Abstract
Na+,K(+)-ATPase has been observed to partially inhibit acidification of early endosomes by increasing membrane potential, whereas chloride channels have been observed to enhance acidification in endosomes and lysosomes. However, little theoretical analysis of the ways in which different pumps and channels may interact has been carried out. We therefore developed quantitative models of endosomal pH regulation based on thermodynamic considerations. We conclude that 1) both size and shape of endosomes will influence steady-state endosomal pH whenever membrane potential due to the pH gradient limits proton pumping, 2) steady-state pH values similar to those observed in early endosomes of living cells can occur in endosomes containing just H(+)-ATPases and Na+,K(+)-ATPases when low endosomal buffering capacities are present, and 3) inclusion of active chloride channels results in predicted pH values well below those observed in vivo. The results support the separation of endocytic compartments into two classes, those (such as early endosomes) whose acidification is limited by attainment of a certain membrane potential, and those (such as lysosomes) whose acidification is limited by the attainment of a certain pH. The theoretical framework and conclusions described are potentially applicable to other membrane-enclosed compartments that are acidified, such as elements of the Golgi apparatus.
Full text
PDF

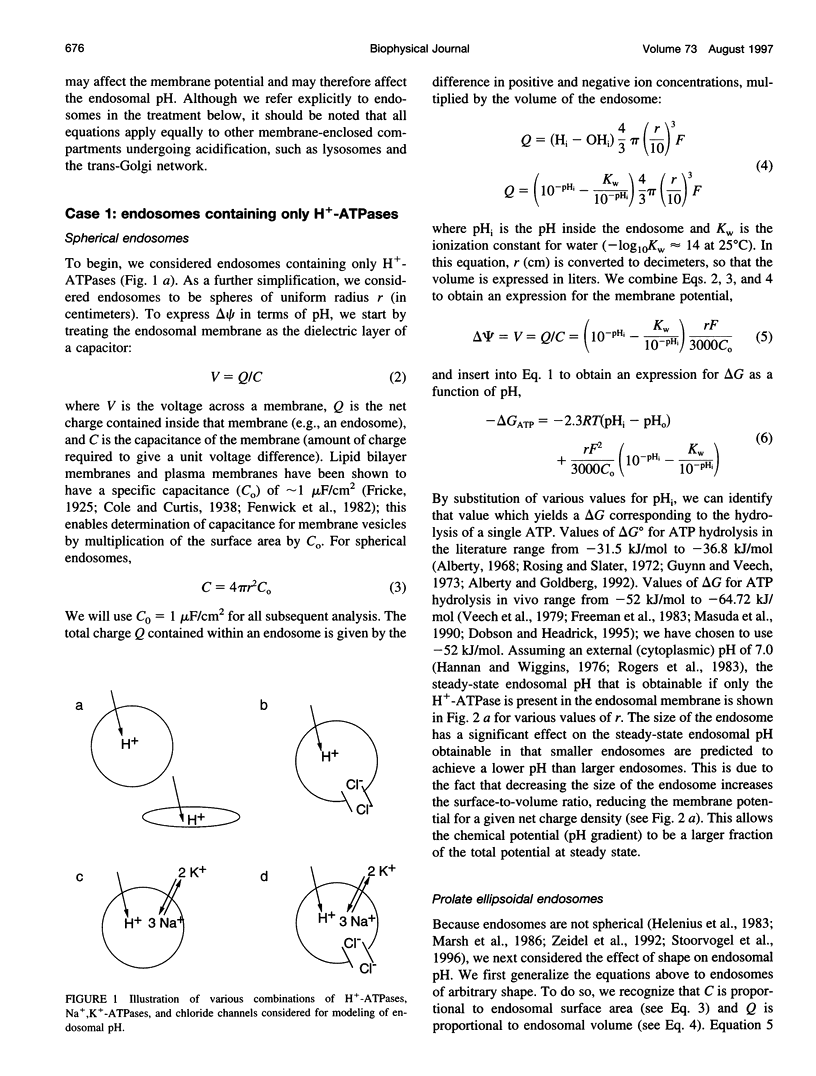
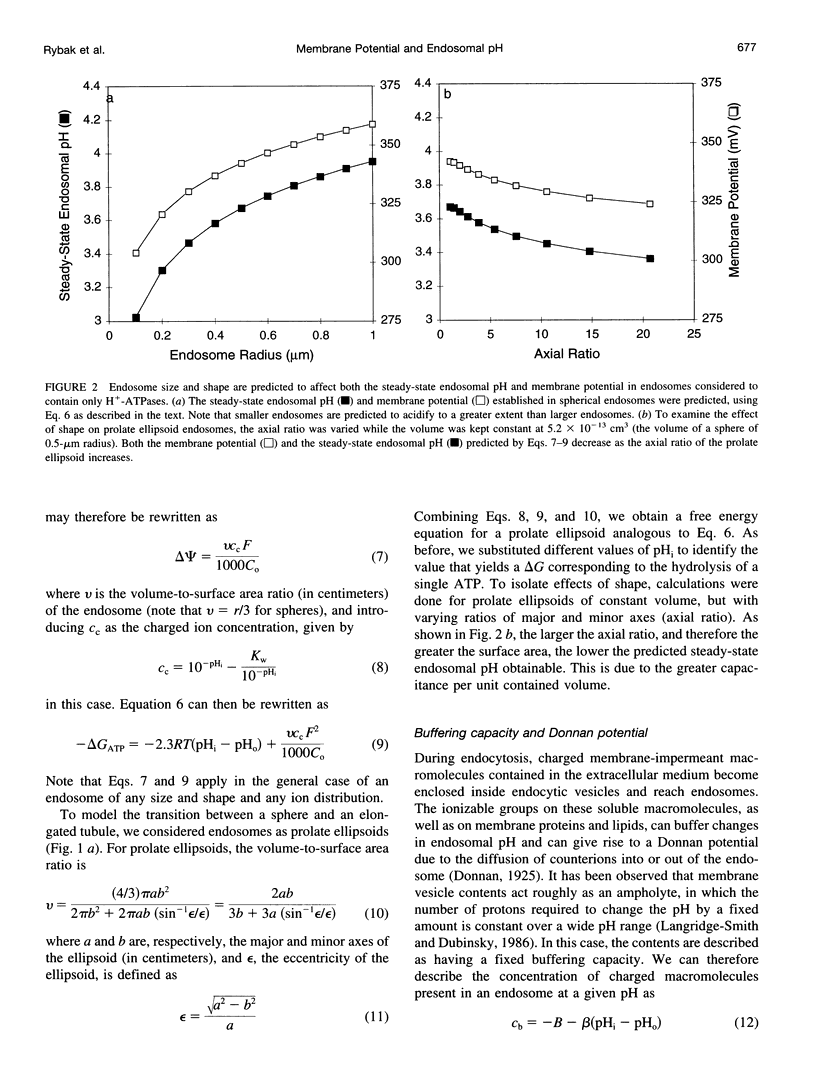
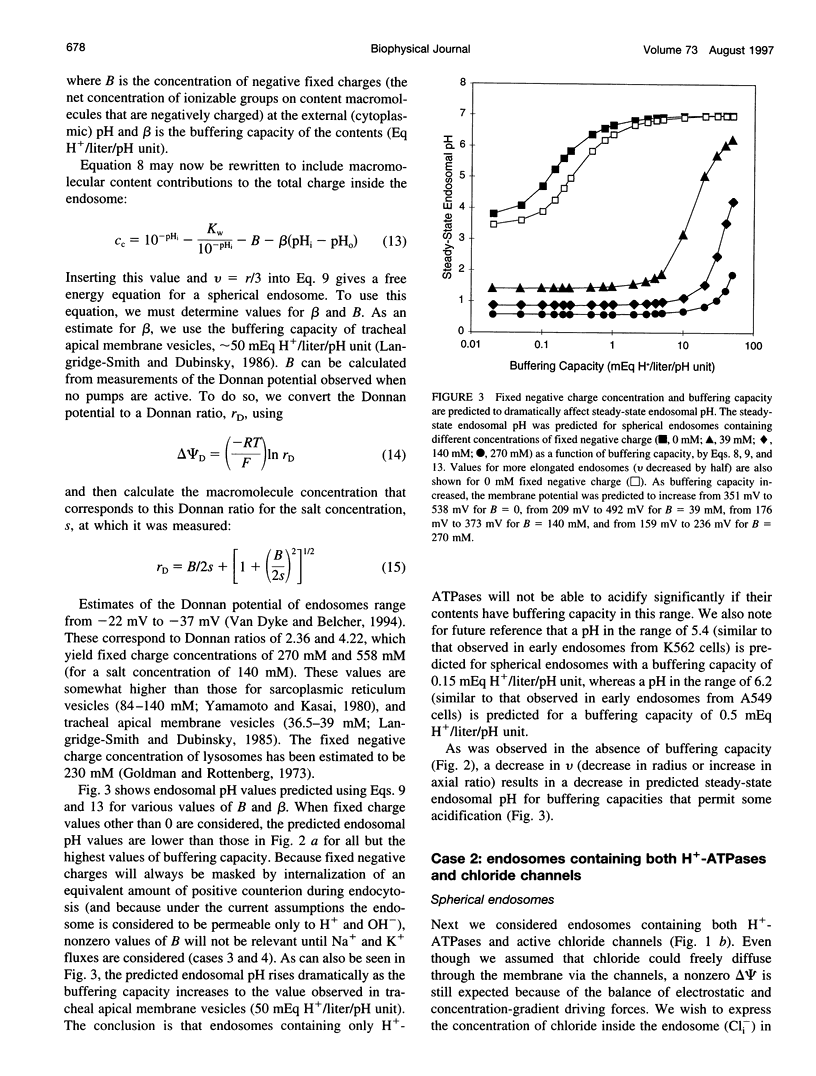
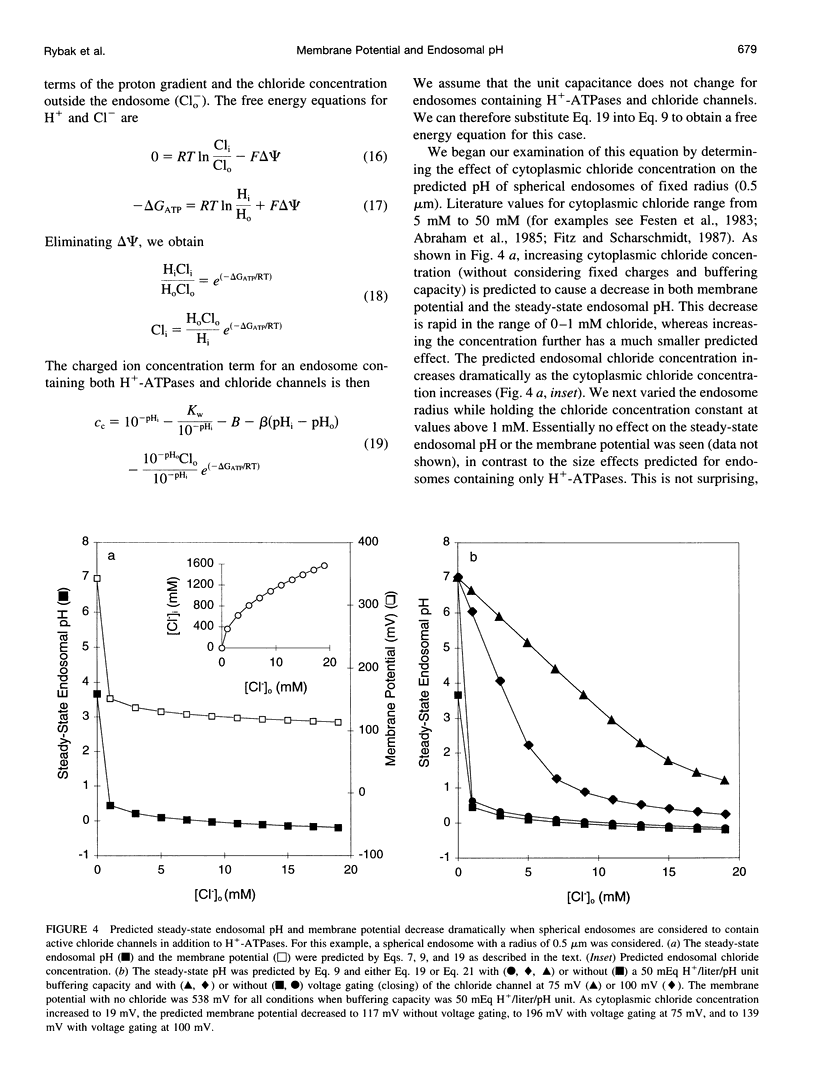





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. H., Breslow J. L., Epstein J., Chang-Sing P., Lechene C. Preparation of individual human diploid fibroblasts and study of ion transport. Am J Physiol. 1985 Jan;248(1 Pt 1):C154–C164. doi: 10.1152/ajpcell.1985.248.1.C154. [DOI] [PubMed] [Google Scholar]
- Alberty R. A. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J Biol Chem. 1968 Apr 10;243(7):1337–1343. [PubMed] [Google Scholar]
- Alberty R. A., Goldberg R. N. Standard thermodynamic formation properties for the adenosine 5'-triphosphate series. Biochemistry. 1992 Nov 3;31(43):10610–10615. doi: 10.1021/bi00158a025. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwer M. S., Hegner D. Role of inorganic electrolytes in bile acid-independent canalicular bile formation. Am J Physiol. 1983 Feb;244(2):G116–G124. doi: 10.1152/ajpgi.1983.244.2.G116. [DOI] [PubMed] [Google Scholar]
- Arai H., Pink S., Forgac M. Interaction of anions and ATP with the coated vesicle proton pump. Biochemistry. 1989 Apr 4;28(7):3075–3082. doi: 10.1021/bi00433a051. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN R. E. Potassium and sodium balance in mammalian red cells. Science. 1954 Sep 17;120(3116):459–460. doi: 10.1126/science.120.3116.459. [DOI] [PubMed] [Google Scholar]
- Bae H. R., Verkman A. S. Protein kinase A regulates chloride conductance in endocytic vesicles from proximal tubule. Nature. 1990 Dec 13;348(6302):637–639. doi: 10.1038/348637a0. [DOI] [PubMed] [Google Scholar]
- Cain C. C., Murphy R. F. A chloroquine-resistant Swiss 3T3 cell line with a defect in late endocytic acidification. J Cell Biol. 1988 Feb;106(2):269–277. doi: 10.1083/jcb.106.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. C., Sipe D. M., Murphy R. F. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):544–548. doi: 10.1073/pnas.86.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Hubbard A. L. Lumenal labeling of rat hepatocyte early endosomes. Presence of multiple membrane receptors and the Na+,K(+)-ATPase. J Biol Chem. 1992 Apr 25;267(12):8213–8221. [PubMed] [Google Scholar]
- Coffey J. W., De Duve C. Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem. 1968 Jun 25;243(12):3255–3263. [PubMed] [Google Scholar]
- Dobson G. P., Headrick J. P. Bioenergetic scaling: metabolic design and body-size constraints in mammals. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7317–7321. doi: 10.1073/pnas.92.16.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festen C. M., Slegers J. F., Van Os C. H. Intracellular activities of chloride, potassium and sodium ions in rabbit corneal epithelium. Biochim Biophys Acta. 1983 Jul 27;732(2):394–404. doi: 10.1016/0005-2736(83)90056-1. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Scharschmidt B. F. Intracellular chloride activity in intact rat liver: relationship to membrane potential and bile flow. Am J Physiol. 1987 May;252(5 Pt 1):G699–G706. doi: 10.1152/ajpgi.1987.252.5.G699. [DOI] [PubMed] [Google Scholar]
- Franciolini F., Petris A. Chloride channels of biological membranes. Biochim Biophys Acta. 1990 May 7;1031(2):247–259. doi: 10.1016/0304-4157(90)90009-2. [DOI] [PubMed] [Google Scholar]
- Freeman D., Bartlett S., Radda G., Ross B. Energetics of sodium transport in the kidney. Saturation transfer 31P-NMR. Biochim Biophys Acta. 1983 Apr 5;762(2):325–336. doi: 10.1016/0167-4889(83)90087-3. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Mâle P., Mellman I. Acidification and ion permeabilities of highly purified rat liver endosomes. J Biol Chem. 1989 Feb 5;264(4):2212–2220. [PubMed] [Google Scholar]
- Fuchs R., Schmid S., Mellman I. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc Natl Acad Sci U S A. 1989 Jan;86(2):539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaete V., Núez M. T., Glass J. Cl-, Na+, and H+ fluxes during the acidification of rabbit reticulocyte endocytic vesicles. J Bioenerg Biomembr. 1991 Feb;23(1):147–160. doi: 10.1007/BF00768844. [DOI] [PubMed] [Google Scholar]
- Goldman R., Rottenberg H. Ion distribution in lysosomal suspensions. FEBS Lett. 1973 Jul 1;33(2):233–238. doi: 10.1016/0014-5793(73)80200-5. [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Veech R. L. The equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. J Biol Chem. 1973 Oct 25;248(20):6966–6972. [PubMed] [Google Scholar]
- Hannan S. F., Wiggins P. M. Intracellular pH of frog sartorius muscle. Biochim Biophys Acta. 1976 Mar 25;428(1):205–222. doi: 10.1016/0304-4165(76)90121-5. [DOI] [PubMed] [Google Scholar]
- Hazelton B. J., Tupper J. T. Intracellular ionic changes in normal and transformed human fibroblasts after extracellular Ca2+ deprivation. Biochem J. 1981 Mar 15;194(3):707–711. doi: 10.1042/bj1940707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killisch I., Steinlein P., Römisch K., Hollinshead R., Beug H., Griffiths G. Characterization of early and late endocytic compartments of the transferrin cycle. Transferrin receptor antibody blocks erythroid differentiation by trapping the receptor in the early endosome. J Cell Sci. 1992 Sep;103(Pt 1):211–232. doi: 10.1242/jcs.103.1.211. [DOI] [PubMed] [Google Scholar]
- Langridge-Smith J. E., Dubinsky W. P. Donnan equilibrium and pH gradient in isolated tracheal apical membrane vesicles. Am J Physiol. 1985 Nov;249(5 Pt 1):C417–C420. doi: 10.1152/ajpcell.1985.249.5.C417. [DOI] [PubMed] [Google Scholar]
- Langridge-Smith J. E., Dubinsky W. P. Relationship of the Donnan potential to the transmembrane pH gradient in tracheal apical membrane vesicles. J Membr Biol. 1986;94(3):197–204. doi: 10.1007/BF01869715. [DOI] [PubMed] [Google Scholar]
- Marsh M., Griffiths G., Dean G. E., Mellman I., Helenius A. Three-dimensional structure of endosomes in BHK-21 cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2899–2903. doi: 10.1073/pnas.83.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T., Dobson G. P., Veech R. L. The Gibbs-Donnan near-equilibrium system of heart. J Biol Chem. 1990 Nov 25;265(33):20321–20334. [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Mulberg A. E., Tulk B. M., Forgac M. Modulation of coated vesicle chloride channel activity and acidification by reversible protein kinase A-dependent phosphorylation. J Biol Chem. 1991 Nov 5;266(31):20590–20593. [PubMed] [Google Scholar]
- Murphy R. F., Powers S., Cantor C. R. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J Cell Biol. 1984 May;98(5):1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M., Ludewig U., Rehfeldt A., Jentsch T. J. Gating of the voltage-dependent chloride channel CIC-0 by the permeant anion. Nature. 1995 Feb 9;373(6514):527–531. doi: 10.1038/373527a0. [DOI] [PubMed] [Google Scholar]
- Pusch M., Steinmeyer K., Jentsch T. J. Low single channel conductance of the major skeletal muscle chloride channel, ClC-1. Biophys J. 1994 Jan;66(1):149–152. doi: 10.1016/S0006-3495(94)80753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M., Bowser R., Murphy R. F. Kinetics and temperature dependence of exposure of endocytosed material to proteolytic enzymes and low pH: evidence for a maturation model for the formation of lysosomes. J Cell Physiol. 1987 May;131(2):200–209. doi: 10.1002/jcp.1041310209. [DOI] [PubMed] [Google Scholar]
- Rogers J., Hesketh T. R., Smith G. A., Metcalfe J. C. Intracellular pH of stimulated thymocytes measured with a new fluorescent indicator. J Biol Chem. 1983 May 25;258(10):5994–5997. [PubMed] [Google Scholar]
- Rosing J., Slater E. C. The value of G degrees for the hydrolysis of ATP. Biochim Biophys Acta. 1972 May 25;267(2):275–290. doi: 10.1016/0005-2728(72)90116-8. [DOI] [PubMed] [Google Scholar]
- Sipe D. M., Jesurum A., Murphy R. F. Absence of Na+,K(+)-ATPase regulation of endosomal acidification in K562 erythroleukemia cells. Analysis via inhibition of transferrin recycling by low temperatures. J Biol Chem. 1991 Feb 25;266(6):3469–3474. [PubMed] [Google Scholar]
- Sipe D. M., Murphy R. F. Binding to cellular receptors results in increased iron release from transferrin at mildly acidic pH. J Biol Chem. 1991 May 5;266(13):8002–8007. [PubMed] [Google Scholar]
- Sipe D. M., Murphy R. F. High-resolution kinetics of transferrin acidification in BALB/c 3T3 cells: exposure to pH 6 followed by temperature-sensitive alkalinization during recycling. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7119–7123. doi: 10.1073/pnas.84.20.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solc C. K., Wine J. J. Swelling-induced and depolarization-induced C1-channels in normal and cystic fibrosis epithelial cells. Am J Physiol. 1991 Oct;261(4 Pt 1):C658–C674. doi: 10.1152/ajpcell.1991.261.4.C658. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Schwappach B., Bens M., Vandewalle A., Jentsch T. J. Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J Biol Chem. 1995 Dec 29;270(52):31172–31177. doi: 10.1074/jbc.270.52.31172. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W., Oorschot V., Geuze H. J. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996 Jan;132(1-2):21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann A., Gründer S., Pusch M., Jentsch T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992 Mar 5;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W. Acidification of rat liver lysosomes: quantitation and comparison with endosomes. Am J Physiol. 1993 Oct;265(4 Pt 1):C901–C917. doi: 10.1152/ajpcell.1993.265.4.C901. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W., Belcher J. D. Acidification of three types of liver endocytic vesicles: similarities and differences. Am J Physiol. 1994 Jan;266(1 Pt 1):C81–C94. doi: 10.1152/ajpcell.1994.266.1.C81. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W. Na+/H+ exchange modulates acidification of early rat liver endocytic vesicles. Am J Physiol. 1995 Oct;269(4 Pt 1):C943–C954. doi: 10.1152/ajpcell.1995.269.4.C943. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W. Proton pump-generated electrochemical gradients in rat liver multivesicular bodies. Quantitation and effects of chloride. J Biol Chem. 1988 Feb 25;263(6):2603–2611. [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Verkman A. S., Alpern R. J. Kinetic transport model for cellular regulation of pH and solute concentration in the renal proximal tubule. Biophys J. 1987 Apr;51(4):533–546. doi: 10.1016/S0006-3495(87)83379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A. E. Kinetic properties of the chloride conductance of frog muscle. J Physiol. 1972 Dec;227(1):291–312. doi: 10.1113/jphysiol.1972.sp010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Kasai M. Donnan potential in sarcoplasmic reticulum vesicles measured by using a fluorescent cyanine dye. J Biochem. 1980 Nov;88(5):1425–1435. doi: 10.1093/oxfordjournals.jbchem.a133112. [DOI] [PubMed] [Google Scholar]
- Yamashiro D. J., Maxfield F. R. Regulation of endocytic processes by pH. Trends Pharmacol Sci. 1988 Jun;9(6):190–193. doi: 10.1016/0165-6147(88)90078-8. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Hammond T., Botelho B., Harris H. W., Jr Functional and structural characterization of endosomes from toad bladder epithelial cells. Am J Physiol. 1992 Jul;263(1 Pt 2):F62–F76. doi: 10.1152/ajprenal.1992.263.1.F62. [DOI] [PubMed] [Google Scholar]
- Zen K., Biwersi J., Periasamy N., Verkman A. S. Second messengers regulate endosomal acidification in Swiss 3T3 fibroblasts. J Cell Biol. 1992 Oct;119(1):99–110. doi: 10.1083/jcb.119.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]


