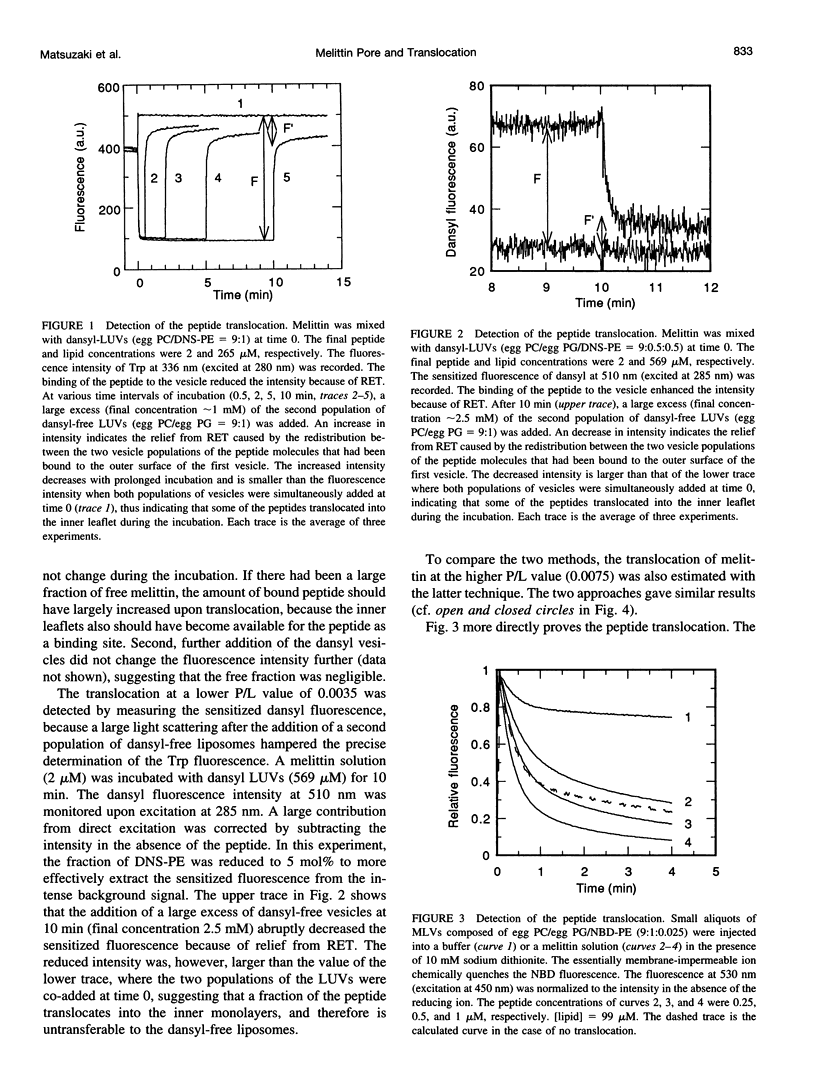
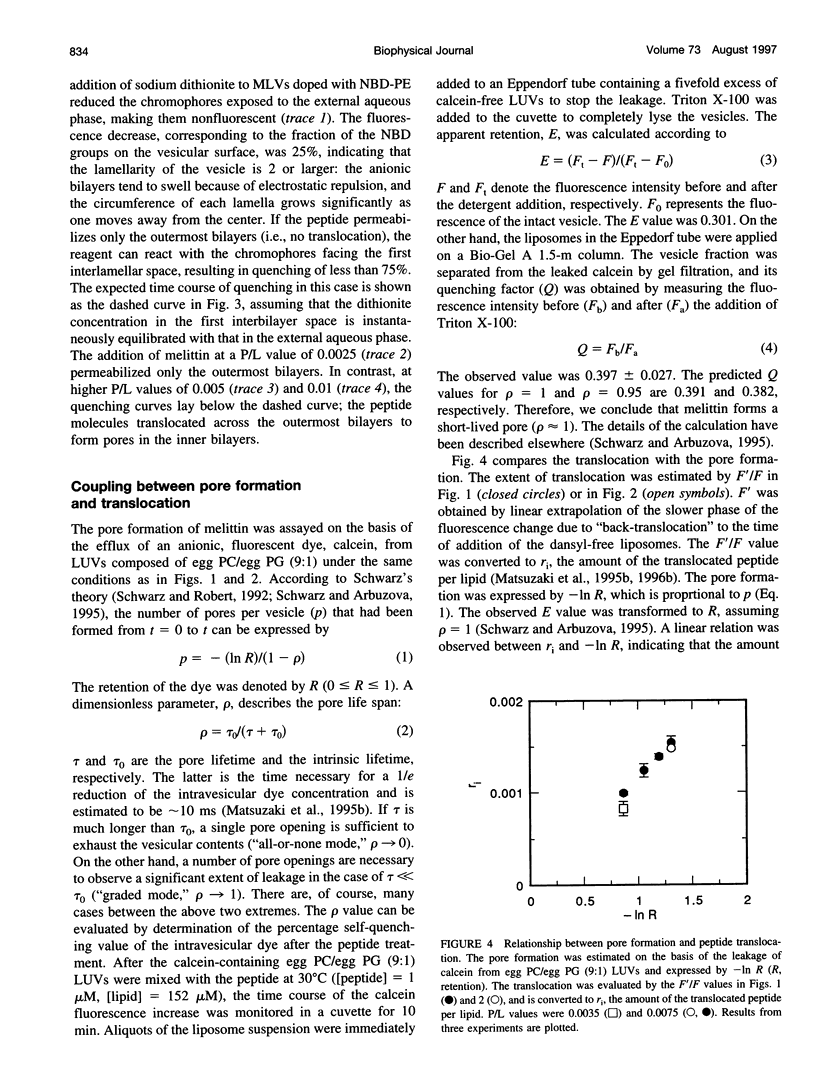
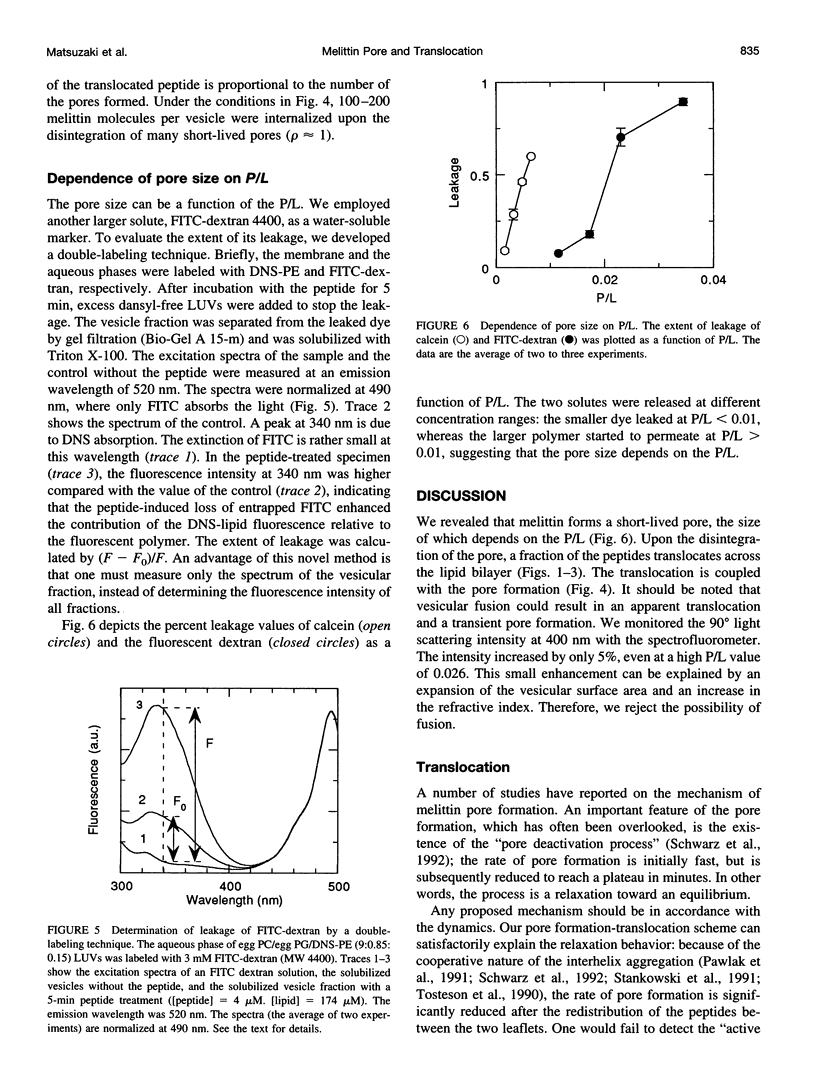
Abstract
Melittin, a bee venom, is a basic amphiphilic peptide, which mainly acts on the lipid matrix of membranes, lysing various cells. To elucidate the molecular mechanism, we investigated its interactions with phospholipid vesicles. The peptide formed a pore with a short lifetime in the membrane, as revealed by the release of an anionic fluorescent dye, calcein, from the liposomes. Our new double-labeling method clarified that the pore size increased with the peptide-to-lipid ratio. Upon the disintegration of the pore, a fraction of the peptides translocated across the bilayer. The pore formation was coupled with the translocation, which was proved by three fluorescence experiments recently developed by our laboratory. A novel model for the melittin pore formation was discussed in comparison with other pore-forming peptides.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbach C., Hubbell W. L. The aggregation state of spin-labeled melittin in solution and bound to phospholipid membranes: evidence that membrane-bound melittin is monomeric. Proteins. 1988;3(4):230–242. doi: 10.1002/prot.340030404. [DOI] [PubMed] [Google Scholar]
- Archer S. J., Ellena J. F., Cafiso D. S. Dynamics and aggregation of the peptide ion channel alamethicin. Measurements using spin-labeled peptides. Biophys J. 1991 Aug;60(2):389–398. doi: 10.1016/S0006-3495(91)82064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Benachir T., Lafleur M. Osmotic and pH transmembrane gradients control the lytic power of melittin. Biophys J. 1996 Feb;70(2):831–840. doi: 10.1016/S0006-3495(96)79622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benachir T., Lafleur M. Study of vesicle leakage induced by melittin. Biochim Biophys Acta. 1995 May 4;1235(2):452–460. doi: 10.1016/0005-2736(95)80035-e. [DOI] [PubMed] [Google Scholar]
- Clague M. J., Cherry R. J. A comparative study of band 3 aggregation in erythrocyte membranes by melittin and other cationic agents. Biochim Biophys Acta. 1989 Mar 27;980(1):93–99. doi: 10.1016/0005-2736(89)90204-6. [DOI] [PubMed] [Google Scholar]
- DeGrado W. F., Musso G. F., Lieber M., Kaiser E. T., Kézdy F. J. Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys J. 1982 Jan;37(1):329–338. doi: 10.1016/S0006-3495(82)84681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey C. E. The actions of melittin on membranes. Biochim Biophys Acta. 1990 May 7;1031(2):143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- Dufourcq J., Faucon J. F., Fourche G., Dasseux J. L., Le Maire M., Gulik-Krzywicki T. Morphological changes of phosphatidylcholine bilayers induced by melittin: vesicularization, fusion, discoidal particles. Biochim Biophys Acta. 1986 Jul 10;859(1):33–48. doi: 10.1016/0005-2736(86)90315-9. [DOI] [PubMed] [Google Scholar]
- Dufton M. J., Hider R. C., Cherry R. J. The influence of melittin on the rotation of band 3 protein in the human erythrocyte membrane. Eur Biophys J. 1984;11(1):17–24. doi: 10.1007/BF00253854. [DOI] [PubMed] [Google Scholar]
- Fattal E., Nir S., Parente R. A., Szoka F. C., Jr Pore-forming peptides induce rapid phospholipid flip-flop in membranes. Biochemistry. 1994 May 31;33(21):6721–6731. doi: 10.1021/bi00187a044. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Frey S., Tamm L. K. Orientation of melittin in phospholipid bilayers. A polarized attenuated total reflection infrared study. Biophys J. 1991 Oct;60(4):922–930. doi: 10.1016/S0006-3495(91)82126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Ludtke S. J., Heller W. T., Huang H. W. Mechanism of alamethicin insertion into lipid bilayers. Biophys J. 1996 Nov;71(5):2669–2679. doi: 10.1016/S0006-3495(96)79458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Ludtke S. J., Worcester D. L., Huang H. W. Neutron scattering in the plane of membranes: structure of alamethicin pores. Biophys J. 1996 Jun;70(6):2659–2666. doi: 10.1016/S0006-3495(96)79835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermetter A., Lakowicz J. R. The aggregation state of mellitin in lipid bilayers. An energy transfer study. J Biol Chem. 1986 Jun 25;261(18):8243–8248. [PubMed] [Google Scholar]
- Hook W. A., Tsuji S., Siraganian R. P. Magainin-2 releases histamine from rat mast cells. Proc Soc Exp Biol Med. 1990 Jan;193(1):50–55. doi: 10.3181/00379727-193-42989. [DOI] [PubMed] [Google Scholar]
- John E., Jähnig F. Aggregation state of melittin in lipid vesicle membranes. Biophys J. 1991 Aug;60(2):319–328. doi: 10.1016/S0006-3495(91)82056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsu T., Ninomiya C., Kuroko M., Kobayashi H., Hirota T., Fujita Y. Action mechanism of amphipathic peptides gramicidin S and melittin on erythrocyte membrane. Biochim Biophys Acta. 1988 Mar 22;939(1):57–63. doi: 10.1016/0005-2736(88)90047-8. [DOI] [PubMed] [Google Scholar]
- Kempf C., Klausner R. D., Weinstein J. N., Van Renswoude J., Pincus M., Blumenthal R. Voltage-dependent trans-bilayer orientation of melittin. J Biol Chem. 1982 Mar 10;257(5):2469–2476. [PubMed] [Google Scholar]
- Ludtke S. J., He K., Heller W. T., Harroun T. A., Yang L., Huang H. W. Membrane pores induced by magainin. Biochemistry. 1996 Oct 29;35(43):13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Murase O., Fujii N., Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996 Sep 3;35(35):11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Murase O., Fujii N., Miyajima K. Translocation of a channel-forming antimicrobial peptide, magainin 2, across lipid bilayers by forming a pore. Biochemistry. 1995 May 16;34(19):6521–6526. doi: 10.1021/bi00019a033. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Murase O., Miyajima K. Kinetics of pore formation by an antimicrobial peptide, magainin 2, in phospholipid bilayers. Biochemistry. 1995 Oct 3;34(39):12553–12559. doi: 10.1021/bi00039a009. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Murase O., Tokuda H., Funakoshi S., Fujii N., Miyajima K. Orientational and aggregational states of magainin 2 in phospholipid bilayers. Biochemistry. 1994 Mar 22;33(11):3342–3349. doi: 10.1021/bi00177a027. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Nakamura A., Murase O., Sugishita K., Fujii N., Miyajima K. Modulation of magainin 2-lipid bilayer interactions by peptide charge. Biochemistry. 1997 Feb 25;36(8):2104–2111. doi: 10.1021/bi961870p. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Yoneyama S., Murase O., Miyajima K. Transbilayer transport of ions and lipids coupled with mastoparan X translocation. Biochemistry. 1996 Jun 25;35(25):8450–8456. doi: 10.1021/bi960342a. [DOI] [PubMed] [Google Scholar]
- Mousli M., Bueb J. L., Bronner C., Rouot B., Landry Y. G protein activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol Sci. 1990 Sep;11(9):358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- Okada A., Wakamatsu K., Miyazawa T., Higashijima T. Vesicle-bound conformation of melittin: transferred nuclear Overhauser enhancement analysis in the presence of perdeuterated phosphatidylcholine vesicles. Biochemistry. 1994 Aug 16;33(32):9438–9446. doi: 10.1021/bi00198a009. [DOI] [PubMed] [Google Scholar]
- Parente R. A., Nir S., Szoka F. C., Jr Mechanism of leakage of phospholipid vesicle contents induced by the peptide GALA. Biochemistry. 1990 Sep 18;29(37):8720–8728. doi: 10.1021/bi00489a031. [DOI] [PubMed] [Google Scholar]
- Pawlak M., Stankowski S., Schwarz G. Melittin induced voltage-dependent conductance in DOPC lipid bilayers. Biochim Biophys Acta. 1991 Feb 11;1062(1):94–102. doi: 10.1016/0005-2736(91)90339-a. [DOI] [PubMed] [Google Scholar]
- Portlock S. H., Clague M. J., Cherry R. J. Leakage of internal markers from erythrocytes and lipid vesicles induced by melittin, gramicidin S and alamethicin: a comparative study. Biochim Biophys Acta. 1990 Nov 30;1030(1):1–10. doi: 10.1016/0005-2736(90)90231-c. [DOI] [PubMed] [Google Scholar]
- Saberwal G., Nagaraj R. Cell-lytic and antibacterial peptides that act by perturbing the barrier function of membranes: facets of their conformational features, structure-function correlations and membrane-perturbing abilities. Biochim Biophys Acta. 1994 Jun 29;1197(2):109–131. doi: 10.1016/0304-4157(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Sansom M. S. The biophysics of peptide models of ion channels. Prog Biophys Mol Biol. 1991;55(3):139–235. doi: 10.1016/0079-6107(91)90004-c. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Arbuzova A. Pore kinetics reflected in the dequenching of a lipid vesicle entrapped fluorescent dye. Biochim Biophys Acta. 1995 Oct 4;1239(1):51–57. doi: 10.1016/0005-2736(95)00134-o. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Beschiaschvili G. Thermodynamic and kinetic studies on the association of melittin with a phospholipid bilayer. Biochim Biophys Acta. 1989 Feb 13;979(1):82–90. doi: 10.1016/0005-2736(89)90526-9. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Robert C. H. Kinetics of pore-mediated release of marker molecules from liposomes or cells. Biophys Chem. 1992 Apr;42(3):291–296. doi: 10.1016/0301-4622(92)80021-v. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Zong R. T., Popescu T. Kinetics of melittin induced pore formation in the membrane of lipid vesicles. Biochim Biophys Acta. 1992 Sep 21;1110(1):97–104. doi: 10.1016/0005-2736(92)90299-2. [DOI] [PubMed] [Google Scholar]
- Stankowski S., Pawlak M., Kaisheva E., Robert C. H., Schwarz G. A combined study of aggregation, membrane affinity and pore activity of natural and modified melittin. Biochim Biophys Acta. 1991 Oct 14;1069(1):77–86. doi: 10.1016/0005-2736(91)90106-i. [DOI] [PubMed] [Google Scholar]
- Steiner H., Hultmark D., Engström A., Bennich H., Boman H. G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981 Jul 16;292(5820):246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C., Weissman L., Eisenberg D. The structure of melittin in the form I crystals and its implication for melittin's lytic and surface activities. Biophys J. 1982 Jan;37(1):353–361. doi: 10.1016/S0006-3495(82)84683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson M. T., Alvarez O., Hubbell W., Bieganski R. M., Attenbach C., Caporales L. H., Levy J. J., Nutt R. F., Rosenblatt M., Tosteson D. C. Primary structure of peptides and ion channels. Role of amino acid side chains in voltage gating of melittin channels. Biophys J. 1990 Dec;58(6):1367–1375. doi: 10.1016/S0006-3495(90)82483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H. Comparison of the conformation and orientation of alamethicin and melittin in lipid membranes. Biochemistry. 1987 Jul 14;26(14):4562–4572. doi: 10.1021/bi00388a060. [DOI] [PubMed] [Google Scholar]



