Actbcg mice exhibit any changes in the structural properties of RBCs. Even though RBCs in Actbcg mice appear morphologically normal overall (Figure 1, right and Figure 2, top), it remains possible that lack of β-actin protein in these cells might result in more subtle morphological changes, or in altered structural integrity that does not strongly affect their resting shape. To test this possibility, we analyzed RBCs from Actbcg mice by scanning electron microscopy (SEM) and scored their size, shape, and uniformity as indicators of altered stiffness and deformability. We also stained them for glycophorin A, a surface marker that enables detailed observation of shape. However, even at this level of detail, RBCs from Actbcg mice appeared normal in size and shape. In addition, we saw no significant changes in the fractions of echinocytes, microcytes, or target cells—the morphological RBC variants that tend to form under stress and are associated with various diseases that affect RBC integrity and stiffness (Figure 4, A, B, and C).
FIGURE 4:
Replacement of β-actin protein with γ-actin does not affect RBC morphology. (A) Characteristic scanning electron micrographs of RBCs from WT and Actbcg mice. (B) Left: quantification of the fractions of different cell shapes in RBC preparations. Diagrams illustrating cell morphologies scored are shown below the X axis; Right: quantification of RBC diameters in both genotypes. Error bars represent SEM, bars are the averages of the individual data points also shown on the chart, each of them the average of all measurements in one biological replicate. n = 6 animals or three biological replicates for each genotype. Significance was calculated by unpaired two-tailed t test without correction for multiple comparisons. Data meet the normal distribution. Colored dots in each chart represent the average for each biological replicate, superimposed on all data points shown in black. Pairs of similar color dots (orange, teal, and purple) represent littermate pairs. (C) Representative fields of view for RBCs stained with antibody to glycophorin A.
To examine RBC morphology and actin filament organization at higher resolution, we performed Zeiss Airyscan confocal imaging of RBCs stained with fluorescently labeled phalloidin to detect actin filaments. We measured cell size, perimeter length, rim and dimple height, and rim/dimple ratio (Figure 5). None of these parameters showed any changes in RBCs derived from Actbcg mice compared with control.
FIGURE 5:
Replacement of β-actin protein with γ-actin does not affect RBC F-actin staining and shape parameters. (A) Maximum intensity projections of Airyscan Z-stacks from WT and mutant mouse RBCs phalloidinstained for F-actin. (B) XZ-plane images of WT and Actbcg (Mutant) RBCs immunostained with Ter119 (membrane marker) were used to measure biconcavity. (C) Quantification of shape parameters. For cell area and perimeter: WT, n = 88 RBCs; mutant, n = 129 RBCs. For rim height, dimple height, or dimple/rim ratios of RBCs: WT, n = 43 RBCs; mutant, n = 48 RBCs. Significance was calculated by unpaired two-tailed t test without correction for multiple comparisons. Data meet the normal distribution. Red lines represent mean values. Scale bars, 2 µm.
Thus, β-actin protein replacement with γ-actin does not affect RBC morphology or shape.
Replacement of β-actin protein with γ-actin does not affect RBC osmotic fragility and deformability
RBCs in the blood stream exist under shear stress induced by the blood flow, and their normal functions depend on their structural stability and ability to withstand pressure. To test their structural stability, we exposed RBCs from WT and Actbcg mice to solutions of different osmolarity. Low osmotic solutions cause hemolysis of RBCs, which can be measured by the release of hemoglobin into the solution. RBCs with increased osmotic fragility are expected to lyse in milder conditions compared with control. However, we detected no differences between RBCs from WT and Actbcg mice in these tests (Figure 6A).
FIGURE 6:

Replacement of β-actin protein with γ-actin does not affect RBC osmotic fragility and deformability. (A) RBC osmolysis curves, n = 5 WT and 5 mutant animals. Individual curves are shown. (B) Ektacytometry assay for RBC deformability, n = 5 WT and 5 mutant animals. Error bars in B represent SEM. See Supplemental Figure S4 for individual curves.
Next, we tested the deformability of RBCs under shear stress using ektacytometry and measured the RBC elongation index under increased pressure. No differences were seen between Actbcg and WT (Figure 6B; Supplemental Figure S4).
Thus, RBCs from Actbcg mice have similar structural properties as compared with control.
Replacement of β-actin protein with γ-actin does not affect platelet morphology, contractility, or hemostasis
In addition to RBCs that constitute the highest cell mass in the blood, the next highest abundance belongs to platelets. Like RBCs, platelets are anucleate and are known to contain around 90% of β-actin, so that platelet preparations are often used as a source of enriched β-actin protein. Platelet actin is highly dynamic and undergoes rapid cycles of disassembly and assembly to induce cell protrusions, adhesion, and myosin-dependent contraction during platelet activation and blood clotting. These dynamic properties (especially myosin binding) have been previously hypothesized to underlie some of the isoform-specific actin differences in nonmuscle cells (Arora et al., 2023; Heissler and Chinthalapudi, 2024; Yu et al., 2024). We tested whether its replacement with γ-actin protein has any effect on the basic properties of platelets.
During isolation in the absence of metabolic inhibitors, platelets partially transition from resting to activated state, forming thin membrane protrusions (filopodia) due to rapid actin polymerization (Hartwig, 1992; Silacci et al., 2004). SEM of platelet preparations enables detailed analysis of this morphological perturbation and detection of any changes in the filopodia length or number that could indicate underlying changes in the actin cytoskeleton. We observed no changes in the overall resting or activated platelet morphology, including the number of filopodia per platelet or their length (Figure 7A).
FIGURE 7:
Replacement of β-actin protein with γ-actin does not affect platelet morphology, contractility, or hemostasis. (A) Top: Characteristic SEM images of isolated platelets; Bottom: quantification of average number of filopodia per platelet, and lengths of protrusions in activated platelets (n = 74 platelets for WT and 87 platelets for Actbcg from three biological replicates). Significance was calculated by unpaired two-tailed Welch's t test without correction for multiple comparisons. Colored dots in each chart represent the average for each biological replicate, superimposed on all data points shown in black. Pairs of similar color dots (orange, maroon, and purple) represent littermate pairs. (B) Left, Macroscopic images of individual contracted blood clots and the average final extent of platelet-driven clot contraction in WT and Actbcg mice. n = 3 biological replicates for each genotype. Significance was calculated by unpaired two-tailed Welch's t test without correction for multiple comparisons. Lines represent the median value. (C) results of the tail bleeding assay. n = 12 biological replicates for each genotype. Significance was calculated by unpaired two-tailed Welch's t test without correction for multiple comparisons. Lines represent the median value.
Platelet functionality was assessed using a blood clot contraction assay, which quantifies the extent of clot shrinkage as a measure of actomyosin-dependent platelet contractility (Tutwiler et al., 2019). There was no difference in the average final extent of clot contraction in the WT (37.3 ± 1.7%) and Actbgc mice (37.0 ± 0.8%; p > 0.05) (Figure 7B). We also detected no changes in platelet aggregation and dense granule secretion in Actbcg mice (Supplemental Figure S5). Accordingly, there were no differences between WT and Actbcg mice in bleeding time and bleeding volume, even though the results were highly variable for both genotypes. This reflects normal hemostasis in the Actbcg mice (Figure 7C).
Thus, replacement of β-actin protein with γ-actin does not impact platelet morphology or function and does not affect hemostasis assessed with the tail bleeding assay.
Replacement of β-actin protein with γ-actin does not affect the composition of the actin cytoskeleton in blood cells
Most of the assays described above measure gross functional changes, expected to occur during major actin cytoskeleton perturbation. It is still possible that β- to γ-actin replacement leads to changes in the binding of actin-associated proteins that may produce subtle changes that are undetectable by the previous assays yet could still be physiologically important. It has been proposed in multiple studies that β- and γ-actin may exhibit changes in interactions with their binding partners that recognize differences in their N-terminal sequence (reviewed in Vedula and Kashina, 2018). Such changes may lead to subtle abnormalities in RBCs and identifying them would point to potential additional tests to perform. To test for potential changes in RBC cytoskeleton composition in Actbcg, we prepared RBC ghosts, the lysed preparations of isolated RBCs that contain only the plasma membrane and associated cytoskeleton (termed the spectrin-based membrane skeleton) while lacking the soluble components, and compared the relative levels of the membrane skeleton actin-binding proteins in WT and Actbcg ghosts using Western blotting (Figure 8). While the levels of β- and γ-actin in these preparations were as expected according to the genotypes, with the mutant mice lacking β-actin protein and exhibiting a corresponding increase in γ-actin expressed from the β-actin gene, the levels of all the major actin-binding proteins in the RBC membrane skeleton were similar between the two preparations. Thus, the replacement of β-actin protein with γ-actin does not induce any changes in the molecular composition of the RBC membrane skeletons.
FIGURE 8:
Replacement of β-actin protein with γ-actin does not affect actin-binding proteins of the spectrin-based membrane skeleton in RBCs. Western blot quantifications of key actin-binding proteins in RBC ghosts. Blot images show individual samples; the bars on top represent the quantification of the signal for each marker normalized to the total protein load. Error bars represent SEM (n = 4 biological replicates for each genotype). Significance was calculated by unpaired two-tailed Welch's t test without correction for multiple comparisons. Lines represent the median value. Bars are the averages of the values in each biological replicates also shown on the chart as dots. The only statistically significant change is between the actin isoforms due to gene editing.
We next analyzed total preparations of cytoskeletons from blood cells, prepared by total cell lysis in nonionic detergent followed by ultracentrifugation to collect the cytoskeletons, using quantitative mass spectrometry. This analysis was performed in biological triplicates, using samples derived in parallel from 3 different mice for each genotype, and peak intensities were calculated for each protein in the sample to enable quantitative comparison. Of note, these preparations were not from purified RBCs and included other blood cell types and thus, by design, were expected to contain a larger number of components.
A total of 542 proteins were identified in these preparations (Supplemental Table S1). None of these proteins exhibited statistically significant changes in preparations from Actbcg mice as compared with control. We manually selected known proteins associated with the actin cytoskeleton, compiling a short list of 47 proteins including known actin-binding partners and proteins involved in actin regulation (Supplemental Table S2), and compared their abundance in the sample measured using intensity-based absolute quantification (iBAQ) and label-free quantification (LFQ) intensities. None of these proteins were differentially abundant between WT and Actbcg mice. To illustrate this, we plotted iBAQ intensities for the 13 key actin-binding proteins with known major roles in actin cytoskeleton maintenance and contractility, averaged for the three biological replicates (Figure 9A). Notably, only a few of these proteins play prominent roles specifically in blood cells, and the levels of these proteins in our preparations were markedly higher than all the others. Again, no difference in the levels of any of these proteins was seen.
FIGURE 9:
Replacement of β-actin protein with γ-actin does not affect actin cytoskeleton composition or de novo translation in blood cells. (A) Mass spectrometry quantification of 13 key structural proteins of the actin cytoskeleton in WT (gray bars) and Actbcg (white bars) blood cell cytoskeleton preparations (see Supplemental Tables S1 and S2 for the full dataset). The image on the right shows a Coomassie Blue stained SDS–PAGE of the total cytoskeleton preparations analyzed. (B) Representative immunoblots with actin isoform antibodies (left) and quantifications (right) of total blood cells treated with MG132 of cycloheximide over time to inhibit protein degradation or translation, respectively. The average (bars) and individual data points (dots) for three biological replicates are shown in the graphs/Error bars represent SEM. Significance was calculated by unpaired two-tailed Welch's t test without correction for multiple comparisons. No significant change over time is seen for either inhibitor treatment for either actin isoform.
β- and γ-actin do not significantly differ in their de novo accumulation rates in blood cells
The combined results from the experiments shown above strongly suggest that β-actin protein replacement with γ-actin protein does not affect actin functionality at the protein level, in either hematopoiesis or in facilitating membrane skeleton functions in mature RBCs and actin cytoskeletal functions in platelets. This leads to the inevitable conclusion that, despite the high enrichment of β-actin in the blood both at the mRNA and protein level, its specific amino acid sequence does not play a key role in these cells. Consequently, we conclude that, contrary to common beliefs, the specific functions of β-actin in the RBCs and platelets are mostly or entirely nucleotide dependent.
β-actin gene possesses a number of nucleotide-level functional elements that could facilitate its unique requirement for mouse survival. While most of these elements have not been functionally dissected, our prior work showed that the mRNA coding sequence of β- and γ-actin, from the first ATG to the stop codon, confers differences in translation dynamics that carry functional consequences for cell migration (Vedula et al., 2021). Protein translation is believed not to contribute significantly to the total protein pool in either platelets or RBCs; however, recent studies demonstrate that some translation in these cells is occurring and may be important for their function (Weyrich et al., 2009; Kumar et al., 2022; Wurtzel et al., 2024).
To test whether β- and γ-actin undergo active translation in the blood, we incubated WB samples (containing all blood cells, with the expected majority of RBCs and platelets) in the presence of the proteasome inhibitor MG132, which suppresses cotranslational and posttranslational degradation of newly synthesized proteins and allows their intracellular accumulation. As a control experiment, we performed the same assay in the presence of translation inhibitor cycloheximide, which inhibits de novo protein synthesis but does not prevent degradation. Actively translating proteins in these assays would accumulate in the presence of MG132, while cycloheximide addition would inhibit this accumulation. However, neither treatment significantly affected the levels of either actin isoform, which remained stable over time regardless of the inhibitor treatment (Figure 9B). This result suggests that β- and γ-actin do not undergo active translation in terminally differentiated blood cells. Thus, translation-level changes resulting from differences in nucleotide coding sequence are unlikely to contribute to actin isoform function in the blood.
DISCUSSION
Our study addresses a long-standing question of the underlying functional mechanisms that drive high β-actin enrichment in RBCs and platelets, the two cell types in which β-actin is the overwhelmingly predominant actin isoform. Prior studies commonly assumed that this enrichment is important because of the specific properties of β-actin protein that make it uniquely suited for the function of these cells. Contrary to these assumptions, our data suggest that β-actin protein is not essential in these cells, and that γ-actin is capable of performing the same functions without impacting lineage specification, cell properties, or actin cytoskeleton function in platelets or RBCs. The interchangeability of β- and γ-actins is particularly striking in mature RBCs where the β-actin filaments form critical linking nodes in the two-dimensional lattice of the spectrin-based membrane skeleton (Mohandas and Gallagher, 2008; Fowler, 2013; Gokhin and Fowler, 2016). These short RBC actin filaments (∼40 nm long) are each associated with eight different actin-binding proteins, located along their length (α1,β1-spectrin, protein 4.1R, Tpm 1.9, Tpm 3.1, dematin), and at their barbed (α,β-adducin) or pointed filament ends (Tmod1 and SH3BGRL2) (Gokhin and Fowler, 2016; Li et al., 2023). Thus, unexpectedly, both the β- and γ-actin proteins appear to satisfy the rigorous structural constraints imposed by the density of these actin-binding proteins on the short actin filament nodes in the spectrin-based membrane skeleton. We conclude that the β- and γ-actin proteins appear to be fully interchangeable in RBC and platelets at the amino acid level, suggesting that the reason for β-actin enrichment in these cells is not functionally related to the specific features of its amino acid sequence.
RBCs and platelets constitute the overwhelming majority of the blood cells, and they share some important properties that set them apart from all the other blood cell types. Both RBCs and platelets are anucleate, and thus actin in these cells functions exclusively in cytoplasmic cytoskeletal structures in platelets, or in the spectrin-based membrane skeleton in RBCs. In both cell types, the actin cytoskeleton integrity and its ability to undergo highly specific rearrangements are central to these cells’ physiological functions. Our data show for the first time that despite a strong bias for β-actin in these cells, their core functions in the actin cytoskeleton can be performed equally well by either of the nonmuscle actin isoforms. Thus, while the reasons and functional consequences of the strong β-actin enrichment in these cells are still unclear, we can now conclude that these reasons are not related to the specific differences in the properties of the actin proteins.
Prior data suggest that β-actin mRNA becomes strongly enriched over any other actin isoforms in blood cell precursors early in hematopoiesis (Supplemental Figures S1 and S2). This enrichment is then maintained through the development of multiple blood cell lineages, keeping β-actin as the strongly dominant actin in these cells. The question of why the enrichment of β-actin mRNA is initiated and maintained remains open. Previous studies showed that mRNA-level differences in the two nonmuscle actins drive their differential intracellular localization via zipcode-mediated targeting (Condeelis and Singer, 2005; Katz et al., 2012; Rodriguez and Kashina, 2018), and it has been increasingly proposed that such localization is important for local translation of actin in different cell types, for example, neurons and cultured fibroblasts. Data from our lab show that nonmuscle actin mRNAs have different translation dynamics due to differences in their coding sequence and repertoire of silent mutations, and that β-actin can be translated at least 2-fold faster than γ-actin—a property linked to the actins’ role in cell adhesion and migration (Vedula et al., 2021). However, none of these mechanisms appear to apply to RBCs and platelets, which are not believed to strongly depend on either mRNA localization or active translation. Indeed, our data show that the overall rate of de novo actin synthesis in the blood cells does not differ dramatically for β- and γ-actin. It is still possible, however, that these mechanisms may contribute at earlier stages of blood cell differentiation, and thus the bias for β-actin protein in the blood is merely a consequence of these early mechanisms. Alternatively, the β-actin gene could become upregulated for reasons independent of RBCs and platelets—for example, to support β-actin–specific features in other blood cell types not included in the present study. Finally, it is possible that β-actin mRNA enrichment in the blood cell lineages occurs coincidentally merely because of its linkage to a prohematopoietic gene cluster and does not carry a specific biological role. Addressing these possibilities constitutes exciting directions for future work.
β-actin is one of the most essential and highly abundant proteins that is ubiquitously expressed in every eukaryotic cell. Unlike its high sequence identity to other actin isoforms, it plays unique roles at the cellular and organismal level that cannot be compensated by other actins (Perrin and Ervasti, 2010; Perrin et al., 2010; Bunnell et al., 2011; Vedula et al., 2017; Patrinostro et al., 2018). Despite decades of study, many unanswered questions still exist around this enigmatic protein. Uncovering the mechanisms and determinants of its unique functions in a variety of functional systems and cell types will uncover new levels of intracellular regulation.
MATERIALS AND METHODS
Request a protocol through Bio-protocol
Animal models
Actbcg mice were generated and maintained as described in (Vedula et al., 2017).
Blood counts and smears were performed by Penn Vet Diagnostic Laboratories according to standard clinical protocols adapted for mice.
Immunofluorescence staining for β and γ-actin
Blood was collected from mice using retroorbital or tail bleeds. A total of 20 µl of WB was mixed with 1 ml of 4% formaldehyde (Electron Microscopy Sciences) and kept overnight at 4°C. After 24 h, the cells were washed thoroughly with PBS and added to poly L-lysine coated dishes allowing the cells to adhere for 1 h at room temperature (RT). Next, cells were treated with 100% ice-cold methanol for 5 min and permeabilized with 0.1% Triton-X for 5 min. The cells were blocked in 1% BSA for 30 min before the addition of primary antibodies (mouse anti-β-actin [Clone 4C2, EMD Millipore] and mouse anti-γ-actin [Clone 2C3, EMD Millipore]) at 1:100 to dilution in PBS. Cells were incubated overnight at 4°C, followed by washes with PBS, and incubated in secondary antibodies for 1 h along with Hoechst stain. Cells were washed and imaged with a confocal microscope.
Western blotting of blood cells for β and γ-actin
Blood was drawn by retroorbital bleed into EDTA microtainers and centrifuged for 5 min at 2400 g to separate the blood cells from the serum. The cell pellet was boiled with 4X SDS sample buffer and then loaded onto a 10% polyacrylamide gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes for Western blotting in a standard 20% methanol Tris-glycine buffer (100 V, 1 h at 4°C). Transfers were stained with Revert 700 Total Protein stain (Catalogue No. 926-11021, LI-COR) and scanned for total protein using Odyssey imager (LI-COR), followed by destaining and blocking in 5% milk for 1 h at RT. Transfers were then incubated in primary antibodies in 5% milk for 1 h followed by washing in PBS and then labeled with secondary antibodies in 5% milk for 1 h. Finally, the transfer membrane was washed and proteins were detected by infrared imaging using Odyssey.
Immunohistochemistry of mouse tissues
For RBC precursor immunohistochemistry staining, 5-μm-thick sections of sternum and spleen were mounted on ProbeOnTM slides (Thermo Fisher Scientific) and stained using a Leica BOND RXm automated platform combined with the Bond Polymer Refine Detection kit (Leica #DS9800). Briefly, after dewaxing and rehydration, sections were pretreated with the epitope retrieval BOND ER2 high pH buffer (Leica #AR9640) for 20 min at 98°C. Endogenous peroxidase was inactivated with 3% hydrogen peroxide for 10 min at RT. Nonspecific tissue–antibody interactions were blocked with Leica PowerVision IHC/ISH Super Blocking solution (PV6122; Leica Biosystems, Deer Park, IL) for 30 min at RT. The same blocking solution also served as a diluent for the primary antibodies. A rabbit monoclonal antibody against the transferrin receptor (TfR1, Abcam, ab214039) was used at a concentration of 1/1000. A biotin-free polymeric IHC detection system consisting of horseradish peroxidase–conjugated antirabbit or antirat IgG was then applied for 25 min at RT. Immunoreactivity was revealed with the diaminobenzidine (DAB) chromogen reaction. Slides were counterstained in hematoxylin, dehydrated in an ethanol series, cleared in xylene, and permanently mounted with a resinous mounting medium (Thermo Fisher Scientific ClearVue coverslipper). Images were acquired using an Olympus BX46 microscope mounted with an Olympus DP28 camera.
Osmotic fragility assay
Blood from mice was collected by the tail or retroorbital bleeds into EDTA microtainers and kept in ice for 1 h. Blood was centrifuged for 5 min at 2400 g to allow separation of serum, white blood cells (WBC), and RBC. RBCs were collected by aspiration from the pellet, and equal aliquotes of RBCs were collected into separate tubes for the experiment. Each aliquote was washed once by resuspension in PBS and centrifugation for 5 min at 2400 g. The PBS supernatant was removed and discarded and the RBCs in each tube were resuspended in equal volumes of NaCl solutions at varying concentrations (0 g/l, 1 g/l, 2 g/l, 3 g/l, 4 g/l, 5 g/l, 6 g/l, 7 g/l, 8 g/l, 9 g/l, and 10 g/l) and incubated for 20 min at RT. After gentle centrifugation at 800 g for 5 min to remove the debris and nonlysed cells, the absorbance of the supernatant was determined at 540 nm.
Shear stress gradient ektacytometry
RBC deformability was assessed using Laser-assisted Optical Rotational Red Cell Analyzer (LORRCA) (R & R Mechatronics, Hoorn, Netherlands) (Hardeman et al., 2001; Ebenuwa et al., 2024). According to the manufacturer's instructions, 50 µL WB, with EDTA, was added to 5 ml iso-osmolar polyvinylpyrrolidone (PVP) solution (pH 7.4, 37°C) (R & R Mechatronics, Hoorn, Netherlands), and mixed by gently inverting the tubes. 1.5 ml mixed solution (PVP + WB) was injected into the instrument cup. For measuring RBC deformability, diffraction patterns of RBCs were captured over a shear stress gradient ranging from 0.3 to 30 Pa (0.3, 0.53, 0.95, 1.69, 3, 5.33, 9.49, 16.87, and 30 Pa) at 37°C with a camera gain of 208 (1,2). The LORRCA Maxsis software (version 5.08) generated red blood cell deformability curves, plotting elongation indices (EI) values against the shear stress gradient. Two key parameters were derived: the maximum elongation index (EI max), and the shear stress needed to reach half-maximal deformability (SS1/2).
Translation rate of actins
Blood was collected and mixed with 3.2% sodium citrate obtained from commercially available vacutainers (BD 363083) in the ratio 1:9, to avoid coagulation. A total of 40 µl of blood was mixed with 250 µl of serum-free media and treated with Cycloheximide (100 µg/ml) and MG-132 (20 µM) while incubating with gentle shaking at 37°C for different timepoints (0 min, 20 min, 40 min, and 60 min) and then subjected to Western blot to analyze the expression of β-actin and ϒ-actin.
Scanning Electron Microscopy (SEM) of RBCs and platelets
Fresh whole citrated mouse blood was centrifuged at 200 g for 5 min at RT to obtain platelet-rich plasma (PRP) and plasma (PRP) and a sedimented RBC pellet.
For SEM of RBCs, a 100-µl aliquot of the sedimented RBCs was resuspended in 1.9 ml of 50 mM Na-cacodylate buffer containing 100 mM NaCl (pH = 7.4) followed by centrifugation at 200 g for 10 min. The cells were resuspended and washed three times in the same buffer. A total of 10 µl of the RBCs suspension at 50% hematocrit was mixed with 490 µl of a fixative containing 2% glutaraldehyde in 50 mM Na-cacodylate and 100 mM NaCl, pH 7.4, and incubated for 5 min at RT. The suspension of fixed RBCs (50 μl) was placed on a polycarbonate filter with 1-µm pore size, kept for 2 h at RT, and then left overnight at 4°C. The fixed and settled RBCs were rinsed with 50 mM cacodylate buffer, pH 7.4, containing 100 mM NaCl, then dehydrated in ethanol at the ascending concentrations of 30–100 vol%, immersed in hexamethyldisilazane and air dried. The dried samples were sputter-coated with gold-palladium (Polaro e5100, Quorum Technologies, UK). Micrographs of RBCs were obtained using an FEI Quanta 250FEG scanning electron microscope (FEI, Hillsboro, OR).
For SEM of platelets, a 50-µl portion of PRP was diluted tenfold with 50 mM Na-cacodylate buffer containing 100 mM NaCl (pH 7.4), after which it was fixed with glutaraldehyde at a final concentration of 2% and deposited on a polycarbonate filter (0.1 μm pore size) by centrifugation at 500 g, 5 min, with fast acceleration and slow deceleration. The fixed and settled platelets were rinsed three times with 50 mM cacodylate buffer, pH 7.4, containing 100 mM NaCl, after which dehydration, drying process, and sputter coating were performed as described for RBCs.
Tail bleeding assay
The distal 5 mm tail segment of 5- to 6-wk-old mice was amputated with a scalpel after administration of anesthesia. The tails were immediately immersed in saline at 37°C. The bleeding time was presented as the sum of bleeding within 15 min of observation, including rebleeding. The time of complete cessation of blood flow (i.e., no bleeding for 1 min) was noted and the bleeding volume was measured by absorbance at 550 nm of the blood:saline mixture in the collection tubes.
Washed platelet preparation and platelet aggregation assay
Blood was collected from the inferior vena cava of anesthetized mice and mixed with 3.8% sodium citrate 9:1 by volume. The WB was centrifuged at 1800 rpm for 5 min. The top layer of the cell suspension was collected and further centrifuged at 800 rpm to collect PRP. Platelets were isolated from PRP by centrifugation at 2800 rpm for 5 min in the presence of 1 μM prostaglandin E1 (PGE1) at RT and then resuspended in HEPES-Tyrode's buffer pH 7.4.
Washed platelets were adjusted to 2 × 108 platelets/ml by using HEPES-Tyrode's buffer and supplemented with 1 mM CaCl2. The aggregation was measured by the turbidimetric method at 37°C in the presence of Chrono-Lume luciferase in a Lumi-Dual aggregometer (Chrono-Log).
Clot contraction/retraction assay
The extent of contraction of clots formed in WB was determined by optical detection of the clot size over time using the Thrombodynamics Analyzer System (HemaCore, Russia) (Tutwiler et al., 2016). Citrated mouse blood samples activated by 5 U/ml thrombin and 4 mM CaCl2 were transferred to a transparent plastic cuvette 7 × 12 × 1 mm that was prelubricated with 4% vol/vol Triton X-100 in 150 mM NaCl to prevent sticking of the clot to the walls of the cuvette. Using the light scatter-based tracking, changes in the clot size during contraction were measured every 15 s over 20 min. Serial images of the shrinking clot were converted into a kinetic curve of clot contraction, from which the extent of contraction, that is the final degree of clot shrinkage after 20 min of clot formation was determined relative to its initial size (Figure 4B).
RBC ghost preparation and Western blotting
WB was obtained from each mouse by retro-orbital bleed. RBC was isolated by sedimentation through 5 volumes of 0.75% Dextran T-500 (10 mM NaHPO4, pH 7.4, 150 mM NaCl, 0.75% Dextran) at 4°C for 1–2 h, followed by washing of cells four times in 20 volumes of ice-cold PBS (10 mM 5 mM NaHPO4, pH 7.4, 150 mM NaCl) at 1500 rpm for 5 min at 4°C (Sui et al., 2017). Ghosts were prepared from packed RBC pellets by hypotonic lysis followed by four washes in 20 volumes of ice-cold Mg++-lysis buffer (5 mM NaHPO4, pH 7.4, 2 mM MgCl2, 1 mM EGTA, 1 mM DTT). A total of 50 µl of packed ghosts were stored frozen at −80°C for Western blotting. Gel samples were prepared by the addition of 200 µl 2x Laemmli sample buffer (Bio-Rad, 1610737) containing 50 mM DTT, protease inhibitor cocktail (1:100; Thermo Fisher Scientific, 78429), and phosphatase inhibitors (1:100; Thermo Fisher Scientific, 78420) and boiled for 5 min at 95°C. A total of 5 µl per sample was loaded onto a 4–20% or 4–12% linear gradient SDS–PAGE mini-gel (Thermo Fisher Scientific, XP04200BOX, XP04120BOX) and electrophoresed at 150 V for 1 h in 1x running buffer (25 mM Tris base, 192 mM glycine, 0.1% SDS), transferred to a nitrocellulose membrane (Amersham Protran, 10600011) in 1x transfer buffer (12.5 mM Tris Base, 96 mM Glycine, and 20% Methanol) in a trans-blot tank (Bio-Rad) at 4°C, 100 V for 2 h. For α1β1-spectrin blots only, 1x transfer buffer was used without methanol, and with the addition of 0.01% SDS. Following the transfer, blots were incubated at 65°C in PBS for 1 h. Total protein staining was performed using the Revert 700 Total Protein Kit (LI-COR, 926-11016) and scanned with a Chemidoc MP Imaging System (Bio-Rad 12003154). Blots were then blocked with Intercept (PBS) Blocking Buffer (LI-COR, 927-70001) for 2 h at RT, then incubated with primary antibodies overnight at 4°C with gentle rocking. Primary antibodies used included mouse anti-β-actin (1:2000, Clone 4C2, EMD Millipore), mouse anti-ɣ-actin (1:2000, Clone 2A3, EMD Millipore), rabbit anti-band 4.1 (1:1000, a gift from Cathy Korsgren and Samuel Lux, Boston Children's Hospital, Boston, MA), sheep anti-Tpm 1.9 (1:1000, MilliporeSigma, AB5441), rabbit anti-α1β1-spectrin (1:2000, a gift from Cathy Korsgren and Samuel Lux, Boston Children's Hospital, Boston, MA), rabbit anti-Tmod1 (1:1000, R174 (Fowler, 1990)), rabbit anti-adducin (1:1000, a gift from Vann Bennett, Duke University, Durham, NC), rabbit anti-Dematin (1:1000, a gift from A. H. Chishti, Tufts University School of Medicine, Boston, MA), and mouse anti-Tpm3.1 (1:1000, Clone 2G10, EMD Millipore). The blots were then washed 3x for 5 min each in TBST (1x TBS + 0.1% Triton X-100) and incubated with IRDye 680RD donkey anti-mouse IgG (LI-COR, 926-68072) and IRDye 800CW donkey anti-rabbit IgG (LI-COR, 925-32213) at 1:20,000 diluted in Intercept (PBS) Blocking Buffer and incubated for 2 h at RT with gentle rocking. Blots were then washed again 3x for 5 min each in TBST before being scanned with a Chemidoc MP Imaging System. Band intensities were quantified using ImageJ and then normalized to total protein staining.
Fluorescence staining for RBC shape measurements
A total of 20 µl of WB was fixed in 1 ml of 4% paraformaldehyde (Electron Microscopy, 15710) overnight at RT with gentle rocking. After washing RBCs 3x in PBS (1000 × g, 5 min), RBCs were permeabilized with 0.3% Triton X-100 (Sigma-Aldrich, 11332481001) in 1x PBS for 15 min, and blocked with 4% BSA (Genesee Scientific, 25-529) and 1% normal goat serum in 1x PBS (blocking buffer) for 4–7 d at 4°C with gentle rocking. RBCs were either stained with Rhodamine Phalloidin (150 nM, Thermo Fisher Scientific, R415) or Alexa Fluor 488 anti-mouse TER-119 (1:100, BioLegend, 116215) diluted in blocking buffer for 2 h at RT with gentle rocking. RBCs were washed 3x in PBS before 7 × 104 cells were cytospun onto glass coverslips at 1200 rpm for 3 min using the Thermo Fisher Scientific Cytospin 4 centrifuge. Coverslips were mounted on slides with Pro ProLong Glass Antifade Mountant (Thermo Fisher Scientific, P36980). Airyscan Z-stack images were acquired using a Zeiss LSM-880 laser scanning confocal microscope (63x oil objective, NA 1.4) with a 0.17 µm Z-step. All images were processed using Zen software (Carl Zeiss) for further analysis. RBC shape measurements were calculated using ImageJ software.
Blood cell cytoskeleton isolation and mass spectrometry
Freshly collected blood was rapidly mixed into a tube recoated with Acid Citrate Dextrose (ACD) buffer and containing ACD buffer at 1:6 ratio (1 part of ACD buffer for 6 parts of blood), and centrifuged at 180 g, 37oC, 15 min. The supernatant PRM and pellet RBC from the spin were separately layered over 1 ml of Ficoll-Paque PLUS media and centrifuged at 400 g/RT/35 min to obtain platelets and RBCs, respectively. In both spins, WBCs formed a cloudy layer at the plasma-Ficoll interface and were collected into a separate tube. Platelets were further collected by centrifugation, and each cell type was then resuspended in F-actin lysis buffer (3 volumes compared with the original fraction volume), homogenized by sonication, and ultracentrifuged at 100,000 × g through 40% sucrose cushion to collect the actin cytoskeleton pellets.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. Yuri Veklich and the Department of Cell and Developmental Biology Imaging Core Facility for the SEM imaging of the blood samples, to N. Adrian Leu for help with animal handling and blood collection, to the members of the Penn Vet Comparative Pathology Core for RBC precursor staining and imaging and to the members of the Penn Vet Diagnostics Laboratories for the clinical analysis of the mouse blood samples. This work was supported by the NIH grant R35 GM122505 to A.K. R.I.L. is supported by the NIH grants P01 HL146373, R01 HL148227 and R01 HL148014. K.P. and M.L. were supported by NIH intramural research program grant ZIA DK 053218-18. RBC Airyscan imaging in the University of Delaware Bioimaging Center was supported by a Core Access Award from the Delaware IDeA Network of Biomedical Research Excellence (NIH/NIGMS P20GM103446) to V.M.F. The veterinary pathologists performing the histopathological analysis are part of the University of Pennsylvania Penn Vet Comparative Pathology Core Facility (RRID:SCR_022438) and are supported by the Abramson Cancer Center Support Grant (P30 CA016520).
Abbreviations used:
- Actbcg
beta-coded gamma actin
- CBC
complete blood count
- CRISPR
clustered regularly interspaced short palindromic repeats
- DAB
diaminobenzidine
- EDTA
ethylene diamine tetraacetate
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- iBAQ
intensity-based absolute quantification
- LFQ
label-free quantification
- mRNA
messenger ribonuicleic acid
- PBS
phosphate buffered saline
- PRP
platelet-rich plasma
- RBC
red blood cells
- RT
room temperature
- SEM
scanning electron microscopy
- WB
whole blood
- WT
wild type.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E24-04-0186) on December 20, 2024.
REFERENCES
- Arora AS, Huang HL,Singh R,Narui Y, Suchenko A, Hatano T, Heissler SM, Balasubramanian MK, Chinthalapudi K (2023). Structural insights into actin isoforms. Elife 12, e82015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell TM, Burbach BJ, Shimizu Y, Ervasti JM (2011). β-actin specifically controls cell growth, migration, and the G-actin pool. Mol Biol Cell 22, 4047–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Baldwin TM, Wong M, Bolden JE, Fairfax KA, Lucas EC, Cole R, Biben C, Morgan C, Ramsay KA, et al. (2019). Haemopedia RNA-seq: A database of gene expression during haematopoiesis in mice and humans. Nucleic Acids Res 47, D780–D785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Singer RH (2005). How and why does beta-actin mRNA target? Biol Cell 97, 97–110. [DOI] [PubMed] [Google Scholar]
- Dugina V, Zwaenepoel I, Gabbiani G, Clement S, Chaponnier C (2009). Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J Cell Sci 122, 2980–2988. [DOI] [PubMed] [Google Scholar]
- Ebenuwa I, Violet PC, Tu H, Lee C, Munyan N, Wang Y, Niyyati M, Patra K, Wilkins KJ, Parrow N, Levine M (2024). Altered RBC deformability in diabetes: Clinical characteristics and RBC pathophysiology. Cardiovasc Diabetol 23, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler VM (1990). Tropomodulin: A cytoskeletal protein that binds to the end of erythrocyte tropomyosin and inhibits tropomyosin binding to actin. J Cell Biol 111, 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler VM (2013). The human erythrocyte plasma membrane: A rosetta stone for decoding membrane-cytoskeleton structure. Curr Top Membr 72, 39–88. [DOI] [PubMed] [Google Scholar]
- Gokhin DS, Fowler VM (2016). Feisty filaments: Actin dynamics in the red blood cell membrane skeleton. Curr Opin Hematol 23, 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeman MR, Dobbe JG, Ince C (2001). The laser-assisted optical rotational cell analyzer (LORCA) as red blood cell aggregometer. Clin Hemorheol Microcirc 25, 1–11. [PubMed] [Google Scholar]
- Hartwig JH (1992). Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol 118, 1421–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissler SM, Chinthalapudi K (2024). Structural and functional mechanisms of actin isoforms. FEBS J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashina AS (2020). Regulation of actin isoforms in cellular and developmental processes. Semin Cell Dev Biol 102, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ZB, Wells AL, Park HY, Wu B, Shenoy SM, Singer RH (2012). β-actin mRNA compartmentalization enhances focal adhesion stability and directs cell migration. Genes Dev 26, 1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SD, Kar D, Akhtar MN, Willard B, Roy D, Hussain T, Rajyaguru PI, Eswarappa SM (2022). Evidence for low-level translation in human erythrocytes. Mol Biol Cell 33, br21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Chen S, Xu K, He MT, Dong MQ, Zhang QC, Gao N (2023). Structural basis of membrane skeleton organization in red blood cells. Cell 186, 1912–1929.e18. [DOI] [PubMed] [Google Scholar]
- Mills EW, Green R, Ingolia NT (2017). Slowed decay of mRNAs enhances platelet specific translation. Blood 129, e38–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N, Gallagher PG (2008). Red cell membrane: Past, present, and future. Blood 112, 3939–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrinostro X, Roy P, Lindsay A, Chamberlain CM, Sundby LJ, Starker CG, Voytas D, Ervasti JM, Perrin BJ (2018). Essential nucleotide- and protein-dependent functions of Actb/beta-actin. Proc Natl Acad Sci U S A 115, 7973–7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin BJ, Ervasti JM (2010). The actin gene family: function follows isoform. Cytoskeleton (Hoboken) 67, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin BJ, Sonnemann KJ, Ervasti JM (2010). β-actin and γ-actin are each dispensable for auditory hair cell development but required for stereocilia maintenance. PLoS Genet 6, e1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Kashina A (2018). Posttranscriptional and posttranslational regulation of actin. Anat Rec (Hoboken) 301, 1991–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D (2004). Gelsolin superfamily proteins: Key regulators of cellular functions. Cell Mol Life Sci 61, 2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, Gokhin DS, Nowak RB, Guo X, An X, Fowler VM (2017). Stabilization of F-actin by tropomyosin isoforms regulates the morphology and mechanical behavior of red blood cells. Mol Biol Cell 28, 2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondeleir D, Drogat B, Slowicka K, Bakkali K, Bartunkova S, Goossens S, Haigh JJ, Ampe C (2013). Beta-actin is involved in modulating erythropoiesis during development by fine-tuning Gata2 expression levels. PLoS One 8, e67855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutwiler V, Litvinov RI, Lozhkin AP, Peshkova AD, Lebedeva T, Ataullakhanov FI, Spiller KL, Cines DB, Weisel JW (2016). Kinetics and mechanics of clot contraction are governed by the molecular and cellular composition of the blood. Blood 127, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutwiler V, Peshkova AD, Le Minh G,Zaitsev S, Litvinov RI, Cines DB, Weisel JW (2019). Blood clot contraction differentially modulates internal and external fibrinolysis. J Thromb Haemost 17, 361–370. [DOI] [PubMed] [Google Scholar]
- Vedula P, Kashina A (2018). The makings of the ‘actin code’: Regulation of actin's biological function at the amino acid and nucleotide level. J Cell Sci 131, jcs215509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedula P, Kurosaka S, Leu NA, Wolf YI, Shabalina SA, Wang J, Sterling S, Dong DW, Kashina A (2017). Diverse functions of homologous actin isoforms are defined by their nucleotide, rather than their amino acid sequence. Elife 6, e31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedula P, Kurosaka S, MacTaggart B, Ni Q, Papoian G, Jiang Y, Dong DW, Kashina A (2021). Different translation dynamics of β- and γ-actin regulates cell migration. Elife 10, e68712. [Google Scholar]
- Weyrich AS, Schwertz H, Kraiss LW, Zimmerman GA (2009). Protein synthesis by platelets: Historical and new perspectives. J Thromb Haemost 7, 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzel JGT, Lazar S, Askari S, Zhao X, Severa J, Ayombil F, Michael JV, Camire RM, McKenzie SE, Stalker TJ, et al. (2024). Plasma growth factors maintain constitutive translation in platelets to regulate reactivity and thrombotic potential. Blood Adv 8, 1550–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CJ, Park YH, An MY, Ryu B, Jung HS (2024). Insights into actin isoform-specific interactions with myosin via computational analysis. Molecules 29, 2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.