Abstract
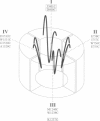
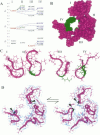
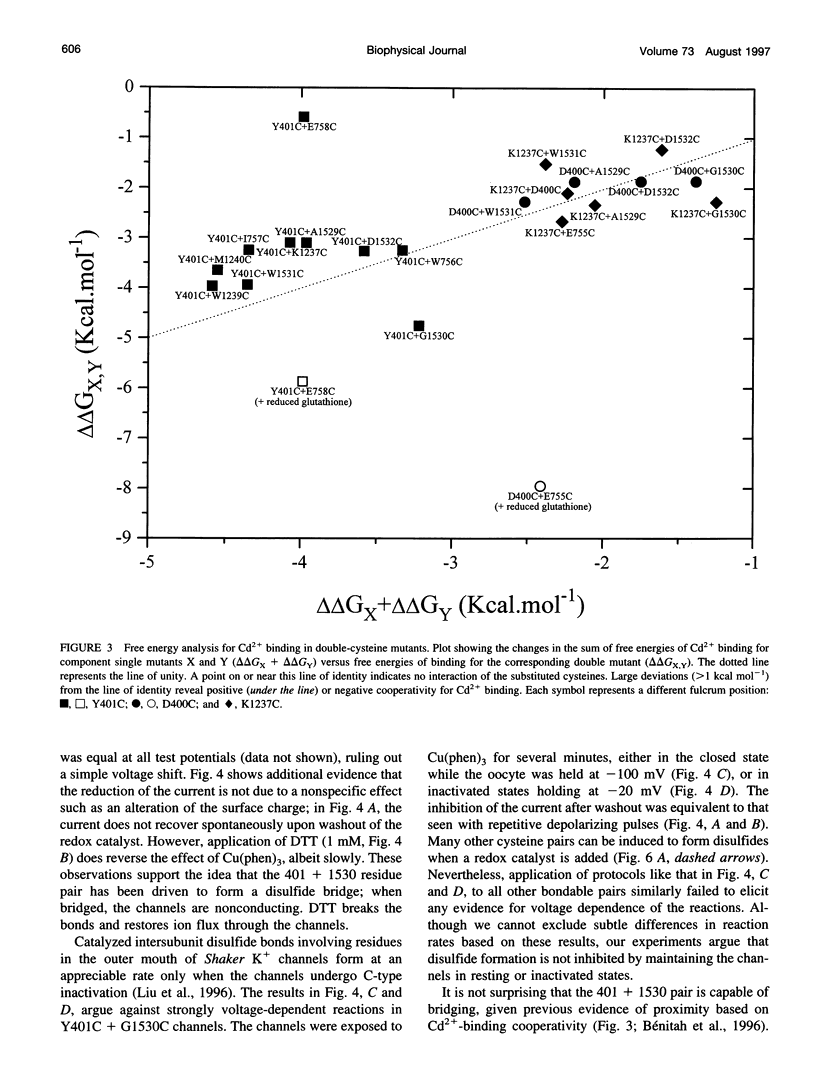
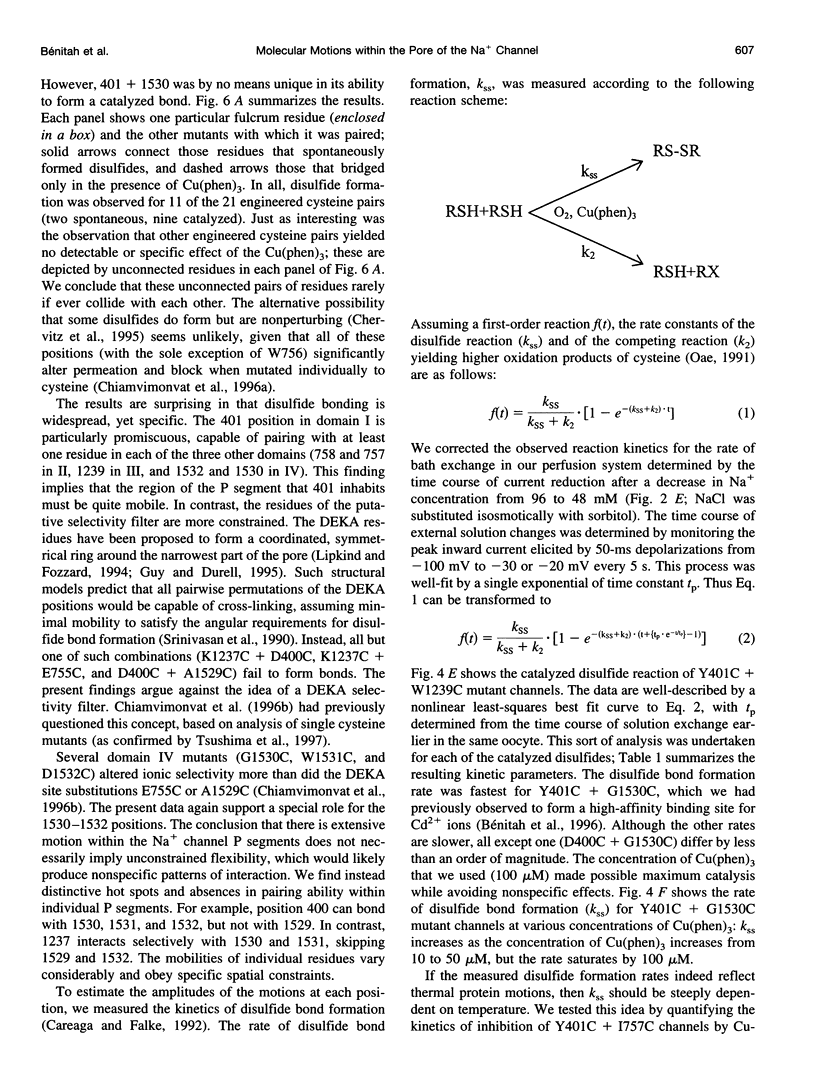
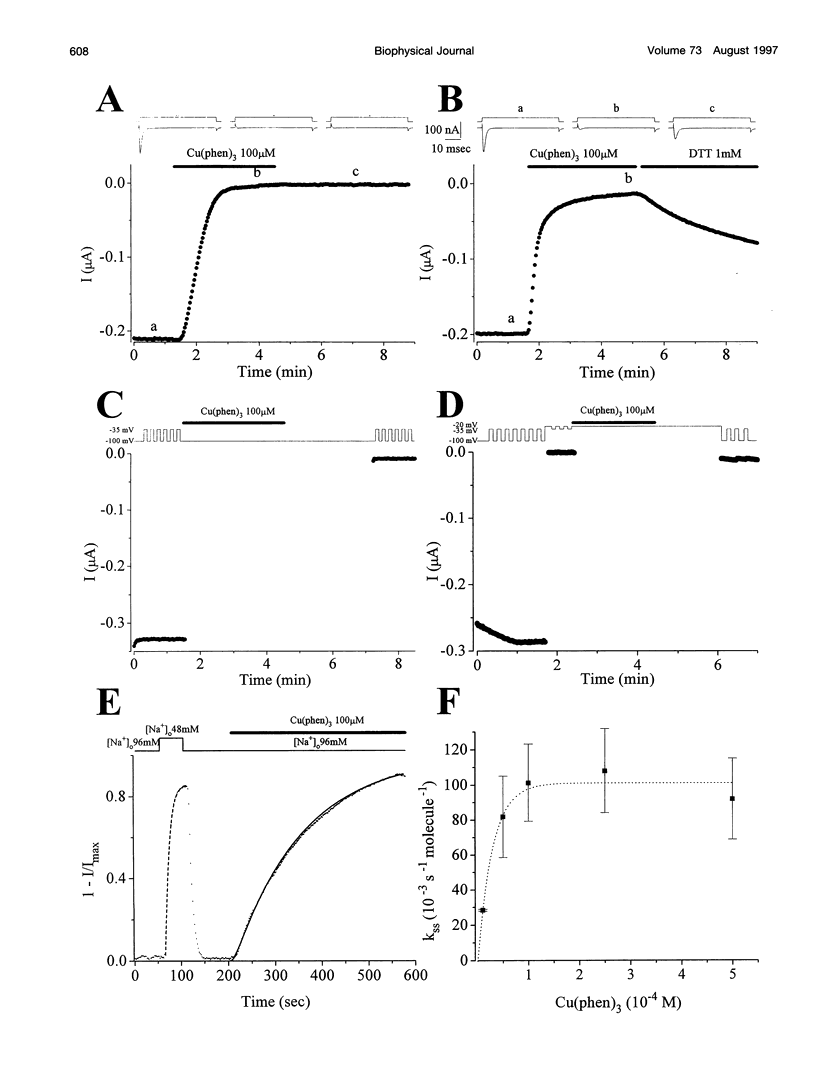
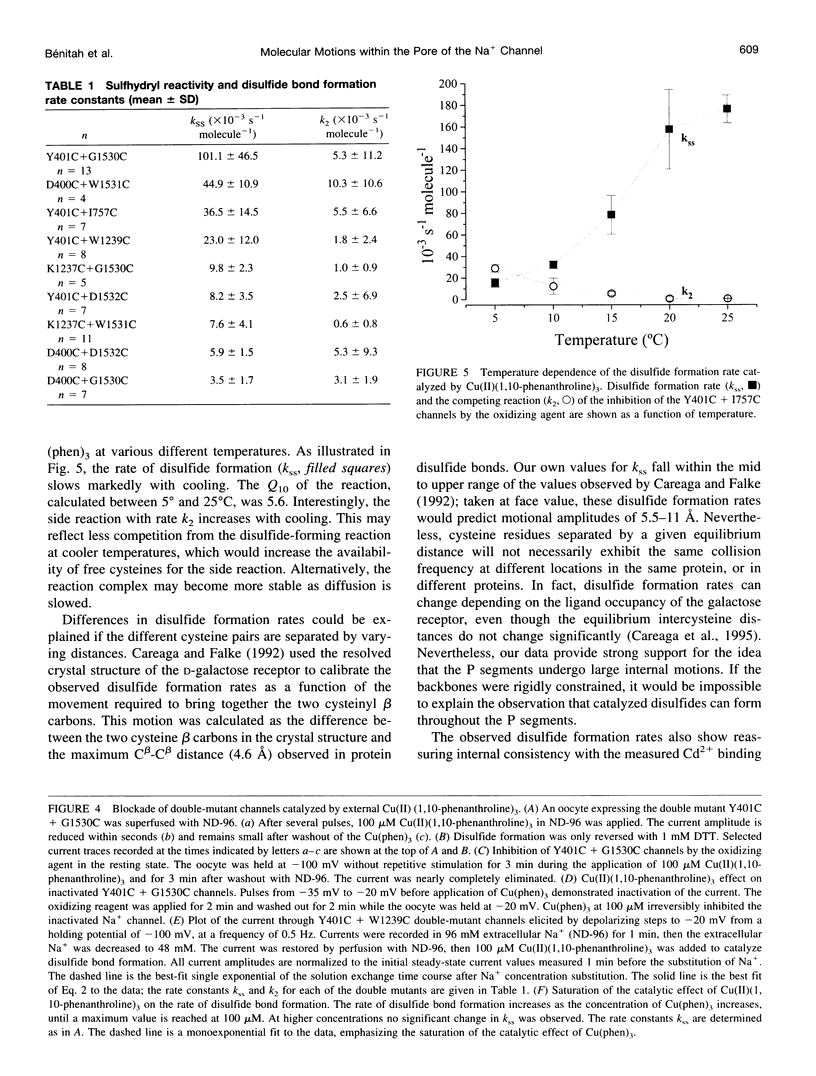
The pores of ion channel proteins are often modeled as static structures. In this view, selectivity reflects rigidly constrained backbone orientations. Such a picture is at variance with the generalization that biological proteins are flexible, capable of major internal motions on biologically relevant time scales. We tested for motions in the sodium channel pore by systematically introducing pairs of cysteine residues throughout the pore-lining segments. Two distinct pairs of residues spontaneously formed disulfide bonds bridging domains I and II. Nine other permutations, involving all four domains, were capable of disulfide bonding in the presence of a redox catalyst. The results are inconsistent with a single fixed backbone structure for the pore; instead, the segments that line the permeation pathway appear capable of sizable motions.
Full text
PDF
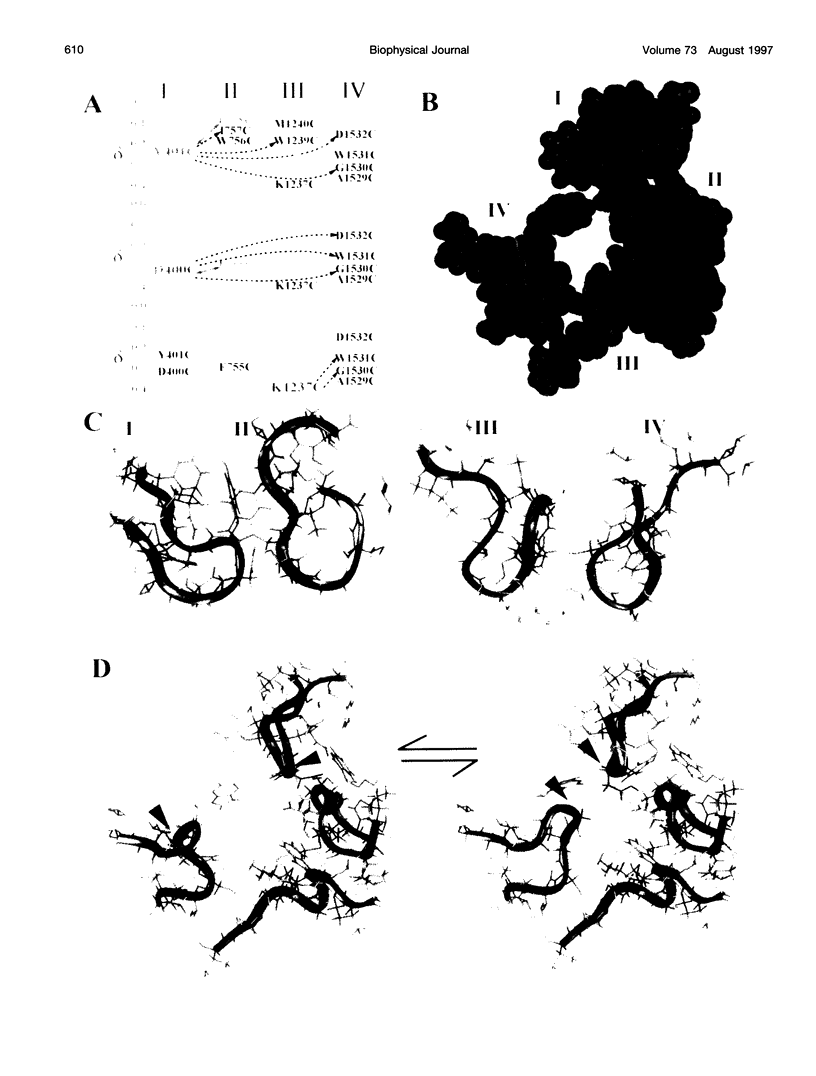



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bénitah J. P., Tomaselli G. F., Marban E. Adjacent pore-lining residues within sodium channels identified by paired cysteine mutagenesis. Proc Natl Acad Sci U S A. 1996 Jul 9;93(14):7392–7396. doi: 10.1073/pnas.93.14.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga C. L., Falke J. J. Thermal motions of surface alpha-helices in the D-galactose chemosensory receptor. Detection by disulfide trapping. J Mol Biol. 1992 Aug 20;226(4):1219–1235. doi: 10.1016/0022-2836(92)91063-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga C. L., Sutherland J., Sabeti J., Falke J. J. Large amplitude twisting motions of an interdomain hinge: a disulfide trapping study of the galactose-glucose binding protein. Biochemistry. 1995 Mar 7;34(9):3048–3055. doi: 10.1021/bi00009a036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. H., Bezprozvanny I., Tsien R. W. Molecular basis of proton block of L-type Ca2+ channels. J Gen Physiol. 1996 Nov;108(5):363–374. doi: 10.1085/jgp.108.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervitz S. A., Lin C. M., Falke J. J. Transmembrane signaling by the aspartate receptor: engineered disulfides reveal static regions of the subunit interface. Biochemistry. 1995 Aug 1;34(30):9722–9733. doi: 10.1021/bi00030a010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamvimonvat N., Pérez-García M. T., Ranjan R., Marban E., Tomaselli G. F. Depth asymmetries of the pore-lining segments of the Na+ channel revealed by cysteine mutagenesis. Neuron. 1996 May;16(5):1037–1047. doi: 10.1016/s0896-6273(00)80127-0. [DOI] [PubMed] [Google Scholar]
- Chiamvimonvat N., Pérez-García M. T., Tomaselli G. F., Marban E. Control of ion flux and selectivity by negatively charged residues in the outer mouth of rat sodium channels. J Physiol. 1996 Feb 15;491(Pt 1):51–59. doi: 10.1113/jphysiol.1996.sp021195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke J. J., Koshland D. E., Jr Global flexibility in a sensory receptor: a site-directed cross-linking approach. Science. 1987 Sep 25;237(4822):1596–1600. doi: 10.1126/science.2820061. [DOI] [PubMed] [Google Scholar]
- Favre I., Moczydlowski E., Schild L. On the structural basis for ionic selectivity among Na+, K+, and Ca2+ in the voltage-gated sodium channel. Biophys J. 1996 Dec;71(6):3110–3125. doi: 10.1016/S0006-3495(96)79505-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Prusak-Sochaczewski E., Zamponi G. W., Becker S., Kularatna A. S., Horn R. Interactions between a pore-blocking peptide and the voltage sensor of the sodium channel: an electrostatic approach to channel geometry. Neuron. 1996 Feb;16(2):407–413. doi: 10.1016/s0896-6273(00)80058-6. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Durell S. R. Structural models of Na+, Ca2+, and K+ channels. Soc Gen Physiol Ser. 1995;50:1–16. [PubMed] [Google Scholar]
- Heinemann S. H., Terlau H., Stühmer W., Imoto K., Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992 Apr 2;356(6368):441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971 Dec;58(6):599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom L. L., De Jongh K. S., Patton D. E., Reber B. F., Offord J., Charbonneau H., Walsh K., Goldin A. L., Catterall W. A. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992 May 8;256(5058):839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- Krovetz H. S., VanDongen H. M., VanDongen A. M. Atomic distance estimates from disulfides and high-affinity metal-binding sites in a K+ channel pore. Biophys J. 1997 Jan;72(1):117–126. doi: 10.1016/S0006-3495(97)78651-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Hess P. Characterization of the high-affinity Ca2+ binding sites in the L-type Ca2+ channel pore in rat phaeochromocytoma cells. J Physiol. 1993 Jul;466:657–682. [PMC free article] [PubMed] [Google Scholar]
- Lipkind G. M., Fozzard H. A. A structural model of the tetrodotoxin and saxitoxin binding site of the Na+ channel. Biophys J. 1994 Jan;66(1):1–13. doi: 10.1016/S0006-3495(94)80746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jurman M. E., Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996 Apr;16(4):859–867. doi: 10.1016/s0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- MULLINS Lj. The penetration of some cations into muscle. J Gen Physiol. 1959 Mar 20;42(4):817–829. doi: 10.1085/jgp.42.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-García M. T., Chiamvimonvat N., Marban E., Tomaselli G. F. Structure of the sodium channel pore revealed by serial cysteine mutagenesis. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):300–304. doi: 10.1073/pnas.93.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-García M. T., Chiamvimonvat N., Ranjan R., Balser J. R., Tomaselli G. F., Marban E. Mechanisms of sodium/calcium selectivity in sodium channels probed by cysteine mutagenesis and sulfhydryl modification. Biophys J. 1997 Mar;72(3):989–996. doi: 10.1016/S0006-3495(97)78751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan N., Sowdhamini R., Ramakrishnan C., Balaram P. Conformations of disulfide bridges in proteins. Int J Pept Protein Res. 1990 Aug;36(2):147–155. doi: 10.1111/j.1399-3011.1990.tb00958.x. [DOI] [PubMed] [Google Scholar]
- Terlau H., Heinemann S. H., Stühmer W., Pusch M., Conti F., Imoto K., Numa S. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 1991 Nov 18;293(1-2):93–96. doi: 10.1016/0014-5793(91)81159-6. [DOI] [PubMed] [Google Scholar]
- Tomaselli G. F., Chiamvimonvat N., Nuss H. B., Balser J. R., Pérez-García M. T., Xu R. H., Orias D. W., Backx P. H., Marban E. A mutation in the pore of the sodium channel alters gating. Biophys J. 1995 May;68(5):1814–1827. doi: 10.1016/S0006-3495(95)80358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer J. S., Cooperman S. S., Tomiko S. A., Zhou J. Y., Crean S. M., Boyle M. B., Kallen R. G., Sheng Z. H., Barchi R. L., Sigworth F. J. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989 Jul;3(1):33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- Tsushima R. G., Li R. A., Backx P. H. Altered ionic selectivity of the sodium channel revealed by cysteine mutations within the pore. J Gen Physiol. 1997 Apr;109(4):463–475. doi: 10.1085/jgp.109.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. P., Felts K. A., Simcox T. G., Braman J. C. A method for the site-directed mono- and multi-mutagenesis of double-stranded DNA. Gene. 1993 Apr 15;126(1):35–41. doi: 10.1016/0378-1119(93)90587-s. [DOI] [PubMed] [Google Scholar]
- Wells J. A. Additivity of mutational effects in proteins. Biochemistry. 1990 Sep 18;29(37):8509–8517. doi: 10.1021/bi00489a001. [DOI] [PubMed] [Google Scholar]
- Yamagishi T., Janecki M., Marban E., Tomaselli G. F. Topology of the P segments in the sodium channel pore revealed by cysteine mutagenesis. Biophys J. 1997 Jul;73(1):195–204. doi: 10.1016/S0006-3495(97)78060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., George A. L., Jr, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996 Jan;16(1):113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- Zhang H. J., Liu Y., Zühlke R. D., Joho R. H. Oxidation of an engineered pore cysteine locks a voltage-gated K+ channel in a nonconducting state. Biophys J. 1996 Dec;71(6):3083–3090. doi: 10.1016/S0006-3495(96)79502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]