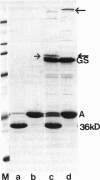
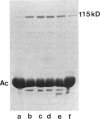
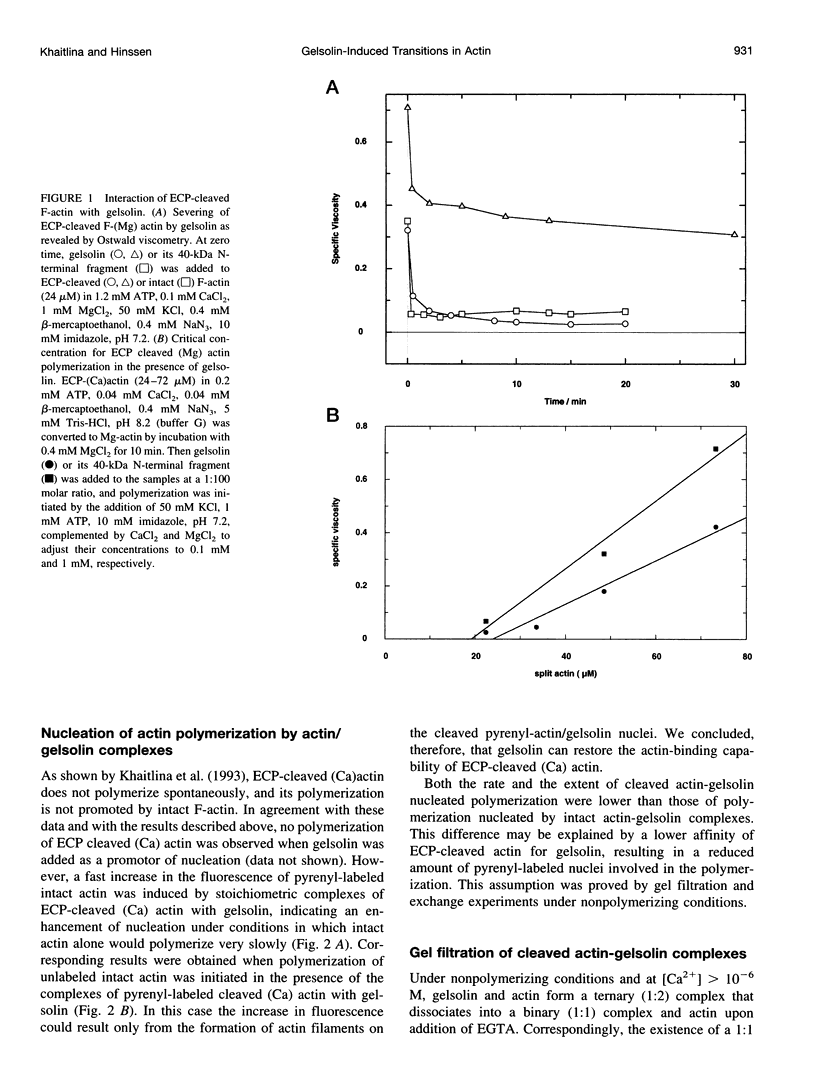
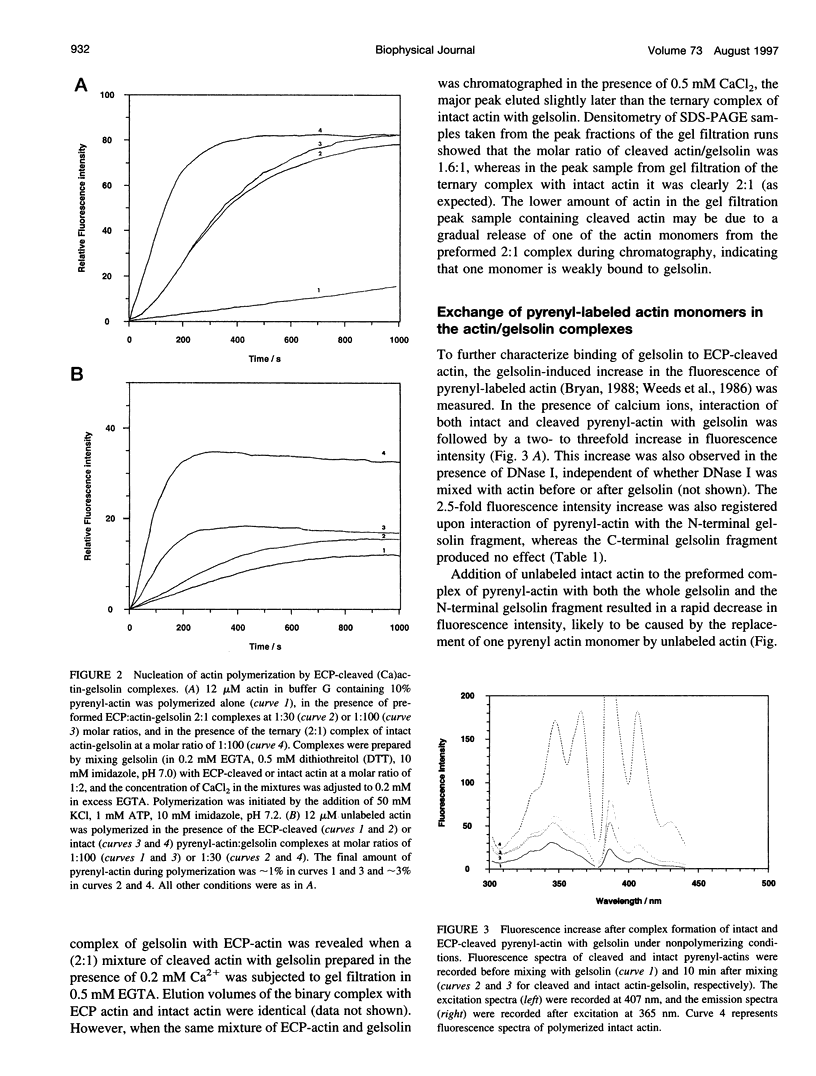
Abstract
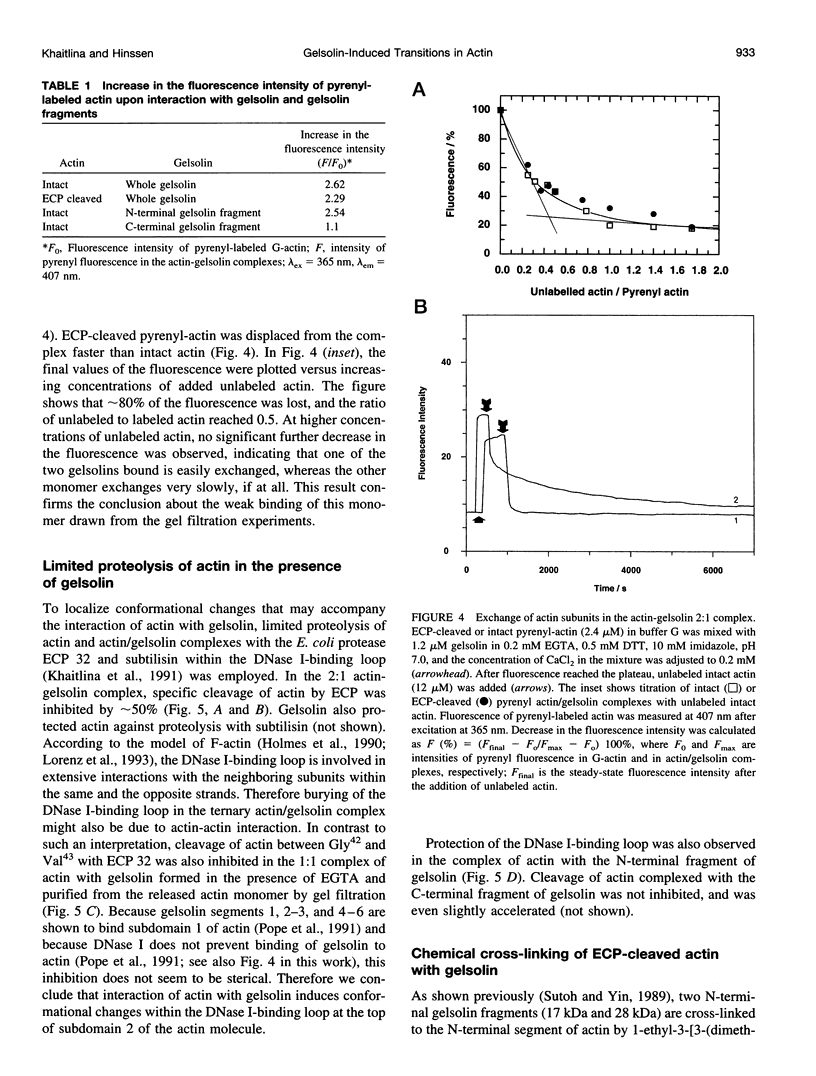
Actin cleaved by the protease from Escherichia coli A2 strain between Gly42 and Val43 (ECP-actin) is no longer polymerizable when it contains Ca2+ as a tightly bound cation, but polymerizes when Mg2+ is bound. We have investigated the interactions of gelsolin with this actin with regard to conformational changes in the actin molecule induced by the binding of gelsolin. ECP-(Ca)actin interacts with gelsolin in a manner similar to that in which it reacts with intact actin, and forms a stoichiometric 2:1 complex. Despite the nonpolymerizability of ECP-(Ca)actin, this complex can act as a nucleus for the polymerization of intact actin, thus indicating that upon interaction with gelsolin, ECP-(Ca)actin undergoes a conformational change that enables its interaction with another actin monomer. By gel filtration and fluorometry it was shown that the binding of at least one of the ECP-cleaved actins to gelsolin is considerably weaker than of intact actin, suggesting that conformational changes in subdomain 2 of actin monomer may directly or allosterically affect actin-gelsolin interactions. On the other hand, interaction with gelsolin changes the conformation of actin within the DNase I-binding loop, as indicated by inhibition of limited proteolysis of actin by ECP and subtilisin. Cross-linking experiments with gelsolin-nucleated actin filaments using N,N-phenylene-bismaleimide (which cross-links adjacent actin monomers between Cys374 and Lys191) reveal that gelsolin causes a significant increase in the yield of the 115-kDa cross-linking product, confirming the evidence that gelsolin stabilizes or changes the conformation of the C-terminal region of the actin molecule, and these changes are propagated from the capped end along the filament. These results allow us to conclude that nucleation of actin polymerization by gelsolin is promoted by conformational changes within subdomain 2 and at the C-terminus of the actin monomer.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan J. Gelsolin has three actin-binding sites. J Cell Biol. 1988 May;106(5):1553–1562. doi: 10.1083/jcb.106.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Kurth M. C. Actin-gelsolin interactions. Evidence for two actin-binding sites. J Biol Chem. 1984 Jun 25;259(12):7480–7487. [PubMed] [Google Scholar]
- Chaponnier C., Janmey P. A., Yin H. L. The actin filament-severing domain of plasma gelsolin. J Cell Biol. 1986 Oct;103(4):1473–1481. doi: 10.1083/jcb.103.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie R. H., Miller C., Cheung P., Goodnight T., Muhlrad A., Reisler E. Structural connectivity in actin: effect of C-terminal modifications on the properties of actin. Biophys J. 1994 Nov;67(5):1957–1964. doi: 10.1016/S0006-3495(94)80678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga M., Phelan J. J. F-actin is intermolecularly crosslinked by N,N'-p-phenylenedimaleimide through lysine-191 and cysteine-374. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6599–6602. doi: 10.1073/pnas.81.21.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg J., Capony J. P., Benyamin Y., Roustan C. Definition of the EGTA-independent interface involved in the serum gelsolin-actin complex. Biochem J. 1993 Aug 1;293(Pt 3):813–817. doi: 10.1042/bj2930813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterkamp T., Weeds A. G., Mannherz H. G. The actin monomers in the ternary gelsolin: 2 actin complex are in an antiparallel orientation. Eur J Biochem. 1993 Dec 1;218(2):507–513. doi: 10.1111/j.1432-1033.1993.tb18403.x. [DOI] [PubMed] [Google Scholar]
- Hinssen H., Small J. V., Sobieszek A. A Ca2+-dependent actin modulator from vertebrate smooth muscle. FEBS Lett. 1984 Jan 23;166(1):90–95. doi: 10.1016/0014-5793(84)80051-4. [DOI] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Khaitlina SYu, Collins J. H., Kuznetsova I. M., Pershina V. P., Synakevich I. G., Turoverov K. K., Usmanova A. M. Physico-chemical properties of actin cleaved with bacterial protease from E. coli A2 strain. FEBS Lett. 1991 Feb 11;279(1):49–51. doi: 10.1016/0014-5793(91)80247-z. [DOI] [PubMed] [Google Scholar]
- Khaitlina S. Y., Moraczewska J., Strzelecka-Gołaszewska H. The actin/actin interactions involving the N-terminus of the DNase-I-binding loop are crucial for stabilization of the actin filament. Eur J Biochem. 1993 Dec 15;218(3):911–920. doi: 10.1111/j.1432-1033.1993.tb18447.x. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Kuznetsova I., Antropova O., Turoverov K., Khaitlina S. Conformational changes in subdomain I of actin induced by proteolytic cleavage within the DNase I-binding loop: energy transfer from tryptophan to AEDANS. FEBS Lett. 1996 Mar 25;383(1-2):105–108. doi: 10.1016/0014-5793(96)00238-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorenz M., Popp D., Holmes K. C. Refinement of the F-actin model against X-ray fiber diffraction data by the use of a directed mutation algorithm. J Mol Biol. 1993 Dec 5;234(3):826–836. doi: 10.1006/jmbi.1993.1628. [DOI] [PubMed] [Google Scholar]
- McLaughlin P. J., Gooch J. T., Mannherz H. G., Weeds A. G. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature. 1993 Aug 19;364(6439):685–692. doi: 10.1038/364685a0. [DOI] [PubMed] [Google Scholar]
- Millonig R., Salvo H., Aebi U. Probing actin polymerization by intermolecular cross-linking. J Cell Biol. 1988 Mar;106(3):785–796. doi: 10.1083/jcb.106.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean C., Hué H. K., Pons F., Roustan C., Benyamin Y. Cation binding sites on actin: a structural relationship between antigenic epitopes and cation exchange. Biochem Biophys Res Commun. 1988 Apr 15;152(1):368–375. doi: 10.1016/s0006-291x(88)80723-x. [DOI] [PubMed] [Google Scholar]
- Orlova A., Egelman E. H. Structural dynamics of F-actin: I. Changes in the C terminus. J Mol Biol. 1995 Feb 3;245(5):582–597. doi: 10.1006/jmbi.1994.0048. [DOI] [PubMed] [Google Scholar]
- Orlova A., Prochniewicz E., Egelman E. H. Structural dynamics of F-actin: II. Cooperativity in structural transitions. J Mol Biol. 1995 Feb 3;245(5):598–607. doi: 10.1006/jmbi.1994.0049. [DOI] [PubMed] [Google Scholar]
- Pope B., Maciver S., Weeds A. Localization of the calcium-sensitive actin monomer binding site in gelsolin to segment 4 and identification of calcium binding sites. Biochemistry. 1995 Feb 7;34(5):1583–1588. doi: 10.1021/bi00005a014. [DOI] [PubMed] [Google Scholar]
- Pope B., Way M., Weeds A. G. Two of the three actin-binding domains of gelsolin bind to the same subdomain of actin. Implications of capping and severing mechanisms. FEBS Lett. 1991 Mar 11;280(1):70–74. doi: 10.1016/0014-5793(91)80206-i. [DOI] [PubMed] [Google Scholar]
- Prochniewicz E., Zhang Q., Janmey P. A., Thomas D. D. Cooperativity in F-actin: binding of gelsolin at the barbed end affects structure and dynamics of the whole filament. J Mol Biol. 1996 Aug 2;260(5):756–766. doi: 10.1006/jmbi.1996.0435. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Strzelecka-Gołaszewska H., Moraczewska J., Khaitlina S. Y., Mossakowska M. Localization of the tightly bound divalent-cation-dependent and nucleotide-dependent conformation changes in G-actin using limited proteolytic digestion. Eur J Biochem. 1993 Feb 1;211(3):731–742. doi: 10.1111/j.1432-1033.1993.tb17603.x. [DOI] [PubMed] [Google Scholar]
- Strzelecka-Gołaszewska H., Mossakowska M., Woźniak A., Moraczewska J., Nakayama H. Long-range conformational effects of proteolytic removal of the last three residues of actin. Biochem J. 1995 Apr 15;307(Pt 2):527–534. doi: 10.1042/bj3070527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K., Yin H. L. End-label fingerprintings show that the N- and C-termini of actin are in the contact site with gelsolin. Biochemistry. 1989 Jun 13;28(12):5269–5275. doi: 10.1021/bi00438a052. [DOI] [PubMed] [Google Scholar]
- Usmanova A. M., Khaitlina S. Iu. Proteaza iz shtamma bakterii E. coli A2, spetsificheski rasshchepliaiushchaia aktin. Biokhimiia. 1989 Aug;54(8):1308–1314. [PubMed] [Google Scholar]
- Weeds A. G., Harris H., Gratzer W., Gooch J. Interactions of pig plasma gelsolin with G-actin. Eur J Biochem. 1986 Nov 17;161(1):77–84. doi: 10.1111/j.1432-1033.1986.tb10126.x. [DOI] [PubMed] [Google Scholar]
- Yin H. L. Calcium and polyphosphoinositide regulation of actin network structure by gelsolin. Adv Exp Med Biol. 1989;255:315–323. doi: 10.1007/978-1-4684-5679-0_35. [DOI] [PubMed] [Google Scholar]