Abstract
Indo-1 fluorescence signals were measured from one extremity of enzymatically isolated skeletal muscle fibers of mice. An original and simple method was developed to allow the measurements to be made under voltage-clamp control: the major part of a single fiber was embedded in silicone grease, so that only a short portion of one end of the fiber, from which the fluorescence measurements were taken, was in contact with the external solution. Membrane potential was held and varied by using a patch-clamp amplifier in whole-cell configuration with a single microelectrode, the tip of which was inserted across the silicone grease within the insulated portion of the fiber. In response to 100-ms depolarizing command pulses to voltages more positive than -40 mV (from a holding potential of -80 mV), clear changes in fluorescence were qualitatively observed to feature a time course of rise and decay expected from a change in intracellular calcium concentration ([Ca2+]i) due to voltage-dependent sarcoplasmic reticulum (SR) calcium release. Although the peak [Ca2+]i elicited by a 100-ms depolarization at 0 or +10 mV varied from fiber to fiber, it could clearly reach a value high enough to saturate Indo-1. The overall results show that this method represents an efficient way of measuring depolarization-induced [Ca2+]i changes in enzymatically dissociated skeletal muscle fibers.
Full text
PDF
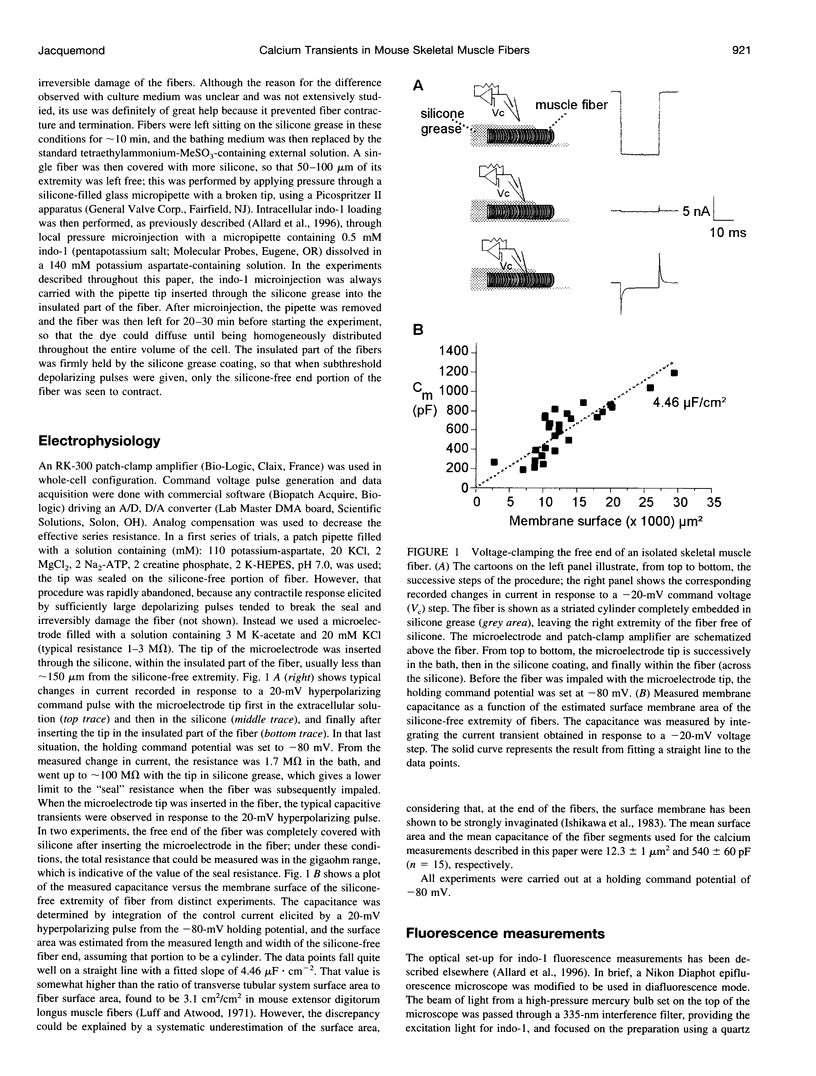
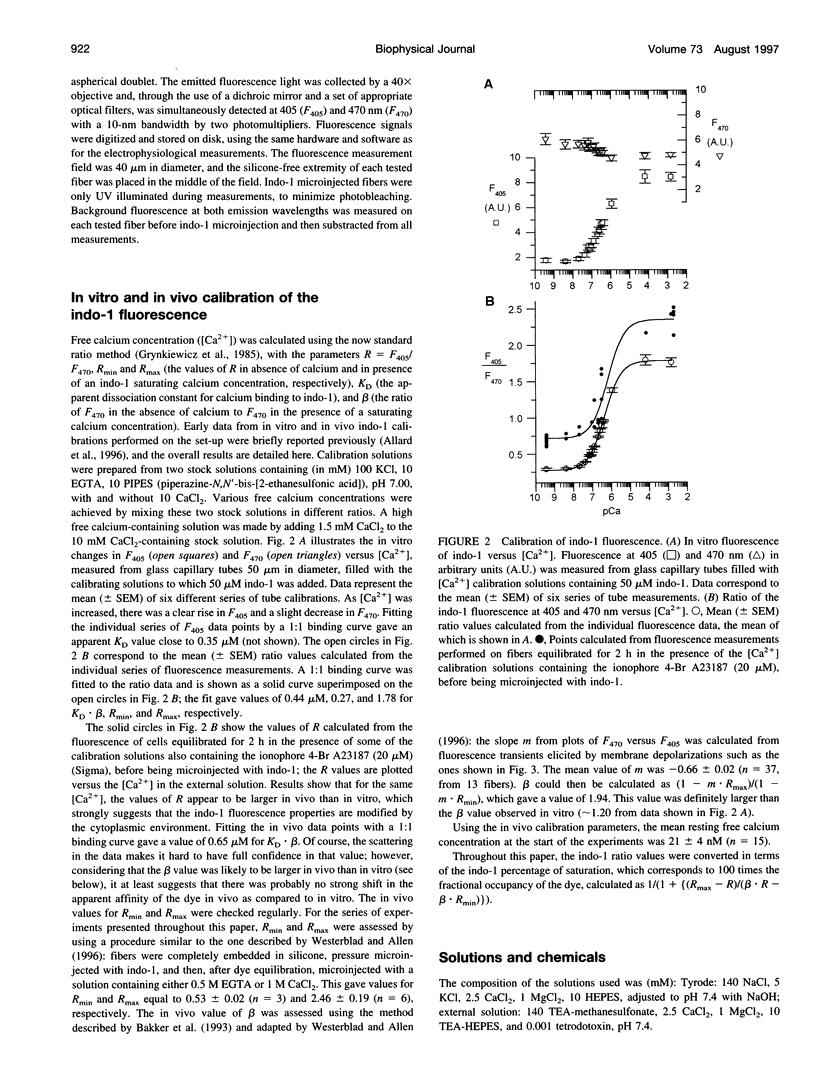
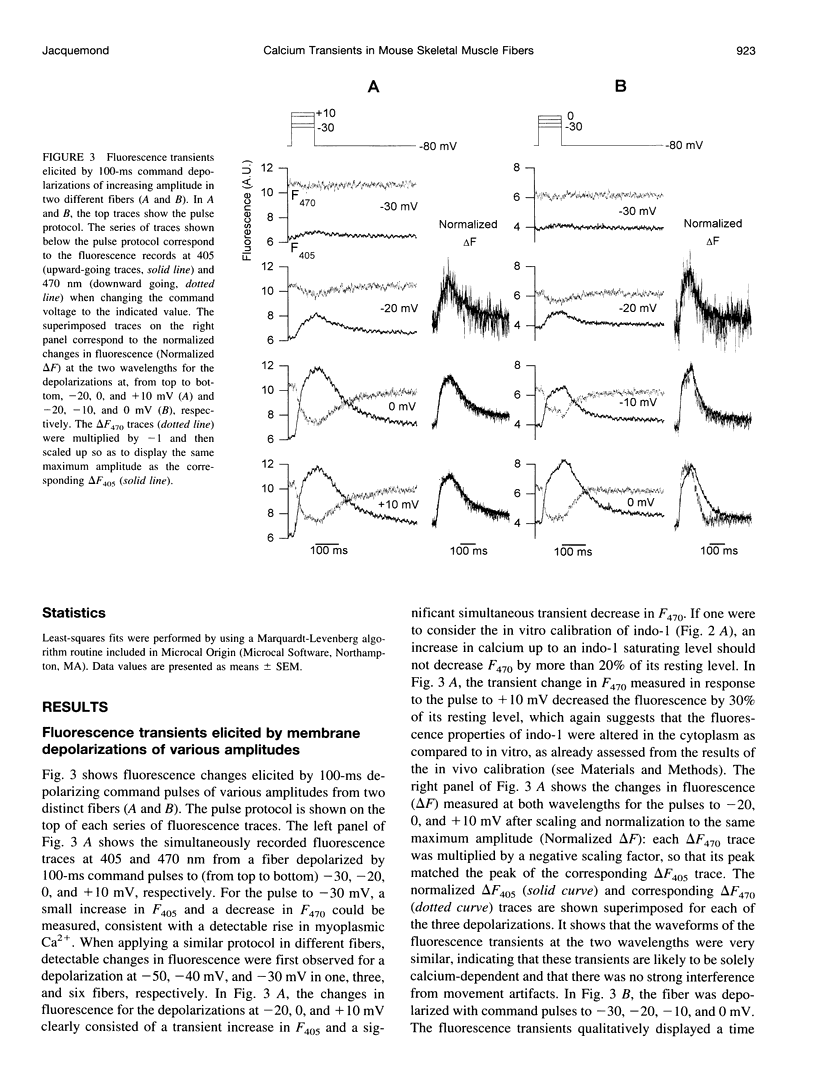
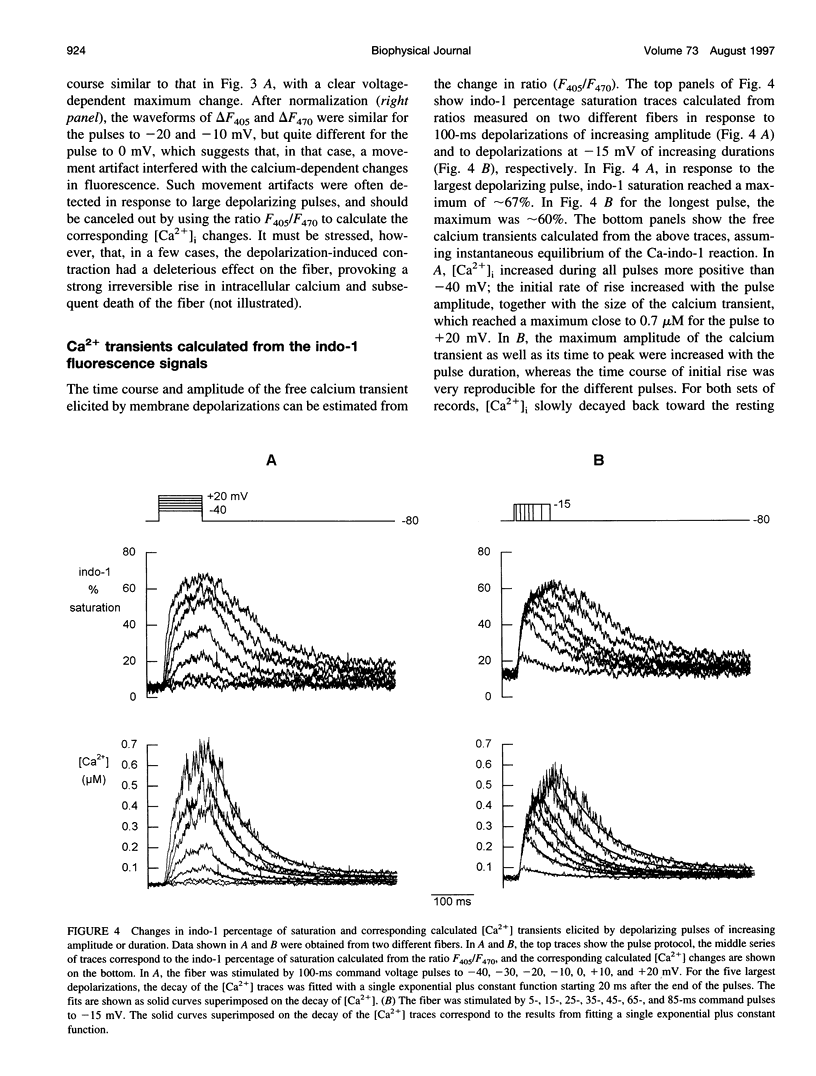


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard B., Bernengo J. C., Rougier O., Jacquemond V. Intracellular Ca2+ changes and Ca2+-activated K+ channel activation induced by acetylcholine at the endplate of mouse skeletal muscle fibres. J Physiol. 1996 Jul 15;494(Pt 2):337–349. doi: 10.1113/jphysiol.1996.sp021496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A. J., Head S. I., Williams D. A., Stephenson D. G. Ca2+ levels in myotubes grown from the skeletal muscle of dystrophic (mdx) and normal mice. J Physiol. 1993 Jan;460:1–13. doi: 10.1113/jphysiol.1993.sp019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A., Betz W. J. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol. 1977 Sep;271(1):25–40. doi: 10.1113/jphysiol.1977.sp011988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. L., Klein M. G., Schneider M. F. Calcium transients in intact rat skeletal muscle fibers in agarose gel. Am J Physiol. 1995 Jul;269(1 Pt 1):C28–C34. doi: 10.1152/ajpcell.1995.269.1.C28. [DOI] [PubMed] [Google Scholar]
- Delbono O. Ca2+ modulation of sarcoplasmic reticulum Ca2+ release in rat skeletal muscle fibers. J Membr Biol. 1995 Jul;146(1):91–99. [PubMed] [Google Scholar]
- Delbono O., Meissner G. Sarcoplasmic reticulum Ca2+ release in rat slow- and fast-twitch muscles. J Membr Biol. 1996 May;151(2):123–130. doi: 10.1007/s002329900063. [DOI] [PubMed] [Google Scholar]
- Delbono O., O'Rourke K. S., Ettinger W. H. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol. 1995 Dec;148(3):211–222. doi: 10.1007/BF00235039. [DOI] [PubMed] [Google Scholar]
- Delbono O., Stefani E. Calcium transients in single mammalian skeletal muscle fibres. J Physiol. 1993 Apr;463:689–707. doi: 10.1113/jphysiol.1993.sp019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., Schneider M. F. Calcium transients and calcium release in rat fast-twitch skeletal muscle fibres. J Physiol. 1993 Apr;463:709–728. doi: 10.1113/jphysiol.1993.sp019618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J., Schneider M. F. Suppression of calcium release by calcium or procaine in voltage clamped rat skeletal muscle fibres. J Physiol. 1995 Jun 1;485(Pt 2):437–445. doi: 10.1113/jphysiol.1995.sp020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Head S. I. Membrane potential, resting calcium and calcium transients in isolated muscle fibres from normal and dystrophic mice. J Physiol. 1993 Sep;469:11–19. doi: 10.1113/jphysiol.1993.sp019801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. P., Timmerman M. P., Bagshaw C. R., Ashley C. C. The kinetics of calcium binding to fura-2 and indo-1. FEBS Lett. 1987 May 25;216(1):35–39. doi: 10.1016/0014-5793(87)80752-4. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Bartschat D. K. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991 May 31;177(1):184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Luff A. R., Atwood H. L. Changes in the sarcoplasmic reticulum and transverse tubular system of fast and slow skeletal muscles of the mouse during postnatal development. J Cell Biol. 1971 Nov;51(21):369–383. doi: 10.1083/jcb.51.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
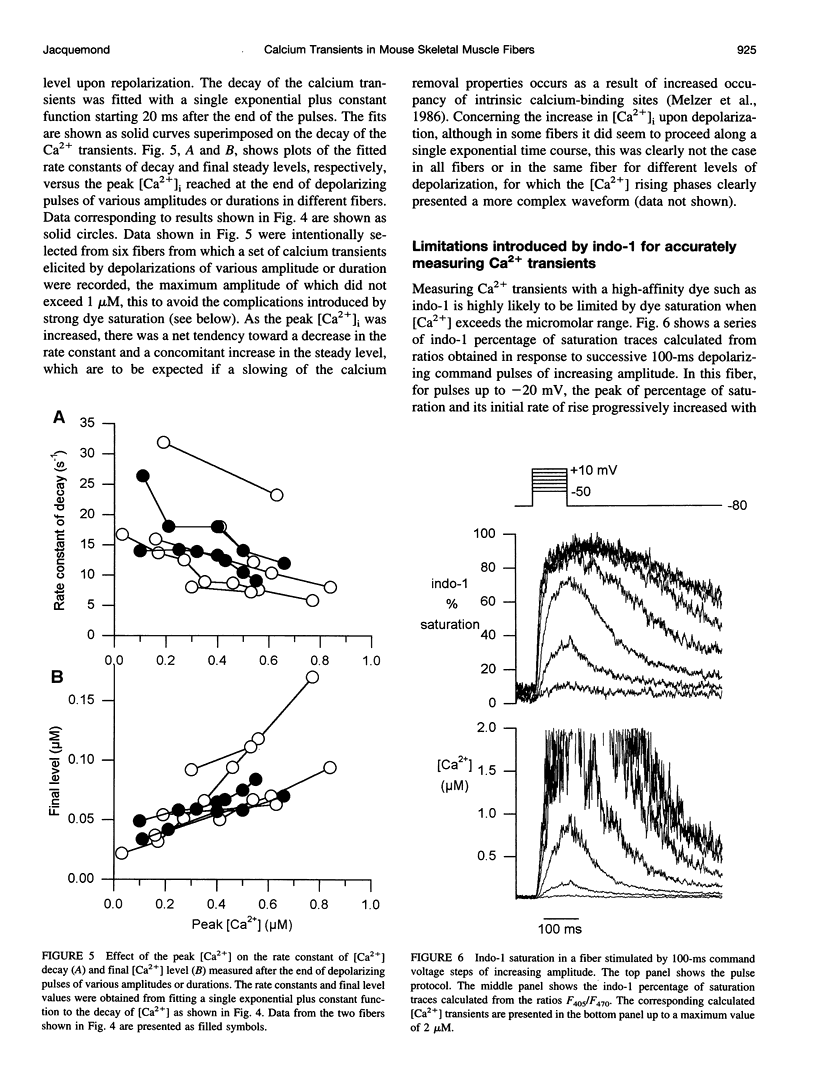
- Melzer W., Herrmann-Frank A., Lüttgau H. C. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995 May 8;1241(1):59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Melzer W., Ríos E., Schneider M. F. The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. J Physiol. 1986 Mar;372:261–292. doi: 10.1113/jphysiol.1986.sp016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F. Control of calcium release in functioning skeletal muscle fibers. Annu Rev Physiol. 1994;56:463–484. doi: 10.1146/annurev.ph.56.030194.002335. [DOI] [PubMed] [Google Scholar]
- Shirokova N., García J., Pizarro G., Ríos E. Ca2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle. J Gen Physiol. 1996 Jan;107(1):1–18. doi: 10.1085/jgp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
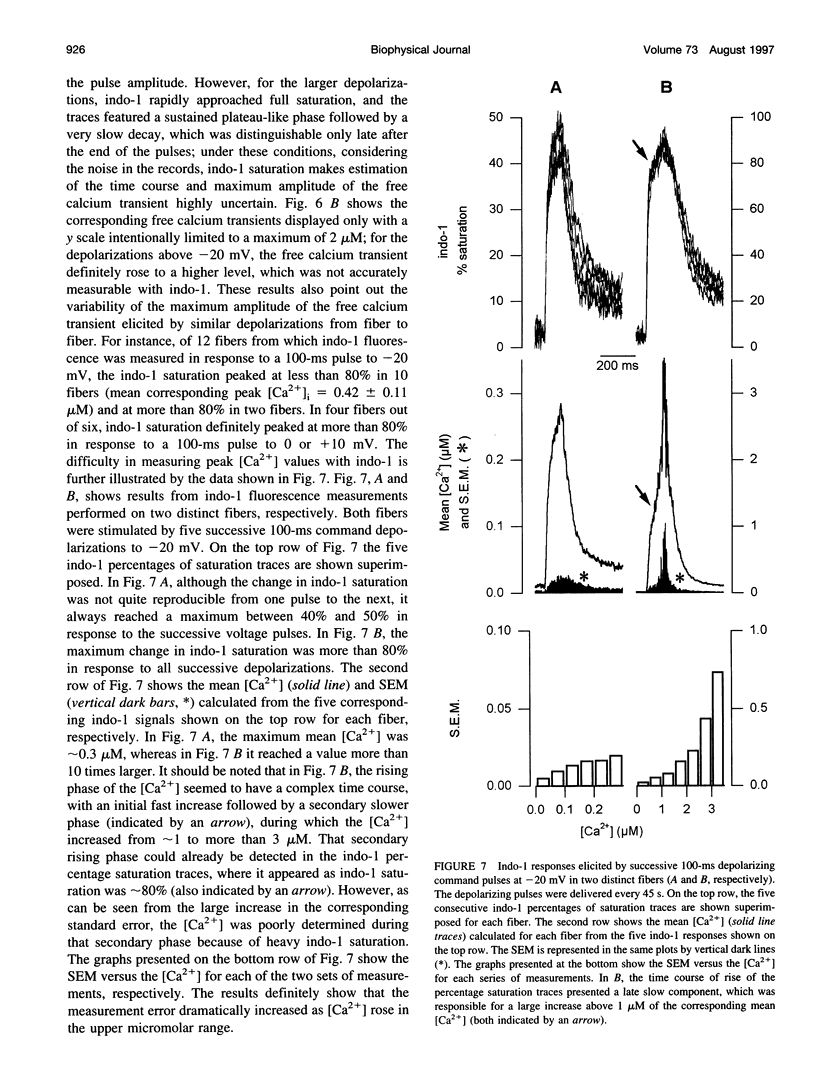
- Turner P. R., Westwood T., Regen C. M., Steinhardt R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988 Oct 20;335(6192):735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991 Sep;98(3):615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Intracellular calibration of the calcium indicator indo-1 in isolated fibers of Xenopus muscle. Biophys J. 1996 Aug;71(2):908–917. doi: 10.1016/S0006-3495(96)79294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Hollingworth S., Baylor S. M. Properties of tri- and tetracarboxylate Ca2+ indicators in frog skeletal muscle fibers. Biophys J. 1996 Feb;70(2):896–916. doi: 10.1016/S0006-3495(96)79633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]



