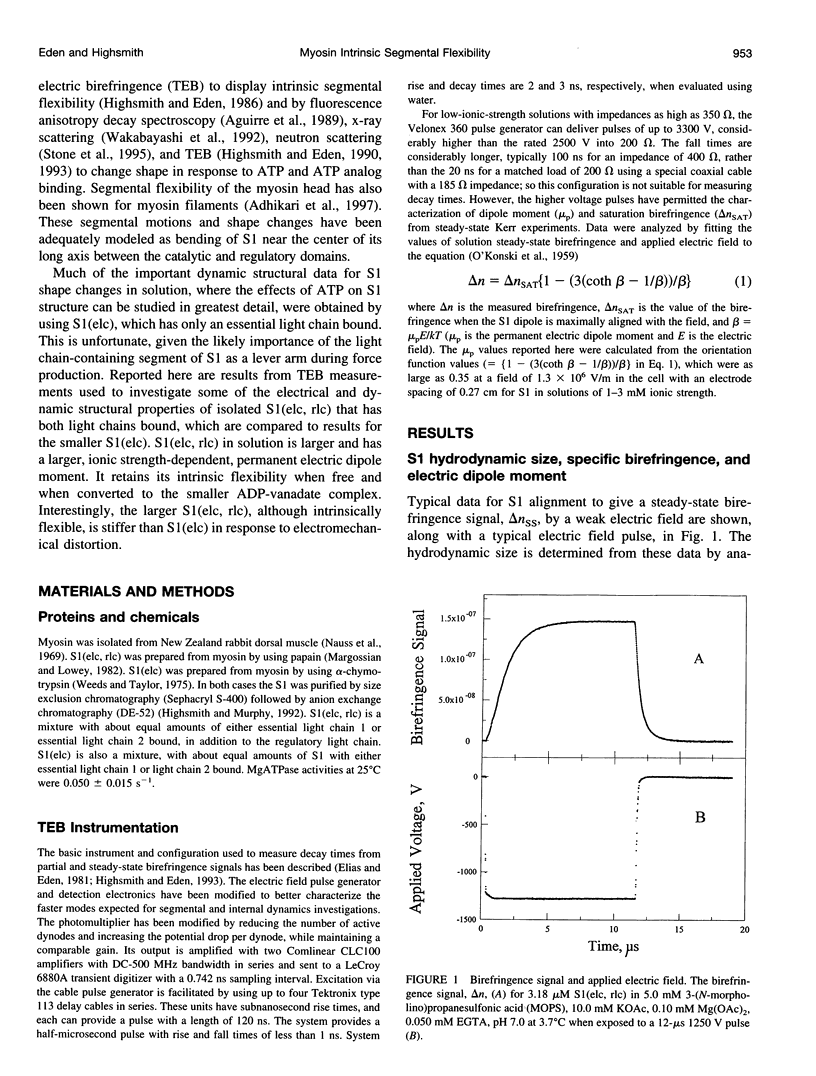
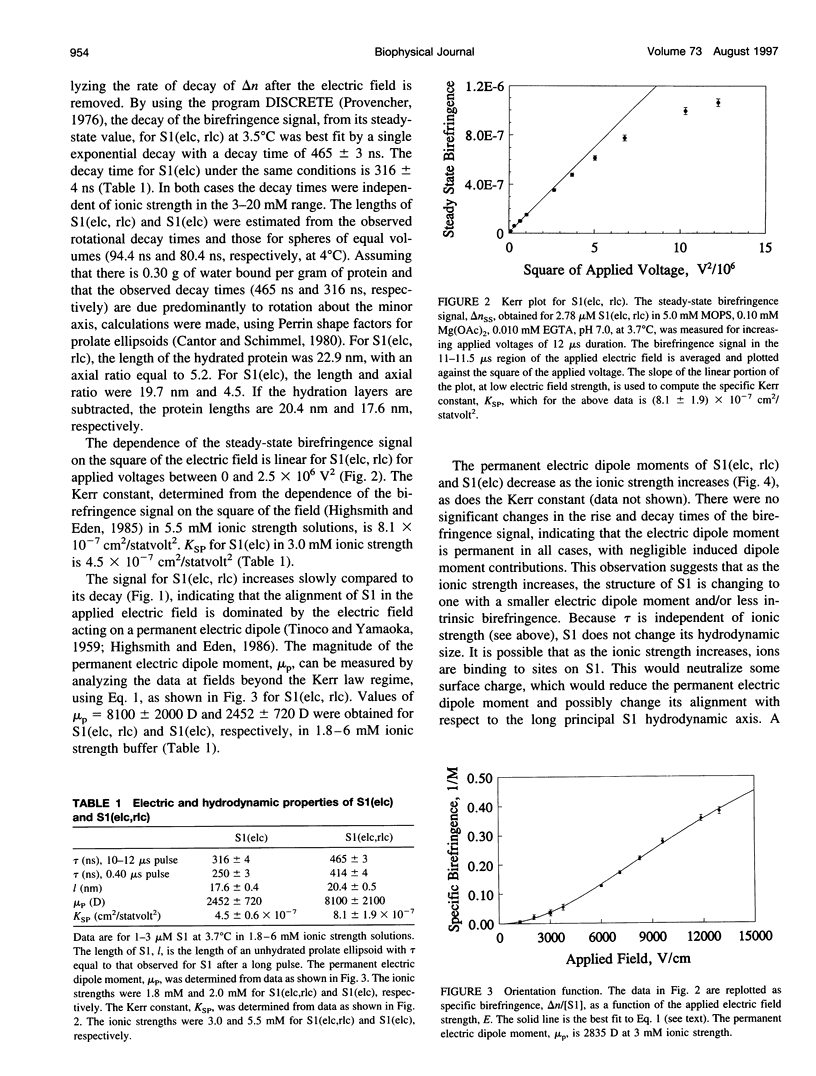
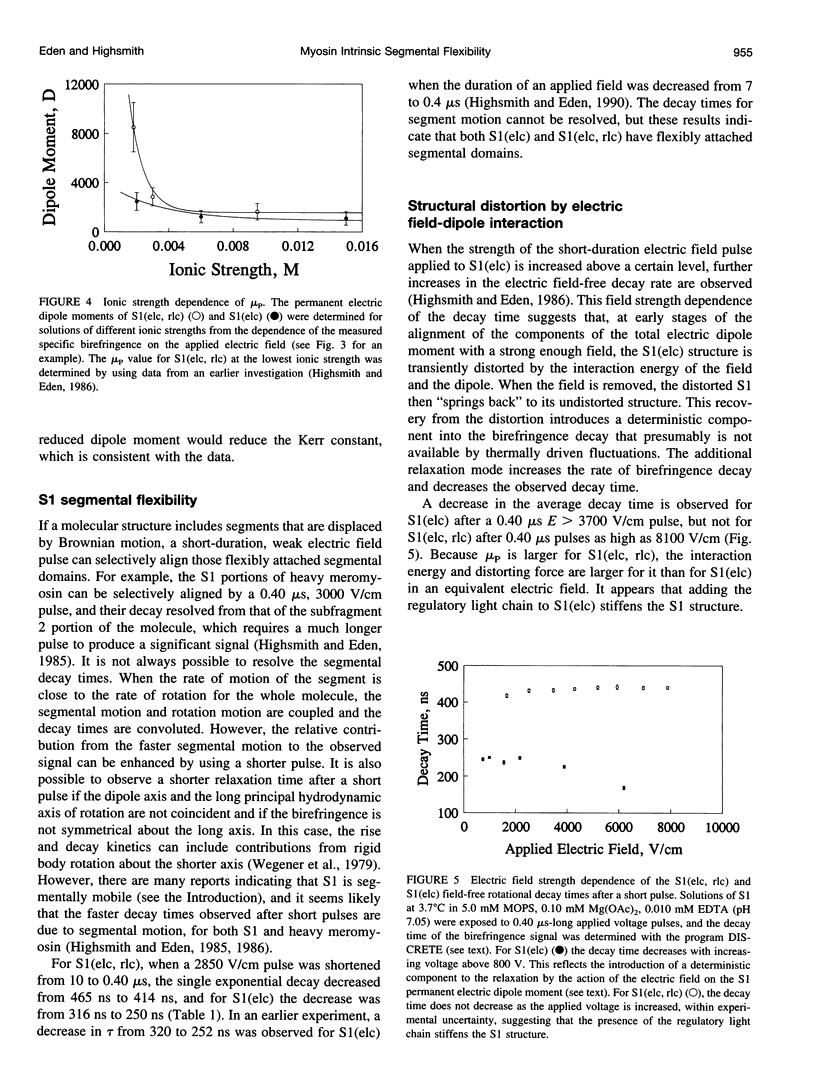
Abstract
The technique of transient electrical birefringence was used to compare some of the electric and structural dynamic properties of myosin subfragment 1 (S1(elc, rlc)), which has both the essential and regulatory light chains bound, to S1(elc), which has only an essential light chain. The rates of rotational Brownian motion indicate that S1(elc, rlc) is larger, as expected. The permanent electric dipole moment of S1(elc, rlc) is also larger, indicating that the regulatory light chain portion of S1(elc, rlc) has a dipole moment and that it is aligned head-to-tail with the dipole moment of the S1(elc) portion. The permanent electric dipoles decrease with increasing ionic strength, apparently because of ion binding to surface charges. Both S1(elc, rlc) and S1(elc) have intrinsic segmental flexibility, as detected by the ability to selectively align segments with a brief weak electric field. However, unlike S1(elc), which can be structurally distorted by the action of a brief strong electric field, S1(elc, rlc) is stiffer and cannot be distorted by fields as high as 7800 V/cm applied to its approximately 8000 D permanent electric dipole moment. The S1 . MgADP . Pi analog S1 . MgADP . Vi is smaller than S1 . MgADP, for both S1(elc, rlc) and S1(elc). Interestingly, the smaller, stiffer S1(elc, rlc) . MgADP . Vi complex retains intrinsic segmental flexibility. These results are discussed within a framework of current hypotheses of force-producing mechanisms that involve S1 segmental motion and/or the loss of cross-bridge flexibility during force production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre R., Lin S. H., Gonsoulin F., Wang C. K., Cheung H. C. Characterization of the ethenoadenosine diphosphate binding site of myosin subfragment 1. Energetics of the equilibrium between two states of nucleotide.S1 and vanadate-induced global conformation changes detected by energy transfer. Biochemistry. 1989 Jan 24;28(2):799–807. doi: 10.1021/bi00428a058. [DOI] [PubMed] [Google Scholar]
- Ajtai K., French A. R., Burghardt T. P. Myosin cross-bridge orientation in rigor and in the presence of nucleotide studied by electron spin resonance. Biophys J. 1989 Sep;56(3):535–541. doi: 10.1016/S0006-3495(89)82700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajtai K., Toft D. J., Burghardt T. P. Path and extent of cross-bridge rotation during muscle contraction. Biochemistry. 1994 May 10;33(18):5382–5391. doi: 10.1021/bi00184a005. [DOI] [PubMed] [Google Scholar]
- Allen T. S., Ling N., Irving M., Goldman Y. E. Orientation changes in myosin regulatory light chains following photorelease of ATP in skinned muscle fibers. Biophys J. 1996 Apr;70(4):1847–1862. doi: 10.1016/S0006-3495(96)79750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson M., Geeves M. A., Kurzawa S. E., Manstein D. J. Myosin motors with artificial lever arms. EMBO J. 1996 Nov 15;15(22):6069–6074. [PMC free article] [PubMed] [Google Scholar]
- Bartels E. M., Cooke P. H., Elliott G. F., Hughes R. A. The myosin molecule--charge response to nucleotide binding. Biochim Biophys Acta. 1993 May 7;1157(1):63–73. doi: 10.1016/0304-4165(93)90079-n. [DOI] [PubMed] [Google Scholar]
- Brenner B., Xu S., Chalovich J. M., Yu L. C. Radial equilibrium lengths of actomyosin cross-bridges in muscle. Biophys J. 1996 Nov;71(5):2751–2758. doi: 10.1016/S0006-3495(96)79468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Díaz Baños F. G., Bordas J., Lowy J., Svensson A. Small segmental rearrangements in the myosin head can explain force generation in muscle. Biophys J. 1996 Aug;71(2):576–589. doi: 10.1016/S0006-3495(96)79292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P. G. Method for the determination of myosin head orientation from EPR spectra. Biophys J. 1994 Jun;66(6):2039–2050. doi: 10.1016/S0006-3495(94)80998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambly B., Franks K., Cooke R. Orientation of spin-labeled light chain-2 exchanged onto myosin cross-bridges in glycerinated muscle fibers. Biophys J. 1991 Jan;59(1):127–138. doi: 10.1016/S0006-3495(91)82205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S., Akasaka K., Konrad M., Goody R., Holmes K., Wade-Jardetzky N., Jardetzky O. Internal motions in myosin. Biochemistry. 1979 Sep 18;18(19):4238–4244. doi: 10.1021/bi00586a031. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Ligand-induced myosin subfragment 1 global conformational change. Biochemistry. 1990 May 1;29(17):4087–4093. doi: 10.1021/bi00469a010. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Myosin subfragment 1 has tertiary structural domains. Biochemistry. 1986 Apr 22;25(8):2237–2242. doi: 10.1021/bi00356a058. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Myosin-ATP chemomechanics. Biochemistry. 1993 Mar 16;32(10):2455–2458. doi: 10.1021/bi00061a001. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Transient electrical birefringence characterization of heavy meromyosin. Biochemistry. 1985 Aug 27;24(18):4917–4924. doi: 10.1021/bi00339a029. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Murphy A. J. Electrostatic changes at the actomyosin-subfragment 1 interface during force-generating reactions. Biochemistry. 1992 Jan 21;31(2):385–389. doi: 10.1021/bi00117a011. [DOI] [PubMed] [Google Scholar]
- Ling N., Shrimpton C., Sleep J., Kendrick-Jones J., Irving M. Fluorescent probes of the orientation of myosin regulatory light chains in relaxed, rigor, and contracting muscle. Biophys J. 1996 Apr;70(4):1836–1846. doi: 10.1016/S0006-3495(96)79749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Waller G. S., Trybus K. M. Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature. 1993 Sep 30;365(6445):454–456. doi: 10.1038/365454a0. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Schneider D. K., Stone D. B. Conformations of myosin subfragment 1 ATPase intermediates from neutron and X-ray scattering. J Mol Biol. 1996 Feb 16;256(1):1–7. doi: 10.1006/jmbi.1996.0063. [DOI] [PubMed] [Google Scholar]
- Naber N., Cooke R. Mobility and orientation of spin probes attached to nucleotides incorporated into actin. Biochemistry. 1994 Apr 5;33(13):3855–3861. doi: 10.1021/bi00179a009. [DOI] [PubMed] [Google Scholar]
- Nauss K. M., Kitagawa S., Gergely J. Pyrophosphate binding to and adenosine triphosphatase activity of myosin and its proteolytic fragments. Implications for the substructure of myosin. J Biol Chem. 1969 Feb 25;244(4):755–765. [PubMed] [Google Scholar]
- Ostap E. M., Thomas D. D. Rotational dynamics of spin-labeled F-actin during activation of myosin S1 ATPase using caged ATP. Biophys J. 1991 Jun;59(6):1235–1241. doi: 10.1016/S0006-3495(91)82338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D., Fajer P. G. Orientation and dynamics of myosin heads in aluminum fluoride induced pre-power stroke states: an EPR study. Biochemistry. 1994 Oct 4;33(39):11993–11999. doi: 10.1021/bi00205a039. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Stone D. B., Schneider D. K., Huang Z., Mendelson R. A. The radius of gyration of native and reductively methylated myosin subfragment-1 from neutron scattering. Biophys J. 1995 Sep;69(3):767–776. doi: 10.1016/S0006-3495(95)79973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. W., Thomas D. D., Goldman Y. E. Transients in orientation of a fluorescent cross-bridge probe following photolysis of caged nucleotides in skeletal muscle fibres. J Mol Biol. 1992 Jan 5;223(1):185–203. doi: 10.1016/0022-2836(92)90725-y. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ramachandran S., Roopnarine O., Hayden D. W., Ostap E. M. The mechanism of force generation in myosin: a disorder-to-order transition, coupled to internal structural changes. Biophys J. 1995 Apr;68(4 Suppl):135S–141S. [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Tokunaga M., Kohno I., Sugimoto Y., Hamanaka T., Takezawa Y., Wakabayashi T., Amemiya Y. Small-angle synchrotron x-ray scattering reveals distinct shape changes of the myosin head during hydrolysis of ATP. Science. 1992 Oct 16;258(5081):443–447. doi: 10.1126/science.1411537. [DOI] [PubMed] [Google Scholar]
- Waller G. S., Ouyang G., Swafford J., Vibert P., Lowey S. A minimal motor domain from chicken skeletal muscle myosin. J Biol Chem. 1995 Jun 23;270(25):15348–15352. doi: 10.1074/jbc.270.25.15348. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Whittaker M., Wilson-Kubalek E. M., Smith J. E., Faust L., Milligan R. A., Sweeney H. L. A 35-A movement of smooth muscle myosin on ADP release. Nature. 1995 Dec 14;378(6558):748–751. doi: 10.1038/378748a0. [DOI] [PubMed] [Google Scholar]


