Abstract
The most common genetic risk factors for Parkinson’s disease (PD) are a set of heterozygous mutant (MT) alleles of the GBA1 gene that encodes β-glucocerebrosidase (GCase), an enzyme normally trafficked through the ER/Golgi apparatus to the lysosomal lumen. We found that half of the GCase in lysosomes from postmortem human GBA-PD brains was present on the lysosomal surface and that this mislocalization depends on a pentapeptide motif in GCase used to target cytosolic protein for degradation by chaperone-mediated autophagy (CMA). MT GCase at the lysosomal surface inhibits CMA, causing accumulation of CMA substrates including α-synuclein. Single-cell transcriptional analysis and proteomics of brains from GBA-PD patients confirmed reduced CMA activity and proteome changes comparable to those in CMA-deficient mouse brain. Loss of the MT GCase CMA motif rescued primary substantia nigra dopaminergic neurons from MT GCase–induced neuronal death. We conclude that MT GBA1 alleles block CMA function and produce α-synuclein accumulation.
Parkinson’s disease–related mutations in β-glucocerebrosidase interfere with selective degradation of α-synuclein by lysosomes.
INTRODUCTION
A broad range of causes and risk factors for Parkinson’s disease (PD) have been identified, but they lead to common pathological sequelae, including α-synuclein aggregation and the degeneration of substantia nigra dopaminergic (DA) neurons. α-Synuclein can be degraded within lysosomes via chaperone-mediated autophagy (CMA), a selective form of autophagy in which proteins are recognized by a specific cytosolic chaperone, hsc70, that delivers them to the lysosomal receptor LAMP2A, resulting in translocation into lysosomes (1). At present, disease-associated alleles of genes coding for five cytosolic proteins related to PD have been reported to cause CMA dysfunction and block lysosomal α-synuclein degradation: leucine-rich repeat kinase 2 (LRRK2), ubiquitin C-terminal hydrolase 1 (UCHL1), vacuolar protein-sorting associated protein 35 (VPS35), DJ-1, and α-synuclein itself (1–5).
β-Glucocerebrosidase (GCase) is a lysosomal enzyme that cleaves glucosylceramide and glucosylsphingomyelin (6). Patients homozygous for mutant (MT) alleles of the glucosylceramidase β (GBA1) gene that codes for GCase develop the lysosomal storage disorder, Gaucher’s disease, while heterozygous expression of MT GBA1 alleles appears in 7 to 20% of PD cases (7). Here, we report that CMA activity is reduced in brains from GBA-PD patients, which display changes in their proteome comparable to those observed in the brains from experimental mouse models with CMA blockage.
There are multiple disease-associated MT GBA1 alleles, and here, we investigate the impact on CMA of the two most common GBA1 alleles associated with PD, N370S [NS] and L444P [LP], as well as the D409H [DH] allele, which is common in particular populations (8). Normally, GCase is folded in the endoplasmic reticulum (ER), trafficked to the Golgi apparatus, and then delivered through vesicular fusion to the lysosomal lumen. Studies by Horowitz and colleagues (9, 10) established that MT GCase is improperly folded and retained in the ER, from where it can be retrotranslocated to the cytosol and delivered for degradation by the ubiquitin proteasome system. We extend these findings by reporting that a fraction of unfolded MT GCase is recognized by hsc70 and binds LAMP2A on the lysosomal surface. The unfolded MT GCase disrupts the normal dynamics of LAMP2A recycling and is poorly translocated into the lysosome. The prolonged binding of MT GCase to the lysosomal surface blocks CMA of other proteins, thus leading to α-synuclein accumulation and α-synuclein–dependent death of substantia nigra DA primary neurons. Last, we detected altered levels of CMA components and overall proteome changes in brains from GBA-PD patients that are compatible with decreased CMA activity. Together, these results suggest how MT GCase may trigger synuclein aggregation by interfering with its lysosomal degradation via CMA, thereby initiating PD pathogenesis.
RESULTS
Aberrant lysosomal trafficking of MT GCase
We first monitored the subcellular distribution of wild-type (WT) and MT GCase in living cells. Previous reports have demonstrated that misfolded MT GCase is retained in the ER from where it can be targeted for degradation upon translocation outside of the ER, in contrast to normal GCase, which is trafficked inside vesicles for fusion and delivery with lysosomes (9, 10). We analyzed available GCase antibodies and found that they were effective for immunoblotting but not for immunolabeling (fig. S1, A and B). Thus, to examine the trafficking of GCase in cells, we stably expressed tagged WT and NS, LP, and DH GCase in SH-SY5Y cells (Fig. 1, A and D) and transfected tagged WT or NS GCase in cultured mouse primary ventral midbrain DA neurons (Fig. 1E). Consistent with previous reports, WT GCase was effectively targeted to lysosomes, but NS, LP, and DH MT GCases were present at lower levels in lysosomes (highlighted with LAMP1) and at higher levels in the ER, as demonstrated by higher levels of colocalization with the ER chaperone protein, BiP (Fig. 1, A to E).
Fig. 1. Differential lysosomal status of WT and MT GCase.
(A to D) SH-SY5Y cells stably expressing myc-tagged WT or indicated MT GCase (NS, N370S; LP, L444P; DH, D409H) were immunolabeled with myc and the lysosomal marker LAMP1 or the ER marker BiP. n = 3 independent experiments. Scale bar, 10 μm. (E) Primary mouse dopamine neuronal cultures were transduced with lentivirus to express human WT or NS GCase constructs tagged with V5 and immunolabeled with V5, LAMP1, and tyrosine hydroxylase (TH). n = 3 independent experiments. Scale bar, 10 μm. (F) Subcellular fractionation of SH-SY5Y expressing indicated MT GCase. Top: Representative immunoblots for the indicated proteins of homogenates (H, Hom) and lysosomes (Lys). Bottom: Quantification of lysosomal GCase content relative to WT and LAMP1 levels, an endosomal/ lysosomal marker: LAMP1 was used as LAMP2A levels are highly altered by cellular stress. n = 3 in each group. (G to I) SH-SY5Y cells expressing WT or indicated MT GCase constructs were subjected to subcellular fractionation to separate ER and cytosol. Representative immunoblots of cytosol and ER for the indicated proteins [(G); only immature cathepsin D (50 kDa) is detected in the ER fractions] and quantification of the amount of each protein in the ER expressed relative to control assigned an arbitrary value of 1 (H) and in the cytosol as percentage of their content in ER (I). n = 3. Values are means ± SEM and individual experiments in (C) to (E). Differences with control were significant for *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Examination of lysosomes isolated from SH-SY5Y cells expressing exogenous GCase indicated that (i) a lower fraction of cellular DH and NS GCases than WT GCase was present in lysosomes (16.4 ± 1.3% and 19.1 ± 0.7% of total cellular content for DH and NS, respectively, compared to 31.9 ± 0.9% in WT; Fig. 1F); (ii) NS, LP, and DH GCases were retained in the ER (Fig. 1, G and H); and (iii) DH and, to a lesser extent, the other two MT GCases were present at high levels in cytosolic fractions (defined as the supernatant of 1-hour centrifugation at 100,000g) (26.2 ± 4.2%, 13.5 ± 2.3%, and 8.3 ± 0.1% of total cellular content for DH, NS, and LP, respectively, compared to 6.1 ± 0.5% in WT; Fig. 1, G and I). These changes in lysosomal distribution were specific to cells expressing MT GCases, as levels of the lysosomal luminal protein cathepsin D in ER and lysosomes and of the lysosomal membrane protein LAMP1 in lysosomes were not different from cells expressing WT GCase (Fig. 1, G and H, and fig. S1, C and D), and cathepsin D was not present in the cytosol in any line (Fig. 1, G and I).
To examine whether the changes in GCase localization were related to its exogenous expression, we then performed similar analysis in induced pluripotent stem cell (iPSC)–derived DA neurons from GCaseWT/NS PD patients and healthy GCaseWT/WT control. We detected a higher content of MT GCase protein in the cytosol and lower amounts in the lysosomes from the GBA-PD line (21.4 ± 3.1% versus 51.4 ± 0.3% of total cellular content in MT and WT, respectively; Fig. 2, A and B). Reduced GCase in lysosomes was not a consequence of lower lysosomal abundance because, on the contrary, we found that the DA neurons derived from WT/NS PD patients displayed more lysosomal compartments (Fig. 2C) with higher LAMP1 and LAMP2A than the control and non-PD carrier lines (Fig. 2D).
Fig. 2. Subcellular distribution of WT and MT GBA in human iPSC-derived DA neurons and fibroblasts and transgenic mouse brains.
(A) Representative immunoblots of ER and cytosol (Cyt) from iPSCs-derived human DA neurons from a healthy control with WT GBA1 (WT/WT) and a pair of twins with heterozygous N370S GBA1 mutation (WT/NS), one with (**) and one without PD (*). (B) Representative immunoblot (top) and quantification of GCase (bottom) in lysosomes isolated from human iPSCs derived from WT/WT and CRISPR-mediated knock-in three isogenic NS/NS MT lines. (C) iPSC-derived DA neurons from a healthy WT/WT and PD WT/NS subject labeled by LysoTracker. Scale bar, 25 μm. (D) iPSC-derived DA neurons from subjects with WT/WT, WT/NS (non-PD), and WT/NS (PD) were FACS-sorted to ~80% purity, and lysosomal markers were analyzed by Western blot. n = 2 clones from each individual. (E to H) Representative immunoblots of cytosol (E) and lysosomes (G and H) from brains of heterozygous L444P GBA1 (WT/LP) and WT mice. Quantification of each protein is shown at the right. n = 3. (I and J) Representative immunoblots (I) and quantification of GCase (J) in homogenates and cytosol from human fibroblasts with WT/WT or WT/LP. (K) Trypsin resistance of GCase in lysosomes isolated from postmortem human brain of control WT/WT and PD cases with NS/LP. Values are means ± SEM. Differences with control were significant for *P < 0.05, **P < 0.01, and ***P < 0.001.
We further confirmed higher than normal levels of cytosolic GCase (Fig. 2, E and F) and lower abundance of the MT GCase in lysosomes isolated from brains of GBA+/L444P mice (13.1 ± 0.5% versus 16.5 ± 0.1% of total cellular content in MT and WT, respectively; Fig. 2G), while other lysosomal enzymes were unchanged (Fig. 2H). Consistently, we also found that fibroblasts from GCaseWT/NS PD patients have higher cytosolic levels of GCase than those from GCaseWT/WT controls (Fig. 2, I and J).
Although isolation of lysosomes from frozen tissues has been challenging, we recently optimized a procedure that preserves the integrity of the lysosomal limiting membrane and lysosomal function (11). Using this method, we analyzed lysosomes isolated from the anterior cingulate samples of healthy individuals and GBA-PD patients. Consistent with our findings in the experimental models, there were lower levels of GCase in PD-GBA lysosomes (Fig. 2K). Because maturation of GCase occurs in lysosomes, the reduced delivery of the enzyme to this compartment was also supported by the observation, consistent with previous reports (12), that levels of GCase activity in brains of PD-GBA patients were ~50% of healthy control levels, while we found normal levels of the lysosomal luminal degradative enzymes hexosaminidase and β-galactosidase (fig. S2, A to C). There was no enhanced accumulation of GCase substrates in PD-GBA brain with the exception of high glucosylceramide in 1 of 13 PD-GBA subjects assayed (fig. S2, D and E). Notably, the GCase associated with the lysosomes isolated from PD-GBA subjects was far more sensitive to trypsin digestion than that in lysosomes isolated from healthy controls, indicating that approximately half of the GCase in PD-GBA subjects was present on the cytosolic side of the lysosomes, whereas no detectable GCase was present on the cytosolic side of lysosomes from healthy controls (0% of control GCase and 47% of PD-GBA GCase associated with lysosomes were trypsin-sensitive, respectively) (Fig. 2K).
Together, these data confirm previous reports of altered ER handling and altered lysosomal trafficking of MT GCases associated with PD (13, 14), indicating that while MT GCase can be delivered to the lysosomal lumen, this conventional trafficking is less efficient than it is for WT GCase, and a large fraction of the MT enzyme instead reaches lysosomes from the cytosolic side of the membrane.
Targeting of GCase for lysosomal degradation via CMA
While a portion of MT GCase that undergoes ER retrotranslocation is degraded by proteasome (9, 15), a portion of the unfolded GCase in the ER might be additionally targeted to lysosomes for degradation. To analyze this possibility, we examined fibroblasts derived from GCaseWT/NS PD patients. We found that a fraction of MT GCase was sensitive to the proteasome inhibitor MG-132 but that a similar fraction was stabilized by treatment with the inhibitors of lysosomal proteolysis, ammonium chloride and leupeptin (Fig. 3, A and B), indicating that MT GCase also undergoes lysosomal degradation. Consistently, inhibition of deubiquitinating enzymes or p97, an ER retrotranslocation system associated with proteasome degradation, only partially stabilized MT GCase, confirming that a fraction of MT GCase was degraded by mechanisms that are independent of the ubiquitin/proteasome system.
Fig. 3. MT GCase binding and uptake by lysosomes.
(A) Immunoblot of human fibroblasts with either WT/WT or WT/NS genotypes incubated with NH4Cl and leupeptin (N/L) or the proteasome inhibitor MG-132 (MG), a pan-deubiquitinase inhibitor (DUBi), or an inhibitor of p97. Ubiquitin is shown as control of inhibitor efficiency and Ponceau staining as loading control. (B) Quantification of the levels of GCase upon the indicated treatments in the indicated human fibroblast genotypes. n = 3. (C) Schematic of the assays for in vitro lysosomal binding and uptake in the isolated lysosomes. (D) Purified and denatured WT GCase and the indicated MTs were incubated with intact isolated lysosomes untreated or treated with proteinase inhibitors (PI). (E) Percentage of denatured GCase bound or taken up by lysosomes. n = 5. (F and G) Association of increasing concentrations of denatured LP (E) or NS (F) GCase proteins with lysosomes. Representative immunoblot (left) and quantification (right). (H and I) Trypsin and proteinase K resistance of WT and different MT GCase incubated with isolated lysosomes as in (A) (H) or of lysosomes from brains of GBA+/L444P and WT mice (I). (J) High exposure of a representative immunoblot of the incubation of denatured GCase proteins with lysosomes to reveal formation of high–molecular weight complex of the proteins at the lysosomal membrane. (K) Intact rat liver lysosomes were incubated with either WT or MT GCase alone (−) or with increasing concentrations of the CMA substrate RNase A at 37°C. Samples were collected by centrifugation and immunoblotted for GCase. Input: 0.04 μg of WT or MT GCase protein (left). Calculation of the amount of GCase associated with lysosomes (right). Values are expressed as percentage of the protein bound when incubated alone. n = 3. Values are means ± SEM. Differences with control were significant for *P < 0.05 and **P < 0.01.
The presence of MT GCase on the surface of lysosomes and its higher cytosolic levels led us to propose the hypothesis that lysosomal degradation of MT GCase could occur by CMA, as macroautophagy, the other major form of mammalian autophagy, delivers substrate proteins directly into the lysosomal lumen. To investigate whether MT GCase is a CMA substrate, we used a well-characterized in vitro assay with isolated lysosomes to analyze the binding of putative CMA substrates to the lysosomal membrane (in lysosomes incubated with purified protein, protein that reaches the lumen upon translocation is rapidly degraded) and translocation of the putative CMA substrate into the lumen of lysosomes pretreated with proteolysis inhibitors to prevent degradation upon translocation (Fig. 3C). We found that denatured unfolded WT and MT GCase proteins, which are the forms expected following retrotranslocation from the ER to cytosol, bound to the surface of isolated intact lysosomes with relatively high efficiency but were poorly internalized by the lysosomes (Fig. 3, D to G). Note that as endogenous luminal lysosomal GCase is glycosylated [sensitive to endoglycosidase H (Endo H)], it displays a higher apparent molecular weight than the unglycosylated exogenously added protein, allowing us to differentiate the protein reaching this compartment from the cytosolic side of the organelle (fig. S3A). Notably, lysosomal uptake of NS and LP GCase was substantially lower than the 20 to 30% internalization typically observed for CMA substrates using this in vitro system (1–5), and uptake of WT and DH GCases was even lower, with only 0.5 to 1.4% of these proteins reaching the lumen (Fig. 3E).
Consistent with its normal localization within the lysosomal lumen, endogenous GCase was protected from trypsin unless the lysosomes were disrupted by Triton X-100 detergent (Fig. 3H). In contrast, but in agreement with our findings in human brain lysosomes (Fig. 2K) and consistent with the presence of bound untranslocated GCase on the cytoplasmic face of lysosomes, most exogenous WT, NS, and LP GCases associated with lysosomes were trypsin-sensitive (Fig. 3H), indicating that it was external on the lysosomal membrane. As further confirmation, proteinase K, which can access both lysosomal membrane and lumen without Triton X-100, completely degraded both endogenous and exogenous lysosome-associated GCases (Fig. 3H). Last, we also confirmed the presence of trypsin-sensitive GCase on the external face of lysosomes isolated from GBA+/L444P mouse brain, indicating that MT, but not WT, GCase was on the cytosolic side of the lysosomes (Fig. 3I). We note that transport of endogenous cytosolic unfolded GCase into lysosomes may be even more inefficient than what we measured with recombinant proteins in the in vitro assay, as cytosolic GCase in human iPSCs from a GCaseWT/NS PD patient was glycosylated (Endo H–sensitive), indicating that it had undergone glycosylation in the ER before its trafficking by the CMA pathway (fig. S3B).
We propose that the lower efficiency of translocation across the lysosomal membrane is responsible for the accumulation of unfolded MT GCase and its subsequent oligomerization. Thus, while nondenatured GCase does not form oligomers on the lysosomal membrane (fig. S3, C and D), denatured unfolded GCase bound to the surface of lysosomes and formed high–molecular weight oligomers (Fig. 3J). Consistent with its normal function as a luminal enzyme and in notable contrast to other CMA substrates, e.g., glyceraldehyde-3-phosphate dehydrogenase (GAPDH), that are readily amenable to degradation once exposed to lysosomal hydrolases, the lysosomal degradation of both unfolded WT and MT GCases was markedly slow (fig. S3, E and F). We also confirmed that GCase proteins presented to lysosomes from the cytosolic side did not disrupt lysosomal integrity (fig. S3G). Together, these findings indicate that unfolded GCases in the ER can be targeted to the lysosome membrane by CMA, but their translocation and degradation inside the lysosome are highly inefficient.
Unfolded GCase blocks CMA
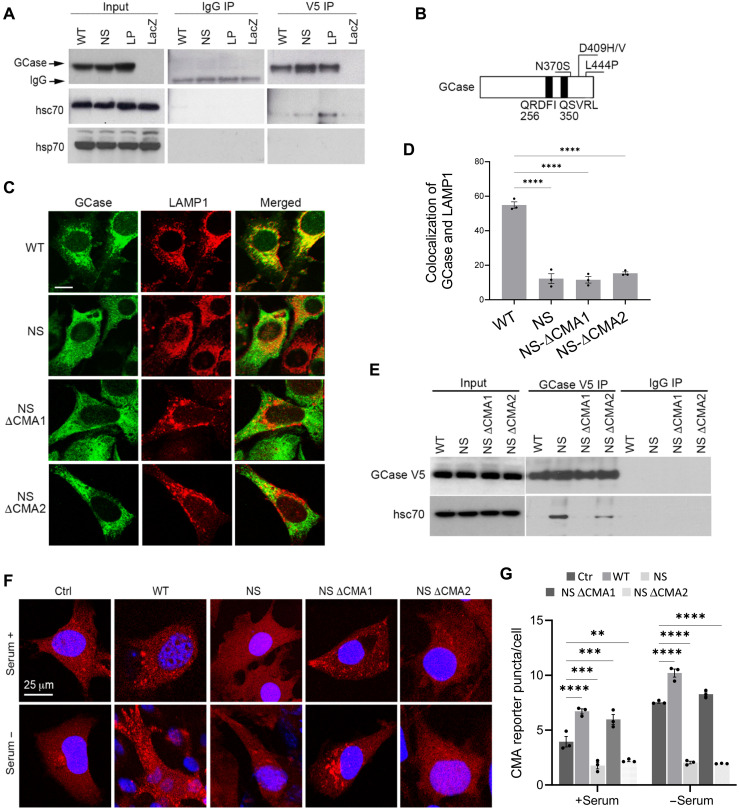
We hypothesized that the strong binding of unfolded GCase to the lysosomal membrane and its poor translocation efficiency might interfere with CMA of other cytosolic proteins. Consistent with this hypothesis, a well-established CMA substrate, ribonuclease A (RNase A), was ineffective at competing with GCase binding at lysosomes (Fig. 3K). To confirm this possible inhibitory effect of MT GCase proteins on CMA activity in intact cells, we used a CMA reporter (KFERQ-PA-mCherry1) (16) in fibroblasts expressing WT or NS, LP, or DH GCase. This reporter is a cytosolic photoactivatable fluorescent protein tagged with the CMA motif that, when delivered to lysosomes via CMA, highlights them as fluorescent puncta (16). Quantification of the number of puncta per cell has been used as a reliable measure of CMA activity. In these cells, CMA is normally active in complete medium but is further induced by nutrient deprivation; consistently, cells in complete medium displayed few puncta, with a small increase when transduced with WT GCase (Fig. 4, A and B). Upon serum withdrawal, control cells and those with WT GCase displayed a ~3-fold increase in CMA, but those with NS, LP, or DH GCase failed to up-regulate CMA (Fig. 4, A and B). Faulty CMA was also confirmed upon measuring the degradation of radiolabeled endogenous CMA proteins by lysosomes (sensitive to the lysosomal inhibitors ammonium chloride and leupeptin) in the same cells, which was inhibited by MT GCase (fig. S4, A and B). We demonstrated that CMA inhibition by MT GCases was not due to protein overexpression, as this also occurred in fibroblasts derived from WT/NS and WT/LP patients, both by the CMA reporter assay (Fig. 4, C and D) and in radiolabeled protein degradation assays (fig. S4, C to E).
Fig. 4. MT GCase blocks CMA.
(A to D) CMA activity in cells transduced with a lentiviral vector carrying the KFERQ-PA-mCherry1 fluorescent reporter. (A and B) NIH3T3 cells stably transduced with AAV carrying GFP (Ctrl), WT GCase, or MT GCase (NS, LP, or DH) in the presence or absence of serum for 12 hours after photoactivation. (C and D) Human fibroblasts from WT/WT, WT/NS, or WT/LP in the presence or absence of serum for 12 hours after photoactivation. Representative micrographs (A and C) and quantification of the average number of puncta per cell in n = 3 independent experiments (B and D). (E to H) NIH3T3 cells expressing lentivirus carrying V5-tagged WT or NS or WT or NS GCase without the leader sequence of the first 39 amino acids, immunolabeled for V5 and LAMP1, n = 3 independent experiments (E and F). The same NIH3T3 lines transduced with a lentiviral vector carrying the KFERQ-PA-mcherry1 CMA reporter in the presence or absence of serum for 12 hours after photoactivation, n = 3 independent experiments (G and H). Values are means ± SEM and individual experiments. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
The first 39 amino acids of GCase provide a leader sequence required for ER localization (17), and we found that deletion of the ER leader sequence increased the cytosolic fraction of GCase from 4% in WT to 15 to 18% (fig. S5A) and significantly reduced its lysosomal abundance (Fig. 4, E and F). We found that deletion of the leader sequence from WT GCase caused it to block CMA, consistent with the hypothesis that unfolded GCase inhibited CMA (Fig. 4, G and H).
The inhibitory effect of MT GCases on CMA was not due to loss of GCase enzymatic activity, as neither cells treated with a GCase inhibitor (fig. S6, A to C) nor GBA1 knockout cells (fig. S6, D and E) exhibited decreased CMA. Thus, MT GCase protein and not the disruption of its enzymatic activity is required for CMA inhibition.
Identification of the CMA-targeting motif in GCase
To act as CMA substrates, proteins must bind hsc70 through a CMA-targeting pentapeptide. NS and LP MT GCases in cells efficiently coimmunoprecipitated hsc70, while WT GCase did not (Fig. 5A). This is consistent with effective WT GCase folding and targeting to lysosomes so that the motif is not accessible to the chaperone. In contrast, misfolded NS or LP GCases that retrotranslocate outside the ER can bind hsc70. We mutated GCase’s two potential pentapeptide CMA-targeting motifs: 256QRDFI260 to 256AADFI26 (NSΔCMA1) and 350QSVRL354 to 350AAVRL354 (NSΔCMA2) (Fig. 5B). NSΔCMA1 and NSΔCMA2 exhibited identical subcellular localization as NS GCase (Fig. 5, C and D), with negligible delivery to lysosomes. NSΔCMA1, however, no longer coimmunoprecipitated with hsc70 (Fig. 5E) and, unlike NS GCase, did not block CMA (Fig. 5, F and G). Thus, the binding of 256QRDFI260 to hsc70 is responsible for CMA targeting.
Fig. 5. Hsc70-mediated lysosomal targeting of MT GCase blocks CMA.
(A) NIH3T3 cells transduced with lentivirus carrying LacZ and WT or MT GCase tagged with V5 were subjected to coimmunoprecipitation using anti-V5 antibodies and probed with V5, hsc70, and hsp70. (B) Scheme of GCase protein depicting the two putative KFERQ-like CMA-targeting motifs and MT GCases. (C and D) NIH3T3 cells transduced with lentivirus carrying WT, NS, NS with the first CMA motif mutated (NSΔCMA1), or NS GCase with the second CMA motif mutated (NSΔCMA2) tagged with V5 were subjected to dual immunolabel for V5 and LAMP1. Representative images. Scale bar, 10 μm. (C) Quantification of the colocalization of GCase and LAMP1, n = 3 independent experiments (D). (E) Coimmunoprecipitation using anti-V5 antibodies in the same cells as (C), probed with V5 and hsc70. (F and G). The same cells were transduced with a lentiviral vector carrying the KFERQ-PA-mCherry1 fluorescent reporter and after photoactivation maintained 12 hours in the presence of serum or in the serum withdrawal condition. (F) Representative micrographs. (G) Number for puncta per cell. n = 3 independent experiments. Values are means ± SEM and individual experiments. Differences with control were significant for **P < 0.01, ***P < 0.001, and ****P < 0.0001.
MT GCase inhibits α-synuclein lysosomal degradation
MT GCase has been reported to block lysosomal degradation and cause α-synuclein accumulation, although the mechanism underlying this action has been unknown (18). We hypothesized that because a fraction of α-synuclein has been shown to undergo degradation through CMA (1), MT GCase blockade of CMA could block CMA-mediated degradation of α-synuclein.
We found in transduced primary cortical neurons that WT GCase overexpression had no effect on α-synuclein levels, but NS, LP, and DH GCase each increased α-synuclein (Fig. 6, A and B). We then measured their effects on the turnover of doxycycline-inducible α-synuclein (19). Four days after doxycycline withdrawal to suppress α-synuclein expression, α-synuclein levels were decreased by 80% (Fig. 6C), indicating a slow turnover of the protein (1, 20). Expression of NS, but not WT, GCase inhibited α-synuclein degradation (Fig. 6D). As expected, NSΔCMA2 GCase also blocked α-synuclein turnover, but NSΔCMA1 GCase, which does not bind hsc70 and does not undergo CMA targeting, had no effect (Fig. 6E). Also, as expected, WT GCase without the leader sequence blocked degradation of α-synuclein (fig. S5, B to D).
Fig. 6. GCase and degradation of α-synuclein and tau.
(A and B) Representative immunoblot (A) and quantification of α-synuclein monomer levels (B) in rat cortical neuronal cultures expressing WT or MT GCase (NS, LP, and DH) for 7 days. (C) α-Synuclein levels in M17 cells at indicated times after 24-hour induction with doxycycline. (D and E) α-Synuclein degradation in the same cells as (C) transduced with lentivirus carrying WT, MT GCase, or MT GCase with the first or second CMA motifs mutated (NSΔCMA1 and NSΔCMA2, respectively). (F) Immunoblots of α-synuclein incubated with lysosomes treated or not with proteinase inhibitors (PI) in the presence of increased concentration of denatured WT and MT GCase. (G and H) Monomers of α-synuclein bound or taken up by lysosomes. (I) Oligomers of α-synuclein bound to lysosomes. (J) GCase proteins bound and taken up by lysosomes in the presence or absence of α-synuclein. (K) Immunoblots of tau incubated with lysosomes treated as in (F). (L and M) Quantification of tau bound or taken up by lysosomes. (N) Quantification of GCase bound or taken up in the presence of tau. (O) Immunoblot (left) and quantification (right) of endogenous levels of tau and GAPDH in homogenate and CMA active lysosomes isolated from brains of GBA+/L444P (LP) and WT mice. (P) Blue native electrophoresis and immunoblot for LAMP2A of lysosomes incubated as in (F) in the presence of PI but with indicated GCase MTs. (Q) Changes in levels of multimeric LAMP2A. n = 3. (R) Viability of ventral midbrain dopamine neuronal cultures from WT or α-synuclein knockout mice transduced with lentivirus carrying WT or NS GCase for 7 days. Values are means ± SEM. Differences with control (*) were significant for *P < 0.05 or **P < 0.01.
In isolated lysosomes, we found that exogenous WT and MT GCase both blocked α-synuclein’s uptake into the lysosome, resulting in increased α-synuclein bound to the outer lysosomal membrane (Fig. 6, F to H), and further elicited α-synuclein oligomerization (Fig. 6, F and I), a feature associated with PD pathology. As with RNase A (Fig. 3K), α-synuclein also did not block GCase binding to the lysosome (Fig. 6, F and J), consistent with strong binding and poor uptake of GCase.
Tau is another CMA substrate that is also a genetic risk factor for PD (21), and tau aggregates, often found in tandem with α-synuclein (22), are particularly prominent in PD patients with cognitive decline, a feature associated with GBA-PD. Tau is translocated into lysosomes more avidly than α-synuclein (23). WT GCase did not interfere with tau uptake, consistent with its negligible luminal translocation (Fig. 6, K and L), but NS GCase significantly inhibited tau uptake by CMA active lysosomes (Fig. 6, K and M). Tau did not interfere with GCase binding, but the combination of tau and GCase at the lysosomal membrane completely blocked GCase’s already inefficient uptake (Fig. 6N). Consistently, we found increased tau levels in GBA+/L444P mouse brain (Fig. 6O), particularly in the lysosomal fraction. This suggests that tau is targeted by CMA to lysosomes but does not undergo translocation/degradation in the presence of the MT GCase (Fig. 6O).
To elucidate how lysosome-bound GCase blocks CMA, we examined the multimerization of LAMP2A at the lysosomal membrane, a step after substrate binding that is required to mediate substrate translocation by CMA. As is typical of CMA substrates including α-synuclein, GCase proteins alone increased LAMP2A multimerization (700 kDa) (24), albeit with different efficiencies. Each of the MT GCases in combination with α-synuclein, however, decreased LAMP2A multimerization, demonstrating that MT GCase impairs the formation of the CMA lysosomal translocation complex required to translocate α-synuclein into CMA lysosomes for degradation (Fig. 6, P and Q).
Neurotoxic effects of MT GCase–induced CMA blockage
To examine whether the combination of MT GCase and α-synuclein could elicit neurotoxicity, we expressed WT and NS GCases in both WT and α-synuclein–deficient cultured murine substantia nigra DA neurons (cocultured with astrocytes derived from rats) (25). WT GCase expression caused no dopamine neuron death, but MT GCase caused neurotoxicity in WT DA neurons, as did NSΔCMA2, which blocks CMA, while toxicity was absent with the NSΔCMA1 mutation that does not block CMA (Fig. 6R). The MT GCase– and NSΔCMA1-mediated loss of DA neurons required expression of neuronal α-synuclein, as dopamine neuron death was absent from neurons obtained from α-synuclein–deficient mice. We conclude that MT GCase–induced DA neurotoxicity required an interaction requiring both MT GCase and α-synuclein (Fig. 6R).
Dysfunctional CMA in brain tissue of GBA-PD patients
To determine whether the cellular and animal models that show that MT GCase blocks CMA are consistent with effects in GBA-PD, we examined well-preserved frozen autopsy tissue dissected from the cingulate gyrus, a region with high levels of α-synuclein aggregates in GBA-PD in the cerebral cortex, from healthy control individuals without neurodegenerative disorders and idiopathic PD and GBA-PD patients (fig. S2; demographics in tables S1 and S2): Note that there are very few surviving substantia nigra DA neurons in PD patients.
As a first step to investigate the status of neuronal CMA in GBA-PD patients, we performed single-nucleus RNA sequencing (snRNA-seq) of the human brain tissues (fig. S7, A and B) and analyzed the transcriptional expression of all known CMA components. We calculated differences in CMA activity based on the weighted average of the expression of each element of the CMA network including effectors and lysosomal and extralysosomal regulators and expressed these as a CMA score (26), an approach previously validated in human disease tissue and in vivo and in vitro experimental disease models (27). Excitatory (pyramidal neurons) and inhibitory (mostly GABAergic interneurons, which produce the neurotransmitter gamma-aminobutyric acid) neurons from both idiopathic and GBA-PD brains displayed a pronounced decrease in CMA score, which was a result of enhanced expression of inhibitory CMA components and reduced expression of most CMA effectors (Fig. 7A). In general, we found that the transcriptional profile of neurons from idiopathic and GBA-PD brains differed from healthy control neurons in relatively few transcripts (only 1.4% of the transcriptome manifest >2-fold changes) (fig. S7, C to E). Some of these changes were common in idiopathic and GBA-PD brains and were related to response to immune signaling (interleukin-initiated), protein transcription, and cholesterol processing (fig. S7, C to E). There were additional changes unique to GBA-PD that were related to metabolism of multibranched polysaccharides as consistent with the normal enzymatic role of GCase (fig. S7, C to E).
Fig. 7. Disruption of CMA phenocopies the proteostasis alterations of GBA-PD human neurons.
(A and B) Normalized gene (A) or protein (B) expression (z scoring within each cell type) of CMA network components (organized in functional groups) (top) and CMA activation score (bottom) of excitatory and inhibitory neurons from brains of healthy controls and idiopathic PD and GBA-PD patients (A) or human iPSC differentiated dopaminergic (hiPSCd DA) neurons from control (WT/WT) or GBA MT (WT/NS) patients. (C) CMA activity in the same cells as (B) transduced with a lentiviral vector carrying the KFERQ-PA-mCherry1 fluorescent reporter 12 hours after photoactivation. Representative images (top) and quantification n = 3 independent experiments (bottom). Individual values per experiment and means ± SEM are shown. Insets show the boxed regions at high magnification to display puncta (arrows). **P < 0.01. (D) Comparison of proteome changes between GBA-PD and healthy control brains and between human iPSC differentiated dopaminergic (DA) neurons from GBA1 WT/WT and GBA1 WT/NS patients in Log2 fold change (FC) in levels of proteins. Red and green indicate proteins that increase or decrease, respectively, in both groups and number of protein in each group are indicated in blue. (E) Changes in protein and mRNA levels of the indicated PD-related genes and genes associated with PD risk in the soluble fraction of brains from GBA-PD patients relative to healthy controls. Values are expressed as log fold change. (F to H) Comparison of proteome changes between GBA-PD and healthy control brains and between L2AKO mice brains relative to their matching controls. Log2 fold change (FC) in levels of proteins (F). Red and green indicate proteins that increase or decrease, respectively, in both groups, Venn diagram of significantly changing proteins in each group (G), and STRING pathway analysis (H) with the proteins changing in both groups. (I and J) Examples of proteins more abundant in the GBA-PD human brain and L2AKO mice brain relative to their respective controls that show decreased association with lysosomes (I) and of proteins with detectable lower levels of both groups that show decreased association with lysosomes and increased abundance in protein aggregates (J). All GO terms are statistically enriched with P < 0.001.
To examine whether the transcriptional changes in the CMA network in GBA-PD–derived neurons also resulted in changes at the protein level, we compared the proteomes of iPSC-derived DA neurons from GBA-PD patients (GCaseWT/NS) and healthy controls (GCaseWT/WT) (fig. S8A). Similar to the snRNA-seq data, cells from GBA-PD patients displayed a lower CMA score than those from healthy control (Fig. 7B). An additional advantage of the iPSC-derived DA neurons is that we can directly assess CMA activity using the KFERQ-PA-mCherry1 fluorescent reporter. Consistent with the CMA score, and consistent with our findings in cells expressing MT GCase (Fig. 4), we found that CMA activity in DA neurons derived from GB-PD patients was significantly lower than in those from healthy control (Fig. 7C).
We next assessed the impact of GBA-PD on the neuronal proteome by comparing proteomic analysis of cingulate cortex from healthy control and GBA-PD patients (fig. S8B), which confirmed a lower CMA score in GBA-PD patient brain (fig. S8C). The top canonical pathways affected by the changes in the GBA-PD proteome are shown in the STRING (search tool for the retrieval of interacting genes/proteins) analysis in fig. S8D. As proteomic analysis cannot distinguish differences in the multiple cell types in the human cortical samples, we compared the results with variations in the proteome between healthy and GBA-PD patient iPSC-derived DA neurons and found that 45.6% of proteins identified as different from controls in the GBA-PD iPSC–derived DA neurons changed in the same direction in the GBA-PD brains compared to healthy individual’s brains (Fig. 7D). We conclude that either proteostasis alterations in GBA-PD extend to most brain cell types or changes in the proteome of DA neurons are particularly pronounced and weighted higher over those in other cell types. Evidence supporting the second possibility is that top canonical pathway analysis of the altered proteins (elevated or reduced) highlighted DA synapses as a down-regulated set in GBA-PD (Fig. 7D, bottom). Also notable were changes in proteins related to proteostasis including protein translation, folding, and degradation (Fig. 7D, bottom). The GBA-PD brains exhibited elevation of 8 PD-related proteins and 15 protein products of genes related with heritable risk of PD from a meta-analysis of genome-wide association studies (Fig. 7E) (28). These changes occurred posttranslationally, as their mRNA levels were mostly unchanged (Fig. 7E). Levels of some PD proteins were unchanged or lower than in control healthy brains, likely due to a higher propensity to aggregate, as the proteomic analysis was performed on the soluble brain fraction. The set of proteins with decreased levels includes GCase itself, with a supersaturation index threefold higher than the average of the full proteome (σu = 0.04). The pool of proteins displaying elevated levels in the GBA-PD brain compared with healthy control exhibited an enrichment of those proteins bearing CMA-targeting motifs (97% versus 72% in the total proteome, P < 0.001), consistent with inhibition of CMA in PD-GBA.
Next, to determine whether any of the changes in the GBA-PD proteome were related to decreased CMA activity, we examined brains of a recently characterized mouse model that is unable to perform CMA [knockout for the CMA limiting protein LAMP2A (L2AKO mouse) (27)]. We found that of nearly 2000 proteins with changing levels in the GBA-PD human brain and the L2AKO mouse brain relative to their respective controls, almost half (42%) changed in the same direction (up-regulated or down-regulated) (Fig. 7, F and G). As for the human GBA-PD brains, RNA-seq analysis revealed that most changes in protein levels occur posttranscriptionally (fig. S7F), consistent with an inhibition of protein degradation. CMA-targeting motifs were present in 90% of the up-regulated proteins in both models. Using STRING analysis of proteins with coincident changes in the patients and the CMA-defective mouse brains, we identified enrichment of proteins involved in glucose and lipid metabolism, which are well-characterized CMA substrates, as well as cell death and neurogenesis and in processes related to vesicular trafficking, including synaptic signaling, endocytosis, and autophagy (Fig. 7H). One of the overrepresented gene ontology (GO) terms was PD, and as in the human GBA-PD brain, we found that the CMA-incompetent mouse brain also displayed elevated levels of 6 PD-related proteins and 10 of the proteins associated with heritable risk of PD (fig. S8E).
We took advantage of the recent proteomic analysis of aggregates and lysosomes isolated from WT and L2AKO mouse brains (27) to confirm that PD-related proteins displaying lower levels in the CMA-incompetent model were detected in the aggregates from L2AKO brains. Many proteins that were elevated in GBA-PD human brains were detected at lower levels in lysosomes from the L2AKO mice compared to control littermates, consistent with their cytosolic accumulation due to deficient lysosomal degradation (Fig. 7I shows representative examples). Similarly, many proteins present at lower levels in GBA-PD human brain tissue and L2AKO brain were also decreased in the L2AKO lysosomes and increased in the aggregate fraction from the same mice (see Fig. 7J for examples and fig. S8F). This is consistent with the immunoblot of total brain homogenates, which demonstrates significantly higher total levels of α-synuclein in the GBA-PD brains (fig. S2, F and G). We conclude that decreased CMA activity associated with GBA-PD genotypes disrupts neuronal proteostasis, leading to accumulation of CMA substrates including other PD-related and prone-to-aggregate proteins.
DISCUSSION
We report that misfolded MT GCase inhibits lysosomal CMA, resulting in a disruption of proteostasis in GBA-PD brain. The data indicate that a fraction of GCase that fails to fold in the ER is efficiently targeted to lysosomes by CMA but blocks the multimerization of LAMP2A. In the case of misfolded MT GCase, this blocks the normal uptake and degradation of other CMA substrates including α-synuclein and so could trigger the buildup of α-synuclein, a process widely suspected as a convergent cause of PD (Fig. 8). These results are consistent with analysis of lysosomes in anterior cingulate brain tissue, a region that displays strong synucleinopathy in GBA-PD. While GBA-PD patients had low levels of lysosomal GCase activity, with the exception of a single sample, they did not display a buildup of the GCase substrates. We found, however, that only subjects heterozygous for MT GCase exhibited GCase on the cytosolic face of lysosomes, where we also detected high levels of established CMA substrates, including α-synuclein.
Fig. 8. MT GCase induces CMA toxicity.
Upon folding in the ER, GCase normally traffics through the Golgi from where it is targeted to lysosomes in the lumen of small vesicles. MT GCase that fails to achieve proper folding is retrotranslocated from the ER into the cytosol for ER-associated degradation (ERAD) by the proteasome. In this study, we found that a fraction of this MT GCase is recognized by hsc70 that targets it to lysosomes for CMA degradation. MT GCase displays a very inefficient lysosomal internalization via the LAMP2A multimeric translocation complex, thus blocking degradation of other CMA substrates including α-synuclein.
Together, these analyses may assist in defining differences between effects of homozygous MT GBA1 alleles that cause Gaucher’s disease, including lysosomal accumulation of GCase substrates, with earlier disease presentation (including in infants), which typically do not include PD, and heterozygous MT GBA1 alleles, which typically do not include obvious pathological substrate accumulation and cause PD at older ages. As we find that unfolded MT GCase is required for CMA blockade, the results further suggest a basis for reports that mice with MT GCase (GBA1D409V/+) feature α-synuclein aggregation, whereas GCase-deficient mice (GBA1+/−) do not (29).
Our results in multiple systems, including control and patient-derived fibroblasts and iPSC-derived DA neurons, a mouse model, and human brain samples, are consistent with studies from Horowitz and colleagues (9, 30) reporting that a variety of Gaucher’s disease mutations, including truncated proteins, drive the accumulation of unfolded GCase in ER labeled by Bip1, a resident chaperone that participates in the unfolded protein response. While unfolded proteins in the ER are generally considered to be eventually degraded by the ubiquitin-proteasome system, CMA active lysosomes are often found in close proximity to the ER membrane, and recent studies demonstrate that CMA contributes to ER homeostasis (31), suggesting the possibility of CMA degradation for those misfolded proteins in the ER that have CMA-targeting motifs.
We found that the CMA targeting of GCase is dependent on the sequence 256QRDFI260. Unfolded GCase strongly binds LAMP2A and inhibits its multimerization, blocking CMA of other substrates including tau and α-synuclein. These observations are consistent with reports of tau and α-synuclein accumulation in GCase PD mouse models (32).
The steps demonstrated in this study could elucidate the accumulation of these proteins and other CMA substrates in the Lewy body pathology of GBA-PD (33, 34). Consistently, expression of MT GCase drives an α-synuclein–dependent death of primary cultured substantia nigra DA neurons, indicating that GCase and α-synuclein interact to produce neurotoxicity. This cell death was dependent on the presence of the CMA motif in GCase, indicating that the toxic interaction was dependent on MT GCase inhibition of CMA. Recent studies from our groups have demonstrated that experimental blockage of CMA in neurons in mice leads to neurodegeneration (27). Neuronal dysfunction in this model was due to the absence of appropriate degradation of a portion of the metastable neuronal proteome, as well as loss of function of the CMA substrates entrapped in aggregates that formed upon CMA blockage, including proteins responsible for normal glycolytic activity, actin dynamics, and endocytosis. The failure of these cellular pathways upon GCase-mediated CMA blockage could also contribute to progression of the disease in GBA-PD patients.
A role for CMA in PD is suggested by reports demonstrating that MT α-synuclein, LRRK2, and now MT GCase each block CMA, while MT UCH-L1 and Vps35 may indirectly impair CMA (3, 5) through changes in the lysosomal system, and sporadic PD patients are reported to exhibit low levels of the CMA LAMP2A receptor (35). It may be that disturbance of lysosomal degradation may underlie a variety of convergent causes of Lewy body–associated disorders (35). As there is a decrease of both CMA and macroautophagy with aging (35, 36), older age, the strongest risk factor for PD, could contribute to the eventual failure of this compensation. It should be noted that the oxidation of cytosolic dopamine has been reported to modify both α-synuclein (37) and GCase (38), and such modifications could contribute to PD’s association with damage to catecholaminergic neurons during older age.
MATERIALS AND METHODS
Animals, cells, and virus
Adult male Wistar rats were used in these studies for subcellular fractionation under animal study protocols approved by the Institutional Animal Care and Use Committee of Columbia University and Albert Einstein College of Medicine. Heterozygous GBA+/L444P mice were obtained from the Mutant Mouse Regional Resource Center (MMRRC) at the University of North Carolina (B6; 129S4-Gbatml/MMRRC stock no. 000117-UNC), and 8-month-old mice were used for the experiments. Where indicated, rodents were starved for 48 hours to activate CMA. NIH3T3 cells were from the American Type Culture Collection. M17 cell lines of α-synuclein under the control of doxycycline were generated as described (19), α-synuclein was induced by adding doxycycline for 24 hours, and then doxycycline was washed out to observe the time-dependent α-synuclein degradation. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, St. Louis, MO) in the presence of 10% fetal bovine serum (FBS) (Sigma-Aldrich), penicillin (50 μg/ml), and streptomycin (50 μg/ml) at 37°C with 5% CO2. Where indicated, cells were washed with Hank’s balanced salt solution (Invitrogen) and subsequently maintained in serum-free medium to activate CMA or macroautophagy. Adeno-associated virus (AAV) carrying either green fluorescent protein (GFP) or different human GCase constructs was from Genzyme. We generated lentivirus carrying GCase mutations by cloning GCase complementary DNA (cDNA) into pLenti7.3/DEST-V5 vector (Invitrogen), and we generated lentivirus according to the user’s manual. Human fibroblasts were obtained from Coriell Institute, and the demographics were described in table S3. Treatments to determine the contribution of different proteolytic systems to GCase degradation were done by incubating cells with 10 mM NH4Cl and 100 μM leupeptin (to inhibit lysosomal proteolysis) or 10 μM MG-132 (to inhibit proteasome). Blockage of deubiquitinases was attained by incubation with the pan-deubiquitinase inhibitor (DUBi) ubiquitin aldehyde (10 μM) (Enzo), and for p97 with 5 μM NMS873 (Tocris), for 12 hours in serum-supplemented medium.
Generation of SH-SY5Y cells stably expressing WT or MT GCase
Human neuroblastoma SH-SY5Y cells were maintained in DMEM plus 10% FBS. To prepare the stable transformed cells, SH-SY5Y cells were transfected with pcDNA3.1-myc-WT-GBA, N370S-GBA, L444P-GBA, and D490H-GBA. Selection was started 2 days later using a medium containing geneticin (700 μg/ml) (G418, Invitrogen). Individual clones were isolated and characterized by Western blot analysis with an antibody against anti-myc or GCase. SH-SY5Y cells were transiently transfected with the target vectors using the Lipofectamine Plus reagent methods (Invitrogen) according to the manufacturer’s instructions.
To generate GCase’s MT plasmids, site-directed mutagenesis was carried out using a QuikChange change site-directed mutagenesis kit (Stratagene) with the following primers: N370S, 5′-CCACAGCATCATCACGAGCCTCCTGTACC-3′ (forward) and 5′-GGTACAGGAGGCTCGTGATGATGCTGTGG-3′ (reverse); L444P, 5′-GTCAGAAGAACGACCCAGACGCAGTGGCAC-3′ (forward) and 5′-GTGCCACTGCGTCTGGGTCGTTCTTCTGAC-3′ (reverse); and D409H, 5′-GTAGACATCACCAAGCACACGTTTTACAAACAGC-3′ (forward) and 5′-GCTGTTTGTAAAACGTGTGCTTGGTGATGTCTAC-3′ (reverse). All mutation sites were confirmed by DNA sequencing analyses.
Postmortem human brains and iPSC-derived DA neurons
Frozen and paraffin-embedded brain tissues from the anterior cingulate gyrus were obtained from the New York Brain Bank. These brains have been characterized extensively and published (35). Each PD subject had cingulate cortical involvement (Braak PD stage ≥ 5). The basic demographic, pathological characterization, and genetic mutations are detailed in tables S1 and S2. All the brains (7 age-matched controls, 12 idiopathic PD cases without GBA1 mutations, and 13 PD cases with heterozygous GBA1 mutations) were subjected to measurement of lysosomal activity and lipid profiles. A subset of subjects (seven age-matched controls and seven PD cases with heterozygous GBA1 mutations) was used to measure lysosomal markers by Western blot analyses. For the Western blot analyses of postmortem PD brains, frozen anterior cingulate gyrus was solubilized in radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich), proteinase inhibitor cocktail tablets (Roche Diagnostics), and phosphatase inhibitor cocktails (Sigma-Aldrich) by sonication for 10 s. The samples were centrifuged, and the supernatants were subjected to Western blot analysis.
For iPSC-derived DA neurons, we selected a control and a pair of twins discordant of PD with heterozygous GBA1 N370S mutation (non-PD and PD). Each subject had two clones of iPSC lines from independent reprogramming. These lines have been extensively characterized and published previously (39). Dopamine neurons were differentiated from iPSCs using a previously published protocol, with neuronal surface marker sorting to achieve ~80% differentiation efficiency (39). For CRISPR-mediated gene knock-in experiments, we used nucleofector electroporation (Lonza) with sfRNA-Cas9-GFP plasmids and single-stranded DNA (ssDNA) oligo with a silent mutation on PAM (NGG) sequence (AGCCTACTGTACCATGTGGTCGGCTGGACCGACTGGAACCTTGCCCTGAACCCCGAAGGAGGAC). iPSC colonies were picked, and GBA N370S mutations were confirmed by Sanger sequencing.
Chemicals
Sources of chemicals and antibodies were as described (2). 3-Methyladenine, ammonium chloride, MG-132, trypsin, and conduritol B epoxide (CBE) were from Sigma-Aldrich; leupeptin was from Calbiochem; Endo H was from Promega; and Triton X-100 was from LabChem Inc. N-hexanoyl-1,7-nitrobenzofurazan (NBD)-glucosylceramide was from Abcam. The antibody against the cytosolic tail of rat and mouse LAMP2A was from Invitrogen (AMC2), and the antibody against mouse total LAMP2 (clone H4B4) and LAMP1 (clone H4A3) was from the Developmental Hybridoma Bank (University of Iowa, Iowa City, IA). Antibodies were from the following: antibody against human LAMP1 was from Santa Cruz Biotechnology, against human LAMP2A was from Abcam, against human GCase was from Novus, against actin was from Sigma-Aldrich, against hsc-70 was from Abcam, against hsp-70 was from Invitrogen, against RNase A was from Rockland Immunochemicals, against hsc70 was from Novus, against human α-synuclein was from BD Biosciences and Sigma-Aldrich, against GAPDH was from Abcam, against total tau (DA9) was a gift from P. Davies (The Feinstein Institute), against BiP was from BD Transduction, against Sec61β was from Thermo Fisher Scientific, and against cathepsin D was from Santa Cruz Biotechnology. LysoTracker used for in vitro studies was from Invitrogen. The purified WT and MT GCases were obtained from Genzyme and GenScript (D409H GCase MT protein), purified tau-441 (2N4R) was obtained from rPeptide, and WT and MT α-synuclein were purified as previously described (1).
Lysosomal isolation and lysosomal subfractionation
Lysosomes with high activity for CMA were isolated by centrifugation of a light mitochondrial-lysosomal fraction in a discontinuous metrizamide density gradient by the modified method described previously (2). Preparations with more than 10% broken lysosomes at the moment of the isolation, measured by β-hexosaminidase latency (40), were discarded.
Intracellular protein turnover
To measure degradation of long-lived proteins, confluent cells were labeled with [3H]leucine (2 μCi/ml) for 48 hours at 37°C and then extensively washed and maintained in complete (10% FBS) or serum-deprived medium containing an excess of unlabeled leucine (2.8 mM) to prevent reutilization of radiolabeled leucine (41). Aliquots of the medium taken at different times were precipitated with trichloroacetic acid (TCA), and proteolysis was measured as the percentage of the initial acid-insoluble radioactivity (protein) transformed into acid-soluble radioactivity (amino acids and small peptides) at the end of the incubation. Total radioactivity incorporated into cellular proteins was determined as the amount of acid-precipitable radioactivity in labeled cells immediately after washing. Where indicated, serum was removed from the medium during the chase, and 20 mM NH4Cl and 100 μM leupeptin were added (41).
Degradation of substrate proteins by intact lysosomes in vitro
Degradation of radiolabeled proteins by isolated intact lysosomes was measured as described previously (41). Briefly, lysosomes isolated from rat livers were incubated with radiolabeled proteins in 3-(N-morpholino) propanesulfonic acid (Mops) buffer [10 mM Mops (pH 7.4), 0.3 M sucrose, 1 mM dithiothreitol, and 5.4 M cysteine] for 30 min at 37°C. Reactions were stopped with 20% TCA and filtered through the Multiscreen Assay System (Millipore, Billerica, MA) and detected in a WinSpectral 1414 liquid scintillation analyzer (PerkinElmer Wallac, Gaithersburg, MD). Proteolysis was measured as the percentage of the initial acid-insoluble radioactivity transformed into acid-soluble radioactivity as described above (2).
Binding and uptake of CMA substrate proteins by isolated lysosomes
Uptake of RNase A and GCase proteins by isolated lysosomes was analyzed as described previously (41). Briefly, freshly isolated intact lysosomes were incubated with the substrate protein in Mops buffer at 37°C for 20 min. Where indicated, lysosomes were preincubated with a cocktail of protease inhibitors for 10 min at 0°C as described before. Lysosomes were collected by centrifugation, washed with Mops buffer, and subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted for the used substrate protein. Binding was calculated from the densitometric analysis as the amount of substrate protein bound to the lysosomal membrane in the absence of protease inhibitors. Uptake was calculated by subtracting the amount of protein associated with lysosomes in the presence (protein bound to the lysosomal membrane and taken up by lysosomes) and absence (protein bound to the lysosomal membrane) of protease inhibitors. GCase proteins were denaturized by incubation in Mops buffer at 95°C for 30 min. Possible aggregates were eliminated by a short spin at 15,000g for 5 min, and the concentration of protein was newly measured.
Neuronal cell culture and neuronal survival assay
Ventral midbrain neurons from postnatal days 0 to 2 mice were dissected, dissociated, and plated on a monolayer of cortical astrocytes at a plating density of ~100,000 cells/cm2, as previously described (42). Mouse neurons were cultured on glass poly-d-lysine–coated coverslips attached to ~0.8-cm2 wells cut into 50-mm dishes. Neurons were transduced with lentivirus carrying either WT or MT GCase at DIV1 (day 1 in vitro), and the DA neuronal density was determined at DIV7 as previously described (41, 42).
Measurement of CMA activity in intact cells
NIH3T3 cell lines were transduced with lentivirus carrying various GCase constructs. GFP was expressed in these cells under a different promoter in the lentivirus. GFP-expressing cells were fluorescence-activated cell sorting (FACS)–sorted and maintained. Cells were transduced with lentivirus carrying the CMA reporter KFERQ-PA-mCherry1 (16). Cells were photoactivated 48 hours after infection by a 405-nm light-emitting diode (LED; Norlux) for 3 min with an intensity of 3.5 mA (current constant). After 8 and 12 hours, cells were fixed with 4% paraformaldehyde and images were acquired with a Leica confocal microscope. Images were prepared using Adobe Photoshop CS6 software (Adobe Systems Inc., Mountain View, CA). CMA activity is determined in this system as a change in the fluorescent pattern from diffuse (cytosolic) to punctate (lysosomal). The number of fluorescent puncta per cell was quantified using ImageJ software [National Institutes of Health (NIH)] in individual frames after thresholding (16). Where indicated, cells plated in glass-bottom 96-well plates were treated, stained with BODIPY 493/503, and fixed, and images were acquired using a high-content microscope (Operetta, PerkinElmer). Images of nine different fields per well were captured, resulting in an average of 1500 to 2000 cells. Nuclei and puncta were identified using the manufacturer’s software. The number of particles/puncta per cell was quantified using “analyze particles” function of ImageJ after thresholding in nonsaturated images.
LAMP2A dynamics
Multimerization of LAMP2A at the lysosomal membrane was studied using blue native electrophoresis (BNE) as previously described. Briefly, isolated lysosomes preincubated with purified WT or MT GCase and α-synuclein were solubilized using 1% octylglucoside (in 20 mM Mops and 150 mM NaCl buffer), and the supernatants after centrifugation were supplemented with nonreducing sample buffer and subjected to BNE on 3 to 12% NativePAGE Bis-Tris Gels (Invitrogen) (24).
Immunofluorescence and immunohistochemistry
Cells grown on coverslips were fixed with 4% paraformaldehyde or methanol, blocked, and then incubated with the primary and corresponding fluorophore-conjugated secondary antibodies. Mounting medium contained DAPI (4′,6-diamidino-2-phenylindole) to highlight the cellular nucleus. Images were acquired and quantified as described previously (43). Formalin-fixed brain tissues were paraffin-embedded and sectioned for immunohistochemistry analysis. Tissue blocks of the anterior cingulate gyrus were sectioned at 7-μm thickness. Antigen retrieval was performed with Trilogy (Cell Marque) in a vegetable steamer for 40-min immunostaining for the desired proteins followed the standard procedures. Cortical neurons were imaged with neurons selected randomly, and fields of 75 μm by 75 μm were photographed in a confocal fluorescent microscope (Leica). The micrographs were analyzed for the area occupied by the LAMP2A-positive puncta per cell. The colocalization of exogenous GCase with LAMP1 or BiP immunoreactivity used ImageJ with a colocalization plugin, adjusting the final intensity of the channels to detect colocalization. Colocalization was calculated by the Pearson’s correlation coefficient.
Proteomic analysis
Cytosolic fractions were lysed using 5% SDS and 50 mM triethylammonium bicarbonate (pH 7.55). Then, samples (100 μg) were digested by means of the ProtiFi S-Trap Mini Spin Column Digestion Protocol. Briefly, proteins were simultaneously reduced and alkylated in the dark [15 mM tris(2-carboxyethyl)phosphine (TCEP) and 30 mM chloroacetamide (CAA) for 30 min at room temperature] and then digested with trypsin (protein:enzyme ratio 1:25 for 1 hour at 47°C) (Promega). Resulting peptides were labeled using a multiplexed, amine-specific iTRAQ Reagent 8-plex kit following the manufacturer’s instructions. Samples were mixed in a 1:1 ratio based on total peptide amount. The final mixture was finally desalted using a Sep-Pak C18 cartridge (Waters) and dried before high-pH reversed-phase high-performance liquid chromatography (HPLC) fractionation. Peptides were prefractionated offline by means of high-pH reversed-phase chromatography using an Ultimate 3000 HPLC system equipped with a sample collector. Briefly, peptides were dissolved in 100 μl of phase A (10 mM NH4OH) and loaded onto an XBridge BEH130 C18 column (3.5 μm, 150 mm length, and 1 mm inside diameter) (Waters). Phase B was 10 mM NH4OH in 90% acetonitrile (ACN). The following gradient (flow rate of 100 μl/min) was used: 0 to 50 min, 0 to 25% B; 50 to 56 min, 25 to 60% B; and 56 to 57 min, 60 to 90% B. Fifty fractions were collected and concatenated into 15 fractions. Liquid chromatography–tandem mass spectrometry (MS/MS) was done by coupling the Ultimate 3000 RSLCnano System (Dionex) to a Q-Exactive HF mass spectrometer (Thermo Fisher Scientific). Peptides were loaded into a trap column (Acclaim PepMap 100, 100 μm by 2 cm, Thermo Fisher Scientific) for 3 min at a flow rate of 10 μl/min in 0.1% Formic acid (FA). Then, peptides were transferred to an analytical column (PepMap RSLC C18, 2 μm, 75 μm by 50 cm, Thermo Fisher Scientific) and separated using a 60-min effective linear gradient (buffer A: 0.1% FA; buffer B: 100% ACN, 0.1% FA) at a flow rate of 250 nl/min. The gradient used was as follows: 0 to 3 min, 2% B; 3 to 5 min, 6% B; 5 to 60 min, 25% B; 60 to 63 min, 33% B; 63 to 65 min, 45% B; 65 to 70 min, 98% B; and 70 to 80 min, 2% B. The peptides were electrosprayed (1.5 kV) into the mass spectrometer through a heated capillary at 300°C and an S-lens radio frequency (RF) level of 50%. The mass spectrometer was operated in a data-dependent mode, with an automatic switch between MS and MS/MS scans using a top 15 method (minimum AGC target 1E3) and a dynamic exclusion of 20 s. MS [350 to 1500 mass/charge ratio (m/z)] and MS/MS spectra were acquired with a resolution of 60,000 and 30,000 full width at half maximum (200 m/z), respectively. Peptides were isolated using a 1.4 Th window and fragmented using higher-energy collisional dissociation at 34% normalized collision energy. The ion target values were 3 × 106 for MS (25-ms maximum injection time) and 1 × 105 for MS/MS (45-ms maximum injection time). Raw files were processed with MaxQuant (v1.6.1.0) using the standard settings against a human protein database (UniProtKB/Swiss-Prot, July 2018, 20,373 sequences) supplemented with contaminants. Lysine side chain and peptide N-terminus labeling with iTRAQ Reagent 8-plex and carbamidomethylation of cysteines were considered as fixed modifications, whereas oxidation of methionines, deamidation of asparagines (N) and glutamines (Q) (NQ), and acetylation of protein N terminal were included as variable modifications. Minimal peptide length was set to seven amino acids, and a maximum of two tryptic missed cleavages were allowed. Results were filtered at 0.01 false discovery rate (peptide and protein levels). Afterward, the “proteinGroup.txt” file was loaded in Prostar (v1.14) (44) using the intensity values for further statistical analysis. A global normalization of log2-transformed intensities across samples was performed using the LOESS function. Missing values were imputed using the algorithms SLSA (for partially observed values) and DetQuantile (for values missing on an entire condition). Pathway analysis was performed using the IPA software (Ingenuity Systems), Reactome (https://reactome.org/), and STRING database (https://stringdb.org/).
Droplet-based snRNA-seq of human brain tissue
The protocol for isolating nuclei from frozen postmortem human brain tissue was adapted from a previous study with modifications (45, 46). All procedures were done on ice or at 4°C. Briefly, postmortem brain tissue was placed in 1500 μl of Nuclei PURE Lysis Buffer (Sigma-Aldrich, NUC201-1KT) and homogenized with a Dounce tissue grinder (Sigma-Aldrich, D8938-1SET) with 20 strokes with pestle A and 15 strokes with pestle B. The homogenized tissue was filtered through a 35-μm cell strainer, centrifuged at 600g for 5 min at 4°C, and washed three times with 1 ml of phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA), 20 mM dithiothreitol, and recombinant RNase inhibitor (0.2 U μl − 1). Then, the nuclei were centrifuged at 600g for 5 min at 4°C and resuspended in 800 μl of PBS containing 0.04% BSA and 1× DAPI, followed by FACS sorting to remove cell debris. The FACS-sorted suspension of DAPI-stained nuclei was counted and diluted to a concentration of 1000 nuclei/μl in PBS containing 0.04% BSA.
For droplet-based snRNA-seq, libraries were prepared with Chromium Single Cell 3′ Reagent Kits v3 (10x Genomics, PN-1000075) according to the manufacturer’s protocol. The snRNA-seq libraries were sequenced on a NovaSeq 6000 sequencer (Illumina) with 100 cycles.
Gene counts were obtained by aligning reads to the hg38 genome with Cell Ranger software (v.3.1.0) (10x Genomics). To account for unspliced nuclear transcripts, reads mapping to pre-mRNA were counted. Cell Ranger 3.1.0 default parameters were used to call cell barcodes. We further removed genes expressed in no more than three cells, cells with unique gene counts more than 9000 or less than 300, cells with unique molecular identifier (UMI) count more than 50,000, and cells with high fraction of mitochondrial reads (>5%). Potential doublet cells were predicted using DoubletFinder (47) for each sample separately, with high-confidence doublets removed. Normalization and clustering were done with Seurat package v3.0.1 (48). Briefly, counts for all nuclei were scaled by the total library size multiplied by a scale factor (10,000) and transformed to log space. A set of 2000 highly variable genes were identified with the FindVariableFeatures function in Seurat package with the default “vst” method. This returned a corrected UMI count matrix, a log-transformed data matrix, and Pearson residuals from the regularized negative binomial regression model. Principal components analysis (PCA) was done on all genes, and t-distributed stochastic neighbor embedding (t-SNE) was run on the top 15 PCs. Cell clusters were identified with the Seurat functions FindNeighbors (using the top 15 PCs) and FindClusters (resolution = 0.1). For each cluster, we assigned a cell type label using statistical enrichment for sets of marker genes (49, 50) and manual evaluation of gene expression for small sets of known marker genes. The subset() function from Seurat was used to subset excitatory and inhibitory neurons, respectively. Standard workflow for visualization and clustering was performed with default assay as “integrated” with 15 PCs used and resolution = 0.1.
Calculation of CMA activation score
Raw counts per mRNA or peptide were used. Before calculations, counts were log-normalized, and each element of the CMA network (26) was attributed a weight and a direction score of +1 or −1 based on the known effect of a given element on CMA activity. The score was calculated as the weighted/directed average of expression counts of every element of the CMA network.
General procedures
Protein-related procedures were as described before (2). Briefly, protein concentration was determined using the Lowry method using BSA as a standard. Cells were solubilized by incubation for 15 min on ice with RIPA buffer [1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, and 0.01 M sodium phosphate (pH 7.2)] containing protease inhibitors. After centrifugation at 16,000g for 15 min, the solubilized portion was collected and subjected to protein determination and immunoblot. Immunoblotting procedures included running samples on SDS-PAGE gels, transferring on nitrocellulose membranes, blockage, and incubation with the relevant primary antibody in 3% BSA. These proteins were visualized by chemiluminescence using peroxidase-conjugated secondary antibodies in the LAS-3000 Imaging System (Fujifilm). ImageJ software (NIH) was used for densitometric quantification. Coimmunoprecipitation was performed after solubilization of cellular extracts in 25 mM tris, 150 mM NaCl, and 0.5% NP-40 in the presence of phosphatase and protease inhibitors. Cells were sonicated and centrifuged at 16,000g for 15 min. Anti-V5 antibodies and protein A beads (Sigma-Aldrich) were used for immunoprecipitations, and beads were washed with buffer with 0.5% NP-40. Protein samples were subjected to Western blot analyses.
Statistical analysis
All numerical results are reported as means ± SEM and represent data from a minimum of three independent experiments unless otherwise stated. We determined the statistical significance of the difference between experimental groups in instances of single comparisons by the two-tailed unpaired Student’s t test of the means with the SigmaPlot software (Jandel Scientific). In instances of multiple means comparisons, we used one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test to determine statistical significance. In the case of the proteomic analysis of the human iPSC-derived DA neurons, we used a pool of three healthy controls and three separate GBA-PD patients and assumed that values came from a normal distribution and calculated the probability to draw the healthy control values from that distribution using t-statistic followed by TDIST function (DF, t stat, 1) to calculate P. All quantification was performed on a blinded basis. Specifically, each group was assigned an arbitrarily label, unrelated to the treatment condition and/or genotypes, and the identity of each group was not revealed until the quantification was completed.
Acknowledgments
Funding: This work was supported by grants from the NIH (K08 NS083738 and R01 NS104423 to S.-H.K., K01 MH096956 to G.T., AG031782 and AG038072 to A.M.C., R01 NS095435 to D.S., R01 NS104390 to G.T., and T32 GM007367 to O.J.L.), the JPB Foundation (to A.M.C., D.S., and L.G.), the Parkinson’s Foundation (to S.-H.K.), the American Parkinson’s Disease Association (to S.-H.K.), Louis V. Gerstner Jr. Scholar Award (to S.-H.K.), and the Backus Foundation (to A.M.C.). The CNIO Proteomics Unit laboratory is a member of Proteored, PRB3 and is supported by grant PT17/0019 of the PE I+D+i 2013-2016 funded by Instituto de Salud Carlos III (ISCIII) and European Regional Development Fund (ERDF).
Author contributions: S.-H.K., I.T., M.M.C., O.J.L., A.M.C., and D.S. designed the study and generated the cell biology results. I.T., A.D., S.-H.K., S.J.H., A.M.C., and D.S. designed the study and generated the biochemical results. E.K. generated neuronal cultures and associated results. A.L. generated results with iPSC-derived neurons. D.K. and H.S.K. generated SH-SY5Y cells expressing GCase. S.K. created the GBA CRISPR knockout lines and helped with biochemical analysis. T.Y. generated the inducible α-synuclein cell line. S.P.S. generated the GCase virus, purified GCase protein, and measured GCase activity. P.X.-E. and J.M. generated the proteomic data and assisted with the analysis. L.F. and L.G. generated snRNA-seq and performed the analysis. M.-K.P. and R.N.A. contributed to the postmortem human brain analysis. S.-H.K., I.T., A.M.C., and D.S. drafted, edited, and revised the manuscript. L.L. and G.T. provided GBA+/L444P MT mice and contributed to antibody validation and data analysis.
Competing interests: S.P.S. is a Sanofi employee and stockholder. R.N.A. has received consultation fees from Avrobio, Caraway, GSK, Merck, Sanofi, and Ono Therapeutics. A.M.C. consults for Life Biosciences, Generian Pharmaceutics, and Cognition Therapeutics. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The snRNA-seq data have been deposited to the Gene Expression Omnibus (GEO) with the dataset accession number GSE189202. All codes used for snRNA-seq data analysis have been archived at Zenodo (https://doi.org/10.5281/zenodo.5780933) and are directly available at https://github.com/lifan36/Kuo-Tasset-et-al-2021. The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD028656.
Supplementary Materials
This PDF file includes:
Figs. S1 to S8
Tables S1 to S3
REFERENCES AND NOTES
- 1.Cuervo A. M., Stefanis L., Fredenburg R., Lansbury P. T., Sulzer D., Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Orenstein S. J., Kuo S. H., Tasset I., Arias E., Koga H., Fernandez-Carasa I., Cortes E., Honig L. S., Dauer W., Consiglio A., Raya A., Sulzer D., Cuervo A. M., Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 16, 394–406 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kabuta T., Furuta A., Aoki S., Furuta K., Wada K., Aberrant interaction between Parkinson disease-associated mutant UCH-L1 and the lysosomal receptor for chaperone-mediated autophagy. J. Biol. Chem. 283, 23731–23738 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B., Cai Z., Tao K., Zeng W., Lu F., Yang R., Feng D., Gao G., Yang Q., Essential control of mitochondrial morphology and function by chaperone-mediated autophagy through degradation of PARK7. Autophagy 12, 1215–1228 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang F. L., Erion J. R., Tian Y., Liu W., Yin D. M., Ye J., Tang B., Mei L., Xiong W. C., VPS35 in dopamine neurons is required for endosome-to-golgi retrieval of Lamp2a, a receptor of chaperone-mediated autophagy that is critical for α-synuclein degradation and prevention of pathogenesis of Parkinson’s disease. J. Neurosci. 35, 10613–10628 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siebert M., Sidransky E., Westbroek W., Glucocerebrosidase is shaking up the synucleinopathies. Brain 137, 1304–1322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidransky E., Nalls M. A., Aasly J. O., Aharon-Peretz J., Annesi G., Barbosa E. R., Bar-Shira A., Berg D., Bras J., Brice A., Chen C. M., Clark L. N., Condroyer C., De Marco E. V., Durr A., Eblan M. J., Fahn S., Farrer M. J., Fung H. C., Gan-Or Z., Gasser T., Gershoni-Baruch R., Giladi N., Griffith A., Gurevich T., Januario C., Kropp P., Lang A. E., Lee-Chen G. J., Lesage S., Marder K., Mata I. F., Mirelman A., Mitsui J., Mizuta I., Nicoletti G., Oliveira C., Ottman R., Orr-Urtreger A., Pereira L. V., Quattrone A., Rogaeva E., Rolfs A., Rosenbaum H., Rozenberg R., Samii A., Samaddar T., Schulte C., Sharma M., Singleton A., Spitz M., Tan E. K., Tayebi N., Toda T., Troiano A. R., Tsuji S., Wittstock M., Wolfsberg T. G., Wu Y. R., Zabetian C. P., Zhao Y., Ziegler S. G., Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361, 1651–1661 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chabas A., Cormand B., Balcells S., Gonzalez-Duarte R., Casanova C., Colomer J., Vilageliu L., Grinberg D., Neuronopathic and non-neuronopathic presentation of Gaucher disease in patients with the third most common mutation (D409H) in Spain. J. Inherit. Metab. Dis. 19, 798–800 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Ron I., Horowitz M., ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum. Mol. Genet. 14, 2387–2398 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Cullen V., Sardi S. P., Ng J., Xu Y. H., Sun Y., Tomlinson J. J., Kolodziej P., Kahn I., Saftig P., Woulfe J., Rochet J. C., Glicksman M. A., Cheng S. H., Grabowski G. A., Shihabuddin L. S., Schlossmacher M. G., Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann. Neurol. 69, 940–953 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Caballero B., Bourdenx M., Luengo E., Diaz A., Sohn P. D., Chen X., Wang C., Juste Y. R., Wegmann S., Patel B., Young Z. T., Kuo S. Y., Rodriguez-Navarro J. A., Shao H., Lopez M. G., Karch C. M., Goate A. M., Gestwicki J. E., Hyman B. T., Gan L., Cuervo A. M., Acetylated tau inhibits chaperone-mediated autophagy and promotes tau pathology propagation in mice. Nat. Commun. 12, 2238 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gegg M. E., Sweet L., Wang B. H., Shihabuddin L. S., Sardi S. P., Schapira A. H., No evidence for substrate accumulation in Parkinson brains with GBA mutations. Mov. Disord. 30, 1085–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurzawa-Akanbi M., Hanson P. S., Blain P. G., Lett D. J., McKeith I. G., Chinnery P. F., Morris C. M., Glucocerebrosidase Mutations alter the endoplasmic reticulum and lysosomes in Lewy body disease. J. Neurochem. 123, 298–309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maor G., Rencus-Lazar S., Filocamo M., Steller H., Segal D., Horowitz M., Unfolded protein response in Gaucher disease: From human to Drosophila. Orphanet J. Rare Dis. 8, 140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ron I., Rapaport D., Horowitz M., Interaction between parkin and mutant glucocerebrosidase variants: A possible link between Parkinson disease and Gaucher disease. Hum. Mol. Genet. 19, 3771–3781 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Koga H., Martinez-Vicente M., Macian F., Verkhusha V. V., Cuervo A. M., A photoconvertible fluorescent reporter to track chaperone-mediated autophagy. Nat. Commun. 2, 386 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasmanik-Chor M., Elroy-Stein O., Aerts H., Agmon V., Gatt S., Horowitz M., Overexpression of human glucocerebrosidase containing different-sized leaders. Biochem. J. 317 ( Pt 1), 81–88 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzulli J. R., Xu Y. H., Sun Y., Knight A. L., McLean P. J., Caldwell G. A., Sidransky E., Grabowski G. A., Krainc D., Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146, 37–52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slone S. R., Lavalley N., McFerrin M., Wang B., Yacoubian T. A., Increased 14-3-3 phosphorylation observed in Parkinson’s disease reduces neuroprotective potential of 14-3-3 proteins. Neurobiol. Dis. 79, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W., Lesuisse C., Xu Y., Troncoso J. C., Price D. L., Lee M. K., Stabilization of α-synuclein protein with aging and familial parkinson’s disease-linked A53T mutation. J. Neurosci. 24, 7400–7409 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Martinez-Vicente M., Kruger U., Kaushik S., Wong E., Mandelkow E. M., Cuervo A. M., Mandelkow E., Tau fragmentation, aggregation and clearance: The dual role of lysosomal processing. Hum. Mol. Genet. 18, 4153–4170 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalaitzakis M. E., Pearce R. K., The morbid anatomy of dementia in Parkinson’s disease. Acta Neuropathol. 118, 587–598 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Caballero B., Wang Y., Diaz A., Tasset I., Juste Y. R., Stiller B., Mandelkow E. M., Mandelkow E., Cuervo A. M., Interplay of pathogenic forms of human tau with different autophagic pathways. Aging Cell 17, e12692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandyopadhyay U., Kaushik S., Varticovski L., Cuervo A. M., The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol. Cell. Biol. 28, 5747–5763 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R. G. W. Staal, S. Rayport, D. Sulzer, Frontiers in neuroengineering amperometric detection of dopamine exocytosis from synaptic terminals, in Electrochemical Methods for Neuroscience, A. C. Michael, L. M. Borland, Eds. (CRC Press/Taylor & Francis, 2007). [PubMed] [Google Scholar]
- 26.Kirchner P., Bourdenx M., Madrigal-Matute J., Tiano S., Diaz A., Bartholdy B. A., Will B., Cuervo A. M., Proteome-wide analysis of chaperone-mediated autophagy targeting motifs. PLOS Biol. 17, e3000301 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourdenx M., Martín-Segura A., Scrivo A., Rodriguez-Navarro J. A., Kaushik S., Tasset I., Diaz A., Storm N. J., Xin Q., Juste Y. R., Stevenson E., Luengo E., Clement C. C., Choi S. J., Krogan N. J., Mosharov E. V., Santambrogio L., Grueninger F., Collin L., Swaney D. L., Sulzer D., Gavathiotis E., Cuervo A. M., Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell 184, 2696–2714.25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nalls M. A., Blauwendraat C., Vallerga C. L., Heilbron K., Bandres-Ciga S., Chang D., Tan M., Kia D. A., Noyce A. J., Xue A., Bras J., Young E., von Coelln R., Simón-Sánchez J., Schulte C., Sharma M., Krohn L., Pihlstrøm L., Siitonen A., Iwaki H., Leonard H., Faghri F., Gibbs J. R., Hernandez D. G., Scholz S. W., Botia J. A., Martinez M., Corvol J. C., Lesage S., Jankovic J., Shulman L. M., Sutherland M., Tienari P., Majamaa K., Toft M., Andreassen O. A., Bangale T., Brice A., Yang J., Gan-Or Z., Gasser T., Heutink P., Shulman J. M., Wood N. W., Hinds D. A., Hardy J. A., Morris H. R., Gratten J., Visscher P. M., Graham R. R., Singleton A. B., Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sardi S. P., Clarke J., Kinnecom C., Tamsett T. J., Li L., Stanek L. M., Passini M. A., Grabowski G. A., Schlossmacher M. G., Sidman R. L., Cheng S. H., Shihabuddin L. S., CNS expression of glucocerebrosidase corrects α-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl. Acad. Sci. U.S.A. 108, 12101–12106 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bendikov-Bar I., Ron I., Filocamo M., Horowitz M., Characterization of the ERAD process of the L444P mutant glucocerebrosidase variant. Blood Cells Mol. Dis. 46, 4–10 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Li W., Zhu J., Dou J., She H., Tao K., Xu H., Yang Q., Mao Z., Phosphorylation of LAMP2A by p38 MAPK couples ER stress to chaperone-mediated autophagy. Nat. Commun. 8, 1763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sardi S. P., Clarke J., Viel C., Chan M., Tamsett T. J., Treleaven C. M., Bu J., Sweet L., Passini M. A., Dodge J. C., Yu W. H., Sidman R. L., Cheng S. H., Shihabuddin L. S., Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc. Natl. Acad. Sci. U.S.A. 110, 3537–3542 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuang D., Leverenz J. B., Lopez O. L., Hamilton R. L., Bennett D. A., Schneider J. A., Buchman A. S., Larson E. B., Crane P. K., Kaye J. A., Kramer P., Woltjer R., Kukull W., Nelson P. T., Jicha G. A., Neltner J. H., Galasko D., Masliah E., Trojanowski J. Q., Schellenberg G. D., Yearout D., Huston H., Fritts-Penniman A., Mata I. F., Wan J. Y., Edwards K. L., Montine T. J., Zabetian C. P., GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology 79, 1944–1950 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins L. M., Williams-Gray C. H., The genetic basis of cognitive impairment and dementia in Parkinson’s disease. Front. Psychol. 7, 89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider J. L., Villarroya J., Diaz-Carretero A., Patel B., Urbanska A. M., Thi M. M., Villarroya F., Santambrogio L., Cuervo A. M., Loss of hepatic chaperone-mediated autophagy accelerates proteostasis failure in aging. Aging Cell 14, 249–264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuervo A. M., Dice J. F., Age-related decline in chaperone-mediated autophagy. J. Biol. Chem. 275, 31505–31513 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Vicente M., Talloczy Z., Kaushik S., Massey A. C., Mazzulli J., Mosharov E. V., Hodara R., Fredenburg R., Wu D. C., Follenzi A., Dauer W., Przedborski S., Ischiropoulos H., Lansbury P. T., Sulzer D., Cuervo A. M., Dopamine-modified α-synuclein blocks chaperone-mediated autophagy. J. Clin. Invest. 118, 777–788 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burbulla L. F., Song P., Mazzulli J. R., Zampese E., Wong Y. C., Jeon S., Santos D. P., Blanz J., Obermaier C. D., Strojny C., Savas J. N., Kiskinis E., Zhuang X., Kruger R., Surmeier D. J., Krainc D., Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357, 1255–1261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodard C. M., Campos B. A., Kuo S. H., Nirenberg M. J., Nestor M. W., Zimmer M., Mosharov E. V., Sulzer D., Zhou H., Paull D., Clark L., Schadt E. E., Sardi S. P., Rubin L., Eggan K., Brock M., Lipnick S., Rao M., Chang S., Li A., Noggle S. A., iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson’s disease. Cell Rep. 9, 1173–1182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storrie B., Madden E., [16] Isolation of subcellular organelles. Methods Enzymol. 182, 203–225 (1990). [DOI] [PubMed] [Google Scholar]
- 41.Patel B., Cuervo A. M., Methods to study chaperone-mediated autophagy. Methods 75, 133–140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosharov E. V., Larsen K. E., Kanter E., Phillips K. A., Wilson K., Schmitz Y., Krantz D. E., Kobayashi K., Edwards R. H., Sulzer D., Interplay between cytosolic dopamine, calcium, and α-synuclein causes selective death of substantia nigra neurons. Neuron 62, 218–229 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klionsky D. J., Abdelmohsen K., Abe A., Abedin M. J., Abeliovich H., Arozena A. A., Adachi H., Adams C. M., Adams P. D., Adeli K., Adhihetty P. J., Adler S. G., Agam G., Agarwal R., Aghi M. K., Agnello M., Agostinis P., Aguilar P. V., Aguirre-Ghiso J., Airoldi E. M., Ait-Si-Ali S., Akematsu T., Akporiaye E. T., Al-Rubeai M., Albaiceta G. M., Albanese C., Albani D., Albert M. L., Aldudo J., Algül H., Alirezaei M., Alloza I., Almasan A., Almonte-Beceril M., Alnemri E. S., Alonso C., Altan-Bonnet N., Altieri D. C., Alvarez S., Alvarez-Erviti L., Alves S., Amadoro G., Amano A., Amantini C., Ambrosio S., Amelio I., Amer A. O., Amessou M., Amon A., An Z., Anania F. A., Andersen S. U., Andley U. P., Andreadi C. K., Andrieu-Abadie N., Anel A., Ann D. K., Anoopkumar-Dukie S., Antonioli M., Aoki H., Apostolova N., Aquila S., Aquilano K., Araki K., Arama E., Aranda A., Araya J., Arcaro A., Arias E., Arimoto H., Ariosa A. R., Armstrong J. L., Arnould T., Arsov I., Asanuma K., Askanas V., Asselin E., Atarashi R., Atherton S. S., Atkin J. D., Attardi L. D., Auberger P., Auburger G., Aurelian L., Autelli R., Avagliano L., Avantaggiati M. L., Avrahami L., Awale S., Azad N., Bachetti T., Backer J. M., Bae D.-H., Bae J.-S., Bae O.-N., Bae S. H., Baehrecke E. H., Baek S.-H., Baghdiguian S., Bagniewska-Zadworna A., Bai H., Bai J., Bai X.-Y., Bailly Y., Balaji K. N., Balduini W., Ballabio A., Balzan R., Banerjee R., Bánhegyi G., Bao H., Barbeau B., Barrachina M. D., Barreiro E., Bartel B., Bartolomé A., Bassham D. C., Bassi M. T., Bast R. C. Jr, Basu A., Batista M. T., Batoko H., Battino M., Bauckman K., Baumgarner B. L., Bayer K. U., Beale R., Beaulieu J.-F., Beck G. R. Jr, Becker C., Beckham J. D., Bédard P.-A., Bednarski P. J., Begley T. J., Behl C., Behrends C., Behrens G. M., Behrns K. E., Bejarano E., Belaid A., Belleudi F., Bénard G., Berchem G., Bergamaschi D., Bergami M., Berkhout B., Berliocchi L., Bernard A., Bernard M., Bernassola F., Bertolotti A., Bess A. S., Besteiro S., Bettuzzi S., Bhalla S., Bhattacharyya S., Bhutia S. K., Biagosch C., Bianchi M. W., Biard-Piechaczyk M., Billes V., Bincoletto C., Bingol B., Bird S. W., Bitoun M., Bjedov I., Blackstone C., Blanc L., Blanco G. A., Blomhoff H. K., Boada-Romero E., Böckler S., Boes M., Boesze-Battaglia K., Boise L. H., Bolino A., Boman A., Bonaldo P., Bordi M., Bosch J., Botana L. M., Botti J., Bou G., Bouché M., Bouchecareilh M., Boucher M.-J., Boulton M. E., Bouret S. G., Boya P., Boyer-Guittaut M., Bozhkov P. V., Brady N., Braga V. M., Brancolini C., Braus G. H., Bravo-San Pedro J. M., Brennan L. A., Bresnick E. H., Brest P., Bridges D., Bringer M.-A., Brini M., Brito G. C., Brodin B., Brookes P. S., Brown E. J., Brown K., Broxmeyer H. E., Bruhat A., Brum P. C., Brumell J. H., Brunetti-Pierri N., Bryson-Richardson R. J., Buch S., Buchan A. M., Budak H., Bulavin D. V., Bultman S. J., Bultynck G., Bumbasirevic V., Burelle Y., Burke R. E., Burmeister M., Bütikofer P., Caberlotto L., Cadwell K., Cahova M., Cai D., Cai J., Cai Q., Calatayud S., Camougrand N., Campanella M., Campbell G. R., Campbell M., Campello S., Candau R., Caniggia I., Cantoni L., Cao L., Caplan A. B., Caraglia M., Cardinali C., Cardoso S. M., Carew J. S., Carleton L. A., Carlin C. R., Carloni S., Carlsson S. R., Carmona-Gutierrez D., Carneiro L. A., Carnevali O., Carra S., Carrier A., Carroll B., Casas C., Casas J., Cassinelli G., Castets P., Castro-Obregon S., Cavallini G., Ceccherini I., Cecconi F., Cederbaum A. I., Ceña V., Cenci S., Cerella C., Cervia D., Cetrullo S., Chaachouay H., Chae H.-J., Chagin A. S., Chai C.-Y., Chakrabarti G., Chamilos G., Chan E. Y., Chan M. T., Chandra D., Chandra P., Chang C.-P., Chang R. C.-C., Chang T. Y., Chatham J. C., Chatterjee S., Chauhan S., Che Y., Cheetham M. E., Cheluvappa R., Chen C.-J., Chen G., Chen G.-C., Chen G., Chen H., Chen J. W., Chen J.-K., Chen M., Chen M., Chen P., Chen Q., Chen Q., Chen S.-D., Chen S., Chen S. S.-L., Chen W., Chen W.-J., Chen W. Q., Chen W., Chen X., Chen Y.-H., Chen Y.-G., Chen Y., Chen Y., Chen Y., Chen Y.-J., Chen Y.-Q., Chen Y., Chen Z., Chen Z., Cheng A., Cheng C. H., Cheng H., Cheong H., Cherry S., Chesney J., Cheung C. H. A., Chevet E., Chi H. C., Chi S.-G., Chiacchiera F., Chiang H.-L., Chiarelli R., Chiariello M., Chieppa M., Chin L.-S., Chiong M., Chiu G. N., Cho D.-H., Cho S.-G., Cho W. C., Cho Y.-Y., Cho Y.-S., Choi A. M., Choi E.-J., Choi E.-K., Choi J., Choi M. E., Choi S.-I., Chou T.-F., Chouaib S., Choubey D., Choubey V., Chow K.-C., Chowdhury K., Chu C. T., Chuang T.-H., Chun T., Chung H., Chung T., Chung Y.-L., Chwae Y.-J., Cianfanelli V., Ciarcia R., Ciechomska I. A., Ciriolo M. R., Cirone M., Claerhout S., Clague M. J., Clària J., Clarke P. G., Clarke R., Clementi E., Cleyrat C., Cnop M., Coccia E. M., Cocco T., Codogno P., Coers J., Cohen E. E., Colecchia D., Coletto L., Coll N. S., Colucci-Guyon E., Comincini S., Condello M., Cook K. L., Coombs G. H., Cooper C. D., Cooper J. M., Coppens I., Corasaniti M. T., Corazzari M., Corbalan R., Corcelle-Termeau E., Cordero M. D., Corral-Ramos C., Corti O., Cossarizza A., Costelli P., Costes S., Cotman S. L., Coto-Montes A., Cottet S., Couve E., Covey L. R., Cowart L. A., Cox J. S., Coxon F. P., Coyne C. B., Cragg M. S., Craven R. J., Crepaldi T., Crespo J. L., Criollo A., Crippa V., Cruz M. T., Cuervo A. M., Cuezva J. M., Cui T., Cutillas P. R., Czaja M. J., Czyzyk-Krzeska M. F., Dagda R. K., Dahmen U., Dai C., Dai W., Dai Y., Dalby K. N., Valle L. D., Dalmasso G., D’Amelio M., Damme M., Darfeuille-Michaud A., Dargemont C., Darley-Usmar V. M., Dasarathy S., Dasgupta B., Dash S., Dass C. R., Davey H. M., Davids L. M., Dávila D., Davis R. J., Dawson T. M., Dawson V. L., Daza P., de Belleroche J., de Figueiredo P., de Figueiredo R. C. B. Q., de la Fuente J., De Martino L., De Matteis A., De Meyer G. R., De Milito A., De Santi M., de Souza W., De Tata V., De Zio D., Debnath J., Dechant R., Decuypere J.-P., Deegan S., Dehay B., Bello B. D., Del Re D. P., Delage-Mourroux R., Delbridge L. M. D., Deldicque L., Delorme-Axford E., Deng Y., Dengjel J., Denizot M., Dent P., Der C. J., Deretic V., Derrien B., Deutsch E., Devarenne T. P., Devenish R. J., Bartolomeo S. D., Daniele N. D., Domenico F. D., Nardo A. D., Paola S. D., Pietro A. D., Renzo L. D., Antonio A. D., Díaz-Araya G., Díaz-Laviada I., Diaz-Meco M. T., Diaz-Nido J., Dickey C. A., Dickson R. C., Diederich M., Digard P., Dikic I., Dinesh-Kumar S. P., Ding C., Ding W.-X., Ding Z., Dini L., Distler J. H. W., Diwan A., Djavaheri-Mergny M., Dmytruk K., Dobson R. C. J., Doetsch V., Dokladny K., Dokudovskaya S., Donadelli M., Dong X. C., Dong X., Dong Z., Donohue T. M. Jr., Doran K. S., D’Orazi G., Dorn G. W. II, Dosenko V., Dridi S., Drucker L., Du J., Du L.-L., Du L., du Toit A., Dua P., Duan L., Duann P., Dubey V. K., Duchen M. R., Duchosal M. A., Duez H., Dugail I., Dumit V. I., Duncan M. C., Dunlop E. A., Dunn W. A. Jr, Dupont N., Dupuis L., Durán R. V., Durcan T. M., Duvezin-Caubet S., Duvvuri U., Eapen V., Ebrahimi-Fakhari D., Echard A., Eckhart L., Edelstein C. L., Edinger A. L., Eichinger L., Eisenberg T., Eisenberg-Lerner A., Eissa N. T., El-Deiry W. S., El-Khoury V., Elazar Z., Eldar-Finkelman H., Elliott C. J. H., Emanuele E., Emmenegger U., Engedal N., Engelbrecht A.-M., Engelender S., Enserink J. M., Erdmann R., Erenpreisa J., Eri R., Eriksen J. L., Erman A., Escalante R., Eskelinen E.-L., Espert L., Esteban-Martínez L., Evans T. J., Fabri M., Fabrias G., Fabrizi C., Facchiano A., Færgeman N. J., Faggioni A., Fairlie W. D., Fan C., Fan D., Fan J., Fang S., Fanto M., Fanzani A., Farkas T., Faure M., Favier F. B., Fearnhead H., Federici M., Fei E., Felizardo T. C., Feng H., Feng Y., Ferguson T. A., Fernández Á. F., Fernandez-Barrena M. G., Fernandez-Checa J. C., Fernández-López A., Fernandez-Zapico M. E., Feron O., Ferraro E., Ferreira-Halder C. V., Fesus L., Feuer R., Fiesel F. C., Filippi-Chiela E. C., Filomeni G., Fimia G. M., Fingert J. H., Finkbeiner S., Finkel T., Fiorito F., Fisher P. B., Flajolet M., Flamigni F., Florey O., Florio S., Andres Floto R., Folini M., Follo C., Fon E. A., Fornai F., Fortunato F., Fraldi A., Franco R., Francois A., François A., Frankel L. B., Fraser I. D. C., Frey N., Freyssenet D. G., Frezza C., Friedman S. L., Frigo D. E., Fu D., Fuentes J. M., Fueyo J., Fujitani Y., Fujiwara Y., Fujiya M., Fukuda M., Fulda S., Fusco C., Gabryel B., Gaestel M., Gailly P., Gajewska M., Galadari S., Galili G., Galindo I., Galindo M. F., Galliciotti G., Galluzzi L., Galluzzi L., Galy V., Gammoh N., Gandy S., Ganesan A. K., Ganesan S., Ganley I. G., Gannagé M., Gao F.-B., Gao F., Gao J.-X., Nannig L. G., Véscovi E. G., Garcia-Macía M., Garcia-Ruiz C., Garg A. D., Garg P. K., Gargini R., Gassen N. C., Gatica D., Gatti E., Gavard J., Gavathiotis E., Ge L., Ge P., Ge S., Gean P.-W., Gelmetti V., Genazzani A. A., Geng J., Genschik P., Gerner L., Gestwicki J. E., Gewirtz D. A., Ghavami S., Ghigo E., Ghosh D., Giammarioli A. M., Giampieri F., Giampietri C., Giatromanolaki A., Gibbings D. J., Gibellini L., Gibson S. B., Ginet V., Giordano A., Giorgini F., Giovannetti E., Girardin S. E., Gispert S., Giuliano S., Gladson C. L., Glavic A., Gleave M., Godefroy N., Gogal R. M. Jr, Gokulan K., Goldman G. H., Goletti D., Goligorsky M. S., Gomes A. V., Gomes L. C., Gomez H., Gomez-Manzano C., Gómez-Sánchez R., Gonçalves D. A. P., Goncu E., Gong Q., Gongora C., Gonzalez C. B., Gonzalez-Alegre P., Gonzalez-Cabo P., González-Polo R. A., Goping I. S., Gorbea C., Gorbunov N. V., Goring D. R., Gorman A. M., Gorski S. M., Goruppi S., Goto-Yamada S., Gotor C., Gottlieb R. A., Gozes I., Gozuacik D., Graba Y., Graef M., Granato G. E., Grant G. D., Grant S., Gravina G. L., Green D. R., Greenhough A., Greenwood M. T., Grimaldi B., Gros F., Grose C., Groulx J.-F., Gruber F., Grumati P., Grune T., Guan J.-L., Guan K.-L., Guerra B., Guillen C., Gulshan K., Gunst J., Guo C., Guo L., Guo M., Guo W., Guo X.-G., Gust A. A., Gustafsson Å. B., Gutierrez E., Gutierrez M. G., Gwak H.-S., Haas A., Haber J. E., Hadano S., Hagedorn M., Hahn D. R., Halayko A. J., Hamacher-Brady A., Hamada K., Hamai A., Hamann A., Hamasaki M., Hamer I., Hamid Q., Hammond E. M., Han F., Han W., Handa J. T., Hanover J. A., Hansen M., Harada M., Harhaji-Trajkovic L., Harper J. W., Harrath A. H., Harris A. L., Harris J., Hasler U., Hasselblatt P., Hasui K., Hawley R. G., Hawley T. S., He C., He C. Y., He F., He G., He R.-R., He X.-H., He Y.-W., He Y.-Y., Heath J. K., Hébert M.-J., Heinzen R. A., Helgason G. V., Hensel M., Henske E. P., Her C., Herman P. K., Hernández A., Hernandez C., Hernández-Tiedra S., Hetz C., Hiesinger P. R., Higaki K., Hilfiker S., Hill B. G., Hill J. A., Hill W. D., Hino K., Hofius D., Hofman P., Höglinger G. U., Höhfeld J., Holz M. K., Hong Y., Hood D. A., Hoozemans J. J. M., Hoppe T., Hsu C., Hsu C.-Y., Hsu L.-C., Hu D., Hu G., Hu H.-M., Hu H., Hu M. C., Hu Y.-C., Hu Z.-W., Hua F., Hua Y., Huang C., Huang H.-L., Huang K.-H., Huang K.-Y., Huang S., Huang S., Huang W.-P., Huang Y.-R., Huang Y., Huang Y., Huber T. B., Huebbe P., Huh W.-K., Hulmi J. J., Hur G. M., Hurley J. H., Husak Z., Hussain S. N. A., Hussain S., Hwang J. J., Hwang S., Hwang T. I. S., Ichihara A., Imai Y., Imbriano C., Inomata M., Into T., Iovane V., Iovanna J. L., Iozzo R. V., Ip N. Y., Irazoqui J. E., Iribarren P., Isaka Y., Isakovic A. J., Ischiropoulos H., Isenberg J. S., Ishaq M., Ishida H., Ishii I., Ishmael J. E., Isidoro C., Isobe K.-i., Isono E., Issazadeh-Navikas S., Itahana K., Itakura E., Ivanov A. I., Iyer A. K. V., Izquierdo J. M., Izumi Y., Izzo V., Jäättelä M., Jaber N., Jackson D. J., Jackson W. T., Jacob T. G., Jacques T. S., Jagannath C., Jain A., Jana N. R., Jang B. K., Jani A., Janji B., Jannig P. R., Jansson P. J., Jean S., Jendrach M., Jeon J.-H., Jessen N., Jeung E.-B., Jia K., Jia L., Jiang H., Jiang H., Jiang L., Jiang T., Jiang X., Jiang X., Jiang X., Jiang Y., Jiang Y., Jiménez A., Jin C., Jin H., Jin L., Jin M., Jin S., Jinwal U. K., Jo E.-K., Johansen T., Johnson D. E., Johnson G. V. W., Johnson J. D., Jonasch E., Jones C., Joosten L. A. B., Jordan J., Joseph A.-M., Joseph B., Joubert A. M., Ju D., Ju J., Juan H.-F., Juenemann K., Juhász G., Jung H. S., Jung J. U., Jung Y.-K., Jungbluth H., Justice M. J., Jutten B., Kaakoush N. O., Kaarniranta K., Kaasik A., Kabuta T., Kaeffer B., Kågedal K., Kahana A., Kajimura S., Kakhlon O., Kalia M., Kalvakolanu D. V., Kamada Y., Kambas K., Kaminskyy V. O., Kampinga H. H., Kandouz M., Kang C., Kang R., Kang T.-C., Kanki T., Kanneganti T.-D., Kanno H., Kanthasamy A. G., Kantorow M., Kaparakis-Liaskos M., Kapuy O., Karantza V., Karim M. R., Karmakar P., Kaser A., Kaushik S., Kawula T., Kaynar A. M., Ke P.-Y., Ke Z.-J., Kehrl J. H., Keller K. E., Kemper J. K., Kenworthy A. K., Kepp O., Kern A., Kesari S., Kessel D., Ketteler R., do Carmo Kettelhut I., Khambu B., Khan M. M., Khandelwal V. K. M., Khare S., Kiang J. G., Kiger A. A., Kihara A., Kim A. L., Kim C. H., Kim D. R., Kim D.-H., Kim E. K., Kim H. Y., Kim H.-R., Kim J.-S., Kim J. H., Kim J. C., Kim J. H., Kim K. W., Kim M. D., Kim M.-M., Kim P. K., Kim S. W., Kim S.-Y., Kim Y.-S., Kim Y., Kimchi A., Kimmelman A. C., Kimura T., King J. S., Kirkegaard K., Kirkin V., Kirshenbaum L. A., Kishi S., Kitajima Y., Kitamoto K., Kitaoka Y., Kitazato K., Kley R. A., Klimecki W. T., Klinkenberg M., Klucken J., Knævelsrud H., Knecht E., Knuppertz L., Ko J.-L., Kobayashi S., Koch J. C., Koechlin-Ramonatxo C., Koenig U., Koh Y. H., Köhler K., Kohlwein S. D., Koike M., Komatsu M., Kominami E., Kong D., Kong H. J., Konstantakou E. G., Kopp B. T., Korcsmaros T., Korhonen L., Korolchuk V. I., Koshkina N. V., Kou Y., Koukourakis M. I., Koumenis C., Kovács A. L., Kovács T., Kovacs W. J., Koya D., Kraft C., Krainc D., Kramer H., Kravic-Stevovic T., Krek W., Kretz-Remy C., Krick R., Krishnamurthy M., Kriston-Vizi J., Kroemer G., Kruer M. C., Kruger R., Ktistakis N. T., Kuchitsu K., Kuhn C., Kumar A. P., Kumar A., Kumar A., Kumar D., Kumar D., Kumar R., Kumar S., Kundu M., Kung H.-J., Kuno A., Kuo S.-H., Kuret J., Kurz T., Kwok T., Kwon T. K., Kwon Y. T., Kyrmizi I., La Spada A. R., Lafont F., Lahm T., Lakkaraju A., Lam T., Lamark T., Lancel S., Landowski T. H., Lane D. J. R., Lane J. D., Lanzi C., Lapaquette P., Lapierre L. R., Laporte J., Laukkarinen J., Laurie G. W., Lavandero S., Lavie L., La Voie M. J., Law B. Y. K., Law H. K.-w., Law K. B., Layfield R., Lazo P. A., Cam L. L., Le Roch K. G., Stunff H. L., Leardkamolkarn V., Lecuit M., Lee B.-H., Lee C.-H., Lee E. F., Lee G. M., Lee H.-J., Lee H., Lee J. K., Lee J., Lee J.-h., Lee J. H., Lee M., Lee M.-S., Lee P. J., Lee S. W., Lee S.-J., Lee S.-J., Lee S. Y., Lee S. H., Lee S. S., Lee S.-J., Lee S., Lee Y.-R., Lee Y. J., Lee Y. H., Leeuwenburgh C., Lefort S., Legouis R., Lei J., Lei Q.-Y., Leib D. A., Leibowitz G., Lekli I., Lemaire S. D., Lemasters J. J., Lemberg M. K., Lemoine A., Leng S., Lenz G., Lenzi P., Lerman L. O., Barbato D. L., Leu J. I.-J., Leung H. Y., Levine B., Lewis P. A., Lezoualc’h F., Li C., Li F., Li F.-J., Li J., Li K., Li L., Li M., Li M., Li Q., Li R., Li S., Li W., Li W., Li X., Li Y., Lian J., Liang C., Liang Q., Liao Y., Liberal J., Liberski P. P., Lie P., Lieberman A. P., Lim H. J., Lim K.-L., Lim K., Lima R. T., Lin C.-S., Lin C.-F., Lin F., Lin F., Lin F.-C., Lin K., Lin K.-H., Lin P.-H., Lin T., Lin W.-W., Lin Y.-S., Lin Y., Linden R., Lindholm D., Lindqvist L. M., Lingor P., Linkermann A., Liotta L. A., Lipinski M. M., Lira V. A., Lisanti M. P., Liton P. B., Liu B., Liu C., Liu C.-F., Liu F., Liu H.-J., Liu J., Liu J.-J., Liu J.-L., Liu K., Liu L., Liu L., Liu Q., Liu R.-Y., Liu S., Liu S., Liu W., Liu X.-D., Liu X., Liu X.-H., Liu X., Liu X., Liu X., Liu Y., Liu Y., Liu Z., Liu Z., Liuzzi J. P., Lizard G., Ljujic M., Lodhi I. J., Logue S. E., Lokeshwar B. L., Long Y. C., Lonial S., Loos B., López-Otín C., López-Vicario C., Lorente M., Lorenzi P. L., Lõrincz P., Los M., Lotze M. T., Lovat P. E., Lu B., Lu B., Lu J., Lu Q., Lu S.-M., Lu S., Lu Y., Luciano F., Luckhart S., Lucocq J. M., Ludovico P., Lugea A., Lukacs N. W., Lum J. J., Lund A. H., Luo H., Luo J., Luo S., Luparello C., Lyons T., Ma J., Ma Y., Ma Y., Ma Z., Machado J., Machado-Santelli G. M., Macian F., MacIntosh G. C., MacKeigan J. P., Macleod K. F., MacMicking J. D., MacMillan-Crow L. A., Madeo F., Madesh M., Madrigal-Matute J., Maeda A., Maeda T., Maegawa G., Maellaro E., Maes H., Magariños M., Maiese K., Maiti T. K., Maiuri L., Maiuri M. C., Maki C. G., Malli R., Malorni W., Maloyan A., Mami-Chouaib F., Man N., Mancias J. D., Mandelkow E.-M., Mandell M. A., Manfredi A. A., Manié S. N., Manzoni C., Mao K., Mao Z., Mao Z.-W., Marambaud P., Marconi A. M., Marelja Z., Marfe G., Margeta M., Margittai E., Mari M., Mariani F. V., Marin C., Marinelli S., Mariño G., Markovic I., Marquez R., Martelli A. M., Martens S., Martin K. R., Martin S. J., Martin S., Martin-Acebes M. A., Martín-Sanz P., Martinand-Mari C., Martinet W., Martinez J., Martinez-Lopez N., Martinez-Outschoorn U., Martínez-Velázquez M., Martinez-Vicente M., Martins W. K., Mashima H., Mastrianni J. A., Matarese G., Matarrese P., Mateo R., Matoba S., Matsumoto N., Matsushita T., Matsuura A., Matsuzawa T., Mattson M. P., Matus S., Maugeri N., Mauvezin C., Mayer A., Maysinger D., Mazzolini G. D., McBrayer M. K., Call K. M., Cormick C. M., McInerney G. M., McIver S. C., Kenna S. M., McMahon J. J., McNeish I. A., Mechta-Grigoriou F., Medema J. P., Medina D. L., Megyeri K., Mehrpour M., Mehta J. L., Mei Y., Meier U.-C., Meijer A. J., Meléndez A., Melino G., Melino S., de Melo E. J. T., Mena M. A., Meneghini M. D., Menendez J. A., Menezes R., Meng L., Meng L.-h., Meng S., Menghini R., Menko A. S., Menna-Barreto R. F. S., Menon M. B., Meraz-Ríos M. A., Merla G., Merlini L., Merlot A. M., Meryk A., Meschini S., Meyer J. N., Mi M.-t., Miao C.-Y., Micale L., Michaeli S., Michiels C., Migliaccio A. R., Mihailidou A. S., Mijaljica D., Mikoshiba K., Milan E., Miller-Fleming L., Mills G. B., Mills I. G., Minakaki G., Minassian B. A., Ming X.-F., Minibayeva F., Minina E. A., Mintern J. D., Minucci S., Miranda-Vizuete A., Mitchell C. H., Miyamoto S., Miyazawa K., Mizushima N., Mnich K., Mograbi B., Mohseni S., Moita L. F., Molinari M., Molinari M., Møller A. B., Mollereau B., Mollinedo F., Mongillo M., Monick M. M., Montagnaro S., Montell C., Moore D. J., Moore M. N., Mora-Rodriguez R., Moreira P. I., Morel E., Morelli M. B., Moreno S., Morgan M. J., Moris A., Moriyasu Y., Morrison J. L., Morrison L. A., Morselli E., Moscat J., Moseley P. L., Mostowy S., Motori E., Mottet D., Mottram J. C., Moussa C. E.-H., Mpakou V. E., Mukhtar H., Mulcahy Levy J. M., Muller S., Muñoz-Moreno R., Muñoz-Pinedo C., Münz C., Murphy M. E., Murray J. T., Murthy A., Mysorekar I. U., Nabi I. R., Nabissi M., Nader G. A., Nagahara Y., Nagai Y., Nagata K., Nagelkerke A., Nagy P., Naidu S. R., Nair S., Nakano H., Nakatogawa H., Nanjundan M., Napolitano G., Naqvi N. I., Nardacci R., Narendra D. P., Narita M., Nascimbeni A. C., Natarajan R., Navegantes L. C., Nawrocki S. T., Nazarko T. Y., Nazarko V. Y., Neill T., Neri L. M., Netea M. G., Netea-Maier R. T., Neves B. M., Ney P. A., Nezis I. P., Nguyen H. T. T., Nguyen H. P., Nicot A.-S., Nilsen H., Nilsson P., Nishimura M., Nishino I., Niso-Santano M., Niu H., Nixon R. A., Njar V. C. O., Noda T., Noegel A. A., Nolte E. M., Norberg E., Norga K. K., Noureini S. K., Notomi S., Notterpek L., Nowikovsky K., Nukina N., Nürnberger T., O’Donnell V. B., O’Dwyer T., O’Dwyer P. J., Oehme I., Oeste C. L., Ogawa M., Ogretmen B., Ogura Y., Oh Y. J., Ohmuraya M., Ohshima T., Ojha R., Okamoto K., Okazaki T., Oliver F. J., Ollinger K., Olsson S., Orban D. P., Ordonez P., Orhon I., Orosz L., O’Rourke E. J., Orozco H., Ortega A. L., Ortona E., Osellame L. D., Oshima J., Oshima S., Osiewacz H. D., Otomo T., Otsu K., Ou J.-h. J., Outeiro T. F., Ouyang D.-y., Ouyang H., Overholtzer M., Ozbun M. A., Ozdinler P. H., Ozpolat B., Pacelli C., Paganetti P., Page G., Pages G., Pagnini U., Pajak B., Pak S. C., Pakos-Zebrucka K., Pakpour N., Palková Z., Palladino F., Pallauf K., Pallet N., Palmieri M., Paludan S. R., Palumbo C., Palumbo S., Pampliega O., Pan H., Pan W., Panaretakis T., Pandey A., Pantazopoulou A., Papackova Z., Papademetrio D. L., Papassideri I., Papini A., Parajuli N., Pardo J., Parekh V. V., Parenti G., Park J.-I., Park J., Park O. K., Parker R., Parlato R., Parys J. B., Parzych K. R., Pasquet J.-M., Pasquier B., Pasumarthi K. B. S., Patschan D., Patterson C., Pattingre S., Pattison S., Pause A., Pavenstädt H., Pavone F., Pedrozo Z., Peña F. J., Peñalva M. A., Pende M., Peng J., Penna F., Penninger J. M., Pensalfini A., Pepe S., Pereira G. J. S., Pereira P. C., la Cruz V. P.-d., Pérez-Pérez M. E., Pérez-Rodríguez D., Pérez-Sala D., Perier C., Perl A., Perlmutter D. H., Perrotta I., Pervaiz S., Pesonen M., Pessin J. E., Peters G. J., Petersen M., Petrache I., Petrof B. J., Petrovski G., Phang J. M., Piacentini M., Pierdominici M., Pierre P., Pierrefite-Carle V., Pietrocola F., Pimentel-Muiños F. X., Pinar M., Pineda B., Pinkas-Kramarski R., Pinti M., Pinton P., Piperdi B., Piret J. M., Platanias L. C., Platta H. W., Plowey E. D., Pöggeler S., Poirot M., Polčic P., Poletti A., Poon A. H., Popelka H., Popova B., Poprawa I., Poulose S. M., Poulton J., Powers S. K., Powers T., Pozuelo-Rubio M., Prak K., Prange R., Prescott M., Priault M., Prince S., Proia R. L., Proikas-Cezanne T., Prokisch H., Promponas V. J., Przyklenk K., Puertollano R., Pugazhenthi S., Puglielli L., Pujol A., Puyal J., Pyeon D., Qi X., Qian W.-b., Qin Z.-H., Qiu Y., Qu Z., Quadrilatero J., Quinn F., Raben N., Rabinowich H., Radogna F., Ragusa M. J., Rahmani M., Raina K., Ramanadham S., Ramesh R., Rami A., Randall-Demllo S., Randow F., Rao H., Rao V. A., Rasmussen B. B., Rasse T. M., Ratovitski E. A., Rautou P.-E., Ray S. K., Razani B., Reed B. H., Reggiori F., Rehm M., Reichert A. S., Rein T., Reiner D. J., Reits E., Ren J., Ren X., Renna M., Reusch J. E. B., Revuelta J. L., Reyes L., Rezaie A. R., Richards R. I., Richardson D. R., Richetta C., Riehle M. A., Rihn B. H., Rikihisa Y., Riley B. E., Rimbach G., Rippo M. R., Ritis K., Rizzi F., Rizzo E., Roach P. J., Robbins J., Roberge M., Roca G., Roccheri M. C., Rocha S., Rodrigues C. M. P., Rodríguez C. I., de Cordoba S. R., Rodriguez-Muela N., Roelofs J., Rogov V. V., Rohn T. T., Rohrer B., Romanelli D., Romani L., Romano P. S., Roncero M. I. G., Rosa J. L., Rosello A., Rosen K. V., Rosenstiel P., Rost-Roszkowska M., Roth K. A., Roué G., Rouis M., Rouschop K. M., Ruan D. T., Ruano D., Rubinsztein D. C., Rucker E. B. III, Rudich A., Rudolf E., Rudolf R., Ruegg M. A., Ruiz-Roldan C., Ruparelia A. A., Rusmini P., Russ D. W., Russo G. L., Russo G., Russo R., Rusten T. E., Ryabovol V., Ryan K. M., Ryter S. W., Sabatini D. M., Sacher M., Sachse C., Sack M. N., Sadoshima J., Saftig P., Sagi-Eisenberg R., Sahni S., Saikumar P., Saito T., Saitoh T., Sakakura K., Sakoh-Nakatogawa M., Sakuraba Y., Salazar-Roa M., Salomoni P., Saluja A. K., Salvaterra P. M., Salvioli R., Samali A., Sanchez A. M. J., Sánchez-Alcázar J. A., Sanchez-Prieto R., Sandri M., Sanjuan M. A., Santaguida S., Santambrogio L., Santoni G., dos Santos C. N., Saran S., Sardiello M., Sargent G., Sarkar P., Sarkar S., Sarrias M. R., Sarwal M. M., Sasakawa C., Sasaki M., Sass M., Sato K., Sato M., Satriano J., Savaraj N., Saveljeva S., Schaefer L., Schaible U. E., Scharl M., Schatzl H. M., Schekman R., Scheper W., Schiavi A., Schipper H. M., Schmeisser H., Schmidt J., Schmitz I., Schneider B. E., Schneider E. M., Schneider J. L., Schon E. A., Schönenberger M. J., Schönthal A. H., Schorderet D. F., Schröder B., Schuck S., Schulze R. J., Schwarten M., Schwarz T. L., Sciarretta S., Scotto K., Scovassi A. I., Screaton R. A., Screen M., Seca H., Sedej S., Segatori L., Segev N., Seglen P. O., Seguí-Simarro J. M., Segura-Aguilar J., Seki E., Seiliez I., Sell C., Semenkovich C. F., Semenza G. L., Sen U., Serra A. L., Serrano-Puebla A., Sesaki H., Setoguchi T., Settembre C., Shacka J. J., Shajahan-Haq A. N., Shapiro I. M., Sharma S., She H., Shen C.-K. J., Shen C.-C., Shen H.-M., Shen S., Shen W., Sheng R., Sheng X., Sheng Z.-H., Shepherd T. G., Shi J., Shi Q., Shi Q., Shi Y., Shibutani S., Shibuya K., Shidoji Y., Shieh J.-J., Shih C.-M., Shimada Y., Shimizu S., Shin D. W., Shinohara M. L., Shintani M., Shintani T., Shioi T., Shirabe K., Shiri-Sverdlov R., Shirihai O., Shore G. C., Shu C.-W., Shukla D., Sibirny A. A., Sica V., Sigurdson C. J., Sigurdsson E. M., Sijwali P. S., Sikorska B., Silveira W. A., Silvente-Poirot S., Silverman G. A., Simak J., Simmet T., Simon A. K., Simon H.-U., Simone C., Simons M., Simonsen A., Singh R., Singh S. V., Singh S. K., Sinha D., Sinha S., Sinicrope F. A., Sirko A., Sirohi K., Sishi B. J. N., Sittler A., Siu P. M., Sivridis E., Skwarska A., Slack R., Slaninová I., Slavov N., Smaili S. S., Smalley K. S. M., Smith D. R., Soenen S. J., Soleimanpour S. A., Solhaug A., Somasundaram K., Son J. H., Sonawane A., Song C., Song F., Song H. K., Song J.-X., Song W., Soo K. Y., Sood A. K., Soong T. W., Soontornniyomkij V., Sorice M., Sotgia F., Soto-Pantoja D. R., Sotthibundhu A., Sousa M. J., Spaink H. P., Span P. N., Spang A., Sparks J. D., Speck P. G., Spector S. A., Spies C. D., Springer W., Clair D. S., Stacchiotti A., Staels B., Stang M. T., Starczynowski D. T., Starokadomskyy P., Steegborn C., Steele J. W., Stefanis L., Steffan J., Stellrecht C. M., Stenmark H., Stepkowski T. M., Stern S. T., Stevens C., Stockwell B. R., Stoka V., Storchova Z., Stork B., Stratoulias V., Stravopodis D. J., Strnad P., Strohecker A. M., Ström A.-L., Stromhaug P., Stulik J., Su Y.-X., Su Z., Subauste C. S., Subramaniam S., Sue C. M., Suh S. W., Sui X., Sukseree S., Sulzer D., Sun F.-L., Sun J., Sun J., Sun S.-Y., Sun Y., Sun Y., Sun Y., Sundaramoorthy V., Sung J., Suzuki H., Suzuki K., Suzuki N., Suzuki T., Suzuki Y. J., Swanson M. S., Swanton C., Swärd K., Swarup G., Sweeney S. T., Sylvester P. W., Szatmari Z., Szegezdi E., Szlosarek P. W., Taegtmeyer H., Tafani M., Taillebourg E., Tait S. W. G., Takacs-Vellai K., Takahashi Y., Takáts S., Takemura G., Takigawa N., Talbot N. J., Tamagno E., Tamburini J., Tan C.-P., Tan L., Tan M. L., Tan M., Tan Y.-J., Tanaka K., Tanaka M., Tang D., Tang D., Tang G., Tanida I., Tanji K., Tannous B. A., Tapia J. A., Tasset-Cuevas I., Tatar M., Tavassoly I., Tavernarakis N., Taylor A., Taylor G. S., Taylor G. A., Paul Taylor J., Taylor M. J., Tchetina E. V., Tee A. R., Teixeira-Clerc F., Telang S., Tencomnao T., Teng B.-B., Teng R.-J., Terro F., Tettamanti G., Theiss A. L., Theron A. E., Thomas K. J., Thomé M. P., Thomes P. G., Thorburn A., Thorner J., Thum T., Thumm M., Thurston T. L. M., Tian L., Till A., Ting J. P.-y., Titorenko V. I., Toker L., Toldo S., Tooze S. A., Topisirovic I., Torgersen M. L., Torosantucci L., Torriglia A., Torrisi M. R., Tournier C., Towns R., Trajkovic V., Travassos L. H., Triola G., Tripathi D. N., Trisciuoglio D., Troncoso R., Trougakos I. P., Truttmann A. C., Tsai K.-J., Tschan M. P., Tseng Y.-H., Tsukuba T., Tsung A., Tsvetkov A. S., Tu S., Tuan H.-Y., Tucci M., Tumbarello D. A., Turk B., Turk V., Turner R. F. B., Tveita A. A., Tyagi S. C., Ubukata M., Uchiyama Y., Udelnow A., Ueno T., Umekawa M., Umemiya-Shirafuji R., Underwood B. R., Ungermann C., Ureshino R. P., Ushioda R., Uversky V. N., Uzcátegui N. L., Vaccari T., Vaccaro M. I., Váchová L., Vakifahmetoglu-Norberg H., Valdor R., Valente E. M., Vallette F., Valverde A. M., Van den Berghe G., Van Den Bosch L., van den Brink G. R., van der Goot F. G., van der Klei I. J., van der Laan L. J. W., van Doorn W. G., van Egmond M., van Golen K. L., Van Kaer L., van Lookeren Campagne M., Vandenabeele P., Vandenberghe W., Vanhorebeek I., Varela-Nieto I., Vasconcelos M. H., Vasko R., Vavvas D. G., Vega-Naredo I., Velasco G., Velentzas A. D., Velentzas P. D., Vellai T., Vellenga E., Vendelbo M. H., Venkatachalam K., Ventura N., Ventura S., Veras P. S. T., Verdier M., Vertessy B. G., Viale A., Vidal M., Vieira H. L. A., Vierstra R. D., Vigneswaran N., Vij N., Vila M., Villar M., Villar V. H., Villarroya J., Vindis C., Viola G., Viscomi M. T., Vitale G., Vogl D. T., Voitsekhovskaja O. V., von Haefen C., von Schwarzenberg K., Voth D. E., Vouret-Craviari V., Vuori K., Vyas J. M., Waeber C., Walker C. L., Walker M. J., Walter J., Wan L., Wan X., Wang B., Wang C., Wang C.-Y., Wang C., Wang C., Wang C., Wang D., Wang F., Wang F., Wang G., Wang H.-J., Wang H., Wang H.-G., Wang H., Wang H.-D., Wang J., Wang J., Wang M., Wang M.-Q., Wang P.-Y., Wang P., Wang R. C., Wang S., Wang T.-F., Wang X., Wang X.-j., Wang X.-W., Wang X., Wang X., Wang Y., Wang Y., Wang Y., Wang Y.-J., Wang Y., Wang Y., Wang Y. T., Wang Y., Wang Z.-N., Wappner P., Ward C., McVeyWard D., Warnes G., Watada H., Watanabe Y., Watase K., Weaver T. E., Weekes C. D., Wei J., Weide T., Weihl C. C., Weindl G., Weis S. N., Wen L., Wen X., Wen Y., Westermann B., Weyand C. M., White A. R., White E., Lindsay Whitton J., Whitworth A. J., Wiels J., Wild F., Wildenberg M. E., Wileman T., Wilkinson D. S., Wilkinson S., Willbold D., Williams C., Williams K., Williamson P. R., Winklhofer K. F., Witkin S. S., Wohlgemuth S. E., Wollert T., Wolvetang E. J., Wong E., Wong G. W., Wong R. W., Wong V. K. W., Woodcock E. A., Wright K. L., Wu C., Wu D., Wu G. S., Wu J., Wu J., Wu M., Wu M., Wu S., Wu W. K. K., Wu Y., Wu Z., Xavier C. P. R., Xavier R. J., Xia G.-X., Xia T., Xia W., Xia Y., Xiao H., Xiao J., Xiao S., Xiao W., Xie C.-M., Xie Z., Xie Z., Xilouri M., Xiong Y., Xu C., Xu C., Xu F., Xu H., Xu H., Xu J., Xu J., Xu J., Xu L., Xu X., Xu Y., Xu Y., Xu Z.-X., Xu Z., Xue Y., Yamada T., Yamamoto A., Yamanaka K., Yamashina S., Yamashiro S., Yan B., Yan B., Yan X., Yan Z., Yanagi Y., Yang D.-S., Yang J.-M., Yang L., Yang M., Yang P.-M., Yang P., Yang Q., Yang W., Yang W. Y., Yang X., Yang Y., Yang Y., Yang Z., Yang Z., Yao M.-C., Yao P. J., Yao X., Yao Z., Yao Z., Yasui L. S., Ye M., Yedvobnick B., Yeganeh B., Yeh E. S., Yeyati P. L., Yi F., Yi L., Yin X.-M., Yip C. K., Yoo Y.-M., Yoo Y. H., Yoon S.-Y., Yoshida K.-I., Yoshimori T., Young K. H., Yu H., Yu J. J., Yu J.-T., Yu J., Yu L., Yu W. H., Yu X.-F., Yu Z., Yuan J., Yuan Z.-M., Yue B. Y. J. T., Yue J., Yue Z., Zacks D. N., Zacksenhaus E., Zaffaroni N., Zaglia T., Zakeri Z., Zecchini V., Zeng J., Zeng M., Zeng Q., Zervos A. S., Zhang D. D., Zhang F., Zhang G., Zhang G.-C., Zhang H., Zhang H., Zhang H., Zhang H., Zhang J., Zhang J., Zhang J., Zhang J., Zhang J.-p., Zhang L., Zhang L., Zhang L., Zhang L., Zhang M.-Y., Zhang X., Zhang X. D., Zhang Y., Zhang Y., Zhang Y., Zhang Y., Zhang Y., Zhao M., Zhao W.-L., Zhao X., Zhao Y. G., Zhao Y., Zhao Y., Zhao Y.-x., Zhao Z., Zhao Z. J., Zheng D., Zheng X.-L., Zheng X., Zhivotovsky B., Zhong Q., Zhou G.-Z., Zhou G., Zhou H., Zhou S.-F., Zhou X.-j., Zhu H., Zhu H., Zhu W.-G., Zhu W., Zhu X.-F., Zhu Y., Zhuang S.-M., Zhuang X., Ziparo E., Zois C. E., Zoladek T., Zong W.-X., Zorzano A., Zughaier S. M., Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wieczorek S., Combes F., Lazar C., Gianetto Q. G., Gatto L., Dorffer A., Hesse A. M., Couté Y., Ferro M., Bruley C., Burger T., DAPAR &ProStaR: software to perform statistical analyses in quantitative discovery proteomics. Bioinformatics 33, 135–136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grubman A., Chew G., Ouyang J. F., Sun G., Choo X. Y., McLean C., Simmons R. K., Buckberry S., Vargas-Landin D. B., Poppe D., Pflueger J., Lister R., Rackham O. J. L., Petretto E., Polo J. M., A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Habib N., Avraham-Davidi I., Basu A., Burks T., Shekhar K., Hofree M., Choudhury S. R., Aguet F., Gelfand E., Ardlie K., Weitz D. A., Rozenblatt-Rosen O., Zhang F., Regev A., Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat. Methods 14, 955–958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGinnis C. S., Patterson D. M., Winkler J., Conrad D. N., Hein M. Y., Srivastava V., Hu J. L., Murrow L. M., Weissman J. S., Werb Z., Chow E. D., Gartner Z. J., MULTI-seq: Sample multiplexing for single-cell RNA sequencing using lipid-tagged indices. Nat. Methods 16, 619–626 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butler A., Hoffman P., Smibert P., Papalexi E., Satija R., Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lake B. B., Chen S., Sos B. C., Fan J., Kaeser G. E., Yung Y. C., Duong T. E., Gao D., Chun J., Kharchenko P. V., Zhang K., Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 36, 70–80 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang P., Zhao D., Lachman H. M., Zheng D., Enriched expression of genes associated with autism spectrum disorders in human inhibitory neurons. Transl. Psychiatry 8, 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S8
Tables S1 to S3