Abstract
Autophagy is an essential cellular recycling process that maintains protein and organelle homeostasis. ATG9A vesicle recruitment is a critical early step in autophagy to initiate autophagosome biogenesis. The mechanisms of ATG9A vesicle recruitment are best understood in the context of starvation-induced nonselective autophagy, whereas less is known about the signals driving ATG9A vesicle recruitment to autophagy initiation sites in the absence of nutrient stress. Here we demonstrate that loss of ATG9A, or the lipid transfer protein ATG2, leads to the accumulation of phosphorylated p62 aggregates in nutrient replete conditions. Furthermore, we show that p62 degradation requires the lipid scramblase activity of ATG9A. Last, we present evidence that polyubiquitin is an essential signal that recruits ATG9A and mediates autophagy foci assembly in nutrient replete cells. Together, our data support a ubiquitin-driven model of ATG9A recruitment and autophagosome formation during basal autophagy.
Although ATG9A is essential for basal autophagy, its impact on global proteome dynamics and mechanism of recruitment to sites of basal autophagy are poorly understood.
The authors found that loss of ATG9A impacts the turnover rate of many proteins, but most prominently p62. Further, loss of ATG9A and its lipid transfer partner ATG2A cause accumulation of large p62 aggregates not found with other ATG gene deletions. Finally, polyubiquitin is required and sufficient to recruit the ATG9A-ATG2A complex to p62-positive sites.
These data support a ubiquitin-driven model of ATG9A recruitment to initiate basal autophagy.
INTRODUCTION
The recycling of cellular material through macroautophagy, referred to here as autophagy, maintains organelle and protein homeostasis. Defects in autophagy are associated with a variety human diseases, including metabolic disorders, neurodegeneration, infectious disease, and autoimmunity (Mizushima and Levine, 2020). Autophagy is characterized by the formation of a double-membrane vesicle called the autophagosome, which engulfs cellular material and ultimately fuses with the lysosome to degrade and recycle its contents (reviewed in Debnath et al., 2023). A variety of proteins regulate autophagosome growth, closure, and fusion with the lysosome. Of the many autophagy-regulating proteins identified, ∼17 are considered essential and carry the “ATG” designation. These ATG proteins are evolutionarily conserved and required for nonselective and selective forms of autophagy (Vargas et al., 2023). One essential yet still incompletely understood protein that acts at the earliest steps of autophagy is ATG9A (Yamamoto et al., 2012; Karanasios et al., 2016).
ATG9A is the only multi-pass transmembrane protein among the core ATG proteins. Under nutrient replete conditions, ATG9A associates with small lipid vesicles that move through the endoplasmic reticulum, Trans-Golgi Network, and endomembrane vesicle systems (Young et al., 2006; Mari et al., 2010; Orsi et al., 2012; Imai et al., 2016; Nishimura et al., 2017). In mitophagy, ATG9A and the ULK1 complex arrive independently at damaged mitochondria (Itakura et al., 2012) and associate via an interaction between the ATG9A C-terminus and the ATG13:ATG101 dimer (Ren et al., 2023). In starvation-induced autophagy, ATG9A vesicles help recruit other ATG proteins and ultimately expand into phagophores (Judith et al., 2019). An emerging model of ATG9A function suggests that ATG9A acts as a lipid scramblase, receiving phospholipids from the lipid transferase ATG2 and distributing these lipids between the inner and outer leaflets of the growing phagophore membrane, thereby regulating autophagosome membrane curvature and lipid composition, which is essential for autophagy progression (Maeda et al., 2020; Matoba et al., 2020; van Vliet et al., 2022).
In contrast to starvation-induced autophagy, less is known about mechanisms of autophagy in the absence of nutrient stress, which we refer to here as basal autophagy—a broad term that encompasses several forms of autophagy that occur in nutrient-replete conditions (Musiwaro et al., 2013). In general, basal autophagy is cargo specific. For example, a form of basal autophagy called aggrephagy prunes toxic protein aggregates from the cell (reviewed in Adriaenssens et al., 2022). As such, defects in aggrephagy are associated with proteinopathy diseases, including Parkinson's disease and amyotrophic lateral sclerosis (Mizushima and Levine, 2020). The specificity of basal autophagy is driven at least in part by autophagy adapter proteins, including p62, TAX1BP1, NBR1, and OPTN, which interact with poly-ubiquitinated substrates and gather them for engulfment by the autophagosome (reviewed in Adriaenssens et al., 2022). For example, through its ubiquitin association domain, p62 binds to polyubiquitinated misfolded proteins and oligomerizes to form p62- and ubiquitin-rich phase-separated droplets, referred to here as ubiquitin-rich condensates, which can be engulfed by autophagosomes (Bjorkoy et al., 2005; Pankiv et al., 2007; Wurzer et al., 2015; Zaffagnini et al., 2018; Turco et al., 2021). Other autophagy adapters are also recruited to these ubiquitin-rich condensates, including NBR1 and TAX1BP1, which likely help package the condensate for engulfment by the autophagosome (Turco et al., 2021). Therefore, in this model, the formation of a ubiquitin-rich condensate seems to be an initial trigger to recruit autophagy machinery, which, in turn, generates the autophagosome in a cargo-directed manner. Interestingly, cell treatments that cause a build-up of ubiquitin-rich condensates trigger a corresponding accumulation of ATG9A at the condensates, suggesting that ATG9A is somehow recruited early to these sites of selective autophagy even in the absence of starvation-induced signaling (Kannangara et al., 2021).
In this study, we used proteomics coupled with D2O labeling to measure the impact of ATG9A deletion on proteome flux in nutrient replete cells. Our results show that loss of ATG9A affects the degradation rates of many proteins, but none more than the autophagy adapter p62. We demonstrate that the loss of ATG9A promotes the accumulation of phosphorylated forms of p62 in large cytoplasmic clusters consistent with ubiquitin-rich condensates. Deletion of other core ATG proteins (ATG13 and ATG5) does not entirely phenocopy ATG9A deletion in regard to p62 phosphorylation and condensate size. However, deletion of ATG2, a partner of ATG9A in mediating lipid transfer and phagophore expansion, phenocopies ATG9A loss, supporting the critical role of this lipid transfer complex in basal autophagy. We also show that p62 flux requires ATG9A lipid scramblase activity and deletion of ATG9A abrogates ATG2A recruitment to ubiquitin-rich condensates. Last, using quantitative live-cell imaging, we show that poly-ubiquitin is required and sufficient to recruit ATG9A to p62-positive clusters.
RESULTS
Quantitative proteome-level measurement of protein turnover in ATG9A-deficient cells
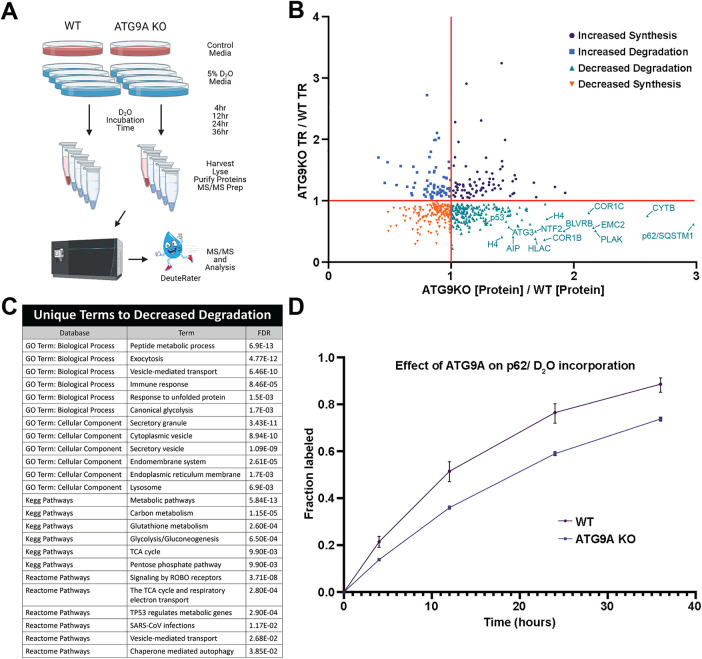
To determine how loss of ATG9A affects protein flux across the proteome during fed conditions, we used a quantitative deuterium labeling LC-MS/MS approach (Mathis et al., 2017; Naylor et al., 2017) to derive synthesis and degradation rates for endogenous proteins in HEK293T wild-type (WT) and HEK293T ATG9A knockout (KO) cells (Figure 1A). Of the 4025 proteins identified, 517 of these were considered to have sufficient quality and quantity within all replicates to be analyzed for turnover rates (Figure 1B). As we expected, the largest fraction of the 517 proteins (208) was for proteins that showed decreased degradation rates in ATG9A KO cells. A gene ontology (GO) term and pathway analysis of these proteins revealed an enrichment in vesicle trafficking pathway and metabolism (Figure 1C). Many notable proteins overlapped with proteins identified as autophagosome-associated via different proteomics methods (Zellner et al., 2021; Danieli et al., 2023; Kurusu et al., 2023). These include multiple isoforms of 14-3-3 proteins, components of the electron transport chain (e.g., ATPase subunits), numerous translation elongation and initiation factors, and HSP90. Across all proteins, we found that the autophagy adapter p62 had the most significantly decreased degradation rate in ATG9A KO cells of all proteins detected (Figure 1, B and D). This strong effect of ATG9A KO on p62 stability is also corroborated by previous CRISPR screens (DeJesus et al., 2016; Shoemaker et al., 2019). Taken together, our proteome flux measurements suggest that ATG9A-dependent basal autophagy targets cytosolic vesicle trafficking and metabolism proteins, and most prominently the autophagy adapter p62.
FIGURE 1:
Quantitative proteome turnover analysis comparing WT and ATG9A KO cells. (A) Schematic showing the workflow using label-free quantitation (control media, n = 3) and metabolic deuterium incorporation (D2O media, n = 3 collected at 4, 12, 24, and 36 h) to derive protein abundance and turnover rates (TR) in WT and ATG9A KO HEK293T cells. (B) Concurrent changes in abundance and turnover, shown as fold differences (ATG9A KO vs. WT cells). The values were derived from a biological triplicate of the experiment in A with error bars omitted for clarity of presentation. (C) Ontologies enriched in the decreased degradation quadrant (positive [Protein] with negative turnover) for ATG9AKO vs. WT as scored by Panther. (D) Metabolic labeling of P62, as an example of the protein turnover data. Error bars represent SD between technical replicates (n = 3) for the fraction of new P62 detected by MS at the indicated time.
Phosphorylated p62 accumulates prominently in cells lacking the ATG9A-ATG2 lipid transfer complex
To determine whether loss of other key autophagy proteins would affect p62 degradation in fed conditions similarly to loss of ATG9A, we knocked out ATG5 and ATG13 in HEK293T cells to inhibit LC3 lipidation-deficient (ATG5 KO) or disrupt formation of the ULK1 complex (ATG13 KO). Quantification of total p62 levels and flux revealed little difference between the ATG9A KO, ATG5 KO, and ATG13 KO lines. However, the ATG9A KO line displayed a prominently upshifted p62 band compared with the other KO lines. (Figure 2, A and B). Using phosphospecific antibodies to detect known phosphorylation sites on p62, including pS269, pS349, and pS403, we confirmed that the upshifted p62 band in ATG9 KO cells is phosphorylated at these sites (Figure 2, C–E). Bafilomcycin treatment had little effect on the turnover of phospho- and unmodified p62, suggesting that loss of ATG9A resulted in the accumulation of a phosphorylated form of p62 that is stalled in the autophagy process, which is not entirely phenocopied by other ATG KOs (Figure 2, C–E).
FIGURE 2:
Phosphorylated p62 accumulates prominently in ATG9A KO cells. (A) Western blot demonstrating the defects of p62 degradation in ATG5, ATG9A, and ATG13 KO cell lines. (B) Quantification of p62 immunoblot signal normalized to actin from three replicates. Error bars represent SD. P values were calculated using a two-tailed Student t test. (C) Immunoblot showing the buildup of phospho-p62 in the indicated ATG protein KO cell lines. (D and E) Quantification (as done in B and C) of pS349 and pS403 on p62 from three biological replicates of immunoblots as shown in D. Error bars represent SD. P values were calculated using a two-tailed Student t test. (F and G) Images depicting the accumulation of pS349 or pS403 p62 in WT and ATG9A KO HEK293T cells (scale bars = 10 µm).
Previous work demonstrated that phosphorylation of p62 at S349 and S403 increased p62 affinity to cargo and/or polyubiquitin (Matsumoto et al., 2011; Pilli et al., 2012; Ichimura et al., 2013; Matsumoto et al., 2015). This suggests these phosphorylation sites mark the pool of p62 at ubiquitin-rich condensates. Supporting this idea, we found that the S349- and S403-phosphorylated forms of p62 were concentrated within large cytosolic puncta consistent in morphology with p62-positive ubiquitin-rich condensates (Figure 2, F–G) (Kannangara et al., 2021). We also observed the autophagy adapter TAX1BP1 at these p62 clusters (Supplemental Figure S1), supporting the idea that these are sites of autophagy initiation in fed cells.
Recent structural and modeling work has suggested that ATG9A is a lipid scramblase that forms a complex with the lipid transfer proteins ATG2A and ATG2B to initiate autophagosome formation (Maeda et al., 2020; Matoba et al., 2020; Ghanbarpour et al., 2021; van Vliet et al., 2022). Together, this ATG9A-ATG2 complex is thought to maintain lipid balance across the autophagosome membrane as it expands. Therefore, given the connected roles ATG2A/B and ATG9A, we hypothesized that loss of ATG2A/B would mimic loss of ATG9A and also lead to the accumulation of phosphorylated p62. To test this idea, we used a U2OS cell line in which a HaloTag is homozygously inserted at the N-terminus of the genomic atg9a locus (Broadbent et al., 2023). HaloTagged ATG9A is fully functional and supports LC3 lipidation and p62 degradation (Broadbent et al., 2023). In this HaloTag-ATG9A U2OS cell line, we deleted ATG2A/B, ATG5, ATG9A, or ATG13 (Broadbent et al., 2023) and confirmed that p62 degradation and phosphorylation showed a similar dependency on ATG9A in these cells (Figure 3, A and B). We found that deletion of ATG2A/B phenocopied loss of ATG9A resulting in an accumulation of phosphorylated p62 (Figure 3, C–F). We also tried to test whether deletion of the ATG9A paralog ATG9B in our ATG9A KO cells would cause an even more severe defect in p62 accumulation. However, we were unable to recover any surviving ATG9A/B double KO cells. Nevertheless, our data suggest that loss of the ATG9A-ATG2 lipid transfer complex causes a significant accumulation of phosphorylated p62 (Figure 3, C–F).
FIGURE 3:
The ATG9A-ATG2 lipid transfer complex is required for degradation of phosphorylated p62. (A) Western Blot demonstrating the defects of p62 degradation in ATG2A/B, ATG5, ATG9A, and ATG13 KO U2OS cell lines. (B) Quantification of the triplicate Western blots in A. Error bars represent SD. P values were calculated using a two-tailed Student t test. (C) Western blot showing the accumulation of phospho-p62 in the indicated ATG KO U2OS cell lines. (D–F) Quantification of the phospho-p62 Western blots in C. Error bars represent SD. P values were calculated using a two-tailed Student t test. (G) Fluorescent gel and western blot showing the degradation of p62 in cells stably expressing WT and M33 Halo-ATG9A variants in the absence and presence of doxycycline. (H) Quantification of triplicate Western blots represented in G. Error bars represent SD. P values were calculated using a two-tailed Student t test.
To determine whether ATG9A scramblase function is required for degradation of the phosphorylated pool of p62, we expressed Halo-ATG9A WT or an ATG9A mutant, termed M33, with reduced lipid scramblase activity (Maeda et al., 2020) in ATG9A KO U2OS cells using an inducible expression cassette. After 24 h of doxycycline-induced ATG9A expression, we found that ATG9A-M33 failed to rescue p62 degradation in the ATG9A KO line (Figure 3, G and H). Together, these results suggest that disruption of the ATG9A-ATG2 lipid transfer complex causes a distinct accumulation of phosphorylated p62 that is not observed by disrupting LC3 lipidation or ULK1 complex formation.
The formation of ubiquitin-rich condensates triggers the recruitment of ATG9A to initiate autophagy
In previous work, we found that perturbations that increase ubiquitin-rich condensate size result in an accumulation of ATG9A at these condensates, which also colocalize with p62, TAX1BP1 and other autophagy markers (Kannangara et al., 2021). However, the molecular mechanism of ATG9A recruitment to these ubiquitin-rich sites remains unclear. Notably, the ULK1 complex scaffold FIP200, which tethers the ULK1 complex to p62 and other adapters at ubiquitin-rich condensates (Ravenhill et al., 2019; Turco et al., 2019; Vargas et al., 2019), is not required for ATG9A accumulation at these sites (Supplemental Figure S2), suggesting that other components of the condensate might recruit ATG9A. ATG9A is observed at the earliest steps of autophagosome growth (Yamamoto et al., 2012; Sawa-Makarska et al., 2020; Ren et al., 2023), which suggests that either the clustering of ATG9A vesicles occurs first to generate nascent autophagosomal membrane to which p62, OPTN, TAX1BP1 and other adapters recruit cargo and downstream autophagy machinery, or, alternatively, the ubiquitin-rich condensates form first to create a platform for ATG9A recruitment. The latter model is supported by evidence that OPTN is required for ATG9A recruitment to artificially induced ubiquitin condensates (Yamano et al., 2020). To distinguish between these two models, we took advantage of HCT-116 cells in which we appended an HA tag in-frame to the C-terminus of the atg9a gene, which allows us to track endogenous ATG9A accumulation at ubiquitin-rich condensates (Kannangara et al., 2021). Treatment of WT cells with wortmannin, which blocks autophagosome growth, resulted in a minor accumulation of small ubiquitin-rich foci positive for p62 and ATG9A (Figure 4, A–C). However, deletion of p62, the scaffold of ubiquitin condensate formation (Ciuffa et al., 2015; Turco et al., 2021), inhibited the formation of large ATG9A puncta and colocalization with ubiquitin (Figure 4, A–C). To define the relationship between p62 cluster formation and ATG9A accumulation further, we deleted ATG13, which allows a clearer visualization of ATG9A accumulation at the p62 clusters/ubiquitin-rich condensates. Deletion of p62 eliminated these large ubiquitin-rich puncta, and reverted ATG9A back to a normal cellular distribution (Figure 4, A–C), supporting the model that formation of ubiquitin-rich condensates precedes ATG9A recruitment.
FIGURE 4:

p62-mediated formation of ubiquitin-rich condensates is required for ATG9A recruitment. (A) Confocal imaging of endogenous p62, ubiquitin and endogenously HA-tagged ATG9A in WT, ATG13 KO and ATG13-p62 double KO (dKO) HCT-116 cells (scale bars = 10 µm). Where indicated, cells were treated for 4 h with 1 µM wortmannin. (B) Quantification of ATG9A-HA and TAX1BP1 colocalization by Pearson's coefficient Quantitation is from three replicates with error bars representing SD. P values were calculated using a two-tailed Student t test for pair-wise comparison. (C) Quantification of ATG9A-HA and ubiquitin colocalization from three biological replicates with error bars representing SD. P values were calculated using a two-tailed Student t test as in B.
To test these models further, we used our recently developed panel of endogenously tagged autophagy proteins to quantitatively measure the formation of autophagy foci by live-cell imaging (Broadbent et al., 2023; Barnaba et al., 2024). Our experiments with these cell lines suggest that the autophagy machinery form foci that rapidly assemble and disassemble, while only a fraction of these foci commit to autophagy and mature into LC3-positive autophagosomes (Broadbent et al., 2023). Why some autophagy foci form only to quickly disassemble while others commit to becoming autophagosomes is unclear. A growing body of literature suggests that polyubiquitin chains attached to misfolded or aggregated proteins are the trigger that nucleates autophagosome formation at ubiquitin-rich condensates (Sun et al., 2018; Zaffagnini et al., 2018; Ravenhill et al., 2019; Turco et al., 2019; Turco et al., 2021). Therefore, we posited that in nutrient replete media, autophagy machinery can form committed foci when tethered to a degradation target, such as polyubiquitin at ubiquitin-rich condensates. To begin testing this idea, we used the E1 ubiquitin-activating enzyme inhibitor MLN7243 (MLN), which potently inhibits the conjugation of polyubiquitin (Figure 5A), while imaging endogenously tagged ATG13 (Halo) as a clearly detectable marker of autophagy foci formation. In addition, we measured the colocalization of Halo-ATG13 with LC3B as an indicator of foci that commit to forming autophagosomes. Strikingly, inhibition of polyubiquitination with the E1 ubiquitin-activating enzyme inhibitor MLN7243 (MLN) reduced the formation of autophagy foci that colocalized with LC3B. The inhibition of polyubiquitination also reduced ATG13 foci that were not colocalized yet with LC3B, suggesting a role for polyubiquitin in the initial formation of autophagy foci (Figure 5B; Supplemental Videos S1 and S2). In addition, we analyzed the lifetime of ATG13 foci, which was largely unchanged in control and MLN-treated cells, perhaps reflecting ATG13 foci that form at already established (prior to MLN treatment) ubiquitin-rich condensates—and once formed, they persist as usual (Figure 5C; Supplemental Videos S1 and S2). Furthermore, in both control and MLN-treated cells, the lifetime of ATG13 foci that colocalized with LC3 was significantly longer than ATG13 that did not colocalize with LC3 signal, consistent with the idea that LC3-positive ATG13 foci have passed a commitment step to form autophagosomes (Figure 5C; Supplemental Videos S1 and S2) (Broadbent et al., 2023).
FIGURE 5:
Poly-ubiquitin is required and sufficient for recruitment of the ATG9A-ATG2A lipid transfer complex. (A) U2OS cells were treated with 10 µM MLN for 2 h, followed by immunoblotting for endogenous ubiquitin. Graph shows quantification of three replicates with P value calculated using a two-tailed Student t test. (B) Rates of ATG13 foci formation divided into colocalized and noncolocalized foci in U2OS cells endogenously expressing Halo-ATG13 and stably expressing GFP-LC3B treated with DMSO or MLN. P values were calculated using a two-tailed Student t test. (C) ATG13 foci length distribution from the data shown in A. P values were calculated using a two-tailed Student t test. (D) Example images showing the difference in recruitment of endogenous Halo-ATG9A to SNAP-p62 aggregates in the presence and absence of MLN. (E) Quantification of the amount of Halo-ATG9A that is recruited to SNAP-P62 aggregates shown in C. P values were calculated using a two-tailed Student t test. (F) Representative images showing the recruitment of Halo-ATG2A to 6x-Ubiquitin-BFP2 aggregates in WT and ATG9AKO cells. (G) Counts of colocalized foci shown in E. (H) kymograph showing representative foci of Halo-ATG2A and 6x-ubiqutin colocalization over-time from the experiment shown in E. P values were calculated using a two-tailed Student t test.
Movie S1.
U2OS cells expressing Halo-ATG13 labeled with JFX650 (left panel, magenta in merge) and GFP-LC3B (center panel, green in merge) in control media imaged at 1 frame per second.
Movie S2.
U2OS cells expressing Halo-ATG13 labeled with JFX650 (left panel, magenta in merge) and GFP-LC3B (center panel, green in merge) treated for 2 hours in media containing 10 µM MLN imaged at 1 frame per second.
Next, we asked whether the inhibition of ubiquitination prevented ATG9A recruitment to p62 clusters. To visualize robust recruitment of ATG9A to large p62 clusters, we used ATG2A/B KO cells in which ATG9A vesicles are recruited to autophagy substrates that fail to be degraded (Olivas et al., 2023). To image ATG9A at these clusters, we used our endogenously-tagged Halo-ATG9A cell line and expressed SNAP-p62 via stable integration into the AAVS1 locus. To distinguish between ATG9A foci that were already present prior to MLN treatment and ATG9A recruited to nascent p62 clusters during MLN treatment, we blocked existing Halo-ATG9A with nonfluorescent HaloTag ligand, followed by incubation with the fluorescent JF646 HaloTag ligand. Using this novel blocking strategy allowed us to only visualize newly synthesized Halo-ATG9A rather than Halo-ATG9A that may already be accumulated at pre-existing p62 clusters. Consistent with our previous observation, inhibition of polyubiquitination reduced recruitment of ATG9A to p62-positive ubiquitin-rich sites (Figure 5, D and E; Supplemental Videos S3 and S4).
Movie S3.
U2OS cells expressing Halo-ATG9 labeled with JFX650 (left panel, magenta in merge) and SNAP-p62 labeled with JF503 (center panel, green in merge) in control media imaged at 1 frame per second.
Movie S4.
U2OS cells expressing Halo-ATG9 labeled with JFX650 (left panel, magenta in merge) and SNAP-p62 labeled with JF503 (center panel, green in merge) after treatment with 10 µM MLN imaged at 1 frame per second.
To test this model further, we asked whether the clustering of polyubiquitin is sufficient to recruit ATG9A. However, in our previous work, we noted difficulty with live-cell imaging of endogenous ATG9A at autophagy foci in cells with an intact autophagy pathway, likely due to the limited number of ATG9A molecules that accumulate at the site of autophagosome formation (Broadbent et al., 2023). To overcome this challenge, we used two different approaches. First, we transiently overexpressed SNAP-tagged ATG9A and 6x-ubiquitin-BFP2 to form artificial ubiquitin-rich condensates. In this experiment, we only observed detectable accumulation of SNAP-ATG9A at 6x-ubiquitin-BFP2 clusters in cells also cooverexpressing OPTN (Supplemental Figure S3), which corroborates the observation by Matsuda and colleagues that OPTN promotes ATG9A localization to artificial ubiquitin clusters (Yamano et al., 2020). Of note, deletion of OPTN alone had no effect on ATG9A recruitment to p62 puncta, suggesting that while OPTN expression may be sufficient to boost ATG9A recruitment to these ubiquitin clusters, it is not required for ATG9A recruitment (Supplemental Figure S4). We also tested the possibility that poly-ubiquitination of ATG9A itself may be required for recruitment to ubiquitin clusters. However, lysine-to-arginine mutations at ATG9A ubiquitination sites (K581 and K838) that were reported as necessary for formation of VPS34-UVRAG complexes (Wang et al., 2022) did not affect ATG9A recruitment to the artificial ubiquitin clusters (Supplemental Figure S3). Last, we imaged endogenously tagged Halo-ATG2A, which detectably accumulates at phagophores, as a marker for the formation of the ATG9A-ATG2 lipid transfer complex. We observed ATG2A recruitment to 6x-ubiquitin-BFP2 condensates, confirming that the 6x-ubiquitin system recruits the lipid transfer complex (Figure 5, F–H; Supplemental Videos S5 and S6). More importantly, the recruitment of ATG2A to 6x-ubiquitin-BFP2 clusters depended on ATG9A (Figure 5, F–H; Supplemental Videos S5 and S6). Taken together, these observations suggest that the formation polyubiquitin condensates, likely together with autophagy adapters like OPTN, recruits the ATG9A-ATG2 lipid transfer complex to initiate autophagosome growth in the context of basal autophagy.
Movie S5.
U2OS cells expressing Halo-ATG2A labeled with JFX650 (left panel, magenta in merge) and 6x-Ubiquitin-BFP (center panel, green in merge) imaged at 1 frame per second.
Movie S6.
ATG9A knock-out U2OS cells expressing Halo-ATG2A labeled with JFX650 (left panel, magenta in merge) and 6x-Ubiquitin-BFP (center panel, green in merge) imaged at 1 frame per second.
DISCUSSION
Our data suggest that deletion of the ATG9A-ATG2 lipid transfer complex affects p62 differently than loss of other ATG proteins (ATG5 and ATG13). Interestingly, in comparing these ATG KO lines, we did not detect a significant difference in the overall rate of p62 degradation, but instead deletion of either ATG2A/B or ATG9A resulted in a build-up of phosphorylated p62 that was not observed to the same degree in cells lacking LC3 conjugation or ULK1 complex activity. The phosphorylated form of p62 has higher affinity for ubiquitin and thus likely marks the pool of p62 within the ubiquitin-rich condensate (Matsumoto et al., 2011; Pilli et al., 2012; Ichimura et al., 2013; Matsumoto et al., 2015). Consistent with this idea, we observed an increase in p62 puncta volume in ATG9A or ATG2A/B KO cell lines compared with ATG5 or ATG13 KO cells. The increase in p62 condensate size upon ATG9A/ATG2 deletion, without a change in p62 turnover rate compared with other ATG KOs, is intriguing. It suggests that the lipid transfer complex may play a role in regulating p62 condensate size. One possibility is that engagement of the lipid transfer complex at the condensate—a commitment step in autophagy initiation—inhibits condensate growth to ensure that condensate size does not exceed the capacity of the autophagosome (Agudo-Canalejo et al., 2021; Turco et al., 2021). Perhaps the ATG9A-ATG2 lipid transfer complex recruits a phosphatase to dephosphorylate p62 and inhibit its ability to recruit additional polyubiquitin into the condensate. Interestingly, the catalytic subunit of protein phosphatase-1 was identified in a proteomic analysis of ATG9A vesicles (Judith et al., 2019). Alternatively, individual autophagosomes could partially degrade condensates, eliminating them in a step-wise process.
Our live-cell imaging experiments suggest that although autophagy foci rapidly assemble and disassemble in the absence of nutrient stress, stable assembly of autophagy foci to form committed autophagosomes occurs at ubiquitin-rich condensates. These observations support the model that ubiquitinated protein condensates recruit ATG proteins to stimulate autophagosome formation (Fujioka and Noda, 2021; Turco et al., 2021). Our work also provides insight into the hierarchy of protein recruitment to the ubiquitin-rich condensates with ATG9A being upstream of ATG2 recruitment to the condensates. Perhaps most interestingly, the live-cell imaging suggests a model in which autophagy machinery “samples” the cellular environment until it senses the condensation of polyubiquitinated cargo, which then triggers autophagosome formation. It seems likely, based on this study and previous work (van Vliet et al., 2022; Broadbent et al., 2023; Olivas et al., 2023), that ATG9A recruitment to ubiquitin-rich condensates provides an early signal to recruit ATG2 and establish a lipid transfer complex between ER subdomains and the expanding ATG9A lipid compartment (van Vliet et al., 2022).
Finally, our data suggest the initiation of autophagy in nutrient replete cells occurs at ubiquitin-rich condensates, which provide a platform to recruit ATG9A and begin assembling the ATG9A-ATG2 lipid transfer complex to initiate autophagosome biogenesis. These data support a growing body of literature supporting the importance of these ubiquitin-rich structures in autophagy initiation (Wurzer et al., 2015; Zaffagnini et al., 2018; Turco et al., 2019; Vargas et al., 2019; Zachari et al., 2019; Kageyama et al., 2021; Mercer et al., 2021; Turco et al., 2021). Because polyubiquitin is a central feature of the diverse substrates of basal autophagy, it seems likely that ubiquitin itself, perhaps cooperatively with autophagy adapters like OPTN (Yamano et al., 2020), interacts directly with ATG9A or another component of the ATG9A vesicle. Our work supports this model but does not entirely rule out other possibilities, including a role for other core autophagy proteins facilitating ATG9A recruitment. Recent structural and molecular studies also demonstrated an interaction between the C-terminus of ATG9A and the ATG13:ATG101 dimer (Kannangara et al., 2021; Ren et al., 2023). ATG13, like FIP200, is dispensable for ATG9A recruitment to ubiquitin-rich condensates in fed conditions (Kannangara et al., 2021). Thus, the question of what mediates ATG9A recruitment/tethering to ubiquitin-rich condensates is still open and should, at least in part, be addressed by defining specific ATG9A domains (or vesicle components) that bind to polyubiquitin, autophagy adapters, or other constituents of the ubiquitin-rich condensate. Going forward, it will be important to precisely define ATG9A interactions with components of ubiquitin-rich condensates and more broadly understand the role of these condensates as a platform for recruitment of other autophagy factors.
MATERIALS AND METHODS
Request a protocol through Bio-protocol
Antibodies, chemicals, and reagents
| Antibodies | Source | Identifier |
|---|---|---|
| Anti-SQSTM1/p62 mouse | Abcam | ab56416 |
| Anti-LC3B rabbit | Cell Signaling Technology | 3868S |
| Anti-actin mouse | Santa Cruz Biotechnology | sc-8432 |
| Antibodies | Source | Identifier |
|---|---|---|
| Anti-β-actin rabbit | Cell Signaling Technology | 4970S |
| Anti-phospho-SQSTM1/p62 (Thr269/Ser272) rabbit | Cell Signaling Technology | 13121S |
| Anti-phospho-SQSTM1/p62 (Ser349) rabbit | Cell Signaling Technology | 16177S |
| Anti-phospho-SQSTM1/p62 (Ser403) rabbit | Cell Signaling Technology | 39786S |
| Anti-Tax1bp1 rabbit | Cell Signaling Technology | 5105S |
| Anti-NBR1 rabbit | Cell Signaling Technology | 48046 (Product discontinued) |
| Anti-NDP52 rabbit | Cell Signaling Technology | 48046 (Product discontinued) |
| Anti-OPTN rabbit | Cell Signaling Technology | 48046 (Product discontinued) |
| Anti-HA-Tag mouse | Cell Signaling Technology | 2367S |
| Anti-HA-Tag goat | Novus Biologicals | NB600-362 |
| NBR1 | Cell Signaling Technology | D2E6 |
| NDP52 | Cell Signaling Technology | D1E4A |
| Anti-Ubiquitin rabbit | Cell Signaling Technology | 3933S (Product discontinued) |
| Anti-rabbit AlexaFluor 488 goat | Abcam | ab150077 |
| Anti-rabbit AlexaFluor 633 goat | Thermo Fisher Scientific | A-21071 |
| Anti-mouse AlexaFluor 488 goat | Thermo Fisher Scientific | A-11001 |
| Anti-mouse AlexaFluor 633 goat | Invitrogen | A-21126 |
| Anti-rabbit AlexaFluor 488 donkey | Thermo Fisher Scientific | A-21206 |
| Anti-mouse AlexaFluor 568 donkey | Thermo Fisher Scientific | A10037 |
| Anti-goat AlexaFluor 633 donkey | Thermo Fisher Scientific | A-21082 |
| Cells | Source | Identifier |
|---|---|---|
| HEK293T | ATCC | |
| HA-ATG9A KI HCT116 | Parent line from ATCC | - |
| U2OS, | ATCC HTB-96 | |
| LentiX-293T | ATCC |
| Chemicals or Reagents | Source | Identifier |
|---|---|---|
| Penicillin-Streptomycin | Life Technologies | 15140122 |
| DMEM | Life Technologies | 11965-092 |
| RPMI | Life Technologies | 11875093 |
| FBS | Genesee Scientific | 25-514 |
| PBS (1X) | Corning | 21-040-CV |
| PEI-MAX | Polysciences | 24765-1 |
| Polybrene | Sigma-Aldrich | TR-1003-G |
| LI-COR Intercept | LI-COR Biosciences | 927-70001 |
| Phosphoblocker Blocking Reagent | Cell Biolabs | AKR-103 |
| Puromycin Dihydrochloride | Thermo Fisher Scientific | A1113802 |
| Wortmannin | Cell Signaling Technology | 9951S |
| DMSO | Thermo Fisher Scientific | D136-1 |
| JFX650(HaloTag) | Grimm et al. 2021 | N/A |
| JF503(SNAP Tag) | Grimm et al. 2015 | N/A |
| 7-bromo heptonal (HaloTag Blocker) | Thermo Fisher Scientific | H54762 |
| AP20187 | APExBIO | B1274 |
| (TAK-243) | Cayman Chemical | 30108 |
| Paraformaldehyde | Sigma-Aldrich | 158127-100 |
| Triton X-100 | Thermo Fisher Scientific | BP151-500 |
| Tween-20 | Thermo Fisher Scientific | BP337-100 |
| SEA BLOCK Blocking Buffer | Thermo Fisher Scientific | 37527 |
| DAPI | Cayman Chemical Company | 14285 |
| Prolong Diamond Antifade mounting reagent | Thermo Fisher Scientific | P36961 |
Plasmids
| Plasmid | Source | Identifier |
|---|---|---|
| pLenti-puro | Addgene | Plasmid #39481 |
| psPAX2 | Addgene | Plasmid #12260 |
| pMD2.G | Addgene | Plasmid #12259 |
| PX459 | Addgene | Plasmid #62988 |
| pBA-mCherry-EGFP-PIM | Addgene | Plasmid #111758 |
| AAVS1-Snap-P62-HRD | ||
| AAVS1-eGFP-LC3B-HRD | ||
| AAVS1 sgRNA |
Primers
| Primer | Sequence (5′-3′) |
|---|---|
| sgRNA ATG13 #1 | 5′-GGACAGCTGCCTGCAGTCGGG-3′ |
| sgRNA ATG13 #2 | 5′-ACACGGTGTACAACAGACTG-3′ |
| sgRNA ATG9A | 5′-CTGTTGGTGCACGTCGCCGAG-3′ |
| sgRNA FIP200 | 5′-CAGGTGCATCTAGAAGACCC-3′ |
| sgRNA ATG5 | 5′-AAGAGTAAGTTATTTGACGT-3′ |
| sgRNA ATG7 | 5′-GAAGCTGAACGAGTATCGGC-3′ |
| sgRNA p62/SQSTM1 | 5′-CGCTACACAAGTCGTAGTCT-3′ |
Cell culture, transfection, and viral transduction
HEK293T cells, HCT116 cells, and their derivatives were cultured in DMEM, supplemented with 10% FBS and Penicillin-Streptomycin (Pen-Strep) at 37°C in a 5% CO2 incubator. U2OS cells were cultured in RPMI media supplemented with FBS and Pen-Strep at 37°C in a 5% CO2. HEK293T and HCT116 cell were transiently transfected using polyethylenimine MAX (PEI-MAX) according to the manufacturer's protocols. U2OS cells were transiently transfected with Lipofectamine 3000 or LONZA according to manufacturer's protocols.
Lentivirus was generated using pLenti-puro (7 µg), psPAX2 (5.25 µg), and pMD2.G (1.75 µg) plasmids that were transfected in LentiX-293T cells within 15 cm plates with PEI-MAX at a 4:1 (µg: µg) ratio with cDNA. Virus was produced for 48 h in the media and collected, centrifuged, and filtered through a sterile 0.45-µm filter (Catalogue No. SE1M003M00). The virus containing medium was then supplemented with 10 µg/ml polybrene and allowed to incubate with the targeted cells for 24 h at 37°C in a 5% CO2 incubator. The cells were washed and split for either chemical selection with puromycin or cell sorting.
Generation of CRISPR-Cas9 KO and knock-in cell lines
To identify appropriate and effective single guide RNAs (sgRNAs), we utilized the CRISPR tool made by Concordet and Haeussler (http://crispor.tefor.net/) (Concordet and Haeussler, 2018). Using genomic exon DNA from the target proteins, we selected the highest scoring sgRNAs calculated by CRISPOR and ordered primers from Eton Bioscience, Inc. Using the cloning protocol defined by Zhang et al. (Cong et al., 2013), we cloned the primers into px330, px459, or px458. We then followed Lipofectamine 3000 or PEI-MAX transfection protocols and either chemically selected or cell sorted to enrich for CRISPR/Cas9-positive cells. After validating the efficiency of the sgRNAs in a mixed population of cells, we selected for single-cell clones through serial dilution or single-cell sorting into 96-well plates. The grown colonies were then tested by Western blot to check for lack of protein expression. U2OS cells stably expressing HaloTag at their respective ATG loci are previously described and published (Broadbent et al., 2023). U2OS cells expressing Halo-ATG2A and an ATG9KO are also published previously (Broadbent et al., 2023). U2OS cells expressing Halo-ATG9A with ATG5KO, ATG2A/BKO, and ATG9KO are published previously (Perez et al., 2022).
Immunoblotting
To prepare whole-cell protein lysates, cells were washed twice and harvested with ice-cold PBS. Cell pellets were resuspended in RIPA lysis buffer (25 mM Tris-HCl [ph 7.5], 75 mM NaCl, 0.5% [wt/vol] Triton X-100, 2.5 mM EDTA, 0.05% [wt/vol] SDS, and 0.25% [wt/vol] Deoxycholate) supplemented with protease and phosphatase inhibitors and incubated for 15 min at 4°C with gentle rotation. The lysed cells were then centrifuged at 21,000 × g for 10 min at 4°C and the supernatant collected. A protein assay is then performed to calculate then approximate protein concentration. Whenever a Western blot was performed and whole cell lysates analyzed, 50 µg of protein was loaded into each well of the 4–5% Criterion TGX Precast Midi Protein Gel. After SDS–PAGE, the separated proteins were transferred onto a nitrocellulose membrane using an iBlot 2. Membranes were allowed to dry completely and then soaked in PBS for 15–30 min and subsequently blocked with either LI-COR Intercept blocking buffer or Phosphoblocker for 1 h at room temperature on a roller. Antibodies were diluted in blocking buffer according to the manufacturer's specifications and allowed to incubate with the membrane in 4°C on a roller overnight. The membranes were washed once, 5 min each, with 0.1% Tween-20/PBS and twice with PBS and then LI-COR secondaries diluted 1:20,000 in PBS were incubated with the cells for 1 h at room temperature on a roller. The membranes were washed again and imaged on the LI-COR Odyssey imaging system. Western blotting quantifications were calculated on Image Studio and statistical analyses were performed in GraphPad Prism 9. For U2OS protein lysates, cells were lysed in 300 µl 1X sample buffer (Bio-Rad) and boiled for 5 min at 95°C and loaded onto an or 4–20% TGX stain-free polyacrylamide gels (Bio-Rad), followed by standard Western blotting procedures. JFX650 was measured on ChemiDoc imaging system (Bio-Rad) using the Cy5 filter set following by the stain-free loading control. Gels were transferred using the Trans-Blot Turbo system (with Turbo transfer buffer, Bio-Rad) and blocked for 30 min in 5% milk. Primary antibody was left overnight at 1:1000-1:3000 concentration. The blot was washed three times in 0.05% PBST and horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary was added at 1:2000 for 1 h (Invitrogen, 31430, 1:2000). U2OS cell Western blot quantification was carried out using the ImageQuant software (Cytiva).
Deuterium exchange
HEK293T WT and HEK293T ATG9A KO cells were seeded into 15 cm plates at ∼15% confluency and allowed to settle and grow overnight. A total of 15 plates per cell line were seeded, totaling 30 plates. The next day, DMEM/10% FBS cell culture medium with or without supplemented D2O (final D2O percentage at 5% to total volume) were added to the cells and allowed to incubate for 4, 12, 24, or 36 h at 37°C. The cells containing 5% D2O cell culture medium were placed in a 5% CO2 incubator containing a 5% D2O water bath. The control cells (incubated in growth medium that did not contain added D2O) were incubated with a water bath containing no extra D2O. At each indicated timepoint, the cells were washed twice with ice-cold PBS and collected in ice cold PBS via cell scraper into a previously weighed 15 ml conical tube. Pellets were centrifuged, supernatant aspirated, and centrifuged again to pull down excess supernatant. After aspiration of the excess supernatant, the tubes were weighed, and pellet size calculated. Cell lysates were generated by RIPA detergent lysis (milligram pellet: µL RIPA lysis buffer at a 1:10 ratio) and protein concentrations calculated for each sample. The proteins were then prepared for mass spectrometry (MS) as described below.
Quantitative LC-MS/MS
After protein concentrations for each sample were calculated, 50 µg of protein from each sample was diluted in 6 M Guanidine/HCl and incubated for 5 min in 95°C. The samples were cooled and then placed in a VWR Centrifugal Filter with a 30 kD molecular weight cutoff (Catalogue No. 82031-534) and centrifuged at 15,000 × g for 15 min. The flow through was discarded and more 6 M Guanidine-HCl was added and washed through the filter by centrifugation at the same speed. A total of 100 µl of 6 M Guanidine/HCl containing 9.2 mM of DTT was added to the filter, vortexed briefly, and incubated at 60°C for 1 h. After the samples were allowed to cool, iodoacetamide was added for a final concentration of ∼20 mM. The samples were protected from light and allowed to incubate at 25°C for 1 h. The samples were then washed with 25 mM ammonium bicarbonate (ABC) three times, with centrifugation at 15,000 × g for 15 min in between and the flow through discarded. The collection tubes were then washed with high performance liquid chromatography (HPLC) grade water three times. Mass spec grade Trypsin was diluted to 1 µg Trypsin:100 µl of 25 mM ABC (1 µg of Trypsin per 50 µg of protein) and added to each sample. The samples were mixed through mild vortexing, spun down briefly to pool the sample on top of the filter, and allowed to incubate on a shaker at 37°C for 18 h. The samples were then centrifuged for 30 min at 15,000 × g, with one more wash through of 25 mM ABC. The filtrant was collected and transferred to vials to be dried by speed-vacuum.
Mass spectrometry data were collected using an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) coupled to an EASY-nLC 1200 liquid chromatography (LC) pump (Thermo Fisher Scientific, Waltham, MA, USA). A capillary reverse-phase column (EASY-spray column pepMap RSLC, C18, 2 µm, 100 Å, 75 µm × 15 cm) was used for separation of peptides. The mobile phase was comprised of buffer A (0.1% formic acid in optima water) and buffer B (optima water and 0.1% formic acid in 80% acetonitrile). The peptides were eluted at 300 nl/min with the following gradients over 2 h: 3–25% B for 80 min; 25–35% B for 20 min; 35–45% B for 8 min; 45–85% B for 2 min and 85% for 8 min. Data were acquired using the top speed method (3 s cycle). A full scan MS at resolution of 120,000 at 200 m/z mass was acquired in the Orbitrap with a target value of 4e5 and a maximum injection time of 60 ms. Peptides with charge states of 2–4 were selected from the top abundant peaks by the quadrupole for high energy collisional dissociation (HCD with normalized energy 29) MS/MS, and the fragment ions were detected in the linear ion trap with target AGC value of 1e4 and a maximum injection time of 250 ms. The dynamic exclusion time was set at 40 s. Precursor ions with ambiguous charge states were not fragmented.
Kinetics data acquisitions were performed in MS-only mode and collected at 60,000 m/z resolution. These settings increase signal intensity, improve signal-to-noise, and give more scan points per elution chromatogram, greatly enhancing kinetic analysis accuracy (Naylor et al., 2017).
Peptide identification and protein label-free quantitation
PEAKS Studio software (version X) was used for de novo sequencing, and database searching to identify proteins in our raw MS data as well as to quantify, filter (quality-control), and normalize our quantitation data for each protein (Zhang et al., 2012). Peptides were identified from MS/MS spectra by searching against the Swiss-Prot human (2020) with a reverse sequence decoy database concatenated. Variables for the search were as follows: enzyme was set as trypsin with one missed cleavage site. Carbamidomethylation of cysteine was set as a fixed modification while N-terminal acetylation and methionine oxidation were set as variable modifications. A false positive rate of 0.01 was required for peptides and proteins. Minimum length of peptide was set to seven amino acids. At least two peptides were required for protein identification. The precursor mass error of 20 ppm was set for the precursor mass, and the mass error was set as 0.3 Da for the MS/MS. Label-free quantitation was enabled with MS1 tolerance ±20 ppm and a MS2 tolerance ±50 ppm, carbamidomethylation of cysteine was set as a fixed modification, while N-terminal acetylation and methionine oxidation were set as variable modifications. Peptide assignments with a false discovery rate less than 1% were included in comparative quantitative analyses and used to generate protein identification files for the quantitative and kinetic analyses. Protein quantitation was performed using the “Label-Free Quantitation” (LFQ) module in the PEAKS software package.
Kinetic protoemics analysis
Protein turnover rates were calculated using the DeuteRater software tool (Naylor et al., 2017). Briefly, data from the mass spectrometer were converted into the .mzML file format using the MSconvet tool (Kessner et al., 2008). Peak picking was used as the first filtering option, no other options were used. An ID file for DeuteRater to use was created using the output files of the PEAKS X Pro (Zhang et al., 2012) peptide identification software. The ID file, .mzML files, and the experimental data (time since the start of labeling for each sample and the final amount of deuterium present) were provided to DeuteRater. DeuteRater calculates a turnover rate based on a rise to plateau fit of the changes in isotopic envelopes as deuterium is incorporated. Default DeuteRater settings were used.
To identify which proteins to include for analysis, a custom scoring system was created. First, peptides associated with each protein needed to be identified in at least six of the 15 total samples (15 for WT and 15 for ATG9A KO), meaning that there needs to be a high enough abundance of peptides identified in both the WT samples and ATG9A KO samples. Second, peptides associated with each protein need to be found in at least four of the five timepoints for WT and ATG9A KO, including the no deuterium control (control, 4, 12, 24, and 36 h). Third, the R2 value calculated for the slope generated from the combined rate graphs by Deuterator (Figure 1D) must be above 0.5. Last, the calculated 95% confidence interval associated with a specific protein found in both the WT and ATG9A KO samples could not overlap. If a protein was found to follow these four criteria, then that protein was included in the analysis found in Figure 1B. All of the above calculations were performed in Microsoft Excel.
Immunofluorescence microscopy
Cells were seeded onto acid-etched coverslips and allowed to culture for 24 h before treatments and/or fixation. When the cells were ready for fixation, the cells were washed with ice-cold PBS three times and incubated with 4% paraformaldehyde/PBS for 10 min at 37°C while protected from light. The cells were subsequently washed with ice-cold PBS three times and then permeabilized with 0.1% Triton X-100/PBS for 10 min at room temperature on a gentle rocker while protected from light. The cells were then washed three times with ice-cold PBS and then blocked with SEA BLOCK Blocking Buffer for 1 h at room temperature on a gentle rocker while protected from light. The blocking buffer was then aspirated from the cells and fresh blocking buffer containing diluted primary antibodies (diluted according to the manufacturer's protocols) was added to the cells. The cells were placed in 4°C on a gentle rocker and allowed to incubate overnight while protected from light. The cells were then washed with ice-cold 0.1% Tween-20/PBS three times, 5 min for each wash, and PBS containing diluted secondaries (diluted according to the manufacturer's protocols) was added to the cells and incubated at room temperature for 1 h on a gentle rocker while protected from light. The cells were subsequently washed three times with ice-cold 0.1% Tween-20/PBS, 5 min for each wash, and either mounted to microscope slides with Prolong Diamond Antifade mounting reagent or incubated with DAPI (1.43 µM final concentration in PBS) for 5 min and then mounted to microscope slides. The slides are then cured overnight at room temperature while protected from light. Images were acquired on a LEICA TCS SP8 confocal microscope fitted with a HC PL APO 63x/1.40 Oil CS2 objective and a HyD detection system (Leica Microsystems).
Quantification of immunofluorescent microscopy
To increase reproducibility, each microscopy experiment was seeded, fixed, and stained on the same day with the same diluted antibodies in blocking buffer or PBS. Furthermore, the images collected had the same laser power and image resolution by set. Each analyzed image was processed using Huygens Essential express deconvolution tool on standard settings. Pearson's coefficient was calculated using colocalization analyzer tool found in Huygens Essential. Threshold intensity values were set at either 1 or 10% of the highest intensity value for each channel, depending on how high the background was. The puncta volume was calculated using the three-dimensional analysis software tool found in LAS-X. For experiments using the U2OS cell lines, the analysis was performed using ICY image analysis software. Statistical Object Distance Analysis (SODA) plugin was used to quantify the colocalization of AT9A foci with P62 foci. All experiments were done in triplicates and pooled into a single analysis (Lagache et al., 2018). The statistical calculations were performed in GraphPad Prism 9.
In-gel and immunoblot assessment of p62 degradation in ATG9A WT and M33 mutant
ATG9AKO edited U2OS cells were transfected using lipofectamine 3000 with 1 µg of a safe harbor AAVS1 locus homology recombination donor containing Halo-ATG9A WT or M33 mutant (K321L, K322L, K323L, T419W) cDNA and 1 µg of AAVS1 targeted sgRNA (Xi et al., 2015; Maeda et al., 2020). Cells were then selected for using puromycin at 1 µg/ml for 2 weeks and sorted on HaloTag florescence. Single-cell clones were grown and single-cell clones expressing similar amounts of ATG9A WT and M33 were chosen for further experiments. The construct knocked into the cells contains a tet-inducible promotor and this was utilized in our experiment. A total of 300,000 cells were plated into a 6-well plate with and without doxycycline at 2 µg/ml. The following day the cells were labeled with JFX650 at 100 nM for 15 min, washed, and lysed in 300 µl 1X sample buffer (Bio-Rad) and boiled for 5 min at 95°C and loaded onto an or 4–20% TGX stain-free polyacrylamide gels (Bio-Rad), followed by standard Western blotting procedures. JFX650 was measured on ChemiDoc imaging system (Bio-Rad) using the Cy5 filter set following by the stain-free loading control. Gels were transferred using the Trans-Blot Turbo system (with Turbo transfer buffer, Bio-Rad) and blocked for 30 min in 5% milk. Primary antibody was left overnight at 1:3000 concentration. The blot was washed three times in 0.05% PBST and HRP-conjugated goat anti-mouse secondary was added at 1:2000 for 1 h (Invitrogen, 31430, 1:2000). U2OS cell Western blot quantification was carried out using the ImageQuant software (Cytiva). Statistical differences were evaluated by two-tailed t test.
Live-cell microscopy
Two microscopes were used for live-cell microscopy in this manuscript. The first is an Olympus microscope (IX83), with a cellTIRF illuminator with four laser lines (100 mW 405 nm, 200 mW 488 nm, 300 mW 561 nm, and 140 mW 640 nm) and an X-Cite TURBO multi-wavelength LED illumination system (Excelitas Technologies). The microscope is equipped with an environmental chamber (cellVivo) to control humidity, temperature, and CO2 level, a 100x TIRF oil-immersion objective (Olympus UApo N, NA = 1.49) with compatible excitation and emission filters. Alternatively, we used an 3i spinning disk confocal microscope equipped with a CSU-W1 confocal spinning disk system (Yokogawa), a 63x/1.46 NA Alpha Plan-Apochromat TIRF objective, four laser lines (100 mW 405 nm, 150 mW 488 nm, 175 mW, 160 mW 561 nm, and 140 mW 638 nm), and an ORCA-Quest qCMOS camera.
Chase-pulse experiment with UAE inhibitor and analysis
Halo-ATG9A C1 cell line was transfected with the AAVS1 safe locus HRD containing SNAP-p62 alongside an sgRNA and selected with puromycin. After selection, 200,000 cells were grown on glass coverslips (170 ± 5 µm, Schott). Coverslips were cleaned with 1M KOH (1 h in a sonicated water bath), rinsed with ddH2O, cleaned with 100% ethanol (1 h in a sonicated water bath), and dried under N2 stream before assembly on a 35 mm diameter imaging dish using epoxy. The day after seeding the HaloTag was blocked using 7-bromo-heptanol at 10 µM for 10 min. The cells were rinsed twice with media and incubated in 2 ml media to deplete excess halo ligand for 5 min. Cells were then treated by adding 2 ml of media containing 100 nM JFX650 Halo ligand and 100 nM JF503 SNAP ligand. Drug treatment was added alongside the dye by adding DMSO or UAE inhibitor MLN7243 at a concentration of 10 µM for 9–10 h. Prior to imaging, we swapped the media for 5 min, maintaining the drug during the wash and then added fresh treated media for imaging. Live-cell imaging was performed on a Olympus TIRF microscope using the 640 nm laser in a Highly Inclined and Laminated Optical sheet (HILO) angle and the Andor iXon 897 Ultra camera was used for signal detection. Image analysis was performed in an unbiased manner using scripts from ImageJ and ICY. Images were first prepped for analysis using ImageJ. Frame 5 of each movie was extracted and a rolling background subtraction of 20 pixels was applied to the image. These images were then analyzed using custom built ICY protocol that identifies foci using a wavelet filter and Gaussian fitting spot detector to identify ROIs and the integrated intensity was quantified across all three replicates. Statistical differences were evaluated by two-tailed t test.
Autophagosome foci kinetics with UAE inhibitor
Halo-ATG13 cells were stably transfected with GFP-LC3. A total of 250,000 cells were then plated on 35 mm dish with 1.5H glass (Cellvis, product #: D35-20-1.5H) and left overnight. The following day, cells were labeled with JFX650 at 100 nM for 15 min and washed for 5 min in media. Cells were then treated with and without UAE inhibitor MLN7243 at 10 µM for 2 h. Live-cell imaging was performed in standard conditions of 5% CO2 and 37°C on the 3i spinning disk confocal microscope Images were acquired at 1 frame every second for 8 min. For image analysis, images were scaled down from 1636 × 1152 to 727 × 512 using the ImageJ “size” function with the recommended settings of a constrained aspect ratio and bilinear downsampling with averaging. The images were then analyzed using a previously established pipeline called K-FOCUS (Barnaba et al., 2023). Briefly, the K-FOCUS pipeline uses cellpose to segment cells and TrackIT to identify single particles and assemble them into tracks (Kuhn et al., 2021; Stringer et al., 2021). K-FOCUS then segregates the tracks into colocalized or noncolocalized fractions. Colocalized tracks are required to be within 3 pixels of each other for at least 10 frames. Statistical differences were evaluated by two-tailed t test.
Live-cell imaging of Halo-ATG2 and 6X-ubiquitin-BFP
Endogenously edited Halo-ATG2A cells expressing WT ATG9A and KO were transfected with HA-6x-ubiquitin-eBFP2 by LONZA electroporation using standard settings for U2OS cells and a homemade buffer of RPMI supplemented with 50 mM bicarbonate. Importantly, ATG9A KO is more prone to forming large aggregates, likely due to having basal nucleation of p62, so any cells that had excessive aggregation were not imaged, keeping the two conditions more similar. Live-cell imaging was performed in standard conditions of 5% CO2 and 37°C on the 3i spinning disk confocal microscope. Images were acquired at 1 frame every second for 8 min. For image analysis, images were scaled down from 1636 × 1152 to 727 × 512 using the ImageJ “size” function with the recommended settings of a constrained aspect ratio and bilinear downsampling with averaging. The images were then analyzed using a previously established pipeline called K-FOCUS (Barnaba et al., 2023). Briefly, the K-FOCUS pipeline uses cellpose to segment cells and TrackIT to identify single particles and assemble them into tracks (Kuhn et al., 2021; Stringer et al., 2021). K-FOCUS then segregates the tracks into colocalized or noncolocalized fractions. Colocalized tracks are required to be within 3 pixels of each other for at least 10 frames. Statistical differences were evaluated by two-tailed t test.
OPTN SE mutant with Halo-ATG9A KR mutant addback
ATG9A KO edited U2OS cells were transfected using lipofectamine 3000 with 1 µg of a safe harbor AAVS1 locus homology recombination donor containing WT ATG9A or KR mutations (K851R and K838R) cDNA and 1 µg of AAVS1 targeted sgRNA. Cells were selected using puromycin at 1 µg/ml for 2 weeks. For the experiment, the selected cells were transfected with lipofectamine 3000 with 1 µg/ml 6x-ubiquitin-BFP and 1 µg/ml OPTN SE. Images were acquired on the Olympus microscope at 1 frame per second over 30 s.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Fritz B. Burns Foundation for generous student and instrument support. We acknowledge the Utah HCS Cell Imaging Core Facility and Anton Classen for assistance with confocoal imaging. We thank the Simmons Center for Cancer Research for student fellowships to C.M.M. and D.M.P. J.L.A. is supported by a National Institutes of Health R01 from NIGMS (NIH R01 GM147310-01) and an American Cancer Society Research Scholar Grant (133550-RSG-19-006-01-CCG). J.C.S. is supported by National Institute for General Medical Sciences (DP2 GM 142307). B.C.N. and J.C.P. are supported by National Institutes of Health R01 from NIA (R01AG066874).
Abbreviations used:
- ATG
Autophagy-related gene
- ULK1
Unc-51-like autophagy-activating kinase
- TAX1BP1
Tax1-binding protein-1
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E24-03-0101) on December 24, 2024.
REFERENCES
- Adriaenssens E, Ferrari L, Martens S (2022). Orchestration of selective autophagy by cargo receptors. Curr Biol 32, R1357–R1371. [DOI] [PubMed] [Google Scholar]
- Agudo-Canalejo J, Schultz SW, Chino H, Migliano SM, Saito C, Koyama-Honda I, Stenmark H, Brech A, May AI, Mizushima N, Knorr RL (2021). Wetting regulates autophagy of phase-separated compartments and the cytosol. Nature 591, 142–146. [DOI] [PubMed] [Google Scholar]
- Barnaba C, Broadbent DG, Kaminsky EG, Perez GI, Schmidt JC (2024). AMPK regulates phagophore-to-autophagosome maturation. J Cell Biol 223, e202309145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnaba C, Broadbent DG, Perez GI, Schmidt JC (2023). AMPK regulates phagophore-to-autophagosome maturation. bioRxiv 2023.2009.2028.559981. [DOI] [PMC free article] [PubMed]
- Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T (2005). p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171, 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent DG, Barnaba C, Perez GI, Schmidt JC (2023). Quantitative analysis of autophagy reveals the role of ATG9 and ATG2 in autophagosome formation. J Cell Biol 222, e202210078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffa R, Lamark T, Tarafder AK, Guesdon A, Rybina S, Hagen WJ, Johansen T, Sachse C (2015). The selective autophagy receptor p62 forms a flexible filamentous helical scaffold. Cell Rep 11, 748–758. [DOI] [PubMed] [Google Scholar]
- Concordet JP, Haeussler M (2018). CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res 46, W242–W245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli A, Vucak G, Baccarini M, Martens S (2023). Sequestration of translation initiation factors in p62 condensates. Cell Rep 42, 113583. [DOI] [PubMed] [Google Scholar]
- Debnath J, Gammoh N, Ryan KM (2023). Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol 24, 560–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus R, Moretti F, McAllister G, Wang Z, Bergman P, Liu S, Frias E, Alford J, Reece-Hoyes JS, Lindeman A, et al. (2016). Functional CRISPR screening identifies the ufmylation pathway as a regulator of SQSTM1/p62. Elife 5, e17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y, Noda NN (2021). Biomolecular condensates in autophagy regulation. Curr Opin Cell Biol 69, 23–29. [DOI] [PubMed] [Google Scholar]
- Ghanbarpour A, Valverde DP, Melia TJ, Reinisch KM (2021). A model for a partnership of lipid transfer proteins and scramblases in membrane expansion and organelle biogenesis. Proc Natl Acad Sci U S A 118, e2101562118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et al. (2013). Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell 51, 618–631. [DOI] [PubMed] [Google Scholar]
- Imai K, Hao F, Fujita N, Tsuji Y, Oe Y, Araki Y, Hamasaki M, Noda T, Yoshimori T (2016). Atg9A trafficking through the recycling endosomes is required for autophagosome formation. J Cell Sci 129, 3781–3791. [DOI] [PubMed] [Google Scholar]
- Itakura E, Kishi-Itakura C, Koyama-Honda I, Mizushima N (2012). Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J Cell Sci 125, 1488–1499. [DOI] [PubMed] [Google Scholar]
- Judith D, Jefferies HBJ, Boeing S, Frith D, Snijders AP, Tooze SA (2019). ATG9A shapes the forming autophagosome through Arfaptin 2 and phosphatidylinositol 4-kinase IIIbeta. J Cell Biol 218, 1634–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama S, Gudmundsson SR, Sou YS, Ichimura Y, Tamura N, Kazuno S, Ueno T, Miura Y, Noshiro D, Abe M, et al. (2021). p62/SQSTM1-droplet serves as a platform for autophagosome formation and anti-oxidative stress response. Nat Commun 12, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara AR, Poole DM, McEwan CM, Youngs JC, Weerasekara VK, Thornock AM, Lazaro MT, Balasooriya ER, Oh LM, Soderblom EJ, et al. (2021). BioID reveals an ATG9A interaction with ATG13-ATG101 in the degradation of p62/SQSTM1-ubiquitin clusters. EMBO Rep 22, e51136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanasios E, Walker SA, Okkenhaug H, Manifava M, Hummel E, Zimmermann H, Ahmed Q, Domart MC, Collinson L, Ktistakis NT (2016). Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles. Nat Commun 7, 12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessner D, Chambers M, Burke R, Agus D, Mallick P (2008). ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics 24, 2534–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn T, Hettich J, Davtyan R, Gebhardt JCM (2021). Single molecule tracking and analysis framework including theory-predicted parameter settings. Sci Rep 11, 9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu R, Fujimoto Y, Morishita H, Noshiro D, Takada S, Yamano K, Tanaka H, Arai R, Kageyama S, Funakoshi T, et al. (2023). Integrated proteomics identifies p62-dependent selective autophagy of the supramolecular vault complex. Dev Cell 58, 1189–1205.e11. [DOI] [PubMed] [Google Scholar]
- Lagache T, Grassart A, Dallongeville S, Faklaris O, Sauvonnet N, Dufour A, Danglot L, Olivo-Marin JC (2018). Mapping molecular assemblies with fluorescence microscopy and object-based spatial statistics. Nat Commun 9, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Yamamoto H, Kinch LN, Garza CM, Takahashi S, Otomo C, Grishin NV, Forli S, Mizushima N, Otomo T (2020). Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Nat Struct Mol Biol 27, 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M, Griffith J, Rieter E, Krishnappa L, Klionsky DJ, Reggiori F (2010). An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol 190, 1005–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis AD, Naylor BC, Carson RH, Evans E, Harwell J, Knecht J, Hexem E, Peelor FF, 3rd, Miller BF, Hamilton KL, et al. (2017). Mechanisms of in vivo ribosome maintenance change in response to nutrient signals. Mol Cell Proteomics 16, 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba K, Kotani T, Tsutsumi A, Tsuji T, Mori T, Noshiro D, Sugita Y, Nomura N, Iwata S, Ohsumi Y, et al. (2020). Atg9 is a lipid scramblase that mediates autophagosomal membrane expansion. Nat Struct Mol Biol 27, 1185–1193. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Shimogori T, Hattori N, Nukina N (2015). TBK1 controls autophagosomal engulfment of polyubiquitinated mitochondria through p62/SQSTM1 phosphorylation. Hum Mol Genet 24, 4429–4442. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Wada K, Okuno M, Kurosawa M, Nukina N (2011). Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol Cell 44, 279–289. [DOI] [PubMed] [Google Scholar]
- Mercer TJ, Ohashi Y, Boeing S, Jefferies HBJ, De Tito S, Flynn H, Tremel S, Zhang W, Wirth M, Frith D, et al. (2021). Phosphoproteomic identification of ULK substrates reveals VPS15-dependent ULK/VPS34 interplay in the regulation of autophagy. EMBO J 40, e105985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B (2020). Autophagy in human diseases. N Engl J Med 383, 1564–1576. [DOI] [PubMed] [Google Scholar]
- Musiwaro P, Smith M, Manifava M, Walker SA, Ktistakis NT (2013). Characteristics and requirements of basal autophagy in HEK 293 cells. Autophagy 9, 1407–1417. [DOI] [PubMed] [Google Scholar]
- Naylor BC, Porter MT, Wilson E, Herring A, Lofthouse S, Hannemann A, Piccolo SR, Rockwood AL, Price JC (2017). DeuteRater: A tool for quantifying peptide isotope precision and kinetic proteomics. Bioinformatics 33, 1514–1520. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Tamura N, Kono N, Shimanaka Y, Arai H, Yamamoto H, Mizushima N (2017). Autophagosome formation is initiated at phosphatidylinositol synthase-enriched ER subdomains. EMBO J 36, 1719–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivas TJ, Wu Y, Yu S, Luan L, Choi P, Guinn ED, Nag S, De Camilli PV, Gupta K, Melia TJ (2023). ATG9 vesicles comprise the seed membrane of mammalian autophagosomes. J Cell Biol 222, e202208088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi A, Razi M, Dooley HC, Robinson D, Weston AE, Collinson LM, Tooze SA (2012). Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Mol Biol Cell 23, 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007). p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282, 24131–24145. [DOI] [PubMed] [Google Scholar]
- Perez GI, Broadbent D, Zarea AA, Dolgikh B, Bernard MP, Withrow A, McGill A, Toomajian V, Thampy LK, Harkema J, et al. (2022). In vitro and in vivo analysis of extracellular vesicle-mediated metastasis using a bright, red-shifted bioluminescent reporter protein. Adv Genet (Hoboken) 3, 2100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilli M, Arko-Mensah J, Ponpuak M, Roberts E, Master S, Mandell MA, Dupont N, Ornatowski W, Jiang S, Bradfute SB, et al. (2012). TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 37, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenhill BJ, Boyle KB, von Muhlinen N, Ellison CJ, Masson GR, Otten EG, Foeglein A, Williams R, Randow F (2019). The cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. Mol Cell 74, 320–329.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Nguyen TN, Lam WK, Buffalo CZ, Lazarou M, Yokom AL, Hurley JH (2023). Structural basis for ATG9A recruitment to the ULK1 complex in mitophagy initiation. Sci Adv 9, eadg2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa-Makarska J, Baumann V, Coudevylle N, von Bulow S, Nogellova V, Abert C, Schuschnig M, Graef M, Hummer G, Martens S (2020). Reconstitution of autophagosome nucleation defines Atg9 vesicles as seeds for membrane formation. Science 369, eaaz7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker CJ, Huang TQ, Weir NR, Polyakov NJ, Schultz SW, Denic V (2019). CRISPR screening using an expanded toolkit of autophagy reporters identifies TMEM41B as a novel autophagy factor. PLoS Biol 17, e2007044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C, Wang T, Michaelos M, Pachitariu M (2021). Cellpose: a generalist algorithm for cellular segmentation. Nat Methods 18, 100–106. [DOI] [PubMed] [Google Scholar]
- Sun D, Wu R, Zheng J, Li P, Yu L (2018). Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res 28, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco E, Savova A, Gere F, Ferrari L, Romanov J, Schuschnig M, Martens S (2021). Reconstitution defines the roles of p62, NBR1 and TAX1BP1 in ubiquitin condensate formation and autophagy initiation. Nat Commun 12, 5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco E, Witt M, Abert C, Bock-Bierbaum T, Su MY, Trapannone R, Sztacho M, Danieli A, Shi X, Zaffagnini G, et al. (2019). FIP200 claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Mol Cell 74, 330–346.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet AR, Chiduza GN, Maslen SL, Pye VE, Joshi D, De Tito S, Jefferies HBJ, Christodoulou E, Roustan C, Punch E, et al. (2022). ATG9A and ATG2A form a heteromeric complex essential for autophagosome formation. Mol Cell 82, 4324–4339.e8. [DOI] [PubMed] [Google Scholar]
- Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T (2023). The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol 24, 167–185. [DOI] [PubMed] [Google Scholar]
- Vargas JNS, Wang C, Bunker E, Hao L, Maric D, Schiavo G, Randow F, Youle RJ (2019). Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Mol Cell 74, 347–362.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Liu TY, Shen CH, Lin SY, Hung CC, Hsu LC, Chen GC (2022). K48/K63-linked polyubiquitination of ATG9A by TRAF6 E3 ligase regulates oxidative stress-induced autophagy. Cell Rep 38, 110354. [DOI] [PubMed] [Google Scholar]
- Wurzer B, Zaffagnini G, Fracchiolla D, Turco E, Abert C, Romanov J, Martens S (2015). Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. Elife 4, e08941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi L, Schmidt JC, Zaug AJ, Ascarrunz DR, Cech TR (2015). A novel two-step genome editing strategy with CRISPR-Cas9 provides new insights into telomerase action and TERT gene expression. Genome Biol 16, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, Kondo-Kakuta C, Ichikawa R, Kinjo M, Ohsumi Y (2012). Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol 198, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano K, Kikuchi R, Kojima W, Hayashida R, Koyano F, Kawawaki J, Shoda T, Demizu Y, Naito M, Tanaka K, Matsuda N (2020). Critical role of mitochondrial ubiquitination and the OPTN-ATG9A axis in mitophagy. J Cell Biol 219, e201912144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AR, Chan EY, Hu XW, Kochl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, Tooze SA (2006). Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci 119, 3888–3900. [DOI] [PubMed] [Google Scholar]
- Zachari M, Gudmundsson SR, Li Z, Manifava M, Shah R, Smith M, Stronge J, Karanasios E, Piunti C, Kishi-Itakura C, et al. (2019). Selective autophagy of mitochondria on a ubiquitin-endoplasmic-reticulum platform. Dev Cell 50, 627–643.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffagnini G, Savova A, Danieli A, Romanov J, Tremel S, Ebner M, Peterbauer T, Sztacho M, Trapannone R, Tarafder AK, et al. (2018). p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J 37, e98308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner S, Schifferer M, Behrends C (2021). Systematically defining selective autophagy receptor-specific cargo using autophagosome content profiling. Mol Cell 81, 1337–1354.e8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xin L, Shan B, Chen W, Xie M, Yuen D, Zhang W, Zhang Z, Lajoie GA, Ma B (2012). PEAKS DB: de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol Cell Proteomics 11, M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]