Abstract
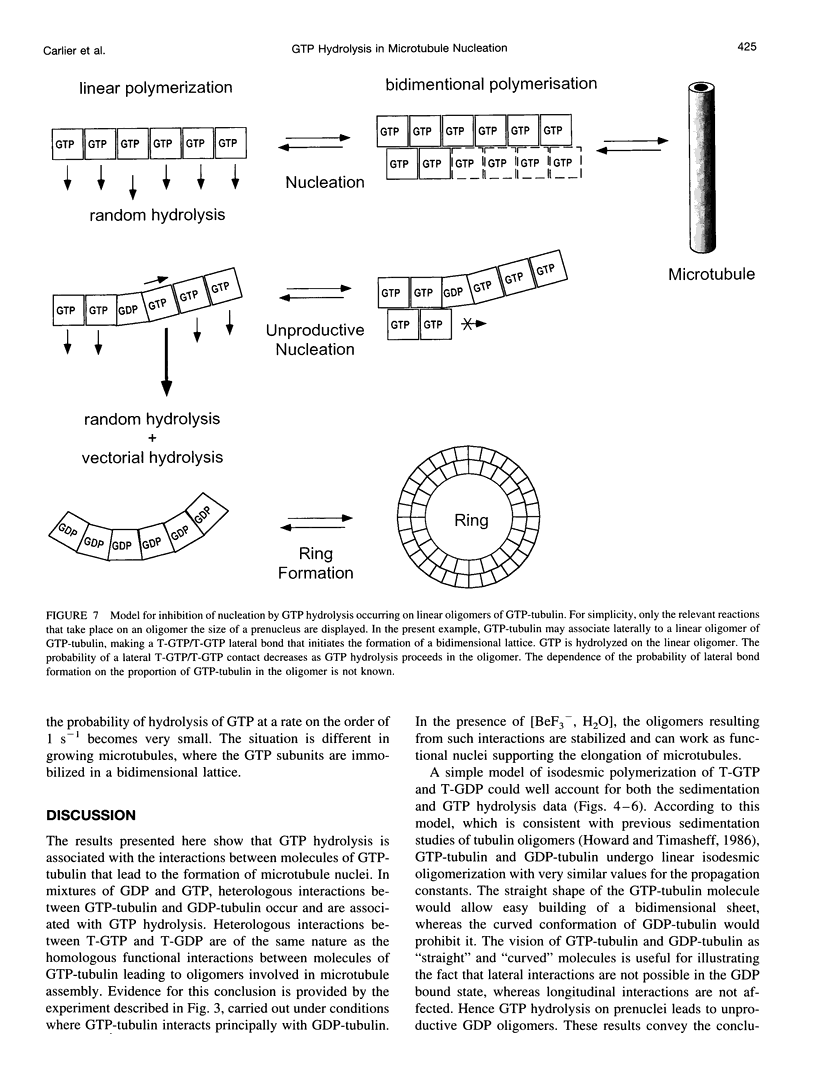
Hydrolysis of GTP is known to accompany microtubule assembly. Here we show that hydrolysis of GTP is also associated with the formation of linear oligomers of tubulin, which are precursors (prenuclei) in microtubule assembly. The hydrolysis of GTP on these linear oligomers inhibits the lateral association of GTP-tubulin that leads to the formation of a bidimensional lattice. Therefore GTP hydrolysis interferes with the nucleation of microtubules. Linear oligomers are also formed in mixtures of GTP-tubulin and GDP-tubulin. The hydrolysis of GTP associated with heterologous interactions between GTP-tubulin and GDP-tubulin in the cooligomer takes place at a threefold faster rate than upon homologous interactions between GTP-tubulins. The implication of these results in a model of vectorial GTP hydrolysis in microtubule assembly is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley P. M., Schilstra M. J., Martin S. R. Microtubule dynamic instability: numerical simulation of microtubule transition properties using a Lateral Cap model. J Cell Sci. 1990 Jan;95(Pt 1):33–48. doi: 10.1242/jcs.95.1.33. [DOI] [PubMed] [Google Scholar]
- Bayley P., Schilstra M., Martin S. A lateral cap model of microtubule dynamic instability. FEBS Lett. 1989 Dec 18;259(1):181–184. doi: 10.1016/0014-5793(89)81523-6. [DOI] [PubMed] [Google Scholar]
- Berne B. J. Interpretation of the light scattering from long rods. J Mol Biol. 1974 Nov 15;89(4):755–758. doi: 10.1016/0022-2836(74)90049-7. [DOI] [PubMed] [Google Scholar]
- Burns R. G. Assembly of chick brain MAP2-tubulin microtubule protein. Characterization of the protein and the MAP2-dependent addition of tubulin dimers. Biochem J. 1991 Jul 1;277(Pt 1):231–238. doi: 10.1042/bj2770231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplow M., Shanks J., Brylawski B. P. Concerning the location of the GTP hydrolysis site on microtubules. Can J Biochem Cell Biol. 1985 Jun;63(6):422–429. doi: 10.1139/o85-061. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Didry D., Melki R., Chabre M., Pantaloni D. Stabilization of microtubules by inorganic phosphate and its structural analogues, the fluoride complexes of aluminum and beryllium. Biochemistry. 1988 May 17;27(10):3555–3559. doi: 10.1021/bi00410a005. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Didry D., Pantaloni D. Microtubule elongation and guanosine 5'-triphosphate hydrolysis. Role of guanine nucleotides in microtubule dynamics. Biochemistry. 1987 Jul 14;26(14):4428–4437. doi: 10.1021/bi00388a036. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Didry D., Simon C., Pantaloni D. Mechanism of GTP hydrolysis in tubulin polymerization: characterization of the kinetic intermediate microtubule-GDP-Pi using phosphate analogues. Biochemistry. 1989 Feb 21;28(4):1783–1791. doi: 10.1021/bi00430a054. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Hill T. L., Chen Y. Interference of GTP hydrolysis in the mechanism of microtubule assembly: an experimental study. Proc Natl Acad Sci U S A. 1984 Feb;81(3):771–775. doi: 10.1073/pnas.81.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F. Nucleotide hydrolysis in cytoskeletal assembly. Curr Opin Cell Biol. 1991 Feb;3(1):12–17. doi: 10.1016/0955-0674(91)90160-z. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Kinetic analysis of cooperativity in tubulin polymerization in the presence of guanosine di- or triphosphate nucleotides. Biochemistry. 1978 May 16;17(10):1908–1915. doi: 10.1021/bi00603a017. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Kinetic analysis of guanosine 5'-triphosphate hydrolysis associated with tubulin polymerization. Biochemistry. 1981 Mar 31;20(7):1918–1924. doi: 10.1021/bi00510a030. [DOI] [PubMed] [Google Scholar]
- Carlier M. F. Role of nucleotide hydrolysis in the dynamics of actin filaments and microtubules. Int Rev Cytol. 1989;115:139–170. doi: 10.1016/s0074-7696(08)60629-4. [DOI] [PubMed] [Google Scholar]
- Chen Y., Hill T. L. Theoretical treatment of microtubules disappearing in solution. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4127–4131. doi: 10.1073/pnas.82.12.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croom H. B., Correia J. J., Baty L. T., Williams R. C., Jr Release of exchangeably bound guanine nucleotides from tubulin in a magnesium-free buffer. Biochemistry. 1985 Jan 29;24(3):768–775. doi: 10.1021/bi00324a035. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty T., Erickson H. P., Pantaloni D. Guanosinetriphosphatase activity of tubulin associated with microtubule assembly. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5372–5376. doi: 10.1073/pnas.74.12.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel D. N., Kirschner M. W. The minimum GTP cap required to stabilize microtubules. Curr Biol. 1994 Dec 1;4(12):1053–1061. doi: 10.1016/s0960-9822(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Engelborghs Y., Van Houtte A. Temperature jump relaxation study of microtubule elongation in the presence of GTP/GDP mixtures. Biophys Chem. 1981 Oct;14(2):195–202. doi: 10.1016/0301-4622(81)85019-3. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Pantaloni D. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophys J. 1981 May;34(2):293–309. doi: 10.1016/S0006-3495(81)84850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. I. Stoichiometry. Biochemistry. 1975 Oct 21;14(21):4559–4566. doi: 10.1021/bi00692a001. [DOI] [PubMed] [Google Scholar]
- Gildersleeve R. F., Cross A. R., Cullen K. E., Fagen A. P., Williams R. C., Jr Microtubules grow and shorten at intrinsically variable rates. J Biol Chem. 1992 Apr 25;267(12):7995–8006. [PubMed] [Google Scholar]
- Hamel E., Lustbader J., Lin C. M. Deoxyguanosine nucleotide analogues: potent stimulators of microtubule nucleation with reduced affinity for the exchangeable nucleotide site of tubulin. Biochemistry. 1984 Oct 23;23(22):5314–5325. doi: 10.1021/bi00317a033. [DOI] [PubMed] [Google Scholar]
- Horio T., Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986 Jun 5;321(6070):605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Howard W. D., Timasheff S. N. GDP state of tubulin: stabilization of double rings. Biochemistry. 1986 Dec 16;25(25):8292–8300. doi: 10.1021/bi00373a025. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Chrétien D., Arnal I., Wade R. H. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(alpha,beta)-methylene-diphosphonate. J Cell Biol. 1995 Jan;128(1-2):117–125. doi: 10.1083/jcb.128.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchnir Fygenson D, Flyvbjerg H, Sneppen K, Libchaber A, Leibler S. Spontaneous nucleation of microtubules. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1995 May;51(5):5058–5063. doi: 10.1103/physreve.51.5058. [DOI] [PubMed] [Google Scholar]
- Margolis R. L. Role of GTP hydrolysis in microtubule treadmilling and assembly. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1586–1590. doi: 10.1073/pnas.78.3.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R., Carlier M. F., Pantaloni D. Direct evidence for GTP and GDP-Pi intermediates in microtubule assembly. Biochemistry. 1990 Sep 25;29(38):8921–8932. doi: 10.1021/bi00490a007. [DOI] [PubMed] [Google Scholar]
- Melki R., Carlier M. F., Pantaloni D., Timasheff S. N. Cold depolymerization of microtubules to double rings: geometric stabilization of assemblies. Biochemistry. 1989 Nov 14;28(23):9143–9152. doi: 10.1021/bi00449a028. [DOI] [PubMed] [Google Scholar]
- Melki R., Carlier M. F. Thermodynamics of tubulin polymerization into zinc sheets: assembly is not regulated by GTP hydrolysis. Biochemistry. 1993 Apr 6;32(13):3405–3413. doi: 10.1021/bi00064a026. [DOI] [PubMed] [Google Scholar]
- Melki R., Fievez S., Carlier M. F. Continuous monitoring of Pi release following nucleotide hydrolysis in actin or tubulin assembly using 2-amino-6-mercapto-7-methylpurine ribonucleoside and purine-nucleoside phosphorylase as an enzyme-linked assay. Biochemistry. 1996 Sep 17;35(37):12038–12045. doi: 10.1021/bi961325o. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- O'Brien E. T., Voter W. A., Erickson H. P. GTP hydrolysis during microtubule assembly. Biochemistry. 1987 Jun 30;26(13):4148–4156. doi: 10.1021/bi00387a061. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., MacDonald E., Jameson J. L., Cuatrecasas P. Role of nucleotides in tubulin polymerization: effect of guanylyl 5'-methylenediphosphonate. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4881–4885. doi: 10.1073/pnas.74.11.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voter W. A., Erickson H. P. The kinetics of microtubule assembly. Evidence for a two-stage nucleation mechanism. J Biol Chem. 1984 Aug 25;259(16):10430–10438. [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J., Dickinson P. J. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 1976 Sep 21;15(19):4248–4254. doi: 10.1021/bi00664a018. [DOI] [PubMed] [Google Scholar]
- Zeeberg B., Caplow M. An isoenergetic exchange mechanism which accounts for tubulin-GDP stabilization of microtubules. J Biol Chem. 1981 Dec 10;256(23):12051–12057. [PubMed] [Google Scholar]



