Abstract
The plasma membrane of the mature guinea pig sperm is segregated into at least four domains of different composition. Previous studies have shown that some proteins localized within these domains are free to diffuse laterally, suggesting that barriers to protein diffusion are responsible for maintaining the nonuniform distribution of at least some surface proteins in mature sperm. The different membrane domains appear sequentially during sperm morphogenesis in the testis and during later passage through the epididymis. To determine when diffusion barriers become functional during sperm development, we examined the diffusion of two proteins that are expressed on the cell surface of developing spermatids and become segregated to different plasma membrane domains during the course of spermiogenesis. Both proteins exhibited rapid lateral diffusion throughout spermiogenesis, even after they become localized to specific regions of the surface membrane. These results suggest that barriers to membrane diffusion form concomitantly with membrane domains during spermiogenesis.
Full text
PDF
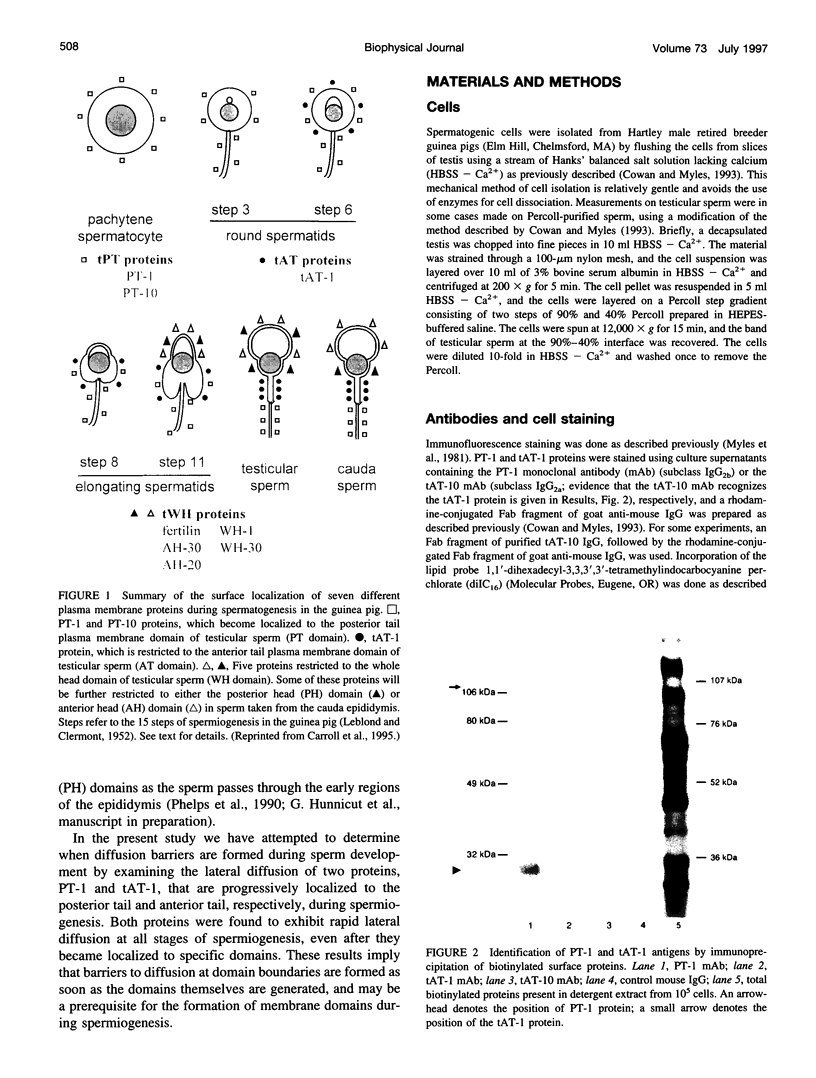


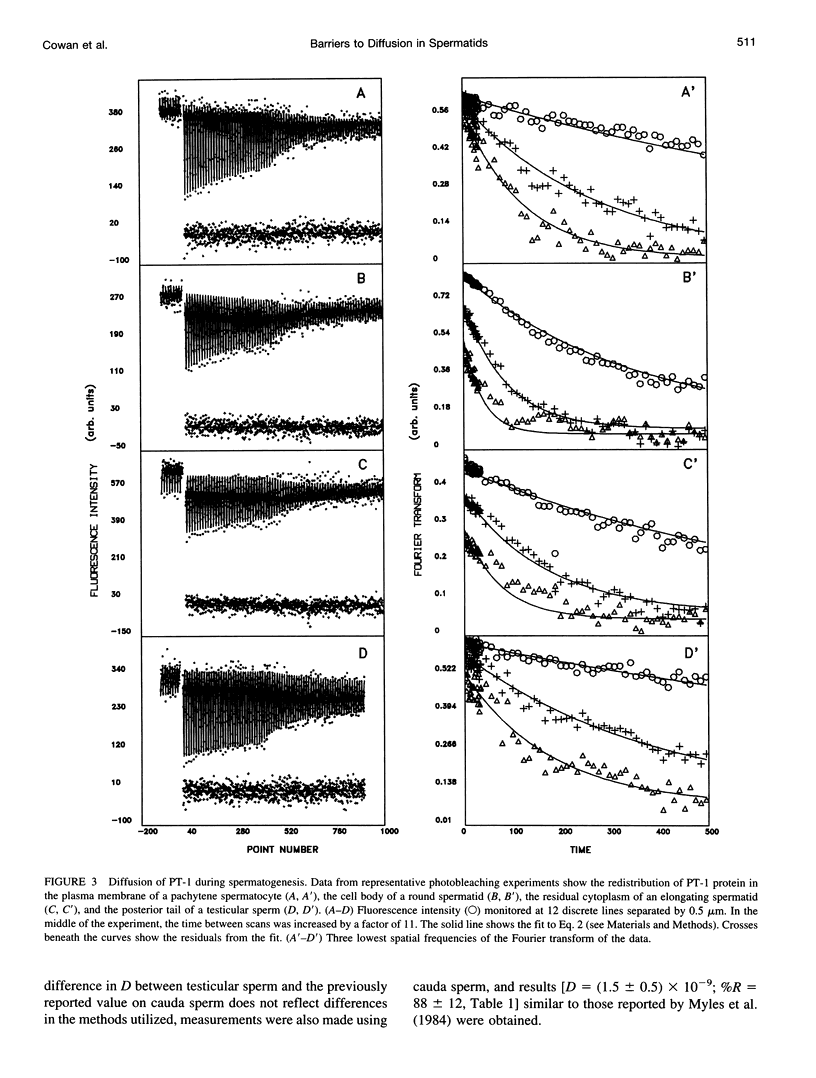

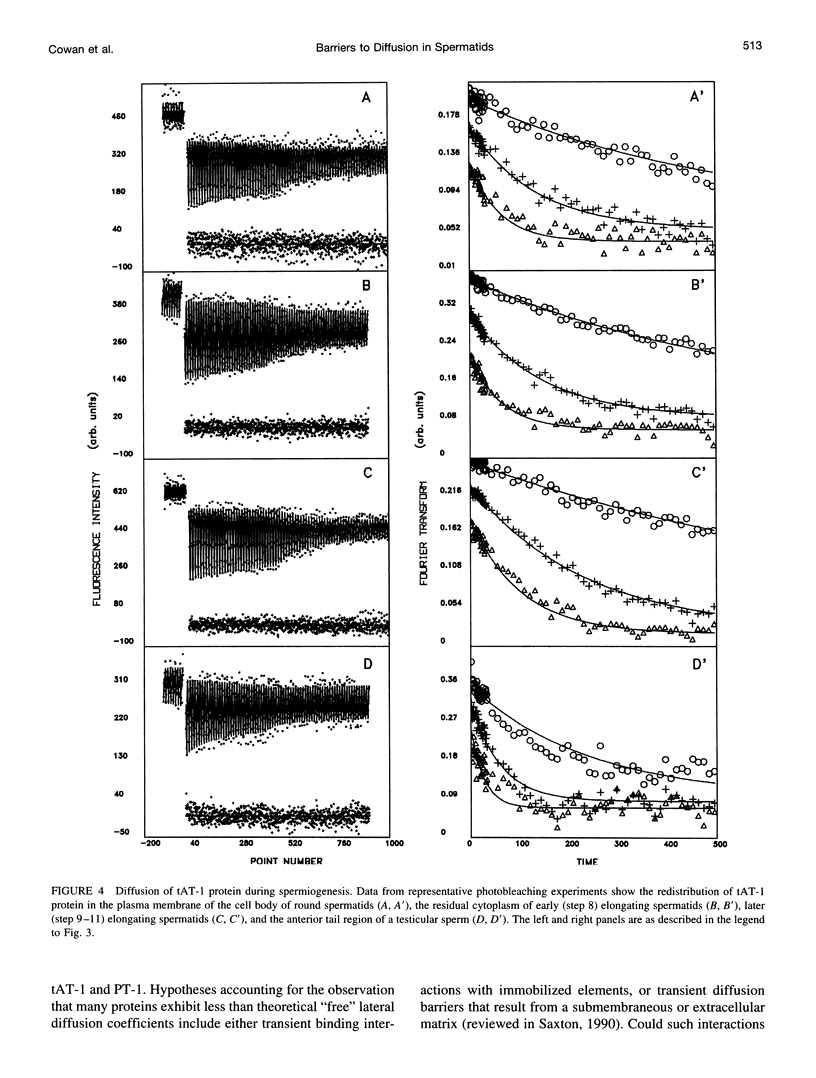



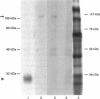
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccetti B., Bigliardi E., Burrini A. G. The cell surface during mammalian spermiogenesis. Dev Biol. 1978 Mar;63(1):187–196. doi: 10.1016/0012-1606(78)90124-0. [DOI] [PubMed] [Google Scholar]
- Bartles J. R. The spermatid plasma membrane comes of age. Trends Cell Biol. 1995 Oct;5(10):400–404. doi: 10.1016/s0962-8924(00)89089-3. [DOI] [PubMed] [Google Scholar]
- Berk D. A., Yuan F., Leunig M., Jain R. K. Fluorescence photobleaching with spatial Fourier analysis: measurement of diffusion in light-scattering media. Biophys J. 1993 Dec;65(6):2428–2436. doi: 10.1016/S0006-3495(93)81326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesario M. M., Bartles J. R. Compartmentalization, processing and redistribution of the plasma membrane protein CE9 on rodent spermatozoa. Relationship of the annulus to domain boundaries in the plasma membrane of the tail. J Cell Sci. 1994 Feb;107(Pt 2):561–570. [PubMed] [Google Scholar]
- Cesario M. M., Ensrud K., Hamilton D. W., Bartles J. R. Biogenesis of the posterior-tail plasma membrane domain of the mammalian spermatozoon: targeting and lateral redistribution of the posterior-tail domain-specific transmembrane protein CE9 during spermiogenesis. Dev Biol. 1995 Jun;169(2):473–486. doi: 10.1006/dbio.1995.1162. [DOI] [PubMed] [Google Scholar]
- Citi S. The molecular organization of tight junctions. J Cell Biol. 1993 May;121(3):485–489. doi: 10.1083/jcb.121.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A. E., Myles D. G. Biogenesis of surface domains during spermiogenesis in the guinea pig. Dev Biol. 1993 Jan;155(1):124–133. doi: 10.1006/dbio.1993.1012. [DOI] [PubMed] [Google Scholar]
- Cowan A. E., Myles D. G., Koppel D. E. Lateral diffusion of the PH-20 protein on guinea pig sperm: evidence that barriers to diffusion maintain plasma membrane domains in mammalian sperm. J Cell Biol. 1987 Apr;104(4):917–923. doi: 10.1083/jcb.104.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C. G., Parton R. G., Simons K. Polarized sorting of glypiated proteins in hippocampal neurons. Nature. 1991 Jan 10;349(6305):158–161. doi: 10.1038/349158a0. [DOI] [PubMed] [Google Scholar]
- Dragsten P. R., Blumenthal R., Handler J. S. Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane? Nature. 1981 Dec 24;294(5843):718–722. doi: 10.1038/294718a0. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Anderson W. A., Phillips D. M. Morphogenetic factors influencing the shape of the sperm head. Dev Biol. 1971 Oct;26(2):220–251. doi: 10.1016/0012-1606(71)90124-2. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Eddy E. M., Phillips D. M. Observations on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis. Biol Reprod. 1970 Feb;2(1):129–153. doi: 10.1095/biolreprod2.1.129. [DOI] [PubMed] [Google Scholar]
- Friend D. S., Fawcett D. W. Membrane differentiations in freeze-fractured mammalian sperm. J Cell Biol. 1974 Nov;63(2 Pt 1):641–664. doi: 10.1083/jcb.63.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S. Sperm maturation: membrane domain boundaries. Ann N Y Acad Sci. 1989;567:208–221. doi: 10.1111/j.1749-6632.1989.tb16472.x. [DOI] [PubMed] [Google Scholar]
- Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993 Dec;123(6 Pt 2):1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Khanin R., Segel L. A. Lipid diffusion in neurons. Nature. 1993 Mar 11;362(6416):119–119. doi: 10.1038/362119a0. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. Breaking through the tight junction barrier. J Cell Biol. 1993 Dec;123(6 Pt 2):1631–1633. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987 Dec;253(6 Pt 1):C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- Jesaitis L. A., Goodenough D. A. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994 Mar;124(6):949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Storrie B., Simons K., Dotti C. G. A functional barrier to movement of lipids in polarized neurons. Nature. 1992 Oct 15;359(6396):647–650. doi: 10.1038/359647a0. [DOI] [PubMed] [Google Scholar]
- Koppel D. E. Association dynamics and lateral transport in biological membranes. J Supramol Struct Cell Biochem. 1981;17(1):61–67. doi: 10.1002/jsscb.380170107. [DOI] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBLOND C. P., CLERMONT Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat. 1952 Mar;90(2):167–215. doi: 10.1002/aja.1000900202. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- Mostov K., Apodaca G., Aroeti B., Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992 Feb;116(3):577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles D. G., Koppel D. E., Cowan A. E., Phelps B. M., Primakoff P. Rearrangement of sperm surface antigens prior to fertilization. Ann N Y Acad Sci. 1987;513:262–273. doi: 10.1111/j.1749-6632.1987.tb25014.x. [DOI] [PubMed] [Google Scholar]
- Myles D. G., Primakoff P., Bellvé A. R. Surface domains of the guinea pig sperm defined with monoclonal antibodies. Cell. 1981 Feb;23(2):433–439. doi: 10.1016/0092-8674(81)90138-0. [DOI] [PubMed] [Google Scholar]
- Myles D. G., Primakoff P., Koppel D. E. A localized surface protein of guinea pig sperm exhibits free diffusion in its domain. J Cell Biol. 1984 May;98(5):1905–1909. doi: 10.1083/jcb.98.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme C. L., Cesario M. M., Myles D. G., Koppel D. E., Bartles J. R. Breaching the diffusion barrier that compartmentalizes the transmembrane glycoprotein CE9 to the posterior-tail plasma membrane domain of the rat spermatozoon. J Cell Biol. 1993 Feb;120(3):687–694. doi: 10.1083/jcb.120.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster D. S., Schneider B. G., Besharse J. C. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci. 1985 Oct;26(10):1386–1404. [PubMed] [Google Scholar]
- Phelps B. M., Koppel D. E., Primakoff P., Myles D. G. Evidence that proteolysis of the surface is an initial step in the mechanism of formation of sperm cell surface domains. J Cell Biol. 1990 Nov;111(5 Pt 1):1839–1847. doi: 10.1083/jcb.111.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps B. M., Primakoff P., Koppel D. E., Low M. G., Myles D. G. Restricted lateral diffusion of PH-20, a PI-anchored sperm membrane protein. Science. 1988 Jun 24;240(4860):1780–1782. doi: 10.1126/science.3381102. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Myles D. G. A map of the guinea pig sperm surface constructed with monoclonal antibodies. Dev Biol. 1983 Aug;98(2):417–428. doi: 10.1016/0012-1606(83)90371-8. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Olson G. Observations on the fine structure of the nuclear ring of the mammalian spermatid. J Ultrastruct Res. 1973 Jun;43(5):438–444. doi: 10.1016/s0022-5320(73)90020-8. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. The membrane skeleton of erythrocytes: models of its effect on lateral diffusion. Int J Biochem. 1990;22(8):801–809. doi: 10.1016/0020-711x(90)90283-9. [DOI] [PubMed] [Google Scholar]
- Sibony M., Segretain D., Gasc J. M. Angiotensin-converting enzyme in murine testis: step-specific expression of the germinal isoform during spermiogenesis. Biol Reprod. 1994 May;50(5):1015–1026. doi: 10.1095/biolreprod50.5.1015. [DOI] [PubMed] [Google Scholar]
- Wang A. Z., Ojakian G. K., Nelson W. J. Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990 Jan;95(Pt 1):153–165. doi: 10.1242/jcs.95.1.153. [DOI] [PubMed] [Google Scholar]
- Wolf D. E., Hagopian S. S., Ishijima S. Changes in sperm plasma membrane lipid diffusibility after hyperactivation during in vitro capacitation in the mouse. J Cell Biol. 1986 Apr;102(4):1372–1377. doi: 10.1083/jcb.102.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Voglmayr J. K. Diffusion and regionalization in membranes of maturing ram spermatozoa. J Cell Biol. 1984 May;98(5):1678–1684. doi: 10.1083/jcb.98.5.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoop M. J., Dotti C. G. Membrane traffic in polarized neurons in culture. J Cell Sci Suppl. 1993;17:85–92. doi: 10.1242/jcs.1993.supplement_17.13. [DOI] [PubMed] [Google Scholar]
- van Meer G., Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986 Jul;5(7):1455–1464. doi: 10.1002/j.1460-2075.1986.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]